Protease Inhibitor Anti-HIV, Lopinavir, Impairs Placental Endocrine Function
Abstract
1. Introduction
2. Results
2.1. Effect of RTV on the Villous Trophoblast
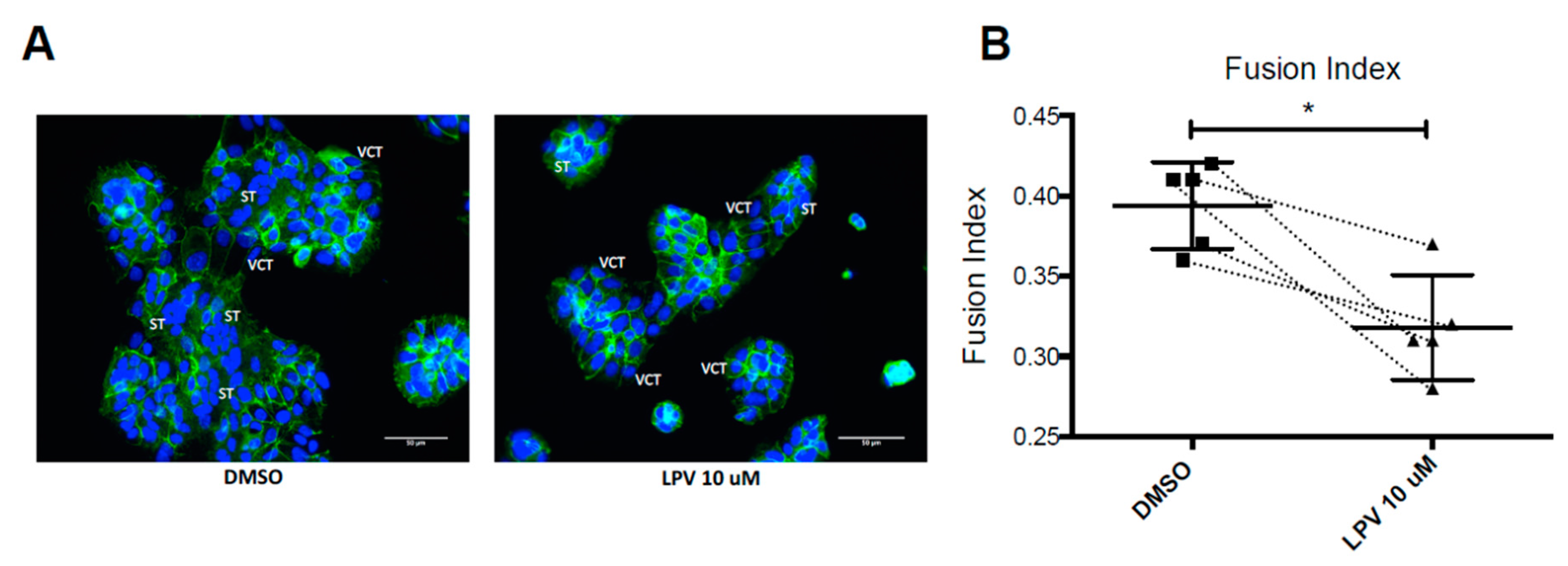
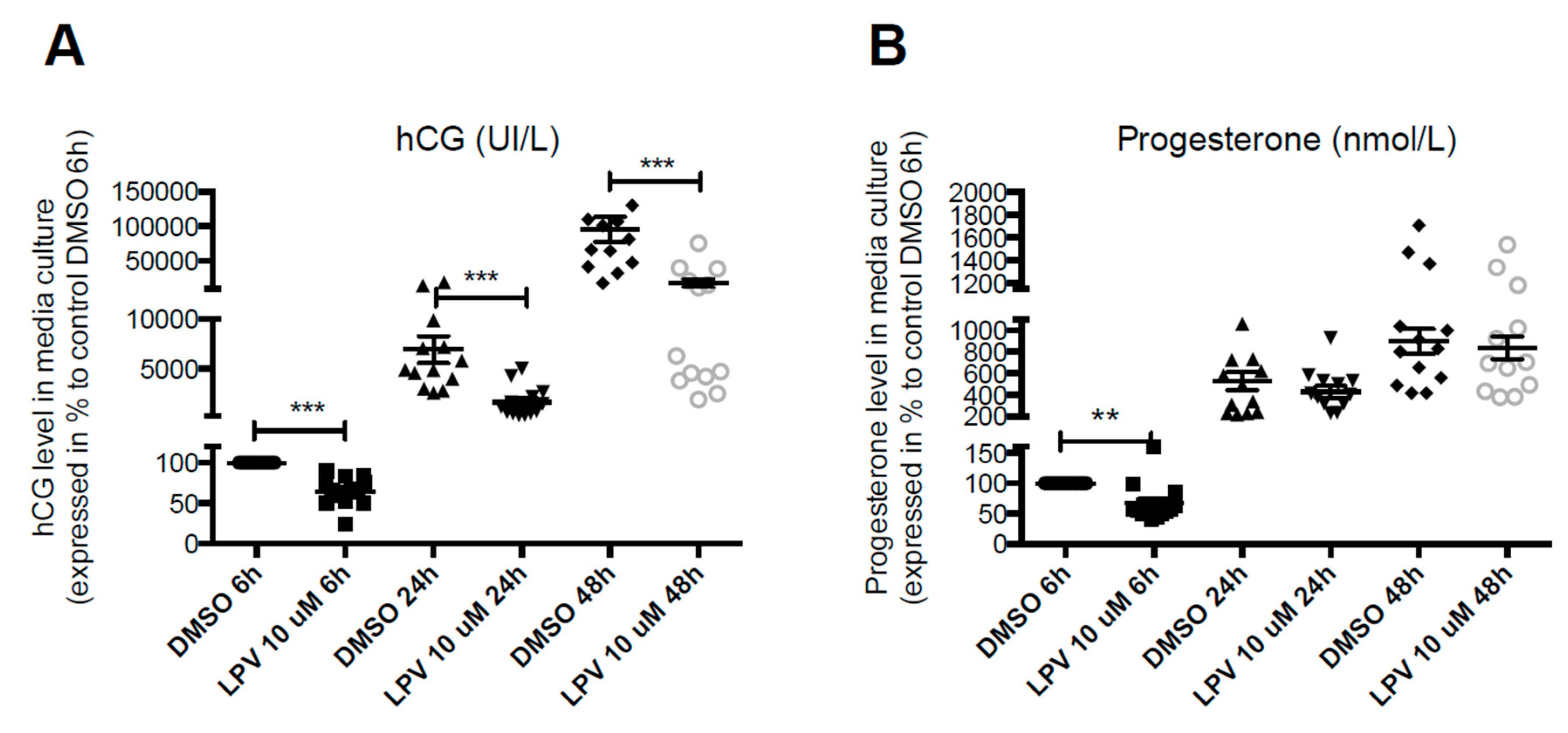
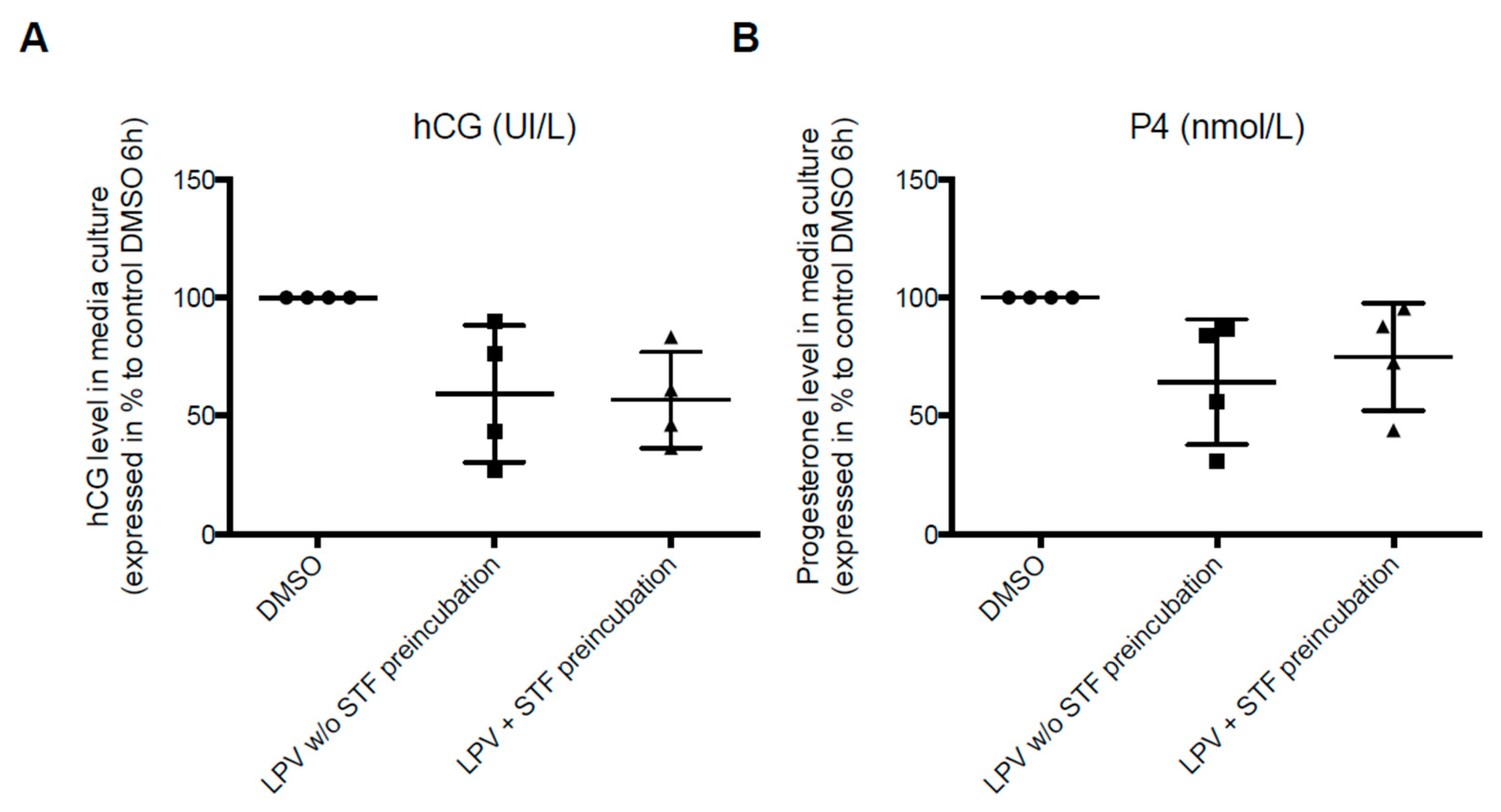
2.2. Effect of LPV on the Villous Trophoblast
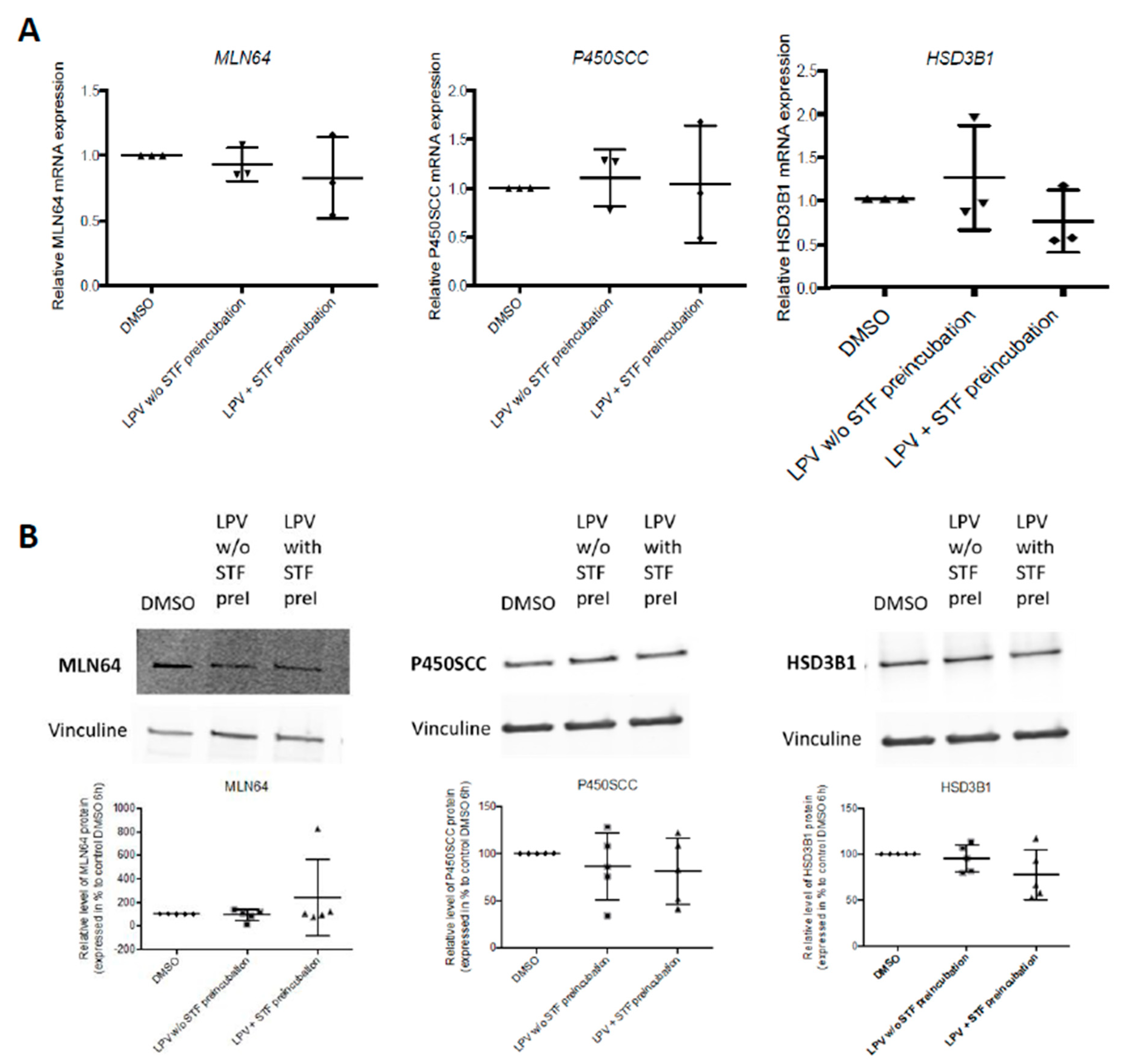
2.3. Expression of Trophoblastic Enzymes Involved in P4 Synthesis during LPV Exposition
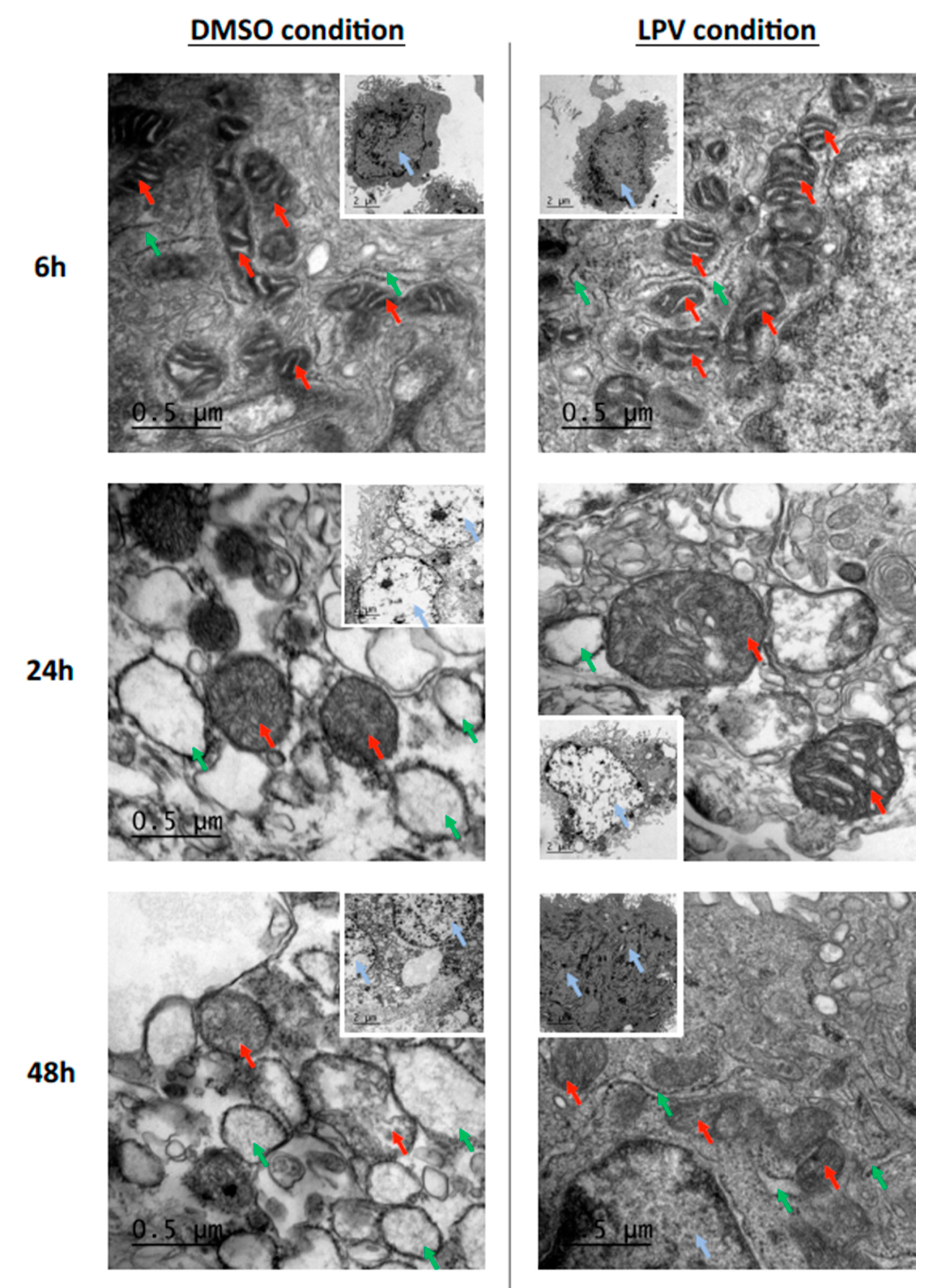
2.4. Trophoblastic Nuclei, Mitochondria and Endoplasmic Reticulum under LPV Treatment
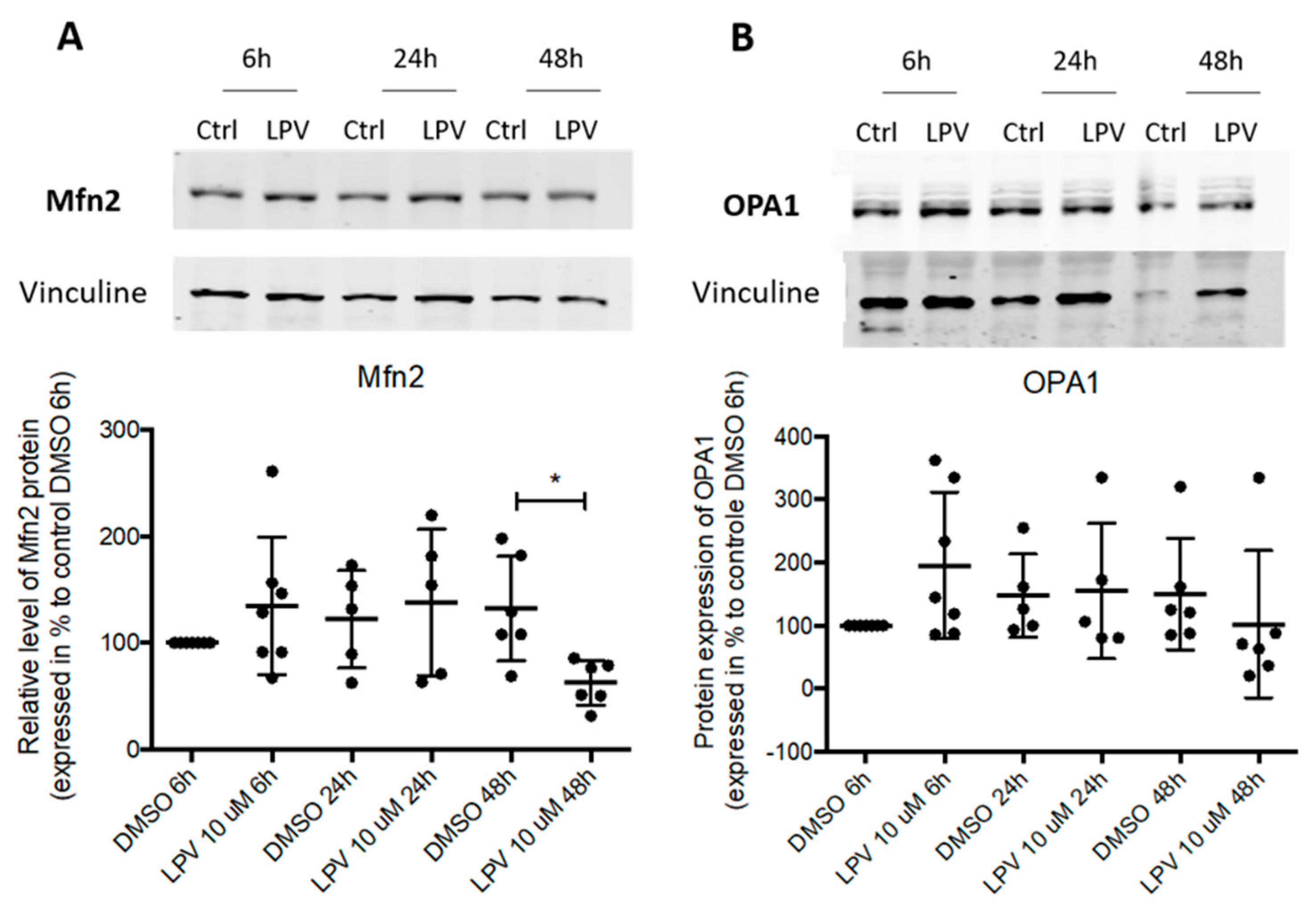
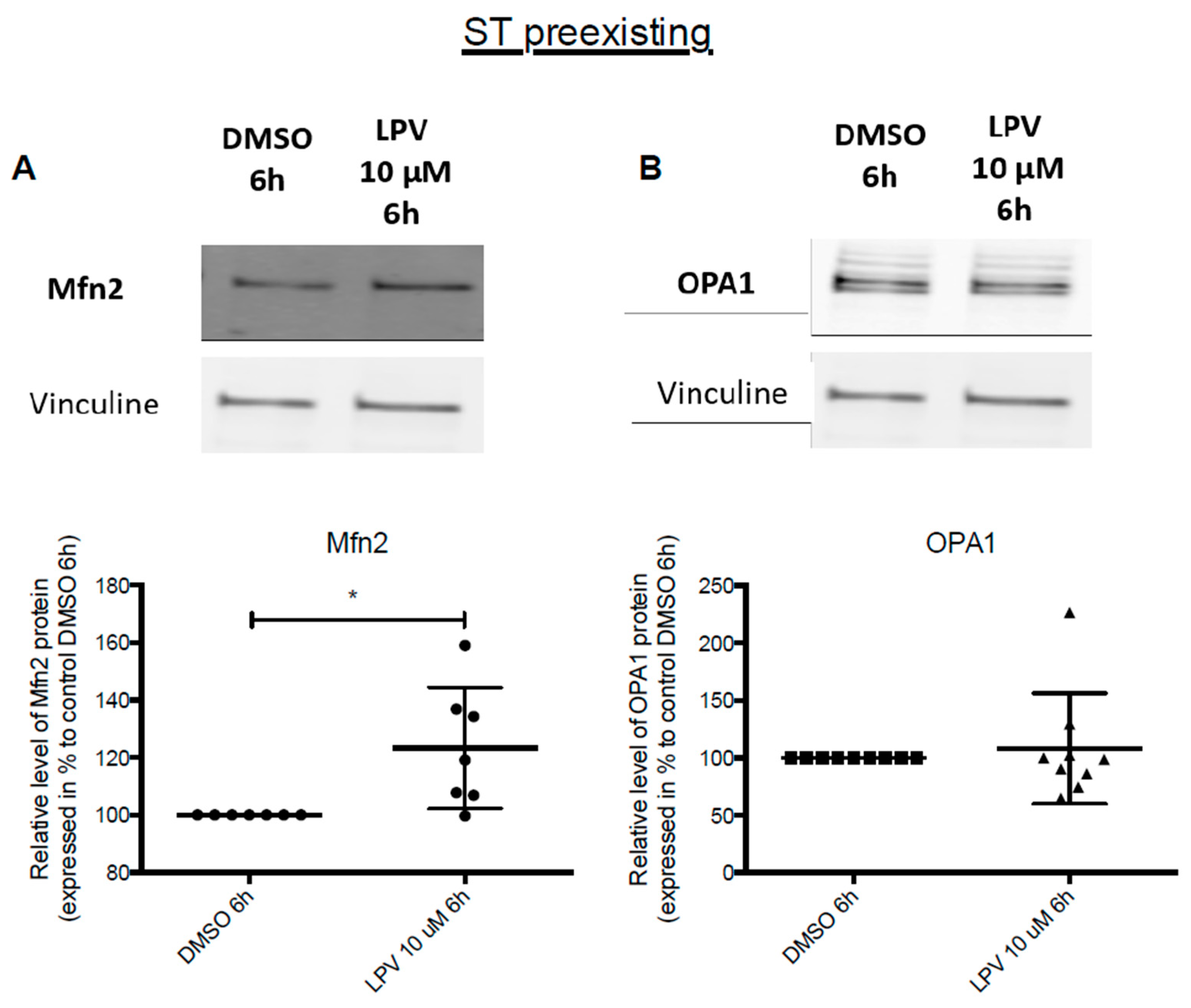
2.5. Mitochondrial Dynamics in Villous Trophoblast under LPV
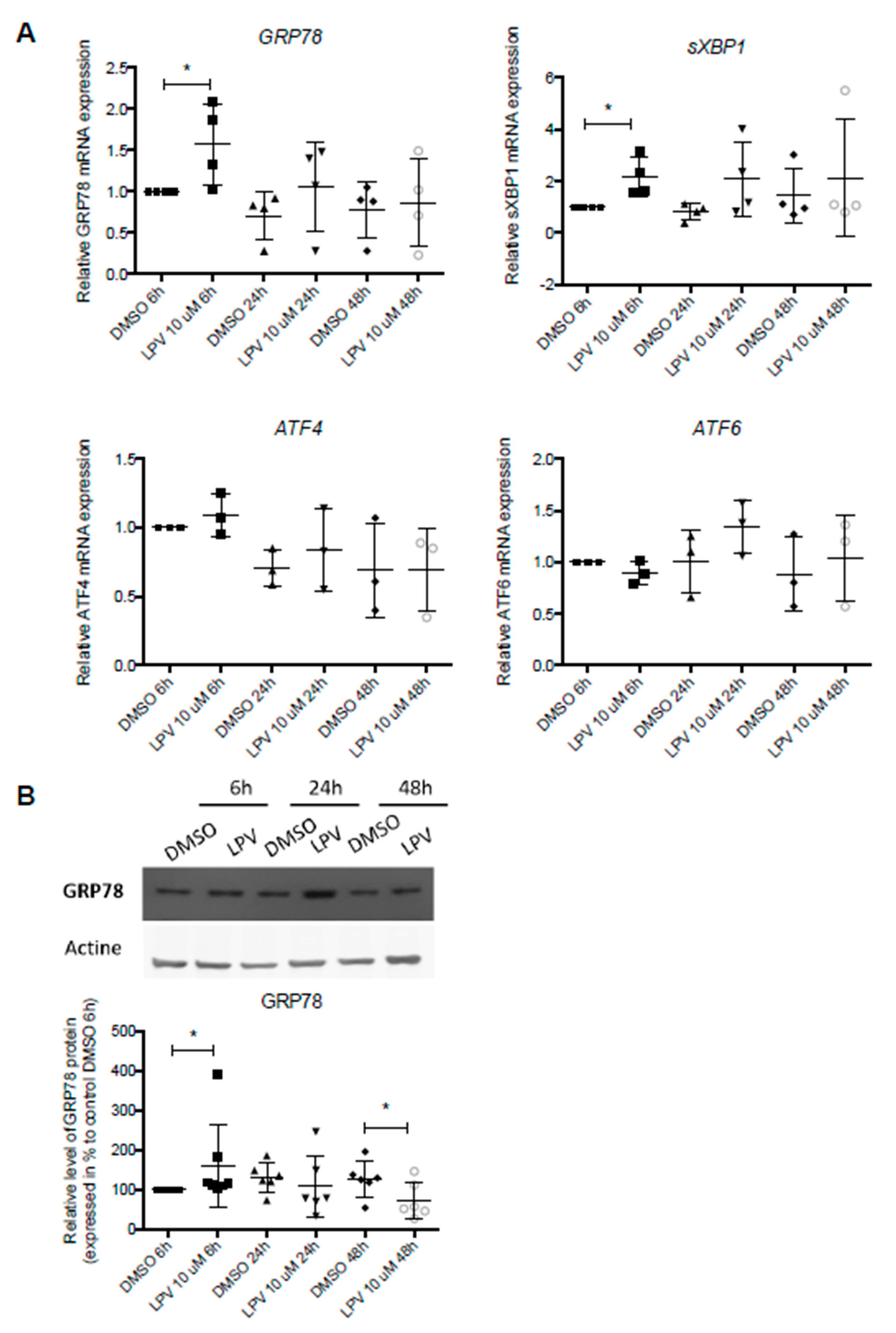
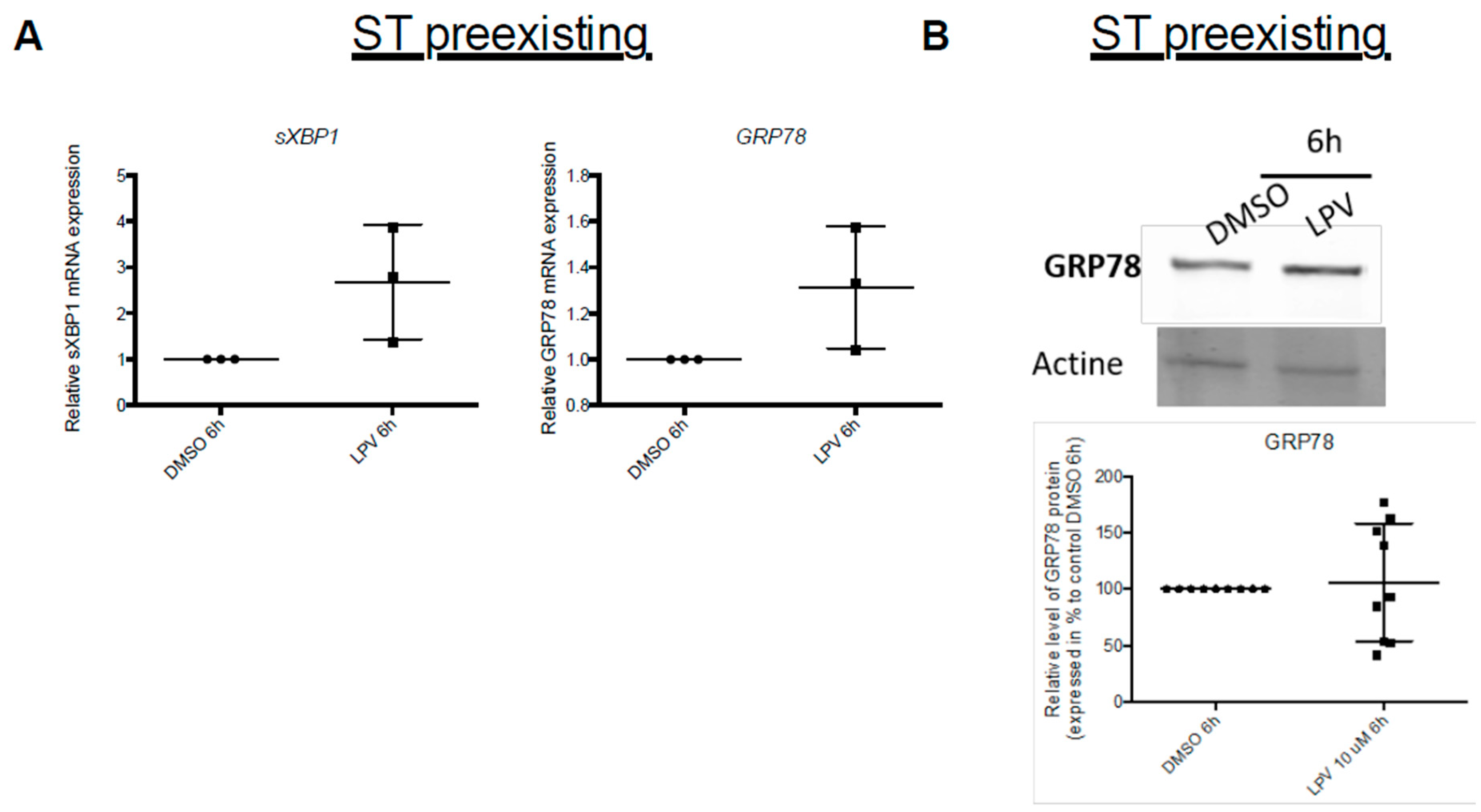
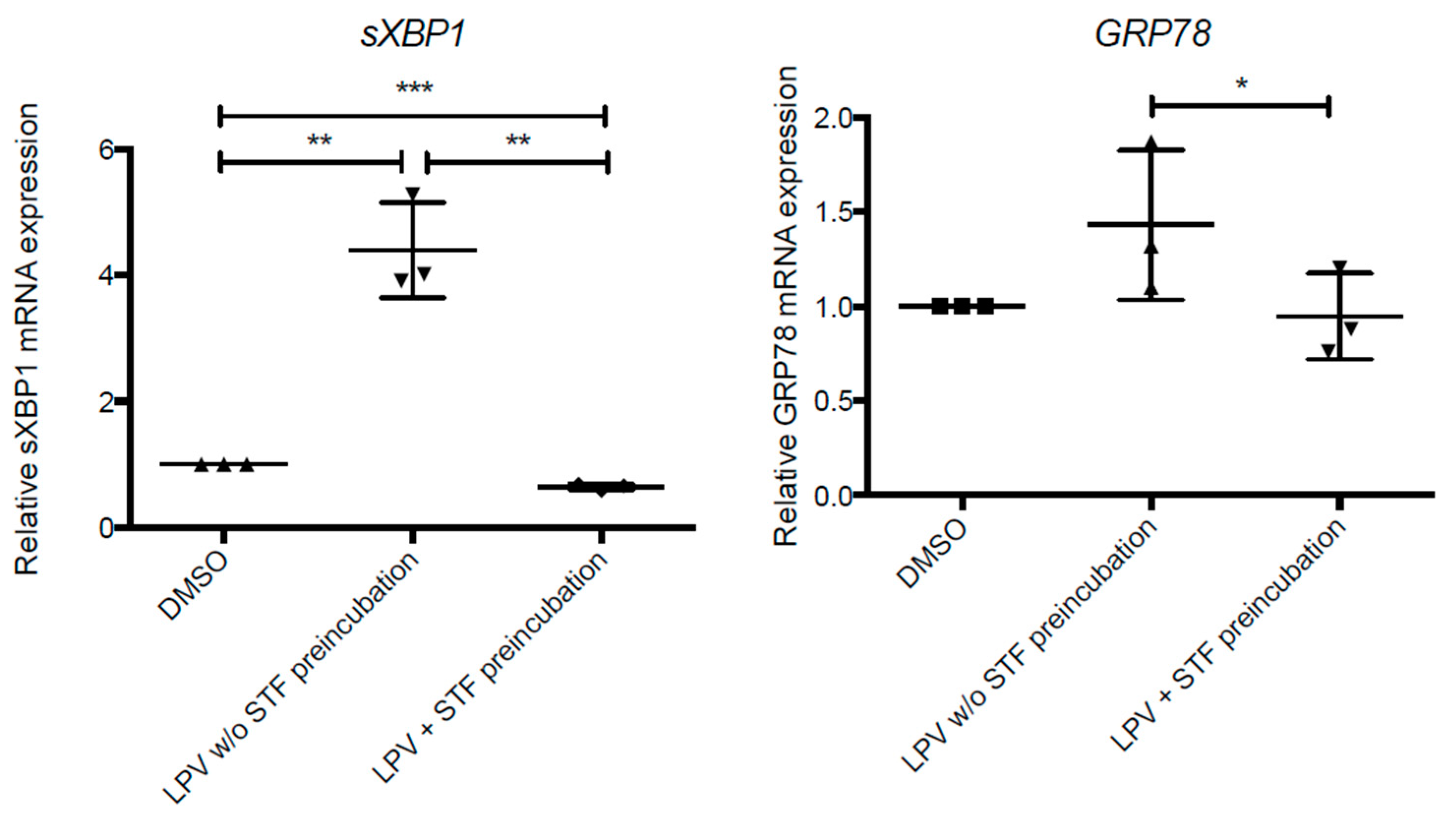
2.6. ER Stress in Villous Trophoblast under LPV
2.7. Effects of LPV on Preformed ST
3. Discussion
4. Materials and Methods
4.1. Placental Tissue Collection
4.2. Isolation and In Vitro Culture of VCT
4.3. Immunofluorescence Microscopy
4.4. Transmission Electronic Microscopy
4.5. Hormones Immuno Assays
4.6. Western Blots
4.7. Reverse Transcription-Quantitative Polymerase Chain Reaction
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Powis, K.M.; Kitch, D.; Ogwu, A.; Hughes, M.D.; Lockman, S.; Leidner, J.; van Widenfelt, E.; Moffat, C.; Moyo, S.; Makhema, J.; et al. Increased Risk of Preterm Delivery among HIV-Infected Women Randomized to Protease Versus Nucleoside Reverse Transcriptase Inhibitor-Based HAART During Pregnancy. J. Infect. Dis. 2011, 204, 506–514. [Google Scholar] [CrossRef]
- Sibiude, J.; Warszawski, J.; Tubiana, R.; Dollfus, C.; Faye, A.; Rouzioux, C.; Teglas, J.-P.; Ekoukou, D.; Blanche, S.; Mandelbrot, L. Premature Delivery in HIV-Infected Women Starting Protease Inhibitor Therapy During Pregnancy: Role of the Ritonavir Boost? Clin. Infect. Dis. 2012, 54, 1348–1360. [Google Scholar] [CrossRef] [PubMed]
- Short, C.-E.S.; Taylor, G.P. Antiretroviral Therapy and Preterm Birth in HIV-Infected Women. Expert Rev. Anti Infect. Ther. 2014, 12, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, D.; Simon, A.; Laborde, K.; Parat, S.; Souchon, P.-F.; Frange, P.; Blanche, S.; Polak, M. Adrenal Enzyme Impairment in Neonates and Adolescents Treated with Ritonavir and Protease Inhibitors for HIV Exposure or Infection. Horm. Res. Paediatr. 2014, 81, 226–231. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.R.; Conroy, A.L.; Gamble, J.L.; Papp, E.; Hawkes, M.; Olwoch, P.; Natureeba, P.; Kamya, M.; Silverman, M.; Cohan, D. Estradiol Levels Are Altered in Human Immunodeficiency Virus-Infected Pregnant Women Randomized to Efavirenz-Versus Lopinavir/Ritonavir-Based Antiretroviral Therapy. Clin. Infect. Dis. 2018, 66, 428. [Google Scholar] [CrossRef]
- Rodway, M.; Zhou, F.; Benoit, J.; Yuen, B.H.; Leung, P.C.K. Differential Effects of 8-Bromo-Cyclic AMP on Human Chorionic Gonadotropin (HCG), Progesterone and Estrogen Production by Term Placental Cells. Life Sci. 1988, 43, 1451–1458. [Google Scholar] [CrossRef]
- Tuckey, R.C. Progesterone Synthesis by the Human Placenta. Placenta 2005, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.C.; Guibourdenche, J.; Frendo, J.L.; Muller, F.; Evain-Brion, D. Human Placental Growth Hormone—A Review. Placenta 2002, 23, S87–S94. [Google Scholar] [CrossRef] [PubMed]
- Fournier, T.; Guibourdenche, J.; Evain-Brion, D. Review: HCGs: Different Sources of Production, Different Glycoforms and Functions. Placenta 2015, 36, S60–S65. [Google Scholar] [CrossRef]
- Fraichard, C.; Bonnet, F.; Garnier, A.; Hébert-Schuster, M.; Bouzerara, A.; Gerbaud, P.; Ferecatu, I.; Fournier, T.; Hernandez, I.; Trabado, S.; et al. Placental Production of Progestins Is Fully Effective in Villous Cytotrophoblasts and Increases with the Syncytiotrophoblast Formation. Mol. Cell. Endocrinol. 2020, 499, 110586. [Google Scholar] [CrossRef]
- Pasqualini, J.R. Enzymes Involved in the Formation and Transformation of Steroid Hormones in the Fetal and Placental Compartments. J. Steroid Biochem. Mol. Biol. 2005, 97, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Pidoux, G.; Gerbaud, P.; Tsatsaris, V.; Marpeau, O.; Ferreira, F.; Meduri, G.; Guibourdenche, J.; Badet, J.; Evain-Brion, D.; Frendo, J.-L. Biochemical Characterization and Modulation of LH/CG-Receptor during Human Trophoblast Differentiation. J. Cell. Physiol. 2007, 212, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Pidoux, G.; Gerbaud, P.; Dompierre, J.; Lygren, B.; Solstad, T.; Evain-Brion, D.; Taskén, K. A PKA–Ezrin–Cx43 Signaling Complex Controls Gap Junction Communication and Thereby Trophoblast Cell Fusion. J. Cell Sci. 2014, 127, 4172–4185. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, P.; Taskén, K.; Pidoux, G. Spatiotemporal Regulation of CAMP Signaling Controls the Human Trophoblast Fusion. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Poidatz, D.; Dos Santos, E.; Gronier, H.; Vialard, F.; Maury, B.; De Mazancourt, P.; Dieudonné, M.-N. Trophoblast Syncytialisation Necessitates Mitochondrial Function through Estrogen-Related Receptor-γ Activation. Mol. Hum. Reprod. 2015, 21, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Bastida-Ruiz, D.; Yart, L.; Wuillemin, C.; Ribaux, P.; Morris, N.; Epiney, M.; Martinez de Tejada, B.; Cohen, M. The Fine-Tuning of Endoplasmic Reticulum Stress Response and Autophagy Activation during Trophoblast Syncytialization. Cell Death Dis. 2019, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Walker, O.S.; Ragos, R.; Wong, M.K.; Adam, M.; Cheung, A.; Raha, S. Reactive Oxygen Species from Mitochondria Impacts Trophoblast Fusion and the Production of Endocrine Hormones by Syncytiotrophoblasts. PLoS ONE 2020, 15, e0229332. [Google Scholar] [CrossRef]
- Martinez, F.; Kiriakidou, M.; Strauss, J.F. Structural and Functional Changes in Mitochondria Associated with Trophoblast Differentiation: Methods to Isolate Enriched Preparations of Syncytiotrophoblast Mitochondria. Endocrinology 1997, 138, 12. [Google Scholar] [CrossRef]
- De Los Rios Castillo, D.; Zarco-Zavala, M.; Olvera-Sanchez, S.; Pardo, J.P.; Juarez, O.; Martinez, F.; Mendoza-Hernandez, G.; García-Trejo, J.J.; Flores-Herrera, O. Atypical Cristae Morphology of Human Syncytiotrophoblast Mitochondria. J. Biol. Chem. 2011, 286, 23911–23919. [Google Scholar] [CrossRef]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial Fusion, Fission, and Mitochondrial Toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef]
- Holland, O.; Dekker Nitert, M.; Gallo, L.A.; Vejzovic, M.; Fisher, J.J.; Perkins, A.V. Review: Placental Mitochondrial Function and Structure in Gestational Disorders. Placenta 2017, 54, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.J.; McKeating, D.R.; Cuffe, J.S.; Bianco-Miotto, T.; Holland, O.J.; Perkins, A.V. Proteomic Analysis of Placental Mitochondria Following Trophoblast Differentiation. Front. Physiol. 2019, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Gaume, B.; Bergmann-Leitner, E.S.; Leitner, W.W.; Robert, E.G.; Catez, F.; Smith, C.L.; Youle, R.J. The Role of Dynamin-Related Protein 1, a Mediator of Mitochondrial Fission, in Apoptosis. Dev. Cell 2001, 1, 515–525. [Google Scholar] [CrossRef]
- Rojo, M.; Legros, F.; Chateau, D.; Lombès, A. Characterization of Mammalian Mitofusins. J. Cell Sci. 2002, 115, 163–1674. [Google Scholar]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 Coordinately Regulate Mitochondrial Fusion and Are Essential for Embryonic Development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef]
- Wasilewski, M.; Semenzato, M.; Rafelski, S.M.; Robbins, J.; Bakardjiev, A.I.; Scorrano, L. Optic Atrophy 1-Dependent Mitochondrial Remodeling Controls Steroidogenesis in Trophoblasts. Curr. Biol. 2012, 22, 1228–1234. [Google Scholar] [CrossRef]
- Cai, H.; Chen, L.; Zhang, M.; Xiang, W.; Su, P. Low Expression of MFN2 Is Associated with Early Unexplained Miscarriage by Regulating Autophagy of Trophoblast Cells. Placenta 2018, 70, 34–40. [Google Scholar] [CrossRef]
- Dal Yontem, F.; Kim, S.; Ding, Z.; Grimm, E.; Ekmekcioglu, S.; Akcakaya, H. Mitochondrial Dynamic Alterations Regulate Melanoma Cell Progression. J. Cell. Biochem. 2019, 120, 2098–2108. [Google Scholar] [CrossRef]
- Shan, A.; Li, M.; Li, X.; Li, Y.; Yan, M.; Xian, P.; Chang, Y.; Chen, X.; Tang, N. BDE-47 Decreases Progesterone Levels in BeWo Cells by Interfering with Mitochondrial Functions and Genes Related to Cholesterol Transport. Chem. Res. Toxicol. 2019, 32, 621–628. [Google Scholar] [CrossRef]
- Bielinska, M.; Boime, I. The Glycoprotein Hormone Family: Structure and Function of the Carbohydrate Chains. In New Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Bastida-Ruiz, D.; Aguilar, E.; Ditisheim, A.; Yart, L.; Cohen, M. Endoplasmic Reticulum Stress Responses in Placentation—A True Balancing Act. Placenta 2017, 57, 163–169. [Google Scholar] [CrossRef]
- Park, H.-J.; Park, S.-J.; Koo, D.-B.; Lee, S.-R.; Kong, I.-K.; Ryoo, J.-W.; Park, Y.-I.; Chang, K.-T.; Lee, D.-S. Progesterone Production Is Affected by Unfolded Protein Response (UPR) Signaling during the Luteal Phase in Mice. Life Sci. 2014, 113, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Papp, E.; Mohammadi, H.; Loutfy, M.R.; Yudin, M.H.; Murphy, K.E.; Walmsley, S.L.; Shah, R.; MacGillivray, J.; Silverman, M.; Serghides, L. HIV Protease Inhibitor Use during Pregnancy Is Associated with Decreased Progesterone Levels, Suggesting a Potential Mechanism Contributing to Fetal Growth Restriction. J. Infect. Dis. 2015, 211, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Mesiano, S.A. Progesterone Signaling in Myometrial Cells: Role in Human Pregnancy and Parturition. Curr. Opin. Physiol. 2020, 13, 117–122. [Google Scholar] [CrossRef]
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Pathology of the Human Placenta, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Handschuh, K.; Guibourdenche, J.; Tsatsaris, V.; Guesnon, M.; Laurendeau, I.; Evain-Brion, D.; Fournier, T. Human Chorionic Gonadotropin Produced by the Invasive Trophoblast but Not the Villous Trophoblast Promotes Cell Invasion and Is Down-Regulated by Peroxisome Proliferator-Activated Receptor-Gamma. Endocrinology 2007, 148, 5011–5019. [Google Scholar] [CrossRef]
- Bustamante, J.; Ramírez-Vélez, R.; Czerniczyniec, A.; Cicerchia, D.; Aguilar de Plata, A.C.; Lores-Arnaiz, S. Oxygen Metabolism in Human Placenta Mitochondria. J. Bioenerg. Biomembr. 2014, 46, 459–469. [Google Scholar] [CrossRef]
- Duarte, A.; Poderoso, C.; Cooke, M.; Soria, G.; Cornejo Maciel, F.; Gottifredi, V.; Podestá, E.J. Mitochondrial Fusion Is Essential for Steroid Biosynthesis. PLoS ONE 2012, 7, e45829. [Google Scholar] [CrossRef]
- Ruiz, M.; Courilleau, D.; Jullian, J.-C.; Fortin, D.; Ventura-Clapier, R.; Blondeau, J.-P.; Garnier, A. A Cardiac-Specific Robotized Cellular Assay Identified Families of Human Ligands as Inducers of PGC-1α Expression and Mitochondrial Biogenesis. PLoS ONE 2012, 7, e46753. [Google Scholar] [CrossRef]
- Guzel, E.; Arlier, S.; Guzeloglu-Kayisli, O.; Tabak, M.S.; Ekiz, T.; Semerci, N.; Larsen, K.; Schatz, F.; Lockwood, C.J.; Kayisli, U.A. Endoplasmic Reticulum Stress and Homeostasis in Reproductive Physiology and Pathology. Int. J. Mol. Sci. 2017, 18, 792. [Google Scholar] [CrossRef]
- Burton, G.J.; Yung, H.W.; Murray, A.J. Mitochondrial—Endoplasmic Reticulum Interactions in the Trophoblast: Stress and Senescence. Placenta 2017, 52, 146–155. [Google Scholar] [CrossRef]
- Taura, M.; Kariya, R.; Kudo, E.; Goto, H.; Iwawaki, T.; Amano, M.; Suico, M.A.; Kai, H.; Mitsuya, H.; Okada, S. Comparative Analysis of ER Stress Response into HIV Protease Inhibitors: Lopinavir but Not Darunavir Induces Potent ER Stress Response via ROS/JNK Pathway. Free Radic. Biol. Med. 2013, 65, 778–788. [Google Scholar] [CrossRef]
- Yeh, R.F.; Gaver, V.E.; Patterson, K.B.; Rezk, N.L.; Baxter-Meheux, F.; Blake, M.J.; Eron, J.J.J.; Klein, C.E.; Rublein, J.C.; Kashuba, A.D.M. Lopinavir/Ritonavir Induces the Hepatic Activity of Cytochrome P450 Enzymes CYP2C9, CYP2C19, and CYP1A2 But Inhibits the Hepatic and Intestinal Activity of CYP3A as Measured by a Phenotyping Drug Cocktail in Healthy Volunteers. JAIDS J. Acquir. Immune Defic. Syndr. 2006, 42, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Besse, A.; Stolze, S.C.; Rasche, L.; Weinhold, N.; Morgan, G.J.; Kraus, M.; Bader, J.; Overkleeft, H.S.; Besse, L.; Driessen, C. Carfilzomib Resistance Due to ABCB1/MDR1 Overexpression Is Overcome by Nelfinavir and Lopinavir in Multiple Myeloma. Leukemia 2018, 32, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Papp, E.; Balogun, K.; Banko, N.; Mohammadi, H.; Loutfy, M.; Yudin, M.H.; Shah, R.; MacGillivray, J.; Murphy, K.E.; Walmsley, S.L.; et al. Low Prolactin and High 20-α-Hydroxysteroid Dehydrogenase Levels Contribute to Lower Progesterone Levels in HIV-Infected Pregnant Women Exposed to Protease Inhibitor–Based Combination Antiretroviral Therapy. J. Infect. Dis. 2016, 213, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Hong-Brown, L.Q.; Brown, C.R.; Huber, D.S.; Lang, C.H. Lopinavir Impairs Protein Synthesis and Induces EEF2 Phosphorylation via the Activation of AMP-Activated Protein Kinase. J. Cell. Biochem. 2008, 105, 814–823. [Google Scholar] [CrossRef]
- Yung, Y.; Maman, E.; Ophir, L.; Rubinstein, N.; Barzilay, E.; Yerushalmi, G.M.; Hourvitz, A. Progesterone Antagonist, RU486, Represses LHCGR Expression and LH/HCG Signaling in Cultured Luteinized Human Mural Granulosa Cells. Gynecol. Endocrinol. 2014, 30, 42–47. [Google Scholar] [CrossRef]
- Guitart-Mampel, M.; Hernandez, A.S.; Moren, C.; Catalan-Garcia, M.; Tobias, E.; Gonzalez-Casacuberta, I.; Juarez-Flores, D.L.; Gatell, J.M.; Cardellach, F.; Milisenda, J.C.; et al. Imbalance in Mitochondrial Dynamics and Apoptosis in Pregnancies among HIV-Infected Women on HAART with Obstetric Complications. J. Antimicrob. Chemother. 2017. [Google Scholar] [CrossRef]
- Ganta, K.K.; Chaubey, B. Endoplasmic Reticulum Stress Leads to Mitochondria-Mediated Apoptosis in Cells Treated with Anti-HIV Protease Inhibitor Ritonavir. Cell Biol. Toxicol. 2019, 35, 189–204. [Google Scholar] [CrossRef]
- Chen, L.; Jarujaron, S.; Wu, X.; Sun, L.; Zha, W.; Liang, G.; Gurley, E.C.; Studer, E.J.; Hylemon, P.B.; Pandak, W.M.; et al. HIV Protease Inhibitor Lopinavir-Induced TNF-a and IL-6 Expression Is Coupled to the Unfolded Protein Response and ERK Signaling Pathways in Macrophages. Biochem. Pharmacol. 2009, 78, 70–77. [Google Scholar] [CrossRef]
- Zha, B.S.; Wan, X.; Zhang, X.; Zha, W.; Zhou, J.; Wabitsch, M.; Wang, G.; Lyall, V.; Hylemon, P.B.; Zhou, H. HIV Protease Inhibitors Disrupt Lipid Metabolism by Activating Endoplasmic Reticulum Stress and Inhibiting Autophagy Activity in Adipocytes. PLoS ONE 2013, 8, e59514. [Google Scholar] [CrossRef]
- Brüning, A.; Kimmich, T.; Brem, G.J.; Buchholtz, M.L.; Mylonas, I.; Kost, B.; Weizsäcker, K.; Gingelmaier, A. Analysis of Endoplasmic Reticulum Stress in Placentas of HIV-Infected Women Treated with Protease Inhibitors. Reprod. Toxicol. 2014, 50, 122–128. [Google Scholar] [CrossRef]
- Gallagher, C.M.; Garri, C.; Cain, E.L.; Ang, K.K.-H.; Wilson, C.G.; Chen, S.; Hearn, B.R.; Jaishankar, P.; Aranda-Diaz, A.; Arkin, M.R.; et al. Ceapins Are a New Class of Unfolded Protein Response Inhibitors, Selectively Targeting the ATF6α Branch. eLife 2016, 5, e11878. [Google Scholar] [CrossRef] [PubMed]
- Alsat, E.; Haziza, J.; Evain-Brion, D. Increase in Epidermal Growth Factor Receptor and Its Messenger Ribonucleic Acid Levels with Differentiation of Human Trophoblast Cells in Culture. J. Cell. Physiol. 1993, 154, 122–128. [Google Scholar] [CrossRef] [PubMed]





| Target Protein | Concentration | Species | Clonality | Reference |
|---|---|---|---|---|
| MLN64 | 1 µg/mL | Rabbit | Polyclonal | ab3478 abcam® |
| P450SCC | 1 µg/mL | Rabbit | Polyclonal | ab75497 abcam® |
| HSD3B1 | 0.18 µg/mL | Rabbit | Monoclonal | ab167417 abcam® |
| Mfn2 | 1 µg/mL | Mouse | Monoclonal | ab56889 abcam® |
| OPA1 | 1 µg/mL | Rabbit | Polyclonal | ab42364 abcam® |
| GRP78 | 1 µg/mL | Rabbit | Polyclonal | G8918 Sigma-Aldrich |
| Actine | 0.2 µg/mL | Mouse | Monoclonal | A5441 Sigma-Aldrich |
| Vinculine | 1 µg/mL | Mouse | Monoclonal | V9131 Sigma-Aldrich |
| Target mRNA | Gene Name | Sequences |
|---|---|---|
| MLN64 | STARD3 | Forward GAGCGATGGTATCTTGCCGC Reverse CTGCAAAGGATTCTGGGGGT |
| P450SCC | CYP11A1 | Forward TTTTTGCCCCTGTTGGATGCA Reverse CCCTGGCGCTCCCCAAAAAT |
| HSD3B1 | HSD3B1 | Forward AGTACGTCCACTCTTCTGTCCA Reverse TTCTCCTTCACCAAGAGGCG |
| GRP78 | GRP78 | Forward CGTGGAGATCATCGCCAAC Reverse ACATAGGACGGCGTGATGC |
| sXBP1 | sXBP1 | Forward CTGAGTCCGAATCAGGTGCAG Reverse ATCCATGGGGAGATGTTCTGG |
| ATF4 | ATF4 | Forward GTTCTCCAGCGACAAGGCTA Reverse ATCCTGCTTGCTGTTGTTGG |
| ATF6 | ATF6 | Forward GAGTATTTTGTCCGCCTGCC Reverse CGGGCTAAAAGGTGACTCCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraichard, C.; Bonnet-Serrano, F.; Laguillier-Morizot, C.; Hebert-Schuster, M.; Lai-Kuen, R.; Sibiude, J.; Fournier, T.; Cohen, M.; Guibourdenche, J. Protease Inhibitor Anti-HIV, Lopinavir, Impairs Placental Endocrine Function. Int. J. Mol. Sci. 2021, 22, 683. https://doi.org/10.3390/ijms22020683
Fraichard C, Bonnet-Serrano F, Laguillier-Morizot C, Hebert-Schuster M, Lai-Kuen R, Sibiude J, Fournier T, Cohen M, Guibourdenche J. Protease Inhibitor Anti-HIV, Lopinavir, Impairs Placental Endocrine Function. International Journal of Molecular Sciences. 2021; 22(2):683. https://doi.org/10.3390/ijms22020683
Chicago/Turabian StyleFraichard, Camille, Fidéline Bonnet-Serrano, Christelle Laguillier-Morizot, Marylise Hebert-Schuster, René Lai-Kuen, Jeanne Sibiude, Thierry Fournier, Marie Cohen, and Jean Guibourdenche. 2021. "Protease Inhibitor Anti-HIV, Lopinavir, Impairs Placental Endocrine Function" International Journal of Molecular Sciences 22, no. 2: 683. https://doi.org/10.3390/ijms22020683
APA StyleFraichard, C., Bonnet-Serrano, F., Laguillier-Morizot, C., Hebert-Schuster, M., Lai-Kuen, R., Sibiude, J., Fournier, T., Cohen, M., & Guibourdenche, J. (2021). Protease Inhibitor Anti-HIV, Lopinavir, Impairs Placental Endocrine Function. International Journal of Molecular Sciences, 22(2), 683. https://doi.org/10.3390/ijms22020683








