Effective Modulation by Lacosamide on Cumulative Inhibition of INa during High-Frequency Stimulation and Recovery of INa Block during Conditioning Pulse Train
Abstract
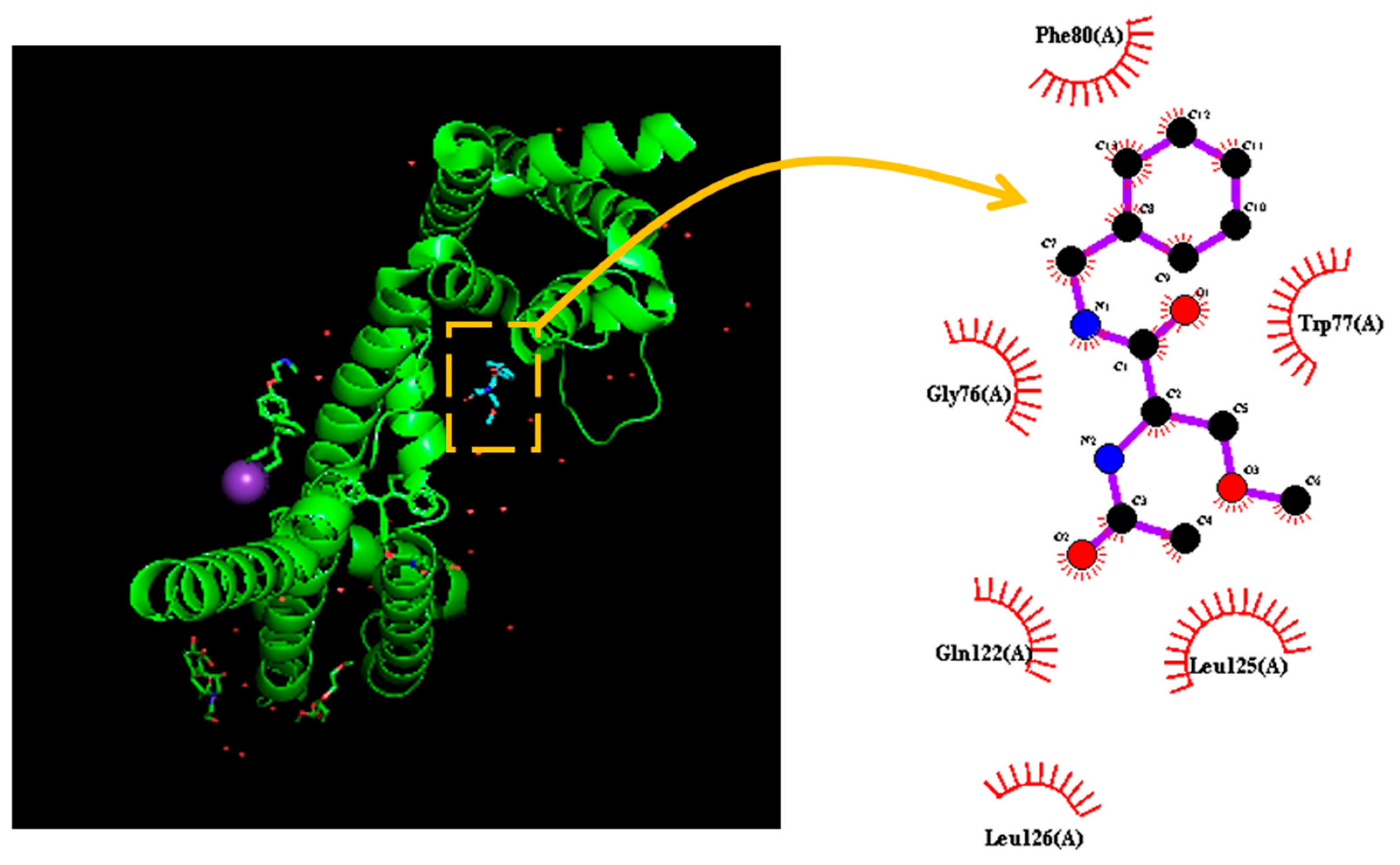
1. Introduction
2. Results
2.1. Effects of LCS on Voltage-Gated Na+ Current (INa)
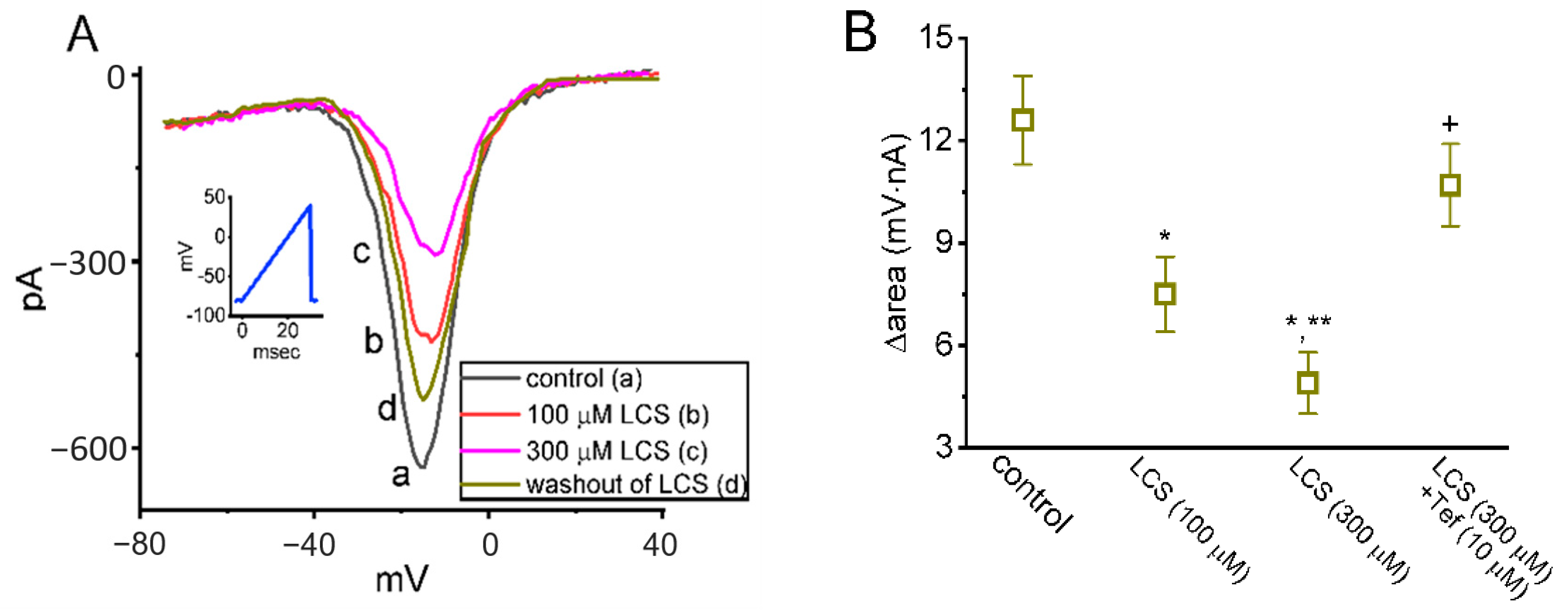
2.2. Effects of LCS on Persistent Na+ Current (INa(P)) Triggered by Isosceles-Triangular Ramp Voltage (Vramp)
2.3. Effect of LCS on Window INa (INa(W)) Elicited by a Short Ascending Vramp
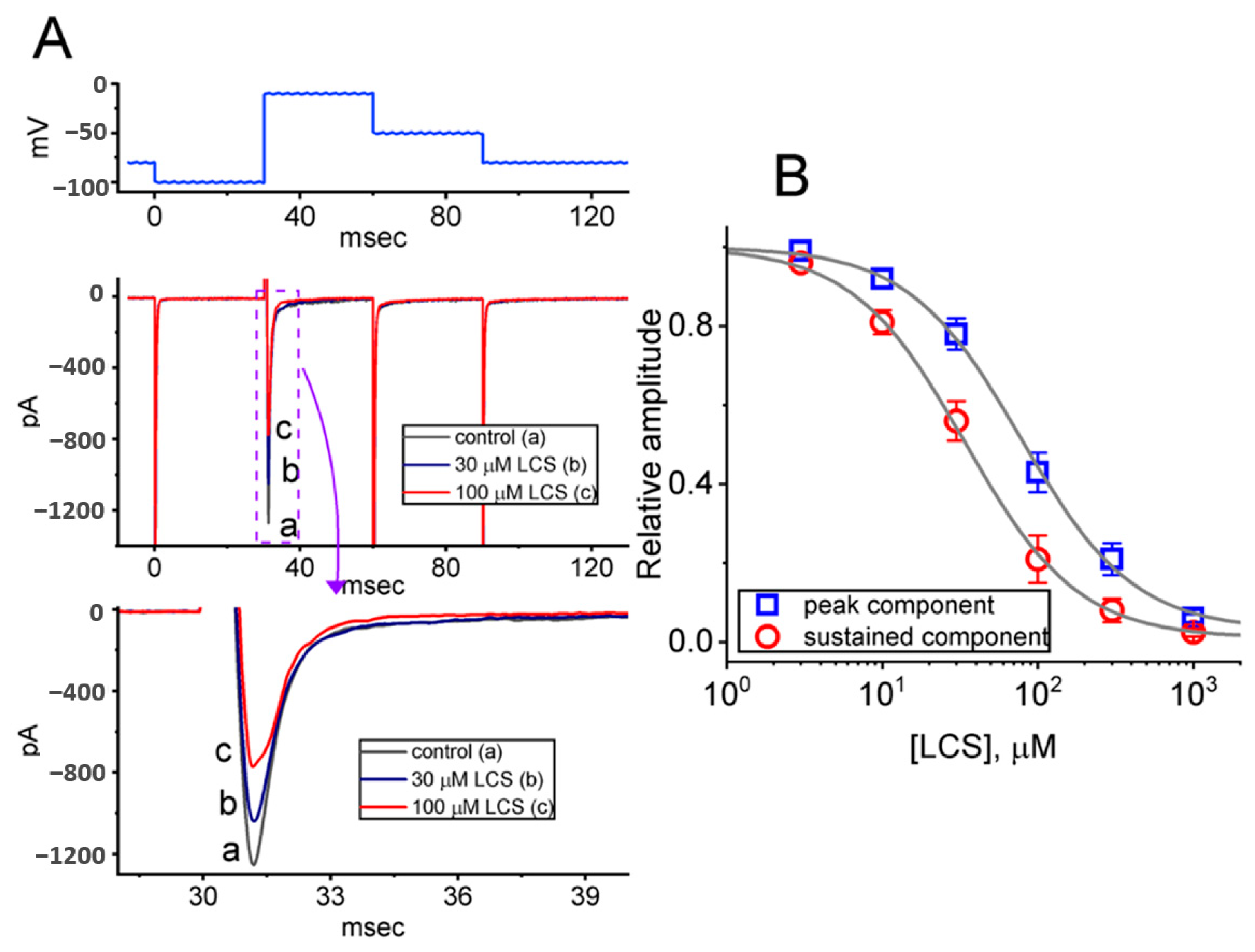
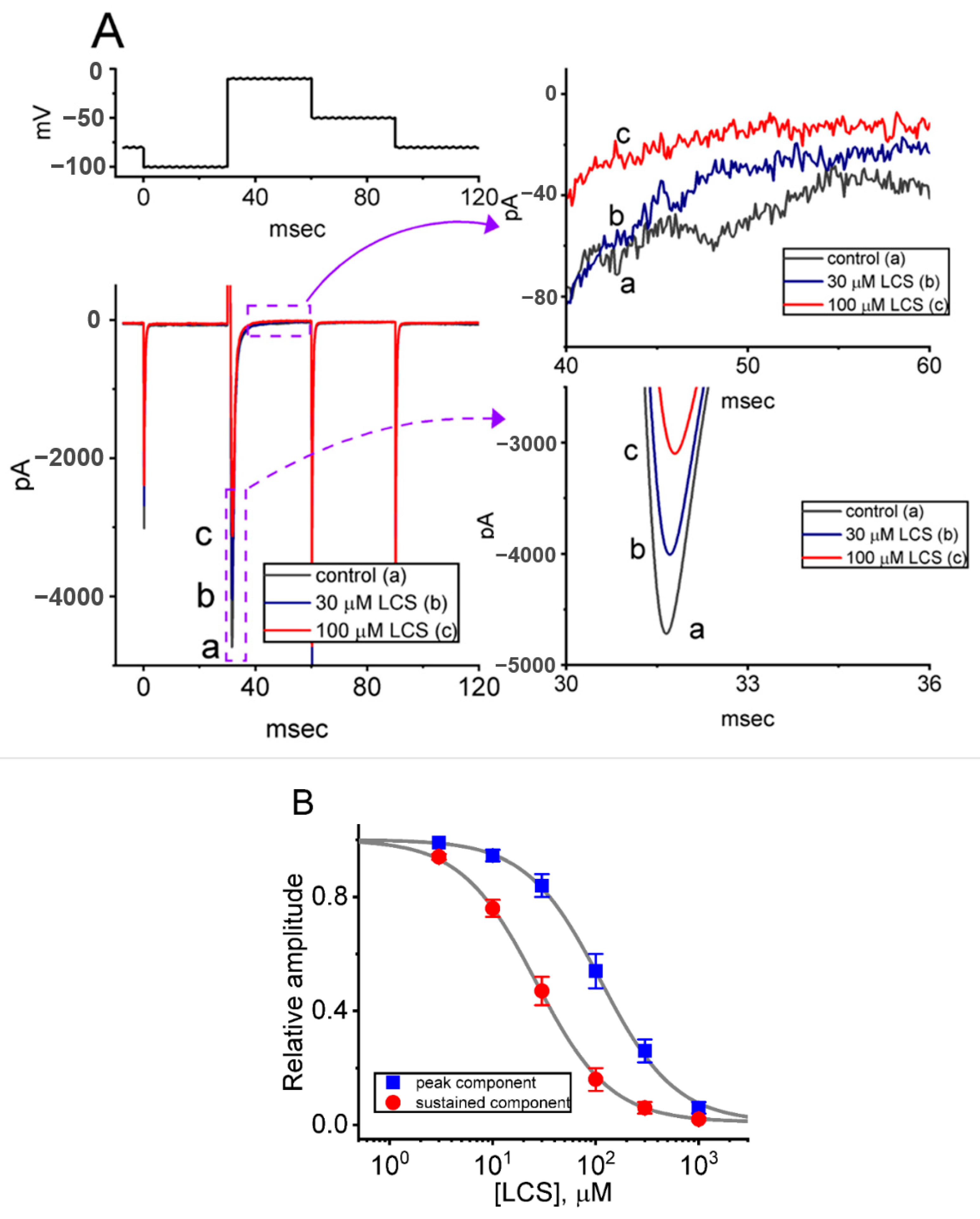
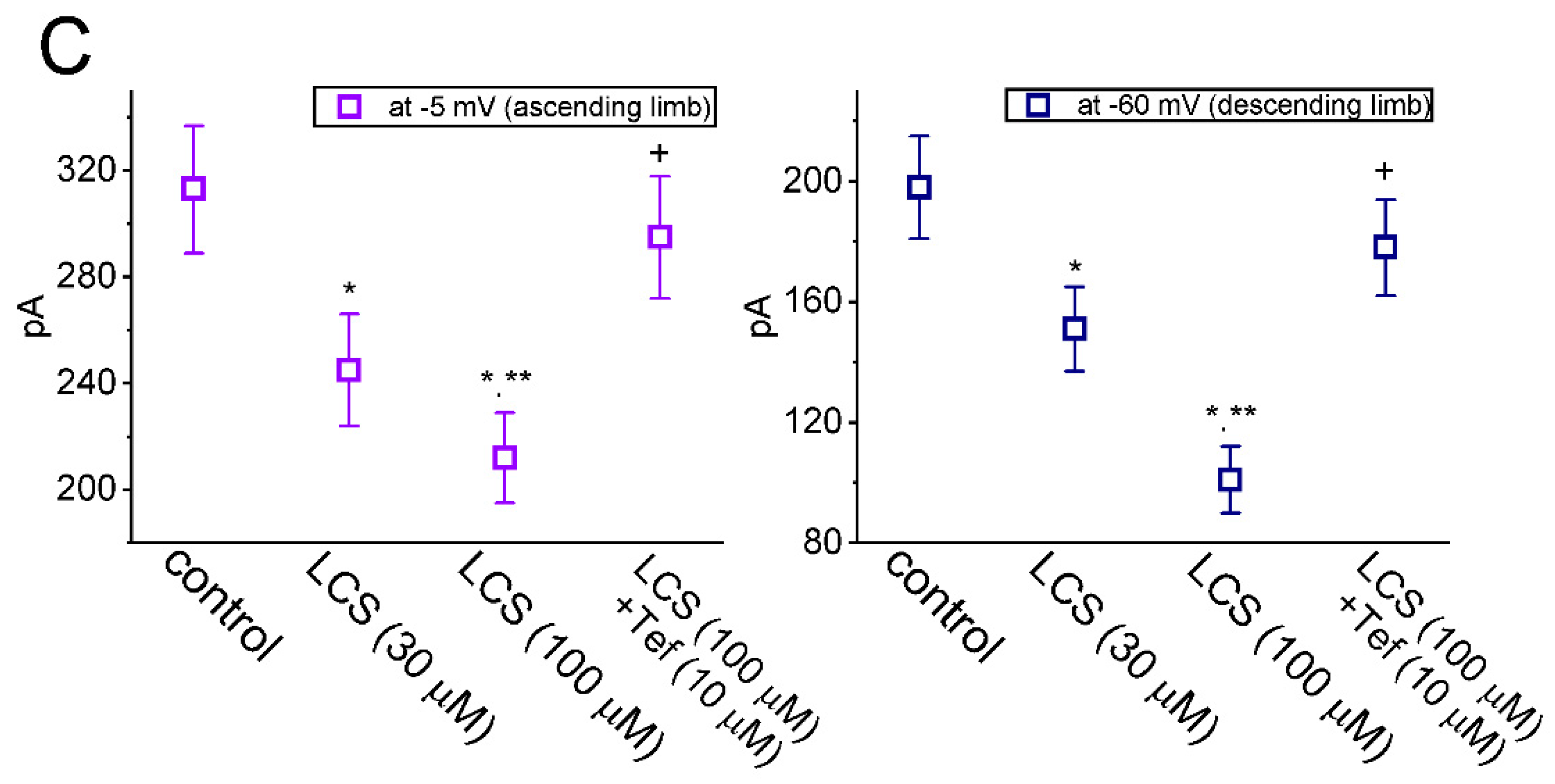
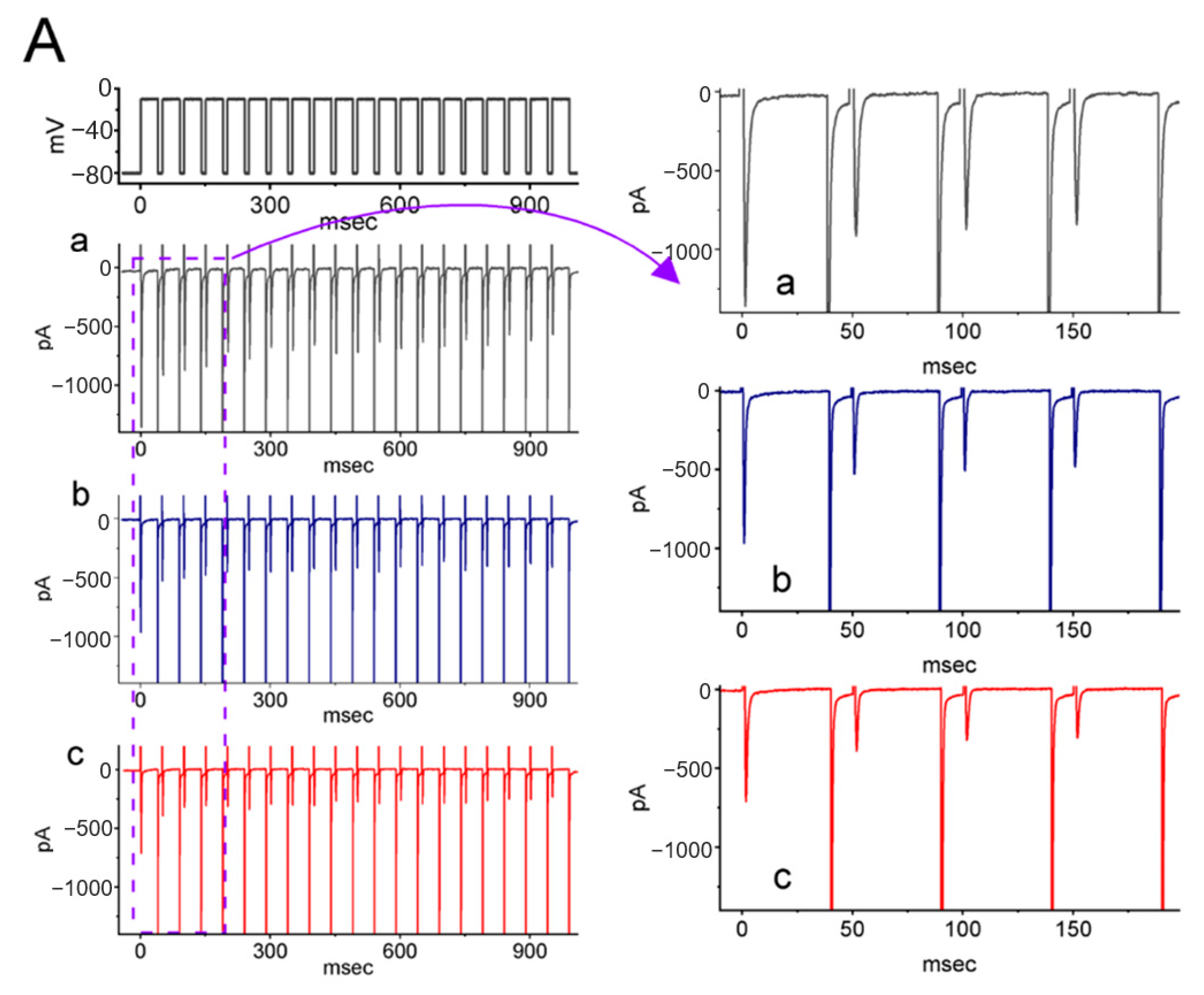
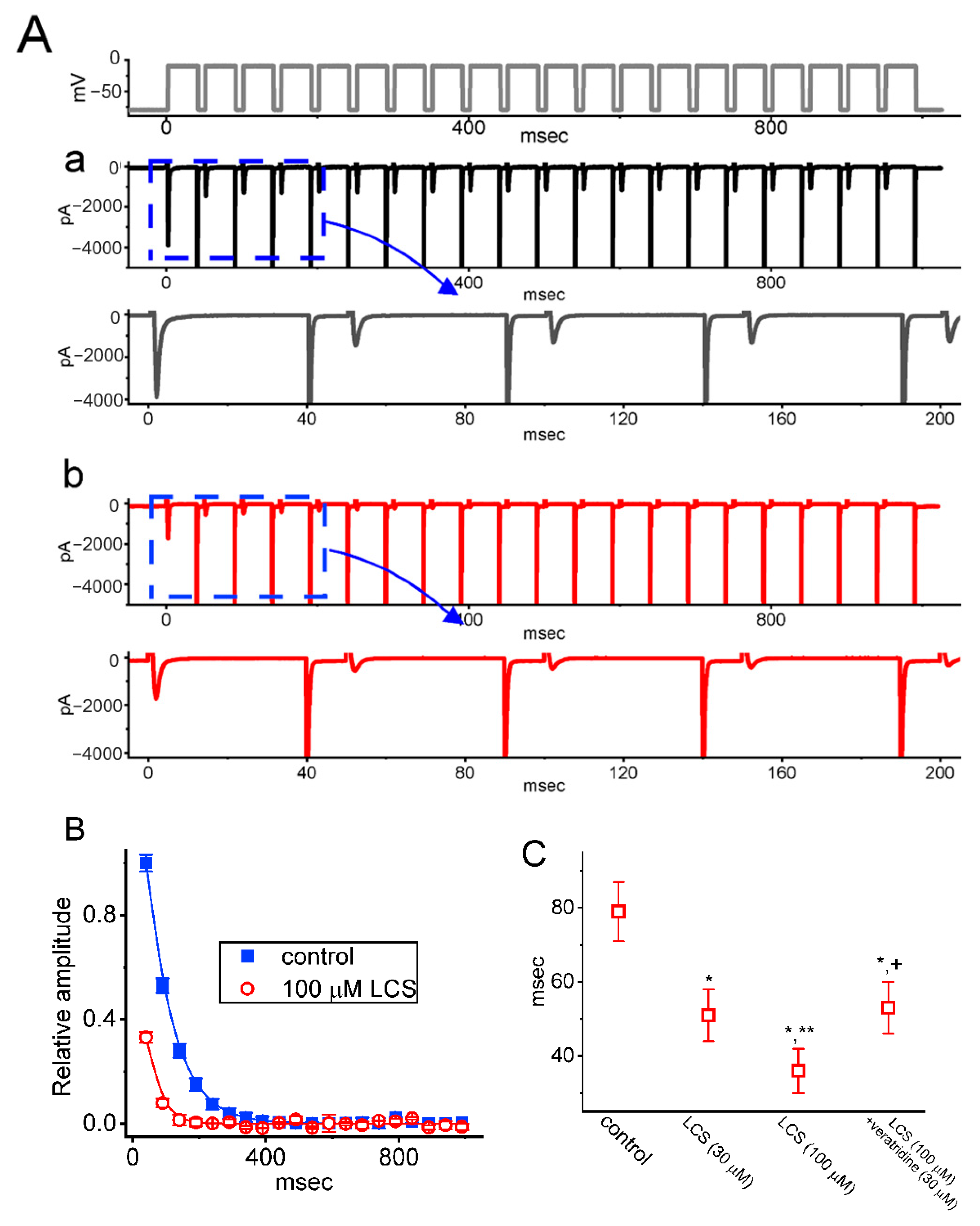
2.4. Effect of LCS on the Cumulative Inhibition of INa during a Train of Depolarizing Pulses
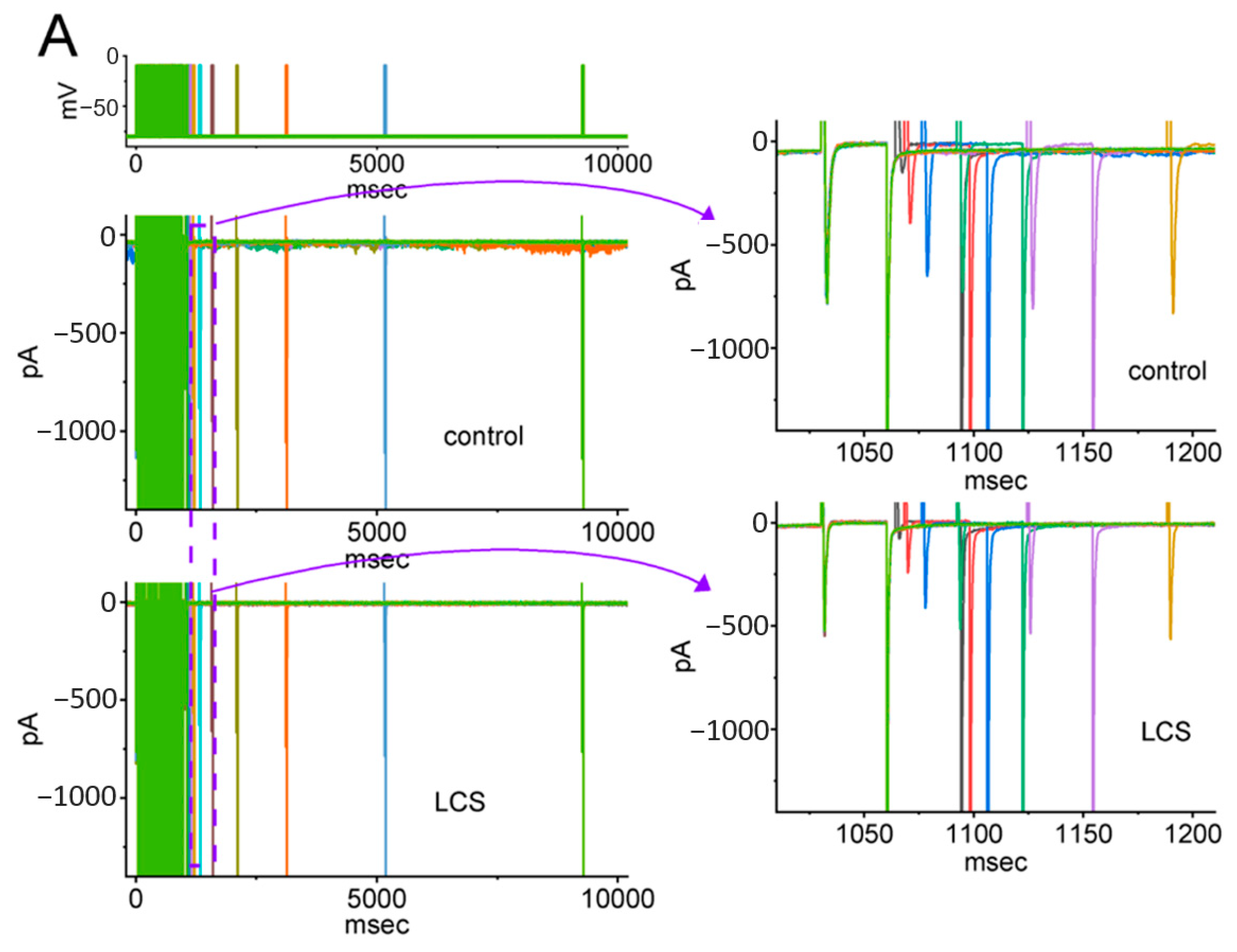
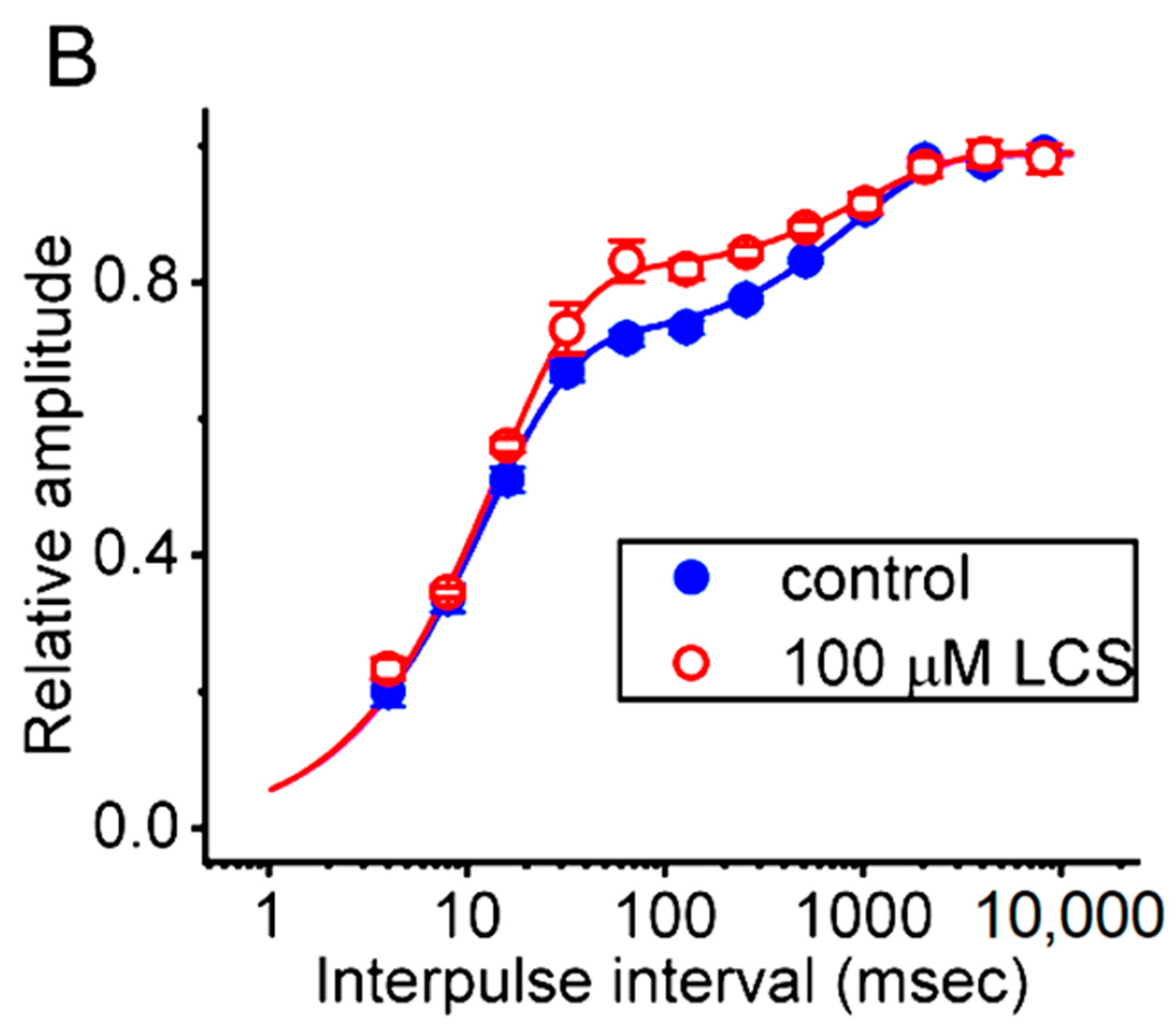
2.5. Modification by LCS of the Recovery Process of INa(T) Inactivation following Conditioning Train of Depolarizing Stimuli
3. Discussion
4. Materials and Methods
4.1. Chemicals, Drugs, and Solutions Used in This Study
4.2. Cell Preparations
4.3. Electrophysiological Measurements
4.4. Data Recordings
4.5. Data Analyses
4.6. Curve-Fitting Approximations and Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Hys(V) | voltage-dependent hysteresis |
| IC50 | concentration required for 50% inhibition |
| INa | voltage-gated Na+ current |
| INa(L) | late Na+ current |
| INa(P) | persistent Na+ current |
| INa(T) | transient (peak) Na+ current |
| INa(W) | window Na+ current |
| LCS | lacosamide (Vimpat®) |
| NaV channel | voltage-gated Na+ channel |
| SEM | standard error of mean |
| τ | time constant |
| τfast | fast time constant |
| τslow | slow time constant |
| TEA | tetraethylammonium chloride |
| Tef | tefluthrin |
| TTX | tetrodotoxin |
| Vramp | ramp voltage |
References
- Labau, J.I.R.; Estacion, M.; Tanaka, B.S.; De Greef, B.T.A.; Hoeijmakers, J.G.J.; Geerts, M.; Gerrits, M.M.; Smeets, H.J.M.; Faber, C.G.; Merkies, I.S.J.; et al. Differential effect of lacosamide on Nav1.7 variants from responsive and non-responsive patients with small fibre neuropathy. Brain 2020, 143, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Casciato, S.; Quarato, P.P.; Gialluisi, A.; D’Aniello, A.; Mascia, A.; Grammaldo, L.G.; Di Gennaro, G. Lacosamide as first add-on or conversion monotherapy: A retrospective real-life study. Epilepsy Behav. 2021, 122, 108128. [Google Scholar] [CrossRef] [PubMed]
- Eilam, A.; Khmeliov, N.; Penker, D.; Gilad, R. Intravenous Lacosamide in Seizure Clusters: Dose and Efficacy. Clin. Neuropharmacol. 2021, 44, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-W.; Moon, J.; Cho, Y.W.; Kim, D.W.; Bin Hong, S.; Kim, D.-Y.; Chang, H.; Yoon, S.H.; Yu, K.-S.; Jang, I.-J.; et al. Tolerability of lacosamide rapid dose titration: A randomized, multicenter, prospective, open-label study. Epilepsy Behav. 2021, 115, 107663. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Vendrell, A.; Teixidor, S.; Sala-Padró, J.; Campoy, S.; Huerta-Villanueva, M. Intravenous lacosamide and phenytoin for the treatment of acute exacerbations of trigeminal neuralgia: A retrospective analysis of 144 cases. Cephalalgia 2022, 42, 3331024221092435. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Lacosamide: A Review in Focal Seizures in Patients with Epilepsy. Drugs 2015, 75, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wilson, S.M.; Brittain, J.M.; Ripsch, M.S.; Salomé, C.; Park, K.D.; White, F.A.; Khanna, R.; Kohn, H. Merging Structural Motifs of Functionalized Amino Acids and α-Aminoamides Results in Novel Anticonvulsant Compounds with Significant Effects on Slow and Fast Inactivation of Voltage-gated Sodium Channels and in the Treatment of Neuropathic Pain. ACS Chem. Neurosci. 2011, 2, 317–322. [Google Scholar] [CrossRef]
- Biton, V. Lacosamide for the treatment of diabetic neuropathic pain. Expert Rev. Neurother. 2008, 8, 1649–1660. [Google Scholar] [CrossRef]
- McCleane, G. Lacosamide for pain. Expert Opin. Investig. Drugs 2010, 19, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Hearn, L.; Derry, S.; Moore, R.A. Lacosamide for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2012, 2012, Cd009318. [Google Scholar] [CrossRef]
- Carmland, M.E.; Kreutzfeldt, M.; Holbech, J.V.; Andersen, N.T.; Jensen, T.S.; Bach, F.W.; Sindrup, S.H.; Finnerup, N.B. Effect of lacosamide in peripheral neuropathic pain: Study protocol for a randomized, placebo-controlled, phenotype-stratified trial. Trials 2019, 20, 588. [Google Scholar] [CrossRef] [PubMed]
- Adamo, D.; Coppola, N.; Pecoraro, G.; Nicolò, M.; Mignogna, M.D. Lacosamide in trigeminal neuralgia: Report of a case refractory to first- and second-generation anticonvulsants. Cranio 2020, 1–5. [Google Scholar] [CrossRef]
- Huang, C.-W.W.; Brown, S.; Pillay, N.; Del Campo, M.; Tellez-Zenteno, J.; McLachlan, R.S. Electroencephalographic and Electrocardiographic Effect of Intravenous Lacosamide in Refractory Focal Epilepsy. J. Clin. Neurophysiol. 2018, 35, 365–369. [Google Scholar] [CrossRef]
- Wu, T.; Chuang, Y.C.; Huang, H.C.; Lim, S.N.; Hsieh, P.F.; Lee, W.T.; Cheng, M.-Y.; Tsai, M.-H.; Jou, S.-B.; Chang, C.-W.; et al. A prospective, multicenter, noninterventional study in Taiwan to evaluate the safety and tolerability of lacosamide as adjunctive therapy for epilepsy in clinical practice. Epilepsy Behav. 2020, 113, 107464. [Google Scholar] [CrossRef] [PubMed]
- Nortey, J.; Smith, D.; Seitzman, G.D.; Gonzales, J.A. Topical Therapeutic Options in Corneal Neuropathic Pain. Front. Pharmacol. 2021, 12, 769909. [Google Scholar] [CrossRef]
- Okanishi, T.; Fujii, Y.; Sakuma, S.; Shiraishi, H.; Motoi, H.; Yazaki, K.; Enoki, H.; Fujimoto, A. Lacosamide monotherapy for the treatment of childhood epilepsy with centrotemporal spikes. Brain Dev. 2022, 44, 380–385. [Google Scholar] [CrossRef]
- Panda, P.K.; Sharawat, I.K.; Dawman, L.; Panda, P.; Kasinathan, A.; Rathaur, V.K. Efficacy and Tolerability of Lacosamide in Lennox-Gastaut Syndrome: A Systematic Review and Meta-analysis. J. Neurosci. Rural. Pract. 2022, 13, 32–42. [Google Scholar] [CrossRef]
- Pozzi, M.; Zanotta, N.; Epifanio, R.; Baldelli, S.; Cattaneo, D.; Clementi, E.; Zucca, C. Lacosamide effectiveness and tolerability in patients with drug-resistant epilepsy and severe disability under polytherapy: Therapy optimization as emerging from an observational study. Epilepsy Behav. 2022, 128, 108598. [Google Scholar] [CrossRef]
- Ragoonanan, D.; Tran, N.; Levesque, M. Safety and Tolerability of Intravenous Push Lacosamide and Levetiracetam. J. Pharm. Pract. 2022, 8971900221087955. [Google Scholar] [CrossRef]
- Behr, C.; Lévesque, M.; Ragsdale, D.; Avoli, M. Lacosamide modulates interictal spiking and high-frequency oscillations in a model of mesial temporal lobe epilepsy. Epilepsy Res. 2015, 115, 8–16. [Google Scholar] [CrossRef]
- Errington, A.C.; Stohr, T.; Heers, C.; Lees, G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol. Pharmacol. 2008, 73, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.K.; Wang, S.-Y. Block of human cardiac sodium channels by lacosamide: Evidence for slow drug binding along the activation pathway. Mol. Pharmacol. 2014, 85, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A.; Tofighy, A.; White, H.S.; Matagne, A.; Wolff, C. Current understanding of the mechanism of action of the antiepileptic drug lacosamide. Epilepsy Res. 2015, 110, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Sumbul, O.; Aygun, H. Electrocorticographic and electrocardiographic evaluation of lacosamide in a penicillin-induced status epilepticus model. Epilepsy Res. 2022, 180, 106866. [Google Scholar] [CrossRef] [PubMed]
- Hamard, J.; Rigal, M.; Gony, M.; Bagheri, H. Lacosamide-induced personality changes: An unexpected adverse effect. Fundam. Clin. Pharmacol. 2022, 36, 224–226. [Google Scholar] [CrossRef]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Catterall, W.A.; Lenaeus, M.J.; Gamal El-Din, T.M. Structure and Pharmacology of Voltage-Gated Sodium and Calcium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 133–154. [Google Scholar] [CrossRef]
- Huang, C.-W.; Hung, T.-Y.; Wu, S.-N. The inhibitory actions by lacosamide, a functionalized amino acid, on voltage-gated Na+ currents. Neuroscience 2015, 287, 125–136. [Google Scholar] [CrossRef]
- Wu, S.N.; Lo, Y.C.; Shen, A.Y.; Chen, B.S. Contribution of non-inactivating Na+ current induced by oxidizing agents to the firing behavior of neuronal action potentials: Experimental and theoretical studies from NG108-15 neuronal cells. Chin. J. Physiol. 2011, 54, 19–29. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Characterization of Direct Perturbations on Voltage-Gated Sodium Current by Esaxerenone, a Nonsteroidal Mineralocorticoid Receptor Blocker. Biomedicines 2021, 9, 549. [Google Scholar] [CrossRef]
- Guérineau, N.C.; Monteil, A.; Lory, P. Sodium background currents in endocrine/neuroendocrine cells: Towards unraveling channel identity and contribution in hormone secretion. Front. Neuroendocrinol. 2021, 63, 100947. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Chuang, C.W.; Cho, H.Y.; Chuang, T.H.; Wu, S.N. The Evidence for Effective Inhibition of I(Na) Produced by Mirogabalin ((1R,5S,6S)-6-(aminomethyl)-3-ethyl-bicyclo[3.2.0]hept-3-ene-6-acetic acid), a Known Blocker of Ca(V) Channels. Int. J. Mol. Sci. 2022, 23, 3845. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.E.; Boucher, P.A.; Joós, B. Left-shifted nav channels in injured bilayer: Primary targets for neuroprotective nav antagonists? Front. Pharmacol. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Taddese, A.; Bean, B.P. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron 2002, 33, 587–600. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Bi, R.-Y.; Zhang, P.; Gan, Y.-H. Veratridine modifies the gating of human voltage-gated sodium channel Nav1.7. Acta Pharmacol. Sin. 2018, 39, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Mistry, A.M.; Kahlig, K.M.; Kearney, J.A.; Xiang, J.; George, A.L.J. Propranolol blocks cardiac and neuronal voltage-gated sodium channels. Front. Pharmacol. 2010, 1, 144. [Google Scholar] [CrossRef]
- Weiser, T.; Qu, Y.; Catterall, W.A.; Scheuer, T. Differential interaction of R-mexiletine with the local anesthetic receptor site on brain and heart sodium channel alpha-subunits. Mol. Pharmacol. 1999, 56, 1238–1244. [Google Scholar] [CrossRef]
- Simasko, S.M. A background sodium conductance is necessary for spontaneous depolarizations in rat pituitary cell line GH3. Am. J. Physiol. 1994, 266 Pt 1, C709–C719. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N.; Chen, B.-S.; Hsu, T.-I.; Peng, H.; Wu, Y.-H.; Lo, Y.-C. Analytical studies of rapidly inactivating and noninactivating sodium currents in differentiated NG108-15 neuronal cells. J. Theor. Biol. 2009, 259, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.C.; Bean, B.P. Incomplete inactivation and rapid recovery of voltage-dependent sodium channels during high-frequency firing in cerebellar Purkinje neurons. J. Neurophysiol. 2011, 105, 860–871. [Google Scholar] [CrossRef]
- Tsai, D.; Morley, J.W.; Suaning, G.J.; Lovell, N.H. Frequency-dependent reduction of voltage-gated sodium current modulates retinal ganglion cell response rate to electrical stimulation. J. Neural Eng. 2011, 8, 066007. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.-R.; Aimar, K.; Ghadiry-Tavi, R.; Yu, A.; Ruben, P. Physiology and Pathophysiology of Sodium Channel Inactivation. Curr. Top Membr. 2016, 78, 479–509. [Google Scholar] [PubMed]
- Navarro, M.A.; Salari, A.; Lin, J.L.; Cowan, L.M.; Penington, N.J.; Milescu, M.; Milescu, L.S. Sodium channels implement a molecular leaky integrator that detects action potentials and regulates neuronal firing. eLife 2020, 9, e54940. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Mizerska, K.; Quirce, S.; Belmonte, C.; Gallar, J.; Acosta, M.D.C.; Meseguer, V. Sodium channel blockers modulate abnormal activity of regenerating nociceptive corneal nerves after surgical lesion. Investig. Opthalmol. Vis. Sci. 2021, 62, 2. [Google Scholar] [CrossRef]
- Sula, A.; Hollingworth, D.; Ng, L.C.; Larmore, M.; DeCaen, P.G.; Wallace, B. A tamoxifen receptor within a voltage-gated sodium channel. Mol. Cell 2021, 81, 1160–1169.e5. [Google Scholar] [CrossRef]


| N | τfast (msec) | τslow (msec) | A | B | |
|---|---|---|---|---|---|
| Control | 8 | 12.2 ± 0.4 | 885 ± 16 | 0.71 ± 0.04 | 0.28 ± 0.02 |
| LCS (30 μM) | 8 | 13.6 ± 0.6 * | 972 ± 17 * | 0.78 ± 0.04 * | 0.22 ± 0.02 * |
| LCS (100 μM) | 8 | 14.1 ± 0.6 * | 1045 ± 19 * | 0.81 ± 0.04 * | 0.18 ± 0.02 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-M.; Lin, Y.-C.; Chiang, C.-W.; Cho, H.-Y.; Chuang, T.-H.; Yu, M.-C.; Wu, S.-N.; Tu, Y.-F. Effective Modulation by Lacosamide on Cumulative Inhibition of INa during High-Frequency Stimulation and Recovery of INa Block during Conditioning Pulse Train. Int. J. Mol. Sci. 2022, 23, 11966. https://doi.org/10.3390/ijms231911966
Wu P-M, Lin Y-C, Chiang C-W, Cho H-Y, Chuang T-H, Yu M-C, Wu S-N, Tu Y-F. Effective Modulation by Lacosamide on Cumulative Inhibition of INa during High-Frequency Stimulation and Recovery of INa Block during Conditioning Pulse Train. International Journal of Molecular Sciences. 2022; 23(19):11966. https://doi.org/10.3390/ijms231911966
Chicago/Turabian StyleWu, Po-Ming, Yu-Ching Lin, Chi-Wu Chiang, Hsin-Yen Cho, Tzu-Hsien Chuang, Meng-Cheng Yu, Sheng-Nan Wu, and Yi-Fang Tu. 2022. "Effective Modulation by Lacosamide on Cumulative Inhibition of INa during High-Frequency Stimulation and Recovery of INa Block during Conditioning Pulse Train" International Journal of Molecular Sciences 23, no. 19: 11966. https://doi.org/10.3390/ijms231911966
APA StyleWu, P.-M., Lin, Y.-C., Chiang, C.-W., Cho, H.-Y., Chuang, T.-H., Yu, M.-C., Wu, S.-N., & Tu, Y.-F. (2022). Effective Modulation by Lacosamide on Cumulative Inhibition of INa during High-Frequency Stimulation and Recovery of INa Block during Conditioning Pulse Train. International Journal of Molecular Sciences, 23(19), 11966. https://doi.org/10.3390/ijms231911966






