Changes in α-Farnesene and Phenolic Metabolism and the Expression of Associated Genes during the Development of Superficial Scald in Two Distinct Pear Cultivars
Abstract
1. Introduction
2. Results
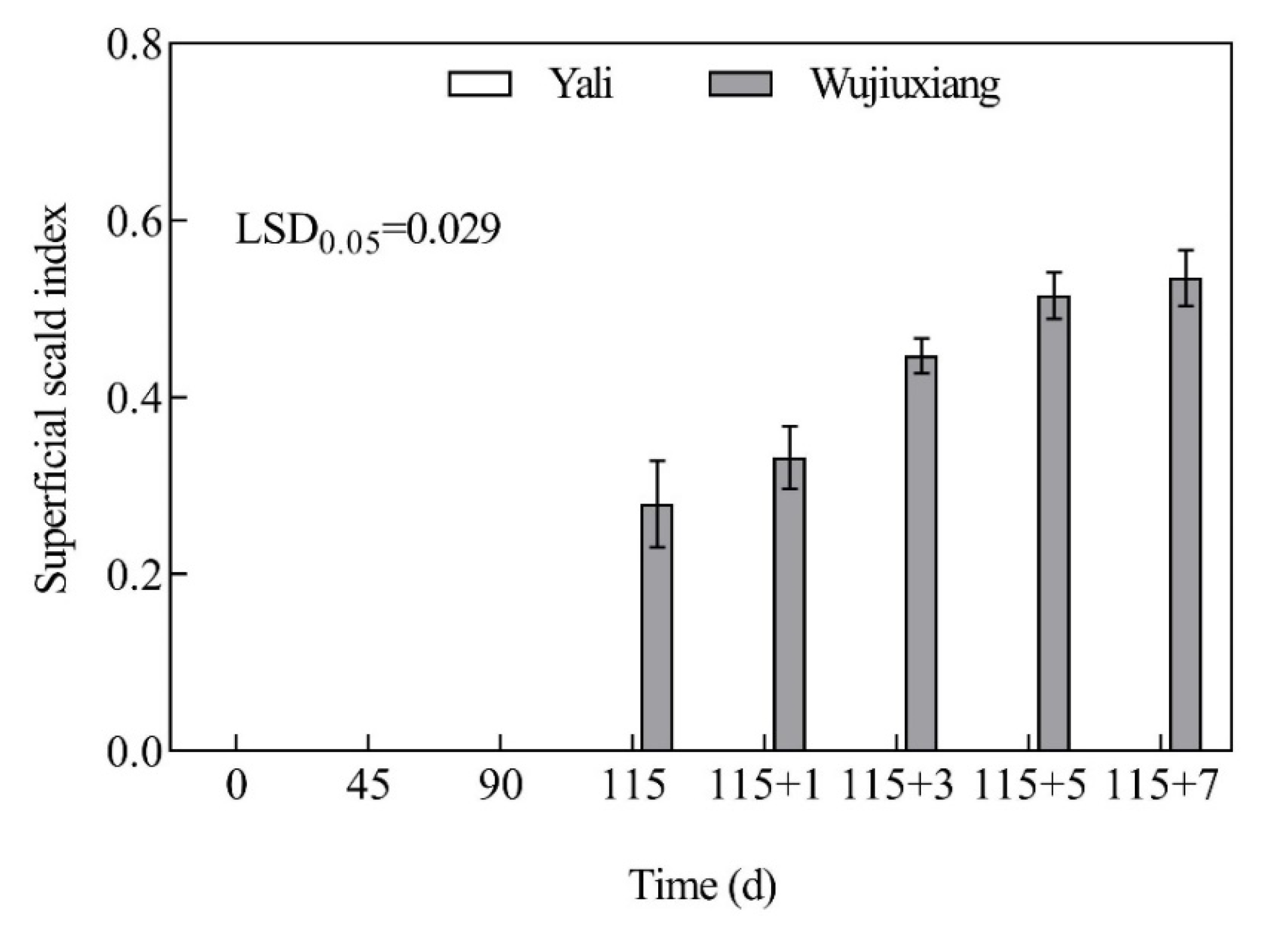
2.1. Changes in the Superficial Scald Index
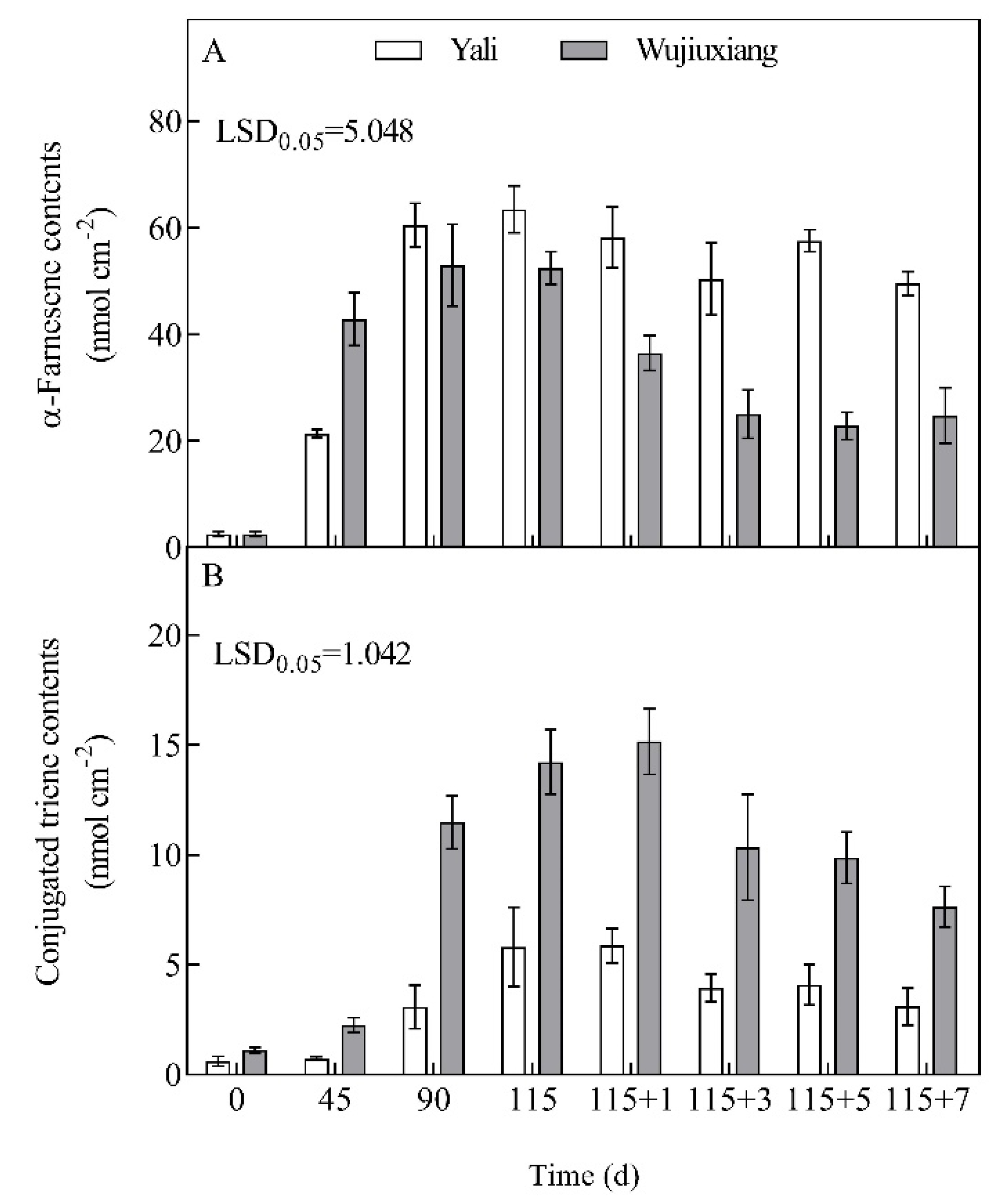
2.2. Changes in the Levels of α-Farnesene and CTols and Their Related Gene Expression
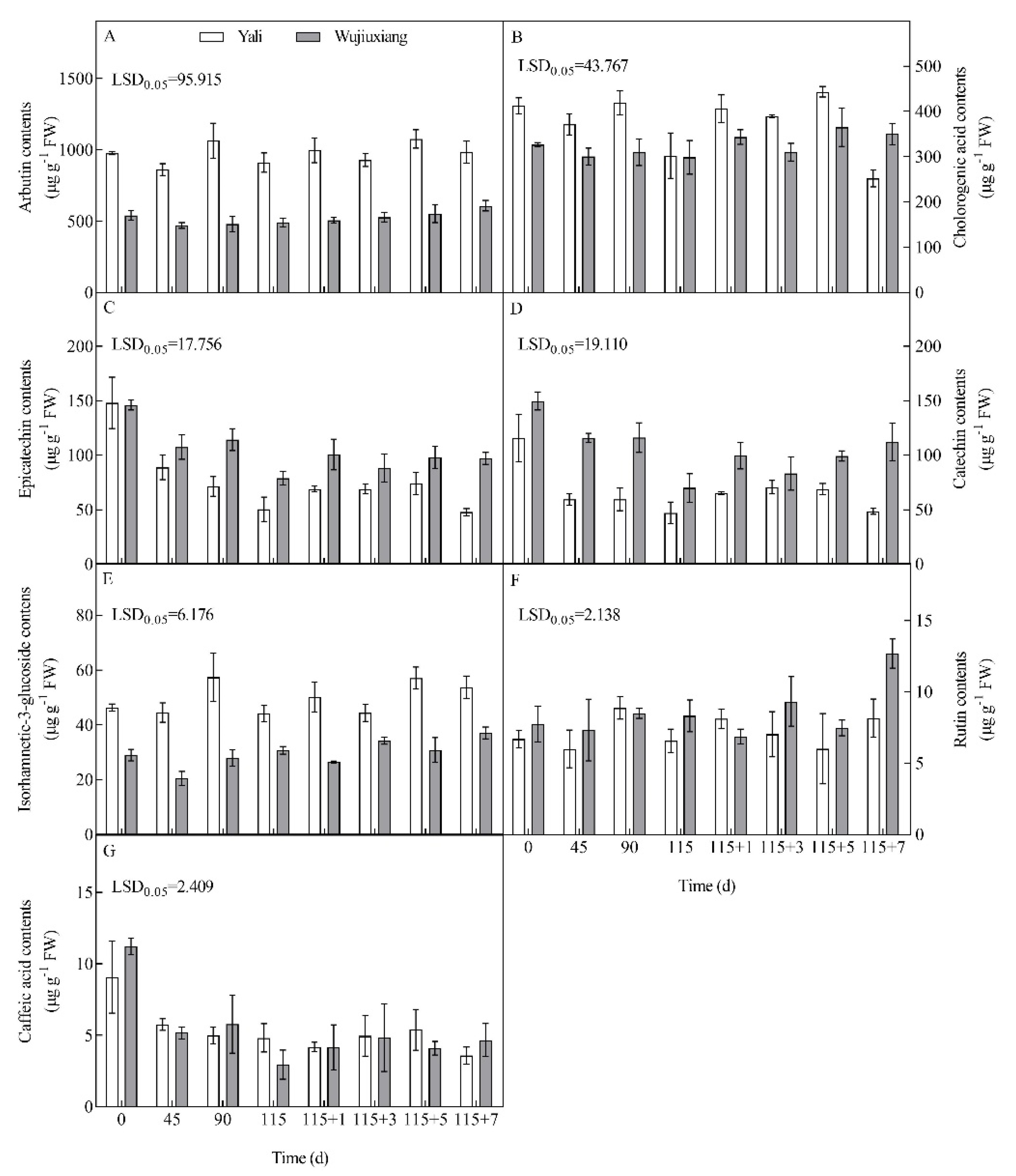
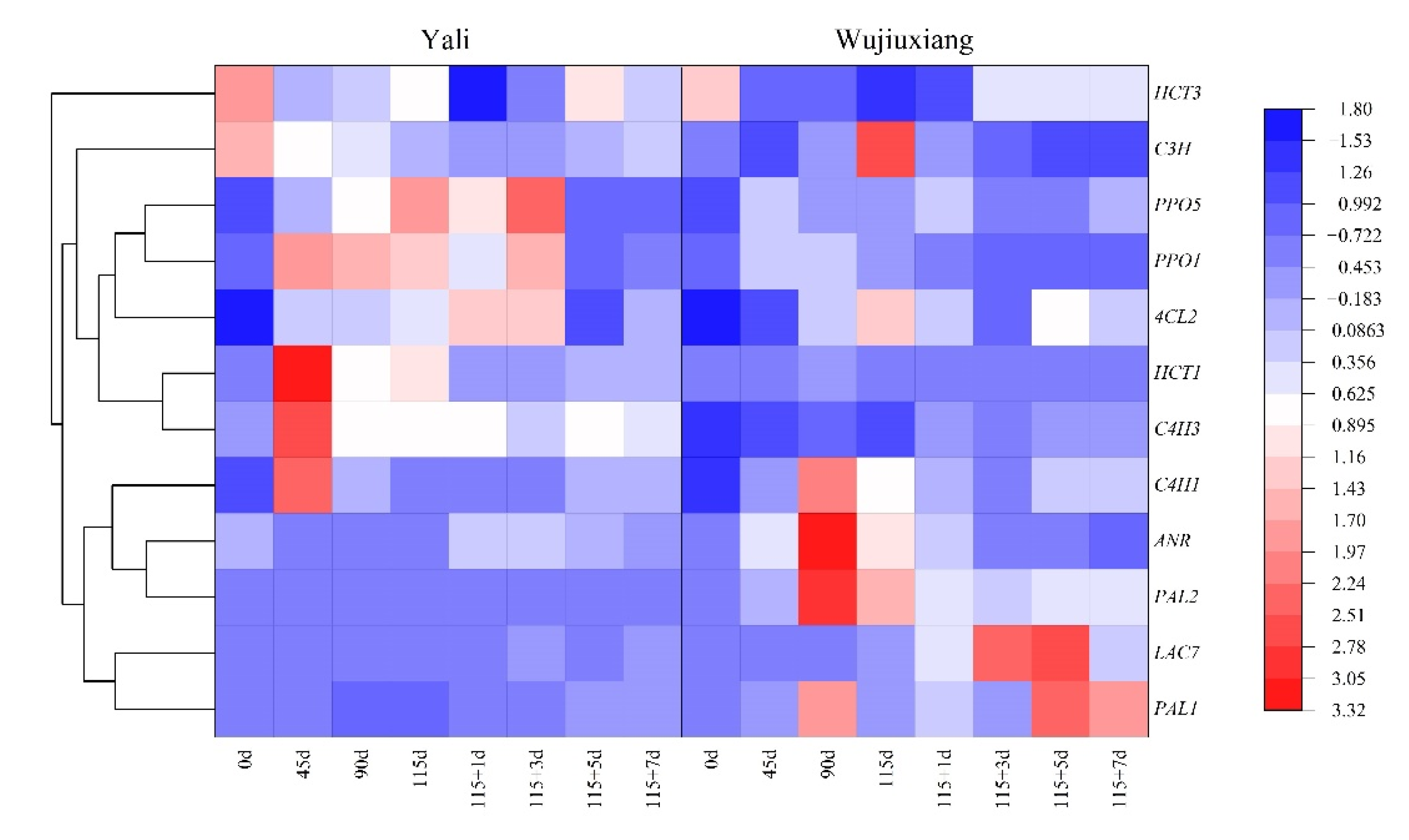
2.3. Changes in the Phenolic Content and Their Related Gene Expression
2.4. Changes in PPO and LAC Gene Expression
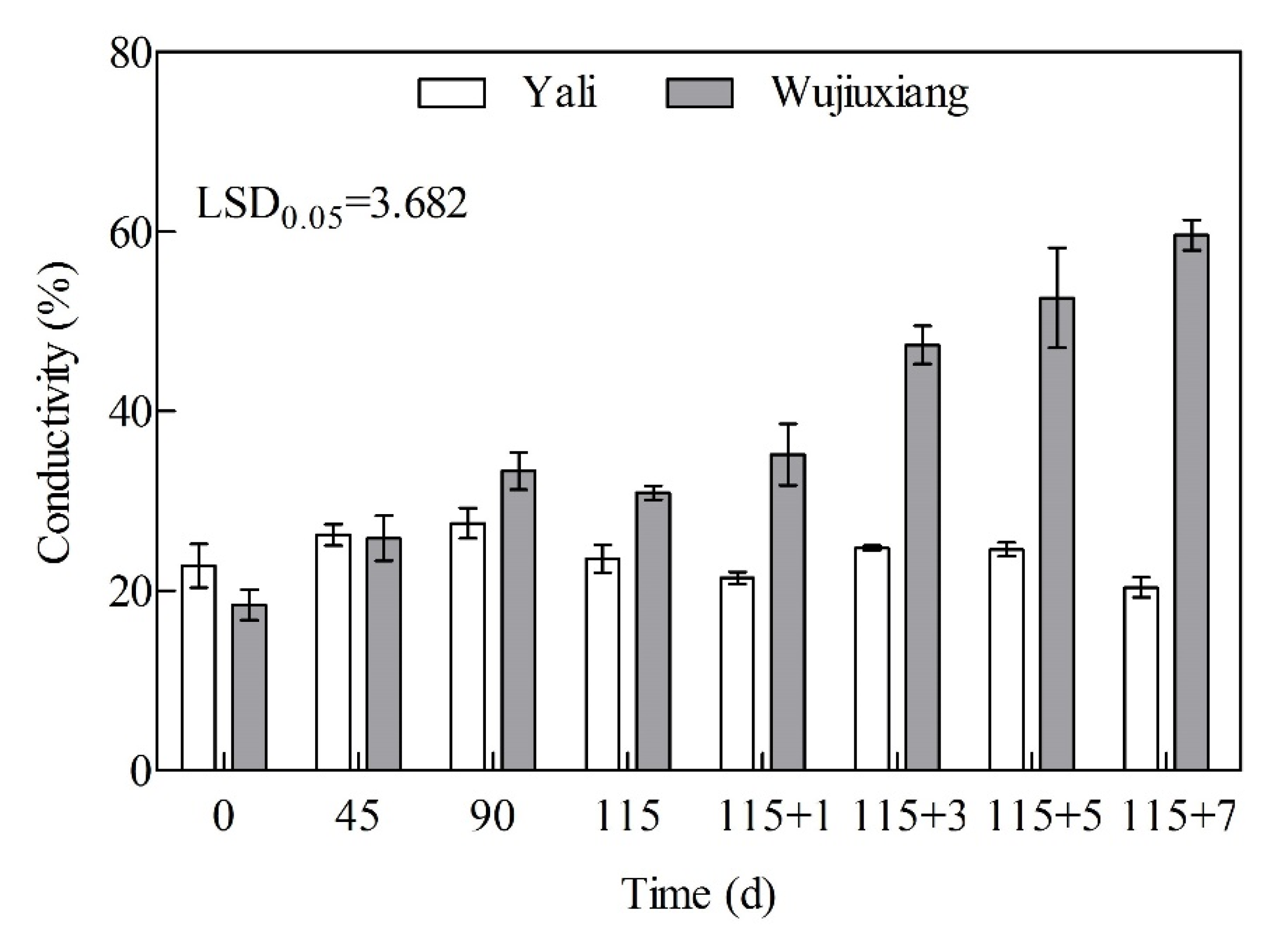
2.5. Changes in Cell Membrane Permeability
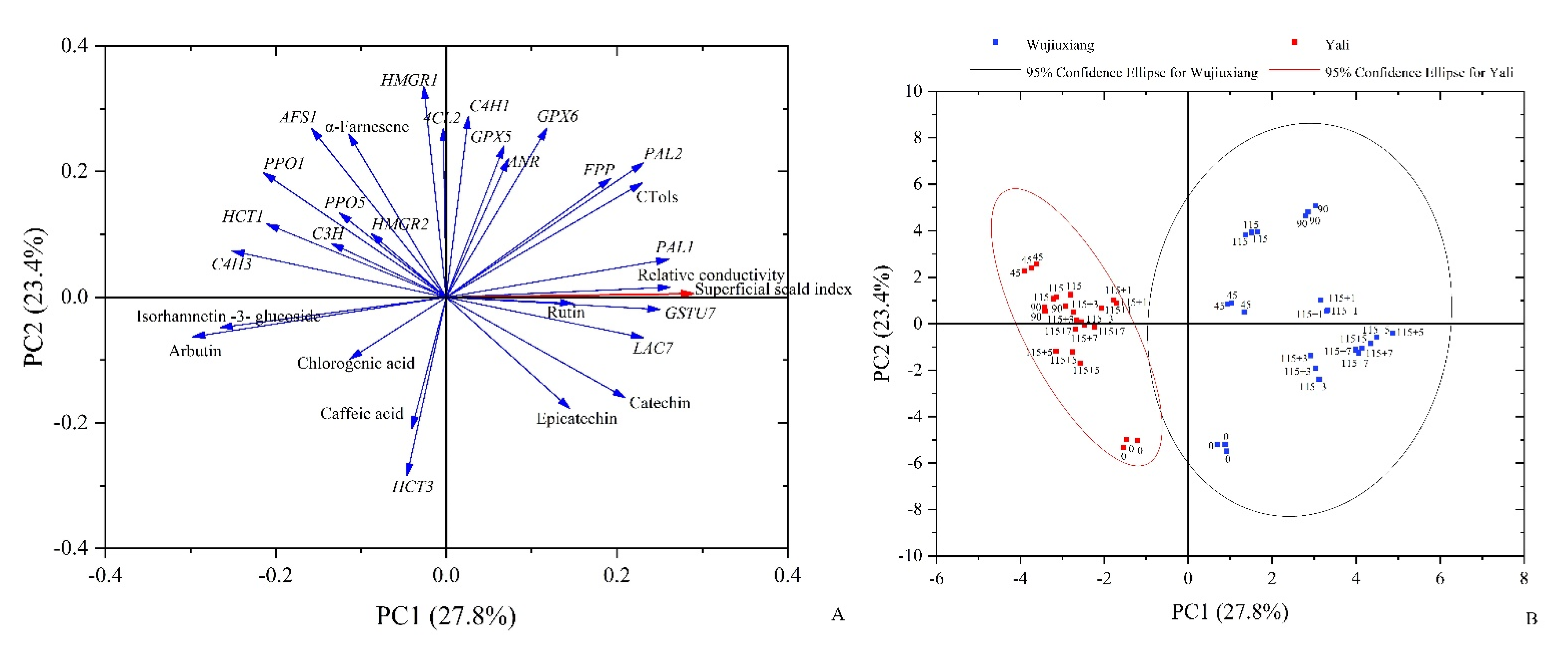
2.6. PCA Analysis
3. Discussion
3.1. CTols Have a Great Influence on the Occurrence of Scald Compared to α-Farnesene
3.2. Phenols and Their Oxidation Reactions
4. Materials and Methods
4.1. Plant Materials
4.2. Experimental Design
4.3. Measurement of the Superficial Scald Index
4.4. Determination of α-Farnesene and CTols Contents
4.5. Estimation of the Phenolic Component Contents
4.6. Relative Conductivity Measurement
4.7. RNA Isolation and Real-Time PCR (RT-PCR) Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gapper, N.E.; Bai, J.; Whitaker, B.D. Inhibition of ethylene-induced α-farnesene synthase gene PcAFS1 expression in ‘d’Anjou’ pears with 1-MCP reduces synthesis and oxidation of α-farnesene and delays development of superficial scald. Postharvest Biol. Technol. 2006, 41, 225–233. [Google Scholar] [CrossRef]
- Calvo, G.; Candan, A.P.; Civello, M.; Giné-Bordonaba, J.; Larrigaudière, C. An insight into the role of fruit maturity at harvest on superficial scald development in ‘Beurré D’Anjou’ pear. Sci. Hortic. 2015, 192, 173–179. [Google Scholar] [CrossRef]
- Hui, W.; Niu, J.; Xu, X.; Guan, J. Evidence supporting the involvement of MHO in the formation of superficial scald in ‘Dangshansuli’ pears. Postharvest Biol. Technol. 2016, 121, 43–50. [Google Scholar] [CrossRef][Green Version]
- Larrigaudière, C.; Candan, A.P.; Giné-Bordonaba, J.; Civello, M.; Calvo, G. Unravelling the physiological basis of superficial scald in pears based on cultivar differences. Sci. Hortic. 2016, 213, 340–345. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y. The combination of ethoxyquin, 1-methylcyclopropene and ethylene treatments controls superficial scald of ‘d’Anjou’ pears with recovery of ripening capacity after long-term controlled atmosphere storage. Postharvest Biol. Technol. 2017, 127, 53–59. [Google Scholar] [CrossRef]
- Zhou, S.; Cheng, Y.; Guan, J. The molecular basis of superficial scald development related to ethylene perception and α-farnesene metabolism in ‘Wujiuxiang’ pear. Sci. Hortic. 2017, 216, 76–82. [Google Scholar] [CrossRef]
- Zhi, H.; Dong, Y. Effect of 1-Methylcyclopropene on Superficial Scald Associated with Ethylene Production, α-Farnesene Catabolism, and Antioxidant System of Over-Mature ‘d’Anjou’ Pears After Long-Term Storage. Food Bioprocess Technol. 2018, 11, 1775–1786. [Google Scholar] [CrossRef]
- Larrigaudière, C.; Lindo-García, V.; Ubach, D.; Giné-Bordonaba, J. 1-Methylcyclopropene and extreme ULO inhibit superficial scald in a different way highlighting the physiological basis of this disorder in pear. Sci. Hortic. 2019, 250, 148–153. [Google Scholar] [CrossRef]
- Giné-Bordonaba, J.; Busatto, N.; Larrigaudière, C.; Lindo-García, V.; Echeverria, G.; Vrhovsek, U.; Farneti, B.; Biasioli, F.; De Quattro, C.; Rossato, M.; et al. Investigation of the transcriptomic and metabolic changes associated with superficial scald physiology impaired by lovastatin and 1-methylcyclopropene in pear fruit (cv. “Blanquilla”). Hortic. Res. 2020, 7, 49. [Google Scholar] [CrossRef]
- Lindo-García, V.; Giné-Bordonaba, J.; Leclerc, C.; Ubach, D.; Larrigaudière, C. The relationship between ethylene- and oxidative-related markers at harvest with the susceptibility of pears to develop superficial scald. Postharvest Biol. Technol. 2020, 163, 111135. [Google Scholar] [CrossRef]
- Busatto, N.; Farneti, B.; Commisso, M.; Bianconi, M.; Iadarola, B.; Zago, E.; Ruperti, B.; Spinelli, F.; Zanella, A.; Velasco, R.; et al. Apple fruit superficial scald resistance mediated by ethylene inhibition is associated with diverse metabolic processes. Plant J. 2018, 93, 270–285. [Google Scholar] [CrossRef]
- Lurie, S.; Watkins, C.B. Superficial scald, its etiology and control. Postharvest Biol. Technol. 2012, 65, 44–60. [Google Scholar] [CrossRef]
- Lurie, S.; Lers, A.; Shacham, Z.; Sonego, L.; Burd, S.; Whitaker, B. Expression of α-Farnesene Synthase AFS1 and 3-Hydroxy-3-methylglutaryl-coenzyme A Reductase HMG2 and HMG3 in Relation to α-Farnesene and Conjugated Trienols in ‘Granny Smith’ Apples Heat or 1-MCP Treated to Prevent Superficial Scald. J. Am. Soc. Hortic. Sci. 2005, 130, 232–236. [Google Scholar] [CrossRef]
- Pechous, S.W.; Watkins, C.B.; Whitaker, B.D. Expression of α-farnesene synthase gene AFS1 in relation to levels of α-farnesene and conjugated trienols in peel tissue of scald-susceptible ‘Law Rome’ and scald-resistant ‘Idared’ apple fruit. Postharvest Biol. Technol. 2005, 35, 125–132. [Google Scholar] [CrossRef]
- Busatto, N.; Farneti, B.; Tadiello, A.; Vrhovsek, U.; Cappellin, L.; Biasioli, F.; Velasco, R.; Costa, G.; Costa, F. Target metabolite and gene transcription profiling during the development of superficial scald in apple (Malus × domestica Borkh). BMC Plant Biol. 2014, 14, 193. [Google Scholar] [CrossRef]
- Wang, L.; Qian, M.; Wang, R.; Wang, L.; Zhang, S. Characterization of the glutathione S-transferase (GST) gene family in Pyrus bretschneideri and their expression pattern upon superficial scald development. Plant Growth Regul. 2018, 86, 211–222. [Google Scholar] [CrossRef]
- Cebulj, A.; Halbwirth, H.; Mikulic-Petkovsek, M.; Veberic, R.; Slatnar, A. The impact of scald development on phenylpropanoid metabolism based on phenol content, enzyme activity, and gene expression analysis. Hortic. Environ. Biotechnol. 2020, 61, 849–858. [Google Scholar] [CrossRef]
- Ding, R.; Che, X.; Liu, H.; Du, B.; Dong, K.; Zhang, Y. Effects of 1-MCP and storage temperature on transcription of mevalonate (MVA) enzyme genes of α-farnesene in ‘White Winter Pearmain’ apples fruit. Sci. Hortic. 2020, 259, 108841. [Google Scholar] [CrossRef]
- Busatto, N.; Giné-Bordonaba, J.; Larrigaudière, C.; Lindo-Garcia, V.; Farneti, B.; Biasioli, F.; Vrhovsek, U.; Costa, F. Molecular and biochemical differences underlying the efficacy of lovastatin in preventing the onset of superficial scald in a susceptible and resistant Pyrus communis L. cultivar. Postharvest Biol. Technol. 2021, 173, 111435. [Google Scholar] [CrossRef]
- Yihui, G.; Song, J.; Du, L.; Vinqvist, M.; Palmer, L.C.; Fillmore, S.; Pang, X.; Zhang, Z. Characterization of laccase from apple fruit during postharvest storage and its response to diphenylamine and 1-methylcyclopropene treatments. Food Chem. 2018, 253, 314–321. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Cheng, Y.-D.; He, J.-G.; Li, L.-M.; Guan, J.-F. Effects of 1-methylcyclopropene and modified atmosphere packaging on fruit quality and superficial scald in Yali pears during storage. J. Integr. Agric. 2018, 17, 1667–1675. [Google Scholar] [CrossRef]
- Zhai, R.; Xiang, F.; Song, J.; Wang, Z.; Yang, C.; Xu, L. Roles of laccase and cultivar-specific phenolic composition in scald-like disorder development in pears. Postharvest Biol. Technol. 2021, 181, 111651. [Google Scholar] [CrossRef]
- Rao, M.V.; Watkins, C.B.; Brown, S.K.; Weeden, N.F. Active oxygen species metabolism in ‘White Angel’ × ‘Rome Beauty’ apple selections resistant and susceptible to superficial scald. J. Am. Soc. Hortic. Sci. 1998, 123, 299–304. [Google Scholar] [CrossRef]
- Whitaker, B.D.; Nock, J.F.; Watkins, C.B. Peel tissue α-farnesene and conjugated trienol concentrations during storage of ‘White Angel’ × ‘Rome Beauty’ hybrid apple selections susceptible and resistant to superficial scald. Postharvest Biol. Technol. 2000, 20, 231–241. [Google Scholar] [CrossRef]
- Li, L.; Xia, Y.; Xu, C.; He, J.; Guan, J. The Incidence of Superficial Scald in “Wujiuxiang” Pears (Pyrus Pyrifolia Cv. Wujiuxiang) during and after Controlled Atmosphere Storage. J. Food Qual. 2016, 39, 201–208. [Google Scholar] [CrossRef]
- Zhou, S.; Li, D.; Cheng, Y.; Guan, J. Characterization of expression and enzyme activity of lipoxygenases during fruit softening and superficial scald development in ‘Wujiuxiang’ pear. J. Appl. Bot. Food Qual. 2016, 89, 307–314. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, Y.; Zhao, Z.; Cheng, Y.; Guan, J. The involvement of phenolic metabolism in superficial scald development in ‘Wujiuxiang’ pear. J. Appl. Bot. Food Qual. 2020, 93, 20–25. [Google Scholar] [CrossRef]
- Rowan, D.D.; Hunt, M.B.; Fielder, S.; Norris, A.J.; Sherburn, M.S. Conjugated Triene Oxidation Products of α-Farnesene Induce Symptoms of Superficial Scald on Stored Apples. J. Agric. Food Chem. 2001, 49, 2780–2787. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, B.D. Oxidation products of alpha-farnesene associated with superficial scald development in ‘d’Anjou pear fruits are conjugated trienols. J. Agric. Food Chem. 2007, 55, 3708–3712. [Google Scholar] [CrossRef] [PubMed]
- Bordonaba, J.G.; Matthieu-Hurtiger, V.; Westercamp, P.; Coureau, C.; Dupille, E.; Larrigaudière, C. Dynamic changes in conjugated trienols during storage may be employed to predict superficial scald in ‘Granny Smith’ apples. LWT-Food Sci. Technol. 2013, 54, 535–541. [Google Scholar] [CrossRef]
- Ding, R.; Du, B.; Zhang, Y. Conjugated trienols and programmed cell death are more closely related to superficial scald than reactive oxygen species in apple fruit stored at low temperature. Sci. Hortic. 2019, 246, 597–603. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Vaghchhipawala, Z.; Li, W.; Asard, H.; Dickman, M.B. Tomato phospholipid hydroperoxide glutathione peroxidase inhibits cell death induced by bax and oxidative stresses in yeast and plants. Plant Physiol. 2004, 135, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Jiang, L.; An, X.; Kang, R.; Yu, M.; Ma, R.; Yu, Z. Potential role of glutathione peroxidase gene family in peach fruit ripening under combined postharvest treatment with heat and 1-MCP. Postharvest Biol. Technol. 2016, 111, 175–184. [Google Scholar] [CrossRef]
- Henry-Kirk, R.A.; McGhie, T.K.; Andre, C.M.; Hellens, R.; Allan, A.C. Transcriptional analysis of apple fruit proanthocyanidin biosynthesis. J. Exp. Bot. 2012, 63, 5437–5450. [Google Scholar] [CrossRef]
- Veltman, R.H.; Larrigaudiere, C.; Wichers, H.; van Schaik, A.; van der Plas, L.; Oosterhaven, J. PPO activity and polyphenol content are not limiting factors during brown core development in pears (Pyrus communis L. cv. Conference). J. Plant Physiol. 1999, 154, 697–702. [Google Scholar] [CrossRef]
- Ju, Z.; Bramlage, W.J. Cuticular phenolics and scald development in ‘Delicious’ apples. J. Am. Soc. Hortic. Sci. 2000, 125, 498–504. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, X.-L.; Luo, H.-H.; Zhou, J.-J.; Gong, Y.-H.; Li, W.-J.; Shi, Z.-W.; He, Q.; Wu, Q.; Li, L.; et al. An intracellular laccase is responsible for epicatechin-mediated anthocyanin degradation in Litchi fruit pericarp. Plant Physiol. 2015, 169, 2391–2408. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, F.; He, Q.; Zhang, X.; Shi, N.; Song, J.; Zhang, Z.; Pang, X. Enzymatic characterization of a laccase from lychee pericarp in relation to browning reveals the mechanisms for fruit color protection. J. Food Process. Preserv. 2018, 42, e13515. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Zhang, Y.; Han, Y.; Li, L.; Yan, S.; Xiao, L. Effect of cooling method on FAD and LOX gene expression and core browning in postharvest Yali pears. Food Sci. 2019, 40, 222–227. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Awad, M.A.; de Jager, A.; van Westing, L.M. Flavonoid and chlorogenic acid levels in apple fruit: Characterisation of variation. Sci. Hortic. 2000, 83, 249–263. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, L.; Zhao, G.; Shen, C.; Yan, H.; Guan, J.; Yang, K. The effects of modified atmosphere packaging on core browning and the expression patterns of PPO and PAL genes in ‘Yali’ pears during cold storage. LWT-Food Sci. Technol. 2015, 60, 1243–1248. [Google Scholar] [CrossRef]
- He, J.-G.; Cheng, Y.-D.; Guan, J.-F.; Ge, W.-Y.; Zhao, Z. Changes of chlorogenic acid content and its synthesis-associated genes expression in Xuehua pear fruit during development. J. Integr. Agric. 2017, 16, 471–477. [Google Scholar] [CrossRef][Green Version]
- Fischer, T.C.; Gosch, C.; Pfeiffer, J.; Halbwirth, H.; Halle, C.; Stich, K.; Forkmann, G. Flavonoid genes of pear (Pyrus communis). Trees 2007, 21, 521–529. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Feng, Y.; Cheng, Y.; Karuppanapandian, T.; Wang, J.; Guan, J. Changes in α-Farnesene and Phenolic Metabolism and the Expression of Associated Genes during the Development of Superficial Scald in Two Distinct Pear Cultivars. Int. J. Mol. Sci. 2022, 23, 12088. https://doi.org/10.3390/ijms232012088
He J, Feng Y, Cheng Y, Karuppanapandian T, Wang J, Guan J. Changes in α-Farnesene and Phenolic Metabolism and the Expression of Associated Genes during the Development of Superficial Scald in Two Distinct Pear Cultivars. International Journal of Molecular Sciences. 2022; 23(20):12088. https://doi.org/10.3390/ijms232012088
Chicago/Turabian StyleHe, Jingang, Yunxiao Feng, Yudou Cheng, Thirupathi Karuppanapandian, Jinxiao Wang, and Junfeng Guan. 2022. "Changes in α-Farnesene and Phenolic Metabolism and the Expression of Associated Genes during the Development of Superficial Scald in Two Distinct Pear Cultivars" International Journal of Molecular Sciences 23, no. 20: 12088. https://doi.org/10.3390/ijms232012088
APA StyleHe, J., Feng, Y., Cheng, Y., Karuppanapandian, T., Wang, J., & Guan, J. (2022). Changes in α-Farnesene and Phenolic Metabolism and the Expression of Associated Genes during the Development of Superficial Scald in Two Distinct Pear Cultivars. International Journal of Molecular Sciences, 23(20), 12088. https://doi.org/10.3390/ijms232012088





