Development and In Vitro and In Vivo Evaluation of an Antineoplastic Copper(II) Compound (Casiopeina III-ia) Loaded in Nonionic Vesicles Using Quality by Design
Abstract
1. Introduction
2. Results
2.1. Quality-by-Design Approach
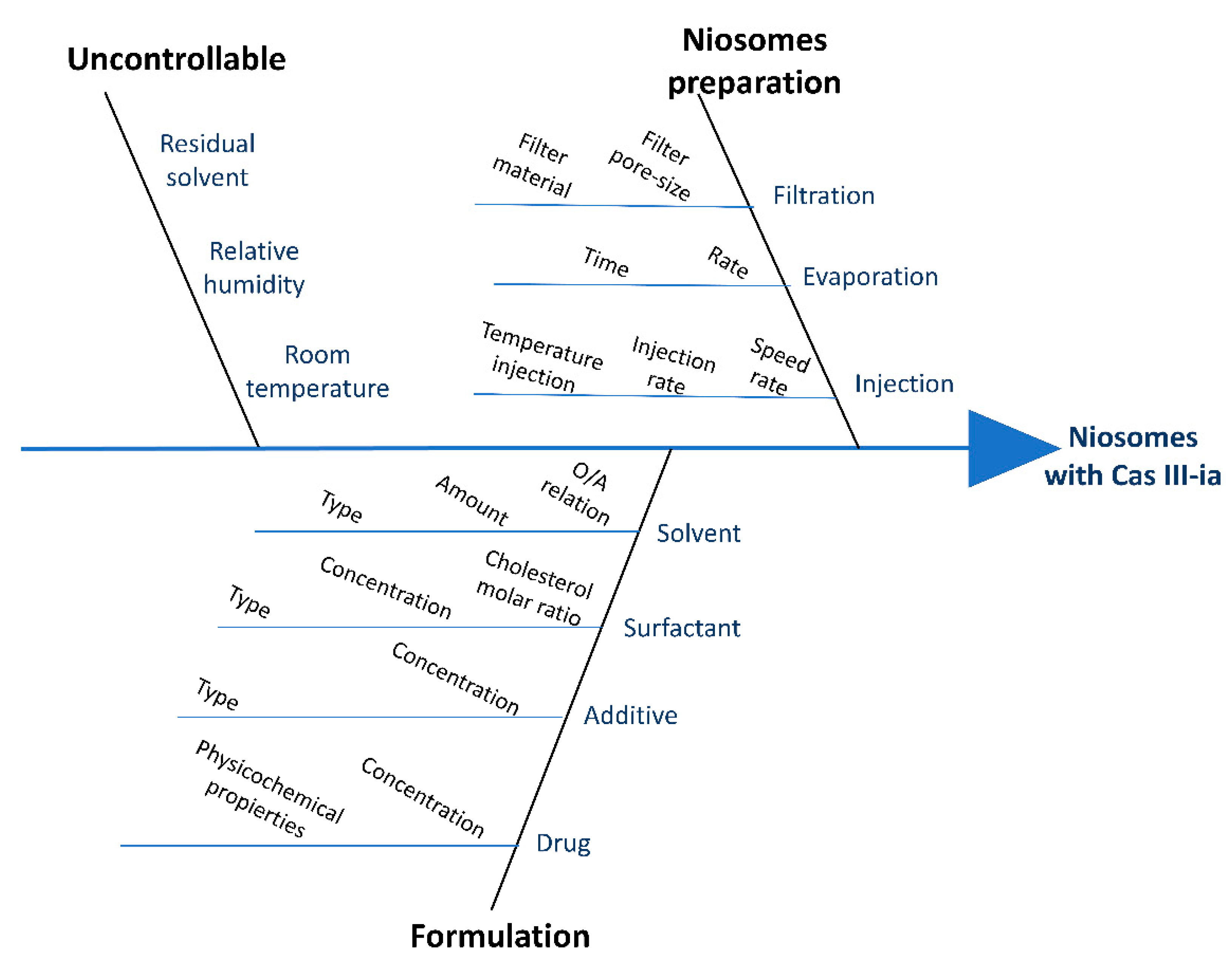
2.1.1. Risk Analysis (RA)
2.1.2. Experimental Design
Plackett–Burman Design
Factorial Design 2k
Central Composite Design
2.2. Preparation of Optimized Nanoformulation
2.3. Optimized Nanoformulation Characterization
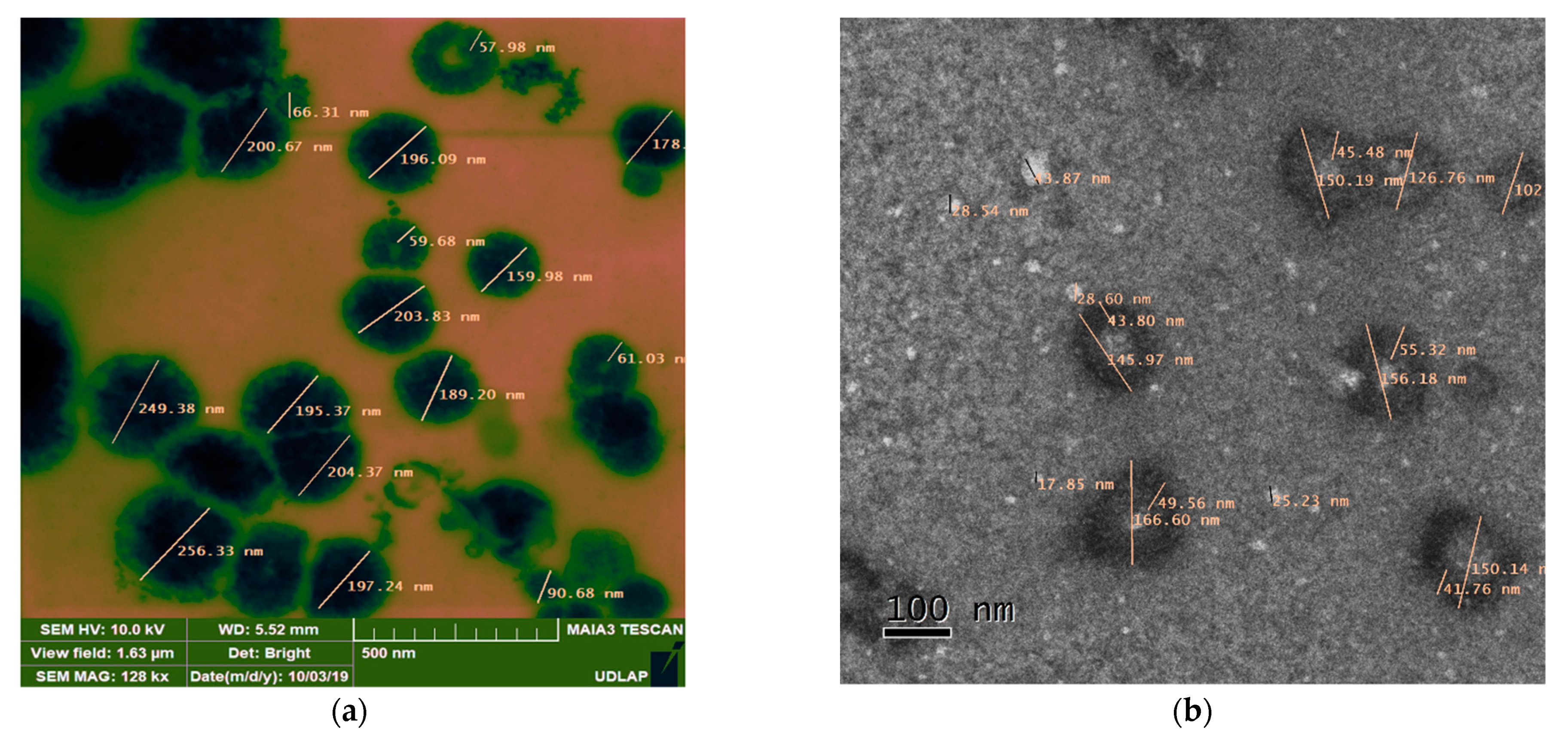
2.3.1. Electronic Microscopy
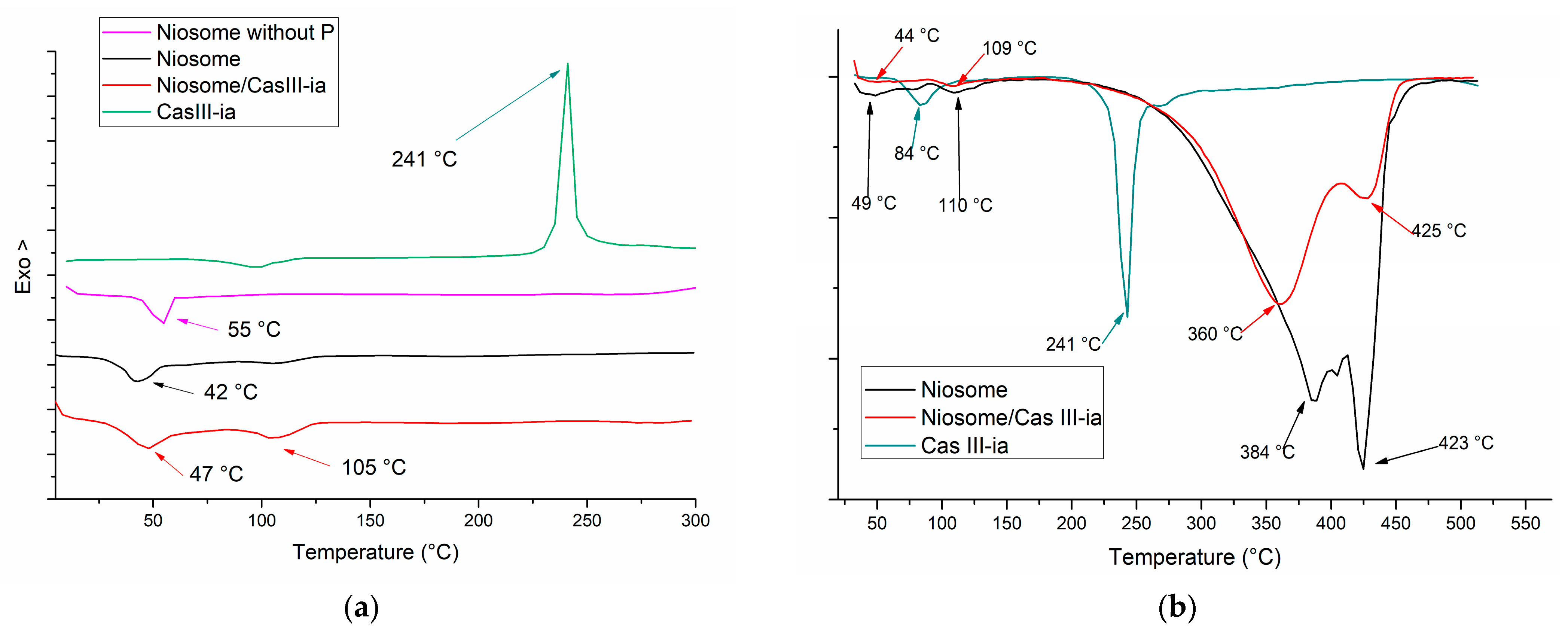
2.3.2. Thermal Analysis
2.3.3. FTIR Studies
2.3.4. Stability Studies
2.3.5. In Vitro Drug Release
2.3.6. Cytotoxicity Assay
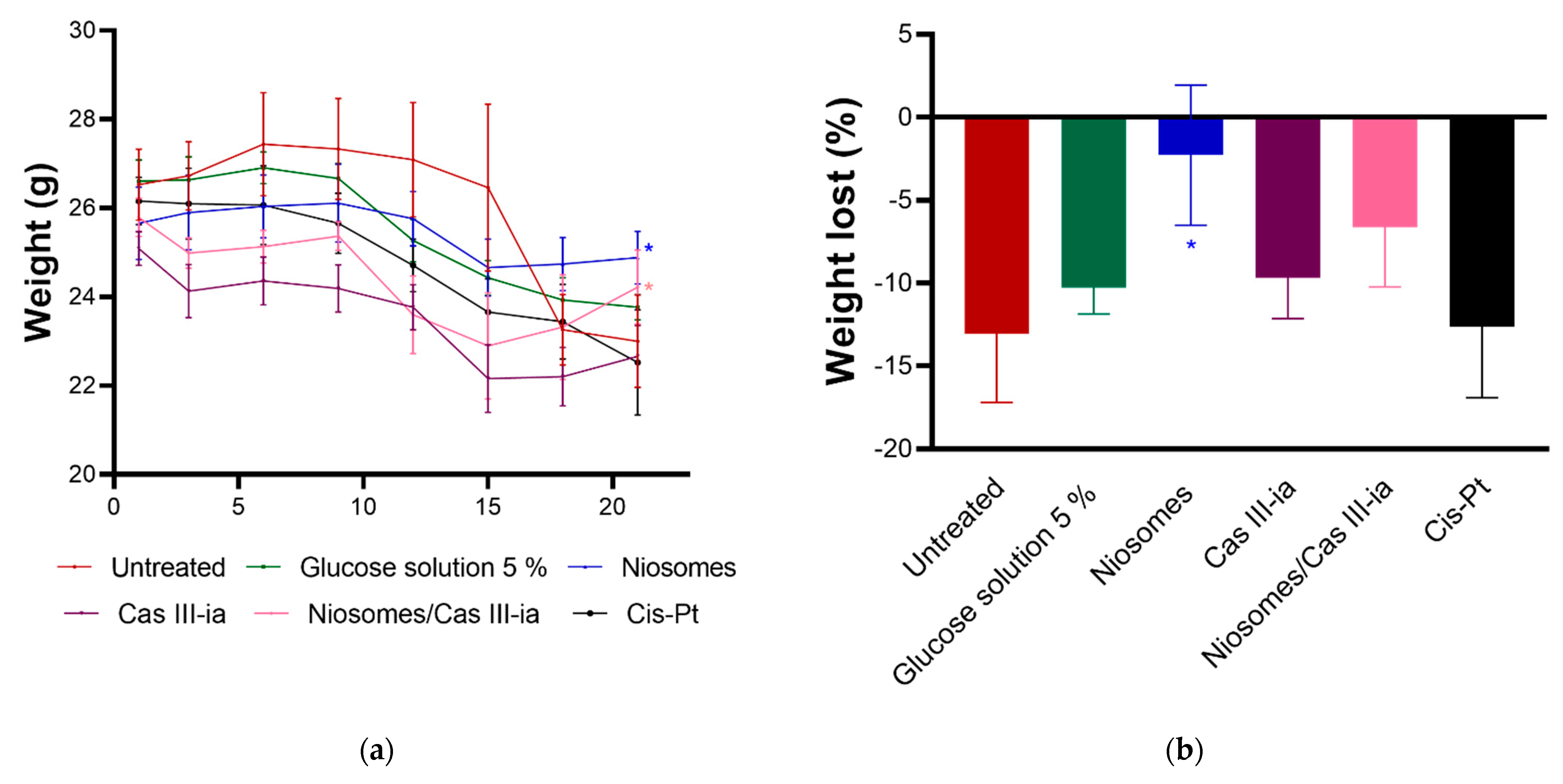
2.3.7. In Vivo Assays
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Quality-by-Design Approach: Risk Analysis
Quality Target Product Profile (QTPP)
Critical Quality Attributes (CQAs)
Critical Process Parameters (CPPs) and Critical Material Attributes (CMAs)
4.2.2. Niosome Formation
4.2.3. Quality-by-Design Approach: Experimental Design (DoE)
Plackett–Burman Design
Factorial Design 2k
Central Composite Design (CCD)
4.2.4. Optimized Formulation: Niosome Characterizations
Particle Size and Polydispersity Index (PDI)
Superficial Charge
Encapsulation Efficiency (EE)
Electronic Microscopy
Fourier Transform Infrared Spectroscopy (FTIR) and Attenuated Total Reflectance Infrared Spectroscopy (ATR-FTIR)
Thermal Analysis
4.2.5. Physical Stability Study
4.2.6. In Vitro Drug Release
4.2.7. In Vitro Cell Assays
4.2.8. In Vivo Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peña, Q.; Wang, A.; Zaremba, O.; Shi, Y.; Scheeren, H.W.; Metselaar, J.M.; Kiessling, F.; Pallares, R.M.; Wuttke, S.; Lammers, T. Metallodrugs in Cancer Nanomedicine. Chem. Soc. Rev. 2022, 51, 2544–2582. [Google Scholar] [CrossRef] [PubMed]
- Allardyce, C.S.; Dyson, P.J. Metal-Based Drugs That Break the Rules. Dalton Trans. 2016, 45, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Proschak, E.; Stark, H.; Merk, D. Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J. Med. Chem. 2019, 62, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.R.; Caleffi-Ferracioli, K.R.; Leite, C.Q.F.; García-Ramos, J.C.; Toledano-Magaña, Y.; Ruiz-Azuara, L.; Siqueira, V.L.D.; Pavan, F.R.; Cardoso, R.F. Potential of Casiopeínas® Copper Complexes and Antituberculosis Drug Combination against Mycobacterium Tuberculosis. Chemotherapy 2016, 61, 249–255. [Google Scholar] [CrossRef]
- Rufino-González, Y.; Ponce-Macotela, M.; García-Ramos, J.C.; Martínez-Gordillo, M.N.; Galindo-Murillo, R.; González-Maciel, A.; Reynoso-Robles, R.; Tovar-Tovar, A.; Flores-Alamo, M.; Toledano-Magaña, Y.; et al. Antigiardiasic Activity of Cu(II) Coordination Compounds: Redox Imbalance and Membrane Damage after a Short Exposure Time. J. Inorg. Biochem. 2019, 195, 83–90. [Google Scholar] [CrossRef]
- Bravo-gómez, M.E.; García-ramos, J.C.; Gracia-mora, I.; Ruiz-azuara, L. Antiproliferative Activity and QSAR Study of Copper (II) Mixed Chelate [Cu(N-N)(acetylacetonato)] NO3 and [Cu(N-N)(glycinato)]NO3 Complexes. J. Inorg. Biochem. 2009, 103, 299–309. [Google Scholar] [CrossRef]
- Castillo-Rodríguez, R.A.; Palencia, G.; Anaya-Rubio, I.; Gallardo-Pérez, J.C.; Jiménez-Farfán, D.; Escamilla-Ramírez, Á.; Zavala-Vega, S.; Cruz-Salgado, A.; Cervantes-Rebolledo, C.; Gracia-Mora, I.; et al. Anti-Proliferative, pro-Apoptotic and Anti-Invasive Effect of the Copper Coordination Compound Cas III-La through the Induction of Reactive Oxygen Species and Regulation of Wnt/β-Catenin Pathway in Glioma. J. Cancer 2021, 12, 5693–5711. [Google Scholar] [CrossRef]
- Resendiz-Acevedo, K.; García-Aguilera, M.E.; Esturau-Escofet, N.; Ruiz-Azuara, L. 1H-NMR Metabolomics Study of the Effect of Cisplatin and Casiopeina IIgly on MDA-MB-231 Breast Tumor Cells. Front. Mol. Biosci. 2021, 8, 742859. [Google Scholar] [CrossRef]
- González-Ballesteros, M.M.; Mejía, C.; Ruiz-Azuara, L. Metallodrugs: An Approach against Invasion and Metastasis in Cancer Treatment. FEBS Open Bio. 2022, 12, 880–899. [Google Scholar] [CrossRef]
- Leal-García, M.; García-Ortuño, L.; Ruiz-Azuara, L.; Gracia-Mora, I.; Luna-Delvillar, J.; Sumano, H. Assessment of Acute Respiratory and Cardiovascular Toxicity of Casiopeinas in Anaesthetized Dogs. Basic Clin. Pharmacol. Toxicol. 2007, 101, 151–158. [Google Scholar] [CrossRef]
- Serment-Guerrero, J.; Cano-Sanchez, P.; Reyes-Perez, E.; Velazquez-Garcia, F.; Bravo-Gomez, M.E.; Ruiz-Azuara, L. Genotoxicity of the Copper Antineoplastic Coordination Complexes Casiopeinas®. Toxicol. Vitr. 2011, 25, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Correia, I.; Borovic, S.; Cavaco, I.; Matos, C.P.; Roy, S.; Santos, H.M.; Fernandes, L.; Capelo, J.L.; Ruiz-Azuara, L.; Pessoa, J.C. Evaluation of the Binding of Four Anti-Tumor Casiopeínas® to Human Serum Albumin. J. Inorg. Biochem. 2017, 175, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Cañas-Alonso, R.C.; Fuentes-Noriega, I.; Ruiz-Azuara, L. Pharmacokinetics of Casiopeína IIgly in Beagle Dog: A Copper Based Compound with Antineoplastic Activity. J. Bioanal. Biomed. 2010, 2, 28–34. [Google Scholar] [CrossRef]
- Miranda-Calderón, J.E.; Macías-Rosales, L.; Gracia-Mora, I.; Ruiz-Azuara, L.; Faustino-Vega, A.; Gracia-Mora, J.; Bernad-Bernad, M.J. Effect of Casiopein III-Ia Loaded into Chitosan Nanoparticles on Tumor Growth Inhibition. J. Drug Deliv. Sci. Technol. 2018, 48, 1–8. [Google Scholar] [CrossRef]
- Masjedi, M.; Montahaei, T. An Illustrated Review on Nonionic Surfactant Vesicles (Niosomes) as an Approach in Modern Drug Delivery: Fabrication, Characterization, Pharmaceutical, and Cosmetic Applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102234. [Google Scholar] [CrossRef]
- Sankhyan, A.; Pawar, P.K. Metformin Loaded Non-Ionic Surfactant Vesicles: Optimization of Formulation, Effect of Process Variables and Characterization. Daru J. Fac. Pharm. 2013, 21, 7. [Google Scholar] [CrossRef]
- Nasr, M.; Mansour, S.; Mortada, N.D. Elshamy, a a Vesicular Aceclofenac Systems: A Comparative Study between Liposomes and Niosomes. J. Microencapsul. 2008, 25, 499–512. [Google Scholar] [CrossRef]
- Sakai, T.; Kurosawa, H.; Okada, T.; Mishima, S. Vesicle Formation in Mixture of a PEO-PPO-PEO Block Copolymer (Pluronic P123) and a Nonionic Surfactant (Span 65) in Water. Colloids. Surfaces A Physicochem. Eng. Asp. 2011, 389, 82–89. [Google Scholar] [CrossRef]
- Manosroi, A.; Wongtrakul, P.; Manosroi, J.; Sakai, H.; Sugawara, F.; Yuasa, M.; Abe, M. Characterization of Vesicles Prepared with Various Non-Ionic Surfactants Mixed with Cholesterol. Colloids Surfaces B Biointerfaces 2003, 30, 129–138. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Jain, R.K. Design Considerations for Nanotherapeutics in Oncology. Nanomedicine 2015, 11, 1893–1907. [Google Scholar] [CrossRef]
- Beddoes, C.M.; Case, C.P.; Briscoe, W.H. Understanding Nanoparticle Cellular Entry: A Physicochemical Perspective. Adv. Colloid. Interface Sci. 2015, 218, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Rawal, M.; Singh, A.; Amiji, M.M. Quality-by-Design Concepts to Improve Nanotechnology-Based Drug Development. Pharm. Res. 2019, 36, 153. [Google Scholar] [CrossRef] [PubMed]
- Bastogne, T. Quality-by-Design of Nanopharmaceuticals—A State of the Art. Nanomedicine 2017, 13, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA) Committee for Human Medicinal Products. ICH Guideline Q8 (R2) on Pharmaceutical Development; European Medicines Agency: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Cacicedo, M.L.; Ruiz, M.C.; Scioli-Montoto, S.; Ruiz, M.E.; Fernández, M.A.; Torres-Sanchez, R.M.; Baran, E.J.; Castro, G.R.; León, I.E. Lipid Nanoparticles-Metvan: Revealing a Novel Way to Deliver a Vanadium Compound to Bone Cancer Cells. New J. Chem. 2019, 43, 17726–17734. [Google Scholar] [CrossRef]
- Patel, V.; Bardoliwala, D.; Lalani, R.; Patil, S.; Ghosh, S.; Javia, A.; Misra, A. Development of a Dry Powder for Inhalation of Nanoparticles Codelivering Cisplatin and ABCC3 SiRNA in Lung Cancer. Ther. Deliv. 2021, 12, 651–670. [Google Scholar] [CrossRef]
- Waghule, T.; Dabholkar, N.; Gorantla, S.; Rapalli, V.K.; Saha, R.N.; Singhvi, G. Quality by Design (QbD) in the Formulation and Optimization of Liquid Crystalline Nanoparticles (LCNPs): A Risk Based Industrial Approach. Biomed. Pharmacother. 2021, 141, 111940. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-Niosomes as Nanoscale Drug Delivery Systems: An Illustrated Review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Waddad, A.Y.; Abbad, S.; Yu, F.; Munyendo, W.L.L.; Wang, J.; Lv, H.; Zhou, J. Formulation, Characterization and Pharmacokinetics of Morin Hydrate Niosomes Prepared from Various Non-Ionic Surfactants. Int. J. Pharm. 2013, 456, 446–458. [Google Scholar] [CrossRef]
- Hao, Y.M.; Li, K. Entrapment and Release Difference Resulting from Hydrogen Bonding Interactions in Niosome. Int. J. Pharm. 2011, 403, 245–253. [Google Scholar] [CrossRef]
- Haroun, M.; Elsewedy, H.S.; Shehata, T.M.; Tratrat, C.; Al Dhubiab, B.E.; Venugopala, K.N.; Almostafa, M.M.; Kochkar, H.; Elnahas, H.M. Significant of Injectable Brucine PEGylated Niosomes in Treatment of MDA Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 71, 103322. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent Advances in Non-Ionic Surfactant Vesicles (Niosomes): Fabrication, Characterization, Pharmaceutical and Cosmetic Applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Shaker, D.S.; Shaker, M.A.; Hanafy, M.S. Cellular Uptake, Cytotoxicity and in-Vivo Evaluation of Tamoxifen Citrate Loaded Niosomes. Int. J. Pharm. 2015, 493, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.B.; El-Shanawany, S.M.; Hamad, M.A.; Elsabahy, M. Niosomes: A Strategy toward Prevention of Clinically Significant Drug Incompatibilities. Sci. Rep. 2017, 7, 6340. [Google Scholar] [CrossRef] [PubMed]
- Rajera, R.; Nagpal, K.; Singh, S.K.; Mishra, D.N. Niosomes: A Controlled and Novel Drug Delivery System. Biol. Pharm. Bull. 2011, 34, 945–953. [Google Scholar] [CrossRef]
- Yang, H.; Deng, A.; Zhang, J.; Wang, J.; Lu, B. Preparation, Characterization and Anticancer Therapeutic Efficacy of Cisplatin-Loaded Niosomes. J. Microencapsul. 2013, 30, 237–244. [Google Scholar] [CrossRef]
- Sharma, V.; Anandhakumar, S.; Sasidharan, M. Self-Degrading Niosomes for Encapsulation of Hydrophilic and Hydrophobic Drugs: An Efficient Carrier for Cancer Multi-Drug Delivery. Mater. Sci. Eng. C 2015, 56, 393–400. [Google Scholar] [CrossRef]
- Sezgin-Bayindir, Z.; Antep, M.N.; Yuksel, N. Development and Characterization of Mixed Niosomes for Oral Delivery Using Candesartan Cilexetil as a Model Poorly Water-Soluble Drug. AAPS PharmSciTech. 2015, 16, 108–117. [Google Scholar] [CrossRef]
- Duong, V.A.; Nguyen, T.T.L.; Maeng, H.J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- European Medicines Agency ICH Guideline Q3C (R6) on Impurities: Guideline for Residual Solvents. Int. Conf. Harmon. Tech. Requir. Regist. Pharm. Hum. Use 2019, 31, 24.
- Schubert, M.A.; Müller-Goymann, C.C. Solvent Injection as a New Approach for Manufacturing Lipid Nanoparticles-Evaluation of the Method and Process Parameters. Eur. J. Pharm. Biopharm. 2003, 55, 125–131. [Google Scholar] [CrossRef]
- Ritwiset, A.; Krongsuk, S.; Johns, J.R. Molecular Structure and Dynamical Properties of Niosome Bilayers with and without Cholesterol Incorporation: A Molecular Dynamics Simulation Study. Appl. Surf. Sci. 2016, 380, 23–31. [Google Scholar] [CrossRef]
- Wilkhu, J.S.; Ouyang, D.; Kirchmeier, M.J.; Anderson, D.E.; Perrie, Y. Investigating the Role of Cholesterol in the Formation of Non-Ionic Surfactant Based Bilayer Vesicles: Thermal Analysis and Molecular Dynamics. Int. J. Pharm. 2014, 461, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S. In Vitro Release Kinetics Model Fitting of Liposomes: An Insight. Chem. Phys. Lipids. 2016, 201, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Hajinezhad, M.R.; Sargazi, S.; Rahdar, A.; Shahraki, S.; Lohrasbi-Nejad, A.; Baino, F. In Vitro and in Vivo Anticancer Effect of PH-Responsive Paclitaxel-Loaded Niosomes. J. Mater. Sci. Mater. Med. 2021, 32, 147. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; Mcmahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, R.; Mazzotta, E. Do Niosomes Have a Place in the Field of Drug Delivery? Expert Opin. Drug Deliv. 2019, 16, 1145–1147. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Ikeda-Imafuku, M.; Wang, L.L.-W.; Rodrigues, D.; Shaha, S.; Zhao, Z.; Mitragotri, S. Strategies to Improve the EPR Effect: A Mechanistic Perspective and Clinical Translation. J. Control. Release 2022, 345, 512–536. [Google Scholar] [CrossRef]
- García-Manrique, P.; Machado, N.D.; Fernández, M.A.; Blanco-López, M.C.; Matos, M.; Gutiérrez, G. Effect of Drug Molecular Weight on Niosomes Size and Encapsulation Efficiency. Colloids Surfaces B Biointerfaces 2020, 186, 110711. [Google Scholar] [CrossRef]
- Akhilesh, D.; Bini, K.B.; Kamath, J. Review on Span-60 Based Non-Ionic Surfactant Vesicles (Niosomes) as Novel Drug Delivery. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 6–12. [Google Scholar]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Adeli-sardou, M. Evaluation of Carum-Loaded Niosomes on Breast Cancer Cells:Physicochemical Properties, In Vitro Cytotoxicity, Flow Cytometric, DNA Fragmentation and Cell Migration Assay. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pardakhty, A.; Varshosaz, J.; Rouholamini, A. In Vitro Study of Polyoxyethylene Alkyl Ether Niosomes for Delivery of Insulin. Int. J. Pharm. 2007, 328, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Mohamed, M.S.; Raveendran, S.; Rochani, A.K.; Maekawa, T.; Kumar, D.S. Formulation, Characterization and Evaluation of Morusin Loaded Niosomes for Potentiation of Anticancer Therapy. RSC Adv. 2018, 8, 32621–32636. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghatak, C.; Banerjee, C.; Mandal, S.; Kuchlyan, J.; Sarkar, N. Spontaneous Transition of Micelle-Vesicle-Micelle in a Mixture of Cationic Surfactant and Anionic Surfactant-like Ionic Liquid: A Pure Nonlipid Small Unilamellar Vesicular Template Used for Solvent and Rotational Relaxation Study. Langmuir 2013, 29, 10066–10076. [Google Scholar] [CrossRef] [PubMed]
- Tavano, L.; Oliviero Rossi, C.; Picci, N.; Muzzalupo, R. Spontaneous Temperature-Sensitive Pluronic(®) based Niosomes: Triggered Drug Release Using Mild Hyperthermia. Int. J. Pharm. 2016, 511, 703–708. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of Polymeric Nanoparticle Size to Improve Therapeutic Delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Teeranachaideekul, V.; Supaperm, T. Effect of Charged and Non-Ionic Membrane Additives on Physicochemical Properties and Stability of Niosomes. AAPS PharmSciTech 2008, 9, 851–859. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Farid, M.; Javidfar, M.; Zabet, N.; Shokoohian, B.; Arki, M.K.; Shpichka, A.; Noorbazargan, H.; Aghdaei, H.A.; Hossein-khannazer, N.; et al. The Optimized Formulation of Tamoxifen-Loaded Niosomes Efficiently Induced Apoptosis and Cell Cycle Arrest in Breast Cancer Cells. AAPS PharmSciTech 2022, 23, 57. [Google Scholar] [CrossRef]
- Ruiz-Azuara, L. Process to Obtain New Mixed Copper Aminoacidate from Methylate Phenathroline Complexes to Be Used as Anticancerigenic Agents. U.S. Patent 5,576,326, 21 April 1992. [Google Scholar]
- Ruiz-Azuara, L. Composición Parental de Casiopeína y Uso de La Misma. IMPI No. Solicitud MX/a/2017/016444, 2017.
- Carvallo-Chaigneau, F.; Trejo-Solís, C.; Gómez-Ruiz, C.; Rodríguez-Aguilera, E.; MacÍas-Rosales, L.; Cortés-Barberena, E.; Cedillo-Peláez, C.; Gracia-Mora, I.; Ruiz-Azuara, L.; Madrid-Marina, V.; et al. Casiopeina III-Ia Induces Apoptosis in HCT-15 Cells in Vitro through Caspase-Dependent Mechanisms and Has Antitumor Effect in Vivo. BioMetals 2008, 21, 17–28. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Parveen, S.; Sahoo, S.K. Polymeric Nanoparticles for Cancer Therapy. J. Drug Target. 2008, 16, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Hussain, T.; Ayub, A.; Rashid, U.; MacRobert, A.J. Nanomaterials in Combating Cancer: Therapeutic Applications and Developments. Nanomedicine 2014, 10, 19–34. [Google Scholar] [CrossRef] [PubMed]
- García-Manrique, P.; Matos, M.; Gutiérrez, G.; Estupiñán, O.R.; Blanco-López, M.C.; Pazos, C. Using Factorial Experimental Design to Prepare Size-Tuned Nanovesicles. Ind. Eng. Chem. Res. 2016, 55, 9164–9175. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of Physicochemical and Surface Properties on in Vivo Fate of Drug Nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef] [PubMed]
- U.S. Departament of Healt and Human Services Food and Drug Administration. Liposome Drug Products Chemistry, Guidance for Industry. 2018. Available online: https://www.fda.gov/media/70837/download (accessed on 18 October 2022).
- Baillie, A.J.; Florence, A.T.; Hume, L.R.; Muirhead, G.T.; Rogerson, A. The Preparation and Properties of Niosomes—Non-ionic Surfactant Vesicles. J. Pharm. Pharmacol. 1985, 37, 863–868. [Google Scholar] [CrossRef]
- Sabry, S.; El hakim Ramadan, A.; Abd elghany, M.; Okda, T.; Hasan, A. Formulation, Characterization, and Evaluation of the Anti-Tumor Activity of Nanosized Galangin Loaded Niosomes on Chemically Induced Hepatocellular Carcinoma in Rats. J. Drug Deliv. Sci. Technol. 2021, 61, 102163. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Vyas, S.P. Non-Ionic Surfactant Based Vesicles (Niosomes) in Drug Delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol Injection Method for Hydrophilic and Lipophilic Drug-Loaded Liposome Preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- Marianecci, C.; Rinaldi, F.; Carafa, M.; Marianecci, C.; Di, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; et al. Niosomes from 80s to Present: The State of the Art. Adv. Colloid. Interface Sci. 2013, 205, 187–206. [Google Scholar] [CrossRef]

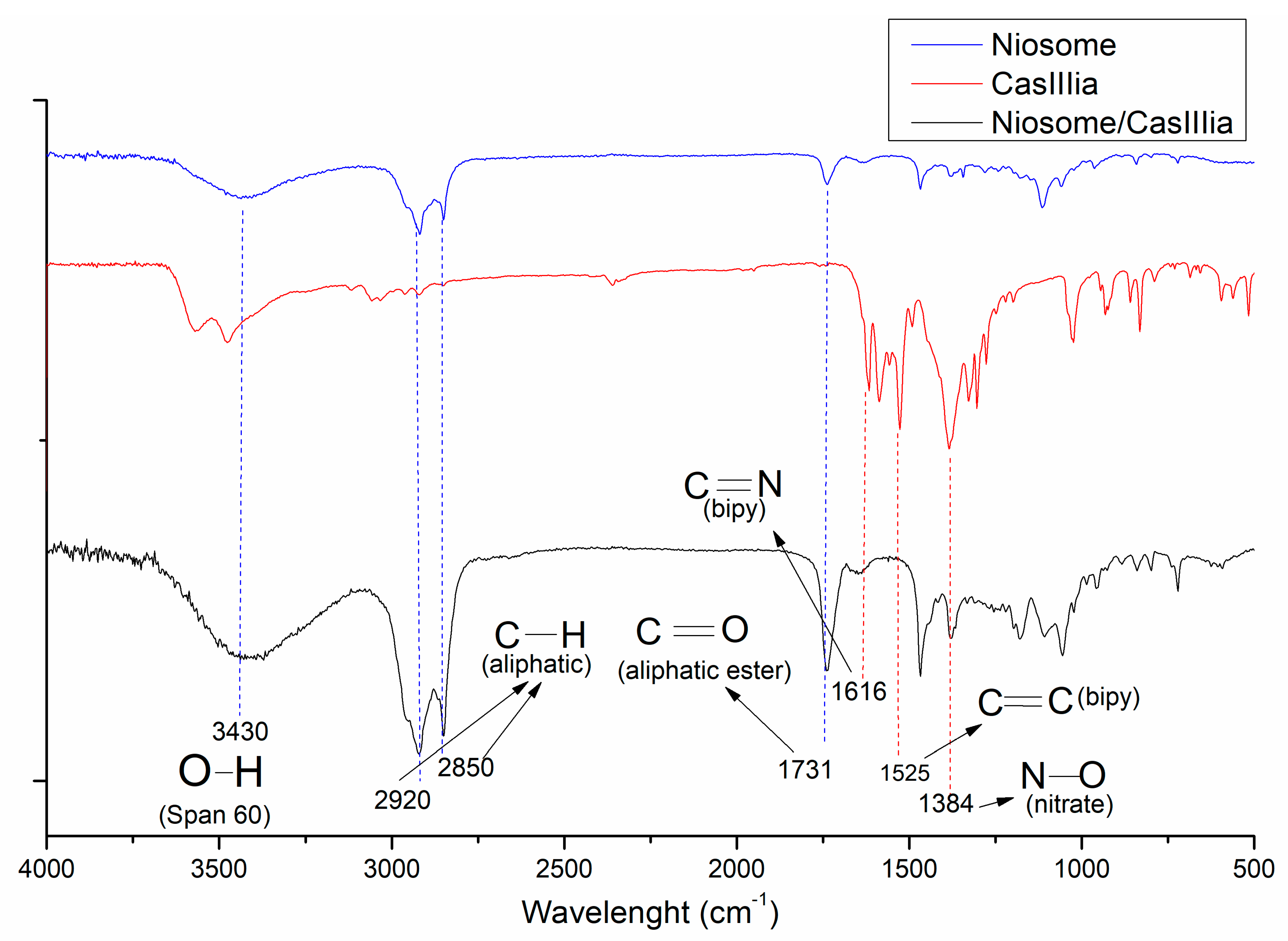
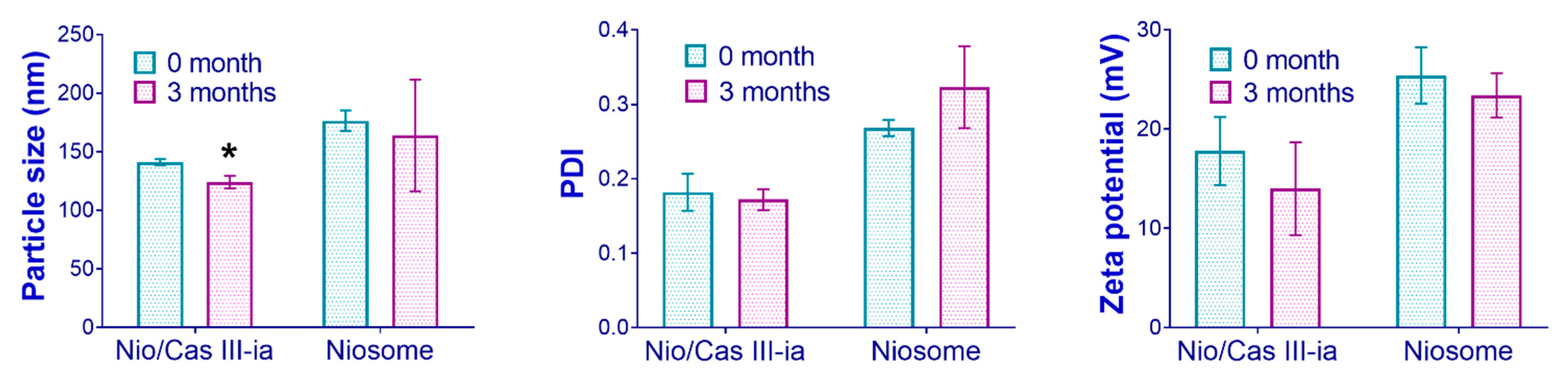

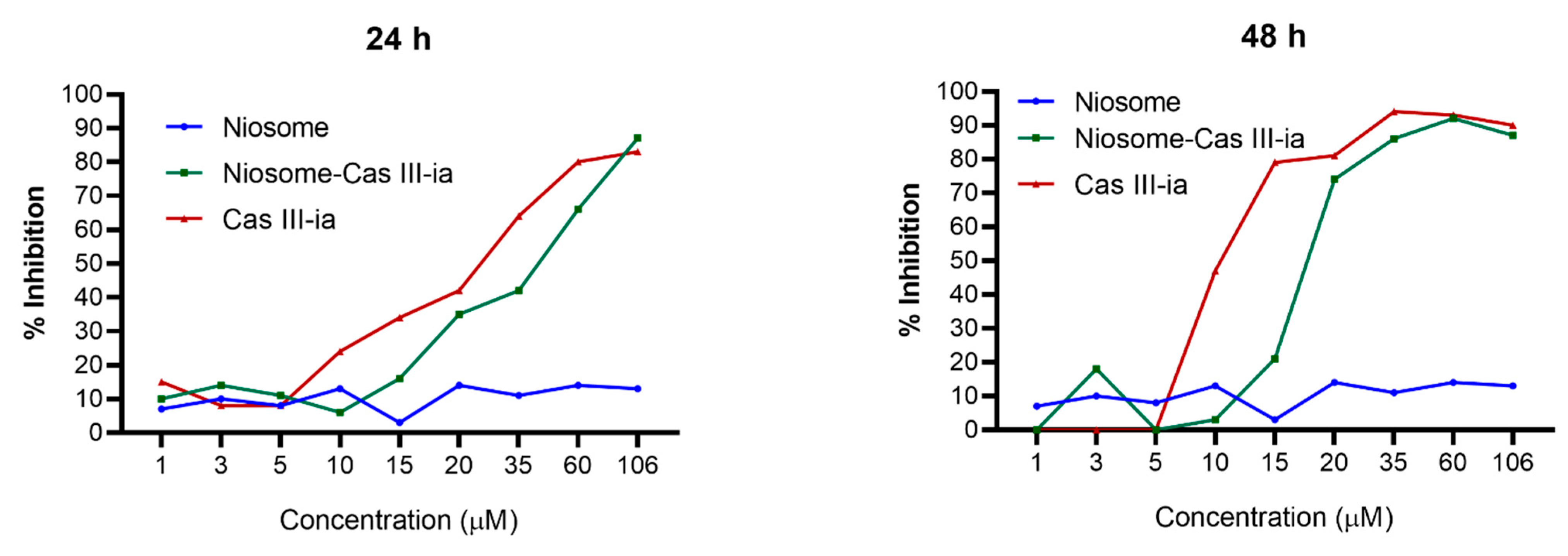
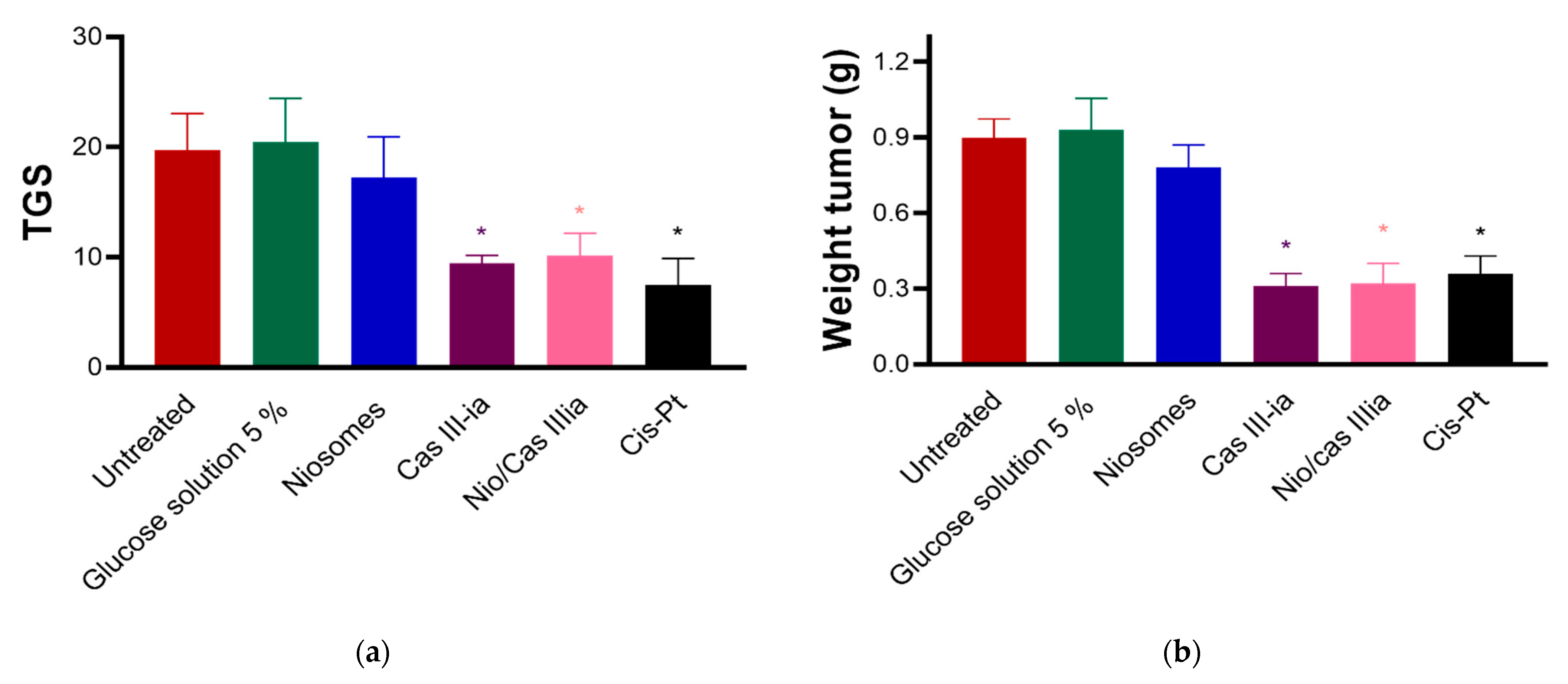
| Drug | Additive (Pluronic F127) | Cholesterol Molar Ratio | Surfactant (Span 60) | Solvent | References | |
|---|---|---|---|---|---|---|
| CQAs | ||||||
| Particle size | High | Low | Low | Medium | High | [28,29,30,31,32] |
| Encapsulation efficiency | High | High | Medium | High | Low | [15,16,30,33,34,35,36] |
| PDI | High | Low | Low | Medium | High | [31] |
| Zeta potential | Medium | High | Low | Medium | Low | [36,37,38] |
| Injection | Evaporation | Filtration | References | |
|---|---|---|---|---|
| CQAs | ||||
| Particle size | High | Low | High | [39,40,41] |
| Encapsulation efficiency | Medium | Low | Medium | [39,41] |
| PDI | High | Low | High | |
| Zeta potential | Medium | Low | Medium |
| Variables Conditions | CQAs Value |
|---|---|
| 697 µM of S/C/P concentration 32 µM of CasIII-ia 170 rpm of speed rate | Maximum EE (40%) Particle size of 150 nm |
| Particle Size (nm) | EE (%) | Zeta Potential (mV) | PDI | |||
|---|---|---|---|---|---|---|
| Observed | Predicted | Observed | Predicted | |||
| Niosomes with CasIII-ia | 150.4 ± 4.66 | 150 ± 19.10 | 39.89 ± 4.99 | 40.44 ± 3.87 | −13.8 ± 1.25 | 0.234 ± 0.01 |
| Niosome blank | 215 ± 1.92 | - | - | - | −23.4 ± 1.95 | 0.231 ± 0.01 |
| QTPP | Target | Justification |
|---|---|---|
| Dosage form | Injectable solution | Patent same dose form. Patent solicitude (MX/a/2017/016444) [61]. |
| Therapeutic indication | Cancer treatment (phase I clinical trial of CasIII-ia) | Casiopeinas have shown activity in tumor lines of cervical (HeLa, SiHa), breast (MCF-7), colon (HCT15), and neuroblastoma (CHP-212) cancers [9]. Moreover, CasIII-ia has shown tumor reduction in a nude mouse xenotransplanted (HCT-15) [62]. |
| Dosage design | A modified-release dosage from a colloidal system | Modified release to favor reaching the site of action of the compound by enhanced permeation and retention effect (EPR) [63,64,65]. |
| Route of administration | Intravenous | Same route of administration as patent. Avoid the first-pass effect [61]. |
| Production method | Solvent ether injection; drug entrapment during niosome formation | In the solvent injection method, the use of sonication is not necessary for the reduction of particle size because it depends on experimental conditions and could be between 50 and 500 nm [28,35]. This represents a lower energy expenditure. Additionally, industrial scalability could result in better feasibility. |
| CQA excipients | Span 60 (sorbitan monostearate), cholesterol and Pluronic F127 (poloxamer 407) | Excipients are critical for niosome formation, entrapment efficiency, zeta potential, and blood circulation time [32,34,66]. |
| Stability | At least 6-month shelf-life at 40 °C and 75% RH; agrees with NOM-073-SSA1-2015 | Measurement of particle size and PDI is related to the quality and safety profile of the preparation [48,67]. |
| Container closure system | Sterile glass vial | To achieve the target shelf-life and to ensure quality during storage [61,68]. |
| CQA | Target | CQA | Justification |
|---|---|---|---|
| Appearance | Colorless liquid | No | Color and appearance are not important for quality. |
| Particle size | 150 nm | Yes | Particle sizes between 20 and 50 nm present a high efficacy and safety. Sizes below 50 nm can suffer fenestration in organs such as the liver and fast-renal clearance. On the other hand, sizes above 200 nm could present spleen retention. Finally, sizes between 100 and 150 nm remain for a longer time in blood circulation [20,48]. |
| Zeta potential | ±30 mV | Yes | The physical stability of vesicular systems depends on the total surface charge. Niosomes in solution require physical stability for storage and systemic circulation [21,48]. |
| Polydispersity index (PDI) | Low | Yes | Niosomes with high PDI values modify the drug release profile, which may increase the toxicity of the drug. |
| Encapsulation efficiency | Maximum | Yes | Encapsulation efficiency diminishes free casiopeina concentration and, consequently, its interaction with blood proteins. |
| Residual solvents | Minimum | Yes | Residual solvents are an important toxic factor. Ether ethylic is a class 3 solvent, in agreement with ICH guideline Q3C. Class 3 solvents have low toxic potential [40]. Available data indicate they are less toxic in acute or short-time studies and negative in genotoxicity studies [40]. |
| Evaporation | Time | 60 min | Estimated time for ethylic ether evaporation |
| Speed rate | 50 rpm | Low agitation is a minor energetic cost | |
| Injection | Temperature | 50 °C | Phase-transition temperature (Tc) of Span 60 is 60 °C. However, cholesterol can reduce phase-transition temperature (Tc) [32]. |
| Injection rate | 40 mL/h | Chiraz et al. reported that the injection rate has no significant effect on the mean vesicle size [72]. | |
| Speed rate | Variable | ||
| Solvent volume | 2.5 mL | Smaller solvent volumes can increase particle size due to fast evaporation. Higher volumes imply higher residual solvent. | |
| Formulation | CasIII-ia concentration | Variable | |
| S/C/P concentration | Variable | ||
| P concentration | 3% | A 2.5–5% additive concentration is recommended. Higher concentrations inhibit niosome formation [73]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Jiménez, Z.; González-Ballesteros, M.; Dávila-Manzanilla, S.G.; Espinoza-Guillén, A.; Ruiz-Azuara, L. Development and In Vitro and In Vivo Evaluation of an Antineoplastic Copper(II) Compound (Casiopeina III-ia) Loaded in Nonionic Vesicles Using Quality by Design. Int. J. Mol. Sci. 2022, 23, 12756. https://doi.org/10.3390/ijms232112756
Aguilar-Jiménez Z, González-Ballesteros M, Dávila-Manzanilla SG, Espinoza-Guillén A, Ruiz-Azuara L. Development and In Vitro and In Vivo Evaluation of an Antineoplastic Copper(II) Compound (Casiopeina III-ia) Loaded in Nonionic Vesicles Using Quality by Design. International Journal of Molecular Sciences. 2022; 23(21):12756. https://doi.org/10.3390/ijms232112756
Chicago/Turabian StyleAguilar-Jiménez, Zenayda, Mauricio González-Ballesteros, Silvia G. Dávila-Manzanilla, Adrián Espinoza-Guillén, and Lena Ruiz-Azuara. 2022. "Development and In Vitro and In Vivo Evaluation of an Antineoplastic Copper(II) Compound (Casiopeina III-ia) Loaded in Nonionic Vesicles Using Quality by Design" International Journal of Molecular Sciences 23, no. 21: 12756. https://doi.org/10.3390/ijms232112756
APA StyleAguilar-Jiménez, Z., González-Ballesteros, M., Dávila-Manzanilla, S. G., Espinoza-Guillén, A., & Ruiz-Azuara, L. (2022). Development and In Vitro and In Vivo Evaluation of an Antineoplastic Copper(II) Compound (Casiopeina III-ia) Loaded in Nonionic Vesicles Using Quality by Design. International Journal of Molecular Sciences, 23(21), 12756. https://doi.org/10.3390/ijms232112756