Novel High Conductive Ceramic Materials Based on Two-Layer Perovskite BaLa2In2O7
Abstract
1. Introduction
2. Results and Discussion
2.1. XRD and SEM Characterization
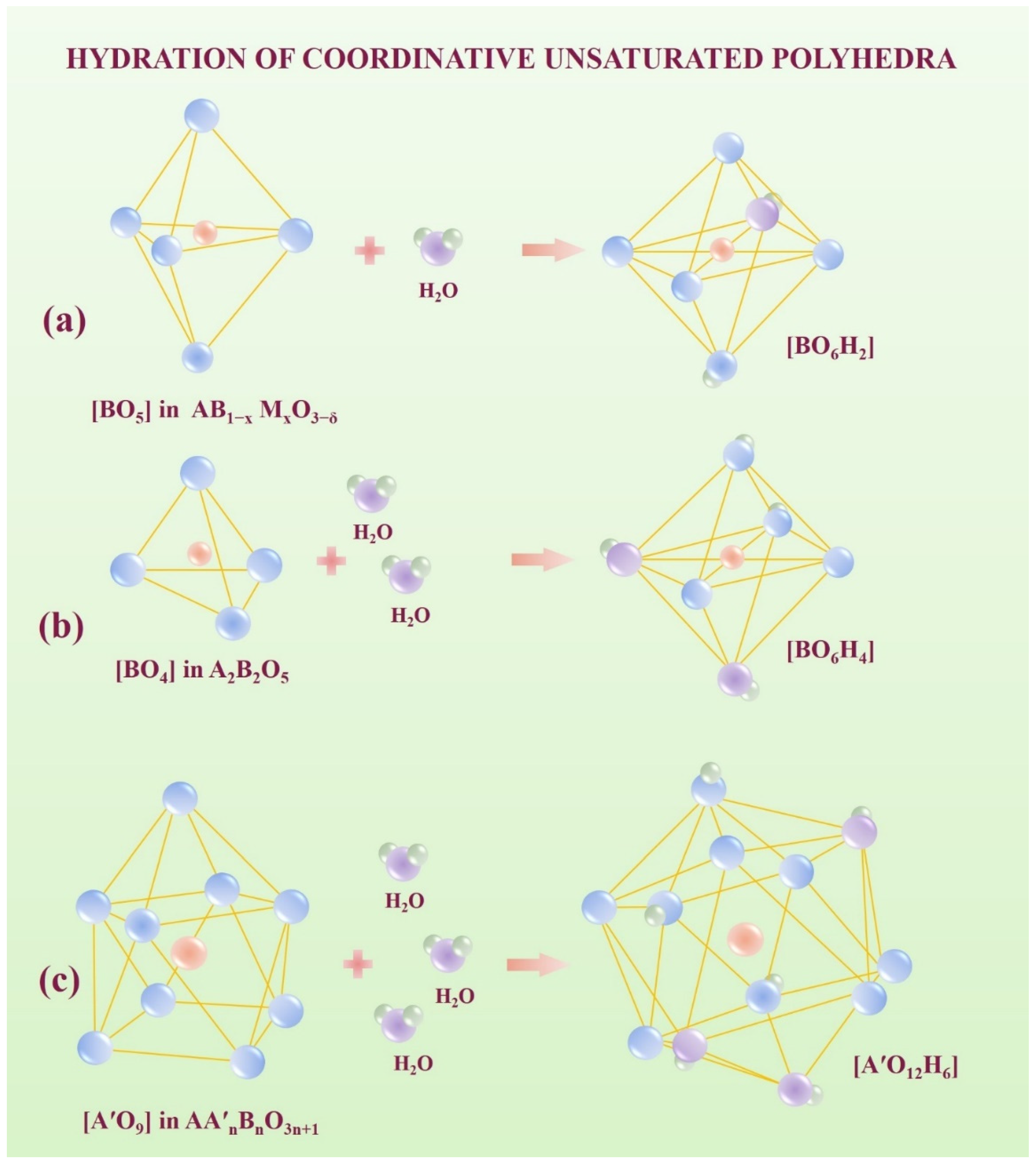
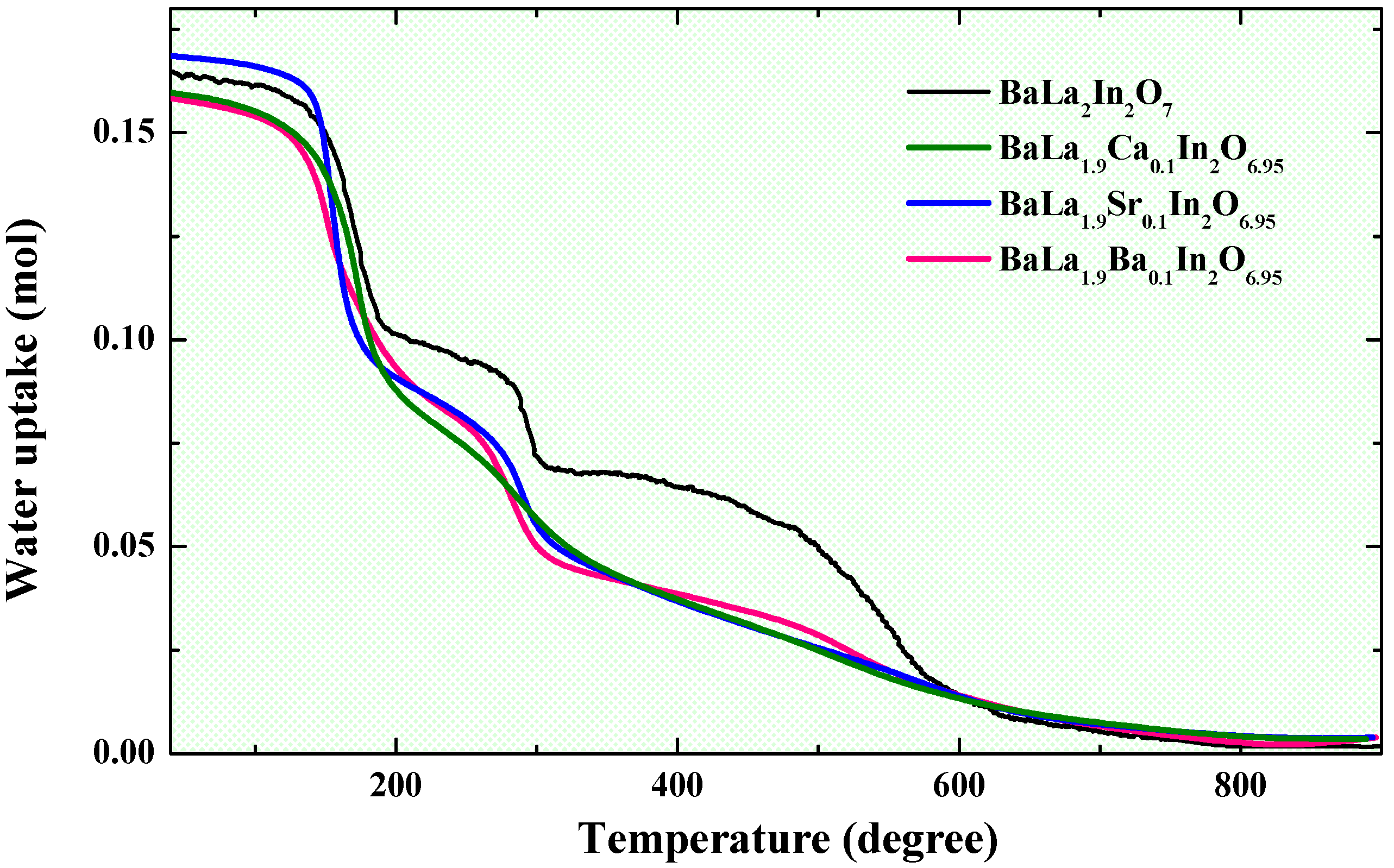
2.2. Water Uptake
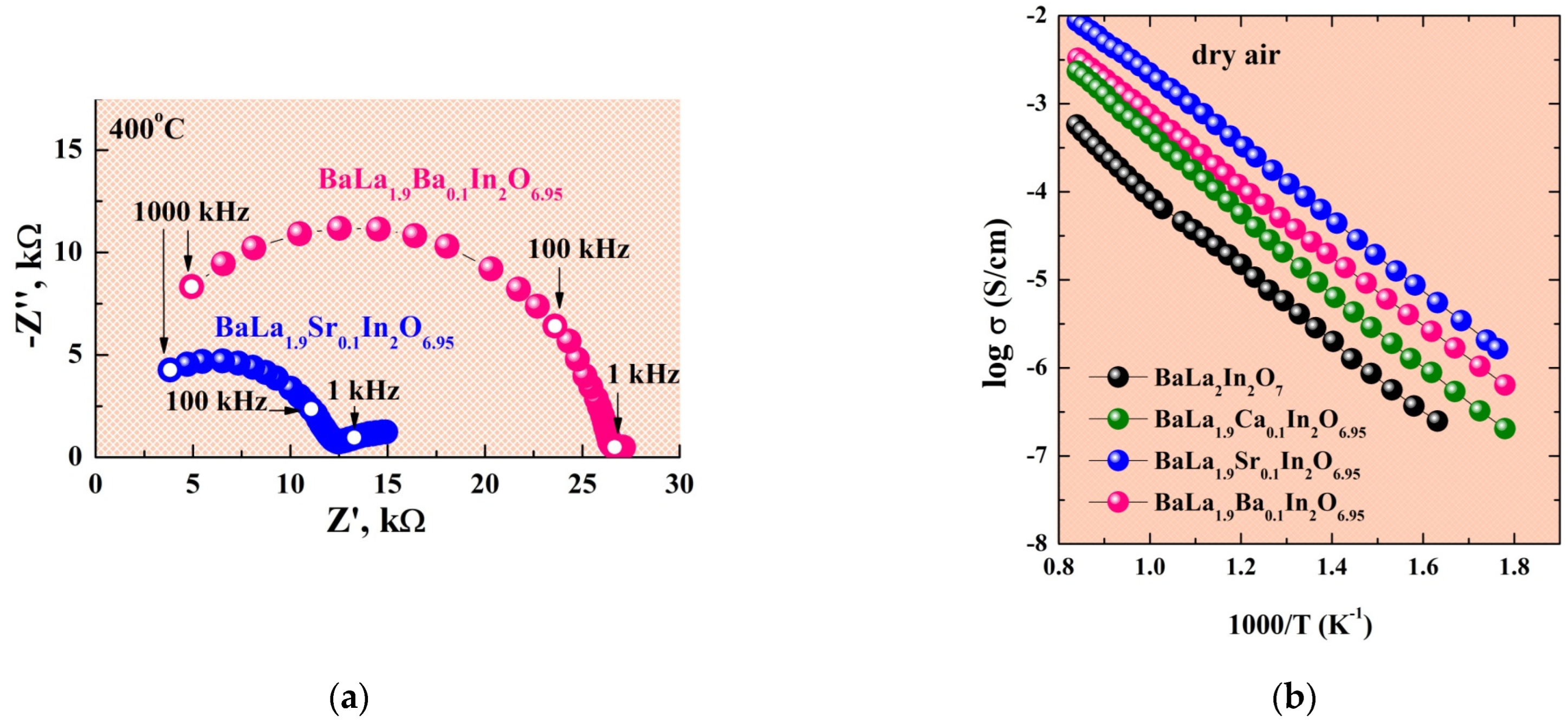
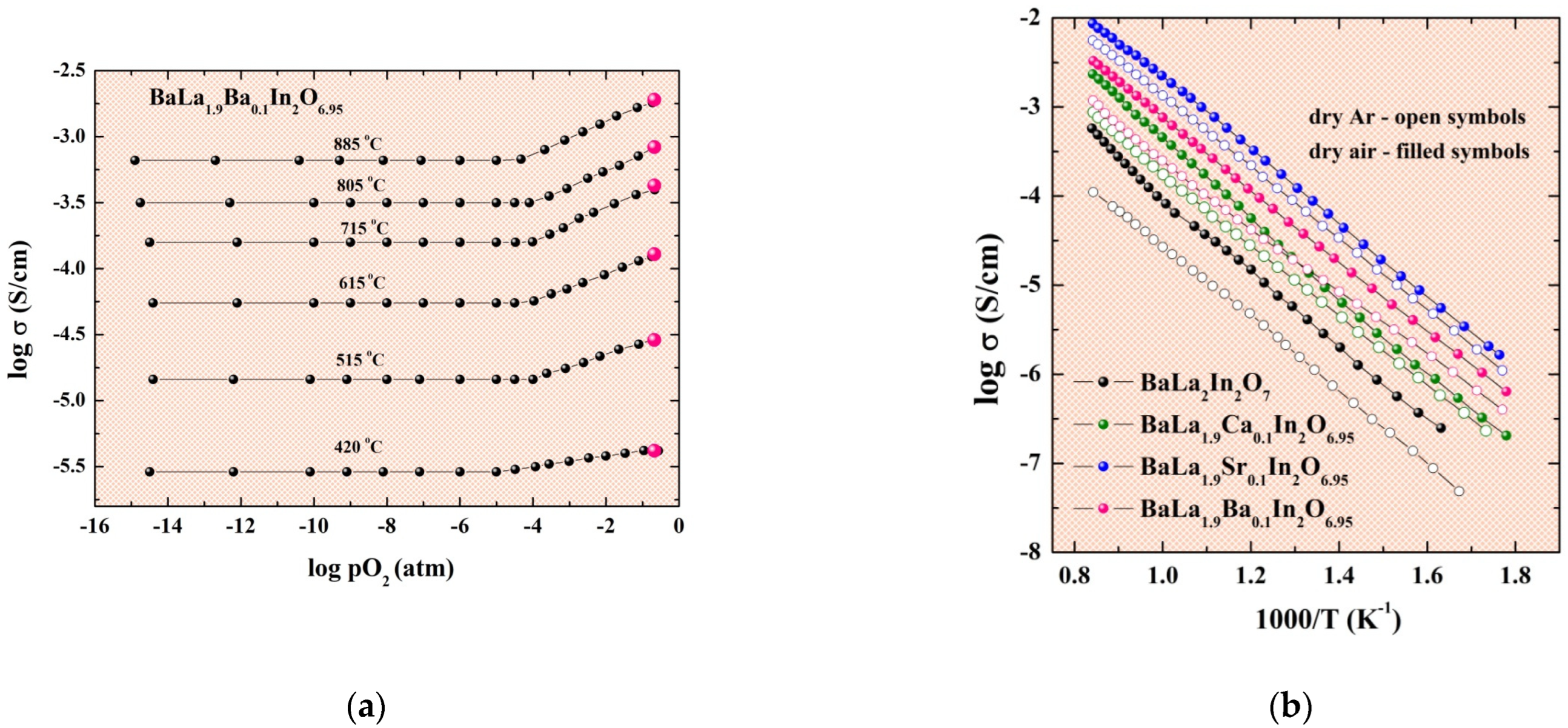
2.3. Electrical Conductivity Investigations under Dry (pH2O = 3.5 × 10−5 atm) Conditions
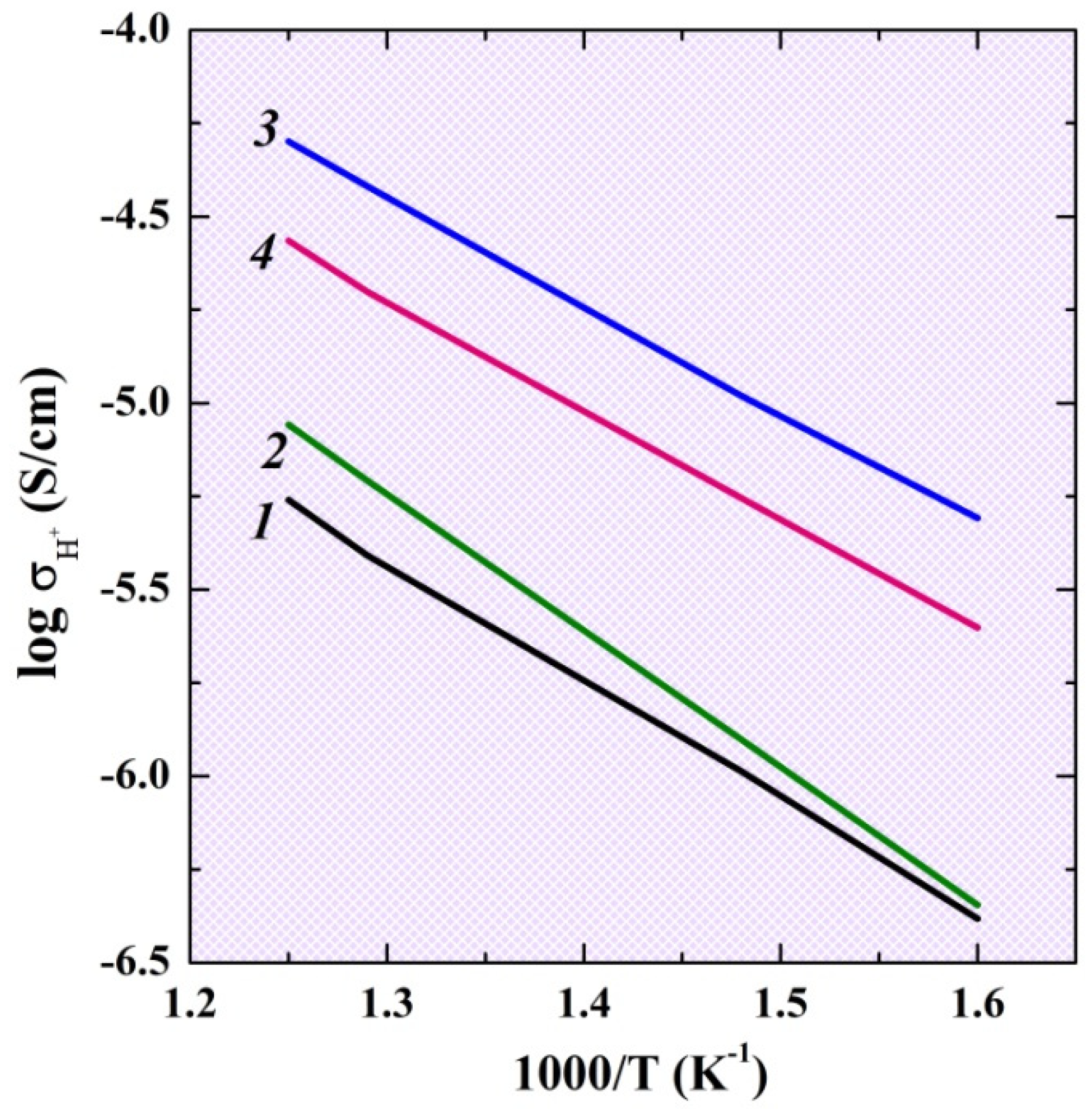
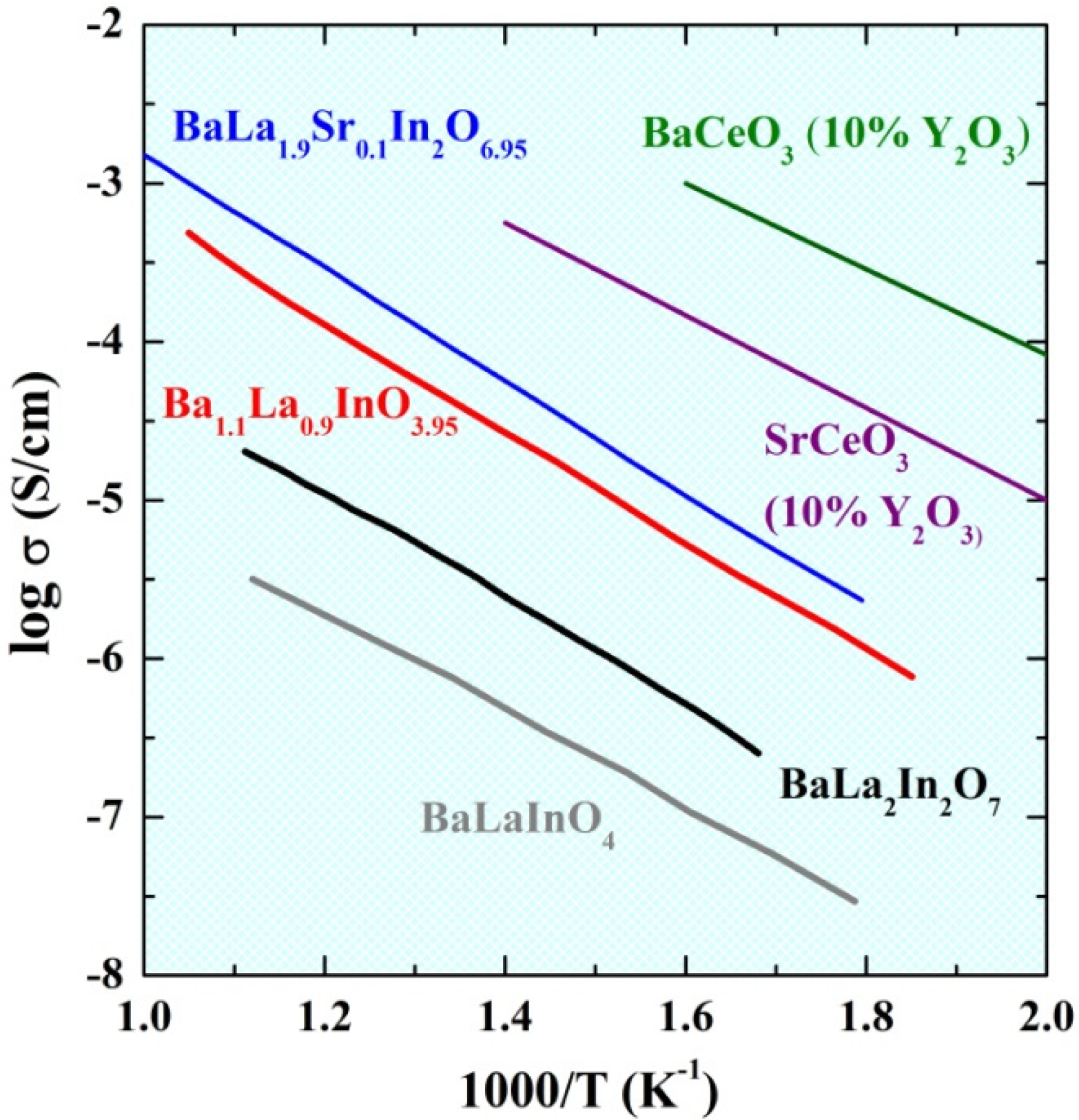
2.4. Electrical Conductivity Investigations under Wet (pH2O = 2 × 10−2 atm) Conditions
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corvalan, C.; Prats, E.V.; Sena, A.; Varangu, L.; Vinci, S. Towards climate resilient and environmentally sustainable health care facilities. Int. J. Environ. Res. Public Health 2020, 17, 8849. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.; Amann, M.; Arnell, N.; Montgomery, H.; Costello, A. The 2020 report of the Lancet countdown on health and climate change: Responding to converging crises. Lancet 2021, 397, 129–170. [Google Scholar] [CrossRef]
- Afroze, S.; Reza, M.S.; Cheok, Q.; Taweekun, J.; Azad, A.K. Solid oxide fuel cell (SOFC); A new approach of energy generation during the pandemic COVID-19. Int. J. Integr. Eng. 2020, 12, 245–256. [Google Scholar] [CrossRef]
- Afroze, S.; Reza, M.S.; Cheok, Q.; Islam, S.N.; Abdalla, A.M.; Taweekun, J.; Azad, A.K.; Khalilpoor, N.; Issakhov, A. Advanced applications of fuel cells during the COVID-19 Pandemic. Int. J. Chem. Eng. 2021, 2021, 5539048. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2019, 45, 3847–3869. [Google Scholar] [CrossRef]
- Easily, R.R.; Chi, Y.; Ibrahiem, D.M.; Chen, Y. Hydrogen strategy in decarbonization era: Egypt as a case study. Int. J. Hydrogen Energy 2022, 47, 18629–18647. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Mansur, M.; Muttaqi, K.M.; Dong, Z.Y.; Blaabjerg, F. Hydrogen energy storage integrated hybrid renewable energy systems: A review analysis for future research directions. Int. J. Hydrogen Energy 2022, 47, 17285–17312. [Google Scholar] [CrossRef]
- Scovell, M.D. Explaining hydrogen energy technology acceptance: A critical review. Int. J. Hydrogen Energy 2022, 47, 10441–104591. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Ji, H.-I.; Lee, J.-H.; Son, J.-W.; Yang, S.; Kim, B.-K. Protonic ceramic electrolysis cells for fuel production: A brief review. J. Korean Ceram. Soc. 2020, 57, 480–494. [Google Scholar] [CrossRef]
- Kim, I.Y.; Ko, J.; Ahn, T.-Y.; Cheong, H.-W.; Yoon, Y.S. Energy materials for energy conversion and storage: Focus on research conducted in Korea. J. Korean Ceram. Soc. 2021, 58, 645–661. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, I.; Kim, K.J.; Yu, H.; Lee, K.T. A brief review of heterostructure electrolytes for high-performance solid oxide fuel cells at reduced temperatures. J. Korean Ceram. Soc. 2022, 59, 131–152. [Google Scholar] [CrossRef]
- Sun, C.; Alonso, J.A.; Bian, J. Recent advances in perovskite-type oxides for energy conversion and storage applications. Adv. Energy Mater. 2020, 11, 2000459. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Ran, R.; Cao, J.; Shao, Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 764–774. [Google Scholar] [CrossRef]
- Yang, B.; Guo, Z.; Wang, J.; Wang, J.; Zhu, T.; Shu, H.; Qiu, G.; Chen, J.; Zhang, J. Solid oxide fuel cell systems fault diagnosis: Critical summarization, classification, and perspectives. J. Energy Storage 2021, 34, 102153. [Google Scholar] [CrossRef]
- Peng, J.; Huang, J.; Wu, X.-L.; Xu, Y.-W.; Chen, H.; Li, X. Solid oxide fuel cell (SOFC) performance evaluation, fault diagnosis and health control: A review. J. Power Sources 2021, 505, 230058. [Google Scholar] [CrossRef]
- Ding, P.; Li, W.; Zhao, H.; Wu, C.; Zhao, L.; Dong, B.; Wang, S. Review on Ruddlesden-Popper perovskites as cathode for solid oxide fuel cells. J. Phys. Mater. 2021, 4, 022002. [Google Scholar] [CrossRef]
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Shen, M.; Ai, F.; Ma, H.; Xu, H.; Zhang, Y. Progress and prospects of reversible solid oxide fuel cell materials. iScience 2021, 24, 103464. [Google Scholar] [CrossRef]
- Kim, S.; Kim, G.; Manthiram, A. A review on infiltration techniques for energy conversion and storage devices: From fundamentals to applications. Sustain. Energy Fuels 2021, 5, 5024–5037. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Chizhova, E.A.; Kharytonau, D.S.; Medvedev, D.A. Layered oxygen-deficient double perovskites as promising cathode materials for solid oxide fuel cells. Materials 2022, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.B.; Motola, M.; Qayyum, S.; Rauf, S.; Khalid, A.; Li, C.-J.; Li, C.-X. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chem. Eng. J. 2022, 428, 132603. [Google Scholar] [CrossRef]
- Im, S.; Lee, J.-H.; Ji, H.-I. PrBa0.5Sr0.5Co1.5Fe0.5O5+δ composite cathode in protonic ceramic fuel cells. J. Korean Ceram. Soc. 2021, 58, 351–358. [Google Scholar] [CrossRef]
- Shim, J.H. Ceramics breakthrough. Nat. Energy 2018, 3, 168–169. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, J.; Zhao, Z.; Amoroso, J.; Tong, J.; Brinkman, K.S. Review: Recent progress in low-temperature proton-conducting ceramics. J. Mater. Sci. 2019, 54, 9291–9312. [Google Scholar] [CrossRef]
- Kim, J.; Sengodan, S.; Kim, S.; Kwon, O.; Bu, Y.; Kim, G. Proton conducting oxides: A review of materials and applications for renewable energy conversion and storage. Renew. Sustain. Energy Rev. 2019, 109, 606–618. [Google Scholar] [CrossRef]
- Medvedev, D.A. Current drawbacks of proton-conducting ceramic materials: How to overcome them for real electrochemical purposes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100549. [Google Scholar] [CrossRef]
- Bello, I.T.; Zhai, S.; He, Q.; Cheng, C.; Dai, Y.; Chen, B.; Zhang, Y.; Ni, M. Materials development and prospective for protonic ceramic fuel cells. Int. J. Energy Res. 2021, 46, 2212–2240. [Google Scholar] [CrossRef]
- Irvine, J.; Rupp, J.L.; Liu, G.; Xu, X.; Haile, S.; Qian, X.; Snyder, A.; Freer, R.; Ekren, D.; Skinner, S.; et al. Roadmap on inorganic perovskites for energy applications. J. Phys. Energy 2021, 3, 031502. [Google Scholar] [CrossRef]
- Hossain, M.K.; Chanda, R.; El-Denglawey, A.; Emrose, T.; Rahman, M.T.; Biswas, M.C.; Hashizume, K. Recent progress in barium zirconate proton conductors for electrochemical hydrogen device applications: A review. Ceram. Int. 2021, 47, 23725–23748. [Google Scholar] [CrossRef]
- Tarasova, N.; Colomban, P.; Animitsa, I. The short-range structure and hydration process of fluorine-substituted double perovskites based on barium-calcium niobate Ba2CaNbO5.5. J. Phys. Chem. Solids 2018, 118, 32–39. [Google Scholar] [CrossRef]
- Cichy, K.; Skubida, W.; Świerczek, K. Structural transformations, water incorporation and transport properties of tin-substituted barium indate. J. Solid State Chem. 2018, 262, 58–67. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. Materials AIILnInO4 with Ruddlesden-Popper structure for electrochemical applications: Relationship between ion (oxygen-ion, proton) conductivity, water uptake and structural changes. Materials 2022, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, N.; Animitsa, I.; Galisheva, A.; Medvedev, D. Layered and hexagonal perovskites as novel classes of proton-conducting solid electrolytes: A focus review. Electrochem. Mater. Technol. 2022, 1, 20221004. [Google Scholar] [CrossRef]
- Fujii, K.; Shiraiwa, M.; Esaki, Y.; Yashima, M.; Kim, S.J.; Lee, S. Improved oxide-ion conductivity of NdBaInO4 by Sr doping. J. Mater. Chem. A 2015, 3, 11985–11990. [Google Scholar] [CrossRef]
- Ishihara, T.; Yan, Y.; Sakai, T.; Ida, S. Oxide ion conductivity in doped NdBaInO4. Solid State Ion. 2016, 288, 262–265. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Lu, F.; Xu, J.; Kuang, X. Acceptor doping and oxygen vacancy migration in layered perovskite NdBaInO4-based mixed conductors. J. Phys. Chem. C 2016, 12, 6416–6426. [Google Scholar] [CrossRef]
- Fujii, K.; Yashima, M. Discovery and development of BaNdInO4—A brief review. J. Ceram. Soc. Japan 2018, 126, 852–859. [Google Scholar] [CrossRef]
- Zhou, Y.; Shiraiwa, M.; Nagao, M.; Fujii, K.; Tanaka, I.; Yashima, M.; Baque, L.; Basbus, J.F.; Mogni, L.V.; Skinner, S.J. Protonic conduction in the BaNdInO4 structure achieved by acceptor doping. Chem. Mater. 2021, 33, 2139–2146. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Effect of acceptor and donor doping on the state of protons in block-layered structures based on BaLaInO4. Solid State Comm. 2021, 323, 114093. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A.; Pryakhina, V. Protonic transport in the new phases BaLaIn0.9M0.1O4.05 (M = Ti, Zr) with Ruddlesden-Popper structure. Solid State Sci. 2020, 101, 106121. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Electrical properties of new protonic conductors Ba1+xLa1−xInO4−0.5x with Ruddlesden-Popper structure. J. Solid State Electrochem. 2020, 24, 1497–1508. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I. Improvement of oxygen-ionic and protonic conductivity of BaLaInO4 through Ti doping. Ionics 2020, 26, 5075–5088. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I. Ba2+/Ti4+-co-doped layered perovskite BaLaInO4: The structure and ionic (O2−, H+) conductivity. Int. J. Hydrogen Energy 2021, 46, 16868–16877. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Belova, K. Simultaneous hetero- and isovalent doping as the strategy for improving transport properties of proton conductors based on BaLaInO4. Materials 2021, 14, 6240. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Korona, D.; Davletbaev, K. Novel proton-conducting layered perovskite based on BaLaInO4 with two different cations in B-sublattice: Synthesis, hydration, ionic (O2+, H+) conductivity. Int. J. Hydrogen Energy 2022, 47, 18972–18982. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Anokhina, I.; Gilev, A.; Cheremisina, P. Novel mid-temperature Y3+ → In3+ doped proton conductors based on the layered perovskite BaLaInO4. Ceram. Int. 2022, 48, 15677–15685. [Google Scholar] [CrossRef]
- Kato, S.; Ogasawara, M.; Sugai, M.; Nakata, S. Synthesis and oxide ion conductivity of new layered perovskite La1−xSr1+xInO4−d. Solid State Ion. 2002, 149, 53–57. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Aguadero, A. Low activation energies for interstitial oxygen conduction in the layered perovskites La1+xSr1−xInO4+d. J. Mater. Chem. A 2015, 3, 17797–17803. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Fernández-Díaz, M.T.; Aguadero, A. Introduction of interstitial oxygen atoms in the layered perovskite LaSrIn1−xBxO4+δ system (B = Zr, Ti). Solid State Ion. 2015, 282, 82–87. [Google Scholar] [CrossRef]
- Troncoso, L.; Mariño, C.; Arce, M.D.; Alonso, J.A. Dual oxygen defects in layered La1.2Sr0.8−xBaxInO4+d (x = 0.2, 0.3) oxide-ion conductors: A neutron diffraction study. Materials 2019, 12, 1624. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, L.; Arce, M.D.; Fernández-Díaz, M.T.; Mogni, L.V.; Alonso, J.A. Water insertion and combined interstitial-vacancy oxygen conduction in the layered perovskites La1.2Sr0.8−xBaxInO4+d. New J. Chem. 2019, 43, 6087–6094. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Korona, D.; Kreimesh, H.; Fedorova, I. Protonic transport in layered perovskites BaLanInnO3n+1 (n = 1, 2) with Ruddlesden-Popper structure. Appl. Sci. 2022, 12, 4082. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Titov, Y.A.; Belyavina, N.M.; Markiv, V.Y.; Slobodyanik, M.S.; Krayevska, Y.A.; Yaschuk, V.P. Synthesis and crystal structure of BaLn2In2O7. Rep. Natl. Acad. Sci. Ukr. 2010, 1, 148–153. [Google Scholar]
- Caldes, M.; Michel, C.; Rouillon, T.; Hervieu, M.; Raveau, B. Novel indates Ln2BaIn2O7, n = 2 members of the Ruddlesden–Popper family (Ln = La, Nd). J. Mater. Chem. 2002, 12, 473–476. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Belova, K.; Egorova, A.; Abakumova, E.; Medvedev, D. Layered perovskites BaM2In2O7 (M = La, Nd): From the structure to the ionic (O2–, H+) conductivity. Materials 2022, 15, 3488. [Google Scholar] [CrossRef]
- He, H.; Huang, X.; Chen, L. Sr-doped LaInO3 and its possible application in a single layer SOFC. Solid State Ion. 2000, 130, 183–193. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-conducting oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
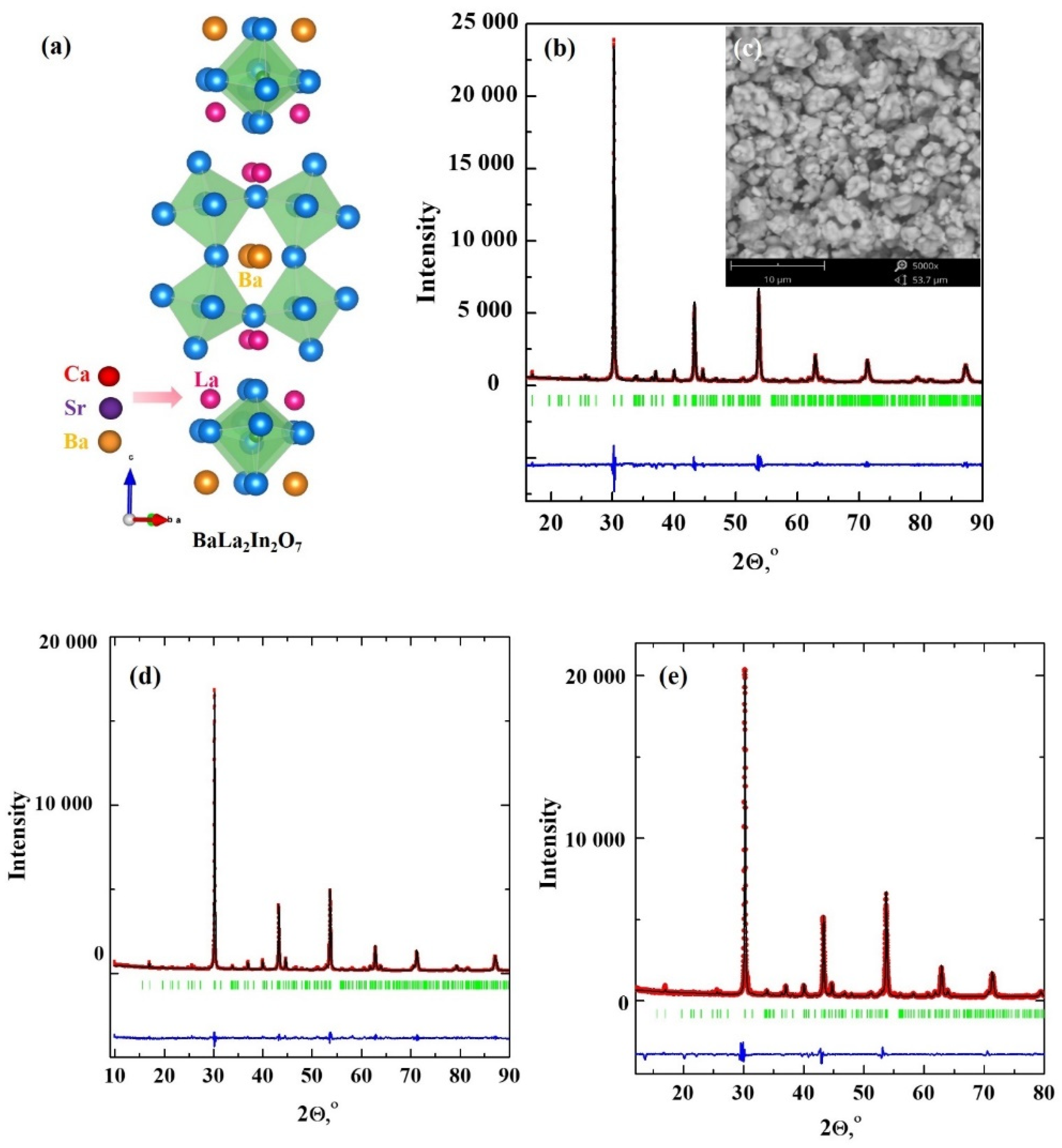

| Sample | a, b (Å) | c (Å) | Vcell (Å3) |
|---|---|---|---|
| BaLa2In2O7 | 5.914 (9) | 20.846 (5) | 729.336 (5) |
| BaLa1.9Ca0.1In2O6.95 | 5.908 (7) | 20.861 (7) | 728.339 (0) |
| BaLa1.9Sr0.1In2O6.95 | 5.916 (3) | 20.870 (4) | 730.518 (3) |
| BaLa1.9Ba0.1In2O6.95 | 5.921 (0) | 20.881 (3) | 732.051 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasova, N.; Bedarkova, A.; Animitsa, I.; Abakumova, E.; Belova, K.; Kreimesh, H. Novel High Conductive Ceramic Materials Based on Two-Layer Perovskite BaLa2In2O7. Int. J. Mol. Sci. 2022, 23, 12813. https://doi.org/10.3390/ijms232112813
Tarasova N, Bedarkova A, Animitsa I, Abakumova E, Belova K, Kreimesh H. Novel High Conductive Ceramic Materials Based on Two-Layer Perovskite BaLa2In2O7. International Journal of Molecular Sciences. 2022; 23(21):12813. https://doi.org/10.3390/ijms232112813
Chicago/Turabian StyleTarasova, Nataliia, Anzhelika Bedarkova, Irina Animitsa, Ekaterina Abakumova, Ksenia Belova, and Hala Kreimesh. 2022. "Novel High Conductive Ceramic Materials Based on Two-Layer Perovskite BaLa2In2O7" International Journal of Molecular Sciences 23, no. 21: 12813. https://doi.org/10.3390/ijms232112813
APA StyleTarasova, N., Bedarkova, A., Animitsa, I., Abakumova, E., Belova, K., & Kreimesh, H. (2022). Novel High Conductive Ceramic Materials Based on Two-Layer Perovskite BaLa2In2O7. International Journal of Molecular Sciences, 23(21), 12813. https://doi.org/10.3390/ijms232112813







