Losartan Potassium and Verapamil Hydrochloride Compound Transdermal Drug Delivery System: Formulation and Characterization
Abstract
1. Introduction
2. Results
2.1. Preselection of Transdermal Systems
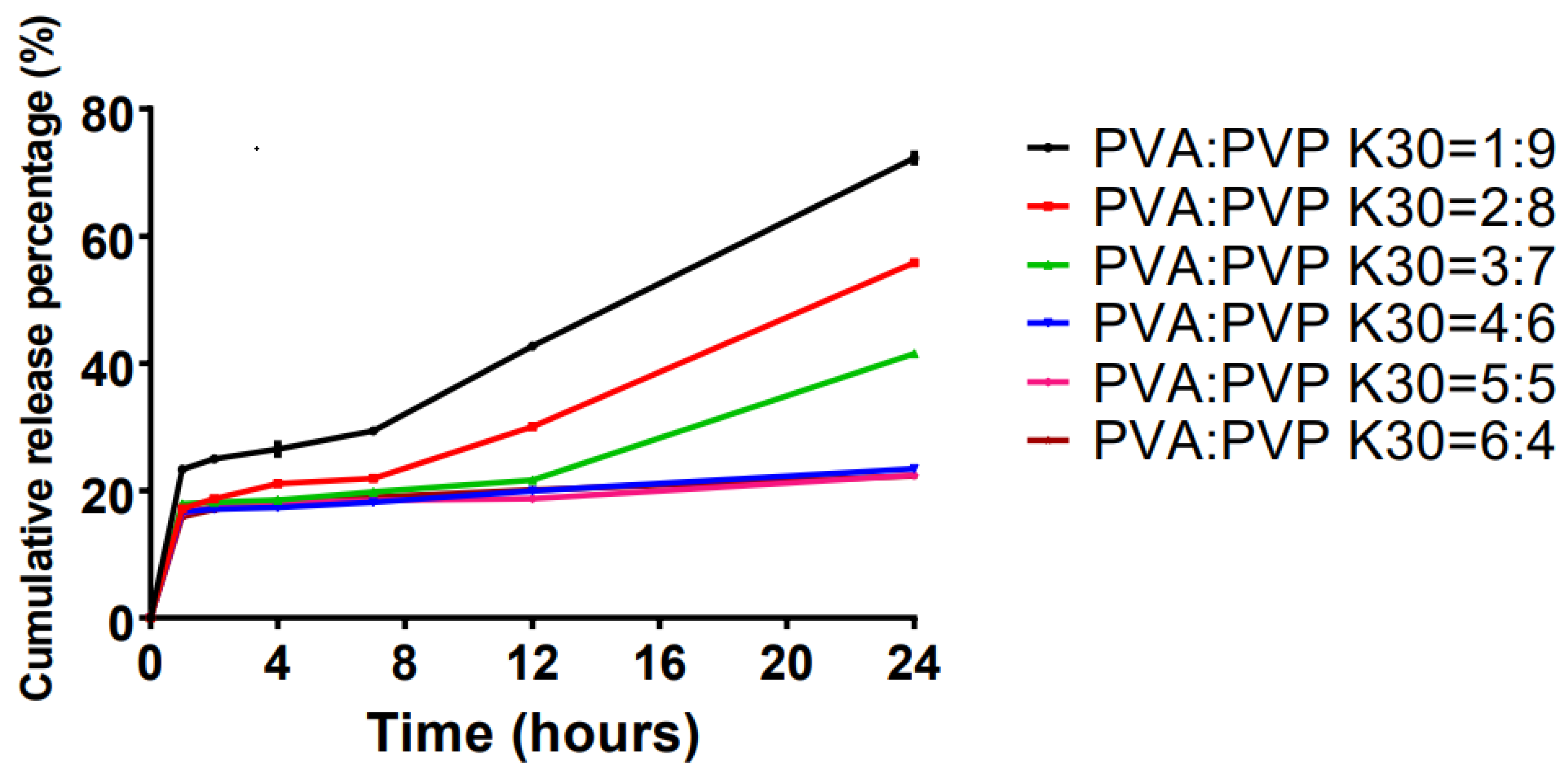
2.2. Effects of Skeleton Materials on the Transdermal Systems
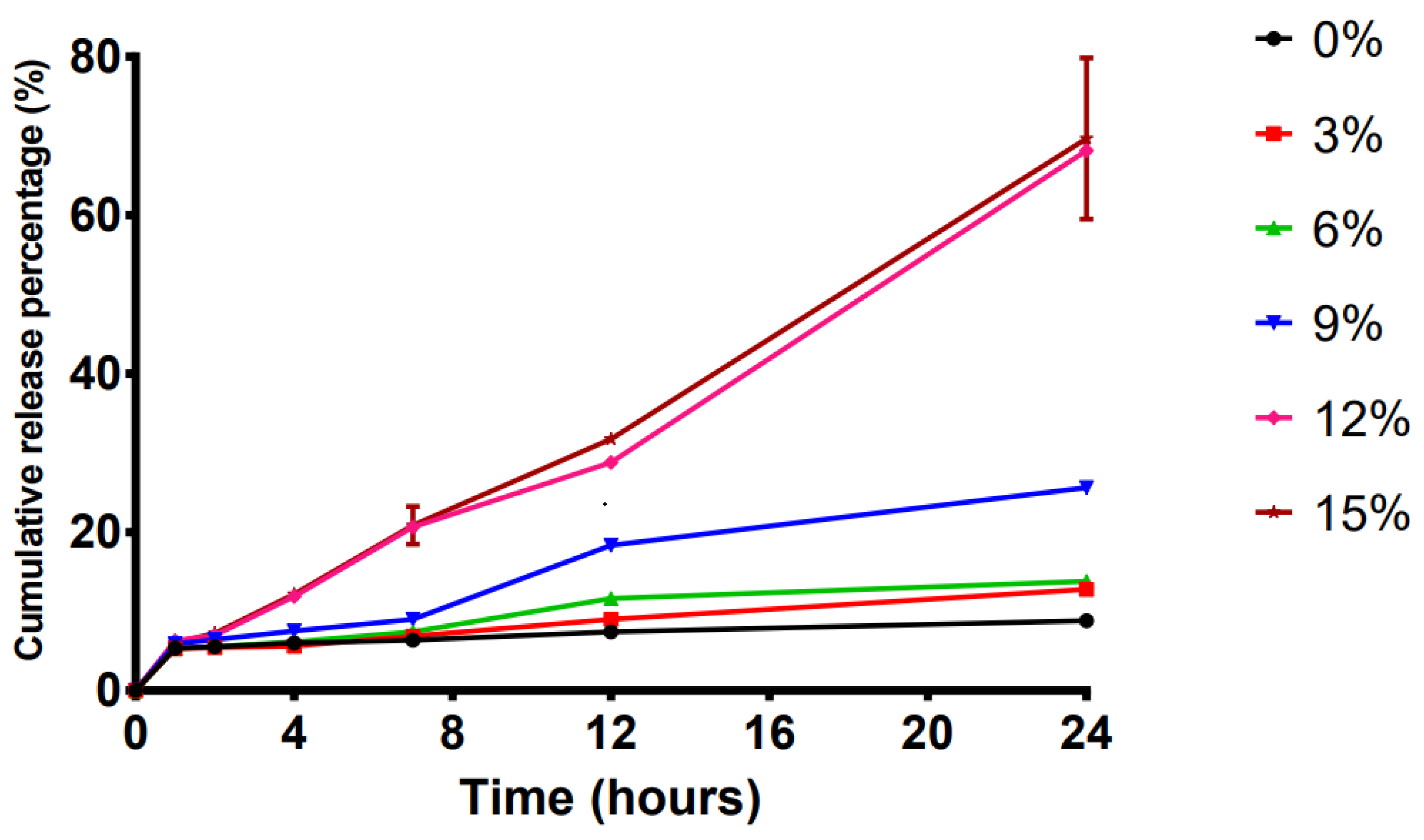
2.3. Effect of the Penetration Enhancer on the Transdermal Systems
2.4. Effects of Polyacrylic Acid Resin II on the Transdermal Systems
2.5. Scanning Electron Microscopy of TDDS
2.6. Stability of TDDS
2.7. Stickiness of the TDDS
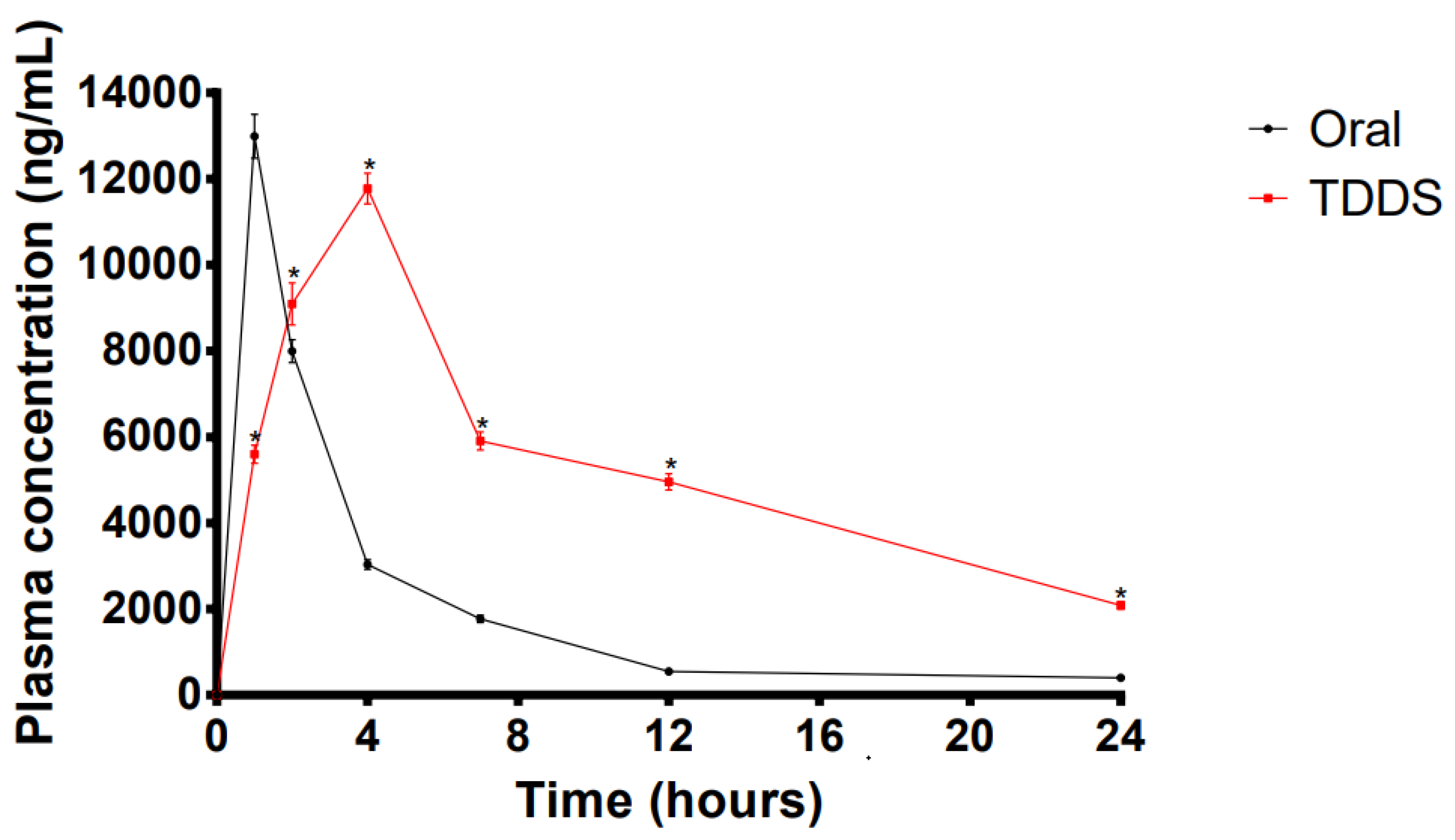
2.8. In Vivo Pharmacokinetic Studies for the TDDS
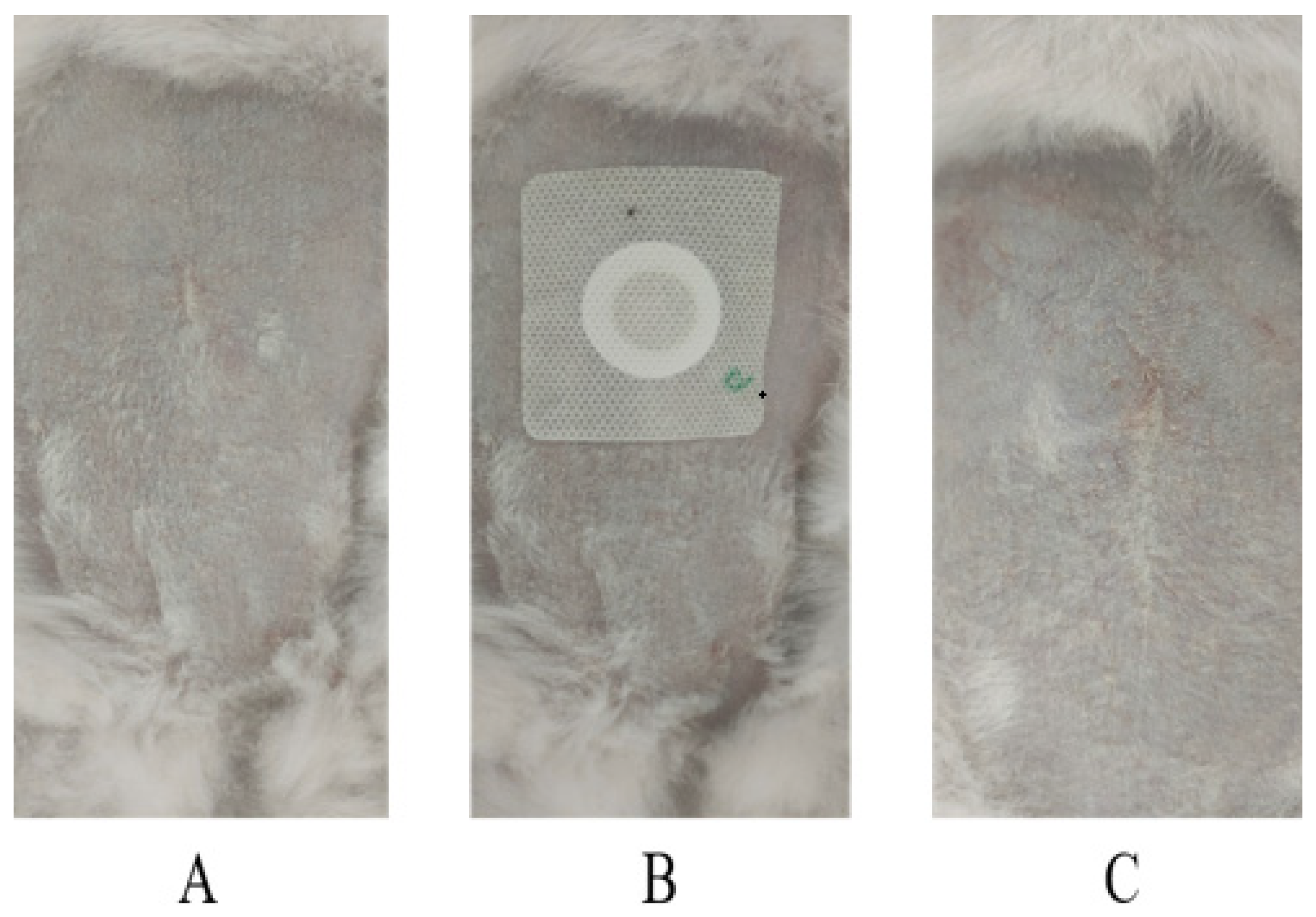
2.9. Skin Irritation Tests
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Drug Evaluation Conditions
4.4. Preparation of Mice Abdominal Skin
4.5. Optimization of Transdermal Systems
4.6. In Vitro Release Studies
4.7. Preparation of TDDS
4.8. Scanning Electron Microscopy Analysis
4.9. Quality of Prepared TDDS
4.9.1. High-Temperature Experiment
4.9.2. Cold Resistance Experiment
4.9.3. Freeze-Thaw Experiment
4.10. Stickiness of the Prepared TDDS
4.10.1. Peel Strength Experiment
4.10.2. Shear Strength Experiment
4.10.3. Hand Adhesion Experiment
4.10.4. In Vivo Pharmacokinetic Studies
4.10.5. Skin Irritation Studies
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poulter, N.R.; Prabhakaran, D.; Caulfield, M. Hypertension. Lancet 2015, 386, 801–812. [Google Scholar] [CrossRef]
- Van Kleef, M.E.; Spiering, W. Hypertension: Overly important but under-controlled. Eur. J. Prev. Cardiol. 2017, 24, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Parto, P.; Krim, S.R. Hypertension and ethnicity. Curr. Opin. Cardiol. 2016, 31, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; White, A.J.; Niehoff, N.M.; O’Brien, K.M.; Sandler, D.P. Airborne metals exposure and risk of hypertension in the Sister Study. Environ. Res. 2020, 191, 110144. [Google Scholar] [CrossRef]
- Collaborators GBDRF. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Ramkanth, S.; Anitha, P.; Gayathri, R.; Mohan, S.; Babu, D. Formulation and design optimization of nano-transferosomes using pioglitazone and eprosartan mesylate for concomitant therapy against diabetes and hypertension. Eur. J. Pharm. Sci. 2021, 162, 105811. [Google Scholar] [CrossRef]
- Azizi, M.; Rossignol, P.; Hulot, J.-S. Emerging Drug Classes and Their Potential Use in Hypertension. Hypertension 2019, 74, 1075–1083. [Google Scholar] [CrossRef]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). J. Am. Med. Assoc. 2014, 311, 507–520. [Google Scholar] [CrossRef]
- Kessing, L.V.; Rytgaard, H.C.; Ekstrom, C.T.; Torp-Pedersen, C.; Berk, M.; Gerds, T.A. Antihypertensive Drugs and Risk of Depression: A Nationwide Population-Based Study. Hypertension 2020, 76, 1263–1279. [Google Scholar] [CrossRef]
- Mancia, G.; Rea, F.; Corrao, G.; Grassi, G. Two-Drug Combinations as First-Step Antihypertensive Treatment. Circ. Res. 2019, 124, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Gorostidi, M.; de la Sierra, A. Combination therapies for hypertension—Why we need to look beyond RAS blockers. Expert Rev. Clin. Pharmacol. 2018, 11, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Law, M.R.; Wald, N.J.; Morris, J.K.; Jordan, R.E. Value of low dose combination treatment with blood pressure lowering drugs: Analysis of 354 randomised trials. Br. Med. J. 2003, 326, 1427. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, I.J.; Kreutz, R.; Olsen, M.H.; E Schutte, A.; Lopez-Jaramillo, P.; Frieden, T.R.; Sliwa, K.; Lackland, D.T.; Brainin, M. Fixed-dose combination antihypertensive medications. Lancet 2019, 394, 637–638. [Google Scholar] [CrossRef]
- Foley, L.; Toney, J.; Barlow, J.W.; O’Connor, M.; Fitzgerald-Hughes, D.; Ramtoola, Z. Investigation of the Physical, Chemical and Microbiological Stability of Losartan Potassium 5 mg/mL Extemporaneous Oral Liquid Suspension. Molecules 2021, 26, 301. [Google Scholar] [CrossRef]
- Rahamathulla, M.; Saisivam, S.; Alshetaili, A.; Hani, U.; Gangadharappa, H.V.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Design and Evaluation of Losartan Potassium Effervescent Floating Matrix Tablets: In Vivo X-ray Imaging and Pharmacokinetic Studies in Albino Rabbits. Polymers 2021, 13, 3476. [Google Scholar] [CrossRef]
- Xing, H.; Luo, X.; Li, Y.; Fan, C.; Liu, N.; Cui, C.; Li, W. Effect of verapamil on the pharmacokinetics of hydroxycamptothecin and its potential mechanism. Pharm. Biol. 2020, 58, 152–156. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, K.A.; Il Kim, Y.; Park, J.Y. Pharmacokinetic and haemodynamic interactions between amlodipine and losartan in human beings. Basic Clin. Pharmacol. Toxicol. 2019, 125, 345–352. [Google Scholar] [CrossRef]
- Chrysant, S.G.; Melino, M.; Karki, S.; Lee, J.; Heyrman, R. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin. Ther. 2008, 30, 587–604. [Google Scholar] [CrossRef]
- Moen, M.D.; Wagstaff, A.J. Losartan: A review of its use in stroke risk reduction in patients with hypertension and left ventricular hypertrophy. Drugs 2005, 65, 2657–2674. [Google Scholar] [CrossRef]
- Mouez, M.A.; Nasr, M.; Abdel-Mottaleb, M.; Geneidi, A.S.; Mansour, S. Composite chitosan-transfersomal vesicles for improved transnasal permeation and bioavailability of verapamil. Int. J. Biol. Macromol. 2016, 93, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Abdel Mouez, M.; Zaki, N.M.; Mansour, S.; Geneidi, A.S. Bioavailability enhancement of verapamil HCl via intranasal chitosan microspheres. Eur. J. Pharm. Sci. 2014, 51, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hu, D.; Xu, H.; Patra, H.K.; Liu, X.; Zhou, Z.; Tang, J.; Slater, N.; Shen, Y. Progress and perspective of microneedle system for anti-cancer drug delivery. Biomaterials 2021, 264, 120410. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Yanagi, M.; Hamada, H. Physical Enhancement? Nanocarrier? Current Progress in Transdermal Drug Delivery. Nanomaterials 2021, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Saeed Al-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.F.; Correia, I.J.; Silva, A.S.; Mano, J.F. Biomaterials for drug delivery patches. Eur. J. Pharm. Sci. 2018, 118, 49–66. [Google Scholar] [CrossRef]
- Hair, P.I.; Keating, G.M.; McKeage, K. Transdermal matrix fentanyl membrane patch (matrifen): In severe cancer-related chronic pain. Drugs 2008, 68, 2001–2009. [Google Scholar] [CrossRef]
- Baig, M.M.F.A.; Naveed, M.; Abbas, M.; Chunxia, W.; Ullah, S.; Hasnat, M.; Shad, A.; Sohail, M.; Khan, G.J.; Ansari, M.T. DNA scaffold nanoparticles coated with HPMC/EC for oral delivery. Int. J. Pharm. 2019, 562, 321–332. [Google Scholar] [CrossRef]
- Ammar, H.O.; Makram, T.S.; Mosallam, S. Effect of Polymers on the Physicochemical Properties and Biological Performance of Fenoprofen Calcium Dihydrate-Triacetyl-beta-Cyclodextrin Complex. Pharmaceutics 2017, 9, 23. [Google Scholar] [CrossRef]
- Allam, A.; Elsabahy, M.; El Badry, M.; Eleraky, N.E. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int. J. Pharm. 2021, 598, 120380. [Google Scholar] [CrossRef]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.A.; Cronemberger, S.; Nogueira, J.C.; et al. Chitosan/hydroxyethyl cellulose inserts for sustained-release of dorzolamide for glaucoma treatment: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 570, 118662. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Q.; Che, Y.; Liu, X.; Dong, C.; Chen, X.; Wang, C. Effect of Na2CO3 on the Microstructure and Macroscopic Properties and Mechanism Analysis of PVA/CMC Composite Film. Polymers 2020, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, M.; Yang, Q.; Liu, Q.; Ye, M.; Yang, G. The Opposed Effects of Polyvinylpyrrolidone K30 on Dissolution and Precipitation for Indomethacin Supersaturating Drug Delivery Systems. AAPS PharmSciTech. 2020, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, D.; Liu, M.; Lin, Q.; Shiu, B.-C. A Study on the Improvement of Using Raw Lacquer and Electrospinning on Properties of PVP Nanofilms. Nanomaterials 2020, 10, 1723. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, A.; Sakai, T.; Sako, K.; Maitani, Y. Polymer combination increased both physical stability and oral absorption of solid dispersions containing a low glass transition temperature drug: Physicochemical characterization and in vivo study. Chem. Pharm. Bull. 2012, 60, 459–464. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Harjoh, N.; Wong, T.W.; Caramella, C. Transdermal insulin delivery with microwave and fatty acids as permeation enhancers. Int. J. Pharm. 2020, 584, 119416. [Google Scholar] [CrossRef]
- Chen, H.-L.; Cai, C.-C.; Ma, J.-Y.; Yu, M.-L.; Zhao, M.-H.; Guo, J.-B.; Xu, H. Effect of the Dispersion States of Azone in Hydroalcoholic Gels on Its Transdermal Permeation Enhancement Efficacy. J. Pharm. Sci. 2018, 107, 1879–1885. [Google Scholar] [CrossRef]
- Handler, A.M.; Marxen, E.; Jacobsen, J.; Janfelt, C. Visualization of the penetration modifying mechanism of laurocapram by Mass Spectrometry Imaging in buccal drug delivery. Eur. J. Pharm. Sci. 2019, 127, 276–281. [Google Scholar] [CrossRef]
- Bozorg, B.D.; Banga, A.K. Effect of Different Pressure-Sensitive Adhesives on Performance Parameters of Matrix-Type Transdermal Delivery Systems. Pharmaceutics 2020, 12, 209. [Google Scholar] [CrossRef]
- Ameen, D.; Michniak-Kohn, B. Development and in vitro evaluation of pressure sensitive adhesive patch for the transdermal delivery of galantamine: Effect of penetration enhancers and crystallization inhibition. Eur. J. Pharm. Biopharm. 2019, 139, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.; Yoshii, A.C.; E Silva, H.R.; Cury, B.S.F.; Chorilli, M. Polyacrylic acid polymers hydrogels intended to topical drug delivery: Preparation and characterization. Pharm. Dev. Technol. 2015, 20, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Arunprasert, K.; Pornpitchanarong, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Aumklad, P.; Patrojanasophon, P. Development and Evaluation of Novel Water-Based Drug-in-Adhesive Patches for the Transdermal Delivery of Ketoprofen. Pharmaceutics 2021, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Stamatas, G.N.; Kollias, N. In vivo documentation of cutaneous inflammation using spectral imaging. J. Biomed. Opt. 2007, 12, 051603. [Google Scholar] [CrossRef] [PubMed]

| First Skeleton Materials (a) | Second Skeleton Materials (b) | Penetration Enhancers (c) | Cumulative Release Rate (%) | |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 19.24 |
| 2 | 1 | 2 | 2 | 23.99 |
| 3 | 1 | 3 | 3 | 24.51 |
| 4 | 1 | 4 | 4 | 26.09 |
| 5 | 1 | 5 | 5 | 28.00 |
| 6 | 2 | 1 | 2 | 41.24 |
| 7 | 2 | 2 | 3 | 40.65 |
| 8 | 2 | 3 | 4 | 28.54 |
| 9 | 2 | 4 | 5 | 44.44 |
| 10 | 2 | 5 | 1 | 40.31 |
| 11 | 3 | 1 | 3 | 41.23 |
| 12 | 3 | 2 | 4 | 38.09 |
| 13 | 3 | 3 | 5 | 42.47 |
| 14 | 3 | 4 | 1 | 41.11 |
| 15 | 3 | 5 | 2 | 33.20 |
| 16 | 4 | 1 | 4 | 47.90 |
| 17 | 4 | 2 | 5 | 41.56 |
| 18 | 4 | 3 | 1 | 42.85 |
| 19 | 4 | 4 | 2 | 36.77 |
| 20 | 4 | 5 | 3 | 36.06 |
| 21 | 5 | 1 | 5 | 41.85 |
| 22 | 5 | 2 | 1 | 32.42 |
| 23 | 5 | 3 | 2 | 52.93 |
| 24 | 5 | 4 | 4 | 78.13 |
| 25 | 5 | 5 | 3 | 46.63 |
| K1 | 121.83 | 191.44 | 175.93 | |
| K2 | 195.18 | 176.71 | 188.13 | |
| K3 | 196.10 | 191.31 | 189.08 | |
| K4 | 205.14 | 226.53 | 218.74 | |
| K5 | 251.95 | 184.20 | 198.31 | |
| k1 | 24.37 | 38.29 | 35.19 | |
| k2 | 39.04 | 35.34 | 37.63 | |
| k3 | 39.22 | 38.26 | 37.82 | |
| k4 | 41.03 | 45.31 | 43.75 | |
| k5 | 50.39 | 36.84 | 39.66 |
| Formulation | PVA:PVP K30 | OA:azone = 2:1 (%, v/v) | Polyacrylic Acid Resin II (%, v/v) |
|---|---|---|---|
| A1 | 1:9 | ||
| A2 | 2:8 | ||
| A3 | 3:7 | ||
| A4 | 4:6 | ||
| A5 | 5:5 | ||
| A6 | 6:4 | ||
| B1 | 0 | ||
| B2 | 3 | ||
| B3 | 6 | ||
| B4 | 9 | ||
| B5 | 12 | ||
| B6 | 15 | ||
| C1 | 0 | ||
| C2 | 2 | ||
| C3 | 5 | ||
| C4 | 7 | ||
| C5 | 10 |
| Parameters | Oral Administration | LP-VPH Compound Patch |
|---|---|---|
| Cmax (ng/mL) | 12,993.02 ± 511.01 | 11,774.14 ± 360.14 |
| Tmax (h) | 1 | 4 |
| AUC(0-t) (ng·h−1·mL−1) | 46,726.65 ± 821.11 | 158,175.20 ± 4206.84 *** |
| MRT (h) | 3.39 ± 0.04 | 4.63 ± 0.64 |
| F (%) | - | 338.51 |
| Administration | Erythema | Edema | ||
|---|---|---|---|---|
| 0 h | 72 h | 0 h | 72 h | |
| Blank patch | 0.00 ± 0.00 | 0.24 ± 0.09 | 0.00 ± 0.00 | 0.17 ± 0.05 |
| Compound patch | 0.00 ± 0.00 | 0.27 ± 0.11 | 0.00 ± 0.00 | 0.19 ± 0.05 |
| Option | Factors | |||
|---|---|---|---|---|
| First Skeleton Materials (a) | Second Skeleton Material (b) | Penetration Enhancers (c) | ||
| Level | 1 | HPMC K4M | HPMC K100M | OA |
| 2 | HPMC K30M | CMC-Na | azone | |
| 3 | HC | EC | OA:azone = 1:1 | |
| 4 | PVP K30 | PVP K30 | OA:azone = 2:1 | |
| 5 | PVA | PVA | OA:azone = 1:2 | |
| Components | Usage (%) |
|---|---|
| PVA | 8.3% (w/w) |
| PVP K30 | 74.7% (w/w) |
| OA:azone = 2:1 | 12% (v/v) |
| polyacrylic acid resin II | 5% (w/w) |
| Symptoms | Skin Condition | Evaluation Score |
|---|---|---|
| erythema | None | 0 |
| Very slight (Almost observable) | 1 | |
| Well defined | 2 | |
| Moderate to severe | 3 | |
| Severe to slight eschar formation | 4 | |
| edema | None | 0 |
| Very slight (Almost observable) | 1 | |
| Slight (Obvious uplift at the edge of the area) | 2 | |
| Moderate (Area edge humps 1 mm) | 3 | |
| Severe (Area edge humps > 1 mm) | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-s.; Sun, Y.-y.; Qin, Z.-c.; Zhang, S.-y.; Chen, W.-b.; Liu, Y.-q. Losartan Potassium and Verapamil Hydrochloride Compound Transdermal Drug Delivery System: Formulation and Characterization. Int. J. Mol. Sci. 2022, 23, 13051. https://doi.org/10.3390/ijms232113051
Chen Y-s, Sun Y-y, Qin Z-c, Zhang S-y, Chen W-b, Liu Y-q. Losartan Potassium and Verapamil Hydrochloride Compound Transdermal Drug Delivery System: Formulation and Characterization. International Journal of Molecular Sciences. 2022; 23(21):13051. https://doi.org/10.3390/ijms232113051
Chicago/Turabian StyleChen, Yu-si, Yi-yang Sun, Zi-chen Qin, Sai-ya Zhang, Wen-bo Chen, and Yan-qiang Liu. 2022. "Losartan Potassium and Verapamil Hydrochloride Compound Transdermal Drug Delivery System: Formulation and Characterization" International Journal of Molecular Sciences 23, no. 21: 13051. https://doi.org/10.3390/ijms232113051
APA StyleChen, Y.-s., Sun, Y.-y., Qin, Z.-c., Zhang, S.-y., Chen, W.-b., & Liu, Y.-q. (2022). Losartan Potassium and Verapamil Hydrochloride Compound Transdermal Drug Delivery System: Formulation and Characterization. International Journal of Molecular Sciences, 23(21), 13051. https://doi.org/10.3390/ijms232113051






