Genome-Wide Identification of Auxin-Responsive GH3 Gene Family in Saccharum and the Expression of ScGH3-1 in Stress Response
Abstract
:1. Introduction
2. Results
2.1. Identification and Characterization of the SsGH3 Gene Family
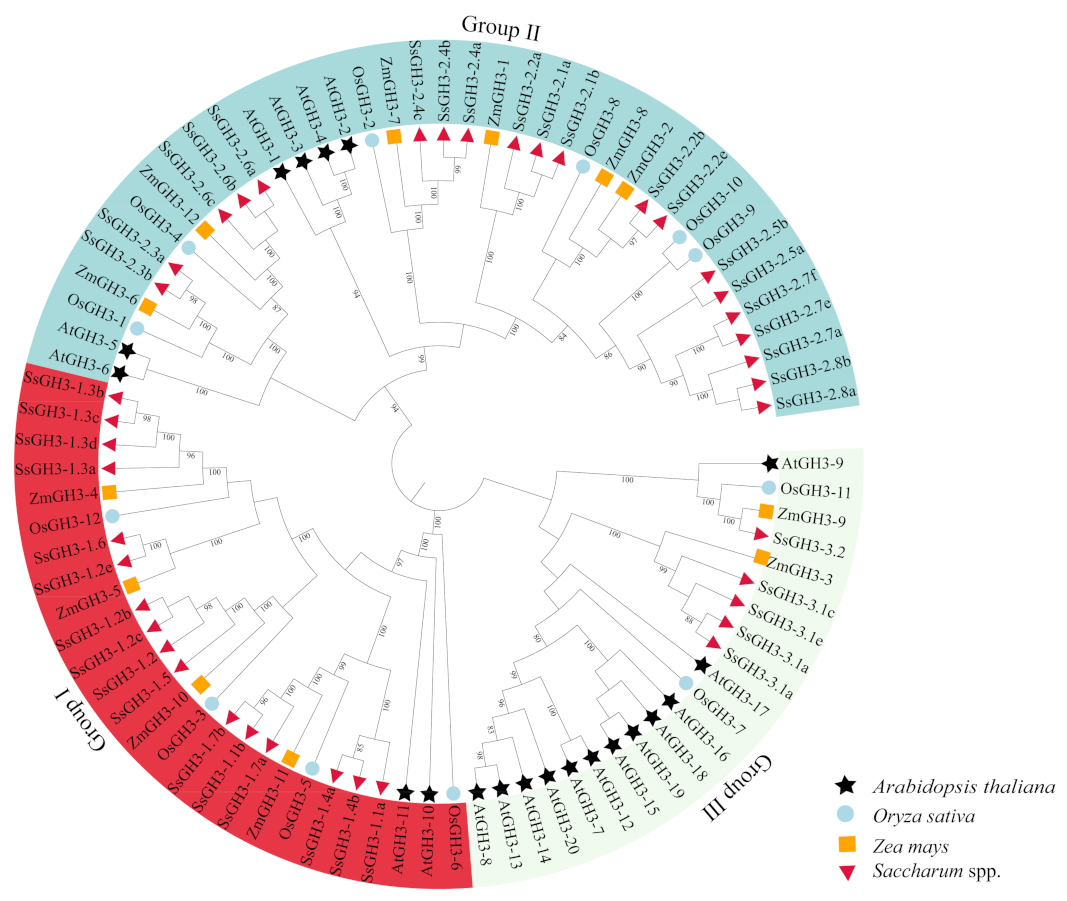
2.2. Phylogenetic Analysis Divided the SsGH3s into Three Sub-Groups
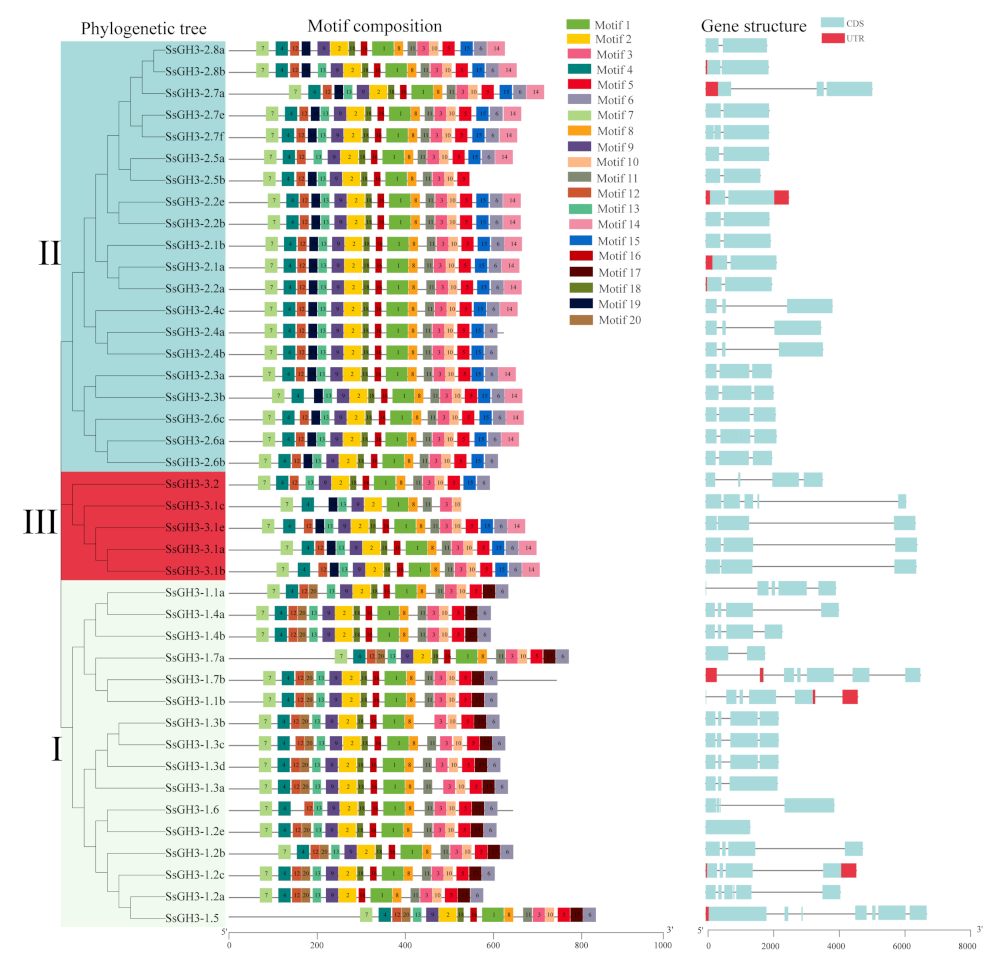
2.3. Phylogenetic, Conserved Motifs, and Gene Structures of the SsGH3 Family Genes
2.4. Chromosomal Location and Synteny Analysis of SsGH3 Gene Family
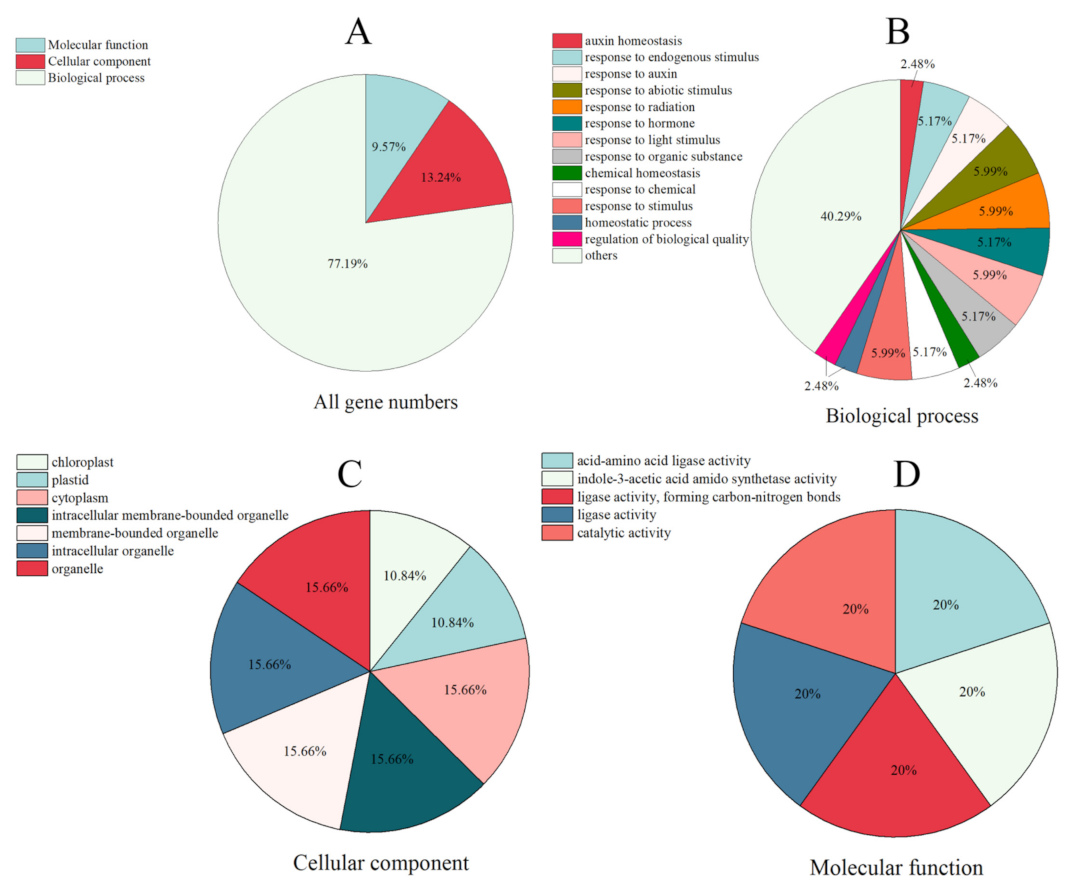
2.5. GO Annotation of SsGH3 Gene Family
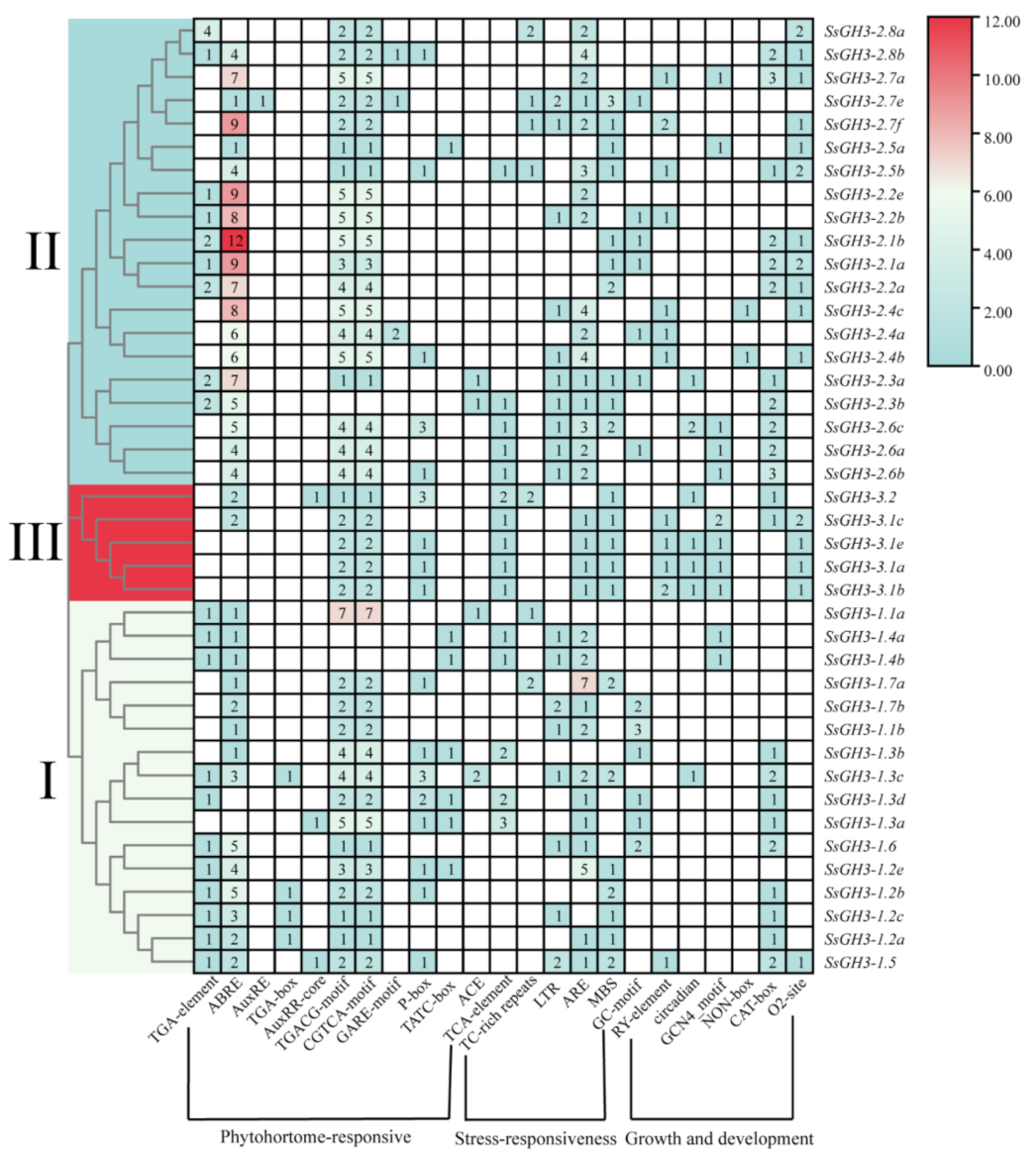
2.6. Cis-Acting Regulatory Elements of SsGH3 Gene Family
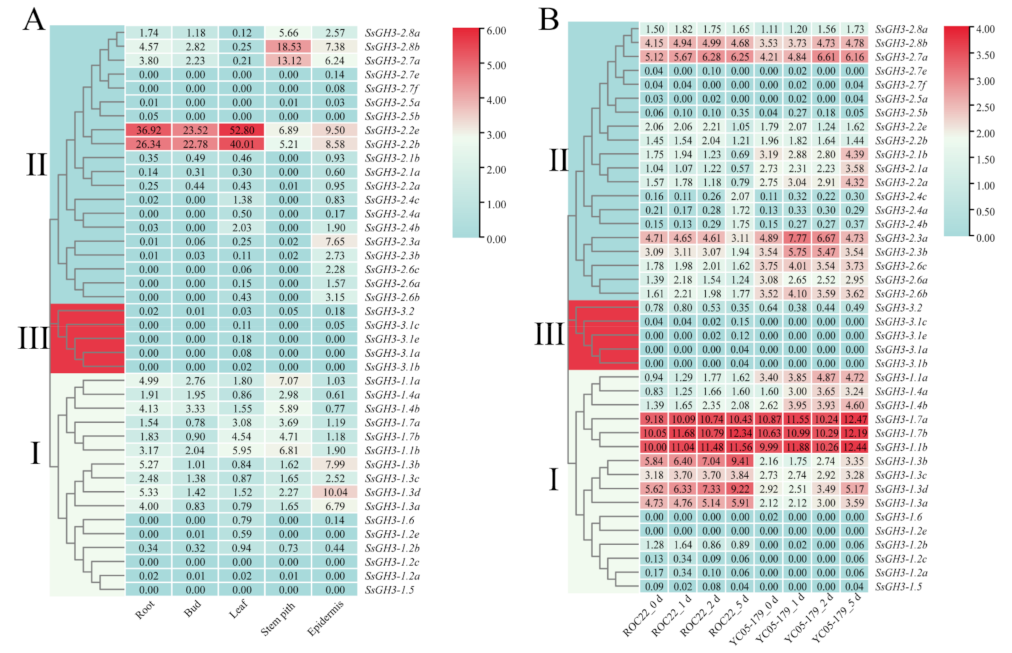
2.7. Expression Patterns of SsGH3 Gene Family, Based on RNA-Seq Databases
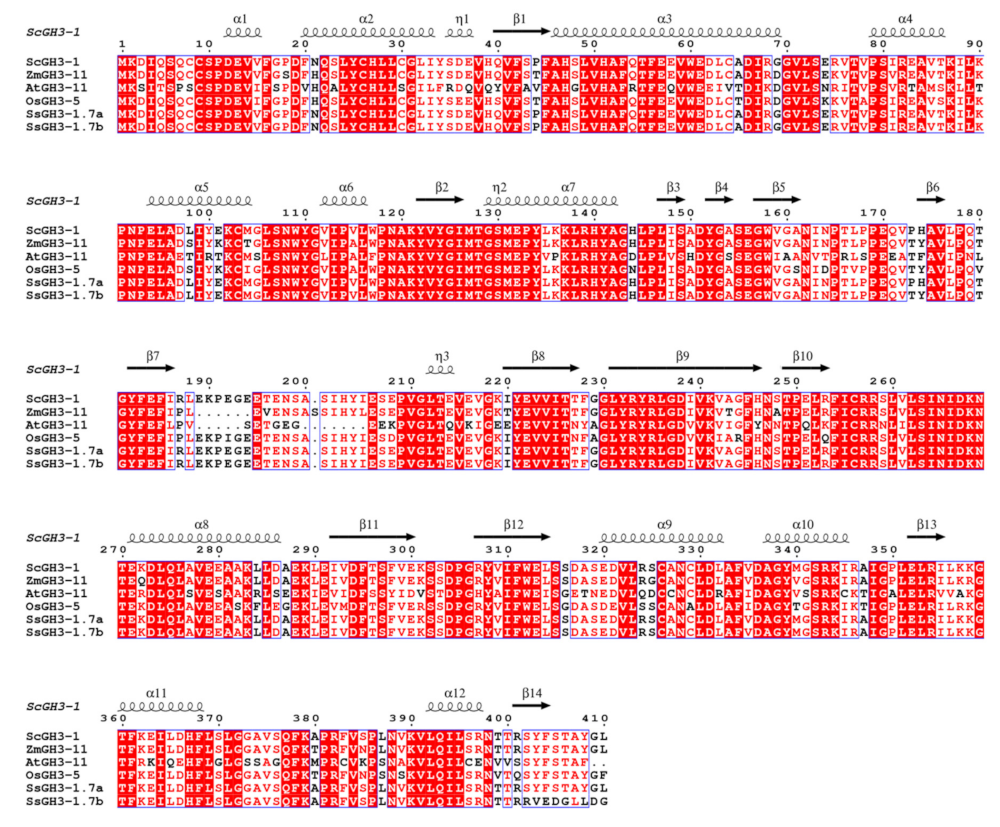
2.8. Cloning and Sequence Analysis of the ScGH3-1 Gene
2.9. ScGH3-1 Is a Nuclear and Membrane Localization Protein
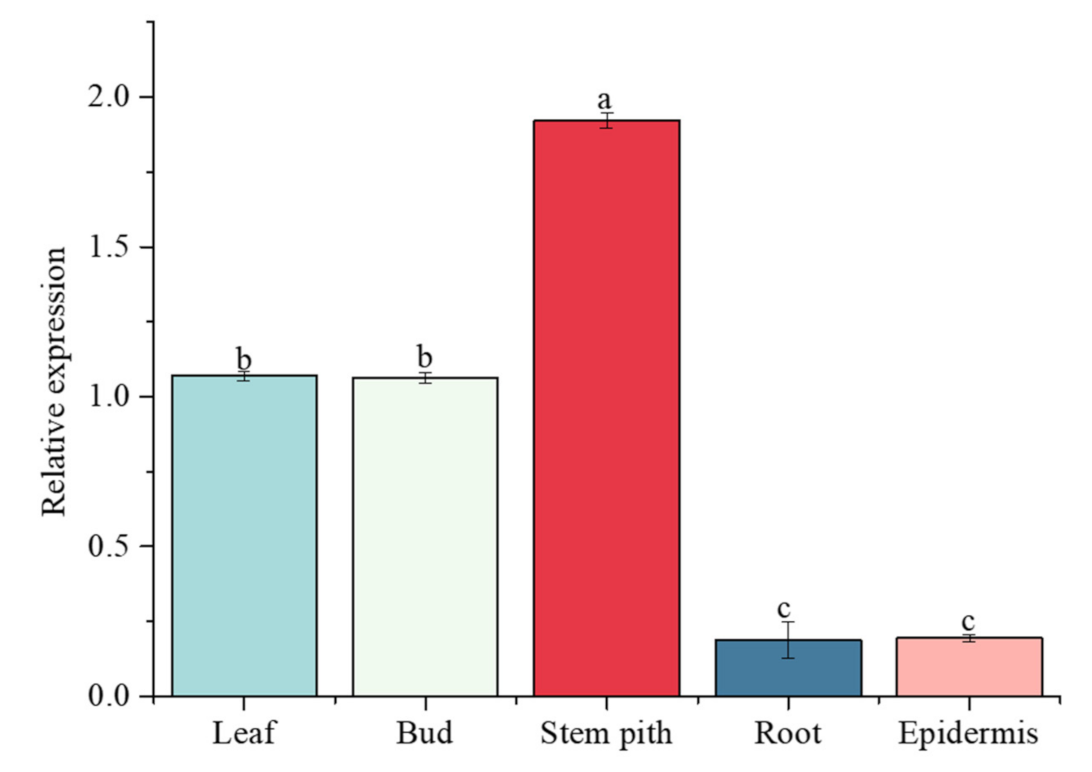
2.10. ScGH3-1 Was Constitutively Expressed in Different Sugarcane Tissues
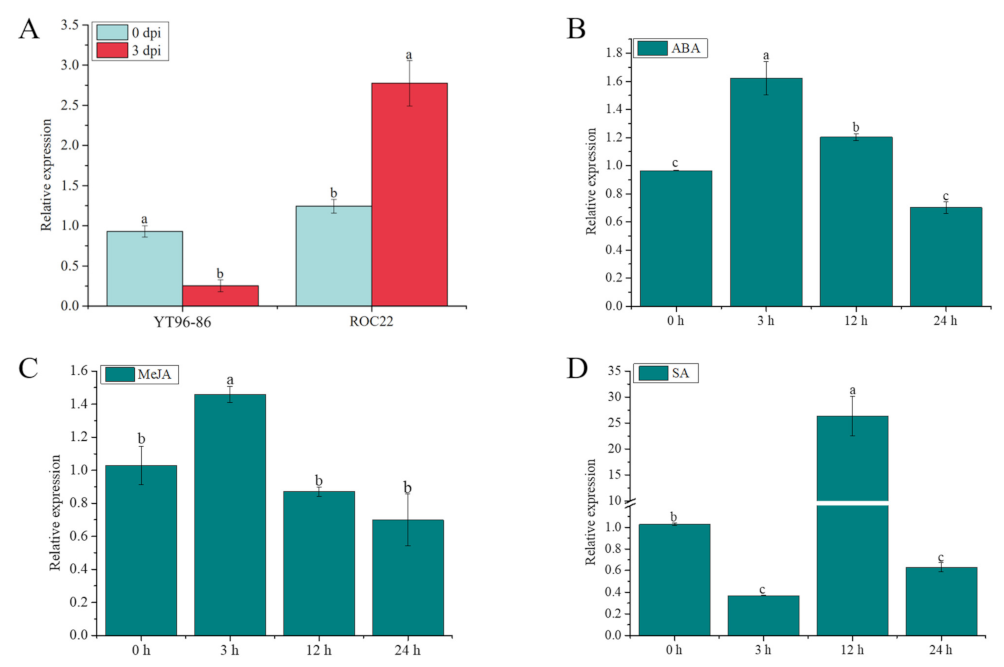
2.11. ScGH3-1 Is Involved in the Response to S. scitamineum, ABA, SA, and MeJA Stresses in Sugarcane
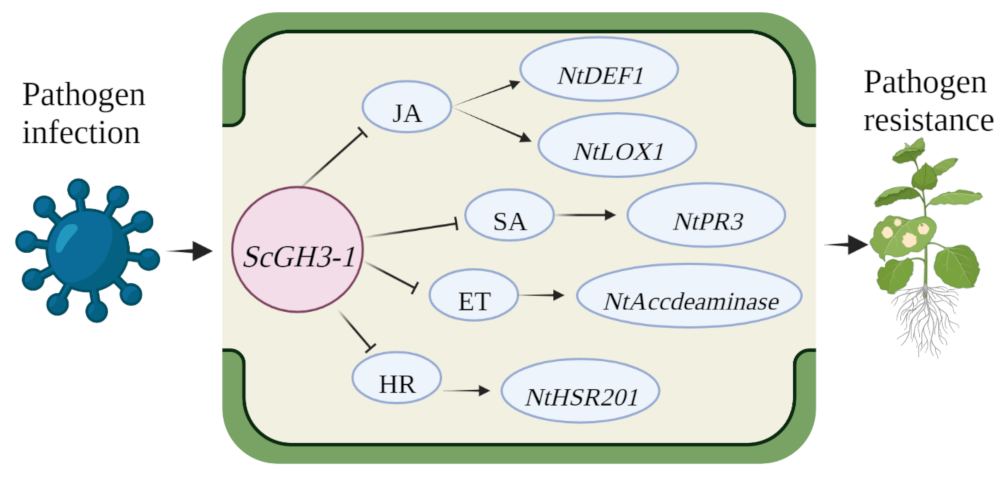
2.12. Transient Overexpression of ScGH3-1 Negatively Regulated the Defense Response of N. benthamiana to Pathogen Infection
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Identification of the SsGH3 Gene Family
4.3. Conserved Motifs and Gene Structures of the SsGH3 Gene Family
4.4. Sequence Features, Gene Duplications, and the Synteny of SsGH3 Family Genes
4.5. GO Annotation of SsGH3 Family Genes
4.6. Cis-Acting Regulatory Elements in the Promoter Regions of SsGH3 Family Genes
4.7. Expression Patterns of SsGH3 Family Genes, Based on the Transcriptome Databases
4.8. Gene Cloning and Bioinformatics Analysis of Sugarcane ScGH3-1 Gene
4.9. Subcellular Localization of Sugarcane ScGH3-1 Gene
4.10. Quantification of the Sugarcane ScGH3-1 Gene by RT-qPCR Analysis
4.11. Transient Expression of the ScGH3-1 Gene in N. benthamiana
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilfoyle, T.J.; Ulmasov, T.; Hagen, G. The ARF family of transcription factors and their role in plant hormone-responsive transcription. Cell. Mol. Life Sci. 1998, 54, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Liscum, E.; Reed, J.W. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 2002, 49, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.G.; Hao, M.Y.; Liu, H.B.; Xiao, J.H.; Li, X.H.; Yuan, M.; Wang, S.P. The group I GH3 family genes encoding JA-Ile synthetase act as positive regulator in the resistance of rice to Xanthomonas oryzae pv. oryzae. Biochem. Biophys. Res. Commun. 2019, 508, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.M.; Hagen, G.; Guilfoyle, T. An auxin-induced polypeptide in dicotyledonous plants. Plant Mol. Biol. 1987, 9, 625–634. [Google Scholar] [CrossRef]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Marién, T.; Maldonado, M.T.; Maldonado, M.C.; Walter, S. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef] [Green Version]
- Zaal, E.J.; Memelink, J.; Mennes, A.M.; Quint, A.; Libbenga, K.R. Auxin-induced mRNA species in tobacco cell cultures. Plant Mol. Biol. 1987, 10, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Zarembinski, T.I.; Theologis, A. Anaerobiosis and plant growth hormones induce two genes encoding 1-aminocyclopropane-1-carboxylate synthase in rice (Oryza sativa L.). Mol. Biol. Cell 1993, 4, 363–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Yin, J.; Zhang, H.; He, Y.; Shuai, S.; Chen, S.; Cao, S.; Li, W.; Ma, D. Genome-wide identification, characterization analysis and expression profiling of auxin-responsive GH3 family genes in wheat (Triticum aestivum L.). Mol. Biol. Rep. 2020, 47, 3885–3907. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, N.; Zhong, T.; Wang, C.; Xu, M.; Ye, J. Identification and characterization of the GH3 gene family in maize. J. Integr. Agr. 2016, 15, 249–261. [Google Scholar] [CrossRef]
- Jez, J.M. Connecting primary and specialized metabolism: Amino acid conjugation of phytohormones by GRETCHEN HAGEN 3 (GH3) acyl acid amido synthetases. Curr. Opin. Plant Biol. 2022, 66, 102194. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Yuen, G.Y.; Lehman, C.C. Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. 1998, 15, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Bakker, P.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overmyer, K.; Tuominen, H.; Kettunen, R.; Betz, C.; Langebartels, C.; Sandermann, H.; Kangasjärvi, J. Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 2000, 12, 1849–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, M.V.; Lee, H.; Creelman, R.A.; Mullet, J.E.; Davis, K.R. Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 2000, 12, 1633–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Li, Q.; Li, Z.; Staswick, P.E.; Wang, M.; Zhu, Y.; He, Z. Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction. Plant Physiol. 2007, 145, 450–464. [Google Scholar] [CrossRef] [Green Version]
- Jagadeeswaran, G.; Raina, S.; Acharya, S.B.; Mosher, S.L.; Appel, H.M.; Schultz, J.C.; Klessig, D.F.; Raina, R. Arabidopsis GH3-LIKE DEFENSE GENE 1 is required for accumulation of salicylic acid, activation of defense responses and resistance to Pseudomonas syringae. Plant J. 2007, 51, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cao, Y.; Huang, L.; Zhao, J.; Xu, C.; Li, X.; Wang, S. Activation of the indole-3-acetic acid–amido synthetase GH3-8 suppresses expansin expression and promotes salicylate-and jasmonate-independent basal immunity in rice. Plant Cell 2008, 20, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Hui, S.; Zhang, M.; Hao, M.; Yuan, M. Rice group I GH3 gene family, positive regulators of bacterial pathogens. Plant Signal. Behav. 2019, 14, e1588659. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, Y.; Deng, X.; Li, S.; Zhang, C.; Li, Y. Comparative genomic and transcriptomic analysis suggests the evolutionary dynamic of GH3 genes in Gramineae crops. Front. Plant Sci. 2019, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Functional Verification of StGH3.1 and StGH3.5 Genes and the Response to Wounding and Disease Resistance of Potato. Doctoral Thesis, Northwest A&F University, Shanxi, China, November 2020. [Google Scholar]
- Lakshmanan, P.; Geijskes, R.J.; Aitken, K.S.; Grof, C.L.; Bonnett, G.D.; Smith, G.R. Sugarcane biotechnology: The challenges and opportunities. Vitr. Cell. Dev. Biol. -Plant 2005, 41, 345–363. [Google Scholar] [CrossRef]
- Garsmeur, O.; Droc, G.; Antonise, R.; Grimwood, J.; Potier, B.; Aitken, K.; Jenkins, J.; Martin, G.; Charron, C. A mosaic monoploid reference sequence for the highly complex genome of sugarcane. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.R.; Yang, L.T. Sugarcane agriculture and sugar industry in China. Sugar Tech. 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Ye, J.; Yang, Y.Y.; Xu, L.P.; Li, Y.R.; Que, Y.X. Economic impact of stem borer-resistant genetically modified sugarcane in Guangxi and Yunnan provinces of China. Sugar Tech. 2016, 18, 537–545. [Google Scholar] [CrossRef]
- Nzioki, H.S.; Jamoza, J.E.; Olweny, C.O.; Rono, J.K. Characterization of physiologic races of sugarcane smut (Ustilago scitaminea) in Kenya. Afr. J. Microbiol. Res. 2010, 4, 1694–1697. [Google Scholar]
- Wang, C.; Li, J.; Zhang, R.; Wang, X.; Shan, H. Research Progress and Prospect of Sugarcane Smut. Agri. Biotech. 2020, 9, 37–40. [Google Scholar]
- Que, Y.X.; Xu, L.P.; Wu, Q.B.; Liu, Y.F.; Ling, H.; Liu, Y.H.; Zhang, Y.Y.; Guo, J.L.; Su, Y.C.; Chen, J.B.; et al. Genome sequencing of Sporisorium scitamineum provides insights into the pathogenic mechanisms of sugarcane smut. BMC Genom. 2014, 15, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, X.; Tang, H.; Zhang, Q.; Hua, X.; Ma, X.; Zhu, F.; Jones, T.; Zhu, X.; Bowers, J.; et al. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L. Nat. Genet. 2018, 50, 1565–1573. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Stone, J.M. Arabidopsis thaliana GH3.9 influences primary root growth. Planta 2007, 226, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Howe, E.S.; Clemente, T.E.; Bass, H.W. Maize histone H2B-mCherry: A new fluorescent chromatin marker for somatic and meiotic chromosome research. DNA Cell Biol. 2012, 31, 925–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, M.; Kaur, N.; Tyagi, A.K.; Khurana, J.P. The auxin-responsive GH3 gene family in rice (Oryza sativa). Funct. Integr. Genom. 2006, 6, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Tiryaki, I.; Rowe, M.L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 2002, 14, 1405–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staswick, P.E.; Tiryaki, I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobuta, K.; Okrent, R.A.; Stoutemyer, M.; Rodibaugh, N.; Kempema, L.; Wildermuth, M.V.; Innes, R.W. The GH3 acyl adenylase family member PBS3 regulates salicylic acid-dependent defense responses in Arabidopsis. Plant Physiol. 2007, 144, 1144–1156. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Zhang, Z.L.; Zou, X.; Huang, J.; Ruas, P.; Thompson, D.; Shen, Q.J. Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 2005, 137, 176–189. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Ding, T.; Su, B.; Jiang, H. Genome-wide identification, characterization and expression analysis of the chalcone synthase family in maize. Int. J. Mol. Sci. 2016, 17, 161. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.L.; Yang, J.; Boykin, L.M.; Zhao, Q.Y.; Wang, Y.J.; Liu, S.S.; Wang, X.W. Developing conversed microsatellite markers and their implications in evolutionary analysis of the Bemisia tabaci complex. Sci. Rep. 2014, 4, 6351. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.B.; Ulmasov, T.; Shi, X.; Hagen, G.; Guiloyle, T.J. Soybean GH3 promoter contains multiple auxin-inducible elements. Plant Cell 1994, 6, 645–657. [Google Scholar]
- Zhang, S.N.; Wang, S.K.; Xu, Y.X.; Yu, C.J.; Shen, C.J.; Qian, Q.; Geisler, M.; Jiang, D.A.; Qi, Y.H. The auxin response factor, OsARF 19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell Environ. 2015, 38, 638–654. [Google Scholar] [CrossRef] [Green Version]
- Westfall, C.S.; Zubieta, C.; Herrmann, J.; Kapp, U.; Nanao, M.H.; Joseph, M.J. Structural basis for prereceptor modulation of plant hormones by GH3 proteins. Science 2012, 336, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, Y.; Zhang, X. Cytochemical localization of peroxidase in the interactions of Populus and Botryosphaeria dothidea. J. Beijing For. Univ. 2008, 6, 107–111. [Google Scholar]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Fan, K.; Wang, Y.; Tian, L.; Zhang, C.; Sun, W.; He, H.; Yu, S. OsGRETCHENHAGEN3-2 modulates rice seed storability via accumulation of abscisic acid and protective substances. Plant Physiol. 2021, 186, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.D.; Kang, B.C.; Jiang, H. A GH3-like gene, CcGH3, isolated from Capsicum chinense L. fruit is regulated by auxin and ethylene. Plant Mol. Biol. 2005, 58, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Riemann, M.; Riemann, M.; Takano, M. Rice Jasmonate Resistant 1 is involved in phytochrome and jasmonate signaling. Plant Cell Environ. 2008, 31, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.S.; Mishra, A.M.; Behari, S.; Husain, M.; Gupta, V.; Rastogi, M.; Gupta, R.K. The role of diffusion-weighted imaging in the differential diagnosis of intracranial cystic mass lesions: A report of 147 lesions. Surg. Neurol. 2006, 66, 246–250. [Google Scholar] [CrossRef]
- Wang, Z.; Kwok, K.; Lui, G.; Zhou, G.J.; Lee, J.S.; Lam, M.; Leung, K. The difference between temperate and tropical saltwater species' acute sensitivity to chemicals is relatively small. Chemosphere 2014, 105, 31–43. [Google Scholar] [CrossRef]
- Ling, H.; Fu, X.; Huang, N.; Zhong, Z.; Su, W.; Lin, W.; Cui, H.; Que, Y. A sugarcane smut fungus effector simulates the host endogenous elicitor peptide to suppress plant immunity. New. Phytol. 2022, 233, 919–933. [Google Scholar] [CrossRef]
- Su, Y.; Guo, J.; Ling, H.; Chen, S.; Wang, S.; Xu, L.; Allan, A.C.; Que, Y. Isolation of a novel peroxisomal catalase gene from sugarcane, which is responsive to biotic and abiotic stresses. PLoS ONE 2014, 9, e84426. [Google Scholar] [CrossRef]
- Liu, F.; Huang, N.; Wang, L.; Ling, H.; Sun, T.; Ahmad, W.; Muhammad, K.; Guo, J.; Xu, L.; Gao, S. A novel L-ascorbate peroxidase 6 gene, ScAPX6, plays an important role in the regulation of response to biotic and abiotic stresses in sugarcane. Front. Plant Sci. 2017, 8, 2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; Castro, E.D.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [Green Version]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talon, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Que, Y.; Su, Y.; Guo, J.; Wu, Q.; Xu, L. A global view of transcriptome dynamics during Sporisorium scitamineum challenge in sugarcane by RNA-Seq. PLoS ONE 2014, 9, e106476. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Pan, Y.-B.; Su, Y.; Zou, W.; Xu, F.; Sun, T.; Grisham, M.P.; Yang, S.; Xu, L.; Que, Y. WGCNA identifies a comprehensive and dynamic gene co-expression network that associates with sugarcane smut resistance. Int. J. Mol. Sci. 2022, 23, 10770. [Google Scholar] [CrossRef]
- Tang, H.; Yu, Q.; Li, Z.; Liu, F.; Su, W.; Zhang, C.; Ling, H.; Luo, J.; Su, Y.; Que, Y. A PIP-mediated osmotic stress signaling cascade plays a positive role in the salt tolerance of sugarcane. BMC Plant Biol. 2021, 21, 589. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Wu, Q.; Guo, J.; Xu, L.; Que, Y. Comprehensive selection of reference genes for gene expression normalization in sugarcane by real time quantitative RT-PCR. PLoS ONE 2014, 9, e97469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, W.; Ren, Y.; Wang, D.; Su, Y.; Feng, J.; Zhang, C.; Tang, H.; Xu, L.; Muhammad, K.; Que, Y. The alcohol dehydrogenase gene family in sugarcane and its involvement in cold stress regulation. BMC Genom. 2020, 21, 521. [Google Scholar] [CrossRef]
- Su, W.; Zhang, C.; Wang, D.; Ren, Y.; Zhang, J.; Zang, S.; Zou, W.; Su, Y.; You, C.; Xu, L.; et al. A comprehensive survey of the aldehyde dehydrogenase gene superfamily in Saccharum and the role of ScALDH2B-1 in the stress response. Environ. Exp. Bot. 2022, 194, 104725. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 -∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Choi, D.S.; Hwang, I.S.; Hwang, B.K. Requirement of the cytosolic interaction between Pathogenesis-Related Protein10 And Leucine-Rich Repeat Protein1 for cell death and defense signaling in pepper. Plant Cell 2012, 24, 1675–1690. [Google Scholar] [CrossRef] [Green Version]
- Sohn, S.I.; Kim, Y.H.; Kim, B.R.; Lee, S.Y.; Lim, C.K.; Hur, J.H.; Lee, J.Y. Transgenic tobacco expressing the hrpN(EP) gene from Erwinia pyrifoliae triggers defense responses against Botrytis cinerea. Mol. Cells 2007, 24, 232–239. [Google Scholar] [PubMed]
- Brogue, K.; Chet, I.; Holliday, M.; Cressman, R.; Biddle, P.; Knowlton, S.; Mauvais, C.J.; Broglie, R. Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 1991, 254, 1194–1197. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Zhuang, R.R.; Chen, Y.T.; Deng, Y.; Cai, T.C.; Wang, S.Y.; Liu, Q.Z.; Tang, R.T.; Shan, S.H.; et al. Overexpression of the peanut CLAVATA1-like leucine-rich repeat receptor-like kinase AhRLK1 confers increased resistance to bacterialwilt in tobacco. J. Exp. Bot. 2019, 70, 5407–5421. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Lin, P.; Zhao, Z.; Wang, D.; Qin, L.; Xu, F.; Su, Y.; Wu, Q.; Que, Y. Genome-Wide Identification of Auxin-Responsive GH3 Gene Family in Saccharum and the Expression of ScGH3-1 in Stress Response. Int. J. Mol. Sci. 2022, 23, 12750. https://doi.org/10.3390/ijms232112750
Zou W, Lin P, Zhao Z, Wang D, Qin L, Xu F, Su Y, Wu Q, Que Y. Genome-Wide Identification of Auxin-Responsive GH3 Gene Family in Saccharum and the Expression of ScGH3-1 in Stress Response. International Journal of Molecular Sciences. 2022; 23(21):12750. https://doi.org/10.3390/ijms232112750
Chicago/Turabian StyleZou, Wenhui, Peixia Lin, Zhennan Zhao, Dongjiao Wang, Liqian Qin, Fu Xu, Yachun Su, Qibin Wu, and Youxiong Que. 2022. "Genome-Wide Identification of Auxin-Responsive GH3 Gene Family in Saccharum and the Expression of ScGH3-1 in Stress Response" International Journal of Molecular Sciences 23, no. 21: 12750. https://doi.org/10.3390/ijms232112750
APA StyleZou, W., Lin, P., Zhao, Z., Wang, D., Qin, L., Xu, F., Su, Y., Wu, Q., & Que, Y. (2022). Genome-Wide Identification of Auxin-Responsive GH3 Gene Family in Saccharum and the Expression of ScGH3-1 in Stress Response. International Journal of Molecular Sciences, 23(21), 12750. https://doi.org/10.3390/ijms232112750






