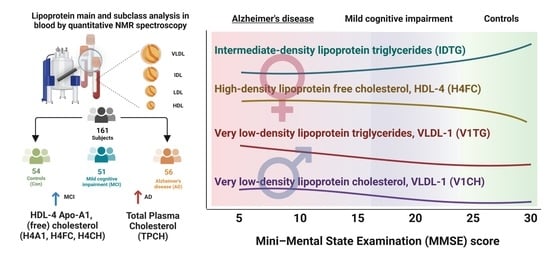
Quantitative NMR-Based Lipoprotein Analysis Identifies Elevated HDL-4 and Triglycerides in the Serum of Alzheimer’s Disease Patients
Abstract
1. Introduction
2. Results
2.1. Cohort Description
2.2. NMR-Based Lipoprotein Variables from Serum
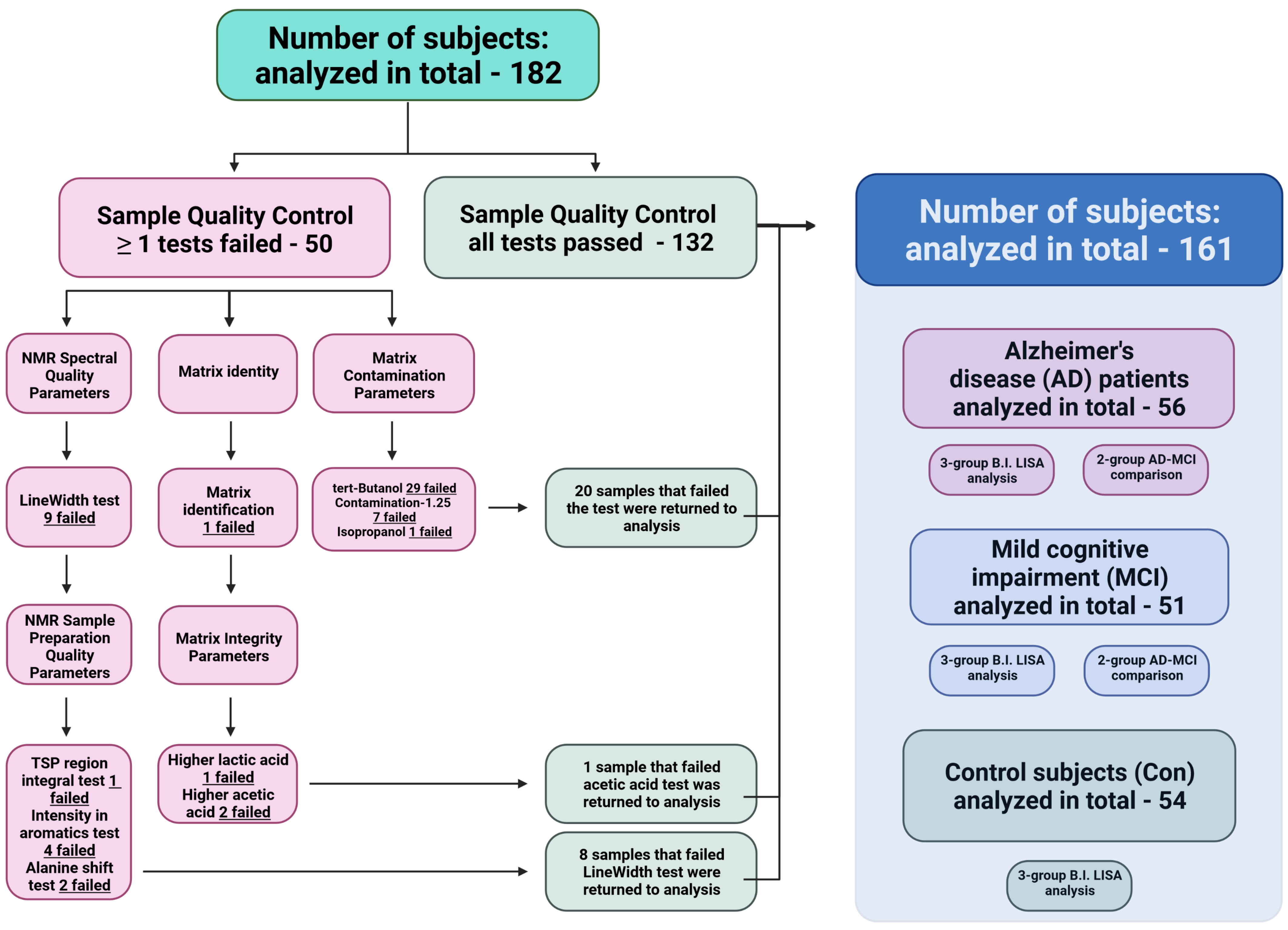
2.3. Qualitative Control (QC) of NMR Spectra Filters Out Nonsuited Datasets
2.4. Univariate Statistics Identified High HDL-4 Parameters and Elevated LDL-2 Cholesterol in the AD Group Only
2.5. Intermediate-Density Lipoprotein (IDL) Triglycerides and Very Low-Density Lipoprotein VLDL Lipoprotein Parameters Are Higher in Dementia Groups via Volcano Plot Analysis That Includes Mini-Mental State Examination MMSE Scores and Age
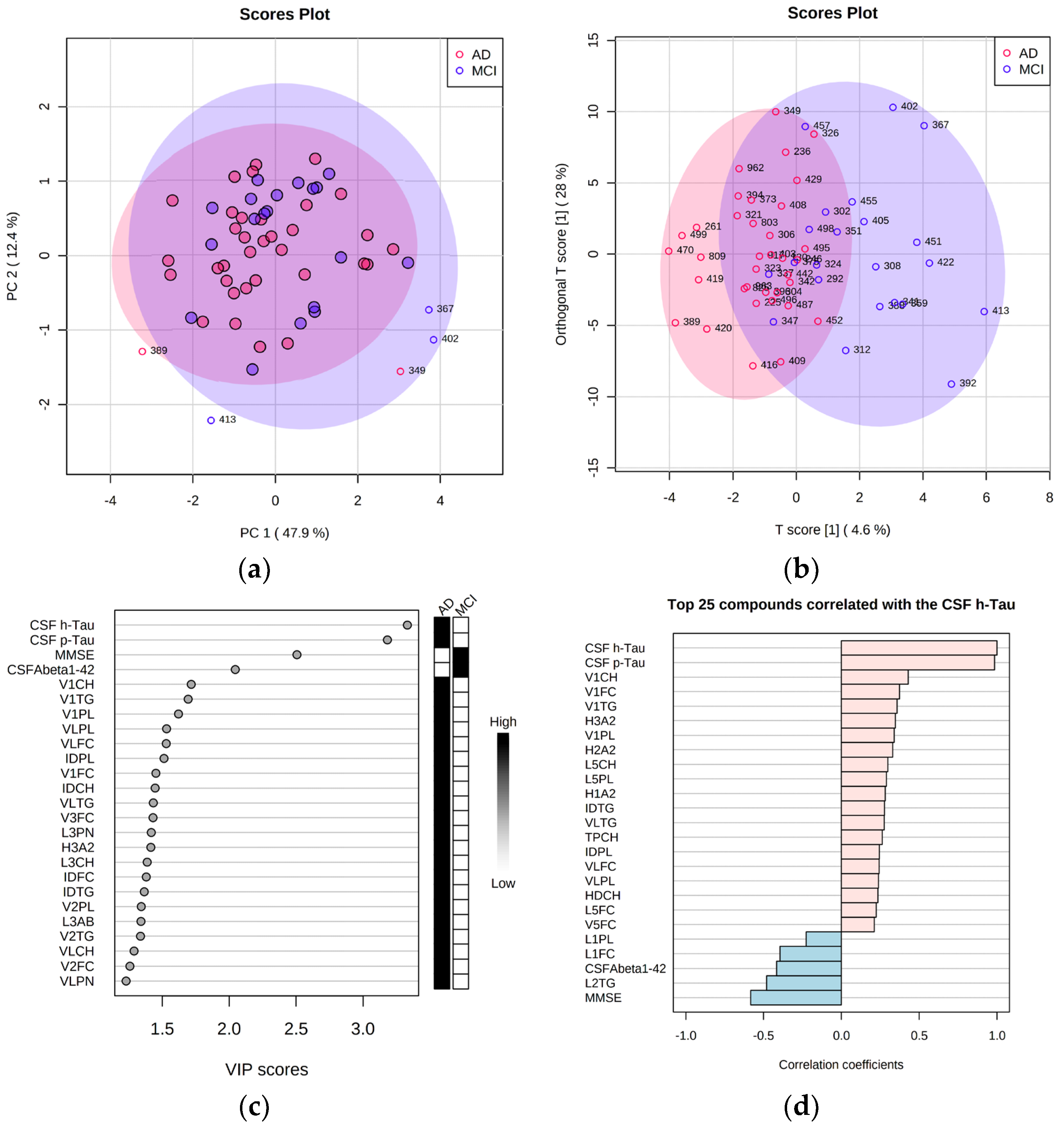
2.6. Adding Cerebrospinal Fluid AD Biomarkers to the Lipoprotein Panels Identifies Increased Triglycerides and VLDL Fraction Variables in AD Samples
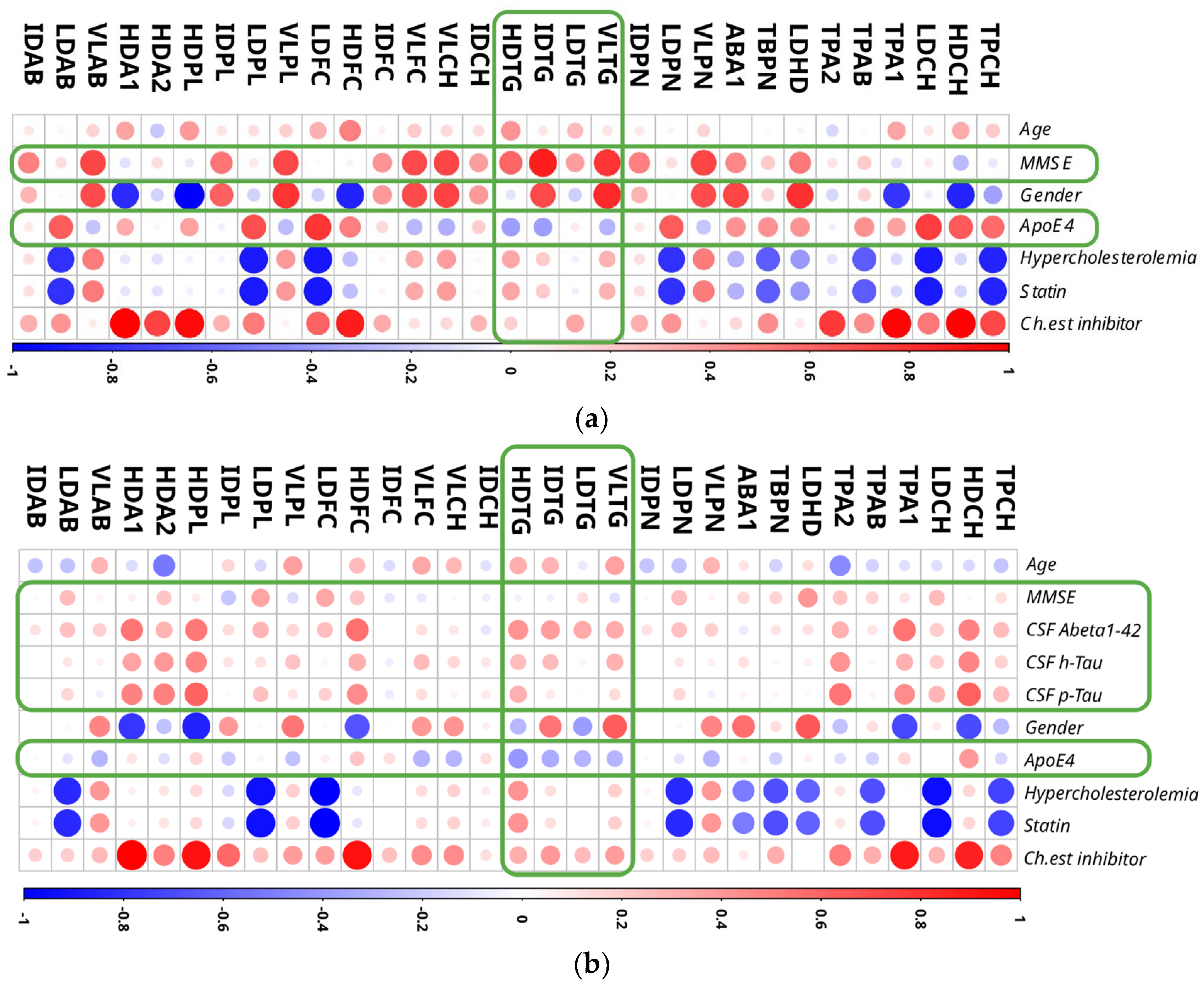
2.7. Correlation of NMR Lipoprotein Data with Clinical Metadata Identifies Triglycerides as Key Variables for the Correlation with Aβ Levels and ApoE4 Status
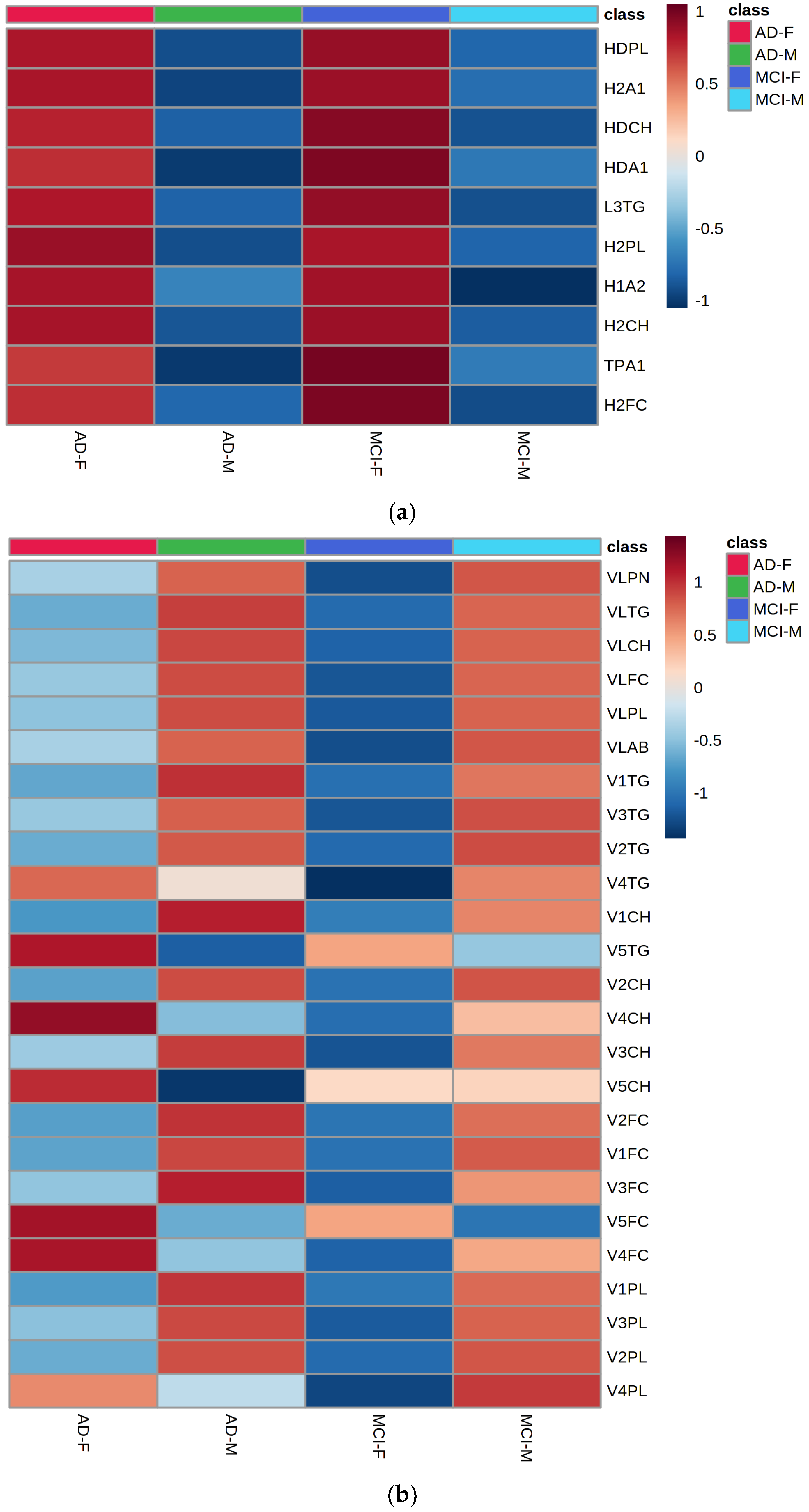
2.8. Gender-Specific Alterations of HDL and VLDL Lipoprotein Parameters Are Mostly Characteristic for AD Patients and Only to a Little Extend for MCI Subjects
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Sample Collection
4.3. NMR Sample Preparation and Experiments
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

| A Variable, AD | p Value, AD | p (FDR Adjusted), AD | VIP (oPLS-DA), AD |
|---|---|---|---|
| H2A1 | 5.87 × 10−6 | 4.88·× 10−4 | 2.114 |
| HDPL | 8.80·× 10−6 | 4.88·× 10−4 | 2.047 |
| L3TG | 1.44·× 10−5 | 4.88·× 10−4 | 2.062 |
| H2PL | 1.76·× 10−5 | 4.88·× 10−4 | 1.913 |
| HDA1 | 2.31·× 10−5 | 5.13·× 10−4 | 1.935 |
| H2CH | 6.55·× 10−5 | 0.0010 | 1.724 |
| HDCH | 1.01·× 10−4 | 0.0014 | 1.750 |
| HDFC | 1.80·× 10−4 | 0.0021 | 1.666 |
| H3CH | 1.97·× 10−4 | 0.0021 | 1.524 |
| H3PL | 2.05·× 10−4 | 0.0021 | 1.754 |
| H1A2 | 2.67·× 10−4 | 0.0025 | 1.823 |
| H1PL | 2.89·× 10−4 | 0.0025 | 1.662 |
| H3A1 | 3.83·× 10−4 | 0.0030 | 1.629 |
| H2FC | 5.14·× 10−4 | 0.0038 | 1.808 |
| H1A1 | 8.89·× 10−4 | 0.0062 | 1.490 |
| H1CH | 0.0010 | 0.0067 | 1.458 |
| H1TG | 0.0014 | 0.0084 | 1.578 |
| H2A2 | 0.0016 | 0.0091 | 1.662 |
| H1FC | 0.0025 | 0.0140 | 1.414 |
| H3FC | 0.0037 | 0.0196 | 1.524 |
| V1CH | 0.0047 | 0.0239 | 1.327 |
| V1PL | 0.0054 | 0.0263 | 1.411 |
| V2FC | 0.0068 | 0.0316 | 1.428 |
| V5TG | 0.0116 | 0.0495 | 1.206 |
| V1TG | 0.0116 | 0.0495 | 1.323 |
| LDHD | 0.0127 | 0.0523 | 1.157 |
| V1FC | 0.0156 | 0.0617 | 1.338 |
| V5CH | 0.0255 | 0.0976 | 1.193 |
| VLTG | 0.0264 | 0.0978 | 1.214 |
| V3FC | 0.0306 | 0.1096 | 1.066 |
| V2PL | 0.0341 | 0.1160 | 1.263 |
| H2TG | 0.0466 | 0.1477 | 1.040 |
| V2TG | 0.0517 | 0.1571 | 1.218 |
| V3PL | 0.0524 | 0.1571 | 1.042 |
| VLPL | 0.0655 | 0.1912 | 1.020 |
| V2CH | 0.0713 | 0.2030 | 1.021 |
| IDTG | 0.0755 | 0.2079 | 0.973 |
| HDTG | 0.0769 | 0.2079 | 1.003 |
| V3CH | 0.0787 | 0.2079 | 0.827 |
| VLCH | 0.0953 | 0.2301 | 0.831 |
| B Variable, Con | p Value, Con | p (FDR Adjusted), Con | VIP (oPLS-DA), Con |
| H1CH | 0.0181 | 0.3750 | 1.755 |
| L2PL | 0.0241 | 0.3750 | 1.601 |
| L2AB | 0.0278 | 0.3750 | 1.682 |
| L2PN | 0.0279 | 0.3750 | 1.682 |
| H2CH | 0.0306 | 0.3750 | 1.465 |
| L2CH | 0.0312 | 0.3750 | 1.530 |
| H1PL | 0.0317 | 0.3750 | 1.733 |
| L2FC | 0.0377 | 0.3750 | 1.532 |
| H1A1 | 0.0483 | 0.3750 | 1.710 |
| H1FC | 0.0508 | 0.3750 | 1.766 |
| H1A2 | 0.0636 | 0.3750 | 1.772 |
| C Variable, MCI | p Value, MCI | p (FDR Adjusted), MCI | VIP (oPLS-DA), MCI |
| H1A2 | 9.37·× 10−5 | 0.0073 | 1.671 |
| H1PL | 1.85·× 10−4 | 0.0073 | 1.548 |
| H1CH | 2.44·× 10−4 | 0.0073 | 1.509 |
| H1FC | 3.50·× 10−4 | 0.0073 | 1.607 |
| H2FC | 3.82·× 10−4 | 0.0073 | 1.675 |
| HDCH | 3.96·× 10−4 | 0.0073 | 1.563 |
| L1FC | 5.99·× 10−4 | 0.0086 | 1.627 |
| H1A1 | 6.19·× 10−4 | 0.0086 | 1.408 |
| HDPL | 8.08·× 10−4 | 0.0098 | 1.541 |
| L2FC | 0.0010 | 0.0098 | 1.430 |
| H2A2 | 0.0011 | 0.0098 | 1.632 |
| H2CH | 0.0012 | 0.0098 | 1.551 |
| L2PL | 0.0012 | 0.0098 | 1.393 |
| H2A1 | 0.0014 | 0.0098 | 1.524 |
| L1AB | 0.0014 | 0.0098 | 1.505 |
| L1PN | 0.0014 | 0.0098 | 1.504 |
| H2PL | 0.0016 | 0.0098 | 1.518 |
| L3TG | 0.0016 | 0.0098 | 1.538 |
| HDFC | 0.0021 | 0.0111 | 1.486 |
| L1PL | 0.0021 | 0.0111 | 1.496 |
| L2AB | 0.0025 | 0.0116 | 1.348 |
| L2PN | 0.0025 | 0.0116 | 1.347 |
| L1CH | 0.0027 | 0.0120 | 1.460 |
| L2CH | 0.0031 | 0.0132 | 1.270 |
| L3FC | 0.0041 | 0.0167 | 1.490 |
| H3FC | 0.0061 | 0.0235 | 1.392 |
| LDFC | 0.0088 | 0.0326 | 1.380 |
| L3PL | 0.0091 | 0.0326 | 1.283 |
| L3PN | 0.0109 | 0.0358 | 1.224 |
| L3AB | 0.0110 | 0.0358 | 1.224 |
| V3PL | 0.0123 | 0.0389 | 0.967 |
| L2TG | 0.0128 | 0.0394 | 1.378 |
| H4TG | 0.0135 | 0.0405 | 0.906 |
| V2FC | 0.0139 | 0.0405 | 1.069 |
| V3CH | 0.0158 | 0.0437 | 0.815 |
| V3FC | 0.0161 | 0.0437 | 0.906 |
| L3CH | 0.0178 | 0.0463 | 1.137 |
| V2PL | 0.0179 | 0.0463 | 1.028 |
| V1PL | 0.0210 | 0.0518 | 1.099 |
| H1TG | 0.0219 | 0.0529 | 0.846 |
| VLPL | 0.0284 | 0.0636 | 0.981 |
| VLAB | 0.0286 | 0.0636 | 0.896 |
| VLPN | 0.0287 | 0.0636 | 0.896 |
| V3TG | 0.0429 | 0.0934 | 0.815 |
| V1FC | 0.0447 | 0.0955 | 1.013 |
| V2TG | 0.0524 | 0.1058 | 0.908 |
| VLTG | 0.0567 | 0.1118 | 0.886 |
| V1CH | 0.0584 | 0.1118 | 0.982 |
| VLCH | 0.0606 | 0.1139 | 0.831 |
| VLFC | 0.0687 | 0.1270 | 0.824 |
| V2CH | 0.0872 | 0.1586 | 0.784 |
| Variable, Female | p Value, Female | p (FDR Adjusted), Female | VIP (PLS-DA), Female |
|---|---|---|---|
| H3FC | 0.0027 | 0.1947 | 0.227 |
| H2FC | 0.0035 | 0.1947 | 0.075 |
| HDA1 | 0.0093 | 0.2594 | 0.074 |
| TPA1 | 0.0110 | 0.2594 | 0.001 |
| HDPL | 0.0139 | 0.2594 | 0.278 |
| HDCH | 0.0145 | 0.2594 | 0.188 |
| H4FC | 0.0164 | 0.2594 | 0.112 |
| H2A1 | 0.0212 | 0.2715 | 0.331 |
| V1FC | 0.0228 | 0.2715 | 0.486 |
| H3CH | 0.0245 | 0.2715 | 0.097 |
| L1FC | 0.0275 | 0.2770 | 0.817 |
| V1PL | 0.0309 | 0.2855 | 1.385 |
| V1TG | 0.0365 | 0.2932 | 0.012 |
| TPA2 | 0.0370 | 0.2932 | 0.098 |
| H2A2 | 0.0452 | 0.3307 | 0.174 |
| HDA2 | 0.0477 | 0.3307 | 0.093 |
| H3PL | 0.0507 | 0.3307 | 0.192 |
| H1A2 | 0.0550 | 0.3390 | 0.449 |
| H4CH | 0.0677 | 0.3666 | 0.089 |
| H3A1 | 0.0709 | 0.3666 | 0.046 |
| V2FC | 0.0729 | 0.3666 | 3.183 |
| HDFC | 0.0746 | 0.3666 | 0.071 |
| L3FC | 0.0760 | 0.3666 | 1.114 |
| VLTG | 0.0844 | 0.3770 | 0.414 |
| V1CH | 0.0895 | 0.3770 | 1.113 |
| H1FC | 0.0906 | 0.3770 | 0.119 |
| V2TG | 0.0917 | 0.3770 | 0.235 |
| V2PL | 0.0956 | 0.3790 | 0.932 |
| Variable, Male | p Value, Male | p (FDR Adjusted), Male | VIP (PLS-DA), Male |
| L2CH | 0.0046 | 0.2436 | 2.759 |
| L2AB | 0.0066 | 0.2436 | 2.274 |
| L2PN | 0.0066 | 0.2436 | 2.275 |
| L2PL | 0.0119 | 0.3304 | 2.151 |
| L3PN | 0.0205 | 0.3664 | 2.848 |
| L3CH | 0.0240 | 0.3664 | 3.185 |
| L2FC | 0.0257 | 0.3664 | 2.255 |
| L3AB | 0.0264 | 0.3664 | 2.772 |
| L3PL | 0.0384 | 0.4734 | 2.410 |
| V5CH | 0.0539 | 0.5987 | 2.935 |
| L3FC | 0.0646 | 0.6520 | 1.921 |
| V5TG | 0.0748 | 0.6922 | 1.189 |
| H4TG | 0.0915 | 0.7814 | 1.216 |
References
- Apostolova, L.G. Alzheimer Disease. Contin. Lifelong Learn. Neurol. 2016, 22, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. J Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J. Lipid metabolism in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.; Zahiri, H.R.; Ceimo, J.; Cooper, K.; Gaul, W.; Connor, D.; Sparks, D.L. Is there a characteristic lipid profile in Alzheimer’s disease? J. Alzheimer’s Dis. 2004, 6, 585–589. [Google Scholar] [CrossRef]
- Leszek, J.; Mikhaylenko, V.E.; Belousov, M.D.; Koutsouraki, E.; Szczechowiak, K.; Kobusiak-Prokopowicz, M.; Mysiak, A.; Diniz, S.B.; Somasundaram, G.S.; Kirkland, E.C.; et al. The Links between Cardiovascular Diseases and Alzheimer’s Disease. Curr. Neuropharmacol. 2021, 19, 152–169. [Google Scholar] [CrossRef]
- Ma, C.; Li, J.; Bao, Z.; Ruan, Q.; Yu, Z. Serum Levels of ApoA1 and ApoA2 Are Associated with Cognitive Status in Older Men. BioMed Res. Int. 2015, 2015, 481621. [Google Scholar] [CrossRef]
- Rao, W.; Zhang, Y.; Li, K.; Zhang, X.Y. Association between cognitive impairment and apolipoprotein A1 or apolipoprotein B levels is regulated by apolipoprotein E variant rs429358 in patients with chronic schizophrenia. Aging 2021, 13, 16353–16366. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, X.; Shen, J.; Jiao, L.; Tong, J.; Zhao, W.; Du, K.; Gong, S.; Liu, M.; Wei, M. Elevated serum TC and LDL-C levels in Alzheimer’s disease and mild cognitive impairment: A meta-analysis study. Brain Res. 2020, 1727, 146554. [Google Scholar] [CrossRef]
- Mattsson, N.; Zetterberg, H.; Hansson, O.; Andreasen, N.; Parnetti, L.; Jonsson, M.; Herukka, S.-K.; van der Flier, W.M.; Blankenstein, M.A.; Ewers, M.; et al. CSF Biomarkers and Incipient Alzheimer Disease in Patients With Mild Cognitive Impairment. JAMA 2009, 302, 385–393. [Google Scholar] [CrossRef]
- Wang, R.; Chen, Z.; Fu, Y.; Wei, X.; Liao, J.; Liu, X.; He, B.; Xu, Y.; Zou, J.; Yang, X.; et al. Plasma Cystatin C and High-Density Lipoprotein Are Important Biomarkers of Alzheimer’s Disease and Vascular Dementia: A Cross-Sectional Study. Front. Aging Neurosci. 2017, 9, 26. [Google Scholar] [CrossRef]
- Kjeldsen, E.W.; Thomassen, J.Q.; Juul Rasmussen, I.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Plasma high-density lipoprotein cholesterol and risk of dementia: Observational and genetic studies. Cardiovasc. Res. 2021, 118, 1330–1343. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Z.; Jia, X.; Zhang, H.; Zhang, H.; Li, J.; Zhang, K. Prediction of Alzheimer’s disease with serum lipid levels in Asian individuals: A meta-analysis. Biomarkers 2019, 24, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wang, F.; Yang, J.; Peng, H.; Li, Y.; Li, B.; Wang, S. Revealing a Novel Landscape of the Association Between Blood Lipid Levels and Alzheimer’s Disease: A Meta-Analysis of a Case-Control Study. Front. Aging Neurosci. 2020, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yang, J.S.; Lee, J.C.; Lee, J.-Y.; Lee, J.-Y.; Kim, E.; Moon, M.H. Lipidomic alterations in lipoproteins of patients with mild cognitive impairment and Alzheimer’s disease by asymmetrical flow field-flow fractionation and nanoflow ultrahigh performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2018, 1568, 91–100. [Google Scholar] [CrossRef]
- Tokuoka, S.M.; Kita, Y.; Shimizu, T.; Oda, Y. Isobaric mass tagging and triple quadrupole mass spectrometry to determine lipid biomarker candidates for Alzheimer’s disease. PLoS ONE 2019, 14, e0226073. [Google Scholar] [CrossRef]
- Oeckl, P.; Otto, M. A Review on MS-Based Blood Biomarkers for Alzheimer’s Disease. Neurol. Ther. 2019, 8, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, J.; Demirkan, A.; Würtz, P.; Draisma, H.H.M.; Haller, T.; Rawal, R.; Vaarhorst, A.; Kangas, A.J.; Lyytikäinen, L.-P.; Pirinen, M.; et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun 2016, 7, 11122. [Google Scholar] [CrossRef] [PubMed]
- Tukiainen, T.; Tynkkynen, T.; Mäkinen, V.-P.; Jylänki, P.; Kangas, A.; Hokkanen, J.; Vehtari, A.; Gröhn, O.; Hallikainen, M.; Soininen, H.; et al. A multi-metabolite analysis of serum by 1H NMR spectroscopy: Early systemic signs of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2008, 375, 356–361. [Google Scholar] [CrossRef]
- Zhang, A.-h.; Sun, H.; Qiu, S.; Wang, X.-j. NMR-based metabolomics coupled with pattern recognition methods in biomarker discovery and disease diagnosis. Magn. Reson. Chem 2013, 51, 549–556. [Google Scholar] [CrossRef]
- Ohtani, R.; Nirengi, S.; Nakamura, M.; Murase, N.; Sainouchi, M.; Kuwata, Y.; Takata, M.; Masuda, Y.; Kotani, K.; Sakane, N. High-Density Lipoprotein Subclasses and Mild Cognitive Impairment: Study of Outcome and aPolipoproteins in Dementia (STOP-Dementia). J. Alzheimer’s Dis. 2018, 66, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Tynkkynen, J.; Chouraki, V.; van der Lee, S.J.; Hernesniemi, J.; Yang, Q.; Li, S.; Beiser, A.; Larson, M.G.; Sääksjärvi, K.; Shipley, M.J.; et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: A prospective study in eight cohorts. Alzheimer’s Dement. 2018, 14, 723–733. [Google Scholar] [CrossRef]
- Teahan, O.; Gamble, S.; Holmes, E.; Waxman, J.; Nicholson, J.K.; Bevan, C.; Keun, H.C. Impact of Analytical Bias in Metabonomic Studies of Human Blood Serum and Plasma. Anal. Chem. 2006, 78, 4307–4318. [Google Scholar] [CrossRef] [PubMed]
- Lodge, S.; Nitschke, P.; Kimhofer, T.; Coudert, J.D.; Begum, S.; Bong, S.-H.; Richards, T.; Edgar, D.; Raby, E.; Spraul, M.; et al. NMR Spectroscopic Windows on the Systemic Effects of SARS-CoV-2 Infection on Plasma Lipoproteins and Metabolites in Relation to Circulating Cytokines. J. Proteome Res. 2021, 20, 1382–1396. [Google Scholar] [CrossRef]
- Burgess, B.L.; McIsaac, S.A.; Naus, K.E.; Chan, J.Y.; Tansley, G.H.K.; Yang, J.; Miao, F.; Ross, C.J.D.; van Eck, M.; Hayden, M.R.; et al. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer’s disease mouse models with abundant Aβ in plasma. Neurobiol. Dis. 2006, 24, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernández-Mijares, A.; González-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar]
- Deng, X.; Saffari, S.E.; Ng, S.Y.E.; Chia, N.; Tan, J.Y.; Choi, X.; Heng, D.L.; Xu, Z.; Tay, K.-Y.; Au, W.-L.; et al. Blood Lipid Biomarkers in Early Parkinson’s Disease and Parkinson’s Disease with Mild Cognitive Impairment. J. Parkinson’s Dis. 2022. preprint. [Google Scholar] [CrossRef]
- Wang, H.; Eckel, R.H. What are lipoproteins doing in the brain? Trends Endocrinol. Metab. 2014, 25, 8–14. [Google Scholar] [CrossRef]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef]
- Balazs, Z.; Panzenboeck, U.; Hammer, A.; Sovic, A.; Quehenberger, O.; Malle, E.; Sattler, W. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated α-tocopherol by an in vitro blood–brain barrier model. J. Neurochem. 2004, 89, 939–950. [Google Scholar] [CrossRef]
- Button, E.B.; Robert, J.; Caffrey, T.M.; Fan, J.; Zhao, W.; Wellington, C.L. HDL from an Alzheimer’s disease perspective. Curr Opin Lipidol. 2019, 30, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Proitsi, P.; Lupton, M.K.; Velayudhan, L.; Newhouse, S.; Fogh, I.; Tsolaki, M.; Daniilidou, M.; Pritchard, M.; Kloszewska, I.; Soininen, H.; et al. Genetic Predisposition to Increased Blood Cholesterol and Triglyceride Lipid Levels and Risk of Alzheimer Disease: A Mendelian Randomization Analysis. PLOS Med. 2014, 11, e1001713. [Google Scholar] [CrossRef]
- Zhou, Z.; Liang, Y.; Zhang, X.; Xu, J.; Lin, J.; Zhang, R.; Kang, K.; Liu, C.; Zhao, C.; Zhao, M. Low-Density Lipoprotein Cholesterol and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2020, 12, 5. [Google Scholar] [CrossRef]
- Nishikawa, O.; Mune, M.; Miyano, M.; Nishide, T.; Nishide, I.; Maeda, A.; Kimura, K.; Takahashi, T.; Kishino, M.; Tone, Y.; et al. Effect of simvastatin on the lipid profile of hemodialysis patients. Kidney Int. 1999, 56, S219–S221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quispe, R.; Martin, S.S.; Jones, S.R. Triglycerides to high-density lipoprotein–cholesterol ratio, glycemic control and cardiovascular risk in obese patients with type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Accardi, G.; Caruso, C.; Colonna-Romano, G.; Camarda, C.; Monastero, R.; Candore, G. Can Alzheimer disease be a form of type 3 diabetes? J. Rejuvenation Res. 2012, 15, 217–221. [Google Scholar] [CrossRef]
- Morrill, S.J.; Gibas, K.J. Ketogenic diet rescues cognition in ApoE4+ patient with mild Alzheimer’s disease: A case study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1187–1191. [Google Scholar] [CrossRef]
- Yip, C.M.; Elton, E.A.; Darabie, A.A.; Morrison, M.R.; McLaurin, J. Cholesterol, a modulator of membrane-associated aβ-fibrillogenesis and neurotoxicity1 1Edited by A. Klug. J. Mol. Biol. 2001, 311, 723–734. [Google Scholar] [CrossRef]
- Kloske, C.M.; Wilcock, D.M. The Important Interface Between Apolipoprotein E and Neuroinflammation in Alzheimer’s Disease. Front. Immunol. 2020, 11, 754. [Google Scholar] [CrossRef]
- Mahley, R.W. Central Nervous System Lipoproteins. Arter. Thromb Vasc Biol 2016, 36, 1305–1315. [Google Scholar] [CrossRef]
- Ramaswamy, G.; Xu, Q.; Huang, Y.; Weisgraber, K.H. Effect of Domain Interaction on Apolipoprotein E Levels in Mouse Brain. J. Neurosci. 2005, 25, 10658–10663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beydoun, M.A.; Beason-Held, L.L.; Kitner-Triolo, M.H.; Beydoun, H.A.; Ferrucci, L.; Resnick, S.M.; Zonderman, A.B. Statins and serum cholesterol’s associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health 2011, 65, 949–957. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, R.J.; Reus, L.M.; De Witte, W.; Tijms, B.M.; Olde Rikkert, M.; Visser, P.J.; Poelmans, G. Genetic overlap between Alzheimer’s disease and blood lipid levels. Neurobiol. Aging 2021, 108, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B.; Duvillard, L.; Pais de Barros, J.P.; Bouillet, B.; Baillot-Rudoni, S.; Rouland, A.; Petit, J.M.; Degrace, P.; Demizieux, L. Liraglutide Increases the Catabolism of Apolipoprotein B100–Containing Lipoproteins in Patients With Type 2 Diabetes and Reduces Proprotein Convertase Subtilisin/Kexin Type 9 Expression. Diabetes Care 2021, 44, 1027–1037. [Google Scholar] [CrossRef]
- Gerl, M.J.; Vaz, W.L.C.; Domingues, N.; Klose, C.; Surma, M.A.; Sampaio, J.L.; Almeida, M.S.; Rodrigues, G.; Araújo-Gonçalves, P.; Ferreira, J.; et al. Cholesterol is Inefficiently Converted to Cholesteryl Esters in the Blood of Cardiovascular Disease Patients. Sci Rep 2018, 8, 14764. [Google Scholar] [CrossRef]
- Hall, K.; Murrell, J.; Ogunniyi, A.; Deeg, M.; Baiyewu, O.; Gao, S.; Gureje, O.; Dickens, J.; Evans, R.; Smith-Gamble, V.; et al. Cholesterol, APOE genotype, and Alzheimer disease. Epidemiol. Study Niger. Yoruba 2006, 66, 223–227. [Google Scholar] [CrossRef]
- Leduc, V.; Bourque, L.; Poirier, J.; Dufour, R. Role of rs3846662 and HMGCR alternative splicing in statin efficacy and baseline lipid levels in familial hypercholesterolemia. Pharm. Genom. 2016, 26, 1–11. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, B.; Yang, Y.; Wang, P.; Yang, Y.; Wang, S. Poorly controlled cholesterol is associated with cognitive impairment in T2DM: A resting-state fMRI study. Lipids Health Dis. 2015, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Matsunaga, A.; Saku, K.; Nakano, S.; Yamada, T. Associations among plasma lipoprotein subfractions as characterized by analytical capillary isotachophoresis, apolipoprotein E phenotype, Alzheimer disease, and mild cognitive impairment. Arter. Thromb. Vasc. Biol. 2004, 24, e144. [Google Scholar] [CrossRef]
- Schilling, S.; Tzourio, C.; Soumaré, A.; Kaffashian, S.; Dartigues, J.-F.; Ancelin, M.-L.; Samieri, C.; Dufouil, C.; Debette, S. Differential associations of plasma lipids with incident dementia and dementia subtypes in the 3C Study: A longitudinal, population-based prospective cohort study. PLOS Med. 2017, 14, e1002265. [Google Scholar] [CrossRef]
- Giacobini, E.; Pepeu, G. Sex and gender differences in the brain cholinergic system and in the response to therapy of Alzheimer disease with cholinesterase inhibitors. J. Curr. Alzheimer Res. 2018, 15, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Montagne, A.; Zhao, Z. Alzheimer’s pathogenic mechanisms and underlying sex difference. Cell. Mol. Life Sci. 2021, 78, 4907–4920. [Google Scholar] [CrossRef] [PubMed]
- Daneshpour, M.S.; Hedayati, M.; Eshraghi, P.; Azizi, F. Association of Apo E gene polymorphism with HDL level in Tehranian population. Eur. J. Lipid Sci. Technol. 2010, 112, 810–816. [Google Scholar] [CrossRef]
- Ancelin, M.-L.; Ripoche, E.; Dupuy, A.-M.; Barberger-Gateau, P.; Auriacombe, S.; Rouaud, O.; Berr, C.; Carrière, I.; Ritchie, K. Sex Differences in the Associations Between Lipid Levels and Incident Dementia. J. Alzheimer’s Dis. 2013, 34, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J.; Volek, J.S.; Liu, Y.; Shachter, N.S.; Contois, J.H.; Fernandez, M.L. Carbohydrate Restriction Alters Lipoprotein Metabolism by Modifying VLDL, LDL, and HDL Subfraction Distribution and Size in Overweight Men. J. Nutr. 2006, 136, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.A.; Doraiswamy, P.M. When Mars Versus Venus is Not a Cliché: Gender Differences in the Neurobiology of Alzheimer’s Disease. Front. Neurol. 2015, 5, 288. [Google Scholar] [CrossRef]
- Campbell, S.C.; Moffatt, R.J.; Stamford, B.A. Smoking and smoking cessation—The relationship between cardiovascular disease and lipoprotein metabolism: A review. Atherosclerosis 2008, 201, 225–235. [Google Scholar] [CrossRef]
- Ghini, V.; Meoni, G.; Pelagatti, L.; Celli, T.; Veneziani, F.; Petrucci, F.; Vannucchi, V.; Bertini, L.; Luchinat, C.; Landini, G.; et al. Profiling metabolites and lipoproteins in COMETA, an Italian cohort of COVID-19 patients. PLoS Pathog 2022, 18, e1010443. [Google Scholar] [CrossRef]
- Rezaei, A.; Neshat, S.; Heshmat-Ghahdarijani, K.J.C.P.i.C. Alterations of lipid profile in COVID-19: A narrative review. 2021, 100907. Current Probl. Cardiol. 2021, 47, 100907. [Google Scholar] [CrossRef]
- Sacks, F.M.; Furtado, J.D.; Jensen, M.K. Protein-based HDL subspecies: Rationale and association with cardiovascular disease, diabetes, stroke, and dementia. Biochim. Et Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159182. [Google Scholar] [CrossRef]
- Dona, A.C.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision High-Throughput Proton NMR Spectroscopy of Human Urine, Serum, and Plasma for Large-Scale Metabolic Phenotyping. Anal Chem 2014, 86, 9887–9894. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, F.; Jensen, L.; Hatch, F. The isolation and quantitative analysis of serum lipoproteins. In Blood Lipids and Lipoproteins: Quantitation, Composition and Metabolism; Nelson, G.J., Ed.; Wiley-Interscience: New York, NY, USA, 1972; pp. 181–274. [Google Scholar]
- Lindgren, F. Preparative ultracentrifugal laboratory procedures and suggestions for lipoprotein analysis. In Analysis of Lipids and Lipoproteins; American Oil Chemists’ Society: Champaign, IL, USA, 1975; pp. 204–224. [Google Scholar]
- Anderson, D.W.; Nichols, A.V.; Forte, T.M.; Lindgren, F.T. Particle distribution of human serum high density lipoproteins. Biochim. Et Biophys. Acta Protein Struct. 1977, 493, 55–68. [Google Scholar] [CrossRef]
- Baumstark, M.W.; Kreutz, W.; Berg, A.; Frey, I.; Keul, J. Structure of human low-density lipoprotein subfractions determined by X-ray small-angle scattering. Biochim. Et Biophys. Acta Protein Struct. Mol. Enzymol. 1990, 1037, 48–57. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; Fayyad, R.; Boekholdt, S.M.; Hovingh, G.K.; Kastelein, J.J.; Melamed, S.; Barter, P.; Waters, D.D.; Ray, K.K. Triglyceride-Rich Lipoprotein Cholesterol and Risk of Cardiovascular Events Among Patients Receiving Statin Therapy in the TNT Trial. Circulation 2018, 138, 770–781. [Google Scholar] [CrossRef]
- Parks, J.S.; Hauser, H. Low density lipoprotein particle size and core cholesteryl ester physical state affect the proton NMR magnetic environment of fatty acid methylene and methyl nuclei. J. Lipid Res. 1996, 37, 1289–1297. [Google Scholar] [CrossRef]
- Knopp, R.H.; Paramsothy, P.; Retzlaff, B.M.; Fish, B.; Walden, C.; Dowdy, A.; Tsunehara, C.; Aikawa, K.; Cheung, M.C. Sex differences in lipoprotein metabolism and dietary response: Basis in hormonal differences and implications for cardiovascular disease. Curr. Cardiol. Rep. 2006, 8, 452–459. [Google Scholar] [CrossRef]
- Yaffe, K.; Barrett-Connor, E.; Lin, F.; Grady, D. Serum Lipoprotein Levels, Statin Use, and Cognitive Function in Older Women. Arch. Neurol. 2002, 59, 378–384. [Google Scholar] [CrossRef]
- Al-Muhtaseb, N.; Al-Yousuf, A.R.; Bajaj, J.S. Plasma lipoproteins and apolipoproteins in insulin-dependent and young non-insulin-dependent Arab women. Acta Diabetol. 1991, 28, 61–69. [Google Scholar] [CrossRef]
- Agardh, C.D.; Björgell, P.; Nilsson-Ehle, P. The effects of tolbutamide on lipoproteins, lipoprotein lipase and hormone-sensitive lipase. Diabetes Res. Clin. Pract. 1999, 46, 99–108. [Google Scholar] [CrossRef]


| Total | Controls (Con) | Mild Cognitive Impairment (MCI) | Alzheimer’s Disease (AD) | |
|---|---|---|---|---|
| patients | 161 | 54 | 51 | 56 |
| female | 71 | 29 | 17 | 25 |
| male | 90 | 25 | 34 | 31 |
| age (mean ± SD) | 70.2 ± 7.4 | 70.4 ± 4.5 | 69.8 ± 8.6 | 70.5 ± 8.5 |
| MMSE (mean ± SD) | 25.3 ± 4.8 | 29.2 ± 0.8 | 26.5 ± 1.5 *** | 20.5 ± 5.0 *** |
| CSF biomarkers J123Marija (Aβ, h-tau, p-tau) measured | ||||
| patients | 58 | 22 | 36 | |
| female | 24 | 7 | 17 | |
| male | 34 | 15 | 19 | |
| age (mean ± SD) | 68.7 ± 8.5 | 68.2 ± 9.3 | 69.0 ± 8.0 | |
| MMSE (mean ± SD) | 23.4 ± 4.3 | 26.5 ± 1.8 | 21.5 ± 4.3 *** | |
| CSF biomarker Aβ (pg/mL, mean ± SD) | 619 ± 287 | 751 ± 378 | 538 ± 177 ** | |
| CSF biomarker h-tau (pg/mL, mean ± SD) | 681 ± 356 | 503 ± 260 | 790 ± 366 *** | |
| CSF biomarker p-tau (pg/mL, mean ± SD) | 86 ± 33 | 69 ± 30 | 96 ± 31 *** | |
| ApoE4 alleles | ||||
| measured (mean ± SD) | 0.49 ± 0.62 | 0.40 ± 0.57 | 0.39 ± 0.57 | 0.70 ± 0.68 ** |
| patients | 152 | 53 | 49 | 50 |
| female | 68 | 29 | 16 | 23 |
| male | 84 | 24 | 33 | 27 |
| age (mean ± SD) | 70.2 ± 7.3 | 70.3 ± 4.5 | 69.9 ± 8.7 | 70.5 ± 8.3 |
| MMSE (mean ± SD) | 25.6 ± 4.4 | 29.2 ± 0.8 | 26.6 ± 1.5 *** | 21.0 ± 4.4 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezhnoy, G.; Laske, C.; Trautwein, C. Quantitative NMR-Based Lipoprotein Analysis Identifies Elevated HDL-4 and Triglycerides in the Serum of Alzheimer’s Disease Patients. Int. J. Mol. Sci. 2022, 23, 12472. https://doi.org/10.3390/ijms232012472
Berezhnoy G, Laske C, Trautwein C. Quantitative NMR-Based Lipoprotein Analysis Identifies Elevated HDL-4 and Triglycerides in the Serum of Alzheimer’s Disease Patients. International Journal of Molecular Sciences. 2022; 23(20):12472. https://doi.org/10.3390/ijms232012472
Chicago/Turabian StyleBerezhnoy, Georgy, Christoph Laske, and Christoph Trautwein. 2022. "Quantitative NMR-Based Lipoprotein Analysis Identifies Elevated HDL-4 and Triglycerides in the Serum of Alzheimer’s Disease Patients" International Journal of Molecular Sciences 23, no. 20: 12472. https://doi.org/10.3390/ijms232012472
APA StyleBerezhnoy, G., Laske, C., & Trautwein, C. (2022). Quantitative NMR-Based Lipoprotein Analysis Identifies Elevated HDL-4 and Triglycerides in the Serum of Alzheimer’s Disease Patients. International Journal of Molecular Sciences, 23(20), 12472. https://doi.org/10.3390/ijms232012472