Mast Cell Cytokines in Acute and Chronic Gingival Tissue Inflammation: Role of IL-33 and IL-37
Abstract
1. Introduction
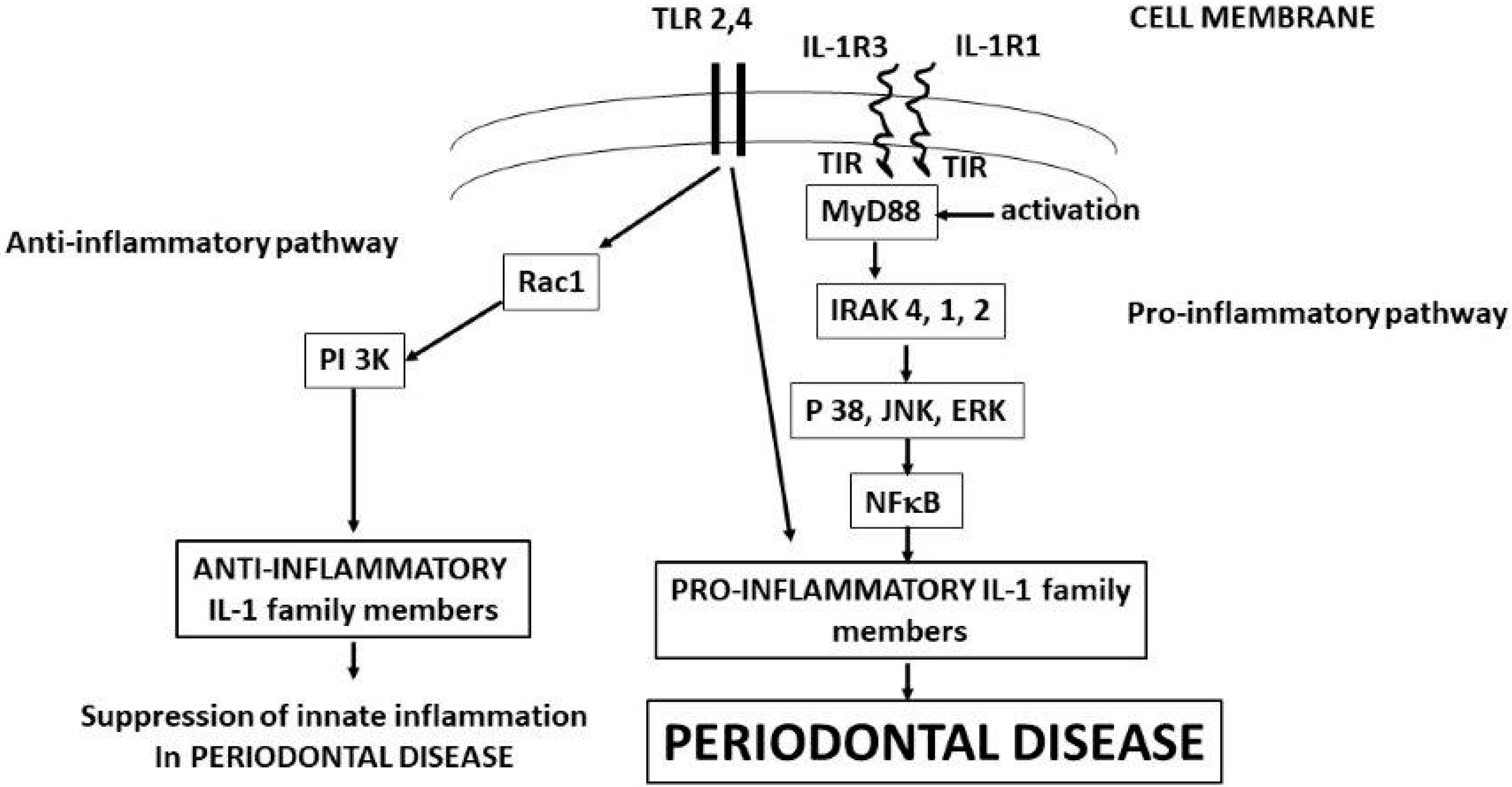
2. IL-1Beta (IL-1β)
3. Interleukin-33 (IL-33)
3.1. IL-33 Expression
3.2. IL-33 in Allergy and Inflammation
3.3. IL-33 in Inflammatory Autoimmune Disease
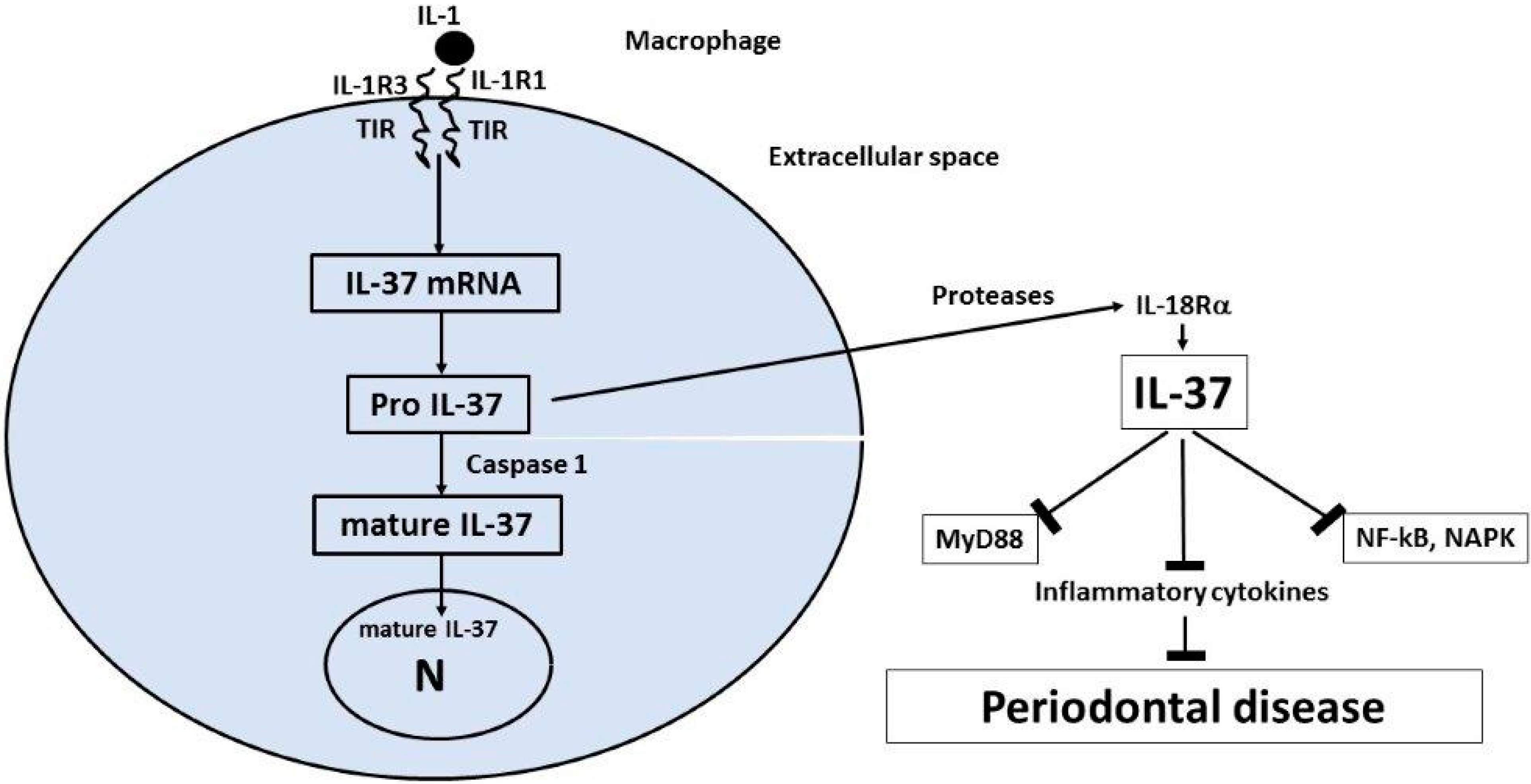
4. Interleukin-37 (IL-37)
5. Conclusions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Eggenhuizen, P.; Ng, B.; Ooi, J. Treg Enhancing Therapies to Treat Autoimmune Diseases. Int. J. Mol. Sci. 2020, 21, 7015. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Kraus, F.W. Autoimmunity and Periodontal Disease. Odontol. Tidskr. 1965, 73, 281–393. [Google Scholar] [PubMed]
- Weyrich, L.S.; Duchene, S.; Soubrier, J.; Arriola, L.; Llamas, B.; Breen, J.; Morris, A.G.; Alt, K.W.; Caramelli, D.; Dresely, V.; et al. Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature 2017, 544, 357–361. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2018, 195, 74–85. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Mowat, A.; Agace, W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Prucsi, Z.; Płonczyńska, A.; Potempa, J.; Sochalska, M. Uncovering the Oral Dysbiotic Microbiota as Masters of Neutrophil Responses in the Pathobiology of Periodontitis. Front. Microbiol. 2021, 12, 729717. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Anusahathien, O.; Singh, G.; Peters, T.; Dolby, A. Immunity to Self-Antigens in Periodontal Disease. J. Periodontol. 1992, 63, 194–199. [Google Scholar] [CrossRef]
- Kaur, G.; Mohindra, K.; Singla, S. Autoimmunity—Basics and link with periodontal disease. Autoimmun. Rev. 2017, 16, 64–71. [Google Scholar] [CrossRef]
- Degasperi, G.R.; Ossick, M.V.; Pinheiro, S.R.L.; Etchegaray, A. Autoimmunity and periodontal disease: Arguing a possible corre-lation. Indian J. Dent. Res. 2020, 31, 615–620. [Google Scholar]
- Berglundh, T.; Donati, M. Aspects of adaptive host response in periodontitis. J. Clin. Periodontol. 2005, 32, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Brun, A.; Nuzzo, A.; Prouvost, B.; Diallo, D.; Hamdan, S.; Meseguer, E.; Guidoux, C.; Lavallée, P.; Amarenco, P.; Lesèche, G.; et al. Oral microbiota and atherothrombotic carotid plaque vulnerability in periodontitis patients. A cross-sectional study. J. Periodontal Res. 2020, 56, 339–350. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef]
- Kajiya, M.; Kurihara, H. Molecular Mechanisms of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 930. [Google Scholar] [CrossRef]
- Fattahi, S.; Sadighi, M.; Faramarzi, M.; Karimifard, E.; Mirzaie, A. Comparison of mast cell counts between the patients with moderate and severe periodontitis. J. Adv. Periodontol. Implant Dent. 2019, 11, 34–38. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Kornman, K.S. Inflammation and Factors That May Regulate Inflammatory Response. J. Periodontol. 2008, 79, 1503–1507. [Google Scholar] [CrossRef]
- Garlet, G.P. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue de-struction viewpoints. J. Dent. Res. 2010, 89, 1349–1363. [Google Scholar] [CrossRef]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major Periodontopathic Pathogen Overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef] [PubMed]
- Latz, E. The inflammasomes: Mechanisms of activation and function. Curr. Opin. Immunol. 2010, 22, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, S.K.; Rathinam, V.A.; Fitzgerald, K.A. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015, 25, 308–315. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Lima, C.A.D.; Vajgel, G.; Sandrin-Garcia, P. The Role of NLRP3 Inflammasome in Lupus Nephritis. Int. J. Mol. Sci. 2021, 22, 12476. [Google Scholar] [CrossRef]
- Saluja, R.; Ketelaar, M.E.; Hawro, T.; Church, M.K.; Maurer, M.; Nawijn, M.C. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol. Immunol. 2015, 63, 80–85. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Petra, A.I.; Taracanova, A.; Panagiotidou, S.; Conti, P. Targeting IL-33 in Autoimmunity and Inflammation. J. Pharmacol. Exp. Ther. 2015, 354, 24–31. [Google Scholar] [CrossRef]
- Liew, F.Y.; Pitman, N.I.; McInnes, I.B. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat. Rev. Immunol. 2010, 10, 103–110. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Simon, A.; Van Der Meer, J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef]
- Arend, W.P.; Palmer, G.; Gabay, C. IL-1, IL-8, and IL-33 families of cytokines. Immunol. Rev. 2008, 223, 20–38. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Tetè, G.; D’orto, B.; Ferrante, L.; Polizzi, E.; Cattoni, F. Role of mast cells in oral inflammation. J. Biol. Regul. Homeost. Agents 2021, 35, 65–70. [Google Scholar]
- Kim, S.R.; Kim, D.I.; Kim, S.; Lee, H.; Lee, K.S.; Cho, S.H.; Lee, Y.C. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis. 2014, 5, e1498. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Di Benedetto, P.; Liakouli, V.; Berardicurti, O.; Carubbi, F.; Ciccia, F.; Alvaro, S.; Triolo, G.; Giacomelli, R. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1beta via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3(NLRP3)-inflammasome activation: A possible implication for therapeutic decision in these patients. Clin. Exp. Immunol. 2015, 182, 35–44. [Google Scholar]
- Inoue, M.; Shinohara, M.L. NLRP3 Inflammasome and MS/EAE. Autoimmune Dis. 2013, 2013, 859145. [Google Scholar] [CrossRef]
- Nakamura, Y.; Franchi, L.; Kambe, N.; Meng, G.; Strober, W.; Nunez, G. Critical role for mast cells in interleukin-1beta-driven skin inflammation associated with an activating mutation in the nlrp3 protein. Immunity 2012, 37, 85–95. [Google Scholar] [CrossRef]
- Carlström, M.; Ekman, A.-K.; Petersson, S.; Söderkvist, P.; Enerbäck, C. Genetic support for the role of the NLRP3 inflammasome in psoriasis susceptibility. Exp. Dermatol. 2012, 21, 932–937. [Google Scholar] [CrossRef]
- Johnston, A.; Xing, X.; Wolterink, L.; Barnes, D.H.; Yin, Z.; Reingold, L.; Kahlenberg, J.M.; Harms, P.W.; Gudjonsson, J.E. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J. Allergy Clin. Immunol. 2016, 140, 109–120. [Google Scholar] [CrossRef]
- Johansen, C.; Moeller, K.; Kragballe, K.; Iversen, L. The Activity of Caspase-1 Is Increased in Lesional Psoriatic Epidermis. J. Investig. Dermatol. 2007, 127, 2857–2864. [Google Scholar] [CrossRef]
- Niebuhr, M.; Baumert, K.; Heratizadeh, A.; Satzger, I.; Werfel, T. Impaired NLRP3 inflammasome expression and function in atopic dermatitis due to Th2 milieu. Allergy 2014, 69, 1058–1067. [Google Scholar] [CrossRef]
- Dowling, J.K.; O’Neill, L.A. Biochemical regulation of the inflammasome. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 424–443. [Google Scholar] [CrossRef]
- Lu, A.; Wu, H. Structural mechanisms of inflammasome assembly. FEBS J. 2014, 282, 435–444. [Google Scholar] [CrossRef]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 inflammasome: A sensor for metabolic danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef]
- Theoharides, T.C. Danger Signals and Inflammation. Clin. Ther. 2016, 38, 996–999. [Google Scholar] [CrossRef]
- Jo, E.-K.; Kim, J.K.; Shin, D.-M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2015, 13, 148–159. [Google Scholar] [CrossRef]
- Franchi, L.; Nunez, G. Immunology. Orchestrating inflammasomes. Science 2012, 337, 1299–1300. [Google Scholar] [CrossRef]
- Dubois, E.A.; Rissmann, R.; Cohen, A.F. Rilonacept and canakinumab. Br. J. Clin. Pharmacol. 2011, 71, 639–641. [Google Scholar] [CrossRef]
- Ruzicka, T.; Hanifin, J.M.; Furue, M.; Pulka, G.; Mlynarczyk, I.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Yoshida, H.; et al. Anti–Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N. Engl. J. Med. 2017, 376, 826–835. [Google Scholar] [CrossRef]
- Finn, D.F.; Walsh, J.J. Twenty-first century mast cell stabilizers. J. Cereb. Blood Flow Metab. 2013, 170, 23–37. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Redegeld, F.A.; Bischoff, S.C.; Gibbs, B.F.; Maurer, M. Mast Cells as Drivers of Disease and Therapeutic Targets. Trends Immunol. 2017, 39, 151–162. [Google Scholar] [CrossRef]
- Weng, Z.; Zhang, B.; Asadi, S.; Sismanopoulos, N.; Butcher, A.; Fu, X.; Katsarou-Katsari, A.; Antoniou, C.; Theoharides, T.C. Quercetin Is More Effective than Cromolyn in Blocking Human Mast Cell Cytokine Release and Inhibits Contact Dermatitis and Photosensitivity in Humans. PLoS ONE 2012, 7, e33805. [Google Scholar] [CrossRef]
- Vieira Dos Santos, R.; Magerl, M.; Martus, P.; Zuberbier, T.; Church, M.K.; Escribano, L.; Maurer, M. Topical sodium cromoglicate relieves allergen- and histamine-induced dermal pruritus. Br. J. Dermatol. 2010, 162, 674–676. [Google Scholar] [CrossRef]
- Barone, A.; Chatelain, S.; Derchi, G.; Di Spirito, F.; Martuscelli, R.; Porzio, M.; Sbordone, L. Antibiotic’s effectiveness after erupted tooth extractions: A retrospective study. Oral Dis. 2020, 26, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Moulin, D.; Donzé, O.; Talabot-Ayer, D.; Mézin, F.; Palmer, G.; Gabay, C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine 2007, 40, 216–225. [Google Scholar] [CrossRef]
- Brusilovsky, M.; Rochman, M.; Azouz, N.P.; Mack, L.E.; Rothenberg, M.E. Uncovering the Secretes of Allergic Inflammation. J. Clin. Investig. 2020, 130, 3419–3421. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.R.; Margulis, A.; Wood, N.; Goldman, S.J.; Kasaian, M.; Chaudhary, D. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Agents Actions 2009, 59, 207–218. [Google Scholar] [CrossRef]
- Halova, I.; Rönnberg, E.; Draberova, L.; Vliagoftis, H.; Nilsson, G.P.; Draber, P. Changing the threshold-Signals and mechanisms of mast cell priming. Immunol. Rev. 2018, 282, 73–86. [Google Scholar] [CrossRef]
- Taracanova, A.; Alevizos, M.; Karagkouni, A.; Weng, Z.; Norwitz, E.; Conti, P.; Leeman, S.E.; Theoharides, T.C. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc. Natl. Acad. Sci. USA 2017, 114, E4002–E4009. [Google Scholar] [CrossRef]
- Rubin, R.H. The indirect effects of cytomegalovirus infection on the outcome of organ transplantation. JAMA 1989, 261, 3607–3609. [Google Scholar] [CrossRef]
- Moussion, C.; Ortega, N.; Girard, J.P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: A novel ‘alarmin’? PLoS ONE 2008, 3, e3331. [Google Scholar] [CrossRef]
- Nile, C.J.; Barksby, E.; Jitprasertwong, P.; Preshaw, P.M.; Taylor, J.J. Expression and regulation of interleukin-33 in human monocytes. Immunology 2010, 130, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Khan, M.M.; Thangavel, R.; Xiong, Z.; Yang, E.; Zaheer, A. Glia maturation factor induces interleukin-33 release from astrocytes: Implications for neurodegenerative diseases. J. Neuroimmune Pharmacol. 2013, 8, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Lukens, J.R.; Gross, J.M.; Kanneganti, T.D. IL-1 family cytokines trigger sterile inflammatory disease. Front. Immunol. 2012, 3, 315. [Google Scholar] [CrossRef]
- Lapérine, O.; Cloitre, A.; Caillon, J.; Huck, O.; Bugueno, I.M.; Pilet, P.; Sourice, S.; le Tilly, E.; Palmer, G.; Davideau, J.; et al. Interleukin-33 and RANK-L Interplay in the Alveolar Bone Loss Associated to Peri-odontitis. PLoS ONE 2016, 11, e0168080. [Google Scholar]
- Pietruczuk, M.; Kraszula, L.; Kupczyk, M.; Kuna, P.; Eusebio, M.J. Dynamics and proliferative capacities of CD8(+)CD28(+)TCRalphabeta(+)CD62L(high) T-cell subsets in healthy and asthmatic subjects. J. Biol. Regul. Homeost. Agents 2021, 35, 485–494. [Google Scholar] [PubMed]
- Cayrol, C.; Girard, J.-P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA 2009, 106, 9021–9026. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, Z. The enigmatic processing and secretion of interleukin-33. Cell. Mol. Immunol. 2010, 7, 260–262. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The Interleukin-1 Family: Back to the Future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Lefrançais, E.; Roga, S.; Gautier, V.; Gonzalez-De-Peredo, A.; Monsarrat, B.; Girard, J.-P.; Cayrol, C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl. Acad. Sci. USA 2012, 109, 1673–1678. [Google Scholar] [CrossRef]
- Faas, M.; Ipseiz, N.; Ackermann, J.; Culemann, S.; Grüneboom, A.; Schröder, F.; Rothe, T.; Scholtysek, C.; Eberhardt, M.; Böttcher, M.; et al. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity 2021, 54, 2531–2546.e5. [Google Scholar] [CrossRef]
- Villarreal, D.O.; Weiner, D.B. Interleukin 33: A switch-hitting cytokine. Curr. Opin. Immunol. 2014, 28, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Salmond, R.J.; Mirchandani, A.S.; Besnard, A.-G.; Bain, C.C.; Thomson, N.C.; Liew, F.Y. IL-33 induces innate lymphoid cell–mediated airway inflammation by activating mammalian target of rapamycin. J. Allergy Clin. Immunol. 2012, 130, 1159–1166.e6. [Google Scholar] [CrossRef] [PubMed]
- Alase, A.; Seltmann, J.; Werfel, T.; Wittmann, M. Interleukin-33 modulates the expression of human beta-defensin 2 in human primary keratinocytes and may influence the susceptibility to bacterial superinfection in acute atopic dermatitis. Br. J. Dermatol. 2012, 167, 1386–1389. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, X.; Zhao, Y.; Chen, F.; Sun, M.; Yang, W.; Chen, L.; Yao, S.; Peniche, A.; Dann, S.M.; et al. Interleukin-33 Promotes REG3gamma Expression in Intestinal Epithelial Cells and Regulates Gut Microbiota. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 21–36. [Google Scholar] [CrossRef]
- Lloyd, C.M. IL-33 family members and asthma—Bridging innate and adaptive immune responses. Curr. Opin. Immunol. 2010, 22, 800–806. [Google Scholar] [CrossRef]
- Kouzaki, H.; Iijima, K.; Kobayashi, T.; O’Grady, S.M.; Kita, H. The Danger Signal, Extracellular ATP, Is a Sensor for an Airborne Allergen and Triggers IL-33 Release and Innate Th2-Type Responses. J. Immunol. 2011, 186, 4375–4387. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, R.; Zhao, G.; Pflugfelder, S.C.; Li, D.-Q. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium. Int. J. Biochem. Cell Biol. 2011, 43, 1383–1391. [Google Scholar] [CrossRef]
- Gaudenzio, N.; Sibilano, R.; Marichal, T.; Starkl, P.; Reber, L.L.; Cenac, N.; McNeil, B.D.; Dong, X.; Hernandez, J.D.; Sagi-Eisenberg, R.; et al. Different activation signals induce distinct mast cell degranulation strategies. J. Clin. Investig. 2016, 126, 3981–3998. [Google Scholar] [CrossRef]
- Seltmann, J.; Werfel, T.; Wittmann, M. Evidence for a regulatory loop between IFN-gamma and IL-33 in skin inflammation. Exp. Dermatol. 2013, 22, 102–107. [Google Scholar] [CrossRef]
- Balato, A.; Lembo, S.; Mattii, M.; Schiattarella, M.; Marino, R.; De Paulis, A.; Balato, N.; Ayala, F. IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Exp. Dermatol. 2012, 21, 892–894. [Google Scholar] [CrossRef]
- Savinko, T.; Karisola, P.; Lehtimäki, S.; Lappeteläinen, A.-M.; Haapakoski, R.; Wolff, H.; Lauerma, A.; Alenius, H. ST2 Regulates Allergic Airway Inflammation and T-Cell Polarization in Epicutaneously Sensitized Mice. J. Investig. Dermatol. 2013, 133, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.; Afonina, I.S.; Mueller, C.; Beyaert, R. Dichotomous Function of IL-33 in Health and Disease: From Biology to Clinical Implications. Biochemical Pharmacology 2018, 148, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Oshio, T.; Komine, M.; Tsuda, H.; Tominaga, S.-I.; Saito, H.; Nakae, S.; Ohtsuki, M. Nuclear expression of IL-33 in epidermal keratinocytes promotes wound healing in mice. J. Dermatol. Sci. 2016, 85, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.; Deredge, D.; Bowers, A.L.; Luchini, A.; Bonsor, D.A.; Beadenkopf, R.; Liotta, L.; Wintrode, P.L.; Sundberg, E.J. IL-1 Family Cytokines Use Distinct Molecular Mechanisms to Signal through Their Shared Co-receptor. Immunity 2017, 47, 510–523.e4. [Google Scholar] [CrossRef]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Rev. Drug. Discov. 2008, 7, 827–840. [Google Scholar] [CrossRef]
- Tare, N.; Li, H.; Morschauser, A.; Cote-Sierra, J.; Ju, G.; Renzetti, L.; Lin, T.A. KU812 cells provide a novel in vitro model of the human IL-33/ST2L axis: Functional responses and identification of signaling pathways. Exp. Cell Res. 2010, 316, 2527–2537. [Google Scholar] [CrossRef]
- Oboki, K.; Nakae, S.; Matsumoto, K.; Saito, H. IL-33 and Airway Inflammation. Allergy Asthma Immunol. Res. 2011, 3, 81–88. [Google Scholar] [CrossRef][Green Version]
- Llop-Guevara, A.; Chu, D.K.; Walker, T.D.; Goncharova, S.; Fattouh, R.; Silver, J.S.; Moore, C.L.; Xie, J.L.; O’Byrne, P.M.; Coyle, A.J.; et al. A GM-CSF/IL-33 Pathway Facilitates Allergic Airway Responses to Sub-Threshold House Dust Mite Exposure. PLoS ONE 2014, 9, e88714. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Neilsen, C.V.; Bryce, P.J. IL-33 Is Produced by Mast Cells and Regulates IgE-Dependent Inflammation. PLoS ONE 2010, 5, e11944. [Google Scholar] [CrossRef]
- Kaur, D.; Gomez, E.; Doe, C.; Berair, R.; Woodman, L.; Saunders, R.M.; Hollins, F.; Rose, F.; Amrani, Y.; May, R.; et al. IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: Airway smooth muscle crosstalk. Allergy 2015, 70, 556–567. [Google Scholar] [CrossRef]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.O. A Large-Scale, Consortium-Based Genomewide Association Study of Asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef]
- Traister, R.S.; Uvalle, C.E.; Hawkins, G.A.; Meyers, D.A.; Bleecker, E.R.; Wenzel, S.E. Phenotypic and genotypic association of epithelial IL1RL1 to human T2-like asthma. J. Allergy Clin. Immunol. 2014, 135, 92–99. [Google Scholar] [CrossRef]
- Pushparaj, P.N.; Tay, H.K.; H’Ng, S.C.; Pitman, N.; Xu, D.; McKenzie, A.; Liew, F.Y.; Melendez, A.J. The cytokine interleukin-33 mediates anaphylactic shock. Proc. Natl. Acad. Sci. USA 2009, 106, 9773–9778. [Google Scholar] [CrossRef]
- Raeiszadeh Jahromi, S.; Mahesh, P.A.; Jayaraj, B.S.; Madhunapantula, S.R.; Holla, A.D.; Vishweswaraiah, S.; Ramachandra, N.B. Serum levels of IL-10, IL-17F and IL-33 in patients with asthma: A case–control study. J. Asthma 2014, 51, 1004–1013. [Google Scholar] [CrossRef]
- Kamijo, S.; Takeda, H.; Tokura, T.; Suzuki, M.; Inui, K.; Hara, M.; Matsuda, H.; Matsuda, A.; Oboki, K.; Ohno, T.; et al. IL-33–Mediated Innate Response and Adaptive Immune Cells Contribute to Maximum Responses of Protease Allergen–Induced Allergic Airway Inflammation. J. Immunol. 2013, 190, 4489–4499. [Google Scholar] [CrossRef]
- Haenuki, Y.; Matsushita, K.; Futatsugi-Yumikura, S.; Ishii, K.J.; Kawagoe, T.; Imoto, Y.; Fujieda, S.; Yasuda, M.; Hisa, Y.; Akira, S.; et al. A critical role of IL-33 in experimental allergic rhinitis. J. Allergy Clin. Immunol. 2012, 130, 184–194.e11. [Google Scholar] [CrossRef]
- Bellomo, R.G.; E Gallenga, C.; Caraffa, A.; Tetè, G.; Ronconi, G.; Conti, P. Anaphylaxis is a rare reaction in COVID-19 vaccination. J. Biol. Regul. Homeost. Agents 2021, 35, 839–842. [Google Scholar] [CrossRef]
- Eiwegger, T.; Akdis, C.A. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur. J. Immunol. 2011, 41, 1535–1538. [Google Scholar] [CrossRef]
- Toki, S.; Goleniewska, K.; Zhang, J.; Zhou, W.; Newcomb, D.C.; Zhou, B.; Kita, H.; Boyd, K.L.; Peebles, R.S., Jr. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy 2020, 75, 1606–1617. [Google Scholar] [CrossRef]
- Louten, J.; Rankin, A.L.; Li, Y.; Murphy, E.E.; Beaumont, M.; Moon, C.; Bourne, P.; McClanahan, T.K.; Pflanz, S.; Malefyt, R.D.W. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int. Immunol. 2011, 23, 307–315. [Google Scholar] [CrossRef]
- Cannavò, S.P.; Bertino, L.; Di Salvo, E.; Papaianni, V.; Ventura-Spagnolo, E.; Gangemi, S. Possible Roles of IL-33 in the Innate-Adaptive Immune Crosstalk of Psoriasis Pathogenesis. Mediat. Inflamm. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alim, M.A.; Peterson, M.; Pejler, G. Do Mast Cells Have a Role in Tendon Healing and Inflammation? Cells 2020, 9, 1134. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa-Mineoka, R.; Okuzawa, Y.; Masuda, K.; Katoh, N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.-C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.J.; et al. A role for IL-25 and IL-33–driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Zhang, B.; Kempuraj, D.; Tagen, M.; Vasiadi, M.; Angelidou, A.; Alysandratos, K.-D.; Kalogeromitros, D.; Asadi, S.; Stavrianeas, N.; et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl. Acad. Sci. USA 2010, 107, 4448–4453. [Google Scholar] [CrossRef]
- Yang, C.; Chen, N.; Tang, X.L.; Qian, X.H.; Cai, C.P. Immunomodulatory effects of IL-33 and IL-25 in an ovalbumin-induced allergic rhinitis mouse model. J. Biol. Regul. Homeost. Agents 2021, 35, 571–581. [Google Scholar] [CrossRef]
- Fux, M.; Pecaric-Petkovic, T.; Odermatt, A.; Hausmann, O.V.; Lorentz, A.; Bischoff, S.C.; Virchow, J.C.; Dahinden, C.A. IL-33 is a mediator rather than a trigger of the acute allergic response in humans. Allergy 2013, 69, 216–222. [Google Scholar] [CrossRef]
- Enoksson, M.; Lyberg, K.; Möller-Westerberg, C.; Fallon, P.G.; Nilsson, G.; Lunderius-Andersson, C. Mast Cells as Sensors of Cell Injury through IL-33 Recognition. J. Immunol. 2011, 186, 2523–2528. [Google Scholar] [CrossRef]
- Lefrancais, E.; Cayrol, C. Mechanisms of IL-33 processing and secretion: Differences and similarities between IL-1 family members. Eur. Cytokine Netw. 2012, 23, 120–127. [Google Scholar] [CrossRef]
- Tung, H.-Y.; Plunkett, B.; Huang, S.-K.; Zhou, Y. Murine Mast Cells Secrete and Respond to Interleukin-33. J. Interf. Cytokine Res. 2014, 34, 141–147. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Wershil, B.K.; Tam, S.-Y.; Costa, J.J. Regulation of Mouse and Human Mast Cell Development, Survival and Function by Stem Cell Factor, the Ligand for the c-kit Receptor. Int. Arch. Allergy Immunol. 1995, 107, 51–53. [Google Scholar] [CrossRef]
- Matsuda, H.; Kannan, Y.; Ushio, H.; Kiso, Y.; Kanemoto, T.; Suzuki, H.; Kitamura, Y. Nerve growth factor induces development of connective tissue-type mast cells in vitro from murine bone marrow cells. J. Exp. Med. 1991, 174, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast cells, mastocytosis and related diseases. N. Engl. J. Med. 2015; in press. [Google Scholar]
- Allakhverdi, Z.; Smith, D.E.; Comeau, M.R.; Delespesse, G. Cutting Edge: The ST2 Ligand IL-33 Potently Activates and Drives Maturation of Human Mast Cells. J. Immunol. 2007, 179, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Iikura, M.; Suto, H.; Kajiwara, N.; Oboki, K.; Ohno, T.; Okayama, Y.; Saito, H.; Galli, S.J.; Nakae, S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab. Investig. 2007, 87, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Compton, R.A.; Lonergan, A.R.; Tsillioni, I.; Conti, P.; Ronconi, G.; Lauritano, D.; Rebeiz, E.E.; Theoharides, T.C. Neurohormonal markers in chronic rhinosinusitis. J. Biol. Regul. Homeost. Agents 2021, 35, 901–908. [Google Scholar]
- Geier, C.B.; Kraupp, S.; Bra, D.; Eibl, M.M.; Farmer, J.R.; Csomos, K.; Walter, J.E.; Wolf, H.M. Reduced Numbers of Circulating Group 2 Innate Lymphoid Cells in Patients with Common Variable Immunodeficiency. Eur. J. Immunol. 2017, 47, 1959–1969. [Google Scholar] [CrossRef]
- Drube, S.; Heink, S.; Walter, S.; Löhn, T.; Grusser, M.; Gerbaulet, A.; Berod, L.; Schons, J.; Dudeck, A.; Freitag, J.; et al. The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood 2010, 115, 3899–3906. [Google Scholar] [CrossRef]
- Hall, G.; Cullen, E.; Sawmynaden, K.; Arnold, J.; Fox, S.; Cowan, R.; Muskett, F.W.; Matthews, D.; Merritt, A.; Kettleborough, C. Structure of a Potential Therapeutic Antibody Bound to Interleukin-16 (IL-16): Mechanistic insights and new therapeutic opportunitieS. J. Biol. Chem. 2016, 291, 16840–16848. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Conti, P. Mast Cells May Regulate the Anti-Inflammatory Activity of IL-37. Int. J. Mol. Sci. 2019, 20, 3701. [Google Scholar] [CrossRef]
- Ho, L.H.; Ohno, T.; Oboki, K.; Kajiwara, N.; Suto, H.; Iikura, M.; Okayama, Y.; Akira, S.; Saito, H.; Galli, S.J.; et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-Fc{epsilon}RI signals. J. Leukoc. Biol. 2007, 82, 1481–1490. [Google Scholar] [CrossRef]
- Nicoletti, M.; Maccauro, G.; Tripodi, D.; Saggini, A.; Potalivo, G.; Castellani, M.; Conti, F.; Rosati, M.; Tomato, E.; Caraffa, A.; et al. Impact of IL-33 on PGD2 Generation by Activated Human Cord Blood-Derived Mast Cell: Lack of Effect on Tryptase Release. Eur. J. Inflamm. 2012, 10, 473–482. [Google Scholar] [CrossRef]
- Kaieda, S.; Wang, J.-X.; Shnayder, R.; Fishgal, N.; Hei, H.; Lee, R.T.; Stevens, R.L.; Nigrovic, P.A. Interleukin-33 Primes Mast Cells for Activation by IgG Immune Complexes. PLoS ONE 2012, 7, e47252. [Google Scholar] [CrossRef] [PubMed]
- Enoksson, M.; Möller-Westerberg, C.; Wicher, G.; Fallon, P.G.; Forsberg-Nilsson, K.; Lunderius-Andersson, C.; Nilsson, G. Intraperitoneal influx of neutrophils in response to IL-33 is mast cell–dependent. Blood 2013, 121, 530–536. [Google Scholar] [CrossRef]
- Franke, K.; Wang, Z.; Zuberbier, T.; Babina, M. Cytokines Stimulated by IL-33 in Human Skin Mast Cells: Involvement of NF-ΚB and P38 at Distinct Levels and Potent Co-Operation with FcεRI and MRGPRX2. Int. J. Mol. Sci. 2021, 22, 3580. [Google Scholar] [CrossRef] [PubMed]
- Blank, U.; Rivera, J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004, 25, 266–273. [Google Scholar] [CrossRef]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef]
- Donelan, J.; Boucher, W.; Papadopoulou, N.; Lytinas, M.; Papaliodis, D.; Dobner, P.; Theoharides, T.C. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc. Natl. Acad. Sci. USA 2006, 103, 7759–7764. [Google Scholar] [CrossRef]
- Zhang, B.; Alysandratos, K.D.; Angelidou, A.; Asadi, S.; Sismanopoulos, N.; Delivanis, D.A.; Weng, Z.; Miniati, A.; Vasiadi, M.; Katsarou-Katsari, A.; et al. Human mast cell degranulation and preformed TNF secretion require mito-chondrial translocation to exocytosis sites: Relevance to atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 1522–1531. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Angelidou, A.; Alysandratos, K.D.; Zhang, B.; Asadi, S.; Francis, K.; Toniato, E.; Kalogeromitros, D. Mast cell activation and autism. Biochim. Biophys. Acta 2012, 1822, 34–41. [Google Scholar] [CrossRef]
- Olszewski, M.B.; Groot, A.J.; Dastych, J.; Knol, E.F. TNF trafficking to human mast cell granules: Mature chain-dependent endocytosis. J. Immunol. 2007, 178, 5701–5709. [Google Scholar] [CrossRef]
- Kunder, C.A.; John, A.L.S.; Li, G.; Leong, K.W.; Berwin, B.; Staats, H.F.; Abraham, S.N. Mast cell–derived particles deliver peripheral signals to remote lymph nodes. J. Exp. Med. 2009, 206, 2455–2467. [Google Scholar] [CrossRef] [PubMed]
- Nakae, S.; Suto, H.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc. Natl. Acad. Sci. USA 2005, 102, 6467–6472. [Google Scholar] [CrossRef]
- Kempuraj, D.; Tagen, M.; Iliopoulou, B.P.; Clemons, A.; Vasiadi, M.; Boucher, W.; House, M.; Wolfberg, A.; Theoharides, T.C. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell-dependent stimulation of Jurkat T cells. J. Cereb. Blood Flow Metab. 2008, 155, 1076–1084. [Google Scholar] [CrossRef]
- Suurmond, J.; Dorjee, A.L.; Boon, M.R.; Knol, E.F.; Huizinga, T.W.; Toes, R.E.; Schuerwegh, A.J. Mast cells are the main interleukin 17-positive cells in anticitrullinated protein anti-body-positive and -negative rheumatoid arthritis and osteoarthritis synovium. Arthritis Res. Ther. 2011, 13, R150. [Google Scholar] [CrossRef] [PubMed]
- Nakae, S.; Suto, H.; Berry, G.J.; Galli, S.J. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in oval-bumin-challenged OTII mice. Blood 2007, 109, 3640–3648. [Google Scholar] [CrossRef]
- Kenna, T.; A Brown, M. The role of IL-17-secreting mast cells in inflammatory joint disease. Nat. Rev. Rheumatol. 2012, 9, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Doe, C.; Woodman, L.; Wan, W.-Y.H.; Sutcliffe, A.; Hollins, F.; Brightling, C. Mast Cell-Airway Smooth Muscle Crosstalk: The Role of Thymic Stromal Lymphopoietin. Chest 2012, 142, 76–85. [Google Scholar] [CrossRef]
- Hsu, C.L.; Chhiba, K.D.; Krier-Burris, R.; Hosakoppal, S.; Berdnikovs, S.; Miller, M.L.; Bryce, P.J. Allergic inflammation is initiated by IL-33-dependent crosstalk be-tween mast cells and basophils. PLoS ONE 2020, 15, e0226701. [Google Scholar] [CrossRef]
- Milovanovic, M.; Volarevic, V.; Radosavljevic, G.; Jovanovic, I.; Pejnovic, N.; Arsenijevic, N.; Lukic, M.L. IL-33/ST2 axis in inflammation and immunopathology. Immunol. Res. 2012, 52, 89–99. [Google Scholar] [CrossRef]
- Pei, C.; Barbour, M.; Fairlie-Clarke, K.J.; Allan, D.; Mu, R.; Jiang, H.-R. Emerging role of interleukin-33 in autoimmune diseases. Immunology 2013, 141, 9–17. [Google Scholar] [CrossRef]
- Hueber, A.J.; Alves-Filho, J.C.; Asquith, D.L.; Michels, C.; Millar, N.L.; Reilly, J.H.; Graham, G.J.; Liew, F.Y.; Miller, A.M.; McInnes, I.B. IL-33 induces skin inflammation with mast cell and neutrophil activation. Eur. J. Immunol. 2011, 41, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; La Rocca, M.; De Bernardo, M.; Rosa, N.; Sbordone, C.; Sbordone, L. Possible Association of Periodontal Disease and Macular Degeneration: A Case-Control Study. Dent. J. 2020, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, L.; Gatta, A.; Farinelli, A.; Scarano, G.; Lumaca, A.; Tinari, N.; Cipollone, F.; Paganelli, R.; Di Gioacchino, M. Allergooncology: An expanding research area. J. Biol. Regul. Homeost. Agents 2020, 34, 319–326. [Google Scholar] [PubMed]
- Yu, S.-L.; Wong, C.-K.; Tam, L.-S. The alarmin functions of high-mobility group box-1 and IL-33 in the pathogenesis of systemic lupus erythematosus. Expert Rev. Clin. Immunol. 2013, 9, 739–749. [Google Scholar] [CrossRef]
- Awada, A.; Nicaise, C.; Ena, S.; Schandéné, L.; Rasschaert, J.; Popescu, I.; Gangji, V.; Soyfoo, M.S. Potential involvement of the IL-33-ST2 axis in the pathogenesis of primary Sjogren’s syndrome. Ann. Rheum. Dis. 2014, 73, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Celik, H.T.; Abusoglu, S.; Burnik, S.F.; Sezer, S.; Serdar, M.A.; Ercan, M.; Uguz, N.; Avcıkucuk, M.; Ceylan, B.; Yildirimkaya, M. Increased serum interleukin-33 levels in patients with Graves’ disease. Endocr. Regul. 2013, 47, 57–64. [Google Scholar] [CrossRef]
- Beltrán, C.J.; Núñez, L.E.; Díaz-Jiménez, D.; Farfan, N.; Candia, E.; Heine, C.; López, F.; González, M.J.; Quera, R.; Hermoso, M.A. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 1097–1107. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Franke, A.; McGovern, D.P.; Barrett, J.C.; Wang, K.; Radford-Smith, G.L.; Ahmad, T.; Lees, C.W.; Balschun, T.; Lee, J.; Roberts, R. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 2010, 42, 1118–1125. [Google Scholar] [CrossRef]
- Hirota, T.; Takahashi, A.; Kubo, M.; Tsunoda, T.; Tomita, K.; Sakashita, M.; Yamada, T.; Fujieda, S.; Tanaka, S.; Doi, S.; et al. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat. Genet. 2012, 44, 1222–1226. [Google Scholar] [CrossRef]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Sismanopoulos, N.; Delivanis, D.-A.; Alysandratos, K.-D.; Angelidou, A.; Therianou, A.; Kalogeromitros, D.; Theoharides, T.C. Mast Cells in Allergic and Inflammatory Diseases. Curr. Pharm. Des. 2012, 18, 2261–2277. [Google Scholar] [CrossRef] [PubMed]
- Rottem, M.; Mekori, Y.A. Mast cells and autoimmunity. Autoimmun. Rev. 2005, 4, 21–27. [Google Scholar] [CrossRef] [PubMed]
- González-de-Olano, D.; Álvarez-Twose, I. Mast Cells as Key Players in Allergy and Inflammation. J. Investig. Allergol. Clin. Immunol. 2018, 28, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Vasiadi, M.; Mondolfi, A.; Alysandratos, K.-D.; Therianou, A.; Katsarou-Katsari, A.; Petrakopoulou, T.; Theoharidis, A.; Miniati, A.; Theoharides, T. Neurotensin serum levels and skin gene expression are increased in atopic dermatitis. Br. J. Dermatol. 2013, 169, 695–699. [Google Scholar] [CrossRef]
- Askenase, P.W. Mast Cells and the Mediation of T-Cell Recruitment in Arthritis. N. Engl. J. Med. 2003, 349, 1294. [Google Scholar] [CrossRef]
- Karagkouni, A.; Alevizos, M.; Theoharides, T.C. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun. Rev. 2013, 12, 947–953. [Google Scholar] [CrossRef]
- Theoharides, T.C. Is a subtype of autism “allergy of the brain”? Clin. Ther. 2013, 35, 584–591. [Google Scholar] [CrossRef]
- Olivera, A.; Beaven, M.A.; Metcalfe, D.D. Mast Cells Signal Their Importance in Health and Disease. J. Allergy Clin. Immunol. 2018, 142, 381–393. [Google Scholar] [CrossRef]
- Xiong, Z.; Thangavel, R.; Kempuraj, D.; Yang, E.; Zaheer, S.; Zaheer, A. Alzheimer’s disease: Evidence for the expression of inter-leukin-33 and its receptor ST2 in the brain. J. Alzheimers Dis. 2014, 40, 297–308. [Google Scholar] [CrossRef]
- Xie, D.; Liu, H.; Xu, F.; Su, W.; Ye, Q.; Yu, F.; Austin, T.J.; Chen, J.; Hu, X. IL33 (Interleukin 33)/ST2 (Interleukin 1 Receptor-like 1) Axis Drives Protective Microglial Responses and Promotes White Matter Integrity after Stroke. Stroke 2021, 52, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.S.; Barger, S.W. Neuroinflammatory Cytokines-The Common Thread in Alzheimer’s Pathogenesis. US Neurol. 2010, 6, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.S. Neuroinflammatory cytokine signaling and Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 770–771. [Google Scholar] [CrossRef]
- Kozauer, N.; Katz, R. Regulatory innovation and drug development for early-stage Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 1169–1171. [Google Scholar] [CrossRef] [PubMed]
- Christophi, G.P.; Gruber, R.C.; Panos, M.; Christophi, R.L.; Jubelt, B.; Massa, P.T. Interleukin-33 upregulation in peripheral leukocytes and CNS of multiple sclerosis patients. Clin. Immunol. 2012, 142, 308–319. [Google Scholar] [CrossRef]
- Zhang, F.; Tossberg, J.T.; Spurlock, C.F.; Yao, S.; Aune, T.M.; Sriram, S. Expression of IL-33 and its epigenetic regulation in multiple sclerosis. Ann. Clin. Transl. Neurol. 2014, 1, 307–318. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Zhang, B. Neuro-Inflammation, blood-brain barrier, seizures and autism. J. Neuroinflammation 2011, 8, 168. [Google Scholar] [CrossRef]
- Hox, V.; Vanoirbeek, J.A.; Alpizar, Y.A.; Voedisch, S.; Callebaut, I.; Bobic, S.; Sharify, A.; De Vooght, V.; Van Gerven, L.; Devos, F.; et al. Crucial Role of Transient Receptor Potential Ankyrin 1 and Mast Cells in Induction of Nonallergic Airway Hyperreactivity in Mice. Am. J. Respir. Crit. Care Med. 2013, 187, 486–493. [Google Scholar] [CrossRef]
- Iossifov, I.; O’Roak, B.J.; Sanders, S.J.; Ronemus, M.; Krumm, N.; Levy, D.; Stessman, H.A.; Witherspoon, K.T.; Vives, L.; Patterson, K.E.; et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014, 515, 216–221. [Google Scholar] [CrossRef]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Ercument Cicek, A.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Angelidou, A.; Alysandratos, K.D.; Asadi, S.; Zhang, B.; Francis, K.; Vasiadi, M.; Kalogeromitros, D.; Theoharides, T.C. Brief Report: “Allergic Symptoms” in children with Autism Spectrum Disorders. More than meets the eye? J. Autism Dev. Disord. 2011, 41, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Autism spectrum disorders and mastocytosis. Int. J. Immunopathol. Pharmacol. 2009, 22, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Angelidou, A.; Alysandratos, K.-D.; Vasiadi, M.; Francis, K.; Asadi, S.; Theoharides, A.; Sideri, K.; Lykouras, L.; Kalogeromitros, D.; et al. Mitochondrial DNA and anti-mitochondrial antibodies in serum of autistic children. J. Neuroinflammation 2010, 7, 80. [Google Scholar] [CrossRef]
- Ashwood, P.; Van de Water, J. A review of autism and the immune response. Clin. Dev. Immunol. 2004, 11, 165–174. [Google Scholar] [CrossRef]
- Gupta, S.; Ellis, S.; Ashar, F.N.; Moes, A.; Bader, J.S.; Zhan, J.; West, A.B.; Arking, D.E. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 2014, 5, 5748. [Google Scholar] [CrossRef]
- Suzuki, K.; Sugihara, G.; Ouchi, Y.; Nakamura, K.; Futatsubashi, M.; Takebayashi, K.; Yoshihara, Y.; Omata, K.; Matsumoto, K.; Tsuchiya, K.; et al. Microglial Activation in Young Adults with Autism Spectrum Disorder. JAMA Psychiatry 2013, 70, 49–58. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Giusti, P. Mast cells, glia and neuroinflammation: Partners in crime? Immunology 2013, 141, 314–327. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Cochrane, D.E. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J. Neuroimmunol. 2004, 146, 1–12. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Enakuaa, S.; Sismanopoulos, N.; Asadi, S.; Papadimas, E.C.; Angelidou, A.; Alysandratos, K.-D. Contribution of stress to asthma worsening through mast cell activation. Ann. Allergy Asthma Immunol. 2012, 109, 14–19. [Google Scholar] [CrossRef]
- Vasiadi, M.; Therianou, A.; Sideri, K.; Smyrnioti, M.; Sismanopoulos, N.; Delivanis, D.A.; Asadi, S.; Katsarou-Katsari, A.; Petrakopoulou, T.; Theoharides, A.; et al. Increased serum CRH levels with decreased skin CRH-R1 gene expression in psoriasis and atopic dermatitis. J. Allergy Clin. Immunol. 2012, 129, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Papadopoulou, N.; Kempuraj, D.; Boucher, W.S.; Sugimoto, K.; Cetrulo, C.L.; Theoharides, T.C. Human Mast Cells Express Corticotropin-Releasing Hormone (CRH) Receptors and CRH Leads to Selective Secretion of Vascular Endothelial Growth Factor. J. Immunol. 2005, 174, 7665–7675. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Donelan, J.M.; Papadopoulou, N.; Cao, J.; Kempuraj, D.; Conti, P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol. Sci. 2004, 25, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A.; Bufler, P. Interleukin-37. Semin. Immunol. 2013, 25, 466–468. [Google Scholar] [CrossRef]
- Kritas, S.K.; Ronconi, G.; Caraffa, A.L.; Gallenga, C.E.; Ross, R.; Conti, P. Mast cells contribute to coronavirus-induced inflammation: New anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents 2020, 34, 9–14. [Google Scholar]
- Conti, P.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Ronconi, G. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: A promising inhibitory strategy. J. Biol. Regul. Homeost. Agents 2020, 34, 1971–1975. [Google Scholar]
- Zhao, M.; Li, Y.; Guo, C.; Wang, L.; Chu, H.; Zhu, F.; Li, Y.; Wang, X.; Wang, Q.; Zhao, W.; et al. IL-37 isoform D downregulates pro-inflammatory cytokines expression in a Smad3-dependent manner. Cell Death Dis. 2018, 9, 582. [Google Scholar] [CrossRef]
- Li, S.; Amo-Aparicio, J.; Neff, C.P.; Tengesdal, I.W.; Azam, T.; Palmer, B.E.; López-Vales, R.; Bufler, P.; Dinarello, C.A. Role for nuclear interleukin-37 in the suppression of innate immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 4456–4461. [Google Scholar] [CrossRef]
- Cavalli, G.; Dinarello, C.A. Suppression of inflammation and acquired immunity by IL-37. Immunol. Rev. 2017, 281, 179–190. [Google Scholar] [CrossRef]
- Kumar, S.; Hanning, C.R.; Brigham-Burke, M.R.; Rieman, D.J.; Lehr, R.; Khandekar, S.; Kirkpatrick, R.B.; Scott, G.F.; Lee, J.C.; Lynch, F.J.; et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 2002, 18, 61–71. [Google Scholar] [CrossRef]
- Nam, S.W.; Kang, S.; Lee, J.H.; Yoo, D.H. Different Features of Interleukin-37 and Interleukin-18 as Disase Activity Markers of Adult-Onset Still’s Disease. J. Clin. Med. 2021, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Liu, J.; Han, B. Reviews of Interleukin-37: Functions, Receptors, and Roles in Diseases. BioMed Res. Int. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Bufler, P.; Azam, T.; Gamboni-Robertson, F.; Reznikov, L.L.; Kumar, S.; Dinarello, C.A.; Kim, S.-H. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc. Natl. Acad. Sci. USA 2002, 99, 13723–13728. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Justice, J.N.; Boyle, K.E.; D’Alessandro, A.; Eisenmesser, E.Z.; Herrera, J.J.; Hansen, K.C.; Nemkov, T.; Stienstra, R.; Garlanda, C.; et al. Interleukin 37 reverses the metabolic cost of inflammation, increases oxidative respiration, and improves exercise tolerance. Proc. Natl. Acad. Sci. USA 2017, 114, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A.; Nold-Petry, C.; Nold, M.; Fujita, M.; Philip, B.; Kim, S.; Bufler, P. Suppression of innate inflammation and immunity by interleukin-37. Eur. J. Immunol. 2016, 46, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Neff, C.P.; Barber, K.; Hong, J.; Luo, Y.; Azam, T.; Palmer, B.E.; Fujita, M.; Garlanda, C.; Mantovani, A.; et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc. Natl. Acad. Sci. USA 2015, 112, 2497–2502. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trimarchi, M.; Lauritano, D.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Frydas, I.; Kritas, S.K.; Calvisi, V.; Conti, P. Mast Cell Cytokines in Acute and Chronic Gingival Tissue Inflammation: Role of IL-33 and IL-37. Int. J. Mol. Sci. 2022, 23, 13242. https://doi.org/10.3390/ijms232113242
Trimarchi M, Lauritano D, Ronconi G, Caraffa A, Gallenga CE, Frydas I, Kritas SK, Calvisi V, Conti P. Mast Cell Cytokines in Acute and Chronic Gingival Tissue Inflammation: Role of IL-33 and IL-37. International Journal of Molecular Sciences. 2022; 23(21):13242. https://doi.org/10.3390/ijms232113242
Chicago/Turabian StyleTrimarchi, Matteo, Dorina Lauritano, Gianpaolo Ronconi, Alessandro Caraffa, Carla E. Gallenga, Ilias Frydas, Spyros K. Kritas, Vittorio Calvisi, and Pio Conti. 2022. "Mast Cell Cytokines in Acute and Chronic Gingival Tissue Inflammation: Role of IL-33 and IL-37" International Journal of Molecular Sciences 23, no. 21: 13242. https://doi.org/10.3390/ijms232113242
APA StyleTrimarchi, M., Lauritano, D., Ronconi, G., Caraffa, A., Gallenga, C. E., Frydas, I., Kritas, S. K., Calvisi, V., & Conti, P. (2022). Mast Cell Cytokines in Acute and Chronic Gingival Tissue Inflammation: Role of IL-33 and IL-37. International Journal of Molecular Sciences, 23(21), 13242. https://doi.org/10.3390/ijms232113242







