Graphene Oxide Loaded on TiO2-Nanotube-Modified Ti Regulates the Behavior of Human Gingival Fibroblasts
Abstract
:1. Introduction
2. Results
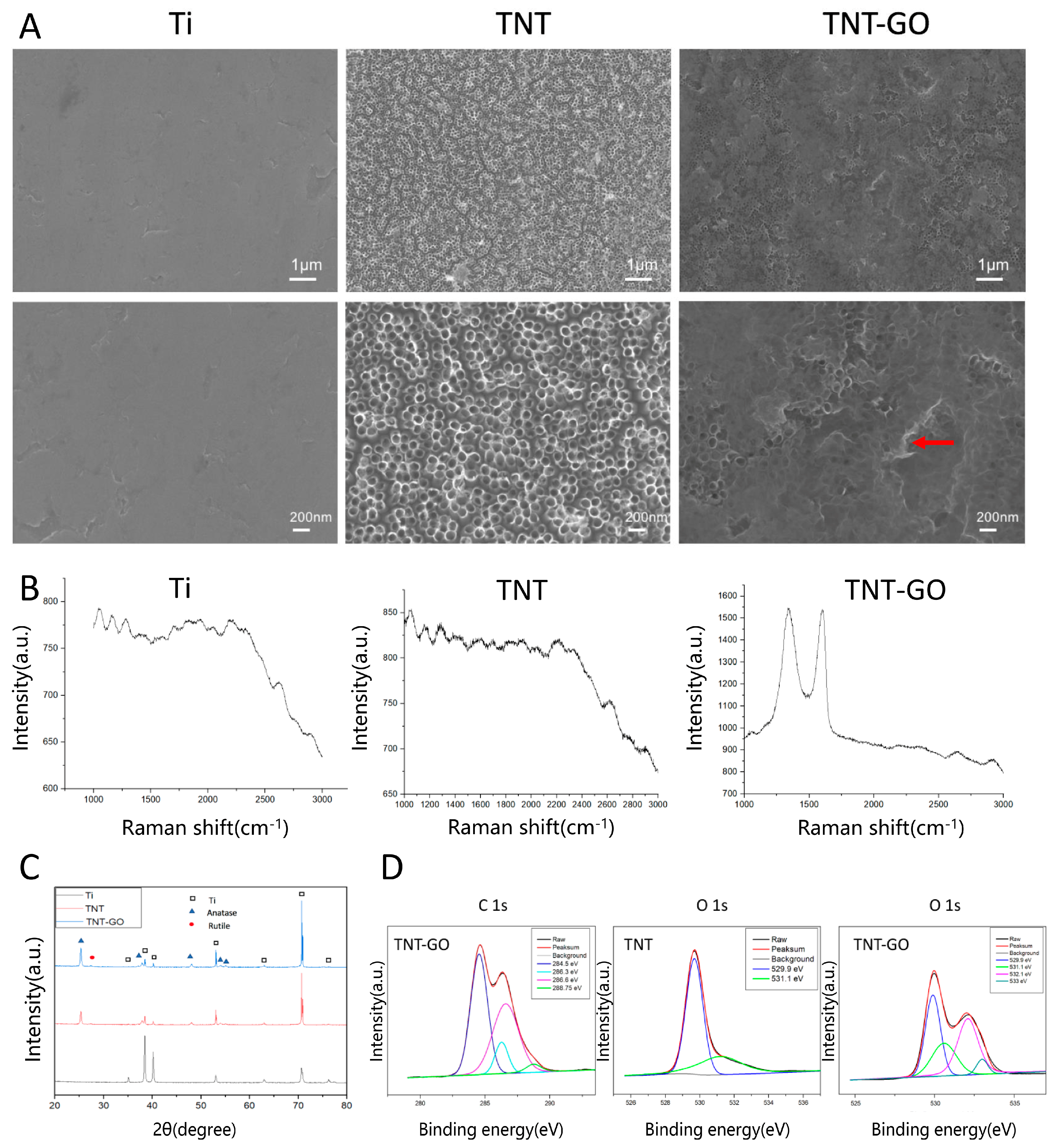
2.1. Surface Properties
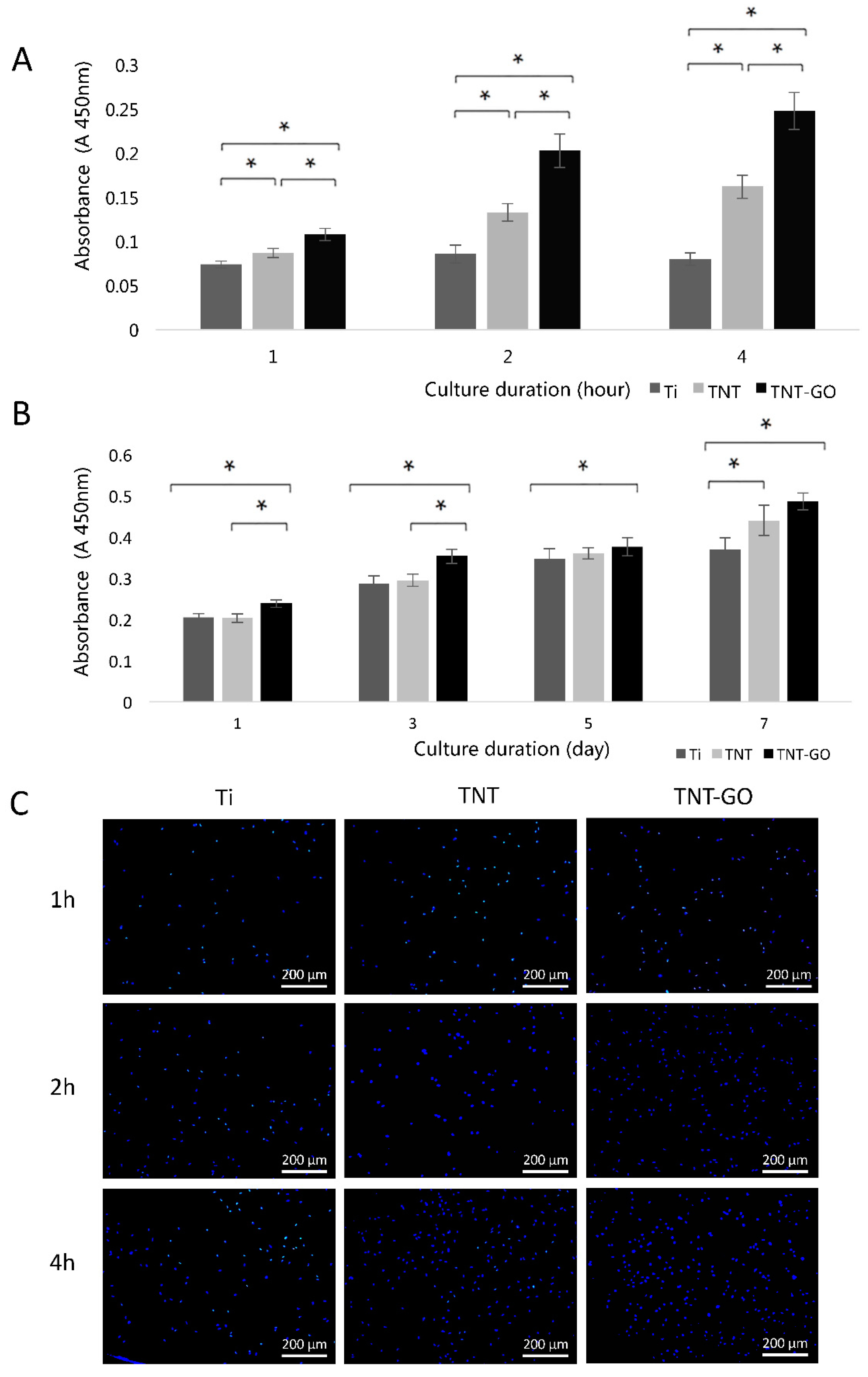
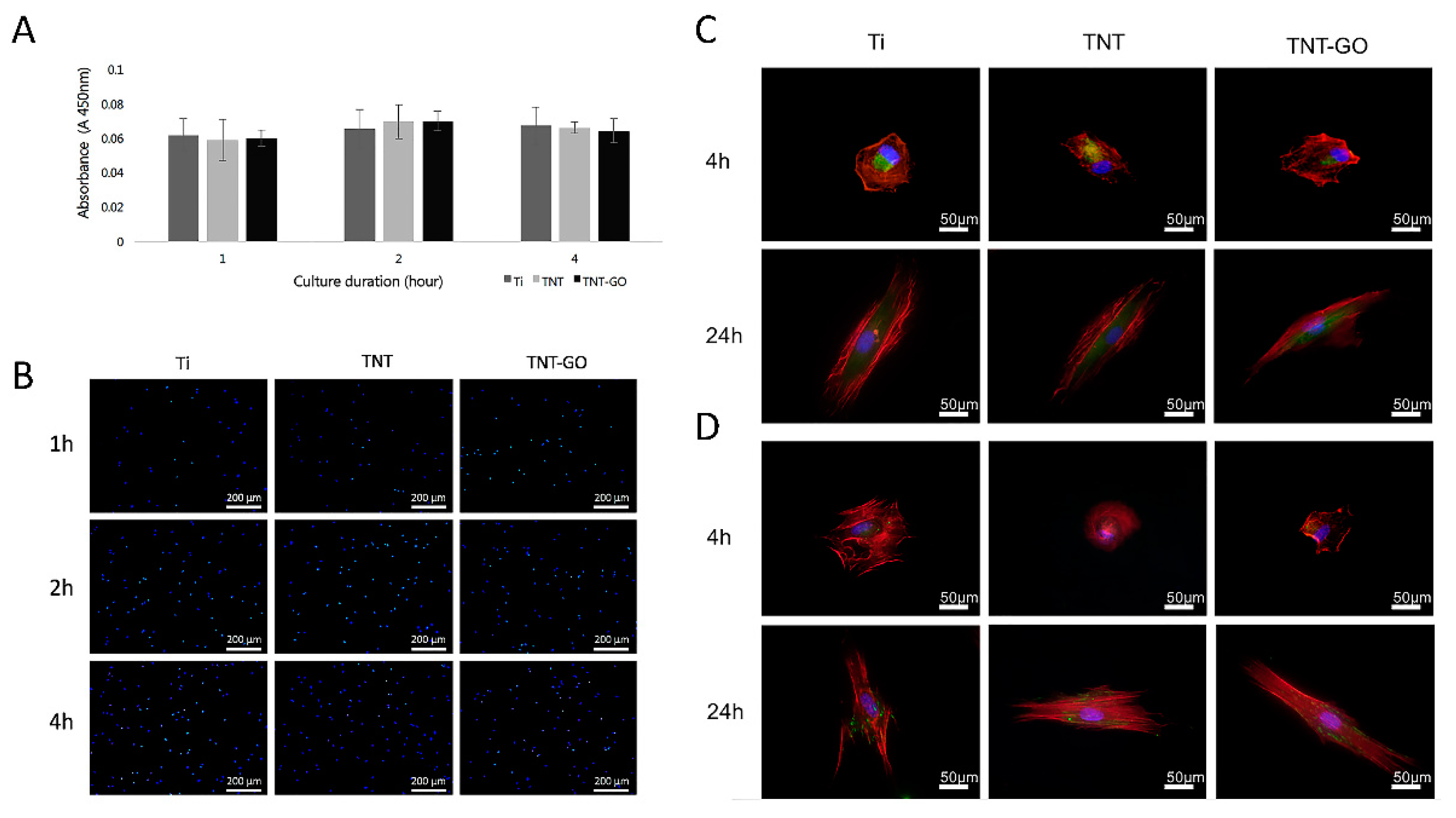
2.2. Cell Adhesion and Proliferation
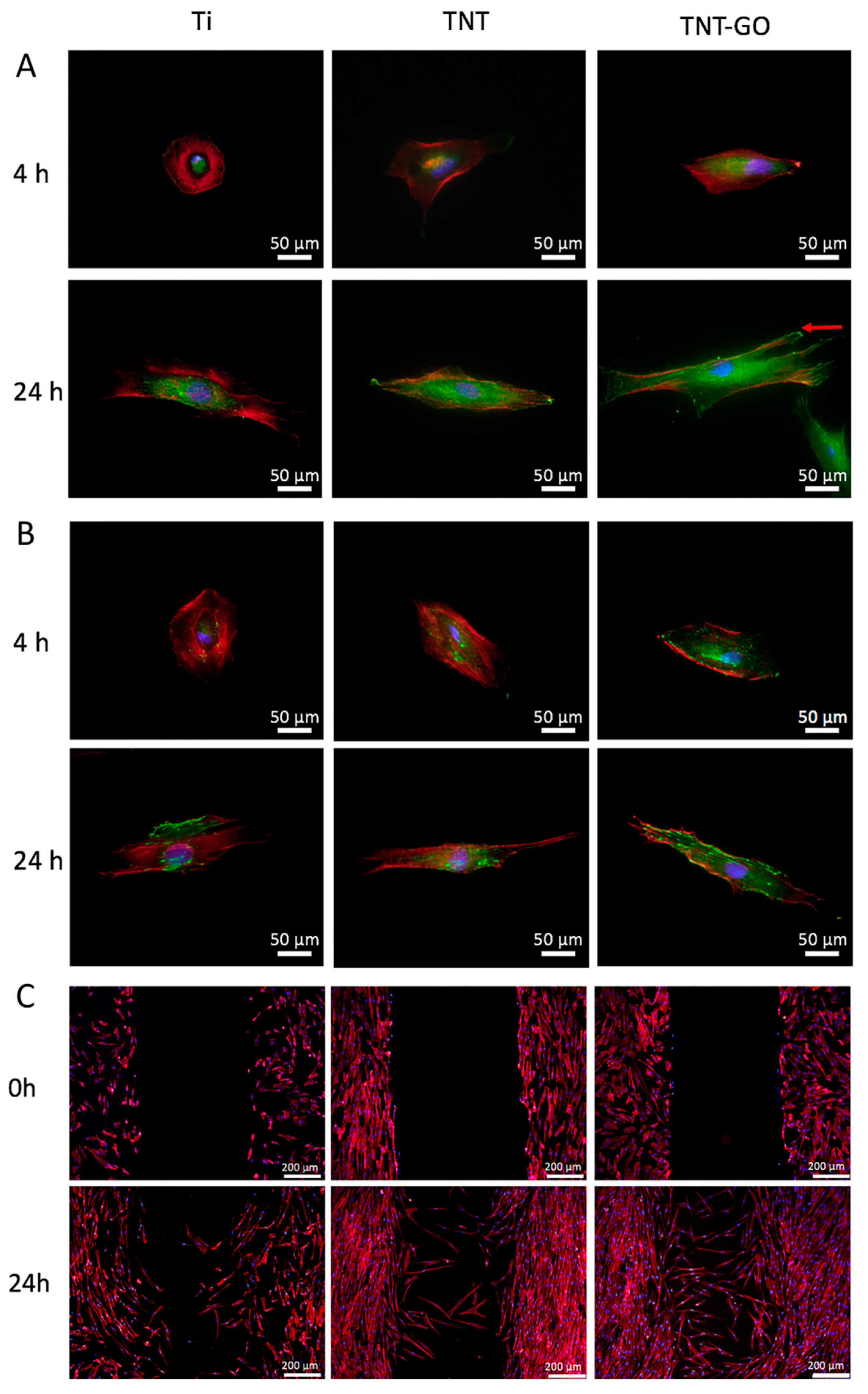
2.3. Cell Morphology
2.4. Immunocytochemistry
2.5. Wound-Healing Assay
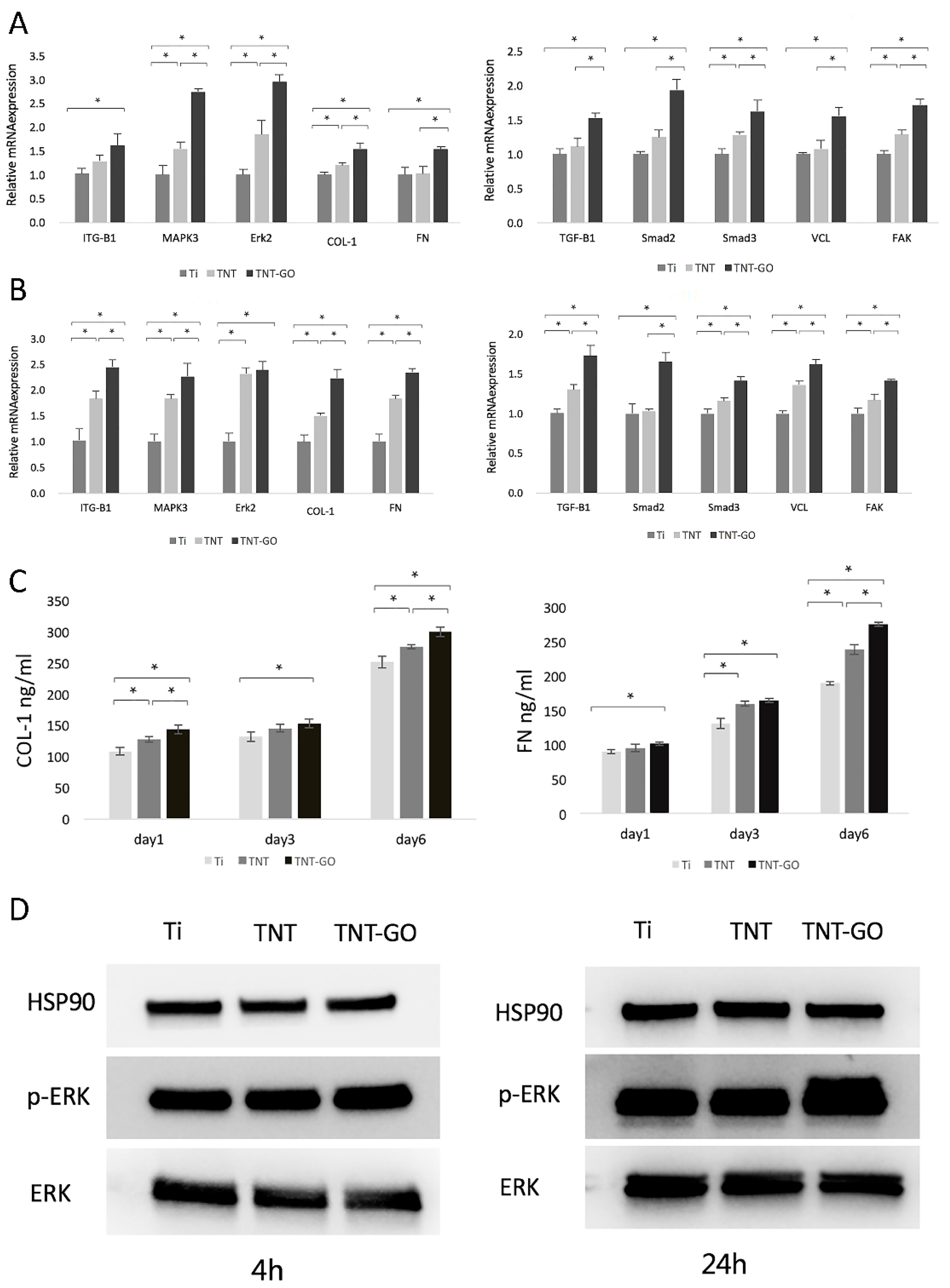
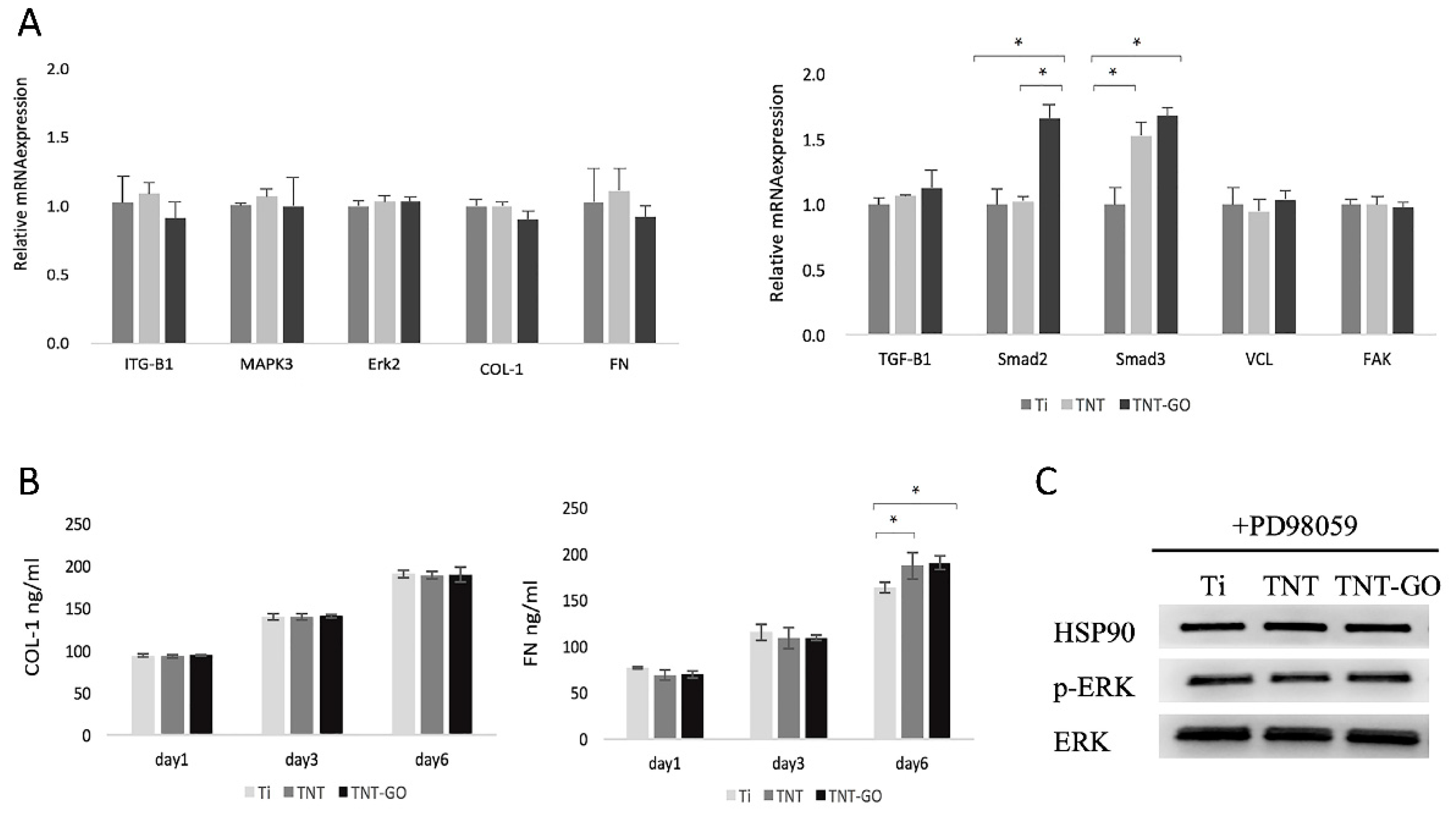
2.6. Gene Expression
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Western Blot
2.9. Response of HGFs after Blocking Erk1/2 Signaling
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Sample Characterization
4.3. Protein Adsorption
4.4. Cell Culture
4.5. Cell Proliferation and Adhesion
4.6. Cell Morphology
4.7. Immunofluorescence
4.8. Wound-Healing Assay
4.9. Western Blot Tests
4.10. ELISA
4.11. Quantitative RT-PCR Analysis
4.12. Responses of HGFs after Blocking Erk1/2 Signaling
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asri, R.; Alias, J.; Zulkifli, F.H.; Kadirgama, K.; Ghani, S.; Shariffuddin], J. A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 2018, 44, 1250–1268. [Google Scholar]
- Domínguez-Trujillo, C.; Peón, E.; Chicardi, E.; Pérez, H.; Rodríguez-Ortiz, J.A.; Pavón, J.J.; García-Couce, J.; Galván, J.C.; García-Moreno, F.; Torres, Y. Sol-gel deposition of hydroxyapatite coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 2018, 333, 158–162. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.-N.; Abughanam, G.; Tran, S.D.; Sheikh, Z.; Mezour, M.A.; Basiri, T.; Xiao, Y.; Cerruti, M.; Siqueira, W.L.; Tamimi, F. Comparative adsorption profiles of basal lamina proteome and gingival cells onto dental and titanium surfaces. Acta Biomater. 2018, 73, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Bobbert, F.S.L.; Lietaert, K.; Eftekhari, A.A.; Pouran, B.; Ahmadi, S.M.; Weinans, H.; Zadpoor, A.A. Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties. Acta Biomater. 2017, 53, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Lu, R.; Wang, X.; Huai, X.; Wang, C.; Wang, Y.; Chen, S. Visible light-induced antibacterial and osteogenic cell proliferation properties of hydrogenated TiO2 nanotubes/Ti foil composite. Nanotechnology 2021, 32, 195101. [Google Scholar] [CrossRef]
- Lu, R.; Wang, C.; Wang, X.; Wang, Y.; Wang, N.; Chou, J.; Li, T.; Zhang, Z.; Ling, Y.; Chen, S. Effects of hydrogenated TiO2 nanotube arrays on protein adsorption and compatibility with osteoblast-like cells. Int. J. Nanomed. 2018, 13, 2037–2049. [Google Scholar] [CrossRef] [Green Version]
- Ivanovski, S.; Lee, R. Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontology 2000 2018, 76, 116–130. [Google Scholar] [CrossRef]
- Rodriguez, X.; Navajas, A.; Vela, X.; Fortuno, A.; Jimenez, J.; Nevins, M. Arrangement of Peri-implant Connective Tissue Fibers Around Platform-Switching Implants with Conical Abutments and Its Relationship to the Underlying Bone: A Human Histologic Study. Int. J. Periodontics Restor. Dent. 2016, 36, 533–540. [Google Scholar]
- Glauser, R.; Schupbach, P.; Gottlow, J.; Dds, C.H.F.H. Periimplant Soft Tissue Barrier at Experimental One-Piece Mini-implants with Different Surface Topography in Humans: A Light-Microscopic Overview and Histometric Analysis. Clin. Implant. Dent. Relat. Res. 2005, 7, s44–s51. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Ayukawa, Y.; Kondo, R.; Oshiro, W.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Soft tissue sealing around dental implants based on histological interpretation. J. Prosthodont. Res. 2016, 60, 3–11. [Google Scholar] [CrossRef]
- Hammerle, C.; Giannobile, W.V. Biology of soft tissue wound healing and regeneration–Consensus Report of Group 1 of the 10th European Workshop on Periodontology. J. Clin. Periodontol. 2014, 41 (Suppl. 15), S1–S5. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, S.; Roffel, S.; Meyer, M.; Gasser, A. Biology of soft tissue repair: Gingival epithelium in wound healing and attachment to the tooth and abutment surface. Eur. Cells Mater. 2019, 38, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Martínez, C.; Martínez, J.; McCulloch, C.A. Role of Fibroblast Populations in Periodontal Wound Healing and Tissue Remodeling. Front. Physiol. 2019, 10, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, W.; Hu, Z.; Ge, S. Healthy and Inflamed Gingival Fibroblasts Differ in Their Inflammatory Response to Porphyromonas gingivalis Lipopolysaccharide. Inflammation 2016, 39, 1842–1852. [Google Scholar] [CrossRef]
- Al Rezk, F.; Trimpou, G.; Lauer, H.C.; Weigl, P.; Krockow, N. Response of soft tissue to different abutment materials with different surface topographies: A review of the literature. Gen Dent 2018, 66, 18–25. [Google Scholar]
- Abdallah, M.N.; Badran, Z.; Ciobanu, O.; Hamdan, N.; Tamimi, F. Strategies for Optimizing the Soft Tissue Seal around Osseointegrated Implants. Adv. Healthc. Mater. 2017, 6, 1700549. [Google Scholar] [CrossRef]
- Zhou, P.; Mao, F.; He, F.; Han, Y.; Li, H.; Chen, J.; Wei, S. Screening the optimal hierarchical micro/nano pattern design for the neck and body surface of titanium implants. Colloids Surf. B Biointerfaces 2019, 178, 515–524. [Google Scholar] [CrossRef]
- Miao, X.; Wang, D.; Xu, L.; Wang, J.; Zeng, D.; Lin, S.; Huang, C.; Liu, X.; Jiang, X. The response of human osteoblasts, epithelial cells, fibroblasts, macrophages and oral bacteria to nanostructured titanium surfaces: A systematic study. Int. J. Nanomed. 2017, 12, 1415–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Long, S.; Mao, F.; Huang, H.; Li, H.; He, F.; Zhang, R.; Ren, L.; Chen, J.; Wei, S. Controlling cell viability and bacterial attachment through fabricating extracellular matrix-like micro/nanostructured surface on titanium implant. Biomed. Mater. 2020, 15, 035002. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Sakurai, K.; Yamada, M. Periodontal-like gingival connective tissue attachment on titanium surface with nano-ordered spikes and pores created by alkali-heat treatment. Dent. Mater. 2015, 31, e116–e130. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Sculean, A.; Bosshardt, D.D.; Miron, R.J. Macrophage behavior and interplay with gingival fibroblasts cultured on six commercially available titanium, zirconium, and titanium-zirconium dental implants. Clin. Oral Investig. 2019, 23, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Lu, R.; Gao, S.; Ling, Y.; Chen, S. Responses of human gingival fibroblasts to superhydrophilic hydrogenated titanium dioxide nanotubes. Colloids Surf. B Biointerfaces 2021, 198, 111489–1114999. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Sager, M.; Wieland, M.; Dard, M.; Becker, J. Histological and immunohistochemical analysis of initial and early subepithelial connective tissue attachment at chemically modified and conventional SLA titanium implants. A Pilot Study Dogs. Clin. Oral Investig. 2007, 11, 245–255. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Q.; Zhang, X.; Qiu, J.; Qian, S.; Liu, X. Co-implantation of magnesium and zinc ions into titanium regulates the behaviors of human gingival fibroblasts. Bioact. Mater. 2020, 6, 64–74. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Tavakkoli Yaraki, M.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 171–185. [Google Scholar] [CrossRef]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Du, Z.; Xiao, L.; Wei, F.; Yang, Y.; Li, M.; Qiu, Y.; Liu, J.; Chen, J.; Xiao, Y. Graphene oxide coated Titanium Surfaces with Osteoimmunomodulatory Role to Enhance Osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110983. [Google Scholar] [CrossRef]
- Tang, L.A.; Lee, W.C.; Shi, H.; Wong, E.Y.; Sadovoy, A.; Gorelik, S.; Hobley, J.; Lim, C.T.; Loh, K.P. Highly wrinkled cross-linked graphene oxide membranes for biological and charge-storage applications. Small 2012, 8, 423–431. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Rodriguez, R.D.; Al-Hamry, A.; Lipovka, A.; Dorozhko, E.; Selyshchev, O.; Ma, B.; Sharma, S.; Mehta, S.K.; Dzhagan, V. Flexible plasmonic graphene oxide/heterostructures for dual-channel detection. Analyst 2019, 144, 3297–3306. [Google Scholar] [CrossRef]
- Canullo, L.; Menini, M.; Santori, G.; Rakic, M.; Sculean, A.; Pesce, P. Titanium abutment surface modifications and peri-implant tissue behavior: A systematic review and meta-analysis. Clin. Oral Investig. 2020, 24, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Mgdm, A.; Gatdm, B.; Cddm, C.; Ftd, D.; Mrm, E. Invitro study of surface alterations to polyetheretherketone and titanium and their effect upon human gingival fibroblasts. J. Prosthet. Dent. 2021, 125, 155–164. [Google Scholar]
- Aktas, O.C.; Metzger, W.; Haidar, A.; Ail, Y.; Nothdurft, F.P. Enhancing Adhesion and Alignment of Human Gingival Fibroblasts on Dental Implants. J. Cranio-Maxillofac. Surg. 2019, 47, 661–667. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Dong, K.; Cui, N.; Lu, T.; Han, Y. Enhanced osteogenic differentiation of osteoblasts on CaTiO3 nanotube film. Colloids Surf. B Biointerfaces 2020, 187, 110773. [Google Scholar] [CrossRef]
- Tan, J.; Zhao, C.; Zhou, J.; Duan, K.; Wang, J.; Lu, X.; Weng, J.; Feng, B. Co-culturing epidermal keratinocytes and dermal fibroblasts on nano-structured titanium surfaces. Mater. Sci. Eng. C. Mater. Biogical Appl. 2017, 78, 288–295. [Google Scholar] [CrossRef]
- Ferrà-Caellas, M.; Llopis-Grimalt, M.A.; Monjo, M.; Ramis, J.M. Tuning Nanopore Diameter of Titanium Surfaces to Improve Human Gingival Fibroblast Response. Int. J. Mol. Sci. 2018, 19, 2881. [Google Scholar] [CrossRef] [Green Version]
- Nothdurft, F.P.; Fontana, D.; Ruppenthal, S.; May, A.; Aktas, C.; Mehraein, Y.; Lipp, P.; Kaestner, L. Differential Behavior of Fibroblasts and Epithelial Cells on Structured Implant Abutment Materials: A Comparison of Materials and Surface Topographies. Clin. Implant. Dent. Relat. Res. 2016, 17, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Linares, A.; Munoz, F.; Permuy, M.; Dard, M.; Blanco, J. Soft tissue histomorphology at implants with a transmucosal modified surface. A Study in Minipigs. Clin. Oral Implant. Res. 2015, 26, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Zigterman, B.; Borre, C.; Braem, A.; Mommaerts, M.Y. Titanium surface modifications and their soft-tissue interface on nonkeratinized soft tissues—A systematic review (Review). Biointerphases 2019, 14, 040802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rausch, M.A.; Shokoohi-Tabrizi, H.; Wehner, C.; Pippenger, B.E.; Andrukhov, O. Impact of Implant Surface Material and Microscale Roughness on the Initial Attachment and Proliferation of Primary Human Gingival Fibroblasts. Biology 2021, 10, 356. [Google Scholar] [CrossRef]
- Newby, S.D.; Masi, T.; Griffin, C.D.; King, W.J.; Chipman, A.; Stephenson, S.; Anderson, D.E.; Biris, A.S.; Bourdo, S.E.; Dhar, M. Functionalized Graphene Nanoparticles Induce Human Mesenchymal Stem Cells to Express Distinct Extracellular Matrix Proteins Mediating Osteogenesis. Int. J. Nanomed. 2020, 15, 2501–2513. [Google Scholar] [CrossRef] [Green Version]
- Bollen, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258. [Google Scholar] [CrossRef]
- Vroman, L. Finding seconds count after contact with blood (and that is all I did). Colloids Surf. B Biointerfaces 2008, 62, 1–4. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Ducheyne, P.; Shapiro, I.M. Effect of serum proteins on osteoblast adhesion to surface-modified bioactive glass and hydroxyapatite. J. Orthop. Res. 2010, 17, 340–345. [Google Scholar] [CrossRef]
- Yala, S.; Boustta, M.; Gallet, O.; Hindie, M.; Carreiras, F.; Benachour, H.; Sidane, D.; Khireddine, H. New synthesis method of HA/P(D, L)LA composites: Study of fibronectin adsorption and their effects in osteoblastic behavior for bone tissue engineering. J. Mater. Sci. Mater. Med. 2016, 27, 140. [Google Scholar] [CrossRef]
- Seth, B.K.; Ray, A.; Banerjee, M.; Bhattacharyya, T.; Basu, S. Structure dependent hydrophobic and hydrophilic interactions between nickel (II) Schiff base complexes and serum albumins: Spectroscopic and docking studies. J. Lumin. 2016, 171, 85–97. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Mielczarski, E.; Rai, B.; Mielczarski, J.A. Quantitative and Qualitative Evaluation of Adsorption/Desorption of Bovine Serum Albumin on Hydrophilic and Hydrophobic Surfaces. Langmuir Acs J. Surf. Colloids 2009, 25, 11614–11620. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Aiyelabegan, H.T.; Sadroddiny, E. Fundamentals of protein and cell interactions in biomaterials. Biomed. Pharmacother. 2017, 88, 956–970. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivodova, V.; Frankova, J.; Ulrichova, J. Osteoblast and gingival fibroblast markers in dental implant studies. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2011, 155, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lu, T.; Wen, J.; Xu, L.; Zeng, D.; Wu, Q.; Cao, L.; Lin, S.; Liu, X.; Jiang, X. Selective responses of human gingival fibroblasts and bacteria on carbon fiber reinforced polyetheretherketone with multilevel nanostructured TiO2. Biomaterials 2016, 83, 207–218. [Google Scholar] [CrossRef]
- Feng, X.; Feng, L.; Jin, M.; Zhai, J.; Jiang, L.; Zhu, D. Reversible Super-hydrophobicity to Super-hydrophilicity Transition of Aligned ZnO Nanorod Films. J. Am. Chem. Soc. 2004, 126, 62–63. [Google Scholar] [CrossRef] [Green Version]
- Marie, P.J. Targeting integrins to promote bone formation and repair. Nat. Rev. Endocrinol. 2013, 9, 288–295. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhou, L.Y.; Qian, S.J.; Gu, Y.X.; Shi, J.Y.; Lai, H.C. Improved response of human gingival fibroblasts to titanium coated with micro-/nano-structured tantalum. Int. J. Implant. Dent. 2021, 7, 36. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.; Ahn, J.H.; Hong, B.H.; Pastorin, G. Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells. Acs. Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [Green Version]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zou, L.; Jiang, L.; Zhao, Z.; Liu, J. Osteoinductive and antimicrobial mechanisms of graphene-based materials for enhancing bone tissue engineering. J. Tissue Eng. Regen. Med. 2021, 15, 915–935. [Google Scholar] [CrossRef] [PubMed]
- Shim, N.; Heo, J. Performance of the Polydopamine-Graphene Oxide Composite Substrate in the Osteogenic Differentiation of Mouse Embryonic Stem Cells. Int. J. Mol. Sci. 2021, 22, 7323. [Google Scholar] [CrossRef] [PubMed]
- Nisio, C.D.; Colli, M.D.; Giacomo, V.D.; Rapino, M.; Zara, S. A dual role for β1 integrin in an in vitro Streptococcus mitis/human gingival fibroblasts co-culture model in response to TEGDMA. Int. Endod. J. 2015, 48, 839–849. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Ren, N.; Li, J.; Qiu, J.; Yan, M.; Liu, H.; Ji, D.; Huang, J.; Yu, J.; Liu, H. Growth and accelerated differentiation of mesenchymal stem cells on graphene-oxide-coated titanate with dexamethasone on surface of titanium implants. Dent. Mater. 2017, 33, 525–535. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Sample | Treatment | Contact Angle (°) | Roughness (nm) |
|---|---|---|---|
| 1 | Ti | 90.9 ± 3.4 | 9.5 ± 1.6 |
| 2 | TNT | 46.3 ± 2.8 | 46.5 ± 2.8 |
| 3 | TNT-GO | 33.9 ± 6.3 | 42.1 ± 3.3 |
| Gene | Primers Sequence (F = Forward, R = Reverse) |
|---|---|
| ITGB1 | F: GCATCCCTGAAAGTCCCAAG R: CAATGGAGAGTGCGTCTGCG |
| MAPK3 | F: GGACCTGATGGAGACTGACCTG R: GTTGGCGGAGTGGATGTACTT |
| Erk2 | F: CTCATCCTCGGAAAACAGACC R: AATGCTGTGTGGACCTTCAGA |
| FN | F: GTGAACGACACATTCCACAAG R: GGTGGAAGTGTGATCCCGTCG |
| Col-I | F: GGCTCCCTCCTAGTCTGTCCT R: GGGAGGAAGCAAAAGACTCT |
| TGF-β | F: ACCTGAACCCGTGTTGCTCT R: CGCCAGGAATTGTTGCTGTA |
| Smad2 | F: AACAGATGGGATGCTTCAGGT R: CCTCACTTGGCTTGCTTCTTT |
| Smad3 | F: GAAGGAGGGTTGACTCAGAAC R: TTTCACACCAGGCACATACTT |
| VCL | F: CGAATCCCAACCATAAGCAC R: CGCACAGTCTCCTTCACAGA |
| FAK | F: CTCCTACTGCCAACCTGGAC R: GCCGACTTCCTTCACCATAG |
| GAPDH | F: ATTTGGTCGTATTGGGCGCC R: ACCTCAACTACATGGTTTAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Wu, K.; Wang, C.; Guo, Y.; Lu, R.; Wang, X.; Chen, S. Graphene Oxide Loaded on TiO2-Nanotube-Modified Ti Regulates the Behavior of Human Gingival Fibroblasts. Int. J. Mol. Sci. 2022, 23, 8723. https://doi.org/10.3390/ijms23158723
Cao X, Wu K, Wang C, Guo Y, Lu R, Wang X, Chen S. Graphene Oxide Loaded on TiO2-Nanotube-Modified Ti Regulates the Behavior of Human Gingival Fibroblasts. International Journal of Molecular Sciences. 2022; 23(15):8723. https://doi.org/10.3390/ijms23158723
Chicago/Turabian StyleCao, Xu, Keyi Wu, Caiyun Wang, Yatong Guo, Ran Lu, Xin Wang, and Su Chen. 2022. "Graphene Oxide Loaded on TiO2-Nanotube-Modified Ti Regulates the Behavior of Human Gingival Fibroblasts" International Journal of Molecular Sciences 23, no. 15: 8723. https://doi.org/10.3390/ijms23158723
APA StyleCao, X., Wu, K., Wang, C., Guo, Y., Lu, R., Wang, X., & Chen, S. (2022). Graphene Oxide Loaded on TiO2-Nanotube-Modified Ti Regulates the Behavior of Human Gingival Fibroblasts. International Journal of Molecular Sciences, 23(15), 8723. https://doi.org/10.3390/ijms23158723





