Plasmonic and Conductive Structures of TCO Films with Embedded Cu Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition and Morphology Analysis
2.2. UV–Vis–NIR Optical Properties
2.3. Electrical and Optical (Egap) Properties
- In all the dry PCSs, the Cu film as deposited was a noncontinuous film or coalesced gradually toward larger particles as a function of thermal annealing;
- In all the wet PCSs, Cu NPs with various sizes were randomly and uniformly distributed on bottom TCOs;
- Cu in all its forms in these structures showed a very high sheet resistance >106 Ω/sq, the sheet resistance of all wet and dry PCSs only depended on the two TCO layers due to a negligible value for the Cu sheet resistance:
2.4. Comparing the best PCSs
3. Materials and Methods
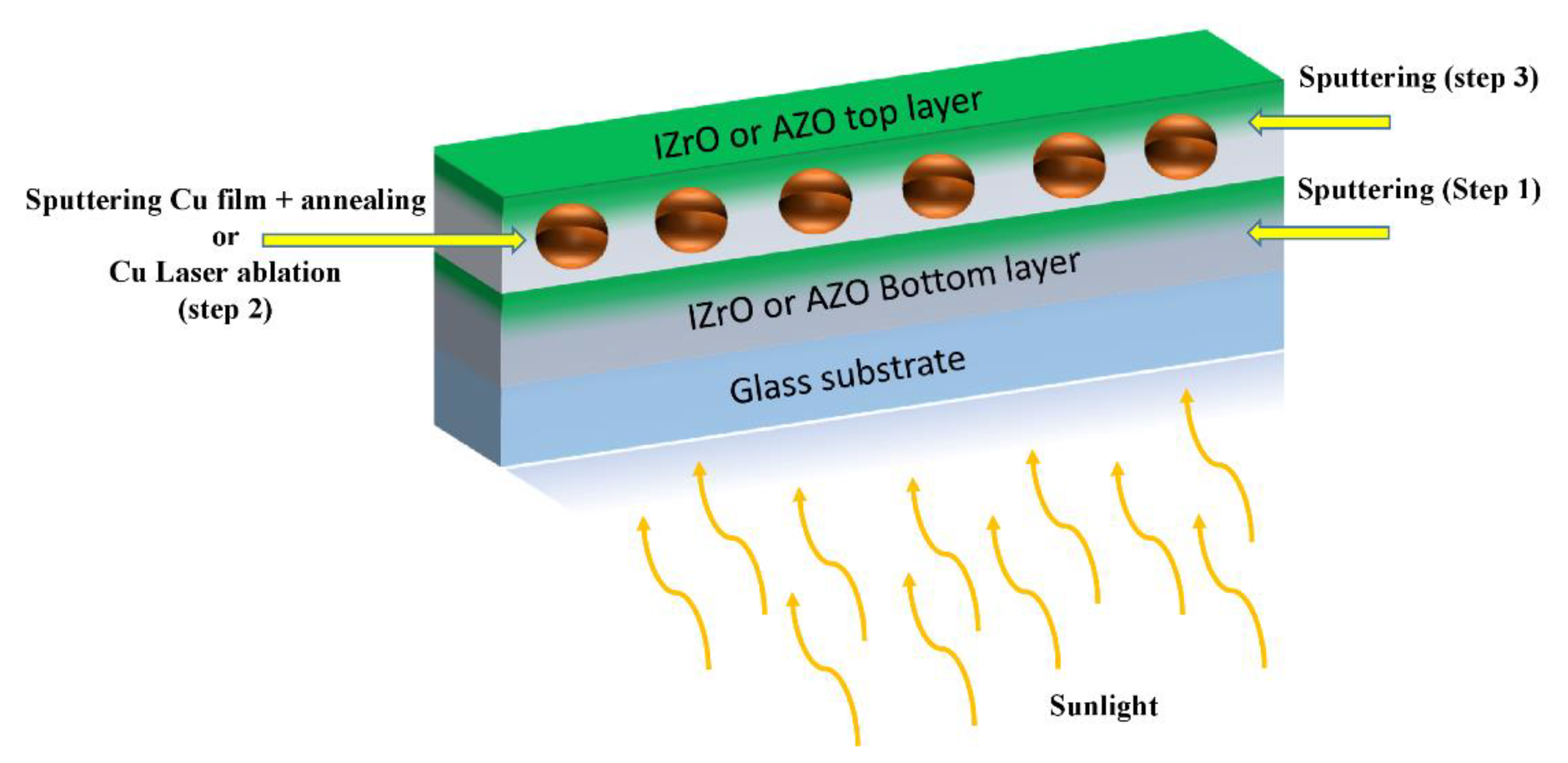
Dry and Wet PCS Preparation
- In the dry configuration, after the deposition of the Cu thin film on the bottom TCO (AZO or IZrO), the Cu nanoparticles were produced by a thermal annealing of the glass substrate/bottom TCO/Cu thin-film stack. This led to an energetically favored set of Cu nanoparticles at temperatures below the melting point. Lastly, the previous Cu nanoparticles were covered with a top TCO (AZO or IZrO) layer.
- In the wet configuration, in the case of laser ablation in a solvent, after using an ultrasonic dispersion process on the produced colloidal solutions in order to avoid agglomeration of nanoparticles, a fraction measuring 100 µL was poured onto the bottom TCO layer. The solvent was evaporated by means of a hot plate at 100 °C for 30 min. This weak thermal process involved the glass substrate/bottom TCO/Cu nanoparticles + solvent stack. After the evaporation of the solvent, the top TCO layer was deposited.
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comunication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. EU Solar Energy Strategy–Brussels. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2022%3A221%3AFIN (accessed on 18 May 2022).
- Kumar, N.M.; Chopra, S.S.; de Oliveira, A.K.; Ahmed, H.; Vaezi, S.; Madukanya, U.E.; Castañón, J.M. Solar PV module technologies. In Photovoltaic Solar Energy Conversion, 1st ed.; Gorjian, S., Shukla, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–78. [Google Scholar]
- Bailey, W.L.; Coleman, M.G.; Harris, C.B.; Lesk, I.A. Texture etching of silicon: Method. U.S. Patent 4,137,123, 30 January 1979. [Google Scholar]
- Campbell, P.; Green, M.A. Light trapping properties of pyramidally textured surfaces. J. Appl. Phys. 1987, 62, 243. [Google Scholar] [CrossRef]
- Campbell, P.; Green, M.A. High performance light trapping textures for monocrystalline silicon solar cells. Sol. Energy Mater. Sol. Cells 2001, 65, 369–375. [Google Scholar] [CrossRef]
- Enrichi, F.; Quandt, A.; Righini, G.C. Plasmonic enhanced solar cells: Summary of possible strategies and recent results. Renew. Sustain. Energy Rev. 2018, 82, 2433–2439. [Google Scholar] [CrossRef]
- De Aberasturi, D.J.; Serrano-Montes, A.B.; Liz-Marzán, L.M. Modern Applications of Plasmonic Nanoparticles: From Energy to Health. Adv. Optical Mater. 2015, 3, 602–617. [Google Scholar] [CrossRef]
- Li, Y.F.; Kou, Z.L.; Jing, F.; Hong-Bo, S. Plasmon-enhanced organic and perovskite solar cells with metal nanoparticles. Nanophotonics 2020, 9, 3111–3133. [Google Scholar] [CrossRef]
- De Souza, M.L.; Corio, P.; Brolo, A.G. Cu nanoparticles enable plasmonic-improved silicon photovoltaic devices. Phys. Chem. Chem. Phys. 2012, 14, 15722–15728. [Google Scholar] [CrossRef]
- Shen, P.; Liu, Y.; Long, Y.; Shen, L.; Kang, B. High-Performance Polymer Solar Cells Enabled by Copper Nanoparticles-Induced Plasmon Resonance Enhancement. J. Phys. Chem. C 2016, 120, 8900–8906. [Google Scholar] [CrossRef]
- Parveen, F.; Sannakki, B.; Mandke, M.V.; Pathan, H.M. Copper nanoparticles: Synthesis methods and its light harvesting performance. Sol. Energy Mater. Sol. Cells 2016, 144, 371–382. [Google Scholar] [CrossRef]
- Zheng, P.; Tang, H.; Liu, B.; Kasani, S.; Huang, L.; Wu, N. Origin of strong and narrow localized surface plasmon resonance of copper nanocubes. Nano Res. 2019, 12, 63–68. [Google Scholar] [CrossRef]
- Kitcometals. Available online: http://www.kitcometals.com/charts/ (accessed on 5 August 2022).
- NASDAQ. Available online: https://www.nasdaq.com/market-activity (accessed on 5 August 2022).
- Chan, G.H.; Zhao, J.; Hicks, E.M.; Schatz, G.C.; Van Duyne, R.P. Plasmonic Properties of Copper Nanoparticles Fabricated by Nanosphere Lithography. Nano Lett. 2007, 7, 1947–1952. [Google Scholar] [CrossRef]
- Sugawa, K.; Tamura, T.; Tahara, H.; Yamaguchi, D.; Akiyama, T.; Otsuki, J.; Kusaka, Y.; Fukuda, N.; Ushijima, H. Metal-Enhanced Fluorescence Platforms Based on Plasmonic Ordered Copper Arrays: Wavelength Dependence of Quenching and Enhancement Effects. ACS Nano 2013, 7, 9997–10010. [Google Scholar] [CrossRef] [PubMed]
- Baruah, P.K.; Sharma, A.K.; Khare, A. Role of confining liquids on the properties of Cu@Cu2O nanoparticles synthesized by pulsed laser ablation and a correlative ablation study of the target surface. RSC Adv. 2019, 9, 15214. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, H.; Li, X.; Muchen, R.; Zeng, H. Localized surface plasmon resonance of Cu nanoparticles by laser ablation in liquid media. RSC Adv. 2015, 5, 79738–79745. [Google Scholar] [CrossRef]
- Rawat, R.; Tiwari, A.; Arun, N.; Rao, S.V.S.N.; Pathak, A.P.; Tripathi, A. Solvents effect on the morphology and stability of cu/cuo nanoparticles synthesized at high fluence laser ablation. ChemistrySelect 2019, 4, 10471–10482. [Google Scholar] [CrossRef]
- Crane, C.C.; Wang, F.; Li, J.; Tao, J.; Zhu, Y.; Chen, J. Synthesis of Copper–Silica Core–Shell Nanostructures with Sharp and Stable Localized Surface Plasmon Resonance. J. Phys. Chem. C 2017, 121, 5684–5692. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Tilaki, R.M.; Iraji Zad, A.; Mahdavi, S.M. Size, composition and optical properties of copper nanoparticles prepared by laser ablation in liquids. Appl. Phys. A 2007, 88, 415–419. [Google Scholar] [CrossRef]
- Ellmer, K. Past achievements and future challenges in the development of optically transparent electrodes. Nat. Photonics 2012, 6, 809–817. [Google Scholar] [CrossRef]
- Boscarino, S.; Torrisi, G.; Crupi, I.; Ruffino, F.; Terrasi, A. Ion irradiation of AZO thin films for flexible electronics. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2017, 392, 14–20. [Google Scholar] [CrossRef]
- Ginley, D.S.; Hosono, H.; Paine, D.C. Handbook of Transparent Conductors; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Ruffino, F.; Grimaldi, M.G. Controlled dewetting as fabrication and patterning strategy for metal nanostructures. Phys. Status Solidi A 2015, 212, 1662–1684. [Google Scholar] [CrossRef]
- Censabella, M.V.; Torrisi, V.; Boninelli, S.; Bongiorno, C.; Grimaldi, M.G.; Ruffino, F. Laser ablation synthesis of mono- and bimetallic Pt and Pd nanoparticles and fabrication of Pt-Pd/Graphene nanocomposites. Appl. Surf. Sci. 2019, 475, 494–503. [Google Scholar] [CrossRef]
- Ghorannevis, Z.; Akbarnejad, E.; Ghoranneviss, M. Structural and morphological properties of ITO thin films grown by magnetron sputtering. J. Theor. Appl. Phys. 2015, 9, 285–290. [Google Scholar] [CrossRef]
- Baladi, A.; Sarraf Mamoory, R. Investigation of different liquid media and ablation times on pulsed laser ablation synthesis of aluminum nanoparticles. Appl. Surf. Sci. 2010, 256, 7559–7564. [Google Scholar] [CrossRef]
- Naser, H.; Alghoul, M.A.; Hossain, M.K.; Asim, N.; Adbullah, M.F.; Ali, M.S.; Alzubi, F.G.; Amin, N. The role of laser ablation technique parameters in synthesis of nanoparticles from different target types. J. Nanoparticle Res. 2019, 21, 249. [Google Scholar] [CrossRef]
- Tauc, J.; Bagley, B.G. (Eds.) Amorphous and Liquid Semiconductors; Springer: New York, NY, USA, 1977. [Google Scholar]
- Mirabella, S.; Agosta, R.; Franzó, G.; Crupi, I.; Miritello, M.; Savio, R.l.; di Stefano, M.A.; di Marco, S.; Simone, F.; Terrasi, A. Light absorption in silicon quantum dots embedded in silica. J. Appl. Phys. 2009, 106, 103505. [Google Scholar] [CrossRef]
- Dasari, S.G.; Nagaraju, P.; Yelsani, V.; Tirumala, S.; Reddy, M.V.R. Nanostructured Indium Oxide Thin Films as a Room Temperature Toluene Sensor. ACS Omega 2021, 6, 17442–17454. [Google Scholar] [CrossRef]
- Tan, B.; Melius, P.; Ziegler, P. A simple gas chromatographic method for the study of organ solvents: Moisture analysis, hygroscopicity, and evaporation. J. Chromatogr. 1982, 20, 213–217. [Google Scholar]
- Xu, J.; Liu, J.B.; Liu, B.X.; Li, S.N.; Wei, S.H.; Huang, B. Design of n-Type Transparent Conducting Oxides: The Case of Transition Metal Doping in In2O3. Adv. Electron. Mater. 2018, 4, 1700553. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liang, Y.C. Physical properties of low temperature sputtering-deposited zirconium-doped indium oxide films at various oxygen partial pressures. Appl. Phys. A 2009, 97, 249–255. [Google Scholar] [CrossRef]
- Rucavado, E.; Landucci, F.; Döbeli, M.; Jeangros, Q.; Boccard, M.; Hessler-Wyser, A.; Ballif, C.; Morales-Masis, M. Zr-doped indium oxide electrodes: Annealing and thickness effects on microstructure and carrier transport. Phys. Rev. Mater. 2019, 3, 084608. [Google Scholar] [CrossRef]
- Aissa, B.; Zakaria, Y.; Shetty, A.R.; Samara, A.; Broussillou, C. High Electron-Mobility of a Transparent and Conductive Zr-Doped In2O3Deposited by Reactive Magnetron Sputtering. In Proceedings of the Conference Record of the IEEE Photovoltaic Specialists Conference, Fort Lauderdale, FL, USA, 20–25 June 2021; pp. 71–73. [Google Scholar]
- Morales-Masis, M.; Rucavado, E.; Monnard, R.; Barraud, L.; Holovsky, J.; Despeisse, Y.; Boccard, M.; Ballif, C. Highly Conductive and Broadband Transparent Zr-Doped In2O3 as Front Electrode for Solar Cells. IEEE J. Photovolt. 2018, 8, 1202–1207. [Google Scholar] [CrossRef]
- Boscarino, S.; Censabella, M.; Micali, M.; Russo, M.; Terrasi, A.; Grimaldi, M.G.; Ruffino, F. Morphology, Electrical and Optical Properties of Cu Nanostructures Embedded in AZO: A Comparison between Dry and Wet Methods. Micromachines 2022, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Aromaa, J.; Kekkonen, M.; Mousapour, M.; Jokilaakso, A.; Lundström, M. The Oxidation of Copper in Air at Temperatures up to 100 °C. Corros. Mater. Degrad. 2021, 2, 625–640. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Biersack, J.P.; Littmark, U. The Stopping and Range of Ions in Solids, Stopping and Ranges of Ions in Matter; Pergamon: New York, NY, USA, 1984; Volume 1. [Google Scholar]
- Morawiec, S.; Mendes, M.J.; Priolo, F.; Crupi, I. Plasmonic nanostructures for light trapping in thin-film solar cells. Mater. Sci. Semicond. Process. 2019, 92, 10–18. [Google Scholar] [CrossRef]
- Temple, T.L.; Bagnall, D.M. Broadband scattering of the solar spectrum by spherical metal nanoparticles. Prog. Photovolt. Res. Appl. 2013, 21, 600–611. [Google Scholar] [CrossRef]
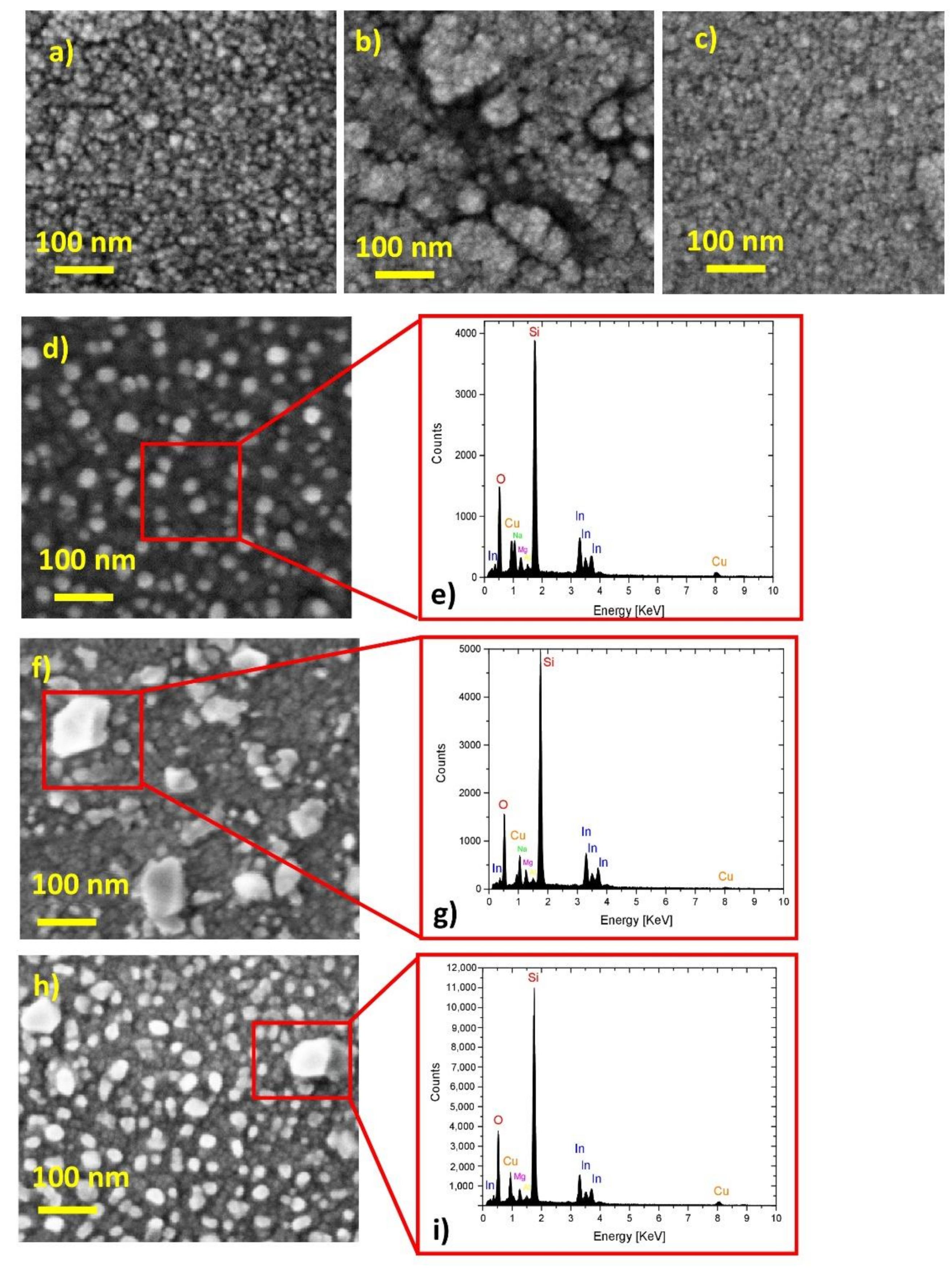
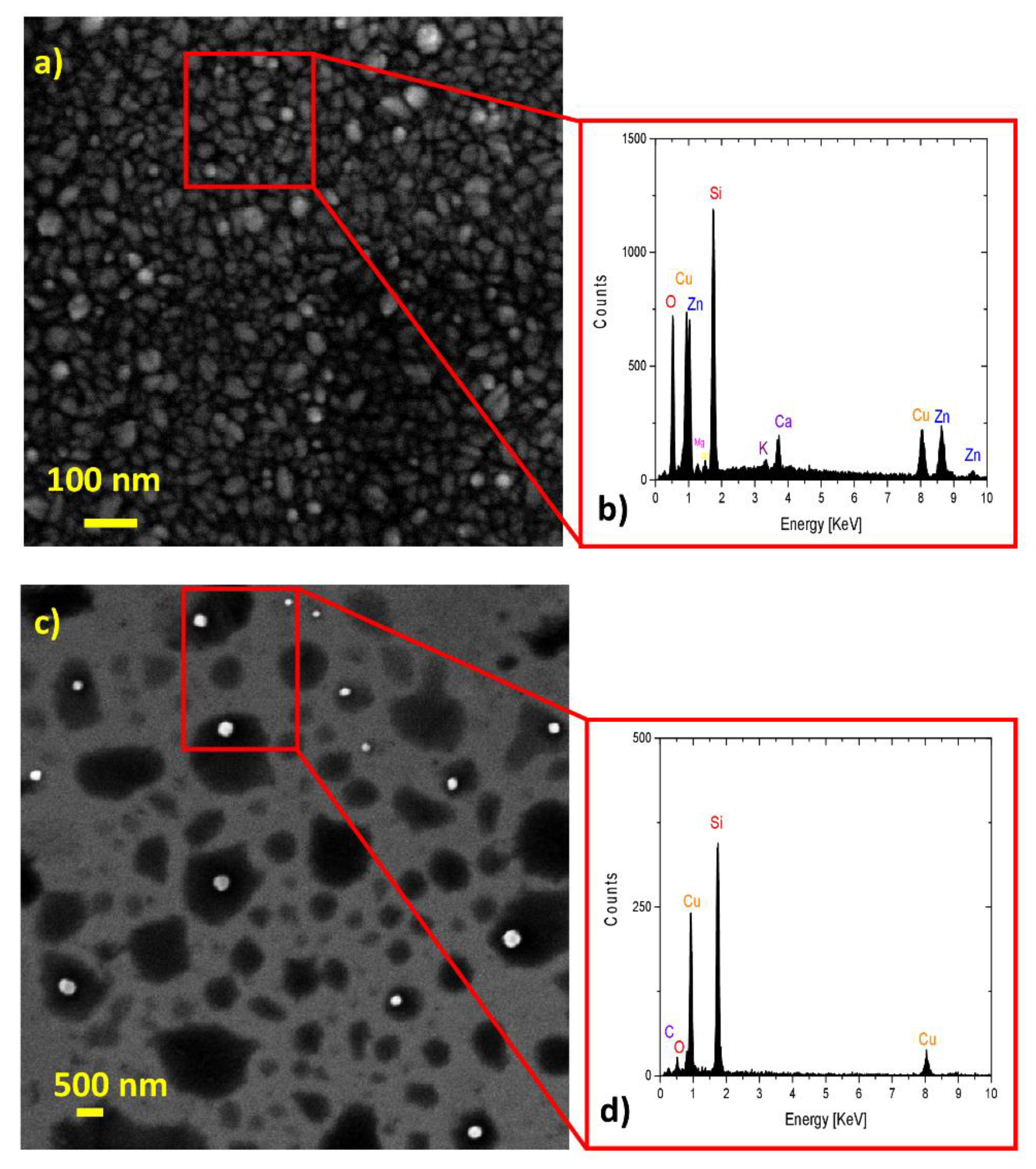
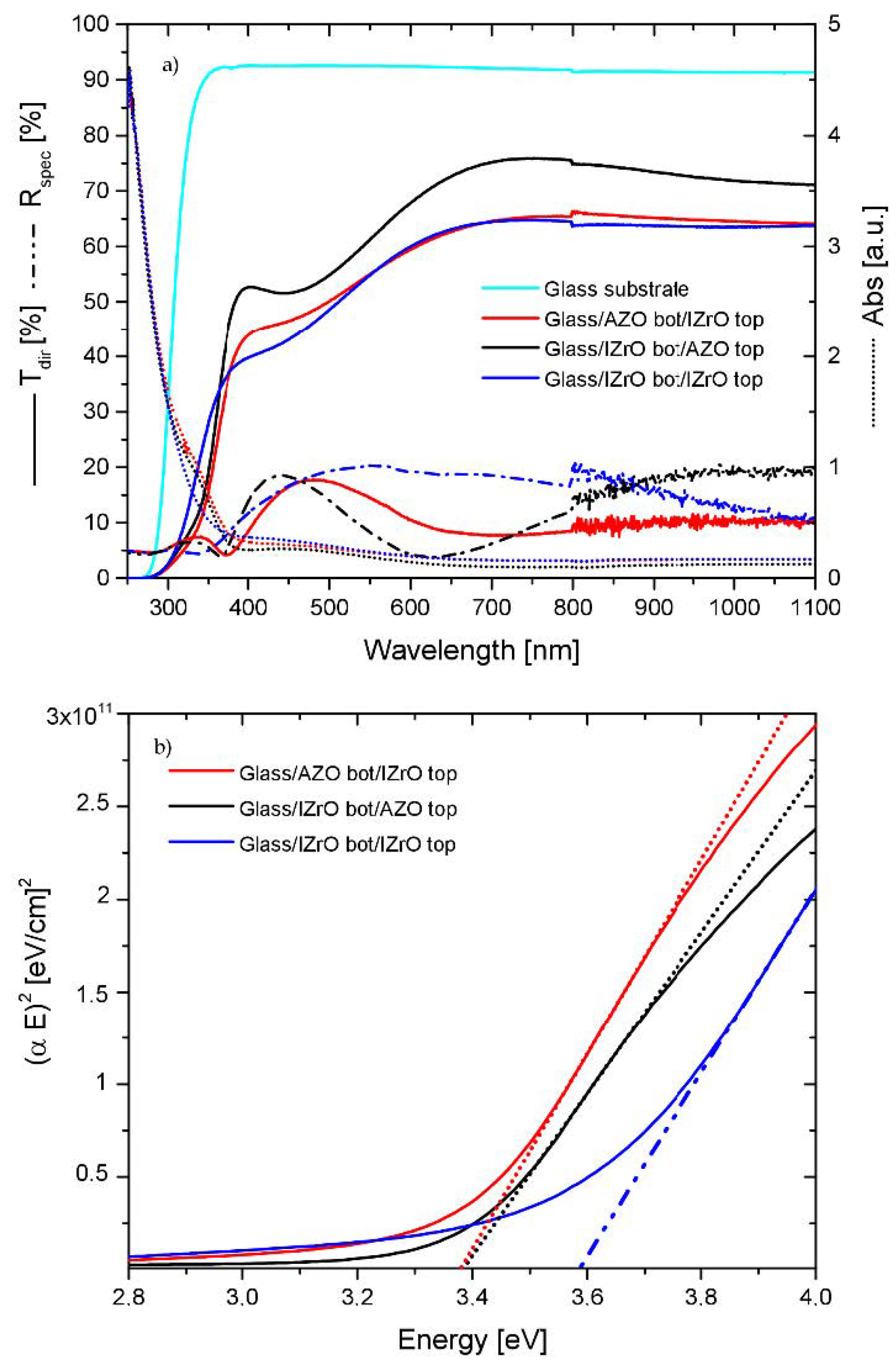
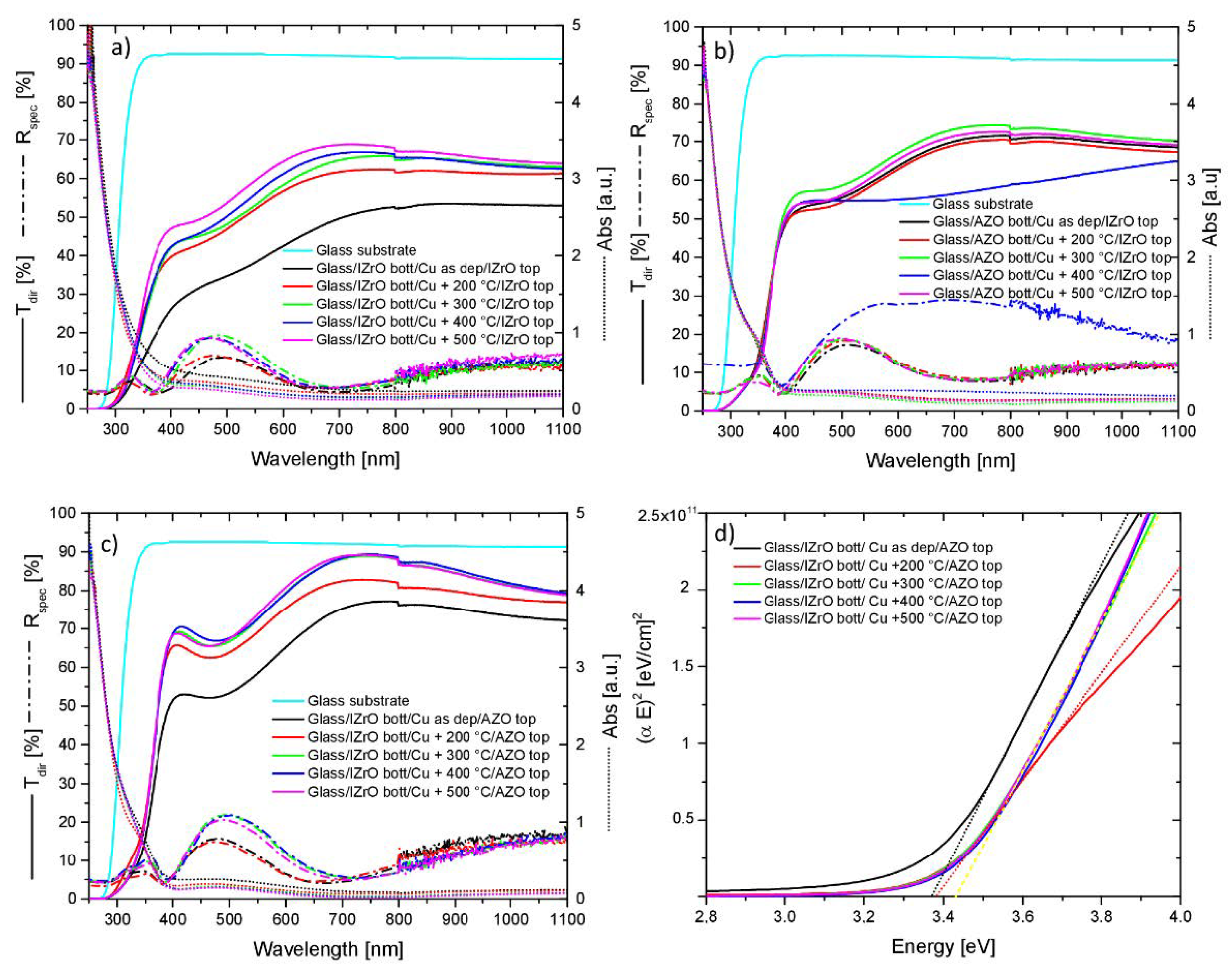
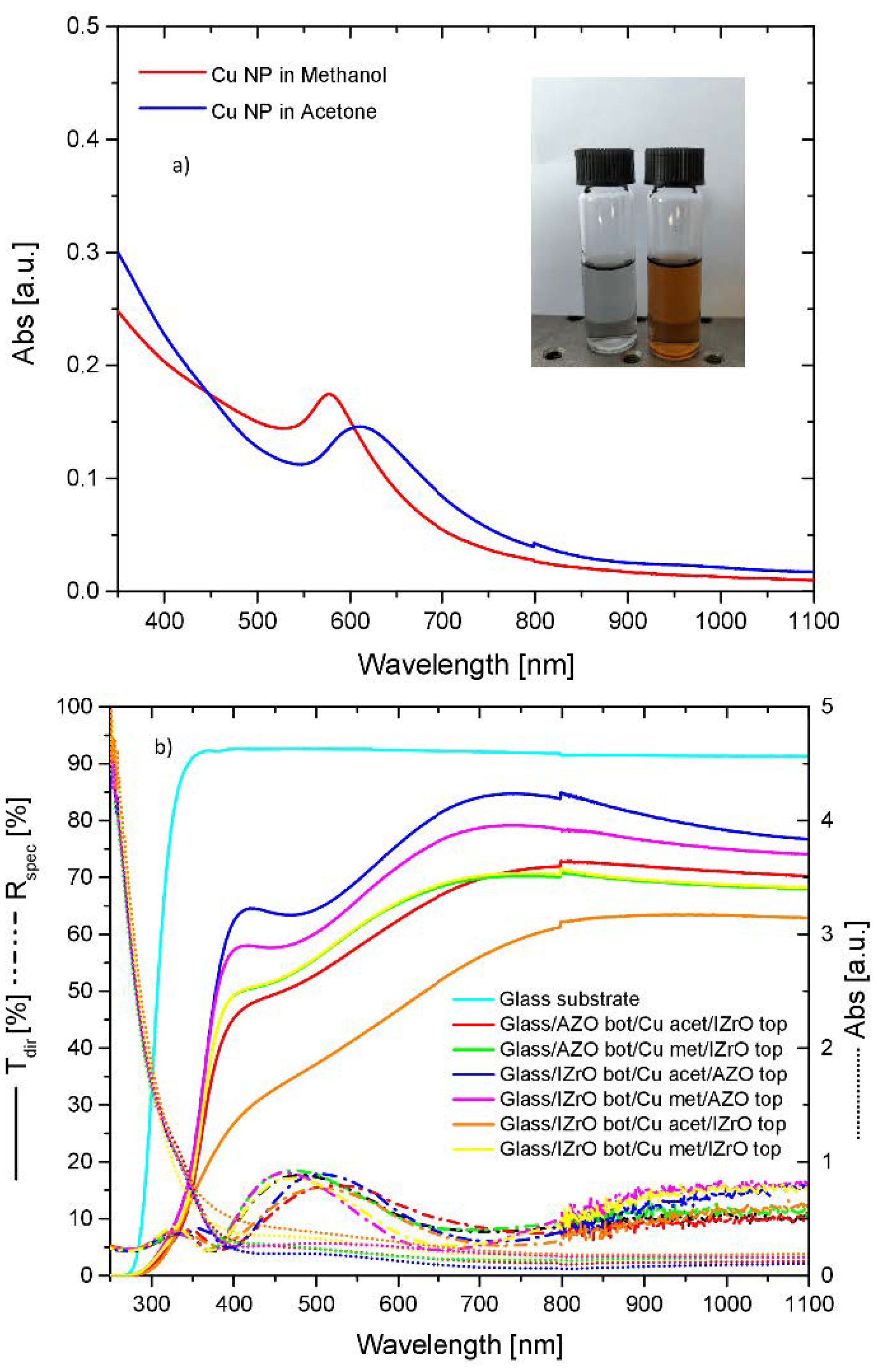
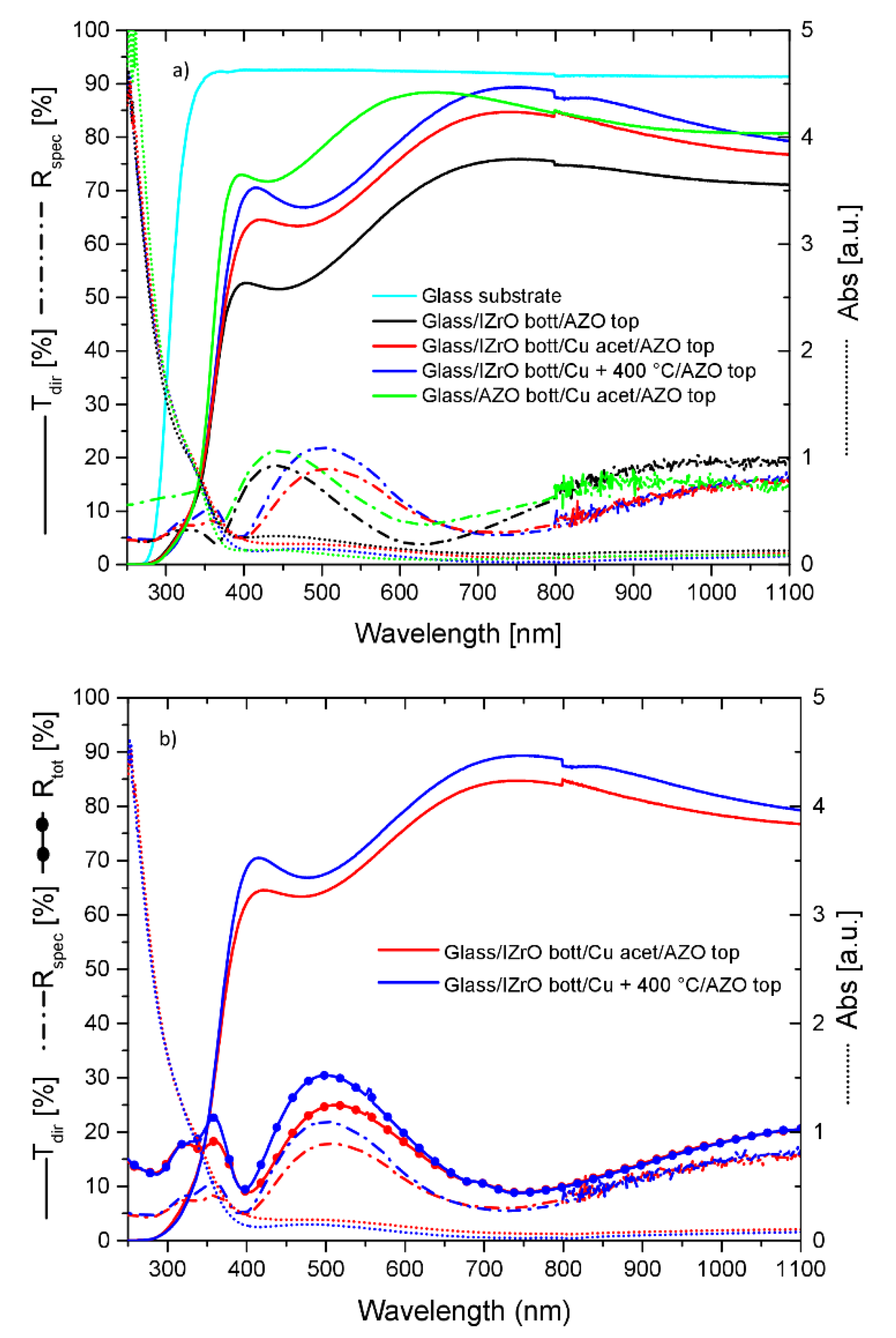
| Samples | Rsh (Ω/Sq) | Egap |
|---|---|---|
| AZO thin film | 1 × 106 | 3.41 |
| IZrO thin film | 243 | 3.58 |
| AZO bott/IZrO top | 235 | 3.38 |
| IZrO bott/AZO top | 236 | 3.38 |
| IZrO bott/IZrO top | 178 | 3.58 |
| Samples | Rsh (Ω/Sq) | Egap |
| AZObott/Cu as dep/IZrOtop | 268 | 3.36 |
| AZObott/Cu + 200 °C/IZrOtop | 279 | 3.37 |
| AZObott/Cu + 300 °C/IZrOtop | 287 | 3.38 |
| AZObott/Cu + 400 °C/IZrOtop | 274 | 3.38 |
| AZObott/Cu + 500 °C/IZrOtop | 277 | 3.40 |
| Samples | Rsh (Ω/Sq) | Egap |
| IZrObott/Cu as dep/AZOtop | 265 | 3.37 |
| IZrObott/Cu + 200 °C/AZOtop | 83 | 3.38 |
| IZrObott/Cu + 300 °C/AZOtop | 1019 | 3.43 |
| IZrObott/Cu + 400 °C/AZOtop | 2093 | 3.43 |
| IZrObott/Cu + 500 °C/AZOtop | 4385 | 3.43 |
| Samples | Rsh (Ω/Sq) | Egap |
| IZrObott/Cu as dep/IZrOtop | 153 | 3.51 |
| IZrObott/Cu + 200 °C/IZrOtop | 87 | 3.62 |
| IZrObott/Cu + 300 °C/IZrOtop | 289 | 3.62 |
| IZrObott/Cu + 400 °C/IZrOtop | 322 | 3.61 |
| IZrObott/Cu + 500 °C/IZrOtop | 521 | 3.64 |
| Samples | Rsh (Ω/Sq) | Egap |
|---|---|---|
| AZObott/Cu acet/IZrOtop | 304 | 3.38 |
| AZObott/Cu met/IZrOtop | 263 | 3.37 |
| IZrObott/Cu acet/AZOtop | 253 | 3.39 |
| IZrObott/Cu met/AZOtop | 215 | 3.38 |
| IZrObott/Cu acet/IZrOtop | 124 | 3.58 |
| IZrObott/Cu met/IZrOtop | 132 | 3.57 |
| Samples | Rsh (Ω/Sq) | Egap | <T>Vis (%) | <T>Vis-NIR (%) |
|---|---|---|---|---|
| IZrObott/AZOtop | 236 | 3.38 | 65.4 | 68.5 |
| IZrObott/Cu Acet/AZOtop | 253 | 3.38 | 74.7 | 76.9 |
| IZrObott/Cu + 400 °C/AZOtop | 2093 | 3.43 | 78.7 | 80.8 |
| AZObott/Cu acet/AZOtop [40] | 2.5 × 106 | 3.44 | 82.8 | 82.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscarino, S.; Iacono, V.; Lo Mastro, A.; Tringali, F.; Terrasi, A.; Grimaldi, M.G.; Ruffino, F. Plasmonic and Conductive Structures of TCO Films with Embedded Cu Nanoparticles. Int. J. Mol. Sci. 2022, 23, 11886. https://doi.org/10.3390/ijms231911886
Boscarino S, Iacono V, Lo Mastro A, Tringali F, Terrasi A, Grimaldi MG, Ruffino F. Plasmonic and Conductive Structures of TCO Films with Embedded Cu Nanoparticles. International Journal of Molecular Sciences. 2022; 23(19):11886. https://doi.org/10.3390/ijms231911886
Chicago/Turabian StyleBoscarino, Stefano, Valentina Iacono, Andrea Lo Mastro, Fiorella Tringali, Antonio Terrasi, Maria Grazia Grimaldi, and Francesco Ruffino. 2022. "Plasmonic and Conductive Structures of TCO Films with Embedded Cu Nanoparticles" International Journal of Molecular Sciences 23, no. 19: 11886. https://doi.org/10.3390/ijms231911886
APA StyleBoscarino, S., Iacono, V., Lo Mastro, A., Tringali, F., Terrasi, A., Grimaldi, M. G., & Ruffino, F. (2022). Plasmonic and Conductive Structures of TCO Films with Embedded Cu Nanoparticles. International Journal of Molecular Sciences, 23(19), 11886. https://doi.org/10.3390/ijms231911886










