Pathogenesis of Hypertension in Metabolic Syndrome: The Role of Fructose and Salt
Abstract
1. Introduction
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [PubMed]
- Misra, A.; Sharma, R.; Gulati, S.; Joshi, S.R.; Sharma, V.; Ghafoorunissa, G.; Ibrahim, A.; Joshi, S.; Laxmaiah, A.; Kurpad, A.; et al. Consensus Dietary Guidelines for Healthy Living and Prevention of Obesity, the Metabolic Syndrome, Diabetes, and Related Disorders in Asian Indians. Diabetes Technol. Ther. 2011, 13, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Sankararaman, S.; Noriega, K.; Velayuthan, S.; Sferra, T.; Martindale, R. Gut Microbiome and Its Impact on Obesity and Obesity-Related Disorders. Curr. Gastroenterol. Rep. 2022, 25, 31–44. [Google Scholar] [CrossRef]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef]
- Pickering, T.G. America the fat: Fast food and fructose. J. Clin. Hypertens. 2003, 5, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Beilin, L.; Huang, R.-C. Childhood obesity, hypertension, the metabolic syndrome and adult cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2008, 35, 409–411. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Wong, A.H.; Hon, K.L. Childhood Obesity: An Updated Review. Curr. Pediatr. Rev. 2022, 18. [Google Scholar] [CrossRef]
- Nehus, E.; Mitsnefes, M. Childhood Obesity and the Metabolic Syndrome. Pediatr. Clin. N. Am. 2018, 66, 31–43. [Google Scholar] [CrossRef]
- Owens, S.; Galloway, R. Childhood Obesity and the Metabolic Syndrome. Curr. Atheroscler. Rep. 2014, 16, 436. [Google Scholar] [CrossRef]
- Park, H.; Jun, S.; Lee, H.A.; Kim, H.S.; Hong, Y.S.; Park, H. The Effect of Childhood Obesity or Sarcopenic Obesity on Metabolic Syndrome Risk in Adolescence: The Ewha Birth and Growth Study. Metabolites 2023, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Waters, H.; Graf, M. America’s Obesity Crisis: The Health and Economic Costs of Excess Weight; Milken Institute: Santa Monica, CA, USA, 2018. [Google Scholar]
- Khan, S.R. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol. Res. 2012, 40, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.H.; Zhang, L.; Wang, P.Y. Prevalence of overweight/obesity and its associations with hypertension, diabetes, dyslipidemia, and metabolic syndrome: A survey in the suburban area of Beijing, 2007. Obes. Facts 2011, 4, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Gelber, R.P.; Gaziano, J.M.; Manson, J.E.; Buring, J.E.; Sesso, H.D. A Prospective Study of Body Mass Index and the Risk of Developing Hypertension in Men. Am. J. Hypertens. 2007, 20, 370–377. [Google Scholar] [CrossRef]
- Kurukulasuriya, L.R.; Stas, S.; Lastra, G.; Manrique, C.; Sowers, J.R. Hypertension in obesity. Med. Clin. N. Am. 2011, 95, 903–917. [Google Scholar] [CrossRef]
- Mikhail, N.; Tuck, M.L. Epidemiological and Clinical Aspects of Obesity Related Hypertension. J. Clin. Hypertens. 2000, 2, 41–45. [Google Scholar]
- Willett, W.C.; Dietz, W.H.; Colditz, G.A. Guidelines for Healthy Weight. N. Engl. J. Med. 1999, 341, 427–434. [Google Scholar] [CrossRef]
- Vlasova, M.; Purhonen, A.K.; Jarvelin, M.R.; Rodilla, E.; Pascual, J.; Herzig, K.H. Role of adipokines in obesity-associated hypertension. Acta Physiol. 2010, 200, 107–127. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Mehta, V.; Onkaramurthy, N.; O’Keefe, J.H. Fructose-induced inflammation and increased cortisol: A new mechanism for how sugar induces visceral adiposity. Prog. Cardiovasc. Dis. 2018, 61, 3–9. [Google Scholar] [CrossRef]
- Lee, M.; Sorn, S.R.; Lee, Y.; Kang, I. Salt Induces Adipogenesis/Lipogenesis and Inflammatory Adipocytokines Secretion in Adipocytes. Int. J. Mol. Sci. 2019, 20, 160. [Google Scholar] [CrossRef]
- Lu, X.; Crowley, S.D. Inflammation in Salt-Sensitive Hypertension and Renal Damage. Curr. Hypertens. Rep. 2018, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.A.; do Carmo, J.M.; Li, X.; Wang, Z.; Mouton, A.J.; Hall, J.E. Role of Hyperinsulinemia and Insulin Resistance in Hypertension: Metabolic Syndrome Revisited. Can. J. Cardiol. 2020, 36, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T. Insulin resistance and salt-sensitive hypertension in metabolic syndrome. Nephrol. Dial. Transplant. 2007, 22, 3102–3107. [Google Scholar] [CrossRef]
- Ihm, S.-H.; Jang, S.-W.; Kim, O.-R.; Chang, K.; Oak, M.-H.; Lee, J.-O.; Lim, D.-Y.; Kim, J.-H. Decaffeinated green tea extract improves hypertension and insulin resistance in a rat model of metabolic syndrome. Atherosclerosis 2012, 224, 377–383. [Google Scholar] [CrossRef]
- Lind, L.; Lithell, H. Hypertension, hyperlipidemia, insulin resistance and obesity: Parts of a metabolic syndrome. Blood Press. Suppl. 1992, 4, 49–54. [Google Scholar]
- Müller-Wieland, D.; Kotzka, J.; Knebel, B.; Krone, W. Metabolic syndrome and hypertension: Pathophysiology and molecular basis of insulin resistance. Basic Res. Cardiol. 1998, 93 (Suppl. S2), 131–134. [Google Scholar] [CrossRef]
- Natali, A.; Ferrannini, E. Hypertension, insulin resistance, and the metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2004, 33, 417–429. [Google Scholar] [CrossRef]
- Said, M.A.; Nafeh, N.; Abdallah, H. Spexin alleviates hypertension, hyperuricaemia, dyslipidemia and insulin resistance in high fructose diet induced metabolic syndrome in rats via enhancing PPAR-ɣ and AMPK and inhibiting IL-6 and TNF-alpha. Arch. Physiol. Biochem. 2021, 1–6. [Google Scholar] [CrossRef]
- Schnackenberg, C.G.; Costell, M.H.; Krosky, D.J.; Cui, J.; Wu, C.W.; Hong, V.S.; Harpel, M.R.; Willette, R.N.; Yue, T.L. Chronic inhibition of 11 beta -hydroxysteroid dehydrogenase type 1 activity decreases hypertension, insulin resistance, and hypertriglyceridemia in metabolic syndrome. Biomed. Res. Int. 2013, 2013, 427640. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Saitoh, Y.; Mizuta, M.; Shiiya, T.; Noma, K.; Mashiba, S.; Kojima, S.; Nakazato, M. Fenofibrate ameliorates insulin resistance, hypertension and novel oxidative stress markers in patients with metabolic syndrome. Obes. Res. Clin. Pract. 2011, 5, e335–e340. [Google Scholar] [CrossRef] [PubMed]
- Kretowicz, M.; Johnson, R.J.; Ishimoto, T.; Nakagawa, T.; Manitius, J. The Impact of Fructose on Renal Function and Blood Pressure. Int. J. Nephrol. 2011, 2011, 315879. [Google Scholar] [CrossRef] [PubMed]
- Student, J.; Sowers, J.; Lockette, W. THIRSTY FOR FRUCTOSE: Arginine Vasopressin, Fructose, and the Pathogenesis of Metabolic and Renal Disease. Front. Cardiovasc. Med. 2022, 9, 883365. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Tomono, Y.; Ito, K.; Furutani, N.; Yoshida, H.; Tada, N. The underlying mechanisms for development of hypertension in the metabolic syndrome. Nutr. J. 2008, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M. Insulin resistance and hypertension: New insights. Kidney Int. 2015, 87, 497–499. [Google Scholar] [CrossRef]
- Sowers, J.R. Insulin resistance and hypertension. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1597–H1602. [Google Scholar] [CrossRef]
- Pina, A.F.; Borges, D.O.; Meneses, M.J.; Branco, P.; Birne, R.; Vilasi, A.; Macedo, M.P. Insulin: Trigger and Target of Renal Functions. Front. Cell Dev. Biol. 2020, 8, 519. [Google Scholar] [CrossRef]
- Strazzullo, P.; Barbato, A.; Galletti, F.; Barba, G.; Siani, A.; Iacone, R.; D’Elia, L.; Russo, O.; Versiero, M.; Farinaro, E.; et al. Abnormalities of renal sodium handling in the metabolic syndrome. Results of the Olivetti Heart Study. J. Hypertens. 2006, 24, 1633–1639. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, H.; Lu, X.; He, D.; Han, Y.; Wang, H.; Zeng, C.; Shi, W. Inhibition of D4 Dopamine Receptors on Insulin Receptor Expression and Effect in Renal Proximal Tubule Cells. J. Am. Heart Assoc. 2016, 5, e002448. [Google Scholar] [CrossRef]
- Pao, A.C. There and back again: Insulin, ENaC, and the cortical collecting duct. Physiol. Rep. 2016, 4, e12809. [Google Scholar] [CrossRef]
- Nizar, J.M.; Dong, W.; McClellan, R.B.; Labarca, M.; Zhou, Y.; Wong, J.; Goens, D.G.; Zhao, M.; Velarde, N.; Bernstein, D.; et al. Na+-sensitive elevation in blood pressure is ENaC independent in diet-induced obesity and insulin resistance. Am. J. Physiol. Renal. Physiol. 2016, 310, F812–F820. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Cooke, C.R.; Andres, R.; Faloona, G.R.; Davis, P.J. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J. Clin. Investig. 1975, 55, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Sechi, L.A. Mechanisms of insulin resistance in rat models of hypertension and their relationships with salt sensitivity. J. Hypertens. 1999, 17, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Fuster, D.G.; Bobulescu, I.A.; Zhang, J.; Wade, J.; Moe, O.W. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am. J. Physiol. Renal. Physiol. 2007, 292, F577–F585. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Satoh, N.; Suzuki, M.; Kume, H.; Homma, Y.; Seki, G.; Horita, S. Stimulatory effect of insulin on renal proximal tubule sodium transport is preserved in type 2 diabetes with nephropathy. Biochem. Biophys. Res. Commun. 2015, 461, 154–158. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamazaki, O.; Shirai, A.; Horita, S.; Satoh, N.; Suzuki, M.; Hamasaki, Y.; Noiri, E.; Kume, H.; Enomoto, Y.; et al. Preserved Na/HCO3 cotransporter sensitivity to insulin may promote hypertension in metabolic syndrome. Kidney Int. 2015, 87, 535–542. [Google Scholar] [CrossRef]
- Nakamura, N.; Matsui, T.; Ishibashi, Y.; Yamagishi, S.-I. Insulin stimulates SGLT2-mediated tubular glucose absorption via oxidative stress generation. Diabetol. Metab. Syndr. 2015, 7, 48. [Google Scholar] [CrossRef]
- Poulsen, S.B.; Fenton, R.; Rieg, T. Sodium-glucose cotransport. Curr. Opin. Nephrol. Hypertens. 2015, 24, 463–469. [Google Scholar] [CrossRef]
- Blass, G.; Klemens, C.A.; Brands, M.W.; Palygin, O.; Staruschenko, A. Postprandial Effects on ENaC-Mediated Sodium Absorption. Sci. Rep. 2019, 9, 4296. [Google Scholar] [CrossRef]
- Chávez-Canales, M.; Arroyo, J.P.; Ko, B.; Vázquez, N.; Bautista, R.; Castañeda-Bueno, M.; Bobadilla, N.A.; Hoover, R.S.; Gamba, G. Insulin increases the functional activity of the renal NaCl cotransporter. J. Hypertens. 2013, 31, 303–311. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Bakris, G. Review: Insulin and endothelin: An interplay contributing to hypertension development? J. Clin. Endocrinol. Metab. 2007, 92, 379–385. [Google Scholar] [CrossRef]
- Engeli, S.; Schling, P.; Gorzelniak, K.; Boschmann, M.; Janke, J.; Ailhaud, G.; Teboul, M.; Massiéra, F.; Sharma, A.M. The adipose-tissue renin-angiotensin-aldosterone system: Role in the metabolic syndrome? Int. J. Biochem. Cell Biol. 2003, 35, 807–825. [Google Scholar] [CrossRef] [PubMed]
- Goodfriend, T.L.; Egan, B.M.; Kelley, D.E. Aldosterone in obesity. Endocr. Res. 1998, 24, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Improving obesity and blood pressure. Hypertens. Res. 2020, 43, 79–89. [Google Scholar] [CrossRef]
- Grassi, G.; Dell’Oro, R.; Facchini, A.; Quarti Trevano, F.; Bolla, G.B.; Mancia, G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J. Hypertens. 2004, 22, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.J.; O’Shaughnessy, B.; O’Neill, S.; Lane, B.; Healy, V. Impact of elevated dietary sodium intake on NAD(P)H oxidase and SOD in the cortex and medulla of the rat kidney. Am. J. Physiol. Integr. Comp. Physiol. 2010, 299, R234–R240. [Google Scholar] [CrossRef]
- Kitiyakara, C.; Chabrashvili, T.; Chen, Y.; Blau, J.; Karber, A.; Aslam, S.; Welch, W.J.; Wilcox, C.S. Salt Intake, Oxidative Stress, and Renal Expression of NADPH Oxidase and Superoxide Dismutase. J. Am. Soc. Nephrol. 2003, 14, 2775–2782. [Google Scholar] [CrossRef]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hébert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef]
- Hansell, P.; Welch, W.J.; Blantz, R.C.; Palm, F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin. Exp. Pharmacol. Physiol. 2013, 40, 123–137. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Grundy, S.M. Inflammation, hypertension, and the metabolic syndrome. JAMA 2003, 290, 3000–3002. [Google Scholar] [CrossRef]
- Sesso, H.D. C-Reactive Protein and the Risk of Developing Hypertension. JAMA 2003, 290, 2945–2951. [Google Scholar] [CrossRef] [PubMed]
- Ertuglu, L.A.; Kirabo, A. Dendritic Cell Epithelial Sodium Channel in Inflammation, Salt-Sensitive Hypertension, and Kidney Damage. Kidney360 2022, 3, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Ertuglu, L.A.; Mutchler, A.P.; Yu, J.; Kirabo, A. Inflammation and oxidative stress in salt sensitive hypertension; The role of the NLRP3 inflammasome. Front. Physiol. 2022, 13, 1096296. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.M.; Ling, Y.H.; Huuskes, B.M.; Ferens, D.M.; Saini, N.; Chan, C.T.; Diep, H.; Kett, M.M.; Samuel, C.S.; Kemp-Harper, B.K.; et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc. Res. 2019, 115, 776–787. [Google Scholar] [CrossRef]
- Pitzer, A.; Elijovich, F.; Laffer, C.L.; Ertuglu, L.A.; Sahinoz, M.; Saleem, M.; Krishnan, J.; Dola, T.; Aden, L.A.; Sheng, Q.; et al. DC ENaC-Dependent Inflammasome Activation Contributes to Salt-Sensitive Hypertension. Circ. Res. 2022, 131, 328–344. [Google Scholar] [CrossRef]
- Lam, J.C.; Ip, M.S. An update on obstructive sleep apnea and the metabolic syndrome. Curr. Opin. Pulm. Med. 2007, 13, 484–489. [Google Scholar] [CrossRef]
- Fujita, T. Mineralocorticoid receptors, salt-sensitive hypertension, and metabolic syndrome. Hypertension 2010, 55, 813–818. [Google Scholar] [CrossRef]
- Preston, R.A.; Afshartous, D.; Caizapanta, E.V.; Materson, B.J.; Rodco, R.; Alonso, E.; Alonso, A.B. Thiazide-Sensitive NCC (Sodium-Chloride Cotransporter) in Human Metabolic Syndrome: Sodium Sensitivity and Potassium-Induced Natriuresis. Hypertension 2021, 77, 447–460. [Google Scholar] [CrossRef]
- Thuzar, M.; Stowasser, M. The mineralocorticoid receptor-an emerging player in metabolic syndrome? J. Hum. Hypertens. 2021, 35, 117–123. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 2395–2399. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Haas, M.E.; Khera, A.V.; Aragam, K.; Chaffin, M.; Klarin, D.; Hindy, G.; Jiang, L.; Wei, W.-Q.; Feng, Q.; et al. A missense variant in Mitochondrial Amidoxime Reducing Component 1 gene and protection against liver disease. PLOS Genet. 2020, 16, e1008629. [Google Scholar] [CrossRef] [PubMed]
- Kamali, Z.; Keaton, J.M.; Javanmard, S.H.; International Consortium of Blood Pressure; Program, M.V.; eQTLGen Consortium; BIOS Consortium; Edwards, T.L.; Snieder, H.; Vaez, A. Large-Scale Multi-Omics Studies Provide New Insights into Blood Pressure Regulation. Int. J. Mol. Sci. 2022, 23, 7557. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. Updating the Mitochondrial Free Radical Theory of Aging: An Integrated View, Key Aspects, and Confounding Concepts. Antioxid. Redox Signal. 2013, 19, 1420–1445. [Google Scholar] [CrossRef]
- Koh, E.H.; Lee, W.J.; Kim, M.-S.; Park, J.-Y.; Lee, I.K.; Lee, K.-U. Intracellular Fatty Acid Metabolism in Skeletal Muscle and Insulin Resistance. Curr. Diabetes Rev. 2005, 1, 331–336. [Google Scholar] [CrossRef]
- Olivetti, G.; Giordano, G.; Corradi, D.; Melissari, M.; Lagrasta, C.; Gambert, S.R.; Anversa, P. Gender differences and aging: Effects on the human heart. J. Am. Coll. Cardiol. 1995, 26, 1068–1079. [Google Scholar] [CrossRef]
- Park, Y.W.; Zhu, S.; Palaniappan, L.; Heshka, S.; Carnethon, M.R.; Heymsfield, S.B. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch. Intern. Med. 2003, 163, 427–436. [Google Scholar] [CrossRef]
- Despres, J.P. Our passive lifestyle, our toxic diet, and the atherogenic/diabetogenic metabolic syndrome: Can we afford to be sedentary and unfit? Circulation 2005, 112, 453–455. [Google Scholar] [CrossRef]
- Gallardo-Alfaro, L.; Bibiloni, M.D.M.; Mascaró, C.M.; Montemayor, S.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; et al. Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study. Nutrients 2020, 12, 1013. [Google Scholar] [CrossRef]
- De Pergola, G.; D’Alessandro, A. Influence of Mediterranean Diet on Blood Pressure. Nutrients 2018, 10, 1700. [Google Scholar] [CrossRef]
- Esposito, K.; Kastorini, C.-M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and metabolic syndrome: An updated systematic review. Rev. Endocr. Metab. Disord. 2013, 14, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Esposito, K. Mediterranean diet and metabolic diseases. Curr. Opin. Infect. Dis. 2008, 19, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Verde, L.; Sulu, C.; Katsiki, N.; Hassapidou, M.; Frias-Toral, E.; Cucalón, G.; Pazderska, A.; Yumuk, V.D.; Colao, A.; et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr. Obes. Rep. 2022, 11, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-M.M.; Steck, S.E.; Fung, T.T.; Zhang, J.; Hazlett, L.J.; Han, K.; Lee, S.-H.; Kwon, H.-S.; Merchant, A.T. Mediterranean diet, Dietary Approaches to Stop Hypertension (DASH) style diet, and metabolic health in U.S. adults. Clin. Nutr. 2017, 36, 1301–1309. [Google Scholar] [CrossRef]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef]
- Madero, M.; Perez-Pozo, S.E.; Jalal, D.; Johnson, R.J.; Sánchez-Lozada, L.G. Dietary Fructose and Hypertension. Curr. Hypertens. Rep. 2011, 13, 29–35. [Google Scholar] [CrossRef]
- Soleimani, M. Dietary fructose, salt absorption and hypertension in metabolic syndrome: Towards a new paradigm. Acta Physiol. 2011, 201, 55–62. [Google Scholar] [CrossRef]
- Soleimani, M.; Alborzi, P. The Role of Salt in the Pathogenesis of Fructose-Induced Hypertension. Int. J. Nephrol. 2011, 2011, 392708. [Google Scholar] [CrossRef]
- Genovesi, S.; Giussani, M.; Orlando, A.; Orgiu, F.; Parati, G. Salt and Sugar: Two Enemies of Healthy Blood Pressure in Children. Nutrients 2021, 13, 697. [Google Scholar] [CrossRef]
- Ha, V.; Sievenpiper, J.L.; de Souza, R.J.; Chiavaroli, L.; Wang, D.D.; Cozma, A.I.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Dibuono, M.; et al. Effect of fructose on blood pressure: A systematic review and meta-analysis of controlled feeding trials. Hypertension 2012, 59, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Jalal, D.I.; Smits, G.; Johnson, R.J.; Chonchol, M. Increased Fructose Associates with Elevated Blood Pressure. J. Am. Soc. Nephrol. 2010, 21, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.E.; Simons, N.; Simons, P.I.; Schaper, N.C.; Feskens, E.J.; van der Ploeg, L.M.; Eynde, M.D.V.D.; Schalkwijk, C.G.; Houben, A.J.; Stehouwer, C.D.; et al. Effects of fructose restriction on blood pressure: Secondary analysis of a double-blind randomized controlled trial. Clin. Nutr. ESPEN 2022, 51, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Barone, S.; Fussell, S.L.; Singh, A.K.; Lucas, F.; Xu, J.; Kim, C.; Wu, X.; Yu, Y.; Amlal, H.; Seidler, U.; et al. Slc2a5 (Glut5) Is Essential for the Absorption of Fructose in the Intestine and Generation of Fructose-induced Hypertension. J. Biol. Chem. 2009, 284, 5056–5066. [Google Scholar] [CrossRef]
- Cui, X.-L.; Jiang, L.; Ferraris, R.P. Regulation of rat intestinal GLUT2 mRNA abundance by luminal and systemic factors. Biochim. Biophys. Acta 2003, 1612, 178–185. [Google Scholar] [CrossRef]
- Ferraris, R.P.; Choe, J.; Patel, C. Intestinal Absorption of Fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef]
- Rieg, J.A.D.; Chavez, S.D.L.M.; Rieg, T. Novel developments in differentiating the role of renal and intestinal sodium hydrogen exchanger 3. Am. J. Physiol. Integr. Comp. Physiol. 2016, 311, R1186–R1191. [Google Scholar] [CrossRef]
- Höglund, P.; Haila, S.; Socha, J.; Tomaszewski, L.; Saarialho-Kere, U.; Karjalainen-Lindsberg, M.-L.; Airola, K.; Holmberg, C.; De La Chapelle, A.; Kere, J. Mutations of the Down–regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat. Genet. 1996, 14, 316–319. [Google Scholar] [CrossRef]
- Kato, A.; Romero, M.F. Regulation of Electroneutral NaCl Absorption by the Small Intestine. Annu. Rev. Physiol. 2011, 73, 261–281. [Google Scholar] [CrossRef]
- Schultheis, P.J.; Clarke, L.L.; Meneton, P.; Miller, M.L.; Soleimani, M.; Gawenis, L.R.; Riddle, T.M.; Duffy, J.J.; Doetschman, T.; Wang, T.; et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat. Genet. 1998, 19, 282–285. [Google Scholar] [CrossRef]
- Schweinfest, C.W.; Spyropoulos, D.D.; Henderson, K.W.; Kim, J.-H.; Chapman, J.M.; Barone, S.; Worrell, R.T.; Wang, Z.; Soleimani, M. slc26a3 (dra)-deficient Mice Display Chloride-losing Diarrhea, Enhanced Colonic Proliferation, and Distinct Up-regulation of Ion Transporters in the Colon. J. Biol. Chem. 2006, 281, 37962–37971. [Google Scholar] [CrossRef] [PubMed]
- Seidler, U.; Rottinghaus, I.; Hillesheim, J.; Chen, M.; Riederer, B.; Krabbenhöft, A.; Engelhardt, R.; Wiemann, M.; Wang, Z.; Barone, S.; et al. Sodium and chloride absorptive defects in the small intestine in Slc26a6 null mice. Pflug. Arch. 2008, 455, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.M.; Simpson, J.E.; Yen, P.; Gill, R.K.; Rigsby, E.V.; Brazill, J.M.; Dudeja, P.K.; Schweinfest, C.W.; Clarke, L.L. Down-regulated in Adenoma Cl/HCO3 Exchanger Couples with Na/H Exchanger 3 for NaCl Absorption in Murine Small Intestine. Gastroenterology 2008, 135, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Petrovic, S.; Mann, E.; Soleimani, M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G573–G579. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Petrovic, S.; Tuo, B.; Riederer, B.; Barone, S.; Lorenz, J.N.; Seidler, U.; Aronson, P.S.; Soleimani, M. Renal and intestinal transport defects in Slc26a6-null mice. Am. J. Physiol. Physiol. 2005, 288, C957–C965. [Google Scholar] [CrossRef]
- Xiao, F.; Yu, Q.; Li, J.; Johansson, M.E.V.; Singh, A.K.; Xia, W.; Riederer, B.; Engelhardt, R.; Montrose, M.; Soleimani, M.; et al. Slc26a3 deficiency is associated with loss of colonic HCO3− secretion, absence of a firm mucus layer and barrier impairment in mice. Acta Physiol. 2014, 211, 161–175. [Google Scholar] [CrossRef]
- Zachos, N.C.; Tse, M.; Donowitz, M. Molecular physiology of intestinal Na+/H+ exchange. Annu. Rev. Physiol. 2005, 67, 411–443. [Google Scholar] [CrossRef]
- Singh, A.K.; Amlal, H.; Haas, P.J.; Dringenberg, U.; Fussell, S.; Barone, S.L.; Engelhardt, R.; Zuo, J.; Seidler, U.; Soleimani, M. Fructose-induced hypertension: Essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int. 2008, 74, 438–447. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Madias, N.E. Sodium and Potassium in the Pathogenesis of Hypertension. N. Engl. J. Med. 2007, 356, 1966–1978. [Google Scholar] [CrossRef]
- Oberleithner, H.; Peters, W.; Kusche-Vihrog, K.; Korte, S.; Schillers, H.; Kliche, K.; Oberleithner, K. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflug. Arch. 2011, 462, 519–528. [Google Scholar] [CrossRef]
- Jhee, J.H.; Park, H.C.; Choi, H.Y. Skin Sodium and Blood Pressure Regulation. Electrolytes Blood Press. 2022, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Polychronopoulou, E.; Braconnier, P.; Burnier, M. New Insights on the Role of Sodium in the Physiological Regulation of Blood Pressure and Development of Hypertension. Front. Cardiovasc. Med. 2019, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Drummer, C.; Hesse, C.; Baisch, F.; Norsk, P.; Elmann-Larsen, B.; Gerzer, R.; Heer, M. Water and sodium balances and their relation to body mass changes in microgravity. Eur. J. Clin. Investig. 2000, 30, 1066–1075. [Google Scholar] [CrossRef]
- Heer, M.; Baisch, F.; Kropp, J.; Gerzer, R.; Drummer, C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am. J. Physiol. Physiol. 2000, 278, F585–F595. [Google Scholar] [CrossRef]
- Rakova, N.; Jüttner, K.; Dahlmann, A.; Schröder, A.; Linz, P.; Kopp, C.; Rauh, M.; Goller, U.; Beck, L.; Agureev, A.; et al. Long-Term Space Flight Simulation Reveals Infradian Rhythmicity in Human Na+ Balance. Cell Metab. 2013, 17, 125–131. [Google Scholar] [CrossRef]
- Foulke-Abel, J.; In, J.; Yin, J.; Zachos, N.C.; Kovbasnjuk, O.; Estes, M.K.; de Jonge, H.; Donowitz, M. Human Enteroids as a Model of Upper Small Intestinal Ion Transport Physiology and Pathophysiology. Gastroenterology 2016, 150, 638–649.e8. [Google Scholar] [CrossRef]
- Hasan, N.M.; Johnson, K.F.; Yin, J.; Baetz, N.W.; Fayad, L.; Sherman, V.; Blutt, S.E.; Estes, M.K.; Kumbhari, V.; Zachos, N.C.; et al. Intestinal stem cell-derived enteroids from morbidly obese patients preserve obesity-related phenotypes: Elevated glucose absorption and gluconeogenesis. Mol. Metab. 2021, 44, 101129. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Cabral, P.D.; Hong, N.J.; Asirwatham, J.; Saez, F.; Garvin, J.L. Fructose reabsorption by rat proximal tubules: Role of Na+-linked cotransporters and the effect of dietary fructose. Am. J. Physiol. Physiol. 2019, 316, F473–F480. [Google Scholar] [CrossRef]
- Ares, G.R.; Kassem, K.M.; Ortiz, P.A. Fructose acutely stimulates NKCC2 activity in rat thick ascending limbs by increasing surface NKCC2 expression. Am. J. Physiol. Renal. Physiol. 2019, 316, F550–F557. [Google Scholar] [CrossRef]
- Bahena-Lopez, J.P.; Rojas-Vega, L.; Chávez-Canales, M.; Bazua-Valenti, S.; Bautista-Pérez, R.; Lee, J.-H.; Madero, M.; Vazquez-Manjarrez, N.; Alquisiras-Burgos, I.; Hernandez-Cruz, A.; et al. Glucose/Fructose Delivery to the Distal Nephron Activates the Sodium-Chloride Cotransporter via the Calcium-Sensing Receptor. J. Am. Soc. Nephrol. 2022, 34, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Cabral, P.D.; Hong, N.J.; Khan, A.H.; Ortiz, P.A.; Beierwaltes, W.H.; Imig, J.; Garvin, J.L. Fructose Stimulates Na/H Exchange Activity and Sensitizes the Proximal Tubule to Angiotensin II. Hypertension 2014, 63, e68–e73. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.; Sato, W.; Reungjui, S.; Heinig, M.; Gersch, M.; Sautin, Y.; Nakagawa, T.; Johnson, R.J. Uric acid, the metabolic syndrome, and renal disease. J. Am. Soc. Nephrol. 2006, 17 (Suppl. S3), S165–S168. [Google Scholar] [CrossRef] [PubMed]
- Flisiński, M.; Brymora, A.; Skoczylas-Makowska, N.; Stefańska, A.; Manitius, J. Fructose-Rich Diet Is a Risk Factor for Metabolic Syndrome, Proximal Tubule Injury and Urolithiasis in Rats. Int. J. Mol. Sci. 2021, 23, 203. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef]
- Lozano, I.; Van der Werf, R.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; et al. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2016, 13, 1–13. [Google Scholar] [CrossRef]
- Zhuhua, Z.; Zhiquan, W.; Zhen, Y.; Yixin, N.; Weiwei, Z.; Xiaoyong, L.; Yueming, L.; Hongmei, Z.; Li, Q.; Qing, S. A novel mice model of metabolic syndrome: The high-fat-high-fructose diet-fed ICR mice. Exp. Anim. 2015, 64, 435–442. [Google Scholar] [CrossRef]
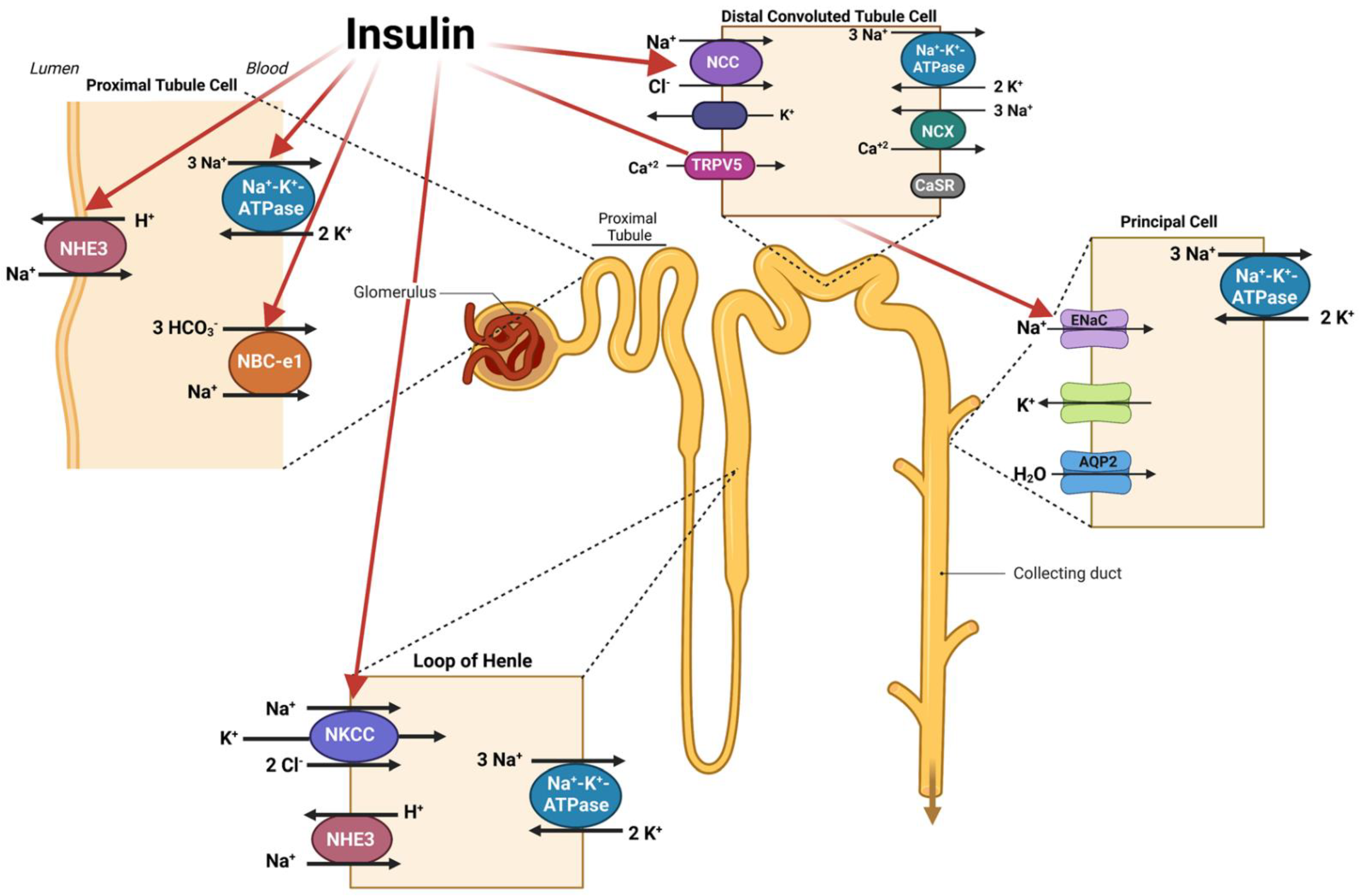
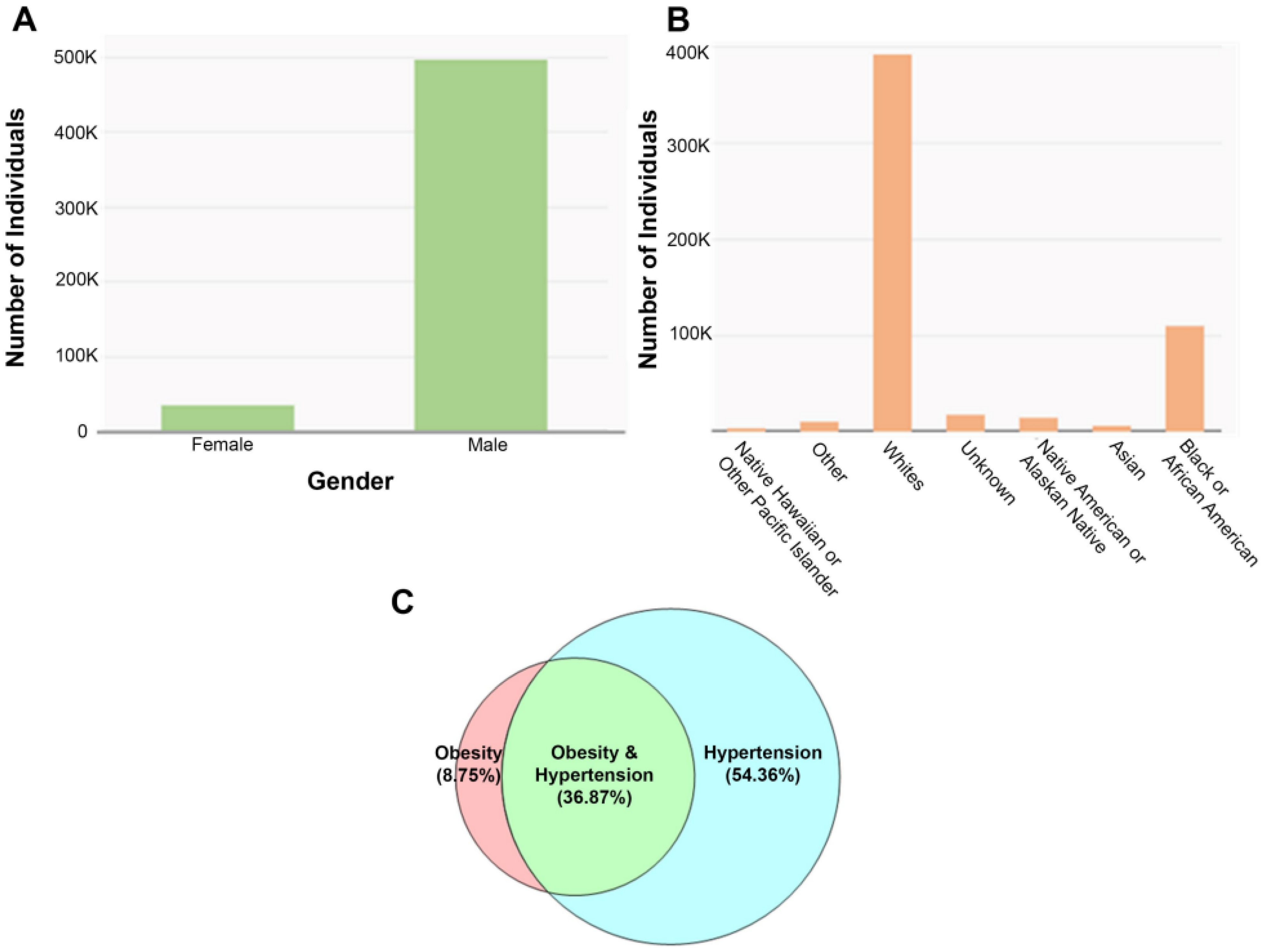

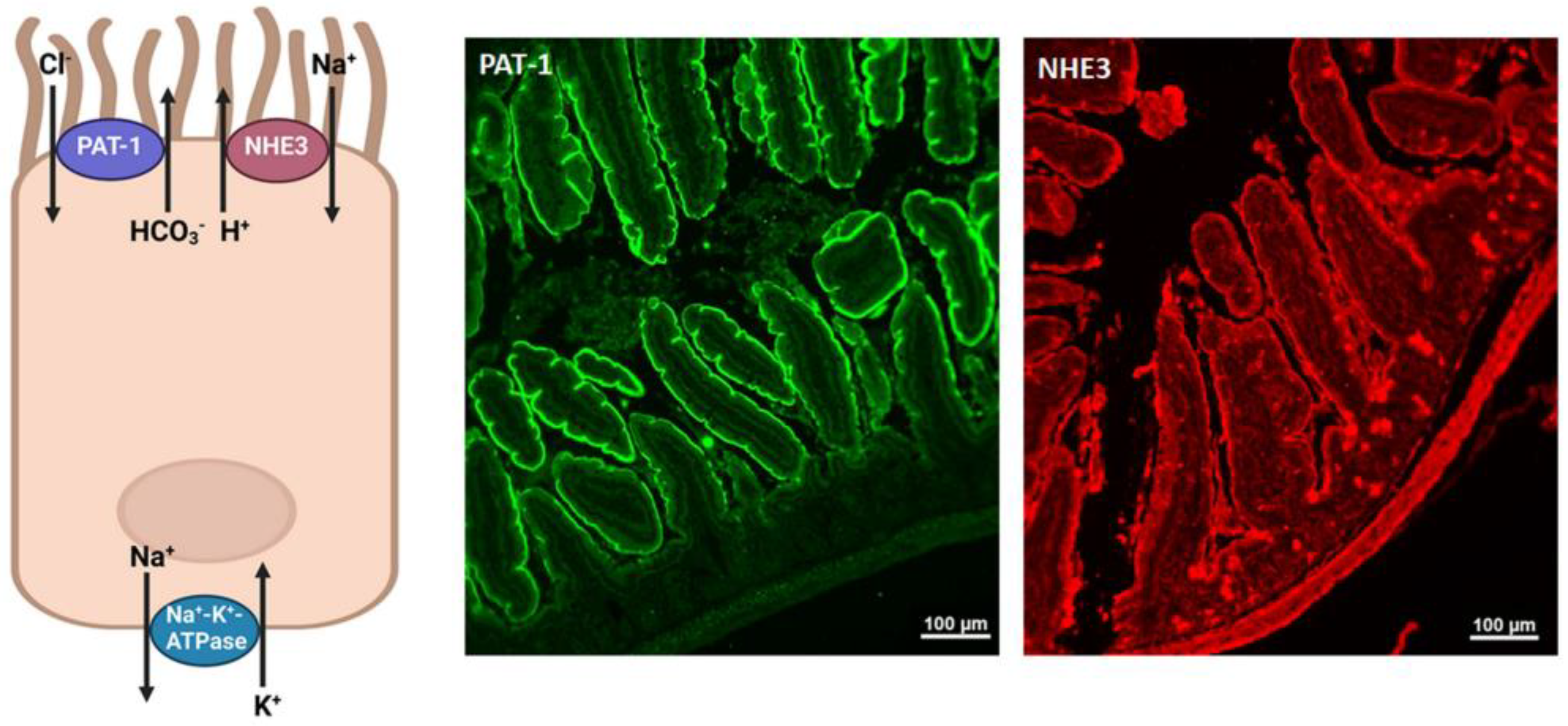
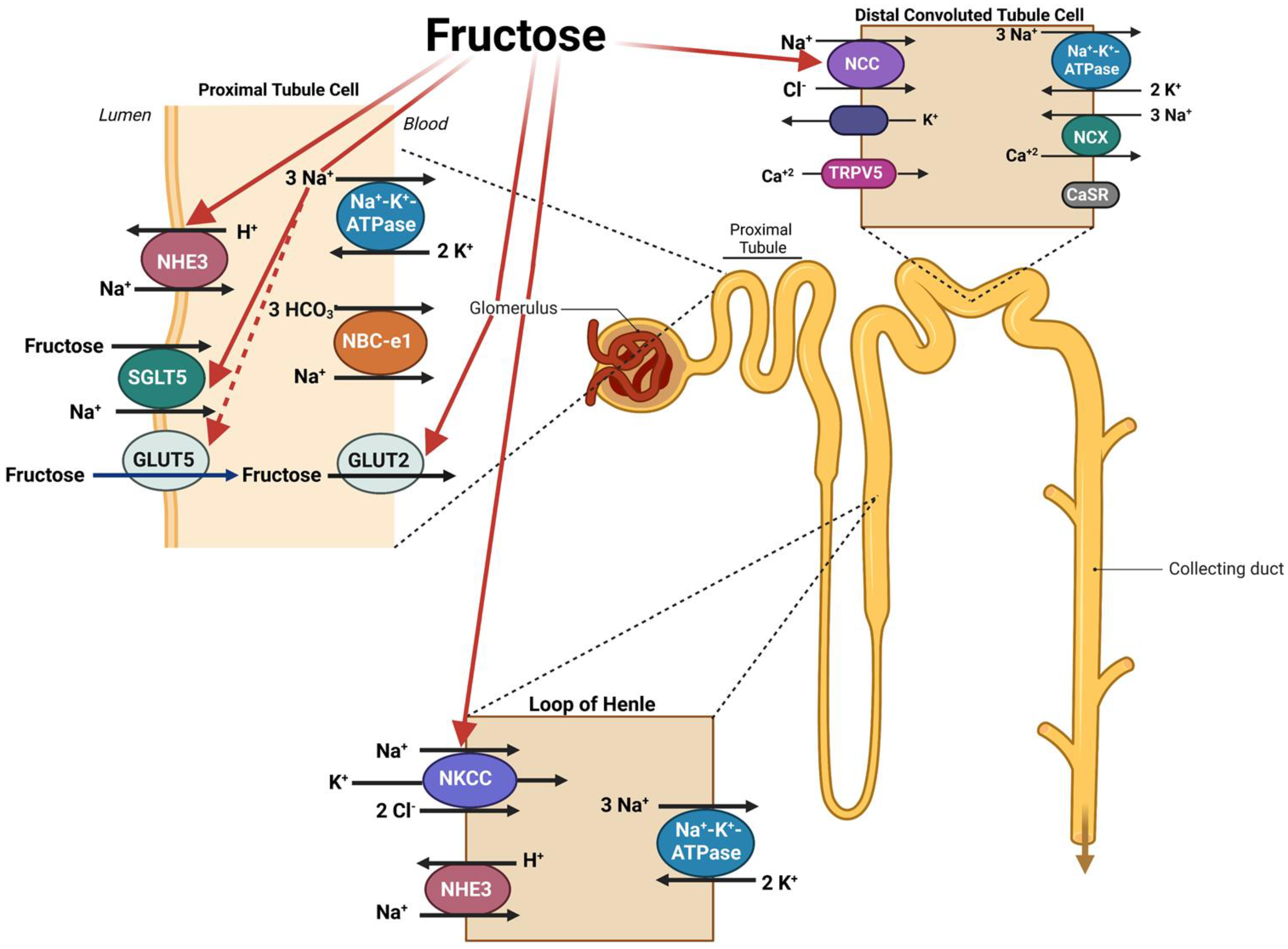
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleimani, M.; Barone, S.; Luo, H.; Zahedi, K. Pathogenesis of Hypertension in Metabolic Syndrome: The Role of Fructose and Salt. Int. J. Mol. Sci. 2023, 24, 4294. https://doi.org/10.3390/ijms24054294
Soleimani M, Barone S, Luo H, Zahedi K. Pathogenesis of Hypertension in Metabolic Syndrome: The Role of Fructose and Salt. International Journal of Molecular Sciences. 2023; 24(5):4294. https://doi.org/10.3390/ijms24054294
Chicago/Turabian StyleSoleimani, Manoocher, Sharon Barone, Henry Luo, and Kamyar Zahedi. 2023. "Pathogenesis of Hypertension in Metabolic Syndrome: The Role of Fructose and Salt" International Journal of Molecular Sciences 24, no. 5: 4294. https://doi.org/10.3390/ijms24054294
APA StyleSoleimani, M., Barone, S., Luo, H., & Zahedi, K. (2023). Pathogenesis of Hypertension in Metabolic Syndrome: The Role of Fructose and Salt. International Journal of Molecular Sciences, 24(5), 4294. https://doi.org/10.3390/ijms24054294







