Erythropoietin Receptor (EPOR) Signaling in the Osteoclast Lineage Contributes to EPO-Induced Bone Loss in Mice
Abstract
1. Introduction
2. Results
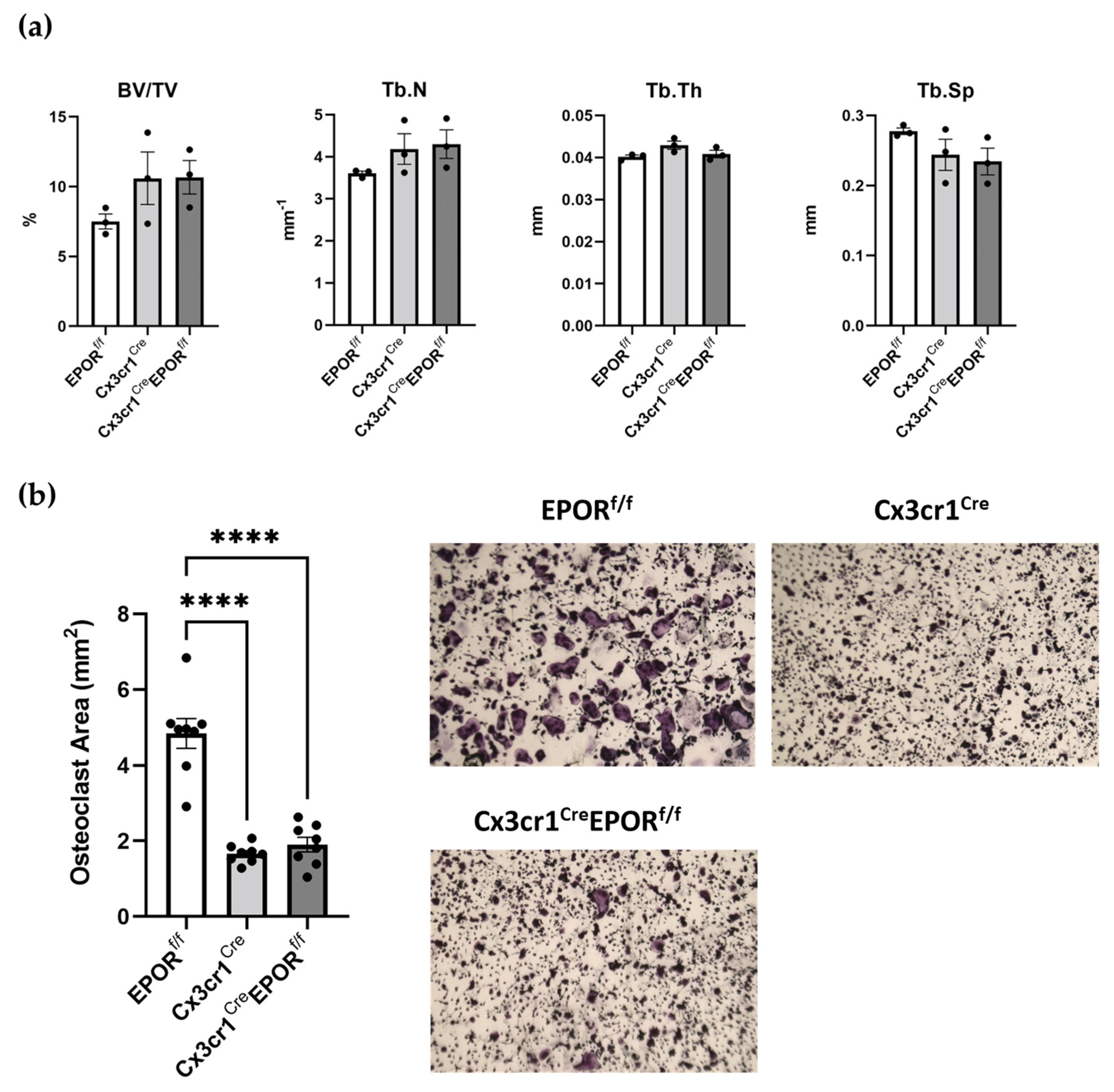
2.1. The Skeletal Effect of EPOR Deletion in the Monocytic Lineage Using Cx3cr1Cre
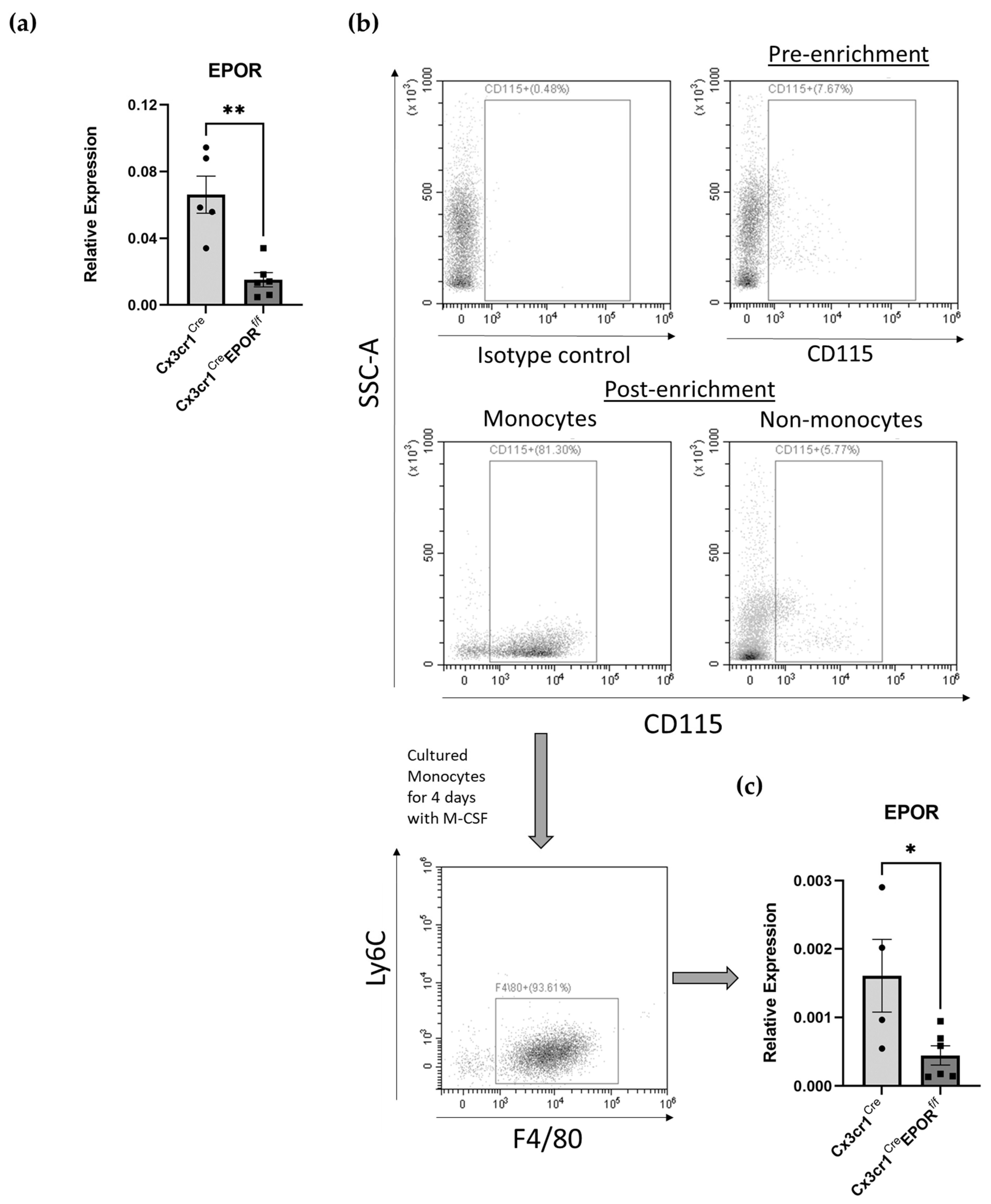
2.2. Confirmation of Conditional EPOR Deletion in the Monocytic Lineage
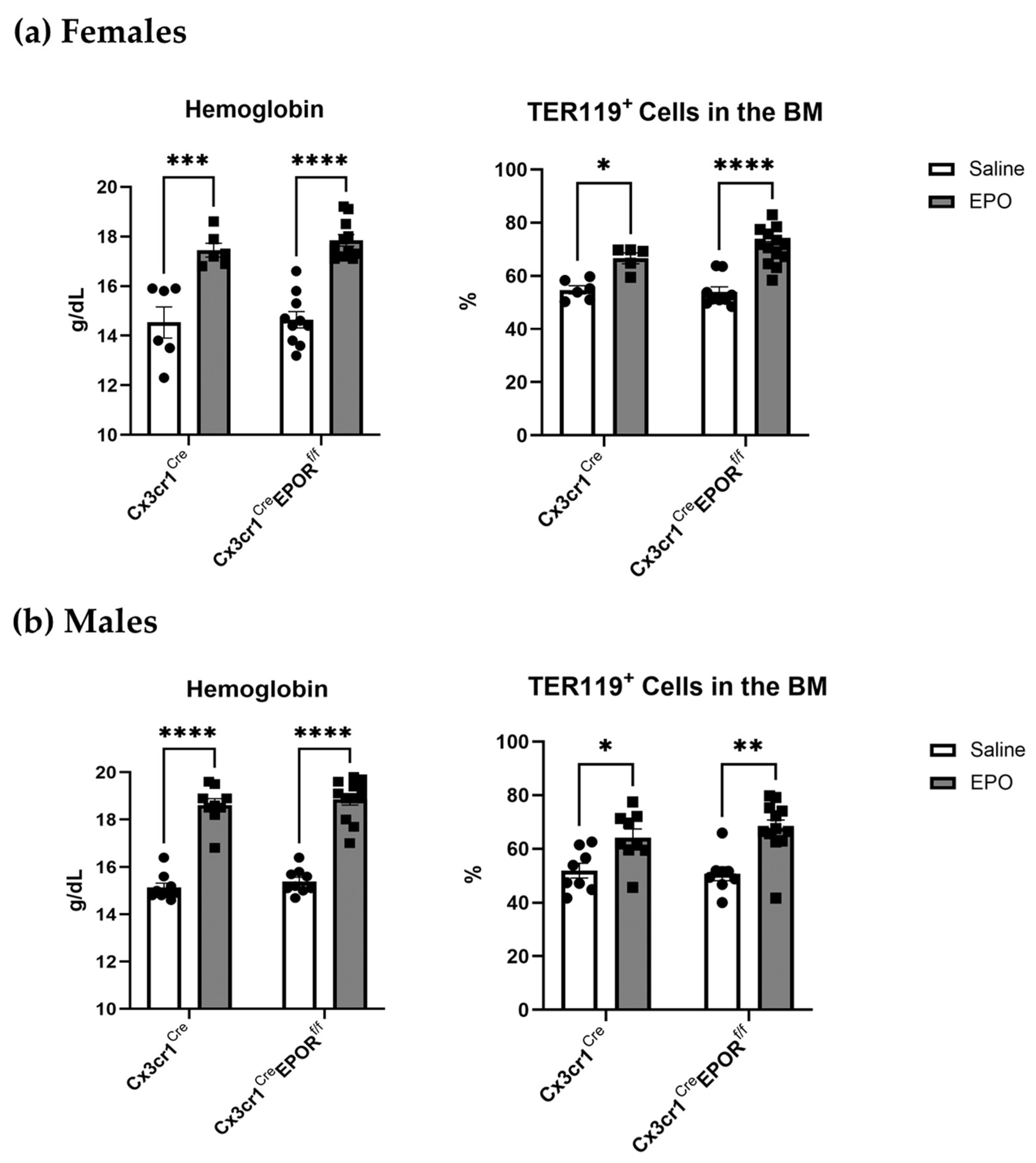
2.3. EPO Erythropoietic Activity Is Preserved in the Cx3cr1Cre EPORf/f Mice
2.4. Cx3cr1Cre EPORf/f Mice Are Partially Protected against EPO-Induced Bone Loss
2.5. Low-Dose EPO Does Not Affect Osteoclast Progenitors
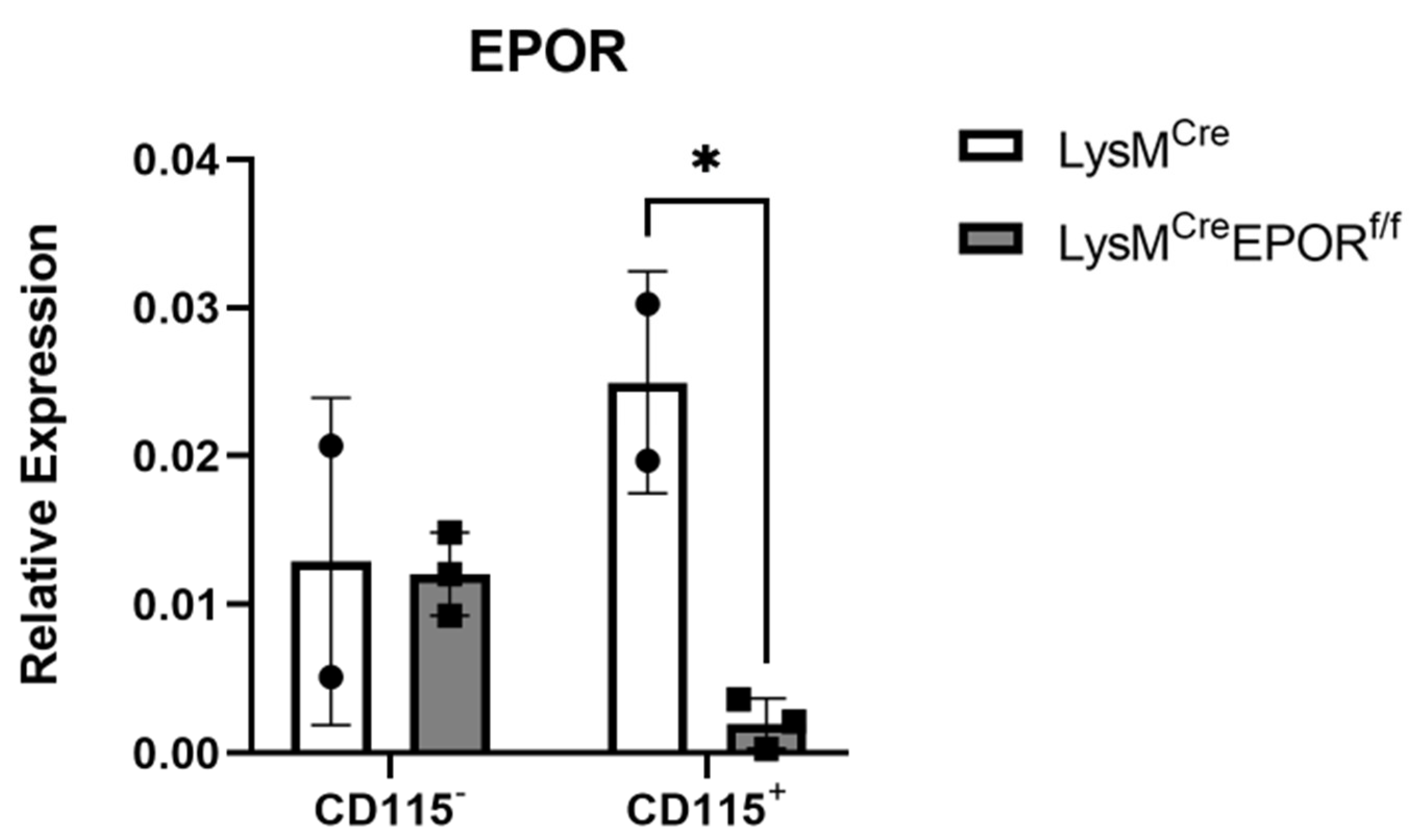
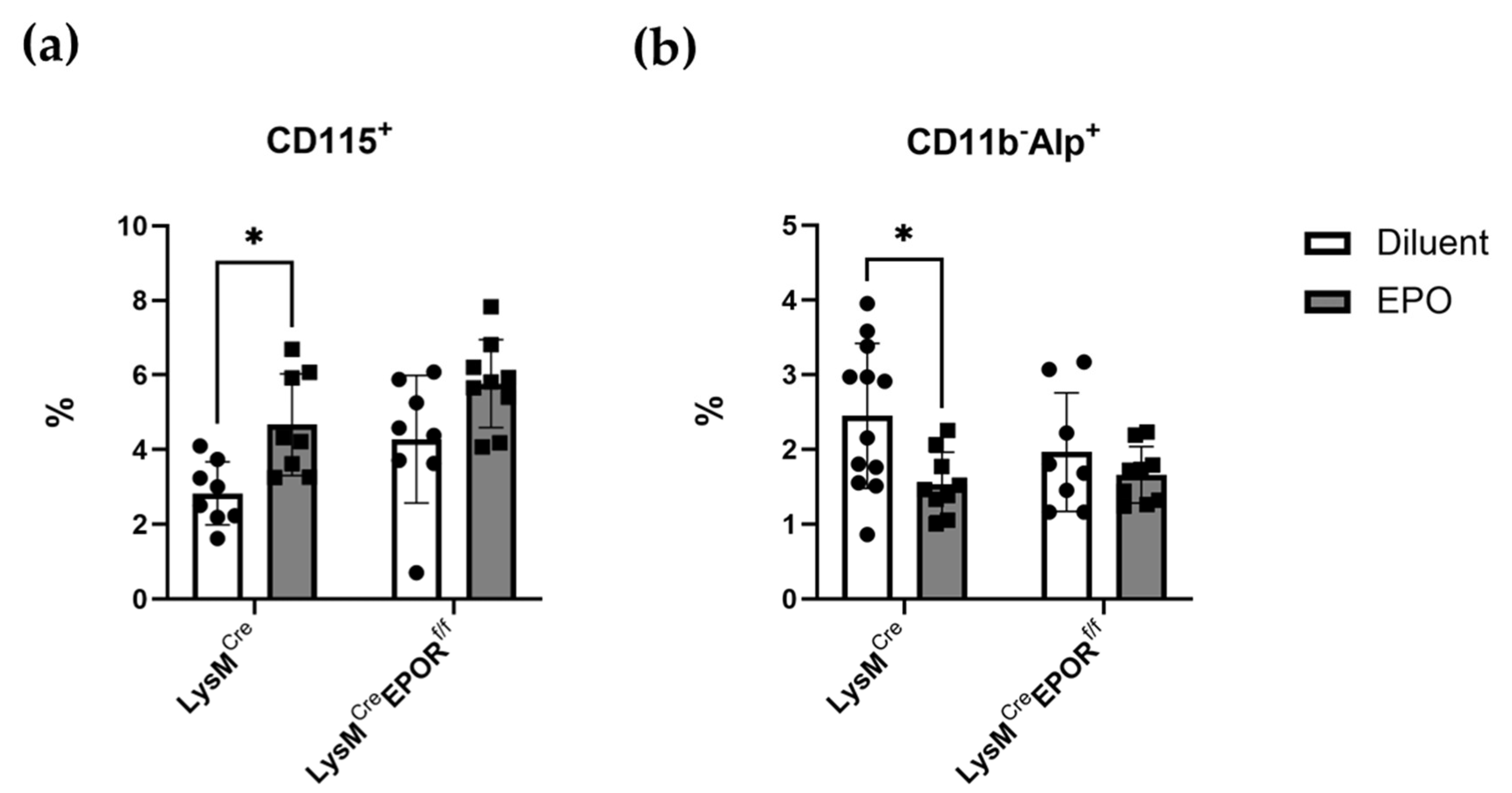
2.6. LysMCre Mediates the Conditional Deletion of EPOR in Preosteoclasts
2.7. The Skeletal Effect of EPO on Both Osteoclast and Osteoblast Precursors Is Partially Mediated by Monocyte EPOR
2.8. Conditional Deletion of EPOR in Preosteoclasts Mitigates EPO-Induced Bone Loss in LysMCreEPORf/f Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Cell Culture
4.3.1. Isolation and Culture of Bone Marrow-Derived Monocytes
4.3.2. Isolation of CD115+ Monocytes
4.4. Microcomputed Tomography (μCT)
4.5. Hemoglobin Levels
4.6. Flow Cytometry
4.7. Real-Time RT PCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Liu, X.; Jaenisch, R.; Lodish, H.F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 1995, 83, 59–67. [Google Scholar] [CrossRef]
- Sasaki, R.; Masuda, S.; Nagao, M. Erythropoietin: Multiple physiological functions and regulation of biosynthesis. Biosci. Biotechnol. Biochem. 2000, 64, 1775–1793. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Lim, S.K.; D’Agati, V.; Costantini, F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996, 10, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Spivak, J.L.; Pham, T.; Isaacs, M.; Hankins, W.D. Erythropoietin is both a mitogen and a survival factor. Blood 1991, 77, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Ramanath, V.; Gupta, D.; Jain, J.; Chaudhary, K.; Nistala, R. Anemia and chronic kidney disease: Making sense of the recent trials. Rev. Recent Clin. Trials 2012, 7, 187–196. [Google Scholar] [CrossRef]
- Wish, J.B. Past, Present, and Future of Chronic Kidney Disease Anemia Management in the United States. Adv. Chronic Kidney Dis. 2009, 16, 101–108. [Google Scholar] [CrossRef]
- Ohashi, Y.; Uemura, Y.; Fujisaka, Y.; Sugiyama, T.; Ohmatsu, H.; Katsumata, N.; Okamoto, R.; Saijo, N.; Hotta, T. Meta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: Individual patient data from Japanese randomized, placebo-controlled trials. Cancer Sci. 2013, 104, 481–485. [Google Scholar] [CrossRef]
- Masuda, S.; Nagao, M.; Takahata, K.; Konishi, Y.; Gallyas, F., Jr.; Tabira, T.; Sasaki, R. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J. Biol. Chem. 1993, 268, 11208–11216. [Google Scholar] [CrossRef]
- Yu, X.; Shacka, J.J.; Eells, J.B.; Suarez-Quian, C.; Przygodzki, R.M.; Beleslin-Cokic, B.; Lin, C.S.; Nikodem, V.M.; Hempstead, B.; Flanders, K.C.; et al. Erythropoietin receptor signalling is required for normal brain development. Development 2002, 129, 505–516. [Google Scholar] [CrossRef]
- Tsai, P.T.; Ohab, J.J.; Kertesz, N.; Groszer, M.; Matter, C.; Gao, J.; Liu, X.; Wu, H.; Carmichael, S.T. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J. Neurosci. 2006, 26, 1269–1274. [Google Scholar] [CrossRef]
- Kertesz, N.; Wu, J.; Chen, T.H.; Sucov, H.M.; Wu, H. The role of erythropoietin in regulating angiogenesis. Dev. Biol. 2004, 276, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, A.; Lee, E.S.; Kessimian, N.; Levinson, R.; Steiner, M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc. Natl. Acad. Sci. USA 1990, 87, 5978–5982. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, M.; Yu, X.; Nicolas-Metral, V.; Pulido, S.M.; Liu, C.; Ruegg, U.T.; Noguchi, C.T. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J. Biol. Chem. 2000, 275, 39754–39761. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Suzuki, N.; Yamamoto, M.; Gassmann, M.; Noguchi, C.T. Endogenous erythropoietin signaling facilitates skeletal muscle repair and recovery following pharmacologically induced damage. FASEB J. 2012, 26, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, L.; Tabak, G.; Gassmann, M.; Mittelman, M.; Neumann, D. Macrophages as novel target cells for erythropoietin. Haematologica 2010, 95, 1823–1831. [Google Scholar] [CrossRef]
- Alnaeeli, M.; Raaka, B.M.; Gavrilova, O.; Teng, R.; Chanturiya, T.; Noguchi, C.T. Erythropoietin signaling: A novel regulator of white adipose tissue inflammation during diet-induced obesity. Diabetes 2014, 63, 2415–2431. [Google Scholar] [CrossRef]
- Suresh, S.; de Castro, L.F.; Dey, S.; Robey, P.G.; Noguchi, C.T. Erythropoietin modulates bone marrow stromal cell differentiation. Bone Res. 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Hiram-Bab, S.; Liron, T.; Deshet-Unger, N.; Mittelman, M.; Gassmann, M.; Rauner, M.; Franke, K.; Wielockx, B.; Neumann, D.; Gabet, Y. Erythropoietin directly stimulates osteoclast precursors and induces bone loss. FASEB J. 2015, 29, 1890–1900. [Google Scholar] [CrossRef]
- Rauner, M.; Franke, K.; Murray, M.; Singh, R.P.; Hiram-Bab, S.; Platzbecker, U.; Gassmann, M.; Socolovsky, M.; Neumann, D.; Gabet, Y.; et al. Increased EPO Levels Are Associated With Bone Loss in Mice Lacking PHD2 in EPO-Producing Cells. J. Bone Min. Res. 2016, 31, 1877–1887. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Jung, Y.; Ziegler, A.M.; Pedersen, E.A.; Wang, J.; Wang, Z.; Song, J.; Lee, C.H.; Sud, S.; Pienta, K.J.; et al. Erythropoietin couples hematopoiesis with bone formation. PLoS ONE 2010, 5, e10853. [Google Scholar] [CrossRef]
- Rauner, M.; Murray, M.; Thiele, S.; Watts, D.; Neumann, D.; Gabet, Y.; Hofbauer, L.C.; Wielockx, B. Epo/EpoR signaling in osteoprogenitor cells is essential for bone homeostasis and Epo-induced bone loss. Bone Res. 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Lee, J.; Noguchi, C.T. Erythropoietin signaling in osteoblasts is required for normal bone formation and for bone loss during erythropoietin-stimulated erythropoiesis. FASEB J. 2020, 34, 11685–11697. [Google Scholar] [CrossRef] [PubMed]
- Rolfing, J.H.; Baatrup, A.; Stiehler, M.; Jensen, J.; Lysdahl, H.; Bunger, C. The osteogenic effect of erythropoietin on human mesenchymal stromal cells is dose-dependent and involves non-hematopoietic receptors and multiple intracellular signaling pathways. Stem Cell Rev. Rep. 2014, 10, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Singbrant, S.; Russell, M.R.; Jovic, T.; Liddicoat, B.; Izon, D.J.; Purton, L.E.; Sims, N.A.; Martin, T.J.; Sankaran, V.G.; Walkley, C.R. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood 2011, 117, 5631–5642. [Google Scholar] [CrossRef]
- Suresh, S.; Alvarez, J.C.; Dey, S.; Noguchi, C.T. Erythropoietin-Induced Changes in Bone and Bone Marrow in Mouse Models of Diet-Induced Obesity. Int. J. Mol. Sci. 2020, 21, 1657. [Google Scholar] [CrossRef]
- Kolomansky, A.; Hiram-Bab, S.; Ben-Califa, N.; Liron, T.; Deshet-Unger, N.; Mittelman, M.; Oster, H.S.; Rauner, M.; Wielockx, B.; Neumann, D.; et al. Erythropoietin Mediated Bone Loss in Mice Is Dose-Dependent and Mostly Irreversible. Int. J. Mol. Sci. 2020, 21, 3817. [Google Scholar] [CrossRef]
- Kristjansdottir, H.L.; Lewerin, C.; Lerner, U.H.; Herlitz, H.; Johansson, P.; Johansson, H.; Karlsson, M.; Lorentzon, M.; Ohlsson, C.; Ljunggren, O.; et al. High Plasma Erythropoietin Predicts Incident Fractures in Elderly Men with Normal Renal Function: The MrOS Sweden Cohort. J. Bone Min. Res. 2020, 35, 298–305. [Google Scholar] [CrossRef]
- Suresh, S.; Wright, E.C.; Wright, D.G.; Abbott, K.C.; Noguchi, C.T. Erythropoietin treatment and the risk of hip fractures in hemodialysis patients. J. Bone Min. Res. 2021, 36, 1211–1219. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Kuo, Y.J. Single-centre cross-sectional study on the impact of cumulative erythropoietin on bone mineral density in maintenance dialysis patients. BMJ Open 2022, 12, e056390. [Google Scholar] [CrossRef]
- Kristjansdottir, H.L.; Mellstrom, D.; Johansson, P.; Karlsson, M.; Vandenput, L.; Lorentzon, M.; Herlitz, H.; Ohlsson, C.; Lerner, U.H.; Lewerin, C. Anemia is associated with increased risk of non-vertebral osteoporotic fractures in elderly men: The MrOS Sweden cohort. Arch. Osteoporos. 2022, 17, 85. [Google Scholar] [CrossRef]
- Hiram-Bab, S.; Neumann, D.; Gabet, Y. Context-Dependent Skeletal Effects of Erythropoietin. Vitam. Horm. 2017, 105, 161–179. [Google Scholar] [PubMed]
- Li, C.; Shi, C.; Kim, J.; Chen, Y.; Ni, S.; Jiang, L.; Zheng, C.; Li, D.; Hou, J.; Taichman, R.S.; et al. Erythropoietin promotes bone formation through EphrinB2/EphB4 signaling. J. Dent. Res. 2015, 94, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef]
- Karsenty, G.; Kronenberg, H.M.; Settembre, C. Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 2009, 25, 629–648. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Osteoclasts: What do they do and how do they do it? Am. J. Pathol. 2007, 170, 427–435. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Xie, X.; Gu, F.; Sui, Z.; Zhang, K.; Yu, T. Recent Advances in Osteoclast Biological Behavior. Front. Cell Dev. Biol. 2021, 9, 788680. [Google Scholar] [CrossRef]
- Merimi, M.; El-Majzoub, R.; Lagneaux, L.; Moussa Agha, D.; Bouhtit, F.; Meuleman, N.; Fahmi, H.; Lewalle, P.; Fayyad-Kazan, M.; Najar, M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front. Cell Dev. Biol. 2021, 9, 661532. [Google Scholar] [CrossRef]
- Tan, S.H.S.; Wong, J.R.Y.; Sim, S.J.Y.; Tjio, C.K.E.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater. Today Bio 2020, 7, 100067. [Google Scholar] [CrossRef]
- Codispoti, B.; Marrelli, M.; Paduano, F.; Tatullo, M. NANOmetric BIO-Banked MSC-Derived Exosome (NANOBIOME) as a Novel Approach to Regenerative Medicine. J. Clin. Med. 2018, 7, 357. [Google Scholar] [CrossRef]
- Deshet-Unger, N.; Kolomansky, A.; Ben-Califa, N.; Hiram-Bab, S.; Gilboa, D.; Liron, T.; Ibrahim, M.; Awida, Z.; Gorodov, A.; Oster, H.S.; et al. Erythropoietin receptor in B cells plays a role in bone remodeling in mice. Theranostics 2020, 10, 8744–8756. [Google Scholar] [CrossRef]
- Hiram-Bab, S.; Neumann, D.; Gabet, Y. Erythropoietin in bone—Controversies and consensus. Cytokine 2017, 89, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Awida, Z.; Bachar, A.; Saed, H.; Gorodov, A.; Ben-Califa, N.; Ibrahim, M.; Kolomansky, A.; Iden, J.A.; Graniewitz Visacovsky, L.; Liron, T.; et al. The Non-Erythropoietic EPO Analogue Cibinetide Inhibits Osteoclastogenesis In Vitro and Increases Bone Mineral Density in Mice. Int. J. Mol. Sci. 2021, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Clausen, B.E.; Burkhardt, C.; Reith, W.; Renkawitz, R.; Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999, 8, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Place, D.E.; Malireddi, R.K.S.; Kim, J.; Vogel, P.; Yamamoto, M.; Kanneganti, T.D. Osteoclast fusion and bone loss are restricted by interferon inducible guanylate binding proteins. Nat. Commun. 2021, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Zhao, H.; Kitaura, H.; Bhattacharyya, S.; Brewer, J.A.; Muglia, L.J.; Ross, F.P.; Teitelbaum, S.L. Glucocorticoids suppress bone formation via the osteoclast. J. Clin. Investig. 2006, 116, 2152–2160. [Google Scholar] [CrossRef]
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef]
- Yahara, Y.; Barrientos, T.; Tang, Y.J.; Puviindran, V.; Nadesan, P.; Zhang, H.; Gibson, J.R.; Gregory, S.G.; Diao, Y.; Xiang, Y.; et al. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat. Cell Biol. 2020, 22, 49–59. [Google Scholar] [CrossRef]
- Dudiki, T.; Nascimento, D.W.; Childs, L.S.; Kareti, S.; Androjna, C.; Zhevlakova, I.; Byzova, T.V. Progressive skeletal defects caused by Kindlin3 deficiency, a model of autosomal recessive osteopetrosis in humans. Bone 2022, 160, 116397. [Google Scholar] [CrossRef]
- Wang, L.; Roth, T.; Nakamura, M.C.; Nissenson, R.A. Female-Specific Role of Progranulin to Suppress Bone Formation. Endocrinology 2019, 160, 2024–2037. [Google Scholar] [CrossRef]
- Hoshino, A.; Ueha, S.; Hanada, S.; Imai, T.; Ito, M.; Yamamoto, K.; Matsushima, K.; Yamaguchi, A.; Iimura, T. Roles of chemokine receptor CX3CR1 in maintaining murine bone homeostasis through the regulation of both osteoblasts and osteoclasts. J. Cell Sci. 2013, 126, 1032–1045. [Google Scholar] [CrossRef]
- Huang, W.; Olsen, B.R. Skeletal defects in Osterix-Cre transgenic mice. Transgenic Res. 2015, 24, 167–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Mishina, Y.; Liu, F. Osterix-Cre transgene causes craniofacial bone development defect. Calcif. Tissue Int. 2015, 96, 129–137. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Zhao, H.; Zhang, H.; Xu, Y.; Wang, S.; Guo, X.; Huang, Y.; Zhang, S.; Han, Y.; et al. Identification and transcriptome analysis of erythroblastic island macrophages. Blood 2019, 134, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Madel, M.B.; Ibanez, L.; Wakkach, A.; de Vries, T.J.; Teti, A.; Apparailly, F.; Blin-Wakkach, C. Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions. Front. Immunol. 2019, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F.; Couasnay, G. Advantages and Limitations of Cre Mouse Lines Used in Skeletal Research. In Skeletal Development and Repair; Methods in Molecular Biology; Humana: New York, NY, USA, 2021; pp. 39–59. [Google Scholar]
- Couasnay, G.; Madel, M.B.; Lim, J.; Lee, B.; Elefteriou, F. Sites of Cre-recombinase activity in mouse lines targeting skeletal cells. J. Bone Min. Res. 2021, 36, 1661–1679. [Google Scholar] [CrossRef]
- Abram, C.L.; Roberge, G.L.; Hu, Y.; Lowell, C.A. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods 2014, 408, 89–100. [Google Scholar] [CrossRef]
- Arandjelovic, S.; Perry, J.S.A.; Zhou, M.; Ceroi, A.; Smirnov, I.; Walk, S.F.; Shankman, L.S.; Cambre, I.; Onengut-Gumuscu, S.; Elewaut, D.; et al. ELMO1 signaling is a promoter of osteoclast function and bone loss. Nat. Commun. 2021, 12, 4974. [Google Scholar] [CrossRef]
- Novak, S.; Roeder, E.; Kalinowski, J.; Jastrzebski, S.; Aguila, H.L.; Lee, S.K.; Kalajzic, I.; Lorenzo, J.A. Osteoclasts Derive Predominantly from Bone Marrow-Resident CX3CR1(+) Precursor Cells in Homeostasis, whereas Circulating CX3CR1(+) Cells Contribute to Osteoclast Development during Fracture Repair. J. Immunol. 2020, 204, 868–878. [Google Scholar] [CrossRef]
- Madel, M.B.; Ibanez, L.; Ciucci, T.; Halper, J.; Rouleau, M.; Boutin, A.; Hue, C.; Duroux-Richard, I.; Apparailly, F.; Garchon, H.J.; et al. Dissecting the phenotypic and functional heterogeneity of mouse inflammatory osteoclasts by the expression of Cx3cr1. Elife 2020, 9, e54493. [Google Scholar] [CrossRef]
- Sadvakassova, G.; Tiedemann, K.; Steer, K.J.D.; Mikolajewicz, N.; Stavnichuk, M.; In-Kyung Lee, I.; Sabirova, Z.; Schranzhofer, M.; Komarova, S.V. Active hematopoiesis triggers exosomal release of PRDX2 that promotes osteoclast formation. Physiol. Rep. 2021, 9, e14745. [Google Scholar] [CrossRef]
- Avneon, M.; Lifshitz, L.; Katz, O.; Prutchi-Sagiv, S.; Gassmann, M.; Mittelman, M.; Neumann, D. Non-erythroid effects of erythropoietin: Are neutrophils a target? Leuk. Res. 2009, 33, 1430–1432. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Gan, W.; Liu, Z.; Shen, Z.; Wang, J.; Shi, R.; Liu, Y.; Liu, Y.; Jiang, M.; Zhang, Z.; et al. Erythropoeitin Signaling in Macrophages Promotes Dying Cell Clearance and Immune Tolerance. Immunity 2016, 44, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Cui, Z.; Gavrilova, O.; Zhang, X.; Gassmann, M.; Noguchi, C.T. Sex-specific brain erythropoietin regulation of mouse metabolism and hypothalamic inflammation. JCI Insight 2020, 5, e134061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rogers, H.M.; Zhang, X.; Noguchi, C.T. Sex difference in mouse metabolic response to erythropoietin. FASEB J. 2017, 31, 2661–2673. [Google Scholar] [CrossRef]
- Yasuda, Y.; Masuda, S.; Chikuma, M.; Inoue, K.; Nagao, M.; Sasaki, R. Estrogen-dependent production of erythropoietin in uterus and its implication in uterine angiogenesis. J. Biol. Chem. 1998, 273, 25381–25387. [Google Scholar] [CrossRef]
- Soliz, J.; Khemiri, H.; Caravagna, C.; Seaborn, T. Erythropoietin and the sex-dimorphic chemoreflex pathway. Adv. Exp. Med. Biol. 2012, 758, 55–62. [Google Scholar]
- Jelkmann, W.; Wiedemann, G. Lack of sex dependence of the serum level of immunoreactive erythropoietin in chronic anemia. Klin. Wochenschr. 1989, 67, 1218. [Google Scholar] [CrossRef]
- Suresh, S.; Rajvanshi, P.K.; Noguchi, C.T. The Many Facets of Erythropoietin Physiologic and Metabolic Response. Front. Physiol. 2019, 10, 1534. [Google Scholar] [CrossRef]
- Lappin, K.M.; Mills, K.I.; Lappin, T.R. Erythropoietin in bone homeostasis-Implications for efficacious anemia therapy. Stem Cells Transl. Med. 2021, 10, 836–843. [Google Scholar] [CrossRef]
- Takeshita, S.; Kaji, K.; Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Min. Res. 2000, 15, 1477–1488. [Google Scholar] [CrossRef]
- Haag, S.M.; Murthy, A. Murine Monocyte and Macrophage Culture. Bio-Protocol 2021, 11, e3928. [Google Scholar] [CrossRef] [PubMed]
- Noh, T.; Gabet, Y.; Cogan, J.; Shi, Y.; Tank, A.; Sasaki, T.; Criswell, B.; Dixon, A.; Lee, C.; Tam, J.; et al. Lef1 haploinsufficient mice display a low turnover and low bone mass phenotype in a gender- and age-specific manner. PLoS ONE 2009, 4, e5438. [Google Scholar] [CrossRef] [PubMed]
- Gabet, Y.; Baniwal, S.K.; Leclerc, N.; Shi, Y.; Kohn-Gabet, A.E.; Cogan, J.; Dixon, A.; Bachar, M.; Guo, L.; Turman, J.E., Jr.; et al. Krox20/EGR2 deficiency accelerates cell growth and differentiation in the monocytic lineage and decreases bone mass. Blood 2010, 116, 3964–3971. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, P.; Koller, B.; Muller, R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif. Tissue Int. 1996, 58, 24–29. [Google Scholar] [CrossRef]
- Neumann, D.; Wikstrom, L.; Watowich, S.S.; Lodish, H.F. Intermediates in degradation of the erythropoietin receptor accumulate and are degraded in lysosomes. J. Biol. Chem. 1993, 268, 13639–13649. [Google Scholar] [CrossRef]
- Yoshimura, A.; D’Andrea, A.D.; Lodish, H.F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc. Natl. Acad. Sci. USA 1990, 87, 4139–4143. [Google Scholar] [CrossRef]



| Antibody | Source | Identifier |
|---|---|---|
| TER-119-APC | BioLegend | Cat#: 116211 |
| CD115-APC | eBioscience | Cat#: 14115282 |
| F4/80-APC | BioLegend | Cat#: 123115 |
| CD11b-APC | BioLegend | Cat#: 101211 |
| CD115-PE | Miltenyi Biotec | Cat#: 130112828 |
| LY6C-PerCP/Cy5.5 | BioLegend | Cat#: 128011 |
| Alkaline Phosphatase (ALPL) | R&D systems | Cat#: AF2910 |
| Goat IgG (H+L)-PE | R&D systems | Cat#: F0107 |
| Anti N-terminus mEPOR | [76,77] | |
| Donkey anti-rabbit IgG H&L-Alexa Fluor® 488 | abcam | Cat#: ab150073 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awida, Z.; Hiram-Bab, S.; Bachar, A.; Saed, H.; Zyc, D.; Gorodov, A.; Ben-Califa, N.; Omari, S.; Omar, J.; Younis, L.; et al. Erythropoietin Receptor (EPOR) Signaling in the Osteoclast Lineage Contributes to EPO-Induced Bone Loss in Mice. Int. J. Mol. Sci. 2022, 23, 12051. https://doi.org/10.3390/ijms231912051
Awida Z, Hiram-Bab S, Bachar A, Saed H, Zyc D, Gorodov A, Ben-Califa N, Omari S, Omar J, Younis L, et al. Erythropoietin Receptor (EPOR) Signaling in the Osteoclast Lineage Contributes to EPO-Induced Bone Loss in Mice. International Journal of Molecular Sciences. 2022; 23(19):12051. https://doi.org/10.3390/ijms231912051
Chicago/Turabian StyleAwida, Zamzam, Sahar Hiram-Bab, Almog Bachar, Hussam Saed, Dan Zyc, Anton Gorodov, Nathalie Ben-Califa, Sewar Omari, Jana Omar, Liana Younis, and et al. 2022. "Erythropoietin Receptor (EPOR) Signaling in the Osteoclast Lineage Contributes to EPO-Induced Bone Loss in Mice" International Journal of Molecular Sciences 23, no. 19: 12051. https://doi.org/10.3390/ijms231912051
APA StyleAwida, Z., Hiram-Bab, S., Bachar, A., Saed, H., Zyc, D., Gorodov, A., Ben-Califa, N., Omari, S., Omar, J., Younis, L., Iden, J. A., Graniewitz Visacovsky, L., Gluzman, I., Liron, T., Raphael-Mizrahi, B., Kolomansky, A., Rauner, M., Wielockx, B., Gabet, Y., & Neumann, D. (2022). Erythropoietin Receptor (EPOR) Signaling in the Osteoclast Lineage Contributes to EPO-Induced Bone Loss in Mice. International Journal of Molecular Sciences, 23(19), 12051. https://doi.org/10.3390/ijms231912051







