Placental Dysfunction in Assisted Reproductive Pregnancies: Perinatal, Neonatal and Adult Life Outcomes
Abstract
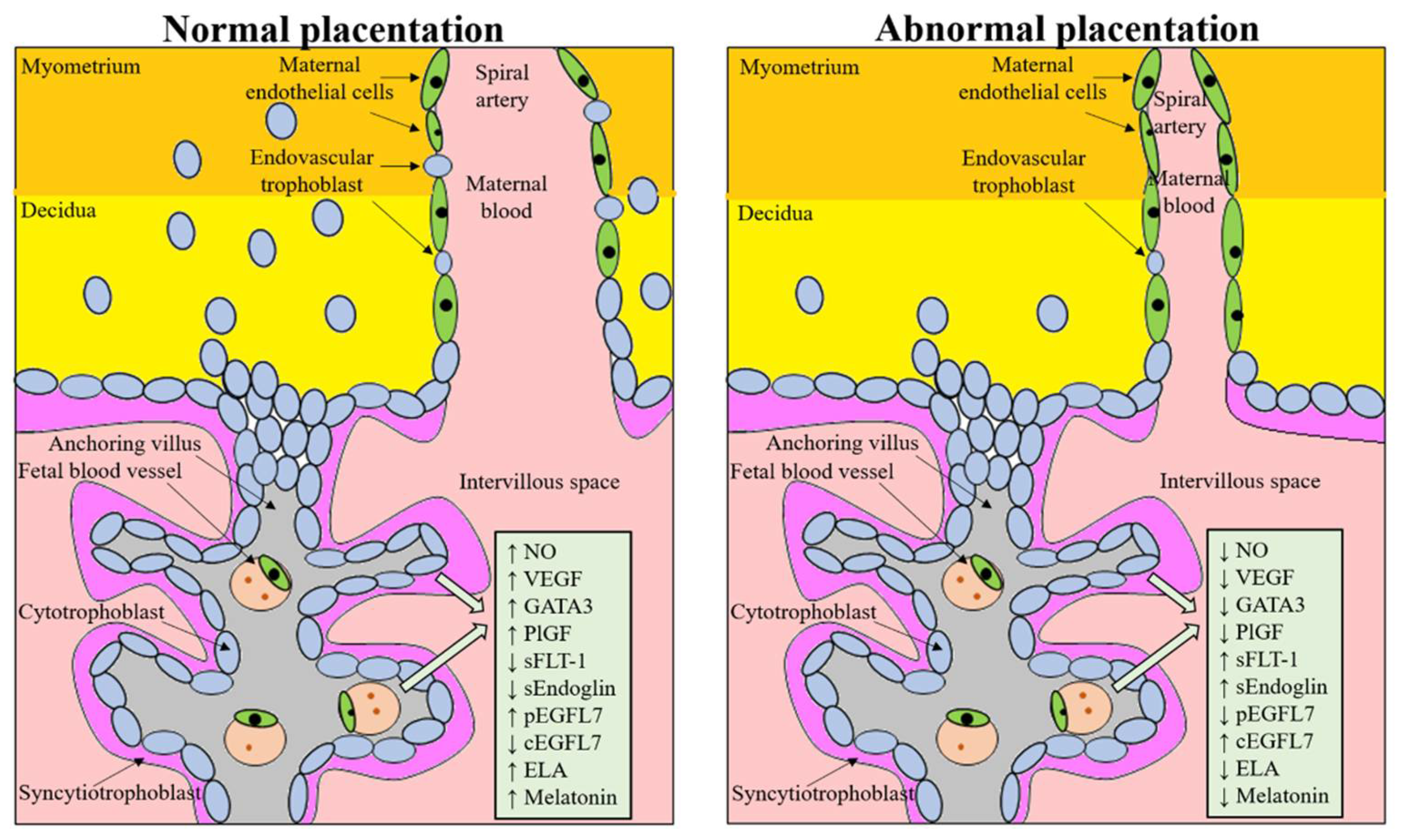
1. Introduction
2. Abnormal Placentation and ART: Molecular Factors and Involved Signaling
3. Pathophysiology of the Placenta in Pregnancy Complications and ART Pregnancies
3.1. Altered Hormonal Milieu Effect on Placental Development
3.2. Epigenetic Changes after ART Techniques Are Associated with Altered Gene Expression in the Placenta and Congenital Imprinting Disorders
3.3. Immune Dysregulation at the Maternal-Fetal Interface
3.4. Mechanical Stress on Embryo and Placental Development (the Case of PGT-A)
4. Metabolic and Cardiovascular Consequences of Placental Dysregulation in Mothers Following ART
5. Health Risk in Infancy as a Consequence of Placental Dysfunction Following ART
6. Simultaneous Action of Factors Dysregulating Normal Placentation
7. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTB | preterm birth |
| LBW | low birth weight |
| PE | preeclampsia |
| FGR | fetal growth restriction |
| PCOS | polycystic ovary syndrome |
| GDM | gestational diabetes mellitus |
| ART | assisted reproductive technology |
| IVF | in vitro fertilization |
| ICSI | intracytoplasmic sperm injection |
| FET | frozen embryo transfer |
| OD | oocyte donation |
| PGT-A | preimplantation genetic testing for aneuploidy |
| HDP | hypertensive disorders of pregnancy |
| VLBW | very low birth weight |
| PlGF | placental growth factor |
| sFLT-1 | soluble fms-like tyrosine kinase 1 |
| EGFR | epidermal growth factor receptor |
| sENDOGLIN | soluble endoglin |
| EGFL7 | epidermal growth factor-like domain 7 |
| TGF-β | transforming growth factor-beta |
| NO | nitric oxide |
| TNF-α | tumor necrosis factor-alpha |
| IL | interleukin |
| SGA | small for gestational age |
| LGA | large for gestational age |
| TH | thyroid hormone |
| LT4 | levothyroxine |
| PIH | pregnancy induced hypertension |
| PGT-M | preimplantation genetic test for monogenic diseases |
| BMP-4 | bone morphogenetic protein-4 |
| CVD | cardiovascular disease |
| PWS | Prader–Willi syndrome |
| AS | Angelman syndrome |
| BWS | Beckwith–Wiedemann syndrome |
| SRS | Silver–Russell syndrome |
| SMM | severe maternal morbidity |
References
- Messerlian, C.; Maclagan, L.; Basso, O. Infertility and the risk of adverse pregnancy outcomes: A systematic review and meta-analysis. Hum. Reprod. 2013, 28, 125–137. [Google Scholar] [CrossRef]
- Healy, D.L.; Breheny, S.; Halliday, J.; Jaques, A.; Rushford, D.; Garrett, C.; Talbot, J.M.; Baker, H.W. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Hum. Reprod. 2010, 25, 265–274. [Google Scholar] [CrossRef]
- Palomba, S.; de Wilde, M.A.; Falbo, A.; Koster, M.P.; La Sala, G.B.; Fauser, B.C. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update 2015, 21, 575–592. [Google Scholar] [CrossRef]
- Vambergue, A.; Fajardy, I. Consequences of gestational and pregestational diabetes on placental function and birth weight. World J. Diabetes 2011, 2, 196–203. [Google Scholar] [CrossRef]
- De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V.; et al. ART in Europe, 2014: Results generated from European registries by ESHRE: The European IVF-monitoring consortium (EIM) for the European society of human reproduction and Embryology (ESHRE). Hum. Reprod. 2018, 33, 1586–1601. [Google Scholar] [CrossRef]
- Helmerhorst, F.M.; Perquin, D.A.M.; Donker, D.; Keirse, M.J.N.C. Perinatal outcome of singletons and twins after assisted conception: A systematic review of controlled studies. BMJ 2004, 328, 261–264. [Google Scholar] [CrossRef]
- Pandey, S.; Shetty, A.; Hamilton, M.; Bhattacharya, S.; Maheshwari, A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 485–503. [Google Scholar] [CrossRef]
- Luke, B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: With an emphasis on US population-based studies. Am. J. Obstet. Gynecol. 2017, 217, 270–281. [Google Scholar] [CrossRef]
- Ginström Ernstad, E.; Wennerholm, U.; Khatibi, A.; Petzold, M.; Bergh, C. Neonatal and maternal outcome after frozen embryo transfer; increased risks in programmed cycles. Am. J. Obstet. Gynecol. 2019, 221, 126.e1–126.e18. [Google Scholar] [CrossRef]
- Saito, K.; Kuwahara, A.; Ishikawa, T.; Morisaki, N.; Miyado, M.; Miyado, K.; Fukami, M.; Miyasaka, N.; Ishihara, O.; Irahara, M.; et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum. Reprod. 2019, 34, 1567–1575. [Google Scholar] [CrossRef]
- Sunderam, S.; Kissin, D.M.; Zhang, Y.; Folger, S.G.; Boulet, S.L.; Warner, L.; Callaghan, W.M.; Barfield, W.D. Assisted reproductive technology surveillance—United States, 2016. MMWR Surveill. Summ. 2019, 68, 1–23. [Google Scholar] [CrossRef]
- Luke, B.; Brown, M.B.; Eisenberg, M.L.; Callan, C.; Botting, B.J.; Pacey, A.; Alastair, G. In vitro fertilization and risk for hypertensive disorders of pregnancy: Associations with treatment parameters. Am. J. Obstet. Gynecol. 2020, 222, 350.e1–350.e13. [Google Scholar] [CrossRef]
- Manna, C. Maternal-Fetal Medicine, Practical Aspects Il; Arduini, D., Palermo, M.S.F., Eds.; AMOLCA Publishing House: Medellin, Colombia, 2021. [Google Scholar]
- Qin, J.; Liu, X.; Sheng, X.; Wang, H.; Gao, S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: A meta- analysis of cohort studies. Fertil. Steril. 2016, 105, 73–85. [Google Scholar] [CrossRef]
- Xiang, M.; Chen, S.; Zhang, X.; Ma, Y. Placental diseases associated with assisted reproductive technology. Reprod. Biol. 2021, 21, 100505. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, C.; Zhang, W. Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil. Steril. 2014, 101, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Haavaldsen, C.; Tanbo, T.; Eskild, A. Placental weight in singleton pregnancies with and without assisted reproductive technology: A population study of 536,567 pregnancies. Hum. Reprod. 2012, 27, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Reig, A.; Seli, E. The association between assisted reproductive technologies and low birth weight. Curr. Opin. Obstet. Gynecol. 2019, 31, 183–187. [Google Scholar] [CrossRef]
- Joy, J.; Gannon, C.; McClure, N.; Cooke, I. Is Assisted Reproduction Associated with Abnormal Placentation? Pediatr. Dev. Pathol. 2012, 15, 306–314. [Google Scholar] [CrossRef]
- Ochoa, E. Alteration of Genomic Imprinting after Assisted Reproductive Technologies and Long-Term Health. Life 2021, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Kopca, T.; Tulay, P. Association of Assisted Reproductive Technology Treatments with Imprinting Disorders. Glob. Med. Genet. 2021, 8, 1–6. [Google Scholar] [CrossRef]
- Fontana, L.; Tabano, S.; Maitz, S.; Colapietro, P.; Garzia, E.; Gerli, A.G.; Sirchia, S.M.; Miozzo, M. Clinical and Molecular Diagnosis of Beckwith-Wiedemann Syndrome with Single- or Multi-Locus Imprinting Disturbance. Int. J. Mol. Sci. 2021, 22, 3445. [Google Scholar] [CrossRef]
- Kroener, L.; Wang, E.T.; Pisarska, M.D. Predisposing Factors to Abnormal First Trimester Placentation and the Impact on Fetal Outcomes. Semin. Reprod. Med. 2016, 34, 27–35. [Google Scholar] [CrossRef]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Fisher, S.J. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 2015, 213 (Suppl. 4), S115–S122. [Google Scholar] [CrossRef]
- Burton, G.J.; Yung, H.W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 2009, 30, S43–S48. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.R.; Walsh, A.J.; Morrow, R.J.; Mullen, J.B.; Lye, S.J.; Ritchie, J.W. Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: Relationship with umbilical artery Doppler waveforms. Am. J. Obstet. Gynecol. 1995, 172, 518–525. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Latest advances in understanding preeclampsia. Science 2005, 308, 1592–1594. [Google Scholar] [CrossRef] [PubMed]
- Benschop, L.; Schalekamp-Timmermans, S.; Broere-Brown, Z.A.; Roeters van Lennep, J.E.; Jaddoe, V.W.V.; Roos-Hesselink, J.W.; Ikram, M.K.; Steegers, E.A.P.; Robert, J.M.; Gandley, R.E. Placental growth factor as an indicator of maternal cardiovascular risk after pregnancy. Circulation 2019, 139, 1698–1709. [Google Scholar] [CrossRef]
- Redman, C.W.; Staff, A.C. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am. J. Obstet. Gynecol. 2015, 213 (Suppl. 4), S9.e1–S9.e4. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Pre-dictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Stepan, H.; Unversucht, A.; Wessel, N.; Faber, R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension 2007, 49, 818–824. [Google Scholar] [CrossRef]
- Hertig, A.; Berkane, N.; Lefevre, G.; Toumi, K.; Marti, H.P.; Capeau, J.; Uzan, S.; Rondeau, E. Maternal serum sFLT-1 concentration is an early and reliable predictive marker of preeclampsia. Clin. Chem. 2004, 50, 1702–1703. [Google Scholar] [CrossRef]
- Tidwell, S.C.; Ho, H.N.; Chiu, W.H.; Torry, R.J.; Torry, D.S. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am. J. Obstet. Gynecol. 2001, 184, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, R.; Mutter, W.P.; Wolf, M.; Levine, R.J.; Taylor, R.N.; Sukhatme, V.P.; Ecker, J.; Karumanchi, S.A. First trimester placental growth factor and solu-ble fms-like tyrosine kinase 1 and risk for preeclampsia. J. Clin. Endocrinol. Metab. 2004, 89, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Hastie, R.; Brownfoot, F.C.; Pritchard, N.; Hannan, N.J.; Cannon, P.; Nguyen, V.; Palmer, K.; Beard, S.; Tong, S.; Kaitu’u-Lino, T.J. EGFR (Epidermal Growth Factor Receptor) Signaling and the Mitochondria Regulate sFlt-1 (Soluble FMS-Like Tyrosine Kinase-1) Secretion. Hypertension 2019, 73, 659–670. [Google Scholar] [CrossRef]
- Vrooman, L.A.; Rhon-Calderon, E.A.; Chao, O.Y.; Nguyen, D.K.; Narapareddy, L.; Dahiya, A.K.; Putt, M.E.; Schultz, R.M.; Bartolomei, M.S. Assisted reproductive technologies induce temporally specific placental defects and the PE risk marker sFLT-1 in mouse. Development 2020, 29, 147. [Google Scholar] [CrossRef]
- Cui, L.; Shu, C.; Liu, Z.; Tong, W.; Cui, M.; Wei, C.; Tang, J.J.; Liu, X.; Hu, J.; Jiang, J.; et al. The expression of serum sEGFR, sFlt-1, sEndoglin and PLGF in preeclampsia. Pregnancy Hypertens. 2018, 13, 127–132. [Google Scholar] [CrossRef]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef]
- Lacko, L.A.; Massimiani, M.; Sones, J.L.; Hurtado, R.; Salvi, S.; Ferrazzani, S.; Davisson, R.L.; Campagnolo, L.; Stuhlmann, H. Novel expression of EGFL7 in placental trophoblast and endothelial cells and its implication in preeclampsia. Mech. Dev. 2014, 133, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Massimiani, M.; Vecchione, L.; Piccirilli, D.; Spitalieri, P.; Amati, F.; Salvi, S.; Ferrazzani, S.; Stuhlmann, H.; Campagnolo, L. Epidermal growth factor-like domain 7 promotes migration and invasion of human trophoblast cells through activation of MAPK, PI3K and NOTCH signaling pathways. Mol. Hum. Reprod. 2015, 21, 435–451. [Google Scholar] [CrossRef][Green Version]
- Lacko, L.A.; Hurtado, R.; Hinds, S.; Poulos, M.G.; Butler, J.M.; Stuhlmann, H. Altered feto-placental vascularization, feto-placental malperfusion and fetal growth restriction in mice with Egfl7 loss of function. Development. 2017, 144, 2469–2479. [Google Scholar] [CrossRef]
- Massimiani, M.; Salvi, S.; Tiralongo, G.M.; Moresi, S.; Stuhlmann, H.; Valensise, H.; Lanzone, A.; Campagnolo, L. Circulating EGFL7 distinguishes between FGR and PE: An observational case-control study. Sci. Rep. 2021, 11, 17919. [Google Scholar] [CrossRef]
- Massimiani, M.; Lacko, L.A.; Burke Swanson, C.S.; Salvi, S.; Argueta, L.B.; Moresi, S.; Ferrazzani, S.; Gelber, S.E.; Baergen, R.N.; Toschi, N.; et al. Increased circulating levels of Epidermal Growth Factor-like Domain 7 in pregnant women affected by preeclampsia. Transl. Res. 2019, 207, 19–29. [Google Scholar] [CrossRef]
- Massimiani, M.; Tiralongo, G.M.; Salvi, S.; Fruci, S.; Lacconi, V.; La Civita, F.; Mancini, M.; Stuhlmann, H.; Valensise, H.; Campagnolo, L. Treatment of pregnancies complicated by intrauterine growth restriction with nitric oxide donors increases placental expression of Epidermal Growth Factor-Like Domain 7 and improves fetal growth: A pilot study. Transl. Res. 2021, 228, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Valensise, H.; Vasapollo, B.; Novelli, G.P.; Giorgi, G.; Verallo, P.; Galante, A.; Arduini, D. Maternal and fetal hemodynamic effects induced by nitric oxide donors and plasma volume expansion in pregnancies with gestational hypertension complicated by intrauterine growth restriction with absent end diastolic flow in the umbilical artery. Ultrasound Obstet. Gynecol. 2008, 31, 55–64. [Google Scholar] [CrossRef]
- Berbets, A.; Koval, H.; Barbe, A.; Albota, O.; Yuzko, O. Melatonin decreases and cytokines increase in women with placental insufficiency. J. Matern.-Fetal Neonatal Med. 2019, 25, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Lupi, L.A., Jr.; Seiva, F.R.; Martinez, M.; Domeniconi, R.F.; Pinheiro, P.F.; Dos Santos, L.D.; Martinez, F.E. Quantitative proteomic profiling reveals that diverse metabolic pathways are influenced by melatonin in an in vivo model of ovarian carcinoma. J. Proteome Res. 2016, 15, 3872–3882. [Google Scholar] [CrossRef]
- De Almeida Chuffa, L.G.; Seiva, F.R.F.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Lupi, L.A. Mitochondrial functions and melatonin: A tour of the reproductive cancers. Cell. Mol. Life Sci. 2019, 76, 837–863. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.T.; Ishizuka, B.; Kudo, Y.; Fusama, S.; Amemiya, A.; Sumi, Y. Detection of melatonin and serotonin N-acetyltransferase and hydroxyindole-Omethyltransferase activities in the rat ovary. Mol. Cell Endocrinol. 1997, 136, 7–14. [Google Scholar] [CrossRef]
- Itoh, M.T.; Ishizuka, B.; Kuribayashi, Y.; Amemiya, A.; Sumi, Y. Melatonin, its precursors, and synthesizing enzyme activities in the human ovary. Mol. Hum. Reprod. 1999, 5, 402–408. [Google Scholar] [CrossRef] [PubMed]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel-Ghaffar, A.E.; Sosa, G.A.; El-Roos, M.E.A.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 2011, 78, 250–262. [Google Scholar] [CrossRef]
- Amireault, P.; Dube, F. Serotonin and its antidepressant-sensitive transport in mouse cumulus-oocyte complexes and early embryos. Biol. Reprod. 2005, 73, 358–365. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Itoh, M.T.; Takahaashi, N.; Tarumi, W.; Ishizuka, B. The rat oocyte synthesises melatonin. Reprod. Fertil. Dev. 2013, 25, 674–682. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tong, J.; Li, W.P.; Chen, Z.J.; Zhang, C. Melaton in concentration in follicular fluid is correlated with antral follicle count (AFC) and in vitro fertilization (IVF) outcomes in women undergoing assisted reproductive technology (ART) procedures. Gynecol. Endocrinol. 2018, 34, 446–450. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Chuffa, L.G.; Seiva, F.R.F.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Lupi, L.A. Clock genes and the role of melatonin in cancer cells: An overview. Melatonin Res. 2019, 2, 133–157. [Google Scholar] [CrossRef]
- Moshkdanian, G.; Moghani-Ghoroghi, F.; Pasbakhsh, P.; Nematollahi-Mahani, S.N.; Najafi, A.; Kashani, S.R. Melatonin upregulates ErbB1 and ErbB4, two primary implantation receptors, in pre-implantation mouse embryos. Iran. J. Basic Med. Sci. 2017, 20, 655–661. [Google Scholar] [CrossRef]
- Pan, B.; Qazi, I.H.; Guo, S.; Yang, J.; Qin, J.; Lv, T.; Zang, S.; Zhang, Y.; Zeng, C.; Meng, Q.; et al. Melatonin improves the first cleavage of parthenogenetic embryos from vitrified-warmed mouse oocytes potentially by promoting cell cycle progression. J. Anim. Sci. Biotechnol. 2021, 12, 84. [Google Scholar] [CrossRef]
- Bourgain, C.; Devroey, P. The endometrium in stimulated cycles for IVF. Hum. Reprod. Update 2003, 9, 515–522. [Google Scholar] [CrossRef]
- Bonagura, T.W.; Pepe, G.J.; Enders, A.C.; Albrecht, E.D. Suppression of extravillous trophoblast vascular endothelial growth factor expression and uterine spiral artery invasion by estrogen during early baboon pregnancy. Endocrinology 2008, 149, 5078–5087. [Google Scholar] [CrossRef]
- Alyasin, A.; Agha-Hosseini, M.; Kabirinasab, M.; Saeidi, H.; Nashtaei, M.S. Serum progesterone levels greater than 32,5 ng/ml on the day of embryo transfer are associated with lower live birth rate after artificial endometrial preparation: A prospective study. Reprod. Biol. Endocrinol. 2021, 19, 24. [Google Scholar] [CrossRef]
- von Versen-Höynck, F.; Schaub, A.M.; Chi, Y.Y.; Chiu, K.H.; Liu, J.; Lingis, M.; Stan Williams, R.; Rhoton-Vlasak, A.; Nichols, W.W.; Fleischmann, R.R.; et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension 2019, 73, 640–649. [Google Scholar] [CrossRef]
- Makhijani, R.; Bartels, C.; Godiwala, P.; Bartolucci, A.; Nulsen, J.; Grow, D.; Benadiva, C.; Engmann, L. Maternal and perinatal outcomes in programmed versus natural vitrified-warmed blastocyst transfer cycles. Reprod. Biomed. Online 2020, 41, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; von Versen-Höynck, F.; Kapphahn, K.I.; Fleischmann, R.R.; Zhao, Q.; Baker, V.L. Maternal and neonatal outcomes associated with trophectoderm biopsy. Fertil. Steril. 2019, 112, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.A.; Leo, C.H.; Senadheera, S.N.; Girling, J.E.; Tare, M.; Parry, L.J. Relaxin deficiency attenuates pregnancy-induced adaptation of the mesenteric artery to angiotensin II in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R847–R857. [Google Scholar] [CrossRef] [PubMed]
- von Versen-Höynck, F.; Strauch, N.K.; Liu, J.; Chi, Y.Y.; Keller-Woods, M.; Conrad, K.P.; Baker, V.L. Effect of mode of conception on maternal serum relaxin, creatinine, and sodium concentrations in an infertile population. Reprod. Sci. 2019, 26, 412–419. [Google Scholar] [CrossRef]
- Obregon, M.J.; Mallol, J.; Pastor, R.; Morreale de Escobar, G.; Escobar del Rey, F. L-thyroxine and 3,5,3’-triiodo-L-thyronine in rat embryos before onset of fetal thyroid function. Endocrinology 1984, 114, 305–307. [Google Scholar] [CrossRef]
- van den Boogaard, E.; Vissenberg, R.; Land, J.A.; van Wely, M.; van der Post, J.A.; Goddijn, M.; Bisschop, P.H. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: A systematic review. Hum. Reprod. Update 2011, 17, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Piccirilli, D.; Baldini, E.; Massimiani, M.; Camaioni, A.; Salustri, A.; Bernardini, R.; Centanni, M.; Ulisse, S.; Moretti, C.; Campagnolo, L. Thyroid hormone regulates protease expression and activation of Notch signaling in implantation and embryo development. J. Endocrinol. 2018, 236, 1–12. [Google Scholar] [CrossRef]
- Dal Lago, A.; Galanti, F.; Miriello, D.; Marcoccia, A.; Massimiani, M.; Campagnolo, L.; Moretti, C.; Rago, R. Positive Impact of Levothyroxine Treatment on Pregnancy Outcome in Euthyroid Women with Thyroid Autoimmunity Affected by Recurrent Miscarriage. J. Clin. Med. 2021, 10, 2105. [Google Scholar] [CrossRef]
- Brosens, I.; Brosens, J.J.; Fusi, L.; Al-Sabbagh, M.; Kuroda, K.; Benagiano, G. Risks of adverse pregnancy outcome in endometriosis. Fertil. Steril. 2012, 98, 30–35. [Google Scholar] [CrossRef]
- Breintoft, K.; Pinnerup, R.; Henriksen, T.; Rytter, D.; Uldbjerg, N.; Forman, A.; Arendt, L. Endometriosis and Risk of Adverse Pregnancy Outcome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 667. [Google Scholar] [CrossRef]
- Jindal, P.; Regan, L.; Fourkala, E.O.; Rai, R.; Moore, G.; Goldin, R.D.; Sebire, N.J. Placental pathology of recurrent spontaneous abortion: The role of histopathological examination of products of conception in routine clinical practice: A mini review. Hum. Reprod. 2007, 22, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Longtine, M.S.; Nelson, D.M. Placental dysfunction and fetal programming: The importance of placental size, shape, histopathology, and molecular composition. Semin. Reprod. Med. 2011, 29, 187–196. [Google Scholar] [CrossRef]
- Palomba, S.; Russo, T.; Falbo, A.; Di Cello, A.; Tolino, A.; Tucci, L.; La Sala, G.B.; Zullo, F. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum. Reprod. 2013, 28, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Russo, T.; Falbo, A.; Di Cello, A.; Amendola, G.; Mazza, R.; Tolino, A.; Zullo, F.; Tucci, L.; La Sala, G.B. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: An experimental case-control study. J. Clin. Endocrinol. Metab. 2012, 97, 2441–2449. [Google Scholar] [CrossRef]
- Palomba, S.; Falbo, A.; Russo, T.; Battista, L.; Tolino, A.; Orio, F.; Zullo, F. Uterine blood flow in pregnant patients with polycystic ovary syndrome: Relationships with clinical outcomes. BJOG 2010, 117, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Sha, T.; Wang, X.; Cheng, W.; Yan, Y. A meta-analysis of pregnancy-related outcomes and complications in women with polycystic ovary syndrome undergoing IVF. Reprod. Biomed. Online 2019, 39, 281–293. [Google Scholar] [CrossRef]
- Maccani, M.A.; Marsit, C.J. Epigenetics in the Placenta. Am. J. Reprod. Immunol. 2009, 62, 78–89. [Google Scholar] [CrossRef]
- Maheshwari, A.; Hamilton, M.; Bhattacharya, S. Should we be promoting embryo transfer at blastocyst stage? Reprod. Biomed. Online 2016, 32, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Katari, S.; Turan, N.; Bibikova, M.; Erinle, O.; Chalian, R.; Foster, M.; Gaughan, J.P.; Coutifaris, C.; Sapienza, C. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum. Mol. Genet. 2009, 18, 3769–3778. [Google Scholar] [CrossRef]
- Nelissen, E.C.; van Montfoort, A.P.; Dumoulin, J.C.; Evers, J.L. Epigenetics and the placenta. Hum. Reprod. Update 2011, 17, 397–417. [Google Scholar] [CrossRef]
- Robins, J.C.; Marsit, C.J.; Padbury, J.F.; Sharma, S.S. Endocrine disruptors, environmental oxygen, epigenetics and pregnancy. Front. Biosci. 2011, 3, 690–700. [Google Scholar] [CrossRef]
- Novakovic, B.; Rakyan, V.; Ng, H.K.; Manuelpillai, U.; Dewi, C.; Wong, N.C.; Morley, R.; Down, T.; Beck, S.; Craig, J.M.; et al. Specific tumour-associated methylation in normal human term placenta and first-trimester cytotrophoblasts. Hum. Reprod. 2008, 14, 547–554. [Google Scholar] [CrossRef]
- de Waal, E.; Mak, W.; Calhoun, S.; Stein, P.; Ord, T.; Krapp, C.; Coutifaris, C.; Schultz, R.M.; Bartolomei, M.S. In vitro culture increases the frequency of stochastic epigenetic errors at imprinted genes in placental tissues from mouse concepti produced through assisted reproductive technologies. Biol. Reprod. 2014, 90, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, S.; Tang, N.; Xiao, X.; Huang, J.; Jiang, F.; Huang, X.; Sun, F.; Wang, X. Assisted reproduction causes reduced fetal growth associated with downregulation of paternally expressed imprinted genes that enhance fetal growth in mice. Biol. Reprod. 2016, 94, 45. [Google Scholar] [CrossRef]
- Xiang, M.; Ma, Y.; Lei, H.; Wen, L.; Chen, S.; Wang, X. In vitro fertilization placenta overgrowth in mice is associated with downregulation of the paternal imprinting gene H19. Mol. Reprod. Dev. 2019, 86, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, F.Z.; Huang, X.; Wang, X.; Tang, N.; Zhu, B.; Li, B. Assisted reproduction causes placental maldevelopment and dysfunction linked to reduced fetal weight in mice. Sci. Rep. 2015, 5, 10596. [Google Scholar] [CrossRef]
- Lee, B.; Kroener, L.L.; Xu, N.; Wang, E.T.; Banks, A.; Williams, J.; Goodarzi, M.O.; Chen, Y.I.; Tang, J.; Wang, Y.; et al. Function and Hormonal Regulation of GATA3 in Human First Trimester Placentation. Biol. Reprod. 2016, 95, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Y.; Zhou, Z.; Sha, J.; Li, Y.; Liu, J. Altered global gene expressions of human placentae subjected to assisted reproductive technology treatments. Placenta 2010, 31, 251–258. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, X.; Liu, J.; Zheng, R.; Yang, R.; Wang, Y.; Sun, L. The placental transcriptome of the first- trimester placenta is affected by in vitro fertilization and embryo transfer. Reprod. Biol. Endocrinol. 2019, 17, 50. [Google Scholar] [CrossRef]
- Kleijkers, S.H.M.; Mantikou, E.; Slappendel, E.; Consten, D.; van Echten-Arends, J.; Wetzels, A.M.; van Wely, M.; Smits, L.J.M.; van Montfoort, A.P.A.; Repping, S.; et al. Influence of embryo culture medium (G5 and HTF) on pregnancy and perinatal outcome after IVF: A multicenter RCT. Hum. Reprod. 2016, 31, 2219–2230. [Google Scholar] [CrossRef]
- Cagnone, G.; Sirard, M.A. The embryonic stress response to in vitro culture: Insight from genomic analysis. Reproduction 2016, 152, R247–R261. [Google Scholar] [CrossRef] [PubMed]
- Salilew-Wondim, D.; Saeed-Zidane, M.; Hoelker, M.; Gebremedhn, S.; Poirier, M.; Pandey, H.O.; Tholen, E.; Neuhoff, C.; Held, E.; Besenfelder, U.; et al. Genome-wide DNA methylation patterns of bovine blastocysts derived from in vivo embryos subjected to in vitro culture before, during or after embryonic genome activation. BMC Genom. 2018, 19, 424. [Google Scholar] [CrossRef]
- Asami, M.; Lam, B.; Ma, M.K.; Rainbow, K.; Braun, S.; VerMilyea, M.D.; Yeo, G.; Perry, A. Human embryonic genome activation initiates at the one-cell stage. Cell Stem Cell 2021. [Google Scholar] [CrossRef]
- Berntsen, S.; Söderström-Anttila, V.; Wennerholm, U.B.; Laivuori, H.; Loft, A.; Oldereid, N.B.; Romundstad, L.B.; Bergh, C.; Pinborg, A. The health of children conceived by ART: “The chicken or the egg?”. Hum. Reprod. Update 2019, 25, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ghosh, J.; Mainigi, M.; Turan, N.; Weinerman, R.; Truongcao, M.; Coutifaris, C.; Sapienza, C. DNA methylation differences between in vitro- and in vivo-conceived children are associated with ART procedures rather than infertility. Clin. Epigenet. 2015, 7, 41. [Google Scholar] [CrossRef]
- Xu, N.; Barlow, G.M.; Cur, J.; Wang, E.T.; Lee, B.; Akhlaghpour, M.; Kroener, L.; Williams, J.; Rotter, J.I.; Chen, Y.I.; et al. Comparison of genome-wide and gene-specific DNA methylation profiling in first trimester chorionic villi from pregnancies conceived with infertility treatments. Reprod. Sci. 2017, 24, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Choux, C.; Carmignac, V.; Bruno, C.; Sagot, P.; Vaiman, D.; Fauque, P. The placenta: Phenotypic and epigenetic modifications induced by Assisted Reproductive Technologies throughout pregnancy. Clin. Epigenet. 2015, 7, 87. [Google Scholar] [CrossRef]
- Ginstrom Ernstad, E.; Bergh, C.; Khatibi, A.; Kallen, K.B.; Westlander, G.; Nilsson, S.; Wennerholm, U.B. Neonatal and maternal outcome after blastocyst transfer: A population-based registry study. Am. J. Obstet. Gynecol. 2016, 214, 378.e1–378.e10. [Google Scholar] [CrossRef]
- Makinen, S.; Soderstrom-Anttila, V.; Vainio, J.; Suikkari, A.M.; Tuuri, T. Does long in vitro culture promote large for gestational age babies? Hum. Reprod. 2013, 28, 828–834. [Google Scholar] [CrossRef]
- Ishihara, O.; Araki, R.; Kuwahara, A.; Itakura, A.; Saito, H.; Adamson, G.D. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: An analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil. Steril. 2014, 101, 128–133. [Google Scholar] [CrossRef]
- Zhu, J.; Lin, S.; Li, M.; Chen, L.; Lian, Y.; Liu, P.; Qiao, J. Effect of in vitro culture period on birthweight of singleton newborn. Hum. Reprod. 2014, 29, 448–454. [Google Scholar] [CrossRef]
- Huang, J.; Yang, X.; Wu, J.; Kuang, Y.; Wang, Y. Impact of Day 7 Blastocyst Transfer on Obstetric and Perinatal Outcome of Singletons Born After Vitrified-Warmed Embryo Transfer. Front. Physiol. 2020, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Pereira, N.; O’Neill, C.; Lu, V.; Rosenwaks, Z.; Palermo, G.D. The safety of intracytoplasmic sperm injection and long-term outcomes. Reproduction 2017, 154, F61–F70. [Google Scholar] [CrossRef] [PubMed]
- Feuer, S.K.; Liu, X.; Donjacour, A.; Simbulan, R.; Maltepe, E.; Rinaudo, P. Transcriptional Signatures throughout Development: The Effects of Mouse Embryo Manipulation In Vitro. Reproduction 2017, 153, 107–122. [Google Scholar] [CrossRef]
- Hiura, H.; Okae, H.; Miyauchi, N.; Sato, F.; Sato, A.; Van De Pette, M.; John, R.M.; Kagami, M.; Nakai, K.; Soejima, H.; et al. Characterization of DNA Methylation Errors in Patients with Imprinting Disorders Conceived by Assisted Reproduction Technologies. Hum. Reprod. 2012, 27, 2541–2548. [Google Scholar] [CrossRef]
- Hiura, H.; Okae, H.; Chiba, H.; Miyauchi, N.; Sato, F.; Sato, A.; Arima, T. Imprinting Methylation Errors in ART. Reprod. Med. Biol. 2014, 13, 193–202. [Google Scholar] [CrossRef]
- Hattori, H.; Hiura, H.; Kitamura, A.; Miyauchi, N.; Kobayashi, N.; Takahashi, S.; Okae, H.; Kyono, K.; Kagami, M.; Ogata, T.; et al. Association of Four Imprinting Disorders and ART. Clin. Epigenet. 2019, 11, 1–12. [Google Scholar] [CrossRef]
- Gundogan, F.; Bianchi, D.W.; Scherjon, S.A.; Roberts, D.J. Placental pathology in egg donor pregnancies. Fertil. Steril. 2010, 93, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Aiello, E.; Pietrolucci, M.E.; Arduini, D. Placental volume and uterine artery Doppler evaluation at 11 + 0 to 13 + 6 weeks’ gestation in pregnancies conceived with in-vitro fertilization: Comparison between autologous and donor oocyte recipients. Ultrasound Obstet. Gynecol. 2016, 47, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Ganer Herman, G.; Tamayev, L.; Feldstein, O.; Bustan, M.; Rachimiel, Z.; Schreiber, L.; Raziel, A.; Bar, J.; Kovo, M. Placental-related disorders of pregnancy and IVF: Does placental histological examination explain the excess risk? Reprod. Biomed. Online 2020, 41, 81–87. [Google Scholar] [CrossRef]
- Munne, S. Status of preimplantation genetic testing and embryo selection. Reprod. Biomed. Online 2018, 37, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Paulson, R.J. Outcome of in vitro fertilization cycles with preimplantation genetic testing for aneuploidies: Let’s be honest with one another. Fertil. Steril. 2019, 112, 1013–1014. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 134: Fetal growth restriction. Obstet. Gynecol. 2013, 121, 1122–1133. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 743: Low dose aspirin use in pregnancy. Obstet. Gynecol. 2018, 132, e44–e52. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 202: Gestational hypertension and pre-eclampsia. Obstet. Gynecol. 2019, 133, 211–218. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists and Society for the Maternal-Fetal Medicine. Obstetrics care consensus: Placenta accreta spectrum. Obstet. Gynecol. 2018, 132, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Luo, K.; He, H.; Lu, C.; Zhang, S.; Tan, Y.; Gong, F.; Lu, G.; Lin, G. Obstetric and neonatal outcomes in blastocyst-stage biopsy with frozen embryo transfer and cleavage-stage biopsy with fresh embryo transfer after preimplantation genetic diagnosis/screening. Fertil. Steril. 2016, 106, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Feldman, B.; Orvieto, R.; Weisel, M.; Aizer, A.; Meyer, R.; Haas, J.; Kirshenbaum, M. Obstetric and Perinatal Outcomes in Pregnancies Conceived After Preimplantation Genetic Testing for Monogenetic Diseases. Obstet. Gynecol. 2020, 136, 782–791. [Google Scholar] [CrossRef]
- Makhijani, R.; Bartels, C.B.; Godiwala, P.; Bartolucci, A.; DiLuigi, A.; Nulsen, J.; Grow, D.; Benadiva, C.; Engmann, L. Impact of trophectoderm biopsy on obstetric and perinatal outcomes following frozen–thawed embryo transfer cycles. Hum. Reprod. 2021, 36, 340–348. [Google Scholar] [CrossRef]
- Starostic, M.R.; Sosina, O.A.; McCoy, R.C. Single-cell analysis of human embryos reveals diverse patterns of aneuploidy and mosaicism. Genome Res. 2020, 30, 814–835. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Pasquale, P.; Brivanlou, A. Preimplantation Genetic Testing for Aneuploidy—A Castle Built on Sand. Trends Mol. Med. 2021, 27, 731–742. [Google Scholar] [CrossRef]
- Gleicher, N.; Vidali, A.; Braverman, J.; Kushnir, V.A.; Albertini, D.F.; Barad, D.H. Further evidence against use of PGS in poor prognosis patients: Report of normal births after transfer of embryos reported as aneuploid. Fertil. Steril. 2015, 104, E59. [Google Scholar] [CrossRef]
- Greco, E.; Minasi, M.G.; Fiorentino, F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N. Engl. J. Med. 2015, 373, 2089–2090. [Google Scholar] [CrossRef] [PubMed]
- Patrizio, P.; Shoham, G.; Shoham, Z.; Leong, M.; Barad, D.H.; Gleicher, N. Worldwide live births following transfer of chromosomally “abnormal” embryos after PGT/A. Results of worldwide web-based survey. J. Assist. Reprod. Genet. 2019, 36, 1599–1607. [Google Scholar] [CrossRef]
- Singla, S.; Iwamoto-Stohl, L.K.; Zhu, M.; Zernicka-Goetz, M. Autophagy-mediated apoptosis eliminates aneuploid cells in a mouse model of chromosome mosaicism. Nat. Commun. 2020, 11, 2958. [Google Scholar] [CrossRef]
- Yang, M.; Rito, T.; Naftaly, J.; Hu, J.; Albertini, D.F.; Barad, D.H.; Brivanlou, A.H.; Gleicher, N. Self-correction of mosaicism in human embryos and gastruloids. Nat. Cell. Biol. 2020, 114, E14–E15. [Google Scholar] [CrossRef]
- Preimplantation Genetic Testing—Good Practice Recommendations of the European Society of Human Reproduction and Embryology (ESHRE). 2020, p. 124. Available online: www.eshre.eu/guidelines (accessed on 23 November 2021).
- Rexhaj, E.; Paoloni-Giacobino, A.; Rimoldi, S.F.; Fuster, D.G.; Anderegg, M.; Somm, E.; Bouillet, E.; Allemann, Y.; Sartori, C.; Scherrer, U. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J. Clin. Investig. 2013, 123, 5052–5060. [Google Scholar] [CrossRef]
- Jayet, P.Y.; Rimoldi, S.F.; Stuber, T.; Salmòn, C.S.; Hutter, D.; Rexhaj, E.; Thalmann, S.; Schwab, M.; Turini, P.; Sartori-Cucchia, C.; et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010, 122, 488–494. [Google Scholar] [CrossRef]
- Sundheimer, L.; Pisarska, M. Abnormal Placentation Associated with Infertility as a Marker of Overall Health. Semin. Reprod. Med. 2017, 35, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.A.; Robertson, W.B.; Dixon, H.G. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet. Gynecol. Annu. 1972, 1, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.G.; Leveno, K.J. Childbearing among older women—The message is cautiously optimistic. N. Engl. J. Med. 1995, 333, 1002–1004. [Google Scholar] [CrossRef]
- Kenny, L.C.; Lavender, T.; McNamee, R.; O’Neill, S.M.; Mills, T.; Khashan, A.S. Advanced maternal age and adverse pregnancy outcome: Evidence from a large contemporary cohort. PLoS ONE 2013, 8, e56583. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Hong, C.; Wang, E.T.; Alexander, C.; Gregory, K.D.; Pisarska, M.D. Pregnancy outcomes in very advanced maternal age pregnancies: The impact of assisted reproductive technology. Fertil. Steril. 2015, 103, 76–80. [Google Scholar] [CrossRef]
- Wang, E.T.; Ozimek, J.A.; Greene, N.; Ramos, L.; Vyas, N.; Kilpatrick, S.J.; Pisarska, M.D. Impact of fertility treatment on severe maternal morbidity. Fertil. Steril. 2016, 106, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Dayan, N.; Joseph, K.S.; Fell, D.B.; Laskin, C.A.; Basso, O.; Park, A.L.; Luo, J.; Guan, J.; Ray, J.G. Infertility treatment and risk of severe maternal morbidity: A propensity score-matched cohort study. CMAJ 2019, 191, E118–E127. [Google Scholar] [CrossRef] [PubMed]
- Dayan, N.; Fell, D.B.; Guo, Y.; Wang, H.; Velez, M.P.; Spitzer, K.; Laskin, C.A. Severe maternal morbidity in women with high BMI in IVF and unassisted singleton pregnancies. Hum. Reprod. 2018, 33, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Piña, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the American Heart Association. J. Am. Coll. Cardiol. 2011, 57, 1404–1423. [Google Scholar] [CrossRef] [PubMed]
- Briana, D.D.; Germanou, K.; Boutsikou, M.; Boutsikou, T.; Athanasopoulos, N.; Marmarinos, A.; Gourgiotis, D.; Malamitsi-Puchner, A. Potential prognostic biomarkers of cardiovascular disease in fetal macrosomia: The impact of gestational diabetes. J. Matern.-Fetal Neonatal. Med. 2018, 31, 895–900. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of chronic adult disease. Acta Paediatr. Suppl. 2004, 93, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Männistö, T.; Mendola, P.; Vääräsmäki, M.; Järvelin, M.R.; Hartikainen, A.L.; Pouta, A.; Suvanto, E. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation 2013, 127, 681–690. [Google Scholar] [CrossRef]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur. J. Epidemiol. 2013, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kvehaugen, A.S.; Dechend, R.; Ramstad, H.B.; Troisi, R.; Fugelseth, D.; Staff, A.C. Endothelial function and circulating biomarkers are disturbed in women and children after PE. Hypertension 2011, 58, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Platt, D.; Papenbrock, T.; Wilkins, A.; Eckert, J.J.; Kwong, W.Y.; Osmond, C.; Hanson, M.; Fleming, T.P. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc. Natl. Acad. Sci. USA 2007, 104, 5449–5454. [Google Scholar] [CrossRef]
- Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Bailey, D.M.; de Marchi, S.F.; McEneny, J.; von Arx, R.; Cerny, D.; Duplain, H.; Germond, M.; et al. Antioxidants improve vascular function in children conceived by assisted reproductive technologies: A randomized double-blind placebo-controlled trial. Eur. J. Prev. Cardiol. 2015, 22, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, L.; Zhao, J.; Wu, F.; Davies, M.J.; Wittert, G.A. Altered Glucose Metabolism in Mouse and Humans Conceived by IVF. Diabetes 2014, 63, 3189–3198. [Google Scholar] [CrossRef]
- Ceelen, M.; Van Weissenbruch, M.M.; Prein, J.; Smit, J.J.; Vermeiden, J.P.W.; Spreeuwenberg, M.; Van Leeuwen, F.E.; Delemarre-Van De Waal, H.A. Growth during Infancy and Early Childhood in Relation to Blood Pressure and Body Fat Measures at Age 8–18 Years of IVF Children and Spontaneously Conceived Controls Born to Subfertile Parents. Hum. Reprod. 2009, 24, 2788–2795. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, X.M.; Jin, L.; Wang, T.T.; Ullah, K.; Sheng, J.Z.; Huang, H.F. Cardiovascular and Metabolic Profiles of Offspring Conceived by Assisted Reproductive Technologies: A Systematic Review and Meta-Analysis. Fertil. Steril. 2017, 107, 622–631. [Google Scholar] [CrossRef]
- Ceelen, M.; Van Weissenbruch, M.M.; Roos, J.C.; Vermeiden, J.P.W.; Van Leeuwen, F.E.; Delemarre-van De Waal, H.A. Body Composition in Children and Adolescents Born after in Vitro Fertilization or Spontaneous Conception. J. Clin. Endocrinol. Metab. 2007, 92, 3417–3423. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, M.; Van Weissenbruch, M.M.; Vermeiden, J.P.W.; Van Leeuwen, F.E.; Delemarre-Van De Waal, H.A. Cardiometabolic Differences in Children Born after In Vitro Fertilization: Follow-up Study. J. Clin. Endocrinol. Metab. 2008, 93, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Forse, T.; Eriksson, J.; Tuomilehto, J.; Reunanen, A.; Osmond, C.; Barker, D. The Fetal and Childhood Growth of Persons Who Develop Type 2 Diabetes. Ann. Intern. Med. 2000, 133, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Garza, C. Potential Mechanisms of Metabolic Imprinting That Lead to Chronic Disease. Am. J. Clin. Nutr. 1999, 69, 179–197. [Google Scholar] [CrossRef]
- Millership, S.J.; Van de Pette, M.; Withers, D.J. Genomic Imprinting and Its Effects on Postnatal Growth and Adult Metabolism. Cell Mol. Life Sci. 2019, 76, 4009–4021. [Google Scholar] [CrossRef]
- Wale, P.L.; Gardner, D.K. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum. Reprod. Update 2016, 22, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Sheehan, P.M.; Brennecke, S.P.; Keogh, R.J. Vessel remodelling, pregnancy hormones and extravillous trophoblast function. Mol. Cell Endocrinol. 2012, 349, 138–144. [Google Scholar] [CrossRef]
- Imudia, A.N.; Awonuga, A.O.; Doyle, J.O.; Kaimal, A.J.; Wright, D.L.; Toth, T.L.; Styer, A.K. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and PE in singleton pregnancies after in vitro fertilization. Fertil. Steril. 2012, 97, 1374–1379. [Google Scholar] [CrossRef]
- Jones, M.L.; Mark, P.J.; Mori, T.A.; Keelan, J.A.; Waddell, B.J. Maternal dietary omega-3 fatty acid supplementation reduces placental oxidative stress and increases fetal and placental growth in the rat. Biol. Reprod. 2013, 88, 37. [Google Scholar] [CrossRef]
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31, S66–S69. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, C.; Wyns, C.; Calhaz-Jorge, C.; de Mouzon, J.; Ferraretti, A.P.; Kupka, M.; Nyboe Andersen, A.; Nygren, K.G.; Goossens, V. 20 years of the European IVF-monitoring Consortium registry: What have we learned? A comparison with registries from two other regions. Hum. Reprod. 2020, 35, 2832–2849. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Norman, R.J.; Wilcox, A.J. Incidence of spontaneous abortion among pregnancies produced by assisted reproductive technology. Hum. Reprod. 2004, 19, 272–277. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manna, C.; Lacconi, V.; Rizzo, G.; De Lorenzo, A.; Massimiani, M. Placental Dysfunction in Assisted Reproductive Pregnancies: Perinatal, Neonatal and Adult Life Outcomes. Int. J. Mol. Sci. 2022, 23, 659. https://doi.org/10.3390/ijms23020659
Manna C, Lacconi V, Rizzo G, De Lorenzo A, Massimiani M. Placental Dysfunction in Assisted Reproductive Pregnancies: Perinatal, Neonatal and Adult Life Outcomes. International Journal of Molecular Sciences. 2022; 23(2):659. https://doi.org/10.3390/ijms23020659
Chicago/Turabian StyleManna, Claudio, Valentina Lacconi, Giuseppe Rizzo, Antonino De Lorenzo, and Micol Massimiani. 2022. "Placental Dysfunction in Assisted Reproductive Pregnancies: Perinatal, Neonatal and Adult Life Outcomes" International Journal of Molecular Sciences 23, no. 2: 659. https://doi.org/10.3390/ijms23020659
APA StyleManna, C., Lacconi, V., Rizzo, G., De Lorenzo, A., & Massimiani, M. (2022). Placental Dysfunction in Assisted Reproductive Pregnancies: Perinatal, Neonatal and Adult Life Outcomes. International Journal of Molecular Sciences, 23(2), 659. https://doi.org/10.3390/ijms23020659







