Characteristics of the Protocols Used in Electrical Pulse Stimulation of Cultured Cells for Mimicking In Vivo Exercise: A Systematic Review, Meta-Analysis, and Meta-Regression
Abstract
1. Introduction
2. Methods
2.1. Searching Process
2.2. Data Extraction
2.3. Meta-Analyses
Metanalysis and Meta-Regression
3. Results
3.1. General Description of Models
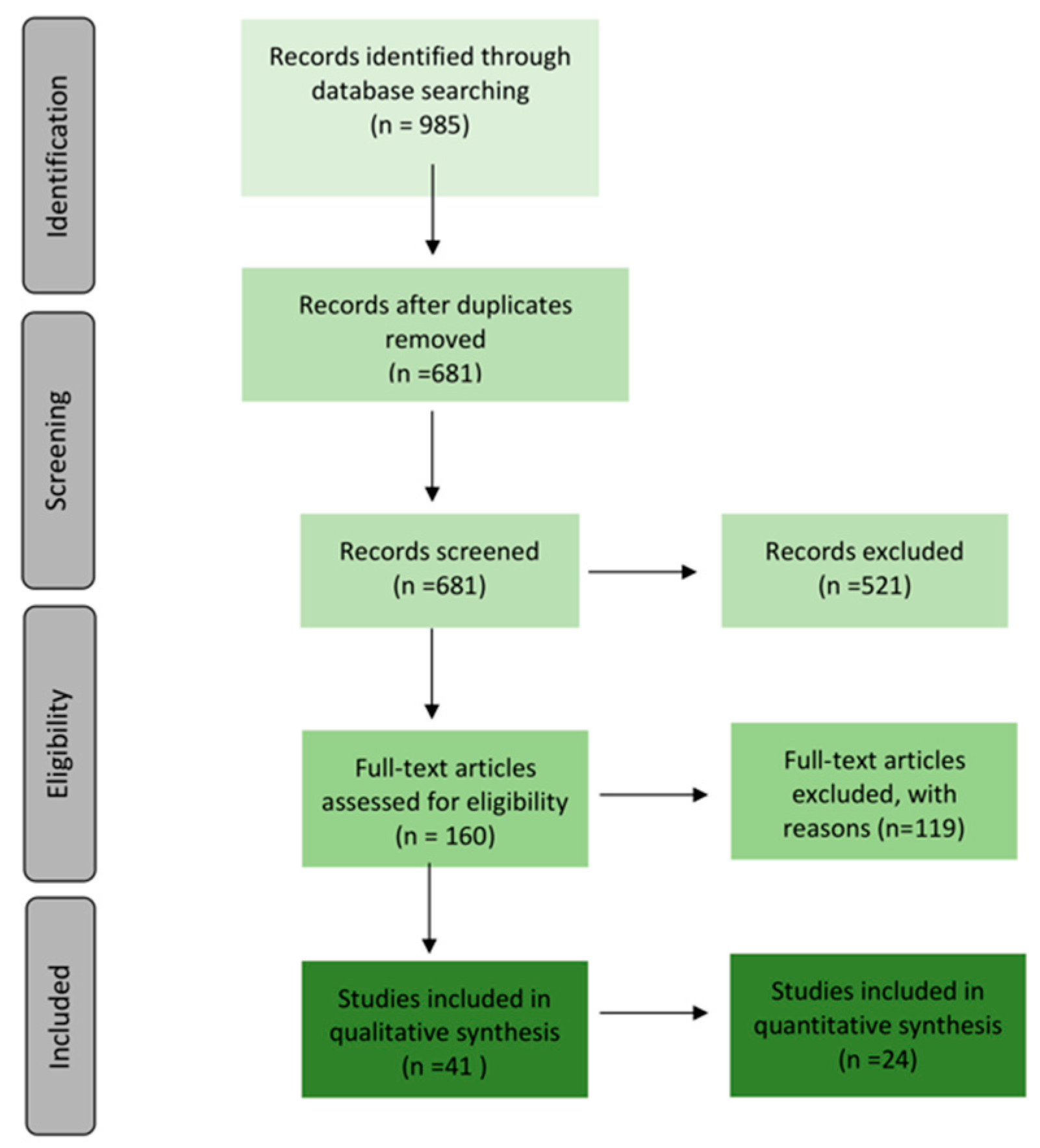
3.1.1. Searching and Selection
3.1.2. Cell Types and Pulse-Stimulator Types
3.2. In Vitro Types of Exercise
3.2.1. Acute and Chronic Exercise
3.2.2. Aerobic, Resistance, and Endurance Training
3.2.3. High-Intensity and Moderate Activity
3.3. In Vivo vs. In Vitro
3.4. Biological Parameters
3.4.1. AMPK Signalling
3.4.2. Glucose Metabolism
3.4.3. Akt Signalling
3.4.4. IL-6 as a Myokine
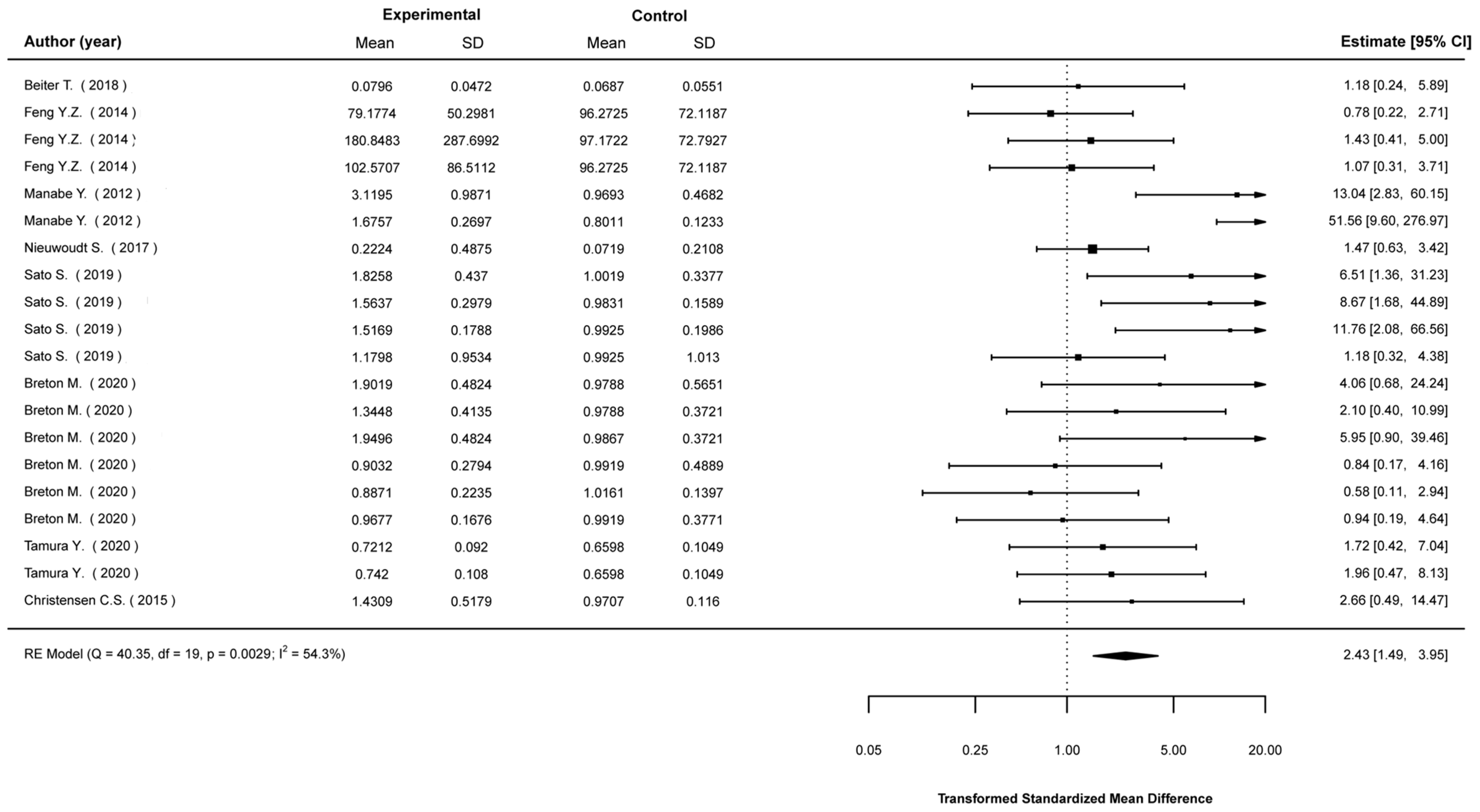
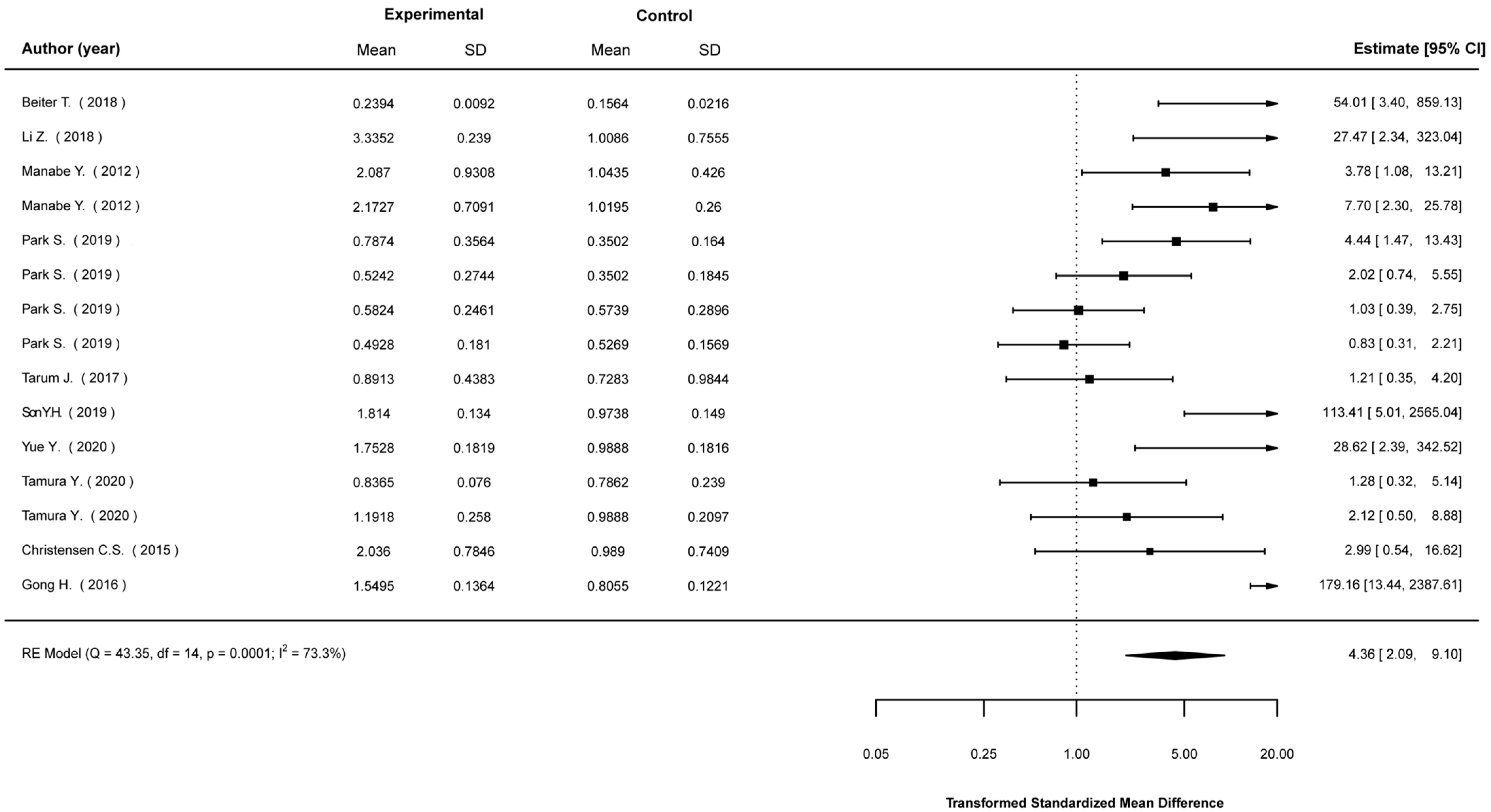
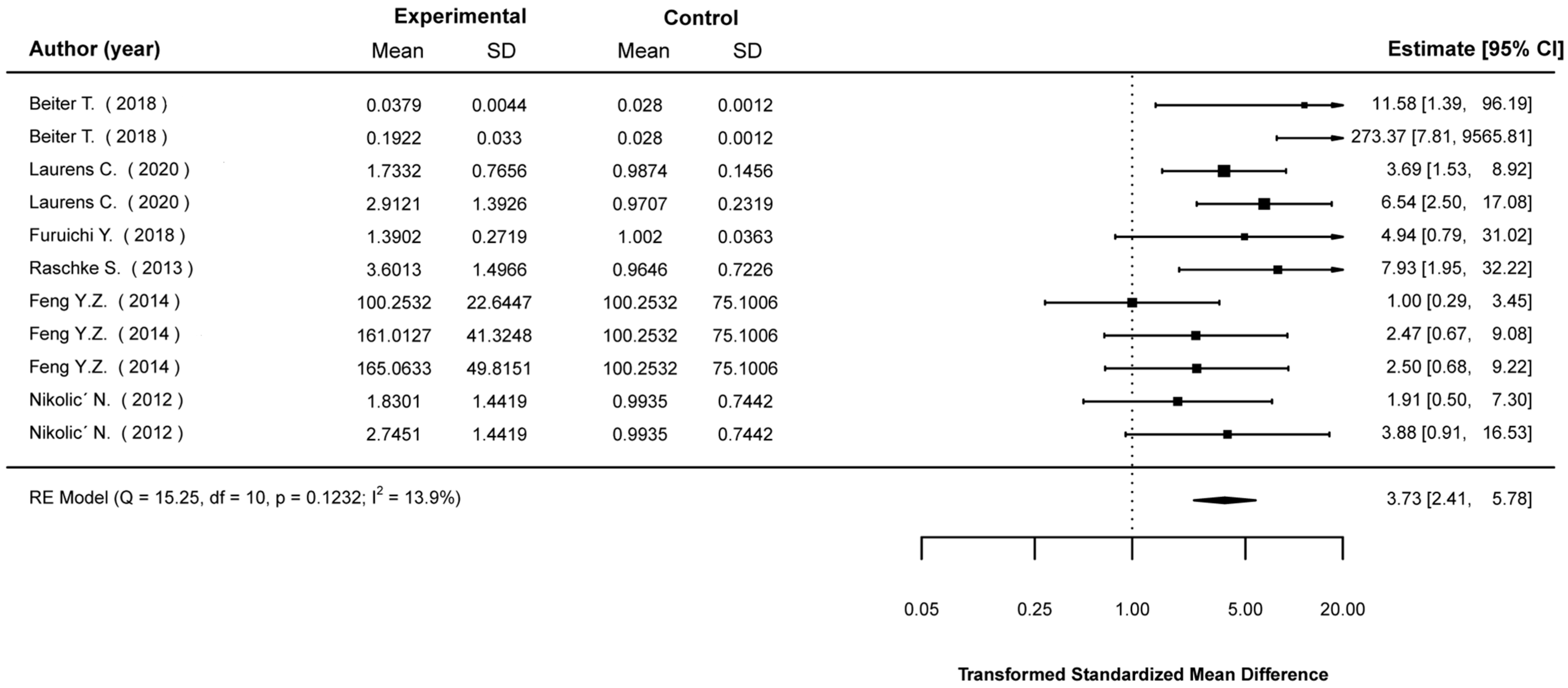
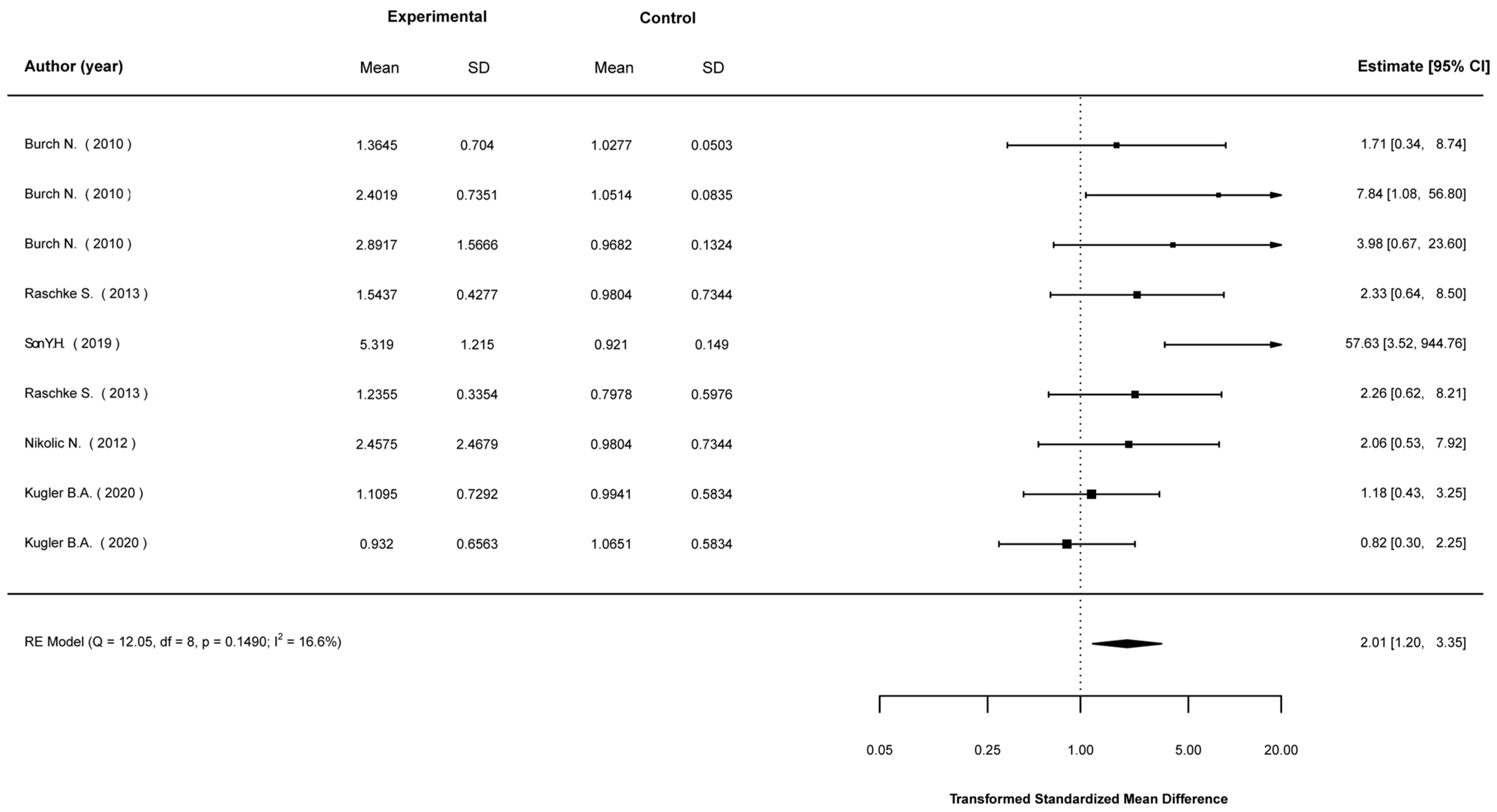
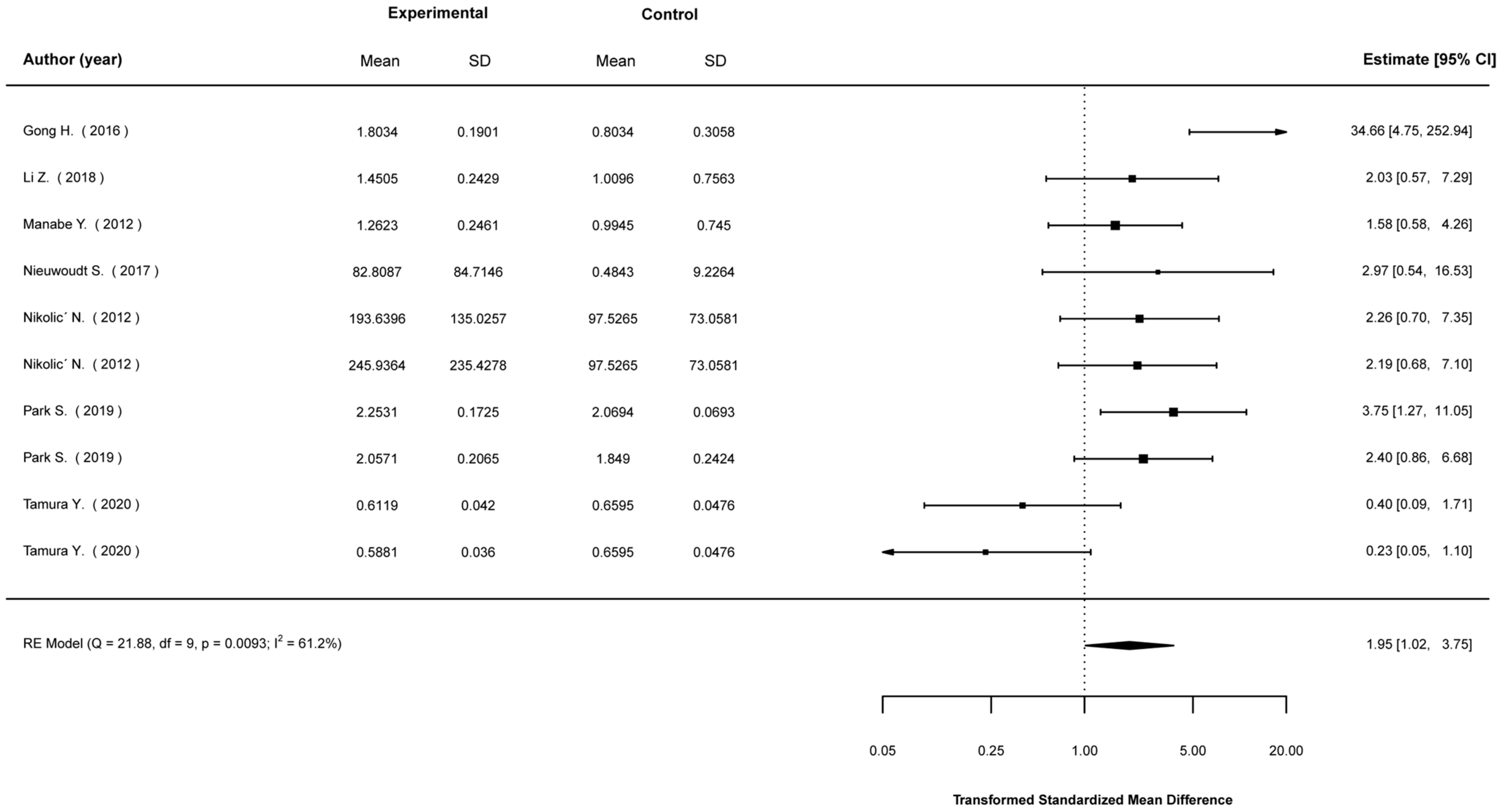
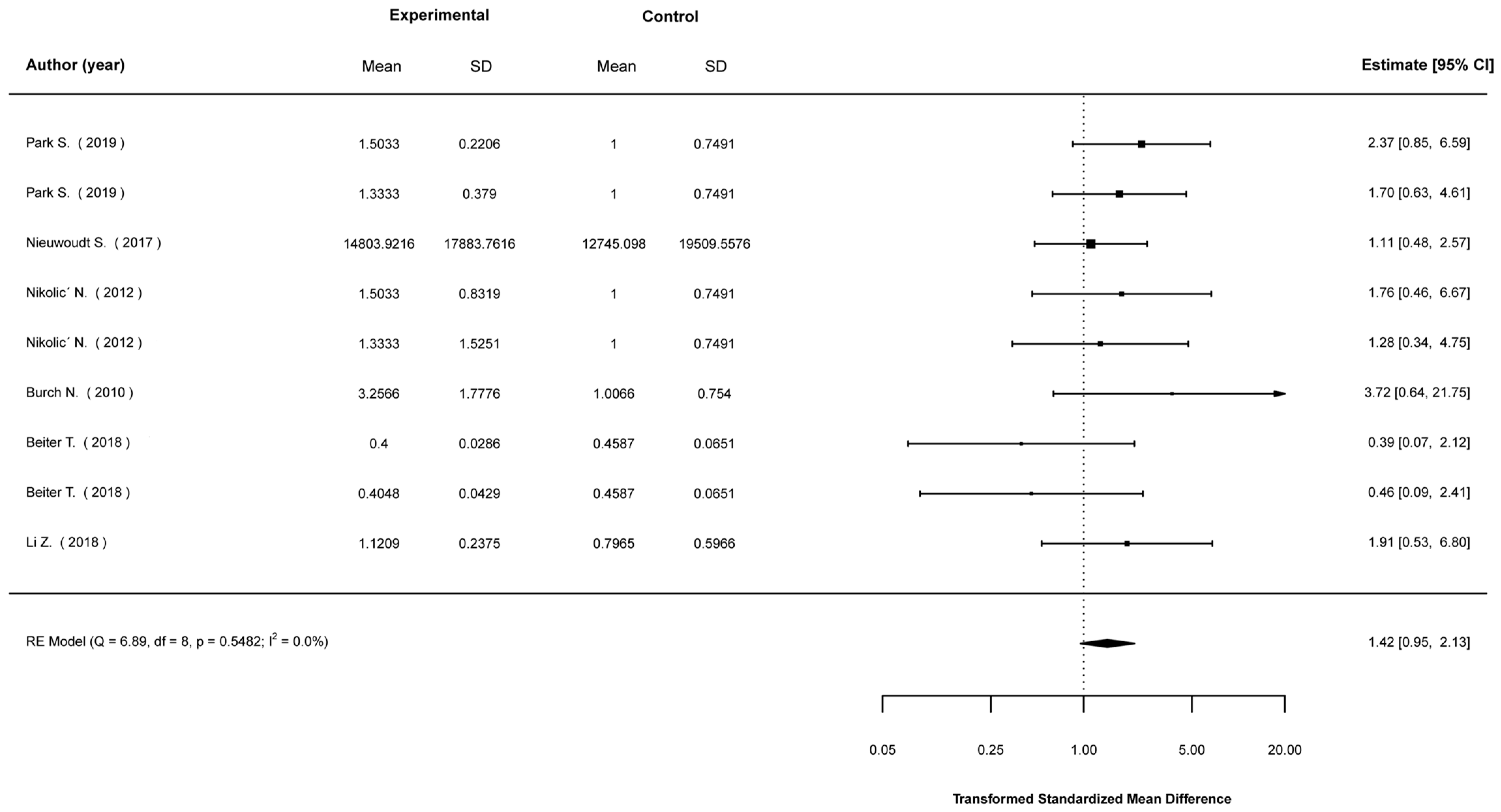
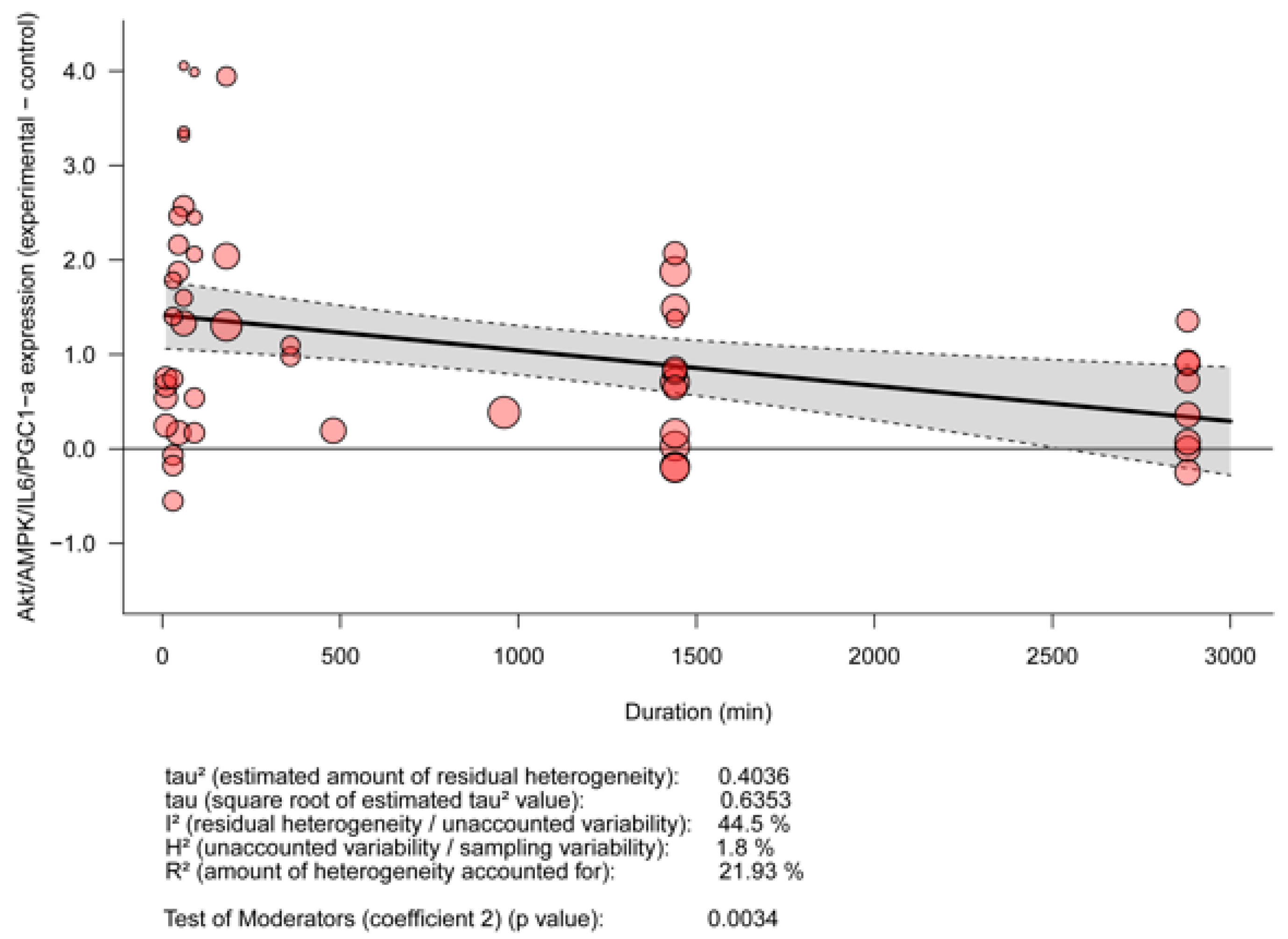
3.5. Meta-Analyses
3.5.1. Mean Differences in Biological Indices between Stimulated and Non-Stimulated Cells
3.5.2. Meta-Regression for the Effect of EPS Depending on Stimulation Duration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pudkasam, S.; Tangalakis, K.; Chinlumprasert, N.; Apostolopoulos, V.; Stojanovska, L. Breast cancer and exercise: The role of adiposity and immune markers. Maturitas 2017, 105, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.T.; Beck, B.R. Exercise, Osteoporosis, and Bone Geometry. Sports 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.A.; Thom, J.M.; Rye, K.-A.; Parmenter, B.J. Aerobic, resistance or combined training: A systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis 2018, 274, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Flouris, A.D.; Bouziotas, C.; Christodoulos, A.D.; Koutedakis, Y. Longitudinal preventive-screening cutoffs for metabolic syndrome in adolescents. Int. J. Obes. 2008, 32, 1506–1512. [Google Scholar] [CrossRef]
- Muscella, A.; Stefàno, E.; Marsigliante, S. The effects of exercise training on lipid metabolism and coronary heart disease. Am. J. Physiol. -Heart Circ. Physiol. 2020, 319, H76–H88. [Google Scholar] [CrossRef]
- da Costa Daniele, T.M.; de Bruin, P.F.C.; de Matos, R.S.; de Bruin, G.S.; Maia Chaves, C.; de Bruin, V.M.S. Exercise effects on brain and behavior in healthy mice, Alzheimer’s disease and Parkinson’s disease model—A systematic review and meta-analysis. Behav. Brain Res. 2020, 383, 112488. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J. Sport Health Sci. 2021, 10, 201–210. [Google Scholar] [CrossRef]
- Nintou, E.; Karligiotou, E.; Vliora, M.; Fatouros, I.G.; Jamurtas, A.Z.; Sakellaridis, N.; Dimas, K.; Flouris, A.D. Effects of In Vitro Muscle Contraction on Thermogenic Protein Levels in Co-Cultured Adipocytes. Life 2021, 11, 1227. [Google Scholar] [CrossRef]
- Lambernd, S.; Taube, A.; Schober, A.; Platzbecker, B.; Gorgens, S.W.; Schlich, R.; Jeruschke, K.; Weiss, J.; Eckardt, K.; Eckel, J. Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia 2012, 55, 1128–1139. [Google Scholar] [CrossRef]
- Song, Y.; Soto, J.; Chen, B.; Yang, L.; Li, S. Cell engineering: Biophysical regulation of the nucleus. Biomaterials 2020, 234, 119743. [Google Scholar] [CrossRef] [PubMed]
- Orfanos, Z.; Godderz, M.P.; Soroka, E.; Godderz, T.; Rumyantseva, A.; van der Ven, P.F.; Hawke, T.J.; Furst, D.O. Breaking sarcomeres by in vitro exercise. Sci. Rep. 2016, 6, 19614. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Eckardt, K.; Bjorklund Holven, K.; Jensen, J.; Eckel, J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS ONE 2013, 8, e62008. [Google Scholar] [CrossRef] [PubMed]
- Evers-van Gogh, I.J.; Alex, S.; Stienstra, R.; Brenkman, A.B.; Kersten, S.; Kalkhoven, E. Electric Pulse Stimulation of Myotubes as an In Vitro Exercise Model: Cell-Mediated and Non-Cell-Mediated Effects. Sci. Rep. 2015, 5, 10944. [Google Scholar] [CrossRef]
- Banan Sadeghian, R.; Ebrahimi, M.; Salehi, S. Electrical stimulation of microengineered skeletal muscle tissue: Effect of stimulus parameters on myotube contractility and maturation. J. Tissue Eng. Regen. Med. 2018, 12, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Beiter, T.; Hudemann, J.; Burgstahler, C.; Niess, A.M.; Munz, B. Effects of extracellular orotic acid on acute contraction-induced adaptation patterns in C2C12 cells. Mol. Cell Biochem. 2018, 448, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Burch, N.; Arnold, A.S.; Item, F.; Summermatter, S.; Brochmann Santana Santos, G.; Christe, M.; Boutellier, U.; Toigo, M.; Handschin, C. Electric pulse stimulation of cultured murine muscle cells reproduces gene expression changes of trained mouse muscle. PLoS ONE 2010, 5, e10970. [Google Scholar] [CrossRef]
- Chaves, A.B.; Miranda, E.R.; Mey, J.T.; Blackburn, B.K.; Fuller, K.N.Z.; Stearns, B.; Ludlow, A.; Williamson, D.L.t.; Houmard, J.A.; Haus, J.M. Exercise reduces the protein abundance of TXNIP and its interacting partner REDD1 in skeletal muscle: Potential role for a PKA-mediated mechanism. J. Appl. Physiol. 2022, 132, 357–366. [Google Scholar] [CrossRef]
- Christensen, C.S.; Christensen, D.P.; Lundh, M.; Dahllof, M.S.; Haase, T.N.; Velasquez, J.M.; Laye, M.J.; Mandrup-Poulsen, T.; Solomon, T.P. Skeletal Muscle to Pancreatic beta-Cell Cross-talk: The Effect of Humoral Mediators Liberated by Muscle Contraction and Acute Exercise on beta-Cell Apoptosis. J. Clin. Endocrinol. Metab. 2015, 100, E1289–E1298. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Nikolic, N.; Bakke, S.S.; Kase, E.T.; Guderud, K.; Hjelmesaeth, J.; Aas, V.; Rustan, A.C.; Thoresen, G.H. Myotubes from lean and severely obese subjects with and without type 2 diabetes respond differently to an in vitro model of exercise. Am J. Physiol. Cell Physiol. 2015, 308, C548–C556. [Google Scholar] [CrossRef]
- Nikolic, N.; Bakke, S.S.; Kase, E.T.; Rudberg, I.; Flo Halle, I.; Rustan, A.C.; Thoresen, G.H.; Aas, V. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS ONE 2012, 7, e33203. [Google Scholar] [CrossRef]
- MCARDLE, A. Contractile activity-induced oxidative stress: Cellular origin and adaptive responses. Am. J. Physiol. Cell Physiol. 2001, 280, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Tarum, J.; Folkesson, M.; Atherton, P.J.; Kadi, F. Electrical pulse stimulation: An in vitro exercise model for the induction of human skeletal muscle cell hypertrophy. A proof-of-concept study. Exp. Physiol. 2017, 102, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Valero-Breton, M.; Warnier, G.; Castro-Sepulveda, M.; Deldicque, L.; Zbinden-Foncea, H. Acute and Chronic Effects of High Frequency Electric Pulse Stimulation on the Akt/mTOR Pathway in Human Primary Myotubes. Front. Bioeng. Biotechnol. 2020, 8, 565679. [Google Scholar] [CrossRef] [PubMed]
- Laurens, C.; Parmar, A.; Murphy, E.; Carper, D.; Lair, B.; Maes, P.; Vion, J.; Boulet, N.; Fontaine, C.; Marquès, M.; et al. Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight 2020, 5, e131870. [Google Scholar] [CrossRef]
- Lambertucci, R.H.; Silveira Ldos, R.; Hirabara, S.M.; Curi, R.; Sweeney, G.; Pithon-Curi, T.C. Effects of moderate electrical stimulation on reactive species production by primary rat skeletal muscle cells: Cross talk between superoxide and nitric oxide production. J. Cell Physiol. 2012, 227, 2511–2518. [Google Scholar] [CrossRef]
- Nikolić, N.; Görgens, S.W.; Thoresen, G.H.; Aas, V.; Eckel, J.; Eckardt, K. Electrical pulse stimulation of cultured skeletal muscle cells as a model for in vitro exercise—Possibilities and limitations. Acta Physiol. 2017, 220, 310–331. [Google Scholar] [CrossRef]
- Manabe, Y.; Miyatake, S.; Takagi, M.; Nakamura, M.; Okeda, A.; Nakano, T.; Hirshman, M.F.; Goodyear, L.J.; Fujii, N.L. Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PLoS ONE 2012, 7, e52592. [Google Scholar] [CrossRef]
- Park, S.; Turner, K.D.; Zheng, D.; Brault, J.J.; Zou, K.; Chaves, A.B.; Nielsen, T.S.; Tanner, C.J.; Treebak, J.T.; Houmard, J.A. Electrical pulse stimulation induces differential responses in insulin action in myotubes from severely obese individuals. J. Physiol. 2019, 597, 449–466. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, C.; Zhang, X.; Zhang, S.; Liu, Q.; Hu, F.; Lv, X.; Li, H.; Yang, J.; Wang, X.; et al. An AMPK/Axin1-Rac1 signaling pathway mediates contraction-regulated glucose uptake in skeletal muscle cells. Am. J. Physiol. -Endocrinol. Metab. 2020, 318, E330–E342. [Google Scholar] [CrossRef]
- Gong, H.; Liu, L.; Ni, C.X.; Zhang, Y.; Su, W.J.; Lian, Y.J.; Peng, W.; Zhang, J.P.; Jiang, C.L. Dexamethasone rapidly inhibits glucose uptake via non-genomic mechanisms in contracting myotubes. Arch. Biochem. Biophys. 2016, 603, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.G.; Voltarelli, V.A.; Tobias, G.C.; de Souza, L.; Borges, G.S.; Paixão, A.O.; de Almeida, N.R.; Bowen, T.S.; Demasi, M.; Miyabara, E.H.; et al. Aerobic Exercise Training and In Vivo Akt Activation Counteract Cancer Cachexia by Inducing a Hypertrophic Profile through eIF-2α Modulation. Cancers 2022, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A. WebPlotDigitizer; Version 4.5; GitHub, Inc.: San Francisco, CA, USA, 2021. [Google Scholar]
- Bonett, D.G. Meta-analytic interval estimation for standardized and unstandardized mean differences. Psychol. Methods 2009, 14, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.J.; Butcher, I.; Assi, V.; Lewis, S.C.; Murray, G.D.; Langhorne, P.; Brady, M.C. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: A systematic review. BMC Med. Res. Methodol. 2018, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.; Solomon, T.P.J. Conditioned media from contracting skeletal muscle potentiates insulin secretion and enhances mitochondrial energy metabolism of pancreatic beta-cells. Metabolism 2019, 91, 1–9. [Google Scholar] [CrossRef]
- Connor, M.K.; Irrcher, I.; Hood, D.A. Contractile activity-induced transcriptional activation of cytochrome C involves Sp1 and is proportional to mitochondrial ATP synthesis in C2C12 muscle cells. J. Biol. Chem. 2001, 276, 15898–15904. [Google Scholar] [CrossRef]
- Fernandez-Verdejo, R.; Vanwynsberghe, A.M.; Hai, T.; Deldicque, L.; Francaux, M. Activating transcription factor 3 regulates chemokine expression in contracting C2C12 myotubes and in mouse skeletal muscle after eccentric exercise. Biochem. Biophys. Res. Commun. 2017, 492, 249–254. [Google Scholar] [CrossRef]
- Fujita, H.; Shimizu, K.; Nagamori, E. Novel method for measuring active tension generation by C2C12 myotube using UV-crosslinked collagen film. Biotechnol. Bioeng. 2010, 106, 482–489. [Google Scholar] [CrossRef]
- Furuichi, Y.; Manabe, Y.; Takagi, M.; Aoki, M.; Fujii, N.L. Evidence for acute contraction-induced myokine secretion by C2C12 myotubes. PLoS ONE 2018, 13, e0206146. [Google Scholar] [CrossRef]
- Guigni, B.A.; Fix, D.K.; Bivona, J.J., 3rd; Palmer, B.M.; Carson, J.A.; Toth, M.J. Electrical stimulation prevents doxorubicin-induced atrophy and mitochondrial loss in cultured myotubes. Am. J. Physiol. -Cell Physiol. 2019, 317, C1213–C1228. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Warabi, E.; Komine, S.; Oh, S.; Shoda, J. Cytoprotective Role of Nrf2 in Electrical Pulse Stimulated C2C12 Myotube. PLoS ONE 2015, 10, e0144835. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Byun, W.S.; Kang, M.J.; Han, J.A.; Moon, J.; Shin, M.J.; Lee, H.J.; Chung, J.H.; Lee, J.S.; Son, C.G.; et al. The myokine meteorin-like (metrnl) improves glucose tolerance in both skeletal muscle cells and mice by targeting AMPKalpha2. FEBS J. 2020, 287, 2087–2104. [Google Scholar] [CrossRef]
- Li, Z.; Yue, Y.; Hu, F.; Zhang, C.; Ma, X.; Li, N.; Qiu, L.; Fu, M.; Chen, L.; Yao, Z.; et al. Electrical pulse stimulation induces GLUT4 translocation in C2C12 myotubes that depends on Rab8A, Rab13, and Rab14. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E478–E493. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.R.W.; Turner, M.C.; Farrington, R.; Player, D.J.; Lewis, M.P. Leucine elicits myotube hypertrophy and enhances maximal contractile force in tissue engineered skeletal muscle in vitro. J. Cell Physiol. 2017, 232, 2788–2797. [Google Scholar] [CrossRef]
- Nakamura, T.; Takagi, S.; Okuzaki, D.; Matsui, S.; Fujisato, T. Hypoxia transactivates cholecystokinin gene expression in 3D-engineered muscle. J. Biosci. Bioeng. 2021, 132, 64–70. [Google Scholar] [CrossRef]
- Nieuwoudt, S.; Mulya, A.; Fealy, C.E.; Martelli, E.; Dasarathy, S.; Naga Prasad, S.V.; Kirwan, J.P. In vitro contraction protects against palmitate-induced insulin resistance in C2C12 myotubes. Am. J. Physiol. Cell Physiol. 2017, 313, C575–C583. [Google Scholar] [CrossRef]
- Pattamaprapanont, P.; Garde, C.; Fabre, O.; Barres, R. Muscle Contraction Induces Acute Hydroxymethylation of the Exercise-Responsive Gene Nr4a3. Front. Endocrinol. 2016, 7, 165. [Google Scholar] [CrossRef]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisloff, U.; Tjonna, A.E.; Raastad, T.; et al. Evidence against a beneficial effect of irisin in humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef]
- Small, L.; Altintas, A.; Laker, R.C.; Ehrlich, A.; Pattamaprapanont, P.; Villarroel, J.; Pillon, N.J.; Zierath, J.R.; Barres, R. Contraction influences Per2 gene expression in skeletal muscle through a calcium-dependent pathway. J. Physiol. 2020, 598, 5739–5752. [Google Scholar] [CrossRef]
- Son, Y.H.; Lee, S.M.; Lee, S.H.; Yoon, J.H.; Kang, J.S.; Yang, Y.R.; Kwon, K.S. Comparative molecular analysis of endurance exercise in vivo with electrically stimulated in vitro myotube contraction. J. Appl. Physiol. 2019, 127, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Kouzaki, K.; Kotani, T.; Nakazato, K. Electrically stimulated contractile activity-induced transcriptomic responses and metabolic remodeling in C2C12 myotubes: Twitch vs. tetanic contractions. Am. J. Physiol. Cell Physiol. 2020, 319, C1029–C1044. [Google Scholar] [CrossRef] [PubMed]
- Thelen, M.H.M. Electrical stimulation of C2C12 myotubes induces contractions and represses thyroid-hormone-dependent transcription of the fast-type sarcoplasmic-reticulum Ca2+-ATPase gene. Biochem. J. 1997, 321, 845–848. [Google Scholar] [CrossRef]
- Sato, S.; Nomura, M.; Yamana, I.; Uchiyama, A.; Furuichi, Y.; Manabe, Y.; Fujii, N.L. A new in vitro muscle contraction model and its application for analysis of mTORC1 signaling in combination with contraction and beta-hydroxy-beta-methylbutyrate administration. Biosci. Biotechnol. Biochem. 2019, 83, 1851–1857. [Google Scholar] [CrossRef]
- Pattwell, D.M.; McArdle, A.; Morgan, J.E.; Patridge, T.A.; Jackson, M.J. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic. Biol. Med. 2004, 37, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Broholm, C.; Laye, M.J.; Brandt, C.; Vadalasetty, R.; Pilegaard, H.; Pedersen, B.K.; Scheele, C. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J. Appl. Physiol. 2011, 111, 251–259. [Google Scholar] [CrossRef]
- Kugler, B.A.; Deng, W.; Francois, B.; Anderson, M.; Hinkley, J.M.; Houmard, J.A.; Gona, P.N.; Zou, K. Distinct Adaptations of Mitochondrial Dynamics to Electrical Pulse Stimulation in Lean and Severely Obese Primary Myotubes. Med. Sci. Sports Exerc. 2021, 53, 1151–1160. [Google Scholar] [CrossRef]
- Løvsletten, N.; Rustan, A.; Laurens, C.; Thoresen, H.; Moro, C.; Nikolić, N. Primary defects in lipid handling and resistance to exercise in myotubes from obese donors with and without type 2 diabetes. Appl. Physiol. Nutr. Metab. 2019, 45, 169–179. [Google Scholar] [CrossRef]
- Scheler, M.; de Angelis, M.H.; Al-Hasani, H.; Haring, H.U.; Weigert, C.; Lehr, S. Methods for proteomics-based analysis of the human muscle secretome using an in vitro exercise model. Methods Mol. Biol. 2015, 1295, 55–64. [Google Scholar] [CrossRef]
- Kubis, H.P.; Scheibe, R.J.; Meissner, J.D.; Hornung, G.; Gros, G. Fast-to-slow transformation and nuclear import/export kinetics of the transcription factor NFATc1 during electrostimulation of rabbit muscle cells in culture. J. Physiol. 2002, 541, 835–847. [Google Scholar] [CrossRef]
- Miyatake, S.; Bilan, P.J.; Pillon, N.J.; Klip, A. Contracting C2C12 myotubes release CCL2 in an NF-κB-dependent manner to induce monocyte chemoattraction. Am. J. Physiol. -Endocrinol. Metab. 2016, 310, E160–E170. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Ruderman, N.B. AMPK and the biochemistry of exercise: Implications for human health and disease. Biochem. J. 2009, 418, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. Exercise-stimulated glucose uptake—Regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017, 13, 133–148. [Google Scholar] [CrossRef]
- Mann, G.; Riddell, M.C.; Adegoke, O.A.J. Effects of Acute Muscle Contraction on the Key Molecules in Insulin and Akt Signaling in Skeletal Muscle in Health and in Insulin Resistant States. Diabetology 2022, 3, 423–446. [Google Scholar] [CrossRef]
- Munoz-Canoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Neufer, P.D.; Bamman, M.M.; Muoio, D.M.; Bouchard, C.; Cooper, D.M.; Goodpaster, B.H.; Booth, F.W.; Kohrt, W.M.; Gerszten, R.E.; Mattson, M.P.; et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metabolism. 2015, 22, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Muise, E.S.; Guan, H.-P.; Liu, J.; Nawrocki, A.R.; Yang, X.; Wang, C.; Rodríguez, C.G.; Zhou, D.; Gorski, J.N.; Kurtz, M.M.; et al. Pharmacological AMPK activation induces transcriptional responses congruent to exercise in skeletal and cardiac muscle, adipose tissues and liver. PLoS ONE 2019, 14, e0211568. [Google Scholar] [CrossRef]
- Sakamoto, K.; Arnolds, D.E.W.; Ekberg, I.; Thorell, A.; Goodyear, L.J. Exercise regulates Akt and glycogen synthase kinase-3 activities in human skeletal muscle. Biochem. Biophys. Res. Commun. 2004, 319, 419–425. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Wolsk-Petersen, E.; Febbraio, M. The metabolic role of IL-6 produced during exercise: Is IL-6 an exercise factor? Proc. Nutr. Soc. 2004, 63, 263–267. [Google Scholar] [CrossRef]
- Uguccioni, G.; D’Souza, D.; Hood, D.A. Regulation of PPARγ Coactivator-1α Function and Expression in Muscle: Effect of Exercise. PPAR Res. 2010, 2010, 937123. [Google Scholar] [CrossRef]
- O’Neill, H.M. AMPK and Exercise: Glucose Uptake and Insulin Sensitivity. Diabetes Metab. J. 2013, 37, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Falasca, M.; Blough, E.R. Akt/protein kinase B in skeletal muscle physiology and pathology. J. Cell. Physiol. 2011, 226, 29–36. [Google Scholar] [CrossRef]
- Chowdhury, S.; Schulz, L.; Palmisano, B.; Singh, P.; Berger, J.M.; Yadav, V.K.; Mera, P.; Ellingsgaard, H.; Hidalgo, J.; Brüning, J.; et al. Muscle-derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J. Clin. Investig. 2020, 130, 2888–2902. [Google Scholar] [CrossRef] [PubMed]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an energy allocator in muscle tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance. Exerc. Immunol. Rev. 2006, 12, 41. [Google Scholar]
- Röhling, M.; Herder, C.; Stemper, T.; Müssig, K. Influence of Acute and Chronic Exercise on Glucose Uptake. J. Diabetes Res. 2016, 2016, 2868652. [Google Scholar] [CrossRef]
- Lee, I.H.; Lee, Y.J.; Seo, H.; Kim, Y.S.; Nam, J.O.; Jeon, B.D.; Kwon, T.D. Study of Muscle Contraction Induced by Electrical Pulse Stimulation and Nitric Oxide in C2c12 Myotube Cells. J. Exerc. Nutr. Biochem. 2018, 22, 22–28. [Google Scholar] [CrossRef]

| Author, Date | Type of Exercise as Defined by the Study Authors | Duration of In Vitro Exercise | In Vivo Protocol | Organism |
|---|---|---|---|---|
| Burch, 2010 [17] | Acute, intermittent, continuous | 90 min = acute, 90 min/4 days = intermittent, 24 h = continuous | Treadmill, at 75% of average distance of exhaustion trial (4 days training, 1 day exhaustion, 2 days rest), 6 weeks total | Mice |
| Fernandez-Verdejo, 2017 [39] | Endurance exercise | 240 min | Treadmill until exhaustion | Mice |
| Lee, 2020 [44] | Acute and chronic exercise | Acute = 1, 3, 6 h chronic = 12, 24, or 36 h | Treadmill 60 min, 5 d/week, 10 m/min | Mice |
| McArdle, 2001 [22] | Aerobic activity | 15 min | ||
| Pattamaprapanont, 2016 [49] | Acute exercise | 30 min | Cycle ergometer at 80% VO2max, 15 min | Healthy males |
| Raschke, 2013 [13] | Regular exercise | 4 to 24 h | Cycle ergometer at 70% VO2max, 60 min | Healthy males |
| Raschke, 2013 [50] | Training model/in humans endurance training | 24 h | Treadmill, at 90% of peak heart rate, 3 d/week for 10 weeks | Healthy males |
| Son, 2019 [52] | Mild endurance exercise | 60 min | Volunteer wheel running daily for 4 weeks | Mice |
| Yue, 2020 [30] | Acute exercise | 60 min | Treadmill, at 75% VO2max, 60 min | Mice |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nintou, E.; Karligiotou, E.; Vliora, M.; Ioannou, L.G.; Flouris, A.D. Characteristics of the Protocols Used in Electrical Pulse Stimulation of Cultured Cells for Mimicking In Vivo Exercise: A Systematic Review, Meta-Analysis, and Meta-Regression. Int. J. Mol. Sci. 2022, 23, 13446. https://doi.org/10.3390/ijms232113446
Nintou E, Karligiotou E, Vliora M, Ioannou LG, Flouris AD. Characteristics of the Protocols Used in Electrical Pulse Stimulation of Cultured Cells for Mimicking In Vivo Exercise: A Systematic Review, Meta-Analysis, and Meta-Regression. International Journal of Molecular Sciences. 2022; 23(21):13446. https://doi.org/10.3390/ijms232113446
Chicago/Turabian StyleNintou, Eleni, Eleni Karligiotou, Maria Vliora, Leonidas G. Ioannou, and Andreas D. Flouris. 2022. "Characteristics of the Protocols Used in Electrical Pulse Stimulation of Cultured Cells for Mimicking In Vivo Exercise: A Systematic Review, Meta-Analysis, and Meta-Regression" International Journal of Molecular Sciences 23, no. 21: 13446. https://doi.org/10.3390/ijms232113446
APA StyleNintou, E., Karligiotou, E., Vliora, M., Ioannou, L. G., & Flouris, A. D. (2022). Characteristics of the Protocols Used in Electrical Pulse Stimulation of Cultured Cells for Mimicking In Vivo Exercise: A Systematic Review, Meta-Analysis, and Meta-Regression. International Journal of Molecular Sciences, 23(21), 13446. https://doi.org/10.3390/ijms232113446









