Lamp1 Deficiency Enhances Sensitivity to α-Synuclein and Oxidative Stress in Drosophila Models of Parkinson Disease
Abstract
1. Introduction
2. Results
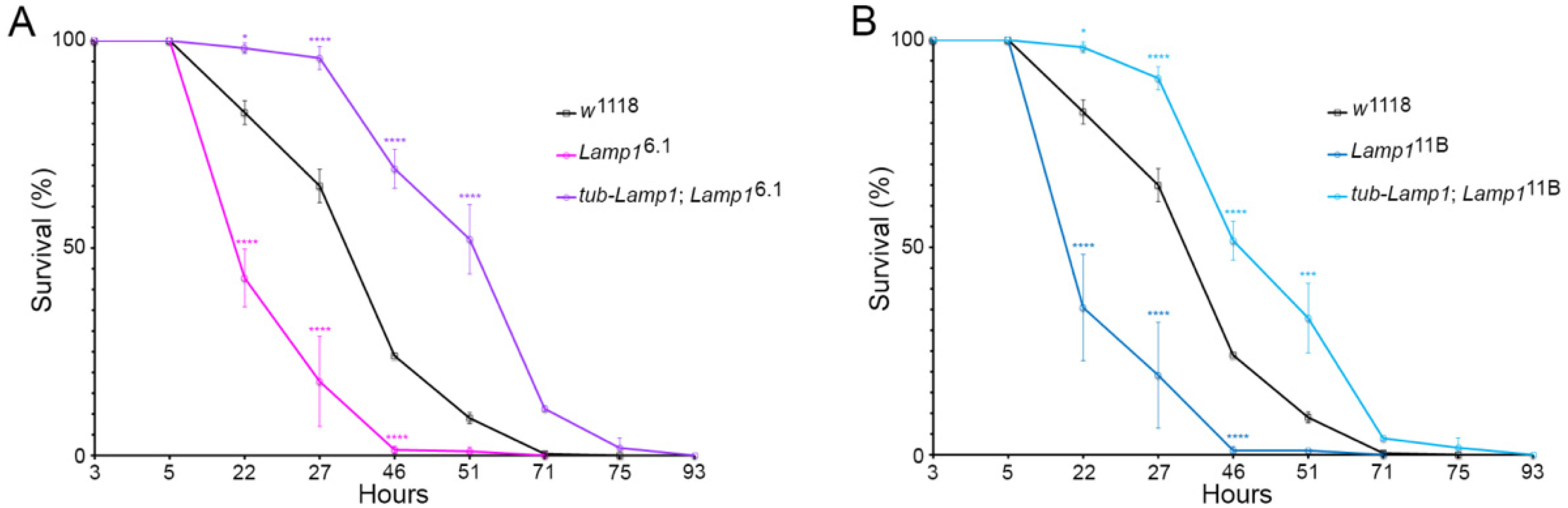
2.1. Drosophila Lamp1 Mutants Are Highly Sensitive to Paraquat Induced Oxidative Stress
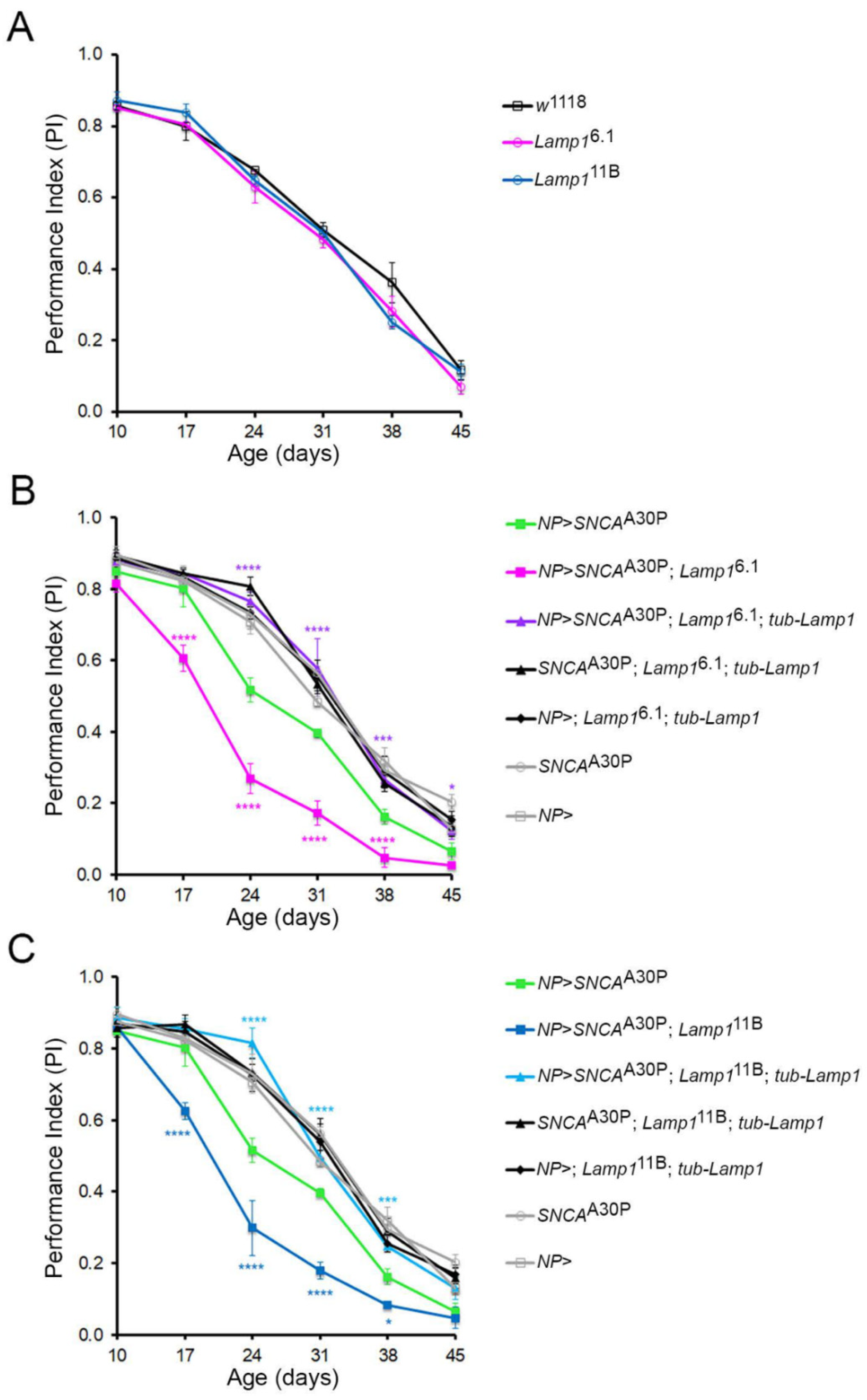
2.2. Lamp1 Deficiency Accelerates the Age-Related Locomotor Defects Induced by Neuronal α-SynA30P Expression
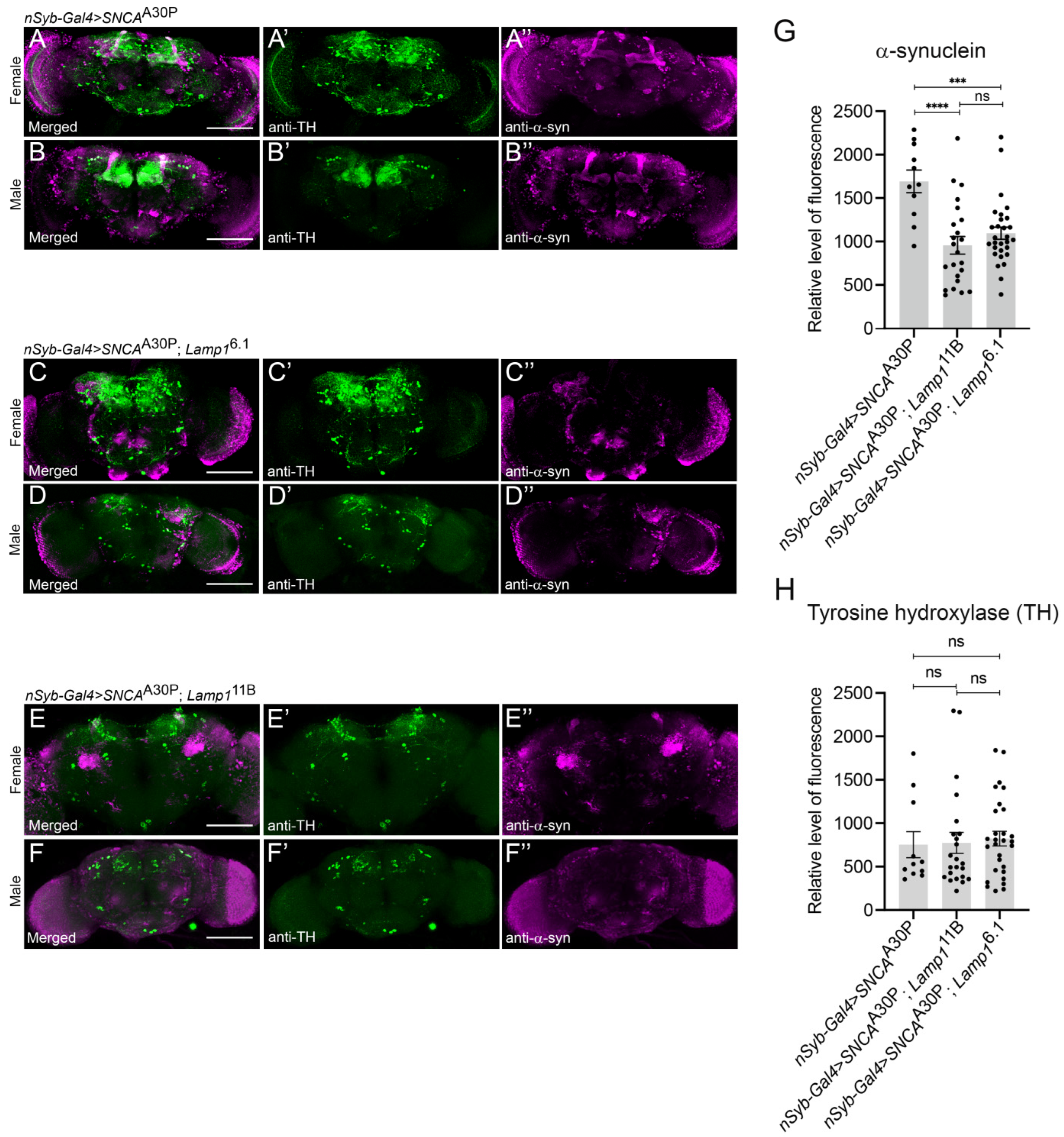
2.3. The Level of Neuronally-Expressed α-SynA30P Is Decreased in Lamp1 Mutant Brains
3. Discussion
4. Materials and Methods
4.1. Drosophila Lines and Culture Methods
4.2. Paraquat Resistance Test
4.3. Locomotion Assay
4.4. Adult Brain Immunostaining
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMA | chaperone-mediated autophagy |
| eMI | endosomal microautophagy |
| LAMP | Lysosomal-associated membrane protein |
| PAM | protocerebral anterior medial |
| PD | Parkinson disease |
| PI | performance index |
| SING | startle-induced negative geotaxis |
| TH | tyrosine hydroxylase |
| tub | tubulin |
| UAS | upstream activating sequence |
| WT | wild-type |
References
- Forno, L.S. Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S.; Ghosh, D.; Dwivedi, S.; Anoop, A.; Mohite, G.M.; Kombrabail, M.; Krishnamoorthy, G.; Maji, S.K. Familial Parkinson disease-associated mutations alter the site-specific microenvironment and dynamics of α-synuclein. J. Biol. Chem. 2015, 290, 7804–7822. [Google Scholar] [CrossRef] [PubMed]
- Feany, M.B.; Bender, W.W. A Drosophila model of Parkinson’s disease. Nature 2000, 404, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Riemensperger, T.; Issa, A.R.; Pech, U.; Coulom, H.; Nguyễn, M.V.; Cassar, M.; Jacquet, M.; Fiala, A.; Birman, S. A single dopamine pathway underlies progressive locomotor deficits in a Drosophila model of Parkinson disease. Cell Rep. 2013, 5, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy [review]. Mol. Aspects Med. 2006, 27, 495–502. [Google Scholar] [CrossRef]
- Wilke, S.; Krausze, J.; Büssow, K. Crystal structure of the conserved domain of the DC lysosomal associated membrane protein: Implications for the lysosomal glycocalyx. BMC Biol. 2012, 10, 62. [Google Scholar] [CrossRef]
- Eskelinen, E.L.; Schmidt, C.K.; Neu, S.; Willenborg, M.; Fuertes, G.; Salvador, N.; Tanaka, Y.; Lüllmann-Rauch, R.; Hartmann, D.; Heeren, J.; et al. Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double deficient fibroblasts. Mol. Biol. Cell 2004, 15, 3132–3145. [Google Scholar] [CrossRef]
- Andrejewski, N.; Punnonen, E.L.; Guhde, G.; Tanaka, Y.; Lullmann-Rauch, R.; Hartmann, D.; von Figura, K.; Saftig, P. Normal lysosomal morphology and function in LAMP-1-deficient mice. J. Biol. Chem. 1999, 274, 12692–12701. [Google Scholar] [CrossRef]
- Tanaka, Y.; Guhde, G.; Suter, A.; Eskelinen, E.L.; Hartmann, D.; Lüllmann-Rauch, R.; Janssen, P.M.L.; Blanz, J.; von Figura, K.; Saftig, P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2 -deficient mice. Nature 2000, 406, 902–906. [Google Scholar] [CrossRef]
- Endo, Y.; Furuta, A.; Nishino, I. Danon disease: A phenotypic expression of LAMP-2 deficiency. Acta Neuropathol. 2015, 129, 391–398. [Google Scholar] [CrossRef]
- Nishino, I.; Fu, J.; Tanji, K.; Yamada, T.; Shimojo, S.; Koori, T.; Mora, M.; Riggs, J.E.; Oh, S.J.; Koga, Y.; et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 2000, 406, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T.J.; Sweet, M.E.; Mestroni, L.; Taylor, M.R. Danon disease—Dysregulation of autophagy in a multisystem disorder with cardiomyopathy. J. Cell Sci. 2016, 129, 2135–2143. [Google Scholar] [CrossRef]
- Chaudhry, N.; Sica, M.; Surabhi, S.; Hernandez, D.S.; Mesquita, A.; Selimovic, A.; Riaz, A.; Lescat, L.; Bai, H.; MacIntosh, G.C.; et al. Lamp1 mediates lipid transport, but is dispensable for autophagy in Drosophila. Autophagy 2022, 10, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Coulom, H.; Birman, S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 2004, 24, 10993–10998. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Bowling, K.; Funderburk, C.; Lawal, H.; Inamdar, A.; Wang, Z.; O’Donnell, J.M. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J. Neurosci. 2007, 27, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Cassar, M.; Issa, A.R.; Riemensperger, T.; Petitgas, C.; Rival, T.; Coulom, H.; Iché-Torres, M.; Han, K.A.; Birman, S. A dopamine receptor contributes to paraquat-induced neurotoxicity in Drosophila. Hum. Mol. Genet. 2015, 24, 197–212. [Google Scholar] [CrossRef]
- Shukla, A.K.; Ratnasekhar, C.; Pragya, P.; Chaouhan, H.S.; Patel, D.K.; Chowdhuri, D.K.; Mudiam, M.K.R. Metabolomic Analysis Provides Insights on Paraquat-Induced Parkinson-Like Symptoms in Drosophila melanogaster. Mol. Neurobiol. 2016, 53, 254–269. [Google Scholar] [CrossRef]
- Nagoshi, E. Drosophila Models of Sporadic Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 3343. [Google Scholar] [CrossRef]
- Hajji, K.; Mteyrek, A.; Sun, J.; Cassar, M.; Mezghani, S.; Leprince, J.; Vaudry, D.; Masmoudi-Kouki, O.; Birman, S. Neuroprotective effects of PACAP against paraquat-induced oxidative stress in the Drosophila central nervous system. Hum. Mol. Genet. 2019, 28, 1905–1918. [Google Scholar] [CrossRef]
- Issa, A.R.; Sun, J.; Petitgas, C.; Mesquita, A.; Dulac, A.; Robin, M.; Mollereau, B.; Jenny, A.; Chérif-Zahar, B.; Birman, S. The lysosomal membrane protein LAMP2A promotes autophagic flux and prevents SNCA-induced Parkinson disease-like symptoms in the Drosophila brain. Autophagy 2018, 14, 1898–1910. [Google Scholar] [CrossRef]
- Scrivo, A.; Bourdenx, M.; Pampliega, O.; Cuervo, A.M. Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol. 2018, 9, 802–815. [Google Scholar] [CrossRef]
- Mak, S.K.; McCormack, A.L.; Manning-Bog, A.B.; Cuervo, A.M.; Di Monte, D.A. Lysosomal degradation of alpha-synuclein in vivo. J. Biol. Chem. 2010, 285, 13621–13629. [Google Scholar] [CrossRef] [PubMed]
- Xilouri, M.; Brekk, O.R.; Kirik, D.; Stefanis, L. LAMP2A as a therapeutic target in Parkinson disease. Autophagy 2013, 9, 2166–2168. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Kikuchi, H.; Fujita, H.; Yamada, D.; Fujiwara, Y.; Kabuta, T.; Nishino, I.; Wada, K.; Uchiyama, Y. Property of lysosomal storage disease associated with midbrain pathology in the central nervous system of Lamp-2-deficient mice. Am. J. Pathol. 2015, 185, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.L.; Ravikumar, B.; Atkins, J.; Skepper, J.N.; Rubinsztein, D.C. Alpha-Synuclein is degraded by both autophagy and the proteasome. J. Biol. Chem. 2003, 278, 25009–25013. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Stefanis, L.; Fredenburg, R.; Lansbury, P.T.; Sulzer, D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 2004, 305, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
- Cascella, R.; Bigi, A.; Cremades, N.; Cecchi, C. Effects of oligomer toxicity, fibril toxicity and fibril spreading in synucleinopathies. Cell Mol. Life Sci. 2022, 79, 174. [Google Scholar] [CrossRef]
- Serysheva, E.; Berhane, H.; Grumolato, L.; Demir, K.; Balmer, S.; Bodak, M.; Boutros, M.; Aaronson, S.; Mlodzik, M.; Jenny, A. Wnk kinases are positive regulators of canonical Wnt/β-catenin signalling. EMBO Rep. 2013, 14, 718–725, Erratum in: EMBO Rep. 2013, 14, 845. [Google Scholar] [CrossRef]
- Rival, T.; Soustelle, L.; Strambi, C.; Besson, M.-T.; Iché, M.; Birman, S. Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr. Biol. 2004, 14, 599–605. [Google Scholar] [CrossRef]
- Riemensperger, T.; Isabel, G.; Coulom, H.; Neuser, K.; Seugnet, L.; Kume, K.; Iché-Torres, M.; Cassar, M.; Strauss, R.; Preat, T.; et al. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. USA 2011, 108, 834–839. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmani, Z.; Surabhi, S.; Rojo-Cortés, F.; Dulac, A.; Jenny, A.; Birman, S. Lamp1 Deficiency Enhances Sensitivity to α-Synuclein and Oxidative Stress in Drosophila Models of Parkinson Disease. Int. J. Mol. Sci. 2022, 23, 13078. https://doi.org/10.3390/ijms232113078
Rahmani Z, Surabhi S, Rojo-Cortés F, Dulac A, Jenny A, Birman S. Lamp1 Deficiency Enhances Sensitivity to α-Synuclein and Oxidative Stress in Drosophila Models of Parkinson Disease. International Journal of Molecular Sciences. 2022; 23(21):13078. https://doi.org/10.3390/ijms232113078
Chicago/Turabian StyleRahmani, Zohra, Satya Surabhi, Francisca Rojo-Cortés, Amina Dulac, Andreas Jenny, and Serge Birman. 2022. "Lamp1 Deficiency Enhances Sensitivity to α-Synuclein and Oxidative Stress in Drosophila Models of Parkinson Disease" International Journal of Molecular Sciences 23, no. 21: 13078. https://doi.org/10.3390/ijms232113078
APA StyleRahmani, Z., Surabhi, S., Rojo-Cortés, F., Dulac, A., Jenny, A., & Birman, S. (2022). Lamp1 Deficiency Enhances Sensitivity to α-Synuclein and Oxidative Stress in Drosophila Models of Parkinson Disease. International Journal of Molecular Sciences, 23(21), 13078. https://doi.org/10.3390/ijms232113078








