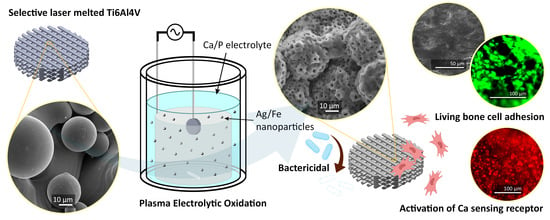
Preventing Antibiotic-Resistant Infections: Additively Manufactured Porous Ti6Al4V Biofunctionalized with Ag and Fe Nanoparticles
Abstract
1. Introduction
2. Results
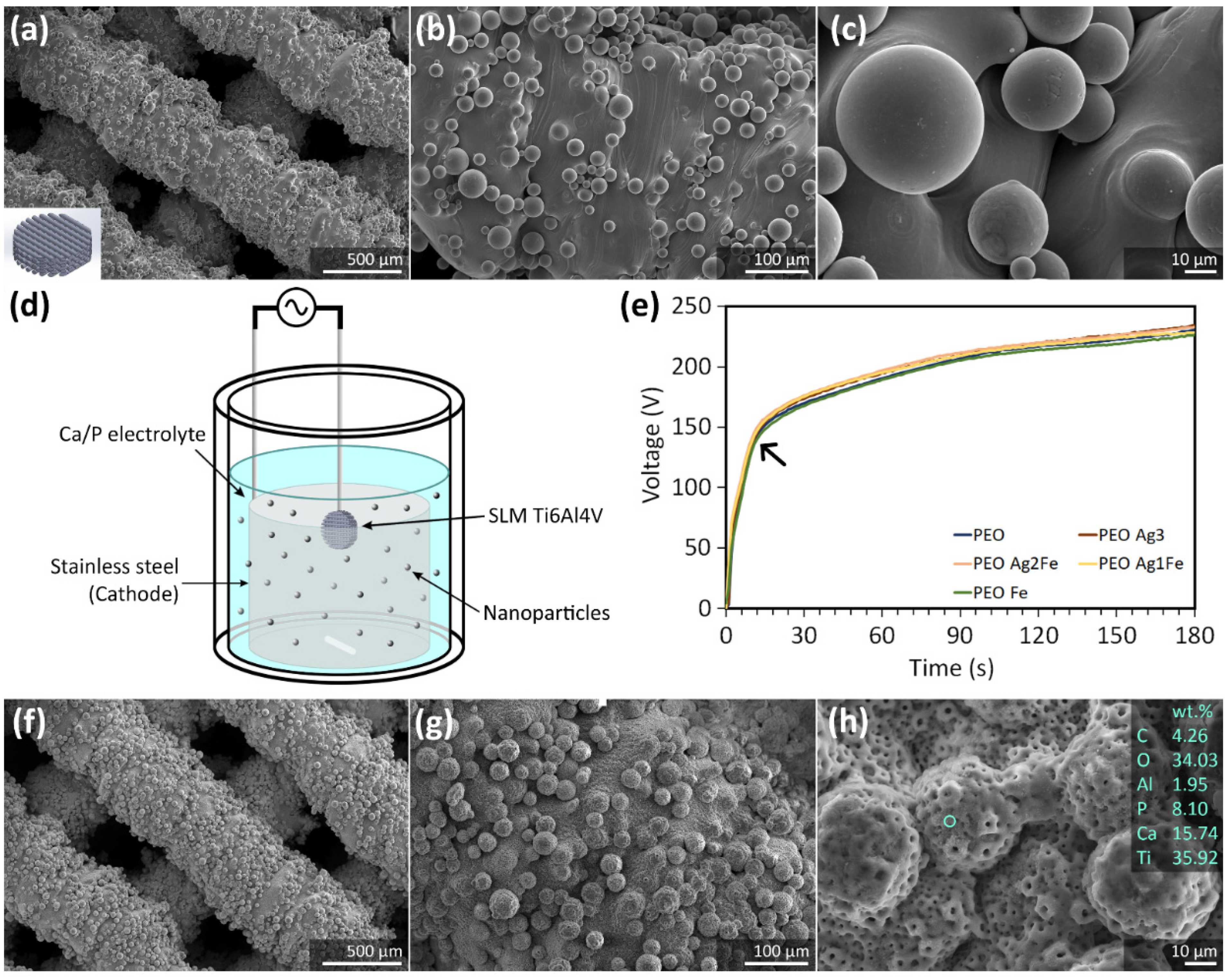
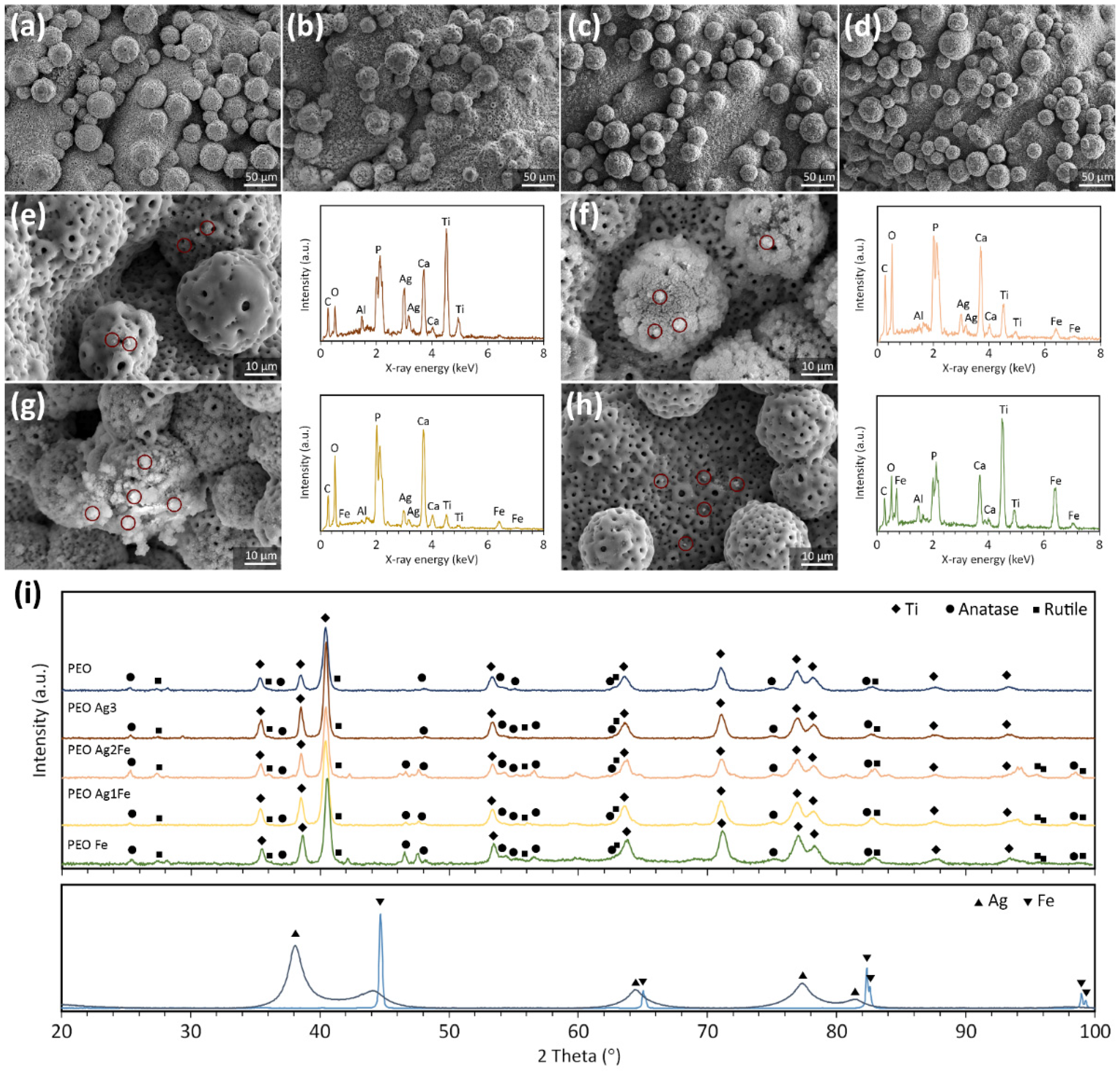
2.1. Surface Biofunctionalization of Ti6Al4V Scaffolds
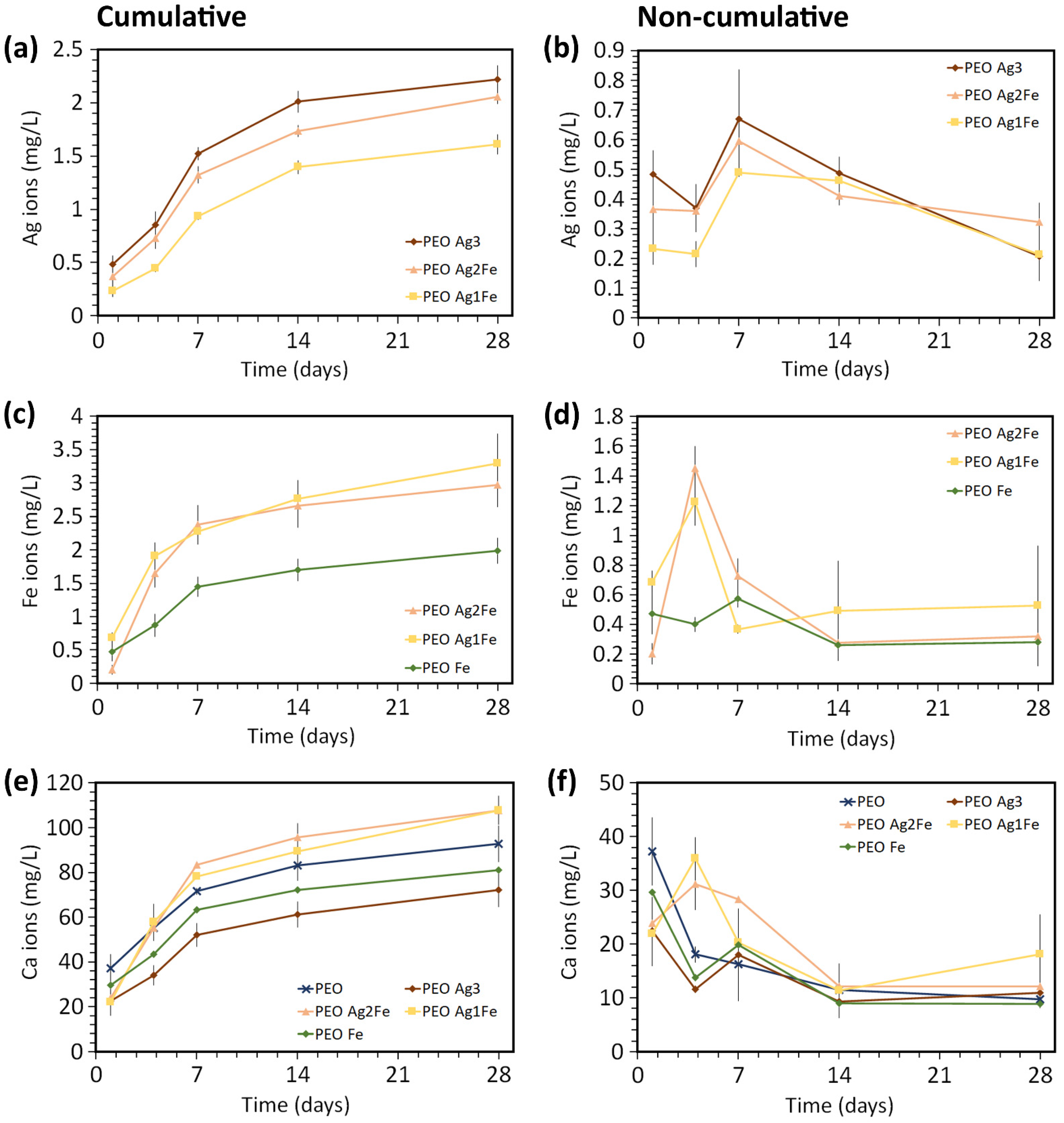
2.2. Ag, Fe, and Ca Ion Release Kinetics
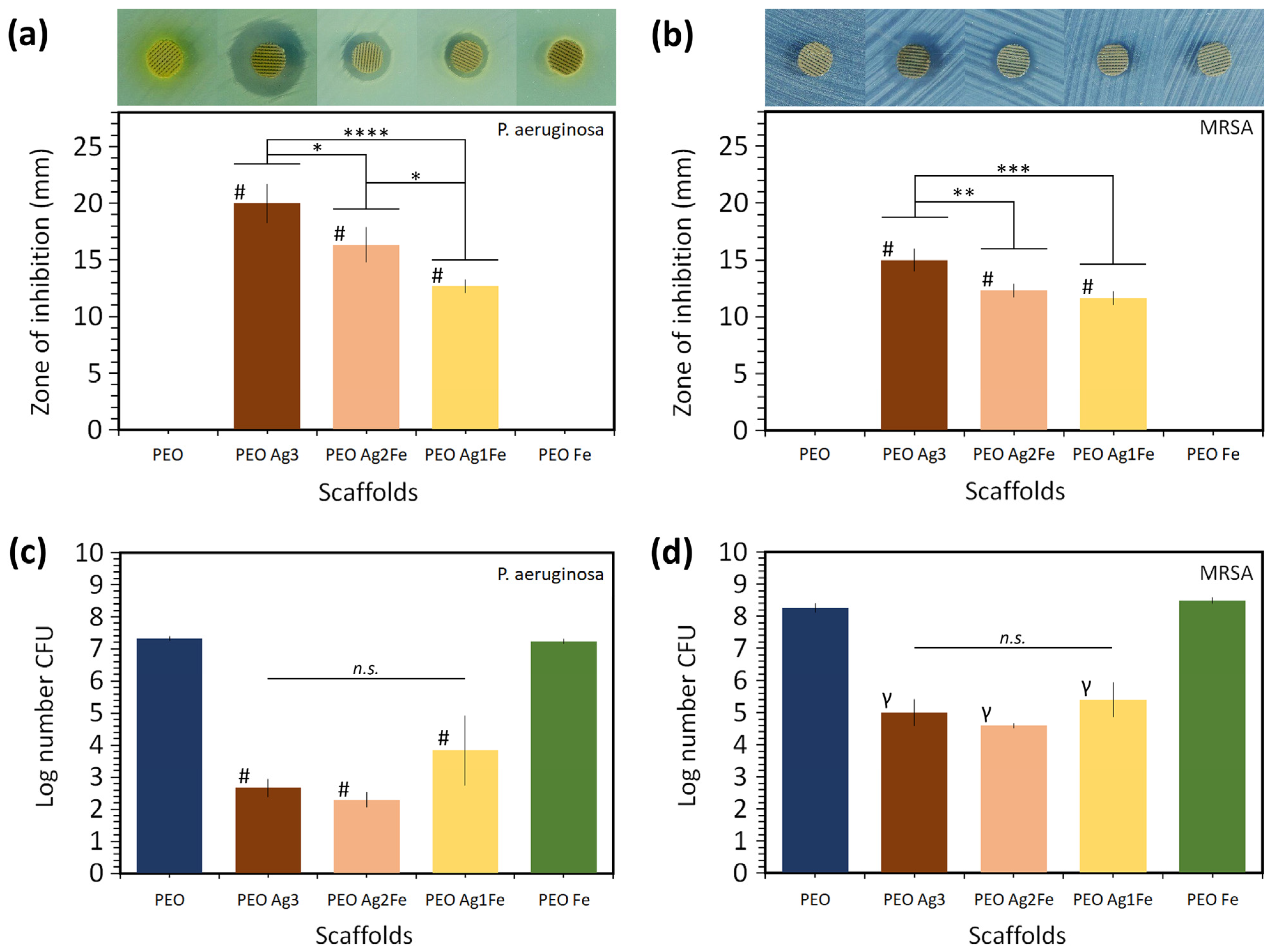
2.3. Antibacterial Properties against P. aeruginosa and MRSA
2.3.1. Zone of Inhibition
2.3.2. Bactericidal Activity
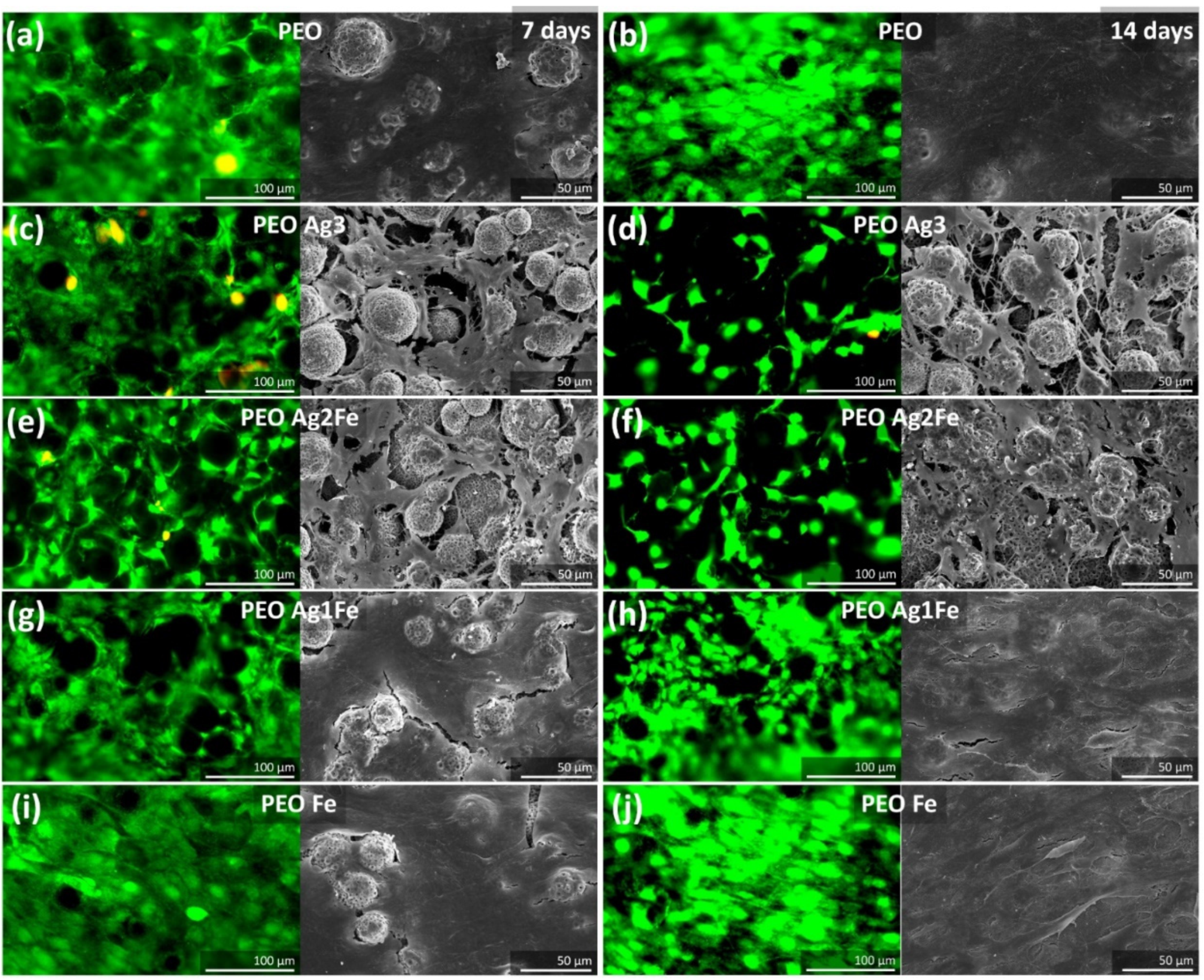
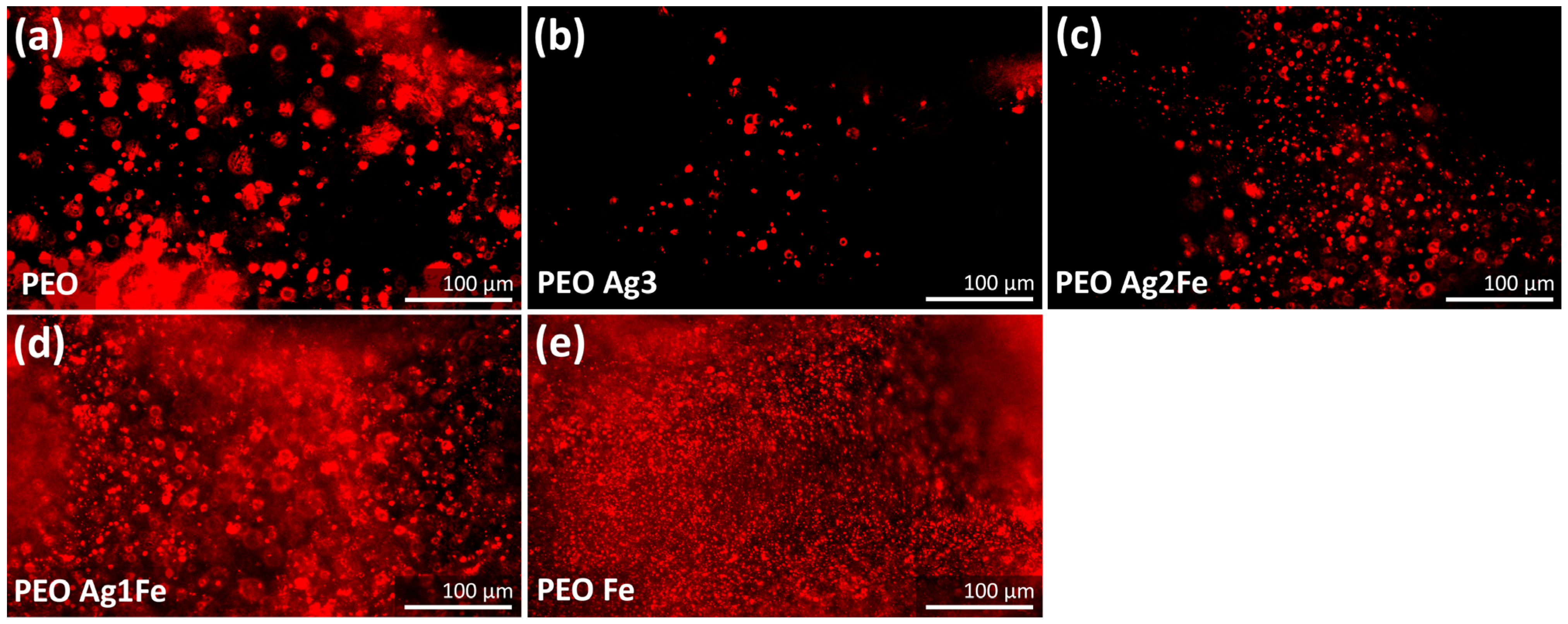
2.4. Cytocompatibility
2.4.1. Cell Proliferation and Morphology
2.4.2. Immunostaining of Phospho-Calcium Sensing Receptors
3. Discussion
4. Materials and Methods
4.1. Scaffold Design and Selective Laser Melting
4.2. Plasma Electrolytic Oxidation (PEO)
4.3. Characterization of Surface Morphology, Porosity, and Phase Composition
4.4. Release of Ag, Fe, and Ca Ions
4.5. Antibacterial Assays
4.5.1. Agar Diffusion
4.5.2. Quantitative Bactericidal Activity
4.6. Cytocompatibility
4.6.1. Preculture of Cells and Cell Seeding
4.6.2. Live/Dead Staining and SEM Imaging
4.6.3. Immunostaining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricciardi, B.F.; Muthukrishnan, G.; Masters, E.; Ninomiya, M.; Lee, C.C.; Schwarz, E.M. Staphylococcus Aureus Evasion of Host Qimmunity in the Setting Ofprosthetic Joint Infection: Biofilm and Beyond. Curr. Rev. Musculoskelet. Med. 2018, 11, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Webster, T.J. Bacteria Antibiotic Resistance: New Challenges and Opportunities for Implant-Associated Orthopedic Infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The Significance of Infection Related to Orthopedic Devices and Issues of Antibiotic Resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, V.; Chittaranjan, S.; Kurian, V.M.; Doble, M. Characteristics of Bacterial Biofilm Associated with Implant Material in Clinical Practice. Polym. J. 2013, 45, 137–152. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant Infections: Adhesion, Biofilm Formation and Immune Evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ehrlich, G.D.; Montanaro, L. Biofilm-Based Implants Infections in Orthopaedics. In Biofilm-Based Healthcare-Associated Infections; Springer: Cham, Switzerland, 2015; Volume I, pp. 29–46. [Google Scholar] [CrossRef]
- Sudduth, J.D.; Moss, J.A.; Spitler, C.A.; Pham, V.L.H.; Jones, L.C.; Brown, J.T.; Bergin, P.F. Open Fractures: Are We Still Treating the Same Types of Infections? Surg. Infect. 2020, 21, 766–772. [Google Scholar] [CrossRef]
- Patrulea, V.; Gan, B.H.; Perron, K.; Cai, X.; Abdel-Sayed, P.; Sublet, E.; Ducret, V.; Nerhot, N.P.; Applegate, L.A.; Borchard, G.; et al. Synergistic Effects of Antimicrobial Peptide Dendrimer-Chitosan Polymer Conjugates against Pseudomonas Aeruginosa. Carbohydr. Polym. 2022, 280, 119025. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Van Hengel, I.A.J.; Putra, N.E.; Tierolf, M.W.A.M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Biofunctionalization of Selective Laser Melted Porous Titanium Using Silver and Zinc Nanoparticles to Prevent Infections by Antibiotic-Resistant Bacteria. Acta Biomater. 2020, 107, 325–337. [Google Scholar] [CrossRef]
- Arabnejad, S.; Burnett Johnston, R.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-Strength Porous Biomaterials for Bone Replacement: A Strategy to Assess the Interplay between Cell Morphology, Mechanical Properties, Bone Ingrowth and Manufacturing Constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Van Hengel, I.A.J.; Gelderman, F.S.A.; Athanasiadis, S.; Minneboo, M.; Weinans, H.; Fluit, A.C.; van der Eerden, B.C.J.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Functionality-Packed Additively Manufactured Porous Titanium Implants. Mater. Today Bio 2020, 7, 100060. [Google Scholar] [CrossRef] [PubMed]
- Amin Yavari, S.; Croes, M.; Akhavan, B.; Jahanmard, F.; Eigenhuis, C.C.; Dadbakhsh, S.; Vogely, H.C.; Bilek, M.M.; Fluit, A.C.; Boel, C.H.E.; et al. Layer by Layer Coating for Bio-Functionalization of Additively Manufactured Meta-Biomaterials. Addit. Manuf. 2020, 32, 100991. [Google Scholar] [CrossRef]
- Bakhshandeh, S.; Gorgin Karaji, Z.; Lietaert, K.; Fluit, A.C.; Boel, C.H.E.; Vogely, H.C.; Vermonden, T.; Hennink, W.E.; Weinans, H.; Zadpoor, A.A.; et al. Simultaneous Delivery of Multiple Antibacterial Agents from Additively Manufactured Porous Biomaterials to Fully Eradicate Planktonic and Adherent Staphylococcus Aureus. ACS Appl. Mater. Interfaces 2017, 9, 25691–25699. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver Nanoparticles: The Powerful Nanoweapon against Multidrug-Resistant Bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Núñez, N.V.; del Turrent, L.C.I.; Padilla, C.R. Bactericidal Effect of Silver Nanoparticles against Multidrug-Resistant Bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The Toxic Effect of Silver Ions and Silver Nanoparticles towards Bacteria and Human Cells Occurs in the Same Concentration Range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Akiyama, T.; Miyamoto, H.; Yonekura, Y.; Tsukamoto, M.; Ando, Y.; Noda, I.; Sonohata, M.; Mawatari, M. Silver Oxide-Containing Hydroxyapatite Coating Has In Vivo Antibacterial Activity in the Rat Tibia. J. Orthop. Res. 2013, 31, 1195–1200. [Google Scholar] [CrossRef]
- Sheehan, E.; McKenna, J.; Mulhall, K.J.; Marks, P.; McCormack, D. Adhesion of Staphylococcus to Orthopaedic Metals, an in Vivo Study. J. Orthop. Res. 2007, 22, 39–43. [Google Scholar] [CrossRef]
- Croes, M.; Bakhshandeh, S.; van Hengel, I.A.J.; Lietaert, K.; van Kessel, K.P.M.; Pouran, B.; van der Wal, B.C.H.; Vogely, H.C.; van Hecke, W.; Fluit, A.C.; et al. Antibacterial and Immunogenic Behavior of Silver Coatings on Additively Manufactured Porous Titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; McShan, D.; Zhang, Y.; Sinha, S.S.; Arslan, Z.; Ray, P.C.; Yu, H. Mechanistic Study of the Synergistic Antibacterial Activity of Combined Silver Nanoparticles and Common Antibiotics. Environ. Sci. Technol. 2016, 50, 8840–8848. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Grumezescu, A.M.; Holban, A.M. Magnetite Nanostructures as Novel Strategies for Anti-Infectious Therapy. Molecules 2014, 19, 12710–12726. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; De Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Pan, W.Y.; Huang, C.C.; Lin, T.T.; Hu, H.Y.; Lin, W.C.; Li, M.J.; Sung, H.W. Synergistic Antibacterial Effects of Localized Heat and Oxidative Stress Caused by Hydroxyl Radicals Mediated by Graphene/Iron Oxide-Based Nanocomposites. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 431–438. [Google Scholar] [CrossRef]
- Grumezescu, V.; Holban, A.M.; Iordache, F.; Socol, G.; Mogoşanu, G.D.; Grumezescu, A.M.; Ficai, A.; Vasile, B.Ş.; Truşcǎ, R.; Chifiriuc, M.C.; et al. MAPLE Fabricated Magnetite@eugenol and (3-Hidroxybutyric Acid-Co-3-Hidroxyvaleric Acid)-Polyvinyl Alcohol Microspheres Coated Surfaces with Anti-Microbial Properties. Appl. Surf. Sci. 2014, 306, 16–22. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, K.; Zhou, Z.; Li, Q.; Shao, L.; Hao, R.Z.; Xiao, R.; Wang, S. Vancomycin-Modified Fe3O4@SiO2@Ag Microflowers as Effective Antimicrobial Agents. Int. J. Nanomed. 2017, 12, 3077–3094. [Google Scholar] [CrossRef]
- Touati, D. Iron and Oxidative Stress in Bacteria. Arch. Biochem. Biophys. 2000, 373, 1–6. [Google Scholar] [CrossRef]
- Tran, N.; Mir, A.; Mallik, D.; Sinha, A.; Nayar, S.; Webster, T.J. Bactericidal Effect of Iron Oxide Nanoparticles on Staphylococcus Aureus. Int. J. Nanomed. 2010, 5, 277–283. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial Reactive Oxygen and Nitrogen Species: Concepts and Controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef]
- Feng, B.; Weng, J.; Yang, B.C.; Qu, S.X.; Zhang, X.D. Characterization of Titanium Surfaces with Calcium and Phosphate and Osteoblast Adhesion. Biomaterials 2004, 25, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Necula, B.S.; van Leeuwen, J.P.T.M.; Fratila-Apachitei, L.E.; Zaat, S.A.J.; Apachitei, I.; Duszczyk, J. In Vitro Cytotoxicity Evaluation of Porous TiO2-Ag Antibacterial Coatings for Human Fetal Osteoblasts. Acta Biomater. 2012, 8, 4191–4197. [Google Scholar] [CrossRef] [PubMed]
- Necula, B.S.; Fratila-Apachitei, L.E.; Zaat, S.A.J.; Apachitei, I.; Duszczyk, J. In Vitro Antibacterial Activity of Porous TiO2-Ag Composite Layers against Methicillin-Resistant Staphylococcus Aureus. Acta Biomater. 2009, 5, 3573–3580. [Google Scholar] [CrossRef]
- Necula, B.S.; Apachitei, I.; Tichelaar, F.D.; Fratila-Apachitei, L.E.; Duszczyk, J. An Electron Microscopical Study on the Growth of TiO2-Ag Antibacterial Coatings on Ti6Al7Nb Biomedical Alloy. Acta Biomater. 2011, 7, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.B.; Baldin, E.K.; Krieger, D.A.; de Castro, V.V.; Aguzzoli, C.; Fonseca, J.C.; Rodrigues, M.; Lopes, M.A.; Malfatti, C. de F. Wear Performance and Osteogenic Differentiation Behavior of Plasma Electrolytic Oxidation Coatings on Ti-6Al-4V Alloys: Potential Application for Bone Tissue Repairs. Surf. Coat. Technol. 2021, 417, 127179. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Gonzalez Tenorio, R.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Matykina, E. Tailoring of Antibacterial and Osteogenic Properties of Ti6Al4V by Plasma Electrolytic Oxidation. Appl. Surf. Sci. 2018, 454, 157–172. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Vail, D.T.P.; Berry, D.J. The Epidemiology of Revision Total Hip Arthroplasty in the United States. J. Bone Jt. Surg. Ser. A 2009, 91, 128–133. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The Epidemiology of Revision Total Knee Arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45–51. [Google Scholar] [CrossRef]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.; Gretzer, C. Aseptic Loosening, Not Only a Question of Wear: A Review of Different Theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; An, Y.H.; Baldassarri, L.; Pirini, V.; Donati, M.E.; Pegreffi, F.; Montanaro, L. Prevalence and Antibiotic Resistance of 15 Minor Staphylococcal Species Colonizing Orthopedic Implants. Int. J. Artif. Organs 2006, 29, 395–401. [Google Scholar] [CrossRef]
- Yuan, Z.; He, Y.; Lin, C.; Liu, P.; Cai, K. Antibacterial Surface Design of Biomedical Titanium Materials for Orthopedic Applications. J. Mater. Sci. Technol. 2021, 78, 51–67. [Google Scholar] [CrossRef]
- Gristina, A.G.; Naylor, P.; Myrvik, Q. Infections from Biomaterials and Implants: A Race for the Surface. Med. Prog. Technol. 1988, 14, 205–224. [Google Scholar] [PubMed]
- Rigo, S.; Cai, C.; Gunkel-Grabole, G.; Maurizi, L.; Zhang, X.; Xu, J.; Palivan, C.G. Nanoscience-Based Strategies to Engineer Antimicrobial Surfaces. Adv. Sci. 2018, 5, 1700892. [Google Scholar] [CrossRef] [PubMed]
- Mi, G.; Shi, D.; Wang, M.; Webster, T.J. Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces. Adv. Healthc. Mater. 2018, 7, 1800103. [Google Scholar] [CrossRef]
- Van Hengel, I.A.J.; Riool, M.; Fratila-Apachitei, L.E.; Witte-Bouma, J.; Farrell, E.; Zadpoor, A.A.; Zaat, S.A.J.; Apachitei, I. Selective Laser Melting Porous Metallic Implants with Immobilized Silver Nanoparticles Kill and Prevent Biofilm Formation by Methicillin-Resistant Staphylococcus Aureus. Biomaterials 2017, 140, 1–15. [Google Scholar] [CrossRef]
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial Resistance to Silver in Wound Care. J. Hosp. Infect. 2005, 60, 1–7. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial Resistance to Silver Nanoparticles and How to Overcome It. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.; Huang, X.; Zhao, L.; Zhang, X.; Wang, L.; Tang, B.; Ma, S.; Chu, P.K. The Effects of Titania Nanotubes with Embedded Silver Oxide Nanoparticles on Bacteria and Osteoblasts. Biomaterials 2014, 35, 4223–4235. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Activity in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 39, 755–756. [Google Scholar] [CrossRef]
- Lozhkomoev, A.S.; Lerner, M.I.; Pervikov, A.V.; Kazantsev, S.O.; Fomenko, A.N. Development of Fe/Cu and Fe/Ag Bimetallic Nanoparticles for Promising Biodegradable Materials with Antimicrobial Effect. Nanotechnologies Russ. 2018, 13, 18–25. [Google Scholar] [CrossRef]
- Padilla-Cruz, A.L.; Garza-Cervantes, J.A.; Vasto-Anzaldo, X.G.; García-Rivas, G.; León-Buitimea, A.; Morones-Ramírez, J.R. Synthesis and Design of Ag–Fe Bimetallic Nanoparticles as Antimicrobial Synergistic Combination Therapies against Clinically Relevant Pathogens. Sci. Rep. 2021, 11, 5351. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Gupta, I.; Chaud, M.V.; Dos Santos, C.A. Broadening the Spectrum of Small-Molecule Antibacterials by Metallic Nanoparticles to Overcome Microbial Resistance. Int. J. Pharm. 2017, 532, 139–148. [Google Scholar] [CrossRef]
- Dalzon, B.; Torres, A.; Diemer, H.; Ravanel, S.; Collin-Faure, V.; Pernet-Gallay, K.; Jouneau, P.H.; Bourguignon, J.; Cianférani, S.; Carrière, M.; et al. How Reversible Are the Effects of Silver Nanoparticles on Macrophages? A Proteomic-Instructed View. Environ. Sci. Nano 2019, 6, 3133–3157. [Google Scholar] [CrossRef]
- Uygur, B.; Craig, G.; Mason, M.D.; Ng, A.K. Cytotoxicity and Genotoxicity of Silver Nanomaterials. NSTI Nanotechnol. 2009, 2, 383–386. [Google Scholar]
- Ratledge, C.; Dover, L.G. Iron Metabolism in Pathogenic Bacteria. Annu. Rev. Microbiol. 2000, 54, 881–941. [Google Scholar] [CrossRef]
- Frawley, E.R.; Fang, F.C. The Ins and Outs of Bacterial Iron Metabolism. Mol. Microbiol. 2014, 93, 609–616. [Google Scholar] [CrossRef]
- Kronstad, J.W.; Caza, M. Shared and Distinct Mechanisms of Iron Acquisition by Bacterial and Fungal Pathogens of Humans. Front. Cell. Infect. Microbiol. 2013, 3, 80. [Google Scholar] [CrossRef]
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.S.; MacDonald, I.C.; Collins, J.J. Potentiating Antibacterial Activity by Predictably Enhancing Endogenous Microbial ROS Production. Nat. Biotechnol. 2013, 31, 160–165. [Google Scholar] [CrossRef]
- Arce Miranda, J.E.; Sotomayor, C.E.; Albesa, I.; Paraje, M.G. Oxidative and Nitrosative Stress in Staphylococcus Aureus Biofilm. FEMS Microbiol. Lett. 2011, 315, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, J.; Wu, Y.; Shen, F.; Yao, M. Biological Responses of Gram-Positive and Gram-Negative Bacteria to NZVI (Fe0), Fe2+ and Fe3+. RSC Adv. 2013, 3, 13835–13842. [Google Scholar] [CrossRef]
- Albesa, I.; Becerra, M.C.; Battán, P.C.; Páez, P.L. Oxidative Stress Involved in the Antibacterial Action of Different Antibiotics. Biochem. Biophys. Res. Commun. 2004, 317, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Hassett, D.J.; Imlay, J.A. Bactericidal Antibiotics and Oxidative Stress: A Radical Proposal. ACS Chem. Biol. 2007, 2, 708–710. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Z.; Yu, Q.; Ma, T. Preparation of Reactive Oxygen Species-Responsive Antibacterial Hydrogels for Efficient Anti-Infection Therapy. Mater. Lett. 2020, 263, 127254. [Google Scholar] [CrossRef]
- Yamamoto, A.; Honma, R.; Sumita, M. Cytotoxicity Evaluation of 43 Metal Salts Using Murine Fibroblasts and Osteoblastic Cells. J. Biomed. Mater. Res. 1998, 39, 331–340. [Google Scholar] [CrossRef]
- Cheshmedzhieva, D.; Ilieva, S.; Permyakov, E.A.; Permyakov, S.E.; Dudev, T. Ca2+/Sr2+ Selectivity in Calcium-Sensing Receptor (CaSR): Implications for Strontium’s Anti-Osteoporosis Effect. Biomolecules 2021, 11, 1576. [Google Scholar] [CrossRef]
- Barradas, A.M.C.; Fernandes, H.A.M.; Groen, N.; Chai, Y.C.; Schrooten, J.; van de Peppel, J.; van Leeuwen, J.P.T.M.; van Blitterswijk, C.A.; De Boer, J. A Calcium-Induced Signaling Cascade Leading to Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stromal Cells. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef]
- Pipino, C.; Tomo, P.D.; Mandatori, D.; Cianci, E.; Lanuti, P.; Cutrona, M.B.; Penolazzi, L.; Pierdomenico, L.; Lambertini, E.; Antonucci, I.; et al. Calcium Sensing Receptor (CaSR) Activation by Calcimimetic R-568 in Human Amniotic Fluid Mesenchymal Stem Cells (HAFMSCs): Correlation with Osteogenic Differentiation. Stem Cells Dev. 2014, 23, 2959–2971. [Google Scholar] [CrossRef]
- Yamauchi, M.; Yamaguchi, T.; Kaji, H.; Sugimoto, T.; Chihara, K. Involvement of Calcium-Sensing Receptor in Osteoblastic Differentiation of Mouse MC3T3-E1 Cells. Am. J. Physiol. Metab. 2005, 288, E608–E616. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chattopadhyay, N.; Kifor, O.; Ye, C.; Vassilev, P.M.; Sanders, J.L.; Brown, E.M. Expression of Extracellular Calcium-Sensing Receptor in Human Osteoblastic MG-63 Cell Line. Am. J. Physiol. Cell Physiol. 2001, 280, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.P.; Yamaguchi, T.; Chattopadhyay, N.; Sanders, J.L.; Vassilev, P.M.; Brown, E.M. Extracellular Calcium-Sensing-Receptor (CaR)-Mediated Opening of an Outward K+ Channel in Murine MC3T3-E1 Osteoblastic Cells: Evidence for Expression of a Functional CaR. Bone 2000, 27, 21–27. [Google Scholar] [CrossRef]
- Dvorak, M.M.; Riccardi, D. Ca2+ as an Extracellular Signal in Bone. Cell Calcium 2004, 35, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Reed, P.; Atilano, M.L.; Alves, R.; Hoiczyk, E.; Sher, X.; Reichmann, N.T.; Pereira, P.M.; Roemer, T.; Filipe, S.R.; Pereira-Leal, J.B.; et al. Staphylococcus Aureus Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance. PLoS Pathog. 2015, 11, e1004891. [Google Scholar] [CrossRef]
| Specimen Group | Calcium Acetate (M) | Calcium Glycerophosphate (M) | Ag NPs (g/L) | Fe NPs (g/L) |
|---|---|---|---|---|
| PEO | 0.15 | 0.2 | - | - |
| PEO Ag3 | 0.15 | 0.2 | 3 | - |
| PEO Ag2Fe | 0.15 | 0.2 | 1.5 | 0.5 |
| PEO Ag1Fe | 0.15 | 0.2 | 1 | 0.5 |
| PEO Fe | 0.15 | 0.2 | - | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra, N.E.; Leeflang, M.A.; Ducret, V.; Patrulea, V.; Fratila-Apachitei, L.E.; Perron, K.; Ye, H.; Zhou, J.; Apachitei, I.; Zadpoor, A.A. Preventing Antibiotic-Resistant Infections: Additively Manufactured Porous Ti6Al4V Biofunctionalized with Ag and Fe Nanoparticles. Int. J. Mol. Sci. 2022, 23, 13239. https://doi.org/10.3390/ijms232113239
Putra NE, Leeflang MA, Ducret V, Patrulea V, Fratila-Apachitei LE, Perron K, Ye H, Zhou J, Apachitei I, Zadpoor AA. Preventing Antibiotic-Resistant Infections: Additively Manufactured Porous Ti6Al4V Biofunctionalized with Ag and Fe Nanoparticles. International Journal of Molecular Sciences. 2022; 23(21):13239. https://doi.org/10.3390/ijms232113239
Chicago/Turabian StylePutra, Niko E., Marius A. Leeflang, Verena Ducret, Viorica Patrulea, Lidy E. Fratila-Apachitei, Karl Perron, Hua Ye, Jie Zhou, Iulian Apachitei, and Amir A. Zadpoor. 2022. "Preventing Antibiotic-Resistant Infections: Additively Manufactured Porous Ti6Al4V Biofunctionalized with Ag and Fe Nanoparticles" International Journal of Molecular Sciences 23, no. 21: 13239. https://doi.org/10.3390/ijms232113239
APA StylePutra, N. E., Leeflang, M. A., Ducret, V., Patrulea, V., Fratila-Apachitei, L. E., Perron, K., Ye, H., Zhou, J., Apachitei, I., & Zadpoor, A. A. (2022). Preventing Antibiotic-Resistant Infections: Additively Manufactured Porous Ti6Al4V Biofunctionalized with Ag and Fe Nanoparticles. International Journal of Molecular Sciences, 23(21), 13239. https://doi.org/10.3390/ijms232113239