Roles of LonP1 in Oral-Maxillofacial Developmental Defects and Tumors: A Novel Insight
Abstract
1. Introduction
2. LonP1 Discovery, Structure and Modulation
2.1. LonP1 Discovery
2.2. LonP1 Structure
2.3. LonP1 Modulation
3. Physiological Functions of LonP1
3.1. LonP1 as a Protease
3.2. LonP1 as a Molecular Chaperone
3.3. LonP1 as a Mitochondrial DNA-Binding Protein
3.4. LonP1 and Mitochondrial Function
3.4.1. LonP1 and Energy Metabolism
3.4.2. LonP1, Mitochondrial Dynamics, and Mitophagy
4. LonP1 in the Oral and Maxillofacial Regions
4.1. LonP1 in Oral and Maxillofacial Tumorigenesis
4.1.1. Roles of LonP1 in Oral and Maxillofacial Tumorigenesis
4.1.2. Mechanisms of LonP1 in Oral and Maxillofacial Tumorigenesis
4.1.3. LonP1 as a Target for Anticancer Therapy
4.2. LonP1 in Oral and Maxillofacial Development
4.2.1. Roles of LonP1 in Oral and Maxillofacial Development
4.2.2. Possible Mechanisms of Action of LonP1 in Oral and Maxillofacial Development
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waxman, L.; Goldberg, A.L. Protease La from Escherichia coli hydrolyzes ATP and proteins in a linked fashion. Proc. Natl. Acad. Sci. USA 1982, 79, 4883–4887. [Google Scholar] [CrossRef]
- Strauss, K.A.; Jinks, R.N.; Puffenberger, E.G.; Venkatesh, S.; Singh, K.; Cheng, I.; Mikita, N.; Thilagavathi, J.; Lee, J.; Sarafianos, S.; et al. CODAS syndrome is associated with mutations of LONP1, encoding mitochondrial AAA+ Lon protease. Am. J. Hum. Genet. 2015, 96, 121–135. [Google Scholar] [CrossRef]
- Cheng, C.W.; Kuo, C.Y.; Fan, C.C.; Fang, W.C.; Jiang, S.S.; Lo, Y.K.; Wang, T.Y.; Kao, M.C.; Lee, A.Y. Overexpression of Lon contributes to survival and aggressive phenotype of cancer cells through mitochondrial complex I-mediated generation of reactive oxygen species. Cell Death Dis. 2013, 4, e681. [Google Scholar] [CrossRef]
- Witkin, E.M. Inherited Differences in Sensitivity to Radiation in Escherichia Coli. Proc. Natl. Acad. Sci. USA 1946, 32, 59–68. [Google Scholar] [CrossRef]
- Howard-Flanders, P.; Simson, E.; Theriot, L. A locus that controls filament formation and sensitivity to radiation in Escherichia coli K-12. Genetics 1964, 49, 237–246. [Google Scholar] [CrossRef]
- Ward, D.E.; Shockley, K.R.; Chang, L.S.; Levy, R.D.; Michel, J.K.; Conners, S.B.; Kelly, R.M. Proteolysis in hyperthermophilic microorganisms. Archaea 2002, 1, 503191. [Google Scholar] [CrossRef]
- van Dijl, J.M.; Kutejová, E.; Suda, K.; Perecko, D.; Schatz, G.; Suzuki, C.K. The ATPase and protease domains of yeast mitochondrial Lon: Roles in proteolysis and respiration-dependent growth. Proc. Natl. Acad. Sci. USA 1998, 95, 10584–10589. [Google Scholar] [CrossRef]
- Rigas, S.; Daras, G.; Laxa, M.; Marathias, N.; Fasseas, C.; Sweetlove, L.J.; Hatzopoulos, P. Role of Lon1 protease in post-germinative growth and maintenance of mitochondrial function in Arabidopsis thaliana. New Phytol. 2009, 181, 588–600. [Google Scholar] [CrossRef]
- Wang, N.; Gottesman, S.; Willingham, M.C.; Gottesman, M.M.; Maurizi, M.R. A human mitochondrial ATP-dependent protease that is highly homologous to bacterial Lon protease. Proc. Natl. Acad. Sci. USA 1993, 90, 11247–11251. [Google Scholar] [CrossRef]
- Desautels, M.; Goldberg, A.L. Demonstration of an ATP-dependent, vanadate-sensitive endoprotease in the matrix of rat liver mitochondria. J. Biol. Chem. 1982, 257, 11673–11679. [Google Scholar] [CrossRef]
- Gottesman, S.; Wickner, S.; Maurizi, M.R. Protein quality control: Triage by chaperones and proteases. Genes Dev. 1997, 11, 815–823. [Google Scholar] [CrossRef]
- De Gaetano, A.; Gibellini, L.; Bianchini, E.; Borella, R.; De Biasi, S.; Nasi, M.; Boraldi, F.; Cossarizza, A.; Pinti, M. Impaired Mitochondrial Morphology and Functionality in Lonp1(wt/-) Mice. J. Clin. Med. 2020, 9, 1783. [Google Scholar] [CrossRef]
- Bota, D.A.; Davies, K.J. Mitochondrial Lon protease in human disease and aging: Including an etiologic classification of Lon-related diseases and disorders. Free Radic. Biol. Med. 2016, 100, 188–198. [Google Scholar] [CrossRef]
- Duman, R.E.; Löwe, J. Crystal Structures of Bacillus subtilis Lon Protease. J. Mol. Biol. 2010, 401, 653–670. [Google Scholar] [CrossRef]
- Rotanova, T.V.; Melnikov, E.E.; Khalatova, A.G.; Makhovskaya, O.V.; Botos, I.; Wlodawer, A.; Gustchina, A. Classification of ATP-dependent proteases Lon and comparison of the active sites of their proteolytic domains. Eur. J. Biochem. 2004, 271, 4865–4871. [Google Scholar] [CrossRef]
- Gur, E. The Lon AAA+ protease. Sub-Cell. Biochem. 2013, 66, 35–51. [Google Scholar] [CrossRef]
- Wlodawer, A.; Sekula, B.; Gustchina, A.; Rotanova, T.V. Structure and the Mode of Activity of Lon Proteases from Diverse Organisms. J. Mol. Biol. 2022, 434, 167504. [Google Scholar] [CrossRef]
- Gibellini, L.; De Gaetano, A.; Mandrioli, M.; Van Tongeren, E.; Bortolotti, C.A.; Cossarizza, A.; Pinti, M. The biology of Lonp1: More than a mitochondrial protease. Int. Rev. Cell Mol. Biol. 2020, 354, 1–61. [Google Scholar] [CrossRef]
- Pomatto, L.C.; Raynes, R.; Davies, K.J. The peroxisomal Lon protease LonP2 in aging and disease: Functions and comparisons with mitochondrial Lon protease LonP1. Biol. Rev. Cambr. Philos. Soc. 2017, 92, 739–753. [Google Scholar] [CrossRef]
- Yokota, S.; Haraguchi, C.M.; Oda, T. Induction of peroxisomal Lon protease in rat liver after di-(2-ethylhexyl)phthalate treatment. Histochem. Cell Biol. 2008, 129, 73–83. [Google Scholar] [CrossRef]
- Okumoto, K.; Kametani, Y.; Fujiki, Y. Two proteases, trypsin domain-containing 1 (Tysnd1) and peroxisomal lon protease (PsLon), cooperatively regulate fatty acid β-oxidation in peroxisomal matrix. J. Biol. Chem. 2011, 286, 44367–44379. [Google Scholar] [CrossRef]
- Walker, C.L.; Pomatto, L.C.D.; Tripathi, D.N.; Davies, K.J.A. Redox Regulation of Homeostasis and Proteostasis in Peroxisomes. Physiol. Rev. 2018, 98, 89–115. [Google Scholar] [CrossRef]
- Cha, S.S.; An, Y.J.; Lee, C.R.; Lee, H.S.; Kim, Y.G.; Kim, S.J.; Kwon, K.K.; De Donatis, G.M.; Lee, J.H.; Maurizi, M.R.; et al. Crystal structure of Lon protease: Molecular architecture of gated entry to a sequestered degradation chamber. EMBO J. 2010, 29, 3520–3530. [Google Scholar] [CrossRef]
- Brown, B.L.; Vieux, E.F.; Kalastavadi, T.; Kim, S.; Chen, J.Z.; Baker, T.A. N domain of the Lon AAA+ protease controls assembly and substrate choice. Protein Sci. Publ. Protein Soc. 2019, 28, 1239–1251. [Google Scholar] [CrossRef]
- García-Nafría, J.; Ondrovičová, G.; Blagova, E.; Levdikov, V.M.; Bauer, J.A.; Suzuki, C.K.; Kutejová, E.; Wilkinson, A.J.; Wilson, K.S. Structure of the catalytic domain of the human mitochondrial Lon protease: Proposed relation of oligomer formation and activity. Protein Sci. 2010, 19, 987–999. [Google Scholar] [CrossRef]
- Kereïche, S.; Kováčik, L.; Bednár, J.; Pevala, V.; Kunová, N.; Ondrovičová, G.; Bauer, J.; Ambro, Ľ.; Bellová, J.; Kutejová, E.; et al. The N-terminal domain plays a crucial role in the structure of a full-length human mitochondrial Lon protease. Sci. Rep. 2016, 6, 33631. [Google Scholar] [CrossRef]
- Shin, M.; Watson, E.R.; Song, A.S.; Mindrebo, J.T.; Novick, S.J.; Griffin, P.R.; Wiseman, R.L.; Lander, G.C. Structures of the human LONP1 protease reveal regulatory steps involved in protease activation. Nat. Commun. 2021, 12, 3239. [Google Scholar] [CrossRef]
- Bahat, A.; Perlberg, S.; Melamed-Book, N.; Isaac, S.; Eden, A.; Lauria, I.; Langer, T.; Orly, J. Transcriptional activation of LON Gene by a new form of mitochondrial stress: A role for the nuclear respiratory factor 2 in StAR overload response (SOR). Mol. Cell. Endocrinol. 2015, 408, 62–72. [Google Scholar] [CrossRef]
- Pinti, M.; Gibellini, L.; De Biasi, S.; Nasi, M.; Roat, E.; O’Connor, J.-E.; Cossarizza, A. Functional characterization of the promoter of the human Lon protease gene. Mitochondrion 2011, 11, 200–206. [Google Scholar] [CrossRef]
- Fukuda, R.; Zhang, H.; Kim, J.-w.; Shimoda, L.; Dang, C.V.; Semenza, G.L. HIF-1 Regulates Cytochrome Oxidase Subunits to Optimize Efficiency of Respiration in Hypoxic Cells. Cell 2007, 129, 111–122. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, M.; Lin, H.; Huang, C.; Shi, X.; Luo, J. Epidermal growth factor up-regulates the transcription of mouse lon homology ATP-dependent protease through extracellular signal-regulated protein kinase- and phosphatidylinositol-3-kinase-dependent pathways. Exp. Cell Res. 2002, 280, 97–106. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bree, A.C.; Liu, J.; Patrick, J.E.; Chien, P.; Kearns, D.B. Adaptor-mediated Lon proteolysis restricts Bacillus subtilis hyperflagellation. Proc. Natl. Acad. Sci. USA 2015, 112, 250–255. [Google Scholar] [CrossRef]
- Kuroda, A.; Nomura, K.; Ohtomo, R.; Kato, J.; Ikeda, T.; Takiguchi, N.; Ohtake, H.; Kornberg, A. Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science 2001, 293, 705–708. [Google Scholar] [CrossRef]
- Lu, B.; Liu, T.; Crosby, J.A.; Thomas-Wohlever, J.; Lee, I.; Suzuki, C.K. The ATP-dependent Lon protease of Mus musculus is a DNA-binding protein that is functionally conserved between yeast and mammals. Gene 2003, 306, 45–55. [Google Scholar] [CrossRef]
- Venkatesh, S.; Lee, J.; Singh, K.; Lee, I.; Suzuki, C.K. Multitasking in the mitochondrion by the ATP-dependent Lon protease. Biochim. Biophys. Acta 2012, 1823, 56–66. [Google Scholar] [CrossRef]
- Bota, D.A.; Davies, K.J.A. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002, 4, 674–680. [Google Scholar] [CrossRef]
- Kita, K.; Suzuki, T.; Ochi, T. Diphenylarsinic Acid Promotes Degradation of Glutaminase C by Mitochondrial Lon Protease. J. Biol. Chem. 2012, 287, 18163–18172. [Google Scholar] [CrossRef]
- Quirós, P.M.; Español, Y.; Acín-Pérez, R.; Rodríguez, F.; Bárcena, C.; Watanabe, K.; Calvo, E.; Loureiro, M.; Fernández-García, M.S.; Fueyo, A.; et al. ATP-Dependent Lon Protease Controls Tumor Bioenergetics by Reprogramming Mitochondrial Activity. Cell Rep. 2014, 8, 542–556. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Goto, M.; Miwa, H.; Suganuma, K.; Tsunekawa-Imai, N.; Shikami, M.; Mizutani, M.; Mizuno, S.; Hanamura, I.; Nitta, M. Adaptation of leukemia cells to hypoxic condition through switching the energy metabolism or avoiding the oxidative stress. BMC Cancer 2014, 14, 76. [Google Scholar] [CrossRef]
- Sepuri, N.B.V.; Angireddy, R.; Srinivasan, S.; Guha, M.; Spear, J.; Lu, B.; Anandatheerthavarada, H.K.; Suzuki, C.K.; Avadhani, N.G. Mitochondrial LON protease-dependent degradation of cytochrome c oxidase subunits under hypoxia and myocardial ischemia. Biochim. Biophys. Acta. Bioenerg. 2017, 1858, 519–528. [Google Scholar] [CrossRef]
- Lin, B.Y.; Zheng, G.T.; Teng, K.W.; Chang, J.Y.; Lee, C.C.; Liao, P.C.; Kao, M.C. TAT-conjugated NDUFS8 can be transduced into mitochondria in a membrane-potential-independent manner and rescue complex I deficiency. Int. J. Mol. Sci. 2021, 22, 6524. [Google Scholar] [CrossRef]
- Teng, H.; Wu, B.; Zhao, K.; Yang, G.; Wu, L.; Wang, R. Oxygen-sensitive mitochondrial accumulation of cystathionine β-synthase mediated by Lon protease. Proc. Natl. Acad. Sci. USA 2013, 110, 12679–12684. [Google Scholar] [CrossRef]
- Tian, Q.; Li, T.; Hou, W.; Zheng, J.; Schrum, L.W.; Bonkovsky, H.L. Lon Peptidase 1 (LONP1)-dependent Breakdown of Mitochondrial 5-Aminolevulinic Acid Synthase Protein by Heme in Human Liver Cells. J. Biol. Chem. 2011, 286, 26424–26430. [Google Scholar] [CrossRef]
- Granot, Z.; Kobiler, O.; Melamed-Book, N.; Eimerl, S.; Bahat, A.; Lu, B.; Braun, S.; Maurizi, M.R.; Suzuki, C.K.; Oppenheim, A.B.; et al. Turnover of mitochondrial steroidogenic acute regulatory (StAR) protein by Lon protease: The unexpected effect of proteasome inhibitors. Mol. Endocrinol. 2007, 21, 2164–2177. [Google Scholar] [CrossRef]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine. 2016, 51, 7–21. [Google Scholar] [CrossRef]
- Greene, A.W.; Grenier, K.; Aguileta, M.A.; Muise, S.; Farazifard, R.; Haque, M.E.; McBride, H.M.; Park, D.S.; Fon, E.A. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 2012, 13, 378–385. [Google Scholar] [CrossRef]
- Jin, S.M.; Youle, R.J. The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy 2013, 9, 1750–1757. [Google Scholar] [CrossRef]
- Kao, T.Y.; Chiu, Y.C.; Fang, W.C.; Cheng, C.W.; Kuo, C.Y.; Juan, H.F.; Wu, S.H.; Lee, A.Y.L. Mitochondrial Lon regulates apoptosis through the association with Hsp60–mtHsp70 complex. Cell Death Dis. 2015, 6, e1642. [Google Scholar] [CrossRef]
- Sung, Y.J.; Kao, T.Y.; Kuo, C.L.; Fan, C.C.; Cheng, A.N.; Fang, W.C.; Chou, H.Y.; Lo, Y.K.; Chen, C.H.; Jiang, S.S.; et al. Mitochondrial Lon sequesters and stabilizes p53 in the matrix to restrain apoptosis under oxidative stress via its chaperone activity. Cell Death Dis. 2018, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Suzuki, C.K.; Wu, S.H. Thermodynamic characterization of specific interactions between the human Lon protease and G-quartet DNA. Nucleic Acids Res. 2008, 36, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.K.; Markovitz, D.M. The Human LON Protease Binds to Mitochondrial Promoters in a Single-Stranded, Site-Specific, Strand-Specific Manner. Biochemistry 1998, 37, 1905–1909. [Google Scholar] [CrossRef]
- Liu, T.; Lu, B.; Lee, I.; Ondrovičová, G.; Kutejová, E.; Suzuki, C.K. DNA and RNA Binding by the Mitochondrial Lon Protease Is Regulated by Nucleotide and Protein Substrate. J. Biol. Chem. 2004, 279, 13902–13910. [Google Scholar] [CrossRef]
- Matsushima, Y.; Kaguni, L.S. Matrix proteases in mitochondrial DNA function. Biochim. Biophys. Acta 2012, 1819, 1080–1087. [Google Scholar] [CrossRef]
- Szczepanowska, K.; Trifunovic, A. Mitochondrial matrix proteases: Quality control and beyond. FEBS J. 2021, 15964. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lee, H.C.; Wang, I.; Hsu, C.H.; Liao, J.H.; Lee, A.Y.; Chen, C.; Wu, S.H. DNA-binding specificity of the Lon protease alpha-domain from Brevibacillus thermoruber WR-249. Biochem. Biophys. Res. Commun. 2009, 388, 62–66. [Google Scholar] [CrossRef]
- Lu, B.; Lee, J.; Nie, X.; Li, M.; Morozov, Y.I.; Venkatesh, S.; Bogenhagen, D.F.; Temiakov, D.; Suzuki, C.K. Phosphorylation of Human TFAM in Mitochondria Impairs DNA Binding and Promotes Degradation by the AAA+ Lon Protease. Mol. Cell 2013, 49, 121–132. [Google Scholar] [CrossRef]
- Matsushima, Y.; Goto, Y.; Kaguni, L.S. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM). Proc. Natl. Acad. Sci. USA 2010, 107, 18410–18415. [Google Scholar] [CrossRef]
- “Reversed” Krebs Cycle Can Feed Tumors. Cancer Discov. 2012, 2, OF2. [CrossRef][Green Version]
- Bikas, A.; Jensen, K.; Patel, A.; Costello, J.; Reynolds, S.M.; Mendonca-Torres, M.C.; Thakur, S.; Klubo-Gwiezdzinska, J.; Ylli, D.; Wartofsky, L.; et al. Cytochrome C Oxidase Subunit 4 (COX4): A Potential Therapeutic Target for the Treatment of Medullary Thyroid Cancer. Cancers 2020, 12, 2548. [Google Scholar] [CrossRef] [PubMed]
- Kocha, K.M.; Reilly, K.; Porplycia, D.S.; McDonald, J.; Snider, T.; Moyes, C.D. Evolution of the oxygen sensitivity of cytochrome c oxidase subunit 4. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R305–R320. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Fu, T.; Guo, Q.; Zhou, D.; Sun, W.; Zhou, Z.; Chen, X.; Zhang, J.; Liu, L.; Xiao, L.; et al. Disuse-associated loss of the protease LONP1 in muscle impairs mitochondrial function and causes reduced skeletal muscle mass and strength. Nat. Commun. 2022, 13, 894. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ding, Q.; Chen, W.; Lin, C.; Ge, L.; Ying, C.; Xu, K.; Wu, Z.; Xu, L.; Ran, J.; et al. LONP1 downregulation with ageing contributes to osteoarthritis via mitochondrial dysfunction. Free Radic. Biol. Med. 2022, 191, 176–190. [Google Scholar] [CrossRef]
- Ghosh, J.C.; Seo, J.H.; Agarwal, E.; Wang, Y.; Kossenkov, A.V.; Tang, H.Y.; Speicher, D.W.; Altieri, D.C. Akt phosphorylation of mitochondrial Lonp1 protease enables oxidative metabolism and advanced tumor traits. Oncogene 2019, 38, 6926–6939. [Google Scholar] [CrossRef]
- Di, K.; Lomeli, N.; Wood, S.D.; Vanderwal, C.D.; Bota, D.A. Mitochondrial Lon is over-expressed in high-grade gliomas, and mediates hypoxic adaptation: Potential role of Lon as a therapeutic target in glioma. Oncotarget 2016, 7, 77457–77467. [Google Scholar] [CrossRef]
- Zhao, K.; Huang, X.; Zhao, W.; Lu, B.; Yang, Z. LONP1-mediated mitochondrial quality control safeguards metabolic shifts in heart development. Development 2022, 149, dev200458. [Google Scholar] [CrossRef]
- Venkatesh, S.; Baljinnyam, E.; Tong, M.; Kashihara, T.; Yan, L.; Liu, T.; Li, H.; Xie, L.H.; Nakamura, M.; Oka, S.I.; et al. Proteomic analysis of mitochondrial biogenesis in cardiomyocytes differentiated from human induced pluripotent stem cells. Am. J. Physiol. Regul. Integr. Comp. physiol. 2021, 320, R547–R562. [Google Scholar] [CrossRef]
- Luo, B.; Wang, M.; Hou, N.; Hu, X.; Jia, G.; Qin, X.; Zuo, X.; Liu, Y.; Luo, K.; Song, W.; et al. ATP-Dependent Lon Protease Contributes to Helicobacter pylori-Induced Gastric Carcinogenesis. Neoplasia 2016, 18, 242–252. [Google Scholar] [CrossRef]
- Gibellini, L.; Losi, L.; De Biasi, S.; Nasi, M.; Lo Tartaro, D.; Pecorini, S.; Patergnani, S.; Pinton, P.; De Gaetano, A.; Carnevale, G.; et al. LonP1 Differently Modulates Mitochondrial Function and Bioenergetics of Primary Versus Metastatic Colon Cancer Cells. Front. Oncol. 2018, 8, 254. [Google Scholar] [CrossRef]
- Nimmo, G.A.M.; Venkatesh, S.; Pandey, A.K.; Marshall, C.R.; Hazrati, L.N.; Blaser, S.; Ahmed, S.; Cameron, J.; Singh, K.; Ray, P.N.; et al. Bi-allelic mutations of LONP1 encoding the mitochondrial LonP1 protease cause pyruvate dehydrogenase deficiency and profound neurodegeneration with progressive cerebellar atrophy. Hum. Mol. Genet. 2019, 28, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, X.; Yu, J.; Tian, Y.; Yang, C.; Chen, Y.; Chen, H.; Ge, H. LonP1 regulates mitochondrial network remodeling through the PINK1/Parkin pathway during myoblast differentiation. Am. J. Physiol. Cell Physiol. 2020, 319, C1020–C1028. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Li, D.; Chen, Z.-X.; Huang, Y.-H.; Gao, W.-Y.; Zheng, B.-Y.; Wang, X.-Z. Hepatitis B Virus X protein elevates Parkin-mediated mitophagy through Lon Peptidase in starvation. Exp. Cell Res. 2018, 368, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.H.; Venkatesh, S.; Li, M.; Lee, J.; Lu, B.; Hilchey, S.P.; Morse, K.M.; Metcalfe, H.M.; Skalska, J.; Andreeff, M.; et al. The mitochondrial ATP-dependent Lon protease: A novel target in lymphoma death mediated by the synthetic triterpenoid CDDO and its derivatives. Blood 2012, 119, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Li, M.; Lu, B.; Zhang, Y.; Lan, L.; Chen, L.; Lu, J. Down-regulating overexpressed human Lon in cervical cancer suppresses cell proliferation and bioenergetics. PLoS ONE 2013, 8, e81084. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, L.; Huang, K.; Wang, R.; Xu, C.; Shi, Y.; Wu, X.; Wu, Z.; Zhang, J.; Chen, L.; et al. Inhibition of Lon blocks cell proliferation, enhances chemosensitivity by promoting apoptosis and decreases cellular bioenergetics of bladder cancer: Potential roles of Lon as a prognostic marker and therapeutic target in baldder cancer. Oncotarget 2014, 5, 11209–11224. [Google Scholar] [CrossRef]
- Wang, B.; Yin, X.; Gan, W.; Pan, F.; Li, S.; Xiang, Z.; Han, X.; Li, D. PRCC-TFE3 fusion-mediated PRKN/parkin-dependent mitophagy promotes cell survival and proliferation in PRCC-TFE3 translocation renal cell carcinoma. Autophagy 2021, 17, 2475–2493. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Bartolomeo, R.; De Biasi, S.; Cormio, A.; Musicco, C.; Carnevale, G.; Pecorini, S.; Nasi, M.; De Pol, A.; et al. Inhibition of Lon protease by triterpenoids alters mitochondria and is associated to cell death in human cancer cells. Oncotarget 2015, 6, 25466–25483. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chou, H.Y.; Chiu, Y.C.; Cheng, A.N.; Fan, C.C.; Chang, Y.N.; Chen, C.H.; Jiang, S.S.; Chen, N.J.; Lee, A.Y. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. 2020, 474, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.N.; Cheng, L.C.; Kuo, C.L.; Lo, Y.K.; Chou, H.Y.; Chen, C.H.; Wang, Y.H.; Chuang, T.H.; Cheng, S.J.; Lee, A.Y. Mitochondrial Lon-induced mtDNA leakage contributes to PD-L1-mediated immunoescape via STING-IFN signaling and extracellular vesicles. J. Immunother. Cancer 2020, 8, 1372. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy-Kanniappan, S.; Geschwind, J.F. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol. Cancer 2013, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Bayot, A.; Basse, N.; Lee, I.; Gareil, M.; Pirotte, B.; Bulteau, A.-L.; Friguet, B.; Reboud-Ravaux, M. Towards the control of intracellular protein turnover: Mitochondrial Lon protease inhibitors versus proteasome inhibitors. Biochimie 2008, 90, 260–269. [Google Scholar] [CrossRef]
- Wang, H.-M.; Cheng, K.-C.; Lin, C.-J.; Hsu, S.-W.; Fang, W.-C.; Hsu, T.-F.; Chiu, C.-C.; Chang, H.-W.; Hsu, C.-H.; Lee, A.Y.-L. Obtusilactone A and (−)-sesamin induce apoptosis in human lung cancer cells by inhibiting mitochondrial Lon protease and activating DNA damage checkpoints. Cancer Sci. 2010, 101, 2612–2620. [Google Scholar] [CrossRef]
- Tangeda, V.; Lo, Y.K.; Babuharisankar, A.P.; Chou, H.-Y.; Kuo, C.-L.; Kao, Y.-H.; Lee, A.Y.-L.; Chang, J.-Y. Lon upregulation contributes to cisplatin resistance by triggering NCLX-mediated mitochondrial Ca2+ release in cancer cells. Cell Death Dis. 2022, 13, 241. [Google Scholar] [CrossRef]
- Shebib, S.M.; Reed, M.H.; Shuckett, E.P.; Cross, H.G.; Perry, J.B.; Chudley, A.E. Newly recognized syndrome of cerebral, ocular, dental, auricular, skeletal anomalies: CODAS syndrome--a case report. Am. J. Med. Genet. 1991, 40, 88–93. [Google Scholar] [CrossRef]
- Dikoglu, E.; Alfaiz, A.; Gorna, M.; Bertola, D.; Chae, J.H.; Cho, T.J.; Derbent, M.; Alanay, Y.; Guran, T.; Kim, O.H.; et al. Mutations in LONP1, a mitochondrial matrix protease, cause CODAS syndrome. Am. J. Med. Genet. Part A 2015, 167, 1501–1509. [Google Scholar] [CrossRef]
- Sha, Z.; Montano, M.M.; Rochon, K.; Mears, J.A.; Deredge, D.; Wintrode, P.; Szweda, L.; Mikita, N.; Lee, I. A structure and function relationship study to identify the impact of the R721G mutation in the human mitochondrial lon protease. Arch. Biochem. Biophys. 2021, 710, 108983. [Google Scholar] [CrossRef]
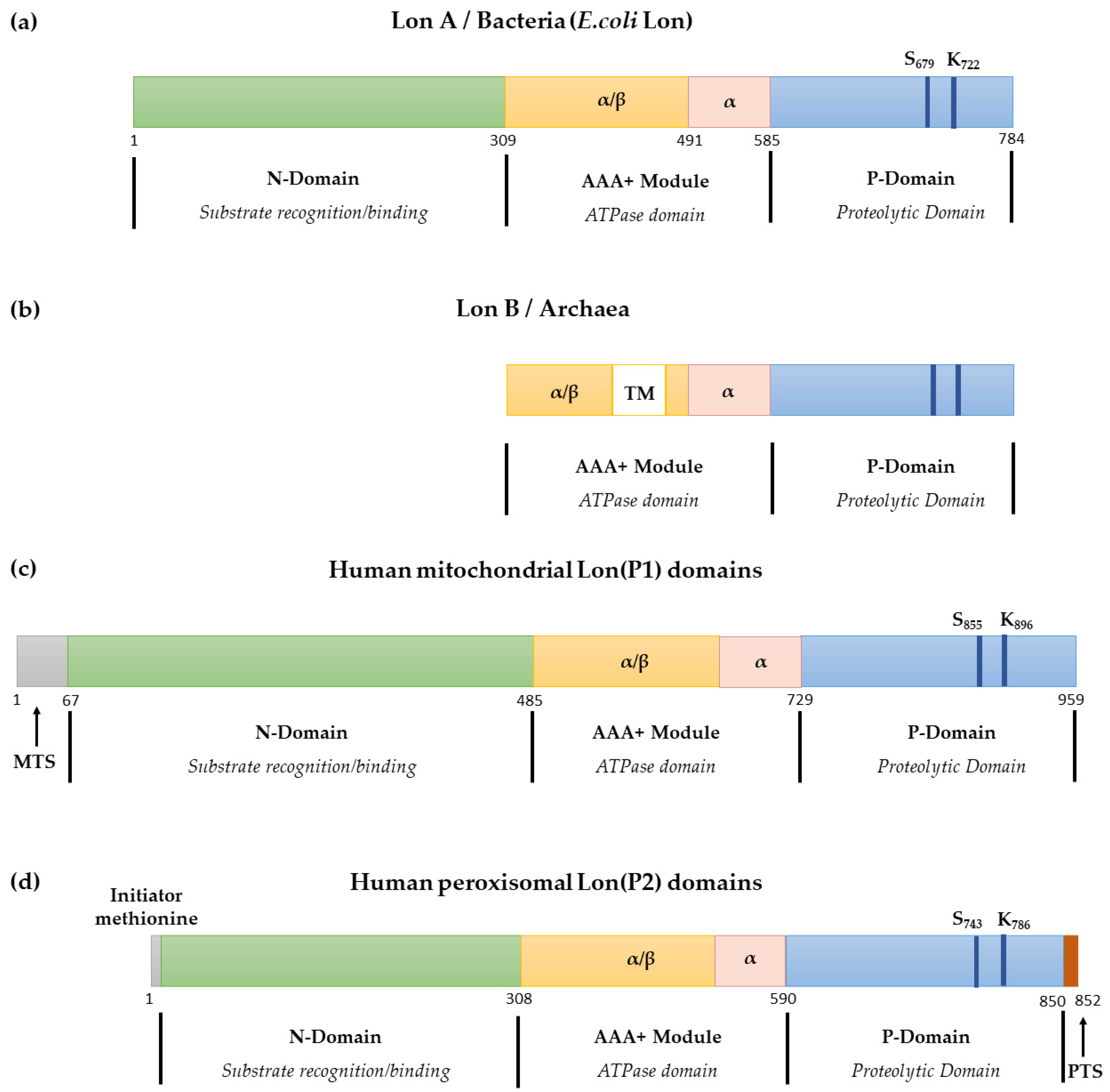
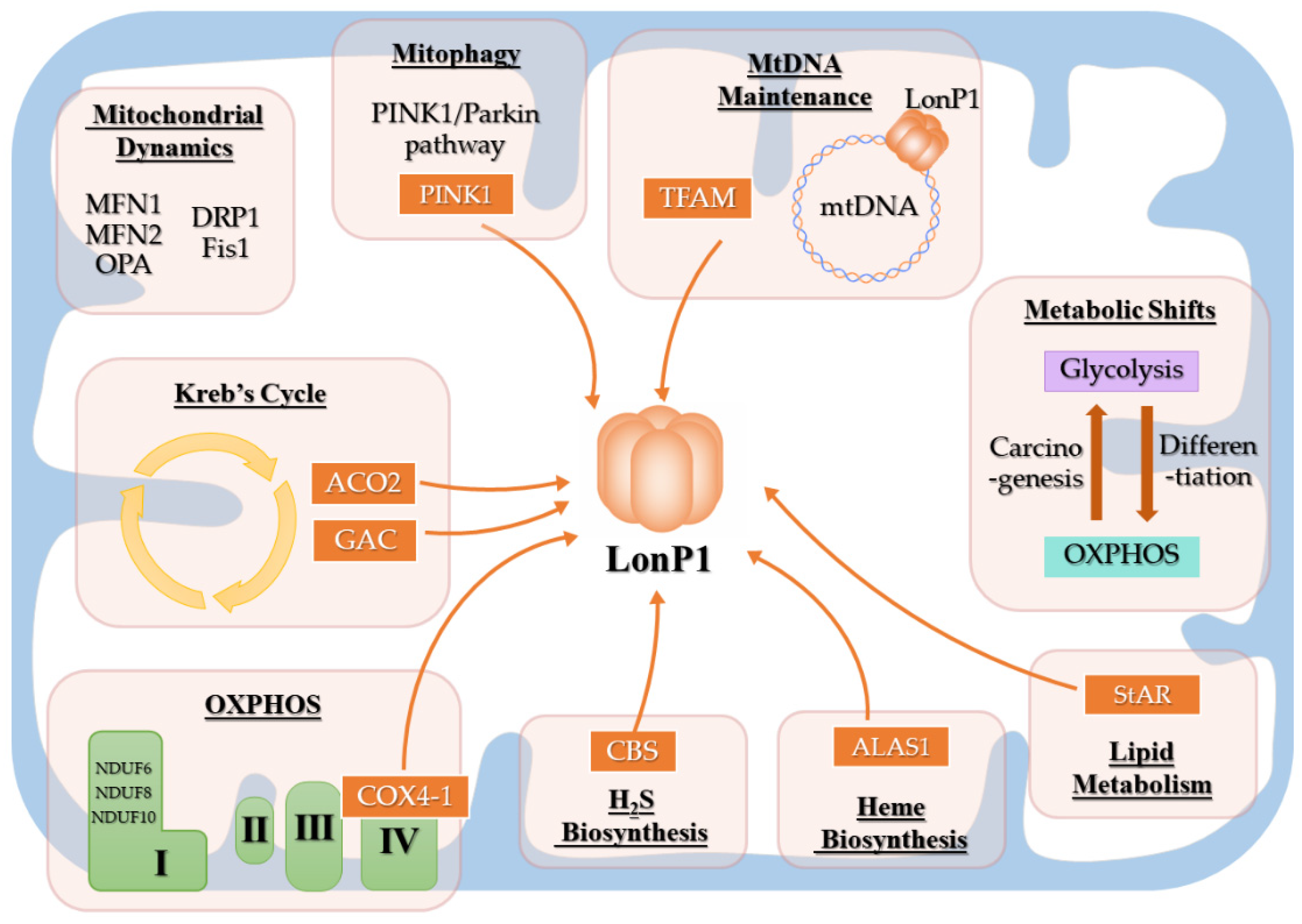
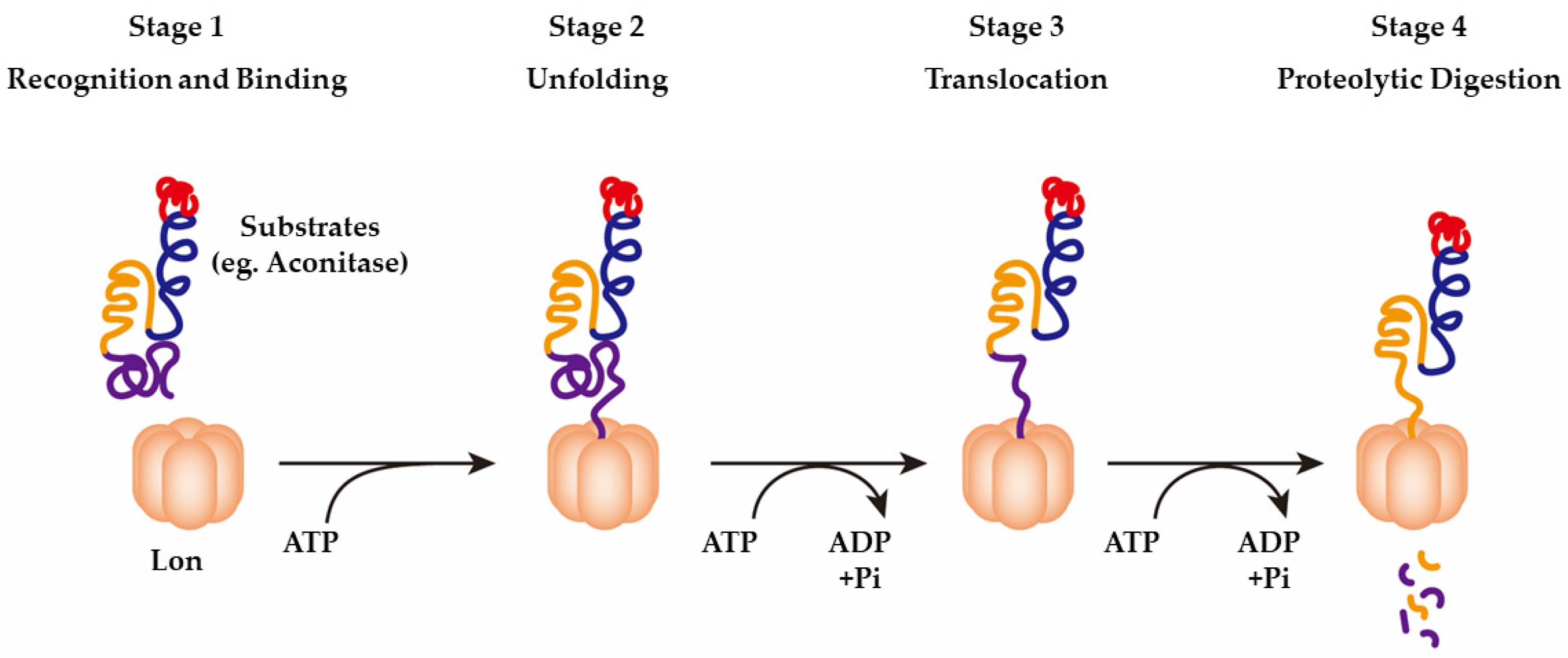
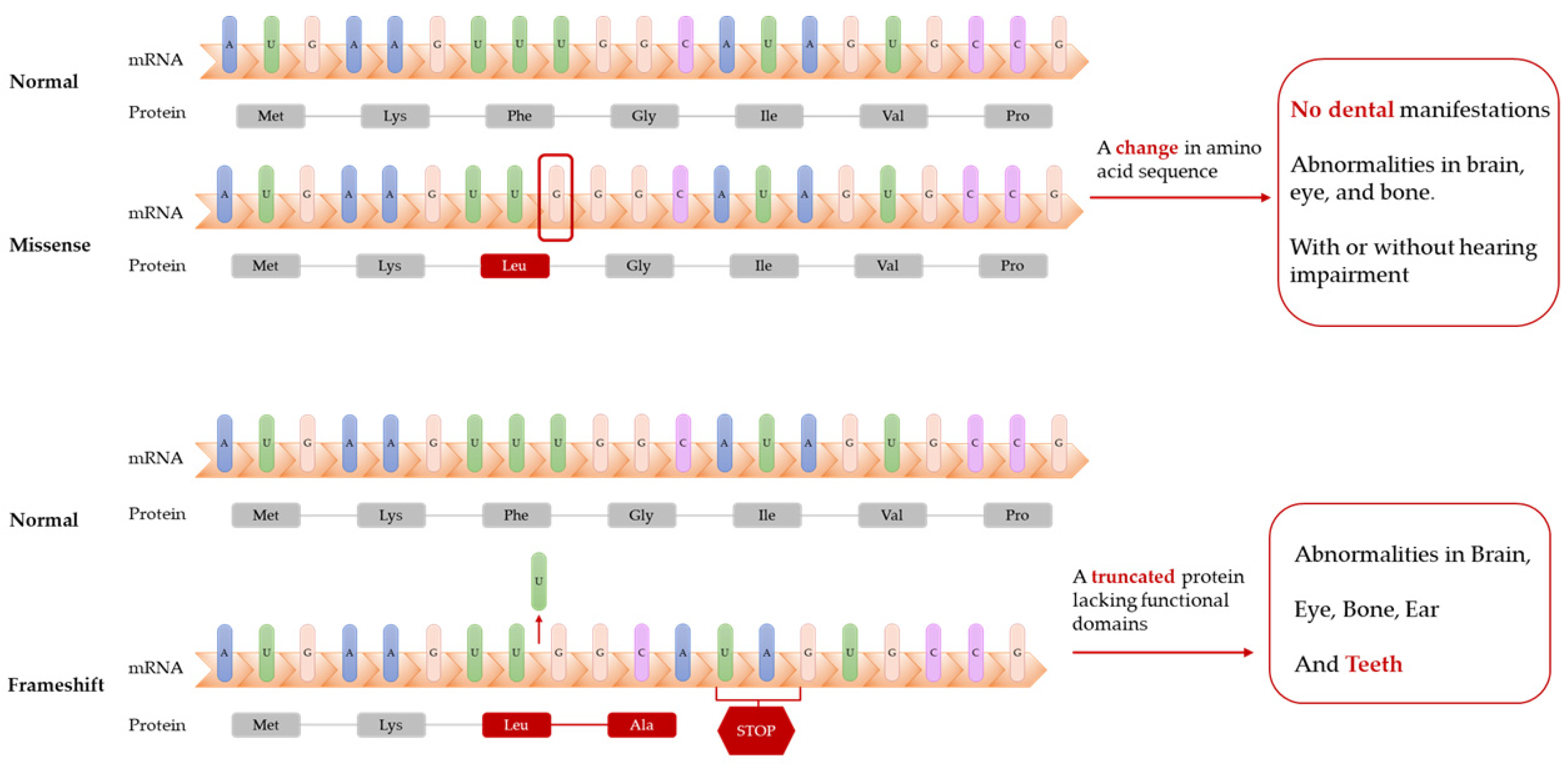
| Publication | Patients Amount | Homozygous/hterozygous | Mutation Type | Mutation Location | LONP1 Variants |
|---|---|---|---|---|---|
| Strauss KA, Am J Hum Genet. 2015 | 1 patient | Homozygous | Unclear | Adjacent to the ATP binding pocket | LONP1 c.2026C > T [p.Pro676Ser] |
| Strauss KA, Am J Hum Genet. 2015 | 8 patients | Homozygous | Unclear | AAA+ module | LONP1 c.2161C > G [p.Arg721Gly] |
| Strauss KA, Am J Hum Genet. 2015 | 1 patient | Heterozygous | Unclear | AAA+ module | LONP1 c.1892C > A/c.2171C > T [p.Ser631Tyr /p.Ala724Val] |
| Dikoglu E, Am J Med Genet A. 2015 | 1 patient | Heterozygous | Missense mutation & shift mutation | Truncated LonP1 and deletion of ATPase and P domains | c.1392G > A p.W464 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Chen, W.; Fan, W.; He, H.; Huang, F. Roles of LonP1 in Oral-Maxillofacial Developmental Defects and Tumors: A Novel Insight. Int. J. Mol. Sci. 2022, 23, 13370. https://doi.org/10.3390/ijms232113370
Ma H, Chen W, Fan W, He H, Huang F. Roles of LonP1 in Oral-Maxillofacial Developmental Defects and Tumors: A Novel Insight. International Journal of Molecular Sciences. 2022; 23(21):13370. https://doi.org/10.3390/ijms232113370
Chicago/Turabian StyleMa, Haozhen, Wanting Chen, Wenguo Fan, Hongwen He, and Fang Huang. 2022. "Roles of LonP1 in Oral-Maxillofacial Developmental Defects and Tumors: A Novel Insight" International Journal of Molecular Sciences 23, no. 21: 13370. https://doi.org/10.3390/ijms232113370
APA StyleMa, H., Chen, W., Fan, W., He, H., & Huang, F. (2022). Roles of LonP1 in Oral-Maxillofacial Developmental Defects and Tumors: A Novel Insight. International Journal of Molecular Sciences, 23(21), 13370. https://doi.org/10.3390/ijms232113370





