Abstract
Tubby-like proteins (TLPs) play important roles in plant growth and development and in responses to abiotic stress. However, TLPs in strawberry remain poorly studied. In this study, eight TLPs were identified in woodland strawberry (Fragaria vesca subspecies vesca ‘Ruegen’). Protein structure analysis revealed that the structure of FvTLPs is highly conserved, but evolutionary and gene structure analyses revealed that the evolutionary pattern of FvTLP family members differs from that of their orthologous genes in Arabidopsis, poplar, and apple. Subcellular localization assays revealed that FvTLPs were localized to the nucleus and plasma membrane. FvTLPs showed no transcriptional activity. Yeast two-hybrid assays revealed that FvTLPs interact with specific FvSKP1s. The expression patterns of FvTLPs in different tissues and under various abiotic stresses (salt, drought, cold, and heat) and hormone treatments (ABA (abscisic acid) and MeJA (methyl jasmonate)) were determined. The expression patterns of FvTLPs indicated that they play a role in regulating growth and development and responses to abiotic stress in F. vesca. The GUS (beta-glucuronidase) activity of FvTLP1pro::GUS plants in GUS activity assays increased under salt and drought stress and abscisic acid treatment. The results of this study provide new insights into the molecular mechanisms underlying the functions of TLPs.
1. Introduction
Tubby-like proteins (TLPs) are ubiquitous in eukaryotes. TLPs are named for their conserved C-terminal tubby domain, which has a unique helix-filled barrel structure [1], and most plant TLPs also carry a conserved N-terminal F-box domain. TLPs have multiple biological roles in plants. Aside from functioning as transcription factors, they are important components of the SCF (SKP1-cullin-1-F-box) complex [2,3]. TLPs have been reported that respond to biotic stress [4,5,6,7]. OsTLP2 might activate disease resistance pathways by regulating the expression of OsWRKY13 [8]. In annual Medicago species, barley, maize, cassava, apple, cotton, soybean, wheat, and Salvia miltiorrhiza, the expression of TLPs is affected by various abiotic stresses and hormone treatments [9,10,11,12,13,14,15,16,17]. TLP1, TLP3, and TLP5 were identified as potential candidate genes for functional studies in cotton because of their putative functions in drought and salt stress responses [18]. In apple, MdTLP7 enhances abiotic stress tolerance, and the tubby domain plays a key role in abiotic stress tolerance [19]. In chickpea, CaTLP1 positively regulates abiotic stress tolerance and enhances abscisic acid (ABA)-mediated stomatal closure by interacting with protein kinases [20,21]. SlTLFP8 enhances tomato water-deficient resistance by modulating stomatal density [22]. SlTLPs may play a role in fruit ripening, and SlTLP1 and SlTLP2 may participate in ethylene-dependent fruit ripening [23]. CsTLP8, AtTLP3, and AtTLP9 function in ABA- and osmotic stress-mediated seed germination [24,25]. In cotton, GhTULP34 negatively regulates osmotic stress tolerance [26]. AtTLP11 might affect seed development by modulating NHL6 expression [27], and AtTLP2 regulates the biosynthesis of homogalacturonan, the major polysaccharides constituent of the Arabidopsis seed coat mucilage, by activating UDP-glucose 4-epimerase 1 (UGE1) [28]. GmTLP8 confers abiotic stress tolerance in plants via stress-responsive genes [15]. ScTLP12 might contribute to the rolling of leaves in rye [29]. However, few studies were performed on TLPs in strawberry, which is a well know berry sensitive to both biotic stress and abiotic stress.
In this study, we identified TLP gene family members in woodland strawberry (Fragaria vesca). Gene and protein structure and phylogenetic relationships were analyzed to clarify the evolutionary pattern of TLP family members in F. vesca. Subcellular localization and transcriptional activity of FvTLPs and protein-protein interactions between FvTLPs and FvSKP1s were analyzed to explore patterns of functional divergence of the TLP family in F. vesca. The cis-elements (cis-acting regulatory DNA elements) and promoters of FvTLPs and the expression of FvTLPs in different tissues and under various treatments were analyzed to determine the functions of FvTLPs. The beta-glucuronidase (GUS) activity of FvTLP1pro::GUS plants was analyzed to verify the expression of FvTLP1. The results of this study enhance our understanding of the functional characteristics of plant TLPs and will aid future studies of the molecular mechanism underlying the functions of TLPs.
2. Results
2.1. Identification of TLP Gene Family Members in F. vesca
The amino acid sequences of AtTLPs and OsTLPs were used to identify TLPs in the whole genome of F. vesca. A total of eight TLPs were identified in Fragaria vesca, and these were named FvTLP1 to FvTLP8 according to their gene IDs. In addition, FvTLP6 and FvTLP8 had two and three spliced transcripts, respectively. PCR amplification revealed that FvTLP8 only has one transcript in ‘Ruegen’. Compared with the coding sequence in the reference genome, the coding sequence of FvTLP2 in ‘Ruegen’ has an 81-bp deletion and two 1bp mutations (Figure S1). Finally, we identified eight TLP genes in ‘Ruegen’ and named them FvTLP1-FvTLP8 according to their gene IDs in the GDR database (Genome Database for Rosaceae, https://www.rosaceae.org, accessed on 6 October 2022), and the two transcripts of FvTLP6 were named FvTLP6a and FvTLP6b (Table 1). The length of the FvTLP-coding sequences ranged from 1077 to 1308 bp, and the length of FvTLP peptides ranged from 358 to 429 aa. The PI (Predicted Isoelectric) of FvTLPs ranged from 9.11 to 9.78, and the MW (Molecular Weight) ranged from 40.1 to 48.9 kD.

Table 1.
Identification and characteristics of TLP genes in Fragaria vesca.
2.2. Phylogenetic and Gene Structure Analysis
To clarify the evolutionary relationships among TLP genes, a phylogenetic tree of TLPs from Arabidopsis, rice, apple, poplar, and F. vesca was constructed. The tree was divided into three phylogenetic subfamilies: A, B, and C; subfamily A could be further divided into subgroups A1, A2, and A3. All FvTLPs belonged to subfamily A, with the exception of FvTLP5 and FvTLP8, which belonged to subfamily B and subfamily C, respectively (Figure 1). The phylogenetic analysis revealed that the TLP proteins of apple and F. vesca showed higher homology compared with those of other species. Conserved domain analysis revealed that FvTLP8 only possesses a tubby domain, and all other FvTLPs possess both the F-box and tubby domain (Table 1 and Figure 1). Amino acid sequence analysis of FvTLPs showed that all FvTLPs possess a conserved membrane-localized site, a phosphatidylinositol 4, 5-bisphosphate (PIP2)-binding site (Figure S2) [30]. We speculated that FvTLPs might be located in the plasma membrane. The study of structural divergence can contribute to our understanding of protein function and evolution [31]. To investigate the diversity of TLP genes in F. vesca, the conserved motifs of FvTLP proteins and the structure of FvTLP genes were analyzed (Figure 2). A total of five conserved motifs were identified in FvTLPs (Figure S3); the conserved motifs 1, 2, 4, and 5 were Tub family domains, and motif 3 was the F-box domain. FvTLP1–FvTLP7 possess all identified motifs, and FvTLP8 possesses motifs 1 and 4. This finding is consistent with the results of the conserved domain analysis. Analysis of gene structure showed that FvTLP8 consisted of nine exons; FvTLP3, FvTLP6a, and FvTLP6b, members of subfamily A2, consisted of five exons; and the other FvTLPs consisted of four exons.
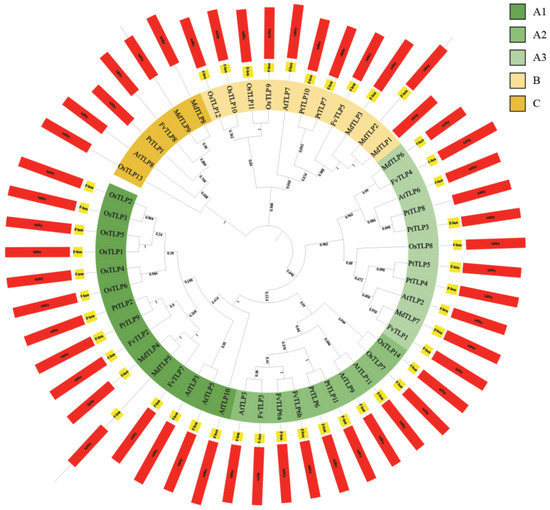
Figure 1.
Phylogenetic tree and conserved domains of TLPs. The phylogenetic tree was constructed using the maximum likelihood method based on multiple sequence alignments of TLP proteins from Arabidopsis thaliana, Oryza sativa, Malus domestica, Populus trichocarpa, and Fragaria vesca. The tree was divided into three phylogenetic subfamilies, which are shown in different colors. Positions of the conserved F-box (yellow rectangle) and tubby (red rectangle) domains are also shown.
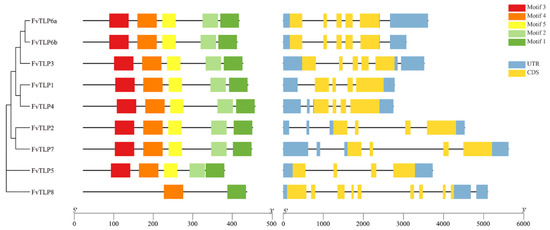
Figure 2.
Conserved motif composition of FvTLPs and gene structure of FvTLPs. Distribution of conserved motifs in FvTLPs (left) and intron–exon structures of FvTLP genes (right). Rectangles of different colors represent different motifs, blue rectangles represent UTRs, black lines represent introns, and ginger rectangles represent exons.
2.3. Cis-Element and Promoter Analysis
Promoters are important molecular tools for functional gene analysis [32] and for exploring the transcriptional regulation of FvTLPs; we identified the cis-elements in putative promoters (Figure 3). Cis-elements related to the light response, development, hormonal responses, and stress responses were unevenly distributed in the promoters of FvTLPs (Figure and Table). The AAGAA-motif and as-1 element are related to development [33]; the AAGAA-motif was detected in all FvTLP promoters, with the exception of the FvTLP5 promoter, and the as-1 element was detected in all FvTLP promoters, with the exception of the FvTLP7 promoter. The RY element is involved in seed-specific regulation and was observed in the FvTLP1, 2, and 4 promoters. E2Fb and MSA-like elements are involved in cell cycle regulation and were only detected in the FvTLP3 promoter. The GCN4_motif is involved in endosperm expression and was present in the FvTLP2 and FvTLP6 promoters. The CAT-box is involved in meristem expression and was only detected in the FvTLP8 promoter. The O2 site is involved in zein metabolism regulation and was observed in the FvTLP5, 6, and 8 promoters. The hormonal-responsive elements in the promoters of FvTLPs included gibberellin-responsive elements (P-box, TATC-box, CARE, GARE-motif, and ERE) [34], salicylic acid element (TCA-element), ABA-responsive element (ABRE), auxin-responsive elements (TGA-element, TGA-box, and AuxRR-core), and MeJA-responsive elements (CGTCA-motif and TGACG-motif). Gibberellin-responsive elements were observed in all FvTLP promoters, and auxin-responsive elements and MeJA-responsive elements were detected in all FvTLP promoters, with the exception of the FvTLP7 promoter. ABREs were detected in all FvTLP promoters, with the exception of the FvTLP6 and FvTLP7 promoters; TCA elements were observed in the FvTLP1,2,5, and 7 promoters. The stress-responsive elements in the promoters of FvTLPs included ARE (anaerobic induction), TC-rich repeats (defense and stress responsiveness), STRE (stress responsiveness), WRE3 (wound-responsive), LTR (low-temperature responsiveness), GC-motif (anoxic specific inducibility), MBS (drought stress responsiveness), WUN-motif (wound-responsive), and DRE core (drought stress responsiveness) elements. The occurrence of other stress-related elements in the promoters of FvTLPs was uneven, with the exception of the WRE3 element, which was present in all FvTLP promoters. Abundant MYC and MYB-binding motifs were observed in the promoters of FvTLPs.
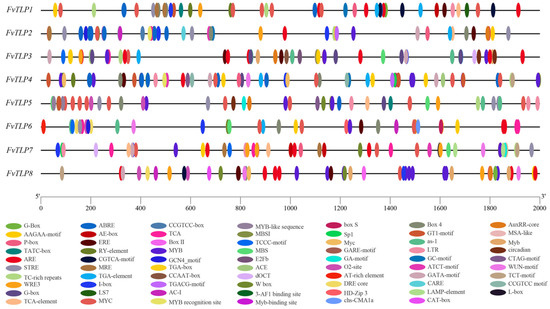
Figure 3.
Cis-element analysis of FvTLP promoters. The distribution of cis-elements in the 2000-bp upstream promoter and the different types of cis-elements are represented by different colors.
2.4. Subcellular Localization and Transcriptional Activity of FvTLPs
Detecting the subcellular localization of FvTLPs is essential for clarifying their functions. To characterize the subcellular localization of FvTLPs, GFP-tagged FvTLPs were expressed in tobacco leaves. The green fluorescence was observed in the nucleus and plasma membrane through confocal microscopy (Figure 4). This demonstrated that all FvTLPs were localized to the nucleus and plasma membrane.
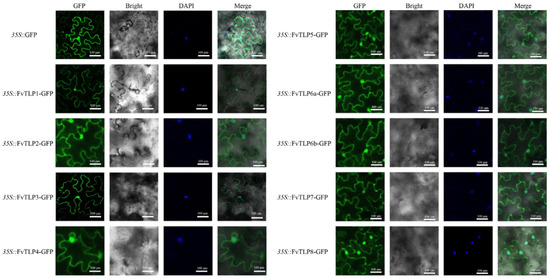
Figure 4.
Subcellular localization of FvTLPs. FvTLP-GFP and GFP were transiently expressed in tobacco epidermal cells and then observed with a confocal microscope. Cell nuclei were stained with DAPI.
Yeast one-hybrid assays were performed to analyze the transcriptional activity of FvTLPs. Yeast cells transformed with the positive control grew well on SD/-Trp-His medium and turned blue in the presence of x-α-Gal, whereas yeast cells with the negative control and FvTLPs did not grow on this medium (Figure 5). This suggested that all FvTLPs have no transcriptional activation function in yeast.
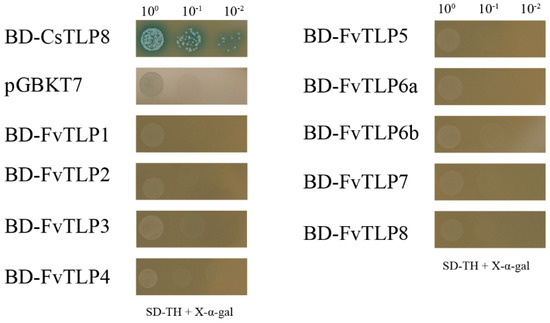
Figure 5.
Transcriptional activity analyses of FvTLPs by yeast one-hybrid assays. Yeast cells transformed with the empty vector pGBKT7 (negative control, NC), pGBKT7-CsTLP8 (positive control, PC), or pGBKT7-FvTLPs were streaked on SD/-Trp/-His/X-α-gal medium.
2.5. FvTLPs Interact with FvSKP1s
Given that the F-box domain is present in the NH2-terminal of FvTLPs, we investigated the interaction between FvTLPs and FvSKP1s using Y2H assays. Y2H assays showed that FvTLP1 interacted with FvSKP1a, FvSKP1c, and FvSKP1d; FvTLP3 interacted with FvSKP1a and FvSKP1c; FvTLP4, 5, 6a, and 6b interacted with FvSKP1c and FvSKP1d; and FvTLP7 interacted with FvSKP1a and FvSKP1d (Figure 6). These findings indicated that FvTLPs could serve as subunits of SCF-type E3 ligase.
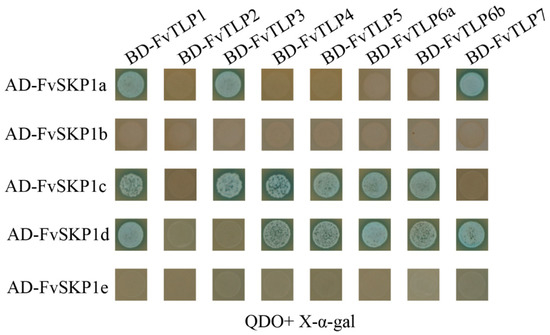
Figure 6.
FvTLPs interact with FvSKP1s. Protein–protein interactions analyzed by yeast two-hybrid assays. Yeast cells transformed with BD- FvTLPs +AD- FvSKP1s were streaked on SD/-Trp/-Leu/-Ade/-His/X-α-gal medium.
2.6. Expression Patterns of FvTLPs in Different Tissues of F. vesca
To explore the functional divergence of FvTLPs, the expression levels of FvTLPs in different tissues were determined. FvTLP1 and FvTLP4 were highly expressed in old leaves and flowers; FvTLP2 and FvTLP7 were highly expressed in the roots; and the expression of FvTLP3 was lower in the roots than in the other tissues. FvTLP5, FvTLP6a, and FvTLP6b were highly expressed in flowers, and FvTLP8 was highly expressed in the petiole (Figure 7A). The expression of FvTLP1, FvTLP6a, FvTLP6b, FvTLP7, and FvTLP8 in achene tissue and the receptacle decreased with fruit ripening. No significant variation was observed in the expression of FvTLP2 and FvTLP5 in achene during the fruit ripening process; however, the expression of these genes decreased in the receptacle as fruits ripened. The expression of FvTLP3 in achene and receptacle increased with fruit ripening. The expression of FvTLP4 decreased in achene tissue but increased in the receptacle as fruits ripened (Figure 7B,C).
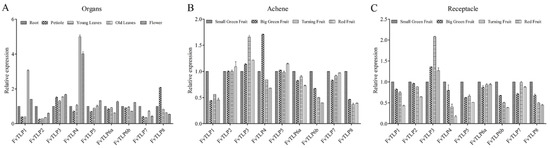
Figure 7.
Expression patterns of TLP genes in Fragaria vesca. (A) Expression profiles of FvTLPs in different tissues (root, petiole, young leaves, old leaves and flower). Expression profiles of FvTLPs in achene (B) and receptacle (C) at different stages of fruit development (small green, big green, turning, and red).
2.7. Responses of FvTLPs to Abiotic Stress and Exogenous Hormones in F. vesca Leaves
To explore the potential function of FvTLPs, the expression of FvTLPs was detected under various abiotic stresses and hormone treatments. Under salt stress, the expression of FvTLP1, FvTLP3, FvTLP4, FvTLP6a, FvTLP7, and FvTLP8 was up-regulated, and the expression of FvTLP2 and FvTLP5 was down-regulated (Figure 8A). The expression of FvTLP1, FvTLP2, FvTLP4, and FvTLP6a was induced by PEG treatment compared with the control (Figure 8B). Under heat stress, the expression of all FvTLPs was induced, with the exception of FvTLP3, and the expression of FvTLP1 and FvTLP4 was markedly induced by heat stress (Figure 8C). Under cold stress, the expression of all FvTLPs was reduced, and the expression of FvTLP1, FvTLP6a, FvTLP6b, and FvTLP8 was significantly inhibited by cold stress. The expression of FvTLP6b was initially down-regulated and later significantly up-regulated (Figure 8D). Under ABA treatment, the expression of all FvTLPs was down-regulated, with the exception of FvTLP1 (Figure 8E). Under MeJA treatment, the expression of FvTLP1, FvTLP2, FvTLP3, FvTLP5, and FvTLP6A was up-regulated, and the expression of FvTLP4, FvTLP7, and FvTLP8 was down-regulated (Figure 8F).
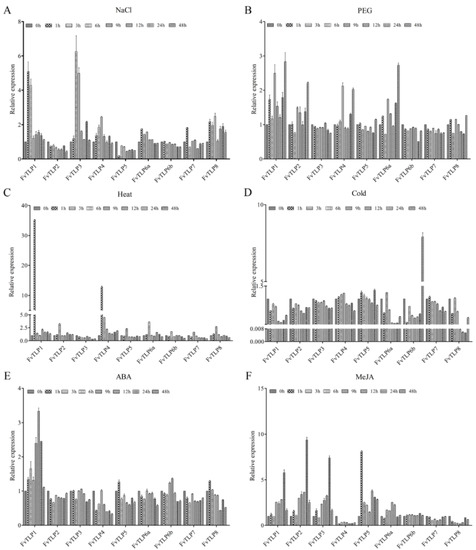
Figure 8.
Responses of FvTLPs to various abiotic stresses and hormone treatments. The expression profile of FvTLPs in the leaves under NaCl (150 mM) treatment (A), PEG6000 (10%) treatment (B), heat (42 °C) treatment (C), cold (4 °C) treatment (D), ABA (100 μM) treatment (E), and MeJA (100 μM) treatment (F).
2.8. FvTLP1 Expression Responds to Abiotic Stress
The expression of FvTLP1 under various abiotic stresses and hormone treatments was assayed by GUS staining of Arabidopsis expressing the GUS reporter gene driven by the FvTLP1 promoter. The GUS expression of FvTLP1pro::GUS plants under NaCl, mannitol, or ABA treatment was significantly higher than that under normal conditions. NaCl, mannitol, and ABA treatment significantly induced GUS expression during seed germination (Figure 9). These results indicated that FvTLP1 might play a role in the response to abiotic stress and in ABA- and osmotic stress-mediated seed germination.
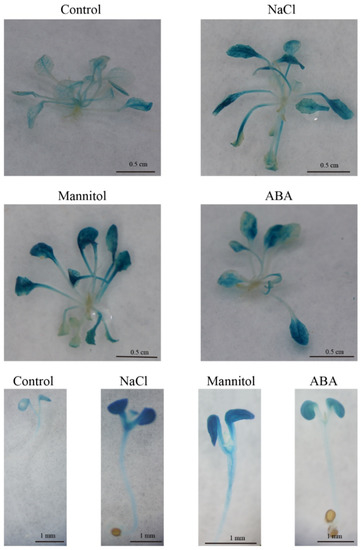
Figure 9.
Histochemical GUS staining of FvTLP1pro::GUS transgenic Arabidopsis. Three-week-old seedlings growing on half-strength MS medium were transferred to half-strength MS medium without or with 150 mM NaCl, 200 mM mannitol, or 100 μM ABA. Seeds of FvTLP1pro::GUS transgenic Arabidopsis were surface-sterilized and germinated on half-strength MS medium supplemented without or with 75 mM NaCl, 75 mM mannitol, or 0.25 μM ABA.
3. Discussion
In this study, we identified eight TLPs in woodland strawberry (F. vesca). The number of TLPs was significantly less in woodland strawberry than in other diploid plants, such as Arabidopsis (11), rice (14), poplar (11), maize (15), tomato (11), and soybean (22) [11,15,23,35], and no paired paralogous TLP genes were identified in F. vesca (Figure 1) as has been observed in TLPs in Arabidopsis, rice, and poplar [35]. Intron/exon structures provide important insights into the evolutionary relationships among members of gene families [36]. The intron/exon structures of FvTLPs significantly differed from those of orthologous genes in Arabidopsis, poplar, and apple. All FvTLPs contained four or five exons, with the exception of FvTLP8, which contained eight exons (Figure 2), and the number of exons in their orthologous genes in Arabidopsis, poplar, and apple ranged from two to nine [13,35]. These results indicated that the evolutionary history of TLP genes in F. vesca differed from that of orthologous genes in other plant species. Although post-transcriptional modifications and variations in the transcripts of a gene are affected by the number of exons, it has been stated that genes with a low number of exons are induced faster in response to external [37,38].
The results of the conserved domain analysis, motif analysis, and amino acid sequence analysis showed that the structure of FvTLPs is conserved (Figure 1, Figure 2 and Figure S2). The subcellular localization of plant TLPs is diverse, as plant TLPs have been reported to be localized to the cell wall, plasma membrane, cytosol, and nucleus [21,22,24]. The PIP2-binding site contributes to the plasma membrane localization of TLPs [30]; all FvTLPs possess a conserved PIP2-binding site in the tubby domain, and all FvTLPs were localized to the plasma membrane (Figure 4). In F. vesca, all FvTLPs possess a conserved F-box domain, with the exception of FvTLP8 (Figure 1). F-box proteins responsible for substrate recognition for the SCF ubiquitin ligase complexes play important roles in plant development, light and phytohormone signaling, mitotic processes, and responses to abiotic stress [39]. In this study, we showed that FvTLPs can interact with specific FvSKP1s (Figure 6), and this has also been observed among TLPs in Arabidopsis, wheat, and cucumber [3,24,25]. These findings indicate that the functions of FvTLPs are conserved.
TLPs have been reported to play important roles in the response to abiotic stress in chickpea, apple, cotton, tomato, cucumber, and soybean [15,19,20,22,25,26]. FvTLPs also play a role in the response to multiple types of stress and hormone treatments. The expression of FvTLP1 was affected by various abiotic stresses and hormone treatments; FvTLP1 expression was strongly induced by heat stress, ABA treatment, and MeJA treatment and strongly reduced by cold stress. The expression of FvTLP2 was induced by heat stress, as well as PEG and MeJA treatment. The expression of FvTLP3 was strongly induced by salt stress and MeJA treatment. The expression of FvTLP4 was strongly induced by heat stress. The expression of FvTLP5 was strongly induced by MeJA treatment. The expression of FvTLP6a was strongly induced by PEG treatment and strongly reduced by cold stress. Under cold treatment, the expression of FvTLP6b was first significantly decreased and then strongly increased. The expression of FvTLP8 was significantly reduced by cold stress and MeJA treatment (Figure 8). These results indicated that FvTLPs might play a role in responses to abiotic stress.
ABA and JA play indispensable roles in controlling leaf senescence [40,41]. The expression levels of FvTLP1 and FvTLP4 were significantly higher in old leaves than in young leaves. The expression of FvTLP1 was strongly induced by ABA and MeJA, and the expression of FvTLP4 was strongly reduced by ABA and MeJA (Figure 7 and Figure 8). These findings indicated that FvTLP1 and FvTLP4 might play a role in regulating leaf senescence via the ABA and JA signaling pathways. SlTLPs have been reported to play a role in ethylene-dependent fruit ripening. The fruit ripening of strawberry is non-climacteric, and ABA and MeJA play a key role in the ripening of strawberry fruit [42,43]. The expression of FvTLP1, FvTLP6b, and FvTLP8 in the achene and receptacle significantly decreased with fruit ripening, whereas the expression of FvTLP3 in the achene and receptacle increased with fruit ripening (Figure 7). The expression of FvTLPs was affected by ABA and MeJA treatment, which suggested that FvTLPs might play a role in fruit ripening via the ABA and JA signaling pathways. Overall, these results indicated that FvTLPs play key roles in regulating the growth and development of strawberries.
Alternative splicing plays a key role in the abiotic stress response by mediating the production of multiple transcripts and increasing proteome complexity; ABA plays a key role in regulating alternative splicing during stress responses [44]. Abiotic stress can also modulate gene expression via alternative splicing pathways [45,46,47,48]. LSM5 (Sm-like protein 5) is involved in the heat shock response by degrading aberrant heat stress-inducible transcripts via mRNA splicing and decapping [49]. HIN1 (HAI1-Interactor 1), a plant-specific RNA-binding protein, promotes growth during drought stress and improves splicing efficiency at intron retention sites [50]. In Arabidopsis, SRAS1 (salt-responsive alternatively spliced gene 1) produces two splicing variants: SRAS1.1 and SRAS1.2. SRAS1.1 and SRAS1.2 exhibit opposite expression patterns in response to salt stress. Salt stress induces the expression of SRAS1.1 and reduces the expression of SRAS1.2; SRAS1.1 increases salt tolerance, and SRAS1.2 decreases salt tolerance. Salt stress-triggered alternative splicing regulates the ratio of SRAS1.1/SRAS1.2, which affects the stability of CSN5A (COP9 signalosome 5A), a core molecular switch between the stress response and development, and regulates the balance between plant development and salt tolerance [51]. In cotton, 93 alternative splicing events have been identified in 29 of 35 GhTULP genes, but the function of the alternative splicing events in GhTULPs in response to environmental stress and other biological processes remains unclear. In this study, FvTLP6 generated two splicing variants: FvTLP6a and FvTLP6b. FvTLP6a and FvTLP6b presented distinct expression patterns under abiotic stress and hormone treatment and at different ripening stages of fruits (Figure 7 and Figure 8). This indicated that FvTLP6a and FvTLP6b might play different roles in abiotic stress responses and fruit ripening regulation.
LTR cis-element has been shown to play key roles in the response to low-temperature stress [52]. MdbHLH33 binds to the promoter of MdCBF2 at the LTR cis-acting element to activate the expression of MdCBF2, which enhances the cold tolerance of transgenic callus [53]. MdbHLH33 can bind to the LTR cis-element of the MdMYBPA1 promoter to activate the expression of MdMYBPA1, which promotes anthocyanin biosynthesis under low-temperature stress [54]. In Lotus corniculatus, the salt-responsive transcription factor LcERF056 enhances salt stress tolerance by binding to the GCC box or DRE of reactive oxygen species-related genes [55]. SlDREBA4 specifically binds to the DRE elements of Hsp (heat shock proteins) genes in response to high-temperature stress [56]. The MBS motif is responsible for ABA and abiotic stress-induced gene expression [57,58]. Low-temperature stress induces the expression of MdMYB2, which binds to the promoter of MdSIZ1 via the MBS motif and enhances the transcription of MdSIZ1, thereby promoting the sumoylation of MdMYB1. Sumoylated MdMYB1 increases anthocyanin accumulation and cold stress tolerance in apple [59]. In S. cerevisiae, the STRE cis-element plays a role in the response to various types of stress [60], and YCP4 regulates the freeze-thaw tolerance of yeast by modulating the activation of the STRE-mediated genes CTT1 and HSP12 [61]. Cis-element and promoter analysis showed that the promoters of FvTLPs contain various stress-related cis-elements, and FvTLPs were expressed in response to various types of abiotic stress (Figure 8). Results of GUS activity assays indicated that FvTLP1 might play a role in ABA- and osmotic stress-mediated seed germination (Figure 9). AtTLP3 and AtTLP9 play redundant roles in regulating ABA- and osmotic stress-mediated seed germination, and CsTLP8 and GhTULP34 negatively regulate seed germination under osmotic stress. The molecular mechanisms underlying these regulatory processes remain unclear. Our results indicate that further study of promoters might provide new insights into the functions of TLPs.
We systematically identified TLP gene family members in woodland strawberry (F. vesca). Gene structure, protein structure, and phylogenetic analyses, along with the results of subcellular localization, transcriptional activity, and protein interaction assays, revealed that the structure and function of TLP genes are highly conserved despite variation in the evolutionary history of TLP genes among plants. Expression patterns of FvTLPs in different tissues and under various abiotic stresses, coupled with GUS activity assays in FvTLP1pro::GUS plants, revealed that FvTLPs play a role in abiotic stress responses in F. vesca. Generally, the results of this study provide new insights into the molecular mechanisms of TLPs.
4. Materials and Methods
4.1. Genome-Scale Identification of FvTLPs
The amino acid sequences of AtTLPs and OsTLPs were downloaded from the TAIR database (http://www.arabidopsis.org/, accessed on 12 December 2020) and RGAP database (http://rice.plantbiology.msu.edu/, accessed on 12 December 2020), respectively. The amino acid sequences of model plants were used as BLAST queries against the woodland strawberry genome (V4.0) to identify TLPs in Fragaria vesca. All the candidate sequences were further verified by a hidden Markov model (HMM) search using the Pfam (http://pfam.xfam.org/, accessed on 5 January 2021) and SMART (http://smart.embl-heidelberg.de/, accessed on 5 January 2021) databases. The coding sequences of FvTLPs were amplified by PCR from ‘Ruegen’ cDNA. Next, the online ExPASy Proteomics Server (http://www.expasy.org/, accessed on 5 March 2021) was used to analyze the physical and chemical characteristics of TLPs, including the number of amino acids, nucleotide data, molecular weight (MW), and isoelectric point (PI).
4.2. Gene Structure, Phylogenetic, and Cis-Element Analysis of FvTLPs
The exon-intron structure of FvTLPs was analyzed using TBtools. The conserved motifs of FvTLPs with default parameters were analyzed using MEME (https://meme.nbcr.net/meme/cgi-bin/meme.cgi, accessed on 9 March 2021), and the maximum number of motifs was set as five. The conserved domains of FvTLPs were analyzed using the Pfam database (http://pfam.xfam.org/, accessed on 12 March 2021). The phylogenetic tree was constructed using the MEGA 10.0 program and modified by iTOL (https://itol.embl.de/, accessed on 21 March 2021). We extracted sequences 2.0 kb upstream from the translation start site of FvTLPs; cis-elements were predicted using the online program PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 26 February 2021) and visualized using TBtools.
4.3. Plant Materials and Treatment
Seeds of woodland strawberry (Fragaria vesca subspecies vesca ‘Ruegen’) were grown in 1-L diameter pots containing Hoagland nutrient solution in a growth chamber (light intensity: approximately 100 mol m−2 s−1; photoperiod: 16 h (light)/8 h (dark); temperature: 25 °C/18 °C; relative humidity: 60%. The 2-month-old plants were treated with either 150 mM NaCl, 10% PEG6000, 100 μM ABA, 100 μM methyl jasmonate (MeJA), cold (4 °C), or heat (40 °C) for 0 h, 1 h, 3 h, 6 h, 9 h, 12 h, 24 h, and 48 h, and the leaves from five individual plants were collected.
4.4. RNA Isolation and qRT-PCR Analysis
The RNA was extracted from the samples following our previously published protocol [62]. Quantitative real-time PCR was performed on a 7500 Real-time PCR System (Applied Biosystems) with the following thermal cycling conditions: 95 °C for 20 s followed by 40 cycles at 95 °C for 5 s, 60 °C for 10 s, and 72 °C for 20 s. A melting curve (61 cycles at 65 °C for 10 s) was generated to determine the specificity of the reaction. The relative gene expression levels were quantified using the 2-△△CT method. FveCHC1 [63] was used as the internal reference gene; the primers of FvTLP genes were designed using Primer Premier 7.0 software. The primer pairs are listed in Supplementary Table S1.
4.5. Subcellular Localization of FvTLPs
The coding sequences of FvTLPs were inserted into the pSuper1300 vector [64] to form SUPERpro: FvTLP-GFP. The fusion constructs were transferred into Agrobacterium tumefaciens strain GV3101 and then injected into tobacco leaves. The fluorescence signal in tobacco epidermal cells was detected by confocal microscopy (FV1000, Olympus, Tokyo, Japan), and cell nuclei were stained with DAPI (4’,6-diamidino-2-phenylindole). The experiment was performed three times.
4.6. Transactivation Activity Assay for FvTLPs in Yeast
The coding sequences of FvTLPs were inserted into the pGBKT7 vector. The fusion constructs were transferred into Y2HGold yeast. Transcriptional activity was assayed as described by Gong et al. [65]. CsTLP8 was used as a positive control, and pGBKT7 plasmid was used as a negative control [25].
4.7. Yeast Two-Hybrid (Y2H) Assays
Y2H assays were performed following the methods of Zhang et al. [66]. Briefly, the coding sequences of FvSKP1s were inserted into the pGADT7 vector. The constructs with FvTLPs-BD and FvSKP1s-AD were then co-transformed into AH109 yeast. Protein interactions were screened on DDO medium (SD/-Leu/-Trp) and QDO medium (SD/-Trp/-Leu/-His/-Ade) supplemented with X-α-galactosidase. The co-transformation of pGADT7-T and pGBKT7-53 was used as positive control, and the co-transformation of pGADT7-T and pGBKT7-Lam was used as negative control (Figure S4).
4.8. Plasmid Construction and Arabidopsis Transformation
The 1600-bp promoter of FvTLP1 was inserted into the pCAMBIA1391 vector (Marker Gene Technologies, Inc., Eugene, OR, USA); the fusion construct was transferred into Agrobacterium tumefaciens strain GV3101 and then integrated into Columbia wild-type Arabidopsis (Col-0) through the floral dipping method. The transgenic lines were identified by resistance to the antibiotic hygromycin B and PCR amplification (Figure S5). T3 transgenic plants were used in subsequent experiments.
4.9. Histochemical Assay for GUS Activity
Transgenic Arabidopsis seedlings were grown on half-strength MS medium, and 3-week-old seedlings were transferred to half-strength MS medium supplemented with 150 mM NaCl, 200 mM mannitol, or 100 μM ABA for two days. Sterilized seeds were sown on half-strength MS medium supplemented with or without 75 mM NaCl, 75 mM mannitol, or 0.25 μM ABA for five days. After the stress treatment, histochemical GUS staining was performed following the method of Jameel et al. [67]. Briefly, the prepared materials were immersed in GUS staining solution buffer and incubated overnight at 37 °C. The plant tissues were then decolorized with 95% ethanol and observed under a light microscope.
4.10. Statistical Analysis
Each experiment was repeated three times. Statistical analyses were conducted using SPSS 20.0 software (IBM Corp. Armonk, NY, USA), and statistical comparisons were performed using a t-test in SPSS.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms231911961/s1.
Author Contributions
S.L., Y.Z., J.D. and J.S. conceived this project and designed the research. S.L. performed most of the experiments. G.W., L.C., R.S., R.W., C.Z., Y.G., H.Z., L.W. and Y.W. participated in this work. S.L. and J.S. analyzed the data. S.L. and J.S. wrote the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from China Agriculture Research System of MOF and MARA (CARS-24-A-14), Beijing Innovation Consortium of Agriculture Research System (BAIC04-2022), Beijing Academy of Agriculture and Forestry Special Fund for the Construction of Scientific and Technological Innovation Capability (KJCX20200114), and Postdoctoral Science Foundation of Beijing Academy of Agricultural and Forestry Sciences under Grant (2020-ZZ-010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Titus, J.B.; Wei-Song, S.; Sandro, S.; Samuel, C.M.; Lawrence, S. Implication of Tubby proteins as transcription factors by structure-based functional analysis. Science 1999, 286, 2119–2125. [Google Scholar]
- Bateman, A.; Finn, R.D.; Sims, P.J.; Wiedmer, T.; Biegert, A.; Soding, J. Phospholipid scramblases and Tubby-like proteins belong to a new superfamily of membrane tethered transcription factors. Bioinformatics 2009, 25, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Dae, Y.K.; Yong, W.S. Interactions between wheat Tubby-like and SKP1-like proteins. Genes Genet. Syst. 2015, 90, 293–304. [Google Scholar]
- Kou, Y.; Qiu, D.; Wang, L.; Li, X.; Wang, S. Molecular analyses of the rice tubby-like protein gene family and their response to bacterial infection. Plant Cell Rep. 2009, 28, 113–121. [Google Scholar] [CrossRef]
- Albuquerque, E.V.S.; Petitot, A.; Da Silva, J.P.; Grossi-de-Sa, M.F.; Fernandez, D. Early responses of coffee immunity-related genes to root-knot nematode infection. Physiol. Mol. Plant Pathol. 2017, 100, 142–150. [Google Scholar] [CrossRef]
- Muthumeena, M.; Ashwin, R.; Ramesh, S.A.; Malathi, P.; Viswanathan, R. Expression profiling of transcription factors (TFs) in sugarcane X Colletotrichum falcatum interaction. J. Plant Biochem. Biotechnol. 2013, 22, 286–294. [Google Scholar]
- Zhang, Y.; Miao, L.; Yang, X.; Jiang, G. Genome-wide characterization and expression of the TLP gene family associated with Colletotrichum gloeosporioides inoculation in Fragaria × ananassa. PeerJ 2022, 10, e12979. [Google Scholar] [CrossRef]
- Cai, M.; Qiu, D.; Yuan, T.; Ding, X.; Li, H.; Duan, L.; Xu, C.; Li, X.; Wang, S. Identification of novel pathogen-responsive cis-elements and their binding proteins in the promoter of OsWRKY13, a gene regulating rice disease resistance. Plant Cell Environ. 2008, 31, 86–96. [Google Scholar] [CrossRef]
- Gharaghani, F.; Rafiei, F.; Mirakhorli, N.; Ebrahimie, E. Differential expression of TLP, ERF1, and R2R3MYB in annual Medicago species under salinity conditions. Genet. Mol. Res. 2015, 14, 10152–10164. [Google Scholar] [CrossRef]
- Janiak, A.; Kwasniewski, M.; Sowa, M.; Kuczyńska, A.; Mikołajczak, K.; Ogrodowicz, P.; Szarejko, I. Insights into barley root transcriptome under mild drought stress with an emphasis on gene expression regulatory mechanisms. Int. J. Mol. Sci. 2019, 20, 6139. [Google Scholar] [CrossRef]
- Yulong, C.; Wei, D.; Baoming, S.; Yang, Z.; Qing, M. Genome-wide identification and comparative analysis of the TUBBY-like protein gene family in maize. Genes Genom. 2016, 38, 25–36. [Google Scholar] [CrossRef]
- Dong, M.; Fan, X.; Pang, X.; Li, Y. Decrypting tubby-like protein gene family of multiple functions in starch root crop cassava. AoB Plants 2019, 11, plz075. [Google Scholar] [CrossRef]
- Xu, J.; Xing, S.; Zhang, Z.; Chen, X.; Wang, X. Genome-wide identification and expression analysis of the tubby-like protein family in the Malus domestica genome. Front. Plant Sci. 2016, 7, 1693. [Google Scholar] [CrossRef] [PubMed]
- Bano, N.; Fakhrah, S.; Mohanty, C.S.; Bag, S.K. Genome-wide identification and evolutionary analysis of Gossypium Tubby-Like Protein (TLP) gene family and expression analyses during salt and drought stress. Front. Plant Sci. 2021, 12, 667929. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Y.; Yu, T.; Hou, Z.; Zheng, J.; Chen, J.; Zhou, Y.; Chen, M.; Fu, J.; Ma, Y.; et al. Comprehensive profiling of Tubby-Like Proteins in soybean and roles of the GmTLP8 gene in abiotic stress responses. Front. Plant Sci. 2022, 13, 844545. [Google Scholar] [CrossRef]
- Altaf, A.; Zada, A.; Hussain, S.; Gull, S.; Ding, Y.; Tao, R.; Zhu, M.; Zhu, X. Genome-wide identification, characterization, and expression analysis of Tubby gene family in wheat (Triticum aestivum L.) under biotic and abiotic stresses. Agronomy 2022, 12, 1121. [Google Scholar] [CrossRef]
- Wang, K.; Cheng, Y.; Yi, L.; He, H.; Zhan, S.; Yang, P. Genome-wide identification of the Tubby-Like Protein (TLPs) family in medicinal model plant Salvia miltiorrhiza. PeerJ 2021, 9, e1140. [Google Scholar] [CrossRef]
- Bano, N.; Fakhrah, S.; Mohanty, C.S.; Bag, S.K. Transcriptome meta-analysis associated targeting hub genes and pathways of drought and salt stress responses in cotton (Gossypium hirsutum): A network biology approach. Front. Plant Sci. 2022, 13, 818472. [Google Scholar] [CrossRef]
- Xu, J.; Xing, S.; Sun, Q.; Zhan, C.; Liu, X.; Zhang, S.; Wang, X. The expression of a tubby-like protein from Malus domestica (MdTLP7) enhances abiotic stress tolerance in Arabidopsis. BMC Plant Biol. 2019, 19, 60–68. [Google Scholar] [CrossRef]
- Wardhan, V.; Pandey, A.; Chakraborty, S.; Chakraborty, N. Chickpea transcription factor CaTLP1 interacts with protein kinases, modulates ROS accumulation and promotes ABA-mediated stomatal closure. Sci. Rep. 2016, 6, 38121. [Google Scholar] [CrossRef]
- Vijay, W.; Kishwer, J.; Sonika, G.; Srinivasarao, C.; Asis, D.; Subhra, C.; Niranjan, C. Overexpression of CaTLP1, a putative transcription factor in chickpea (Cicer arietinum L.), promotes stress tolerance. Plant Mol. Biol. 2012, 79, 479–493. [Google Scholar]
- Li, S.; Zhang, J.; Liu, L.; Wang, Z.; Li, Y.; Guo, L.; Li, Y.; Zhang, X.; Ren, S.; Zhao, B.; et al. SlTLFP8 reduces water loss to improve water-use efficiency by modulating cell size and stomatal density via endoreduplication. Plant Cell Environ. 2020, 43, 2666–2679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, X.; Su, D.; Feng, Y.; Zhao, H.; Deng, H.; Liu, M. Comprehensive profiling of Tubby-Like Protein expression uncovers ripening-related TLP genes in tomato (Solanum lycopersicum). Int. J. Mol. Sci. 2020, 21, 1000. [Google Scholar] [CrossRef]
- Bao, Y.; Song, W.; Jin, Y.; Jiang, C.; Yang, Y.; Li, B.; Huang, W.; Liu, H.; Zhang, H. Characterization of Arabidopsis Tubby-like proteins and redundant function of AtTLP3 and AtTLP9 in plant response to ABA and osmotic stress. Plant Mol. Biol. 2014, 86, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Z.; Wang, F.; Lv, H.; Cao, M.; Zhang, N.; Li, F.; Wang, H.; Li, X.; Yuan, X.; et al. A tubby-like protein CsTLP8 acts in the ABA signaling pathway and negatively regulates osmotic stresses tolerance during seed germination. BMC Plant Biol. 2021, 21, 340. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Cao, X.; Chen, B.; Ma, C.; Lv, J.; Sun, Z.; Qiao, K.; Zhu, L.; Zhang, C.; et al. GhTULP34, a member of tubby-like proteins, interacts with GhSKP1A to negatively regulate plant osmotic stress. Genomics 2021, 113, 462–474. [Google Scholar] [CrossRef]
- Song, W.; Cheng, Z.; Guo, X.; Yu, C.; Wang, H.; Wang, J.; Li, B.; Zhang, H.; Bao, Y. Overexpression of NHL6 affects seed production in transgenic Arabidopsis plants. Plant Growth Regul. 2019, 88, 41–47. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Ahmed, R.I.; Wang, Y.; Hu, R.; Zhou, G.; Kong, Y. Tubby-like Protein 2 regulates homogalacturonan biosynthesis in Arabidopsis seed coat mucilage. Plant Mol. Biol. 2019, 99, 421–436. [Google Scholar] [CrossRef]
- Myśków, B.; Góralska, M.; Lenarczyk, N.; Czyczyło-Mysza, I.; Stojałowski, S. Putative candidate genes responsible for leaf rolling in rye (Secale cereale L.). BMC Genet. 2018, 19, 57. [Google Scholar] [CrossRef]
- Santagata, S.; Boggon, T.J.; Baird, C.L.; Gomez, C.A.; Zhao, J.; Shan, W.S.; Myszka, D.G.; Shapiro, L. G-protein signaling through Tubby proteins. Science 2001, 292, 2041–2050. [Google Scholar] [CrossRef]
- Zea, D.J.; Monzon, A.M.; Parisi, G.; Marino-Buslje, C. How is structural divergence related to evolutionary information? Mol. Phylogenet. Evol. 2018, 127, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Sun, Q.; Kong, Y. Genome-wide mining seed-specific candidate genes from peanut for promoter cloning. PLoS ONE 2019, 14, e214025. [Google Scholar] [CrossRef] [PubMed]
- Ain-Ali, Q.; Mushtaq, N.; Amir, R.; Gul, A.; Tahir, M.; Munir, F. Genome-wide promoter analysis, homology modeling and protein interaction network of Dehydration Responsive Element Binding (DREB) gene family in Solanum tuberosum. PLoS ONE 2021, 16, e261215. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X.; Li, G.; Kumar, S.; Sun, Z.; Li, Y.; Guo, W.; Yang, J.; Hou, H. Genome-wide identification of the Nramp gene family in Spirodela polyrhiza and expression analysis under cadmium stress. Int. J. Mol. Sci. 2021, 22, 6414. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Wang, X.; Gu, S.; Yu, J.; Liang, G.; Yan, C.; Xu, C. Genomewide comparative phylogenetic and molecular evolutionary analysis of tubby-like protein family in Arabidopsis, rice, and poplar. Genomics 2008, 92, 246–253. [Google Scholar] [CrossRef]
- Márquez, Y.; Mantica, F.; Cozzuto, L.; Burguera, D.; Hermoso-Pulido, A.; Ponomarenko, J.; Roy, S.W.; Irimia, M. ExOrthist: A tool to infer exon orthologies at any evolutionary distance. Genome Biol. 2021, 22, 239. [Google Scholar] [CrossRef]
- Koralewski, T.E.; Krutovsky, K.V. Evolution of exon-intron structure and alternative splicing. PLoS ONE 2011, 6, e18055. [Google Scholar] [CrossRef]
- Heidari, P.; Puresmaeli, F.; Mora-Poblete, F. Genome-wide identification and molecular evolution of the Magnesium Transporter (MGT) gene family in Citrullus lanatus and Cucumis sativus. Agronomy 2022, 12, 2253. [Google Scholar] [CrossRef]
- Al-Saharin, R.; Hellmann, H.; Mooney, S. Plant E3 ligases and their role in abiotic stress response. Cells 2022, 11, 890. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Yuan, J.J.; Xiao, C.C.; Li, G.X.; Yan, J.Y.; Zheng, S.J.; Ding, Z.J. RING-box proteins regulate leaf senescence and stomatal closure via repression of ABA transporter gene ABCG40. J. Integr. Plant Biol. 2022, 64, 979–994. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, M.; Li, Y.; Zhang, J.; Su, H.; Cao, M.; Liu, Z.; Zhang, X.; Zhao, B.; Guo, Y.; et al. The transcription factor SlWRKY37 positively regulates jasmonic acid- and dark-induced leaf senescence in tomato. J. Exp. Bot. 2022, erac258. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Dai, Z.; Zeng, B.; Li, J.; Ouyang, J.; Kang, L.; Wang, W.; Jia, W. Autocatalytic biosynthesis of abscisic acid and its synergistic action with auxin to regulate strawberry fruit ripening. Hortic. Res. 2022, 9, uhab076. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Bigotes, A.; Figueroa, P.M.; Figueroa, C.R. Jasmonate metabolism and its relationship with abscisic acid during strawberry fruit development and ripening. J. Plant Growth Regul. 2018, 37, 101–113. [Google Scholar] [CrossRef]
- Laloum, T.; Martín, G.; Duque, P. Alternative splicing control of abiotic stress responses. Trends Plant Sci. 2018, 23, 140–150. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Cheng, Z.; Zeng, C.; Li, W.; Zhang, L.; Peng, M. Large-scale analysis of the cassava transcriptome reveals the impact of cold stress on alternative splicing. J. Exp. Bot. 2019, 71, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Du, Q.; Campbell, M.; Yu, B.; Walia, H.; Zhang, C. Genome-wide discovery of natural variation in pre-mRNA splicing and prioritising causal alternative splicing to salt stress response in rice. New Phytol. 2021, 230, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, S.R.; Danilevskaya, O.N.; Meng, X.; Beatty, M.; Zastrow-Hayes, G.; Harris, C.; Van Allen, B.; Habben, J.; Li, B. Genome-wide analysis of alternative splicing during development and drought stress in maize. Plant Physiol. 2015, 170, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Serrano, N.; Gao, G.; Atia, M.; Mokhtar, M.; Woo, Y.H.; Bazin, J.; Veluchamy, A.; Benhamed, M.; Crespi, M.; et al. Thermopriming triggers splicing memory in Arabidopsis. J. Exp. Bot. 2018, 69, 2659–2675. [Google Scholar] [CrossRef]
- Okamoto, M.; Matsui, A.; Tanaka, M.; Morosawa, T.; Ishida, J.; Iida, K.; Mochizuki, Y.; Toyoda, T.; Seki, M. Sm-Like Protein-mediated RNA metabolism is required for heat stress tolerance in Arabidopsis. Front. Plant Sci. 2016, 7, 1079. [Google Scholar] [CrossRef]
- Chong, G.L.; Foo, M.H.; Lin, W.; Wong, M.M.; Verslues, P.E. Highly ABA-Induced 1 (HAI1)-Interacting protein HIN1 and drought acclimation-enhanced splicing efficiency at intron retention sites. Proc. Natl. Acad. Sci. USA 2019, 116, 22376–22385. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Guo, Q.; Liu, P.; Li, Y.; Wu, C.; Yang, G.; Huang, J.; Zhang, S.; Zheng, C.; et al. Salt responsive alternative splicing of a RING finger E3 ligase modulates the salt stress tolerance by fine-tuning the balance of COP9 signalosome subunit 5A. PLoS Genet. 2021, 17, e1009898. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zheng, C.; Ji, G.; Jiang, P. Synergistic effects of HSE and LTR elements from HSP70 gene promoter of Ulva prolifera (Ulvophyceae, Chlorophyta) upon temperature induction1. J. Phycol. 2019, 55, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, N.; Wang, Y.; Jiang, S.; Fang, H.; Zhang, J.; Su, M.; Zuo, W.; Xu, L.; Zhang, Z.; et al. Overexpression of the transcription factor MdbHLH33 increases cold tolerance of transgenic apple callus. Plant Cell Tissue Organ Cult. 2018, 134, 131–140. [Google Scholar] [CrossRef]
- Wang, N.; Qu, C.; Jiang, S.; Chen, Z.; Xu, H.; Fang, H.; Su, M.; Zhang, J.; Wang, Y.; Liu, W.; et al. The proanthocyanidin-specific transcription factor MdMYBPA1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples. Plant J. 2018, 96, 39–55. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Z.; Hu, X.; Xiong, J.; Hu, L.; Xu, Y.; Tang, Y.; Wu, Y. The key regulator LcERF056 enhances salt tolerance by modulating reactive oxygen species-related genes in Lotus corniculatus. BMC Plant Biol. 2021, 21, 605. [Google Scholar] [CrossRef]
- Mao, L.; Deng, M.; Jiang, S.; Zhu, H.; Yang, Z.; Yue, Y.; Zhao, K. Characterization of the DREBA4-type transcription factor (SlDREBA4), which contributes to heat tolerance in tomatoes. Front. Plant Sci. 2020, 11, 554520. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, M.; Guo, Z.; Zhu, X.; Xia, Z. Identification of a 119-bp promoter of the maize sulfite oxidase gene (ZmSO) That confers high-level gene expression and ABA or drought inducibility in transgenic plants. Int. J. Mol. Sci. 2019, 20, 3326. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Li, J.; Li, H.; Zhang, G. The functional and regulatory mechanisms of the Thellungiella salsuginea Ascorbate Peroxidase 6 (TsAPX6) in response to salinity and water deficit stresses. PLoS ONE 2016, 11, e154042. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, L.; Gao, H.; Wang, X.; Li, Z.; Li, Y. The transcription factor MdMYB2 influences cold tolerance and anthocyanin accumulation by activating SUMO E3 ligase MdSIZ1 in apple. Plant Physiol. 2022, 189, 2044–2060. [Google Scholar] [CrossRef]
- Freitas, F.Z.; Virgilio, S.; Cupertino, F.B.; Kowbel, D.J.; Fioramonte, M.; Gozzo, F.C.; Glass, N.L.; Bertolini, M.C. The SEB-1 transcription factor binds to the STRE motif in Neurospora crassa and Regulates a Variety of Cellular Processes Including the Stress Response and Reserve Carbohydrate Metabolism. G3-Genes Genomes Genet. 2016, 6, 1327–1343. [Google Scholar] [CrossRef]
- Kim, H. Disruption of YCP4 enhances freeze-thaw tolerance in Saccharomyces cerevisiae. Biotechnol. Lett. 2022, 44, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, R.; Liu, H.; Chang, L.; Li, S.; Zhao, M.; Shennan, C.; Lei, J.; Dong, J.; Zhong, C.; et al. Complete chloroplast genome sequencing of ten wild Fragaria species in China provides evidence for phylogenetic evolution of Fragaria. Genomics 2021, 113, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, X.; Lin, Y.; Wang, X.; Yan, Z.; Wang, Q.; Ding, J.; Gu, T.; Li, Y. Identification of reference genes for transcript normalization in various tissue types and seedlings subjected to different abiotic stresses of woodland strawberry Fragaria vesca. Sci. Hortic. 2020, 261, 108840. [Google Scholar] [CrossRef]
- Liang, Y.; Jiang, C.; Liu, Y.; Gao, Y.; Lu, J.; Aiwaili, P.; Fei, Z.; Jiang, C.; Hong, B.; Ma, C.; et al. Auxin regulates sucrose transport to repress petal abscission in rose (Rosa hybrida). Plant Cell 2020, 32, 3485–3499. [Google Scholar] [CrossRef]
- Gong, Z.; Luo, Y.; Zhang, W.; Jian, W.; Zhang, L.; Gao, X.; Hu, X.; Yuan, Y.; Wu, M.; Xu, X.; et al. A SlMYB75-centred transcriptional cascade regulates trichome formation and sesquiterpene accumulation in tomato. J. Exp. Bot. 2021, 72, 3806–3820. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Chen, X.; Zhao, L.; Liu, X.; Wang, Y.; Wu, G.; Xia, C.; Zhang, L.; Kong, X. The protein phosphatase 2C clade A TaPP2CA interact with calcium-dependent protein kinases, TaCDPK5/TaCDPK9–1, that phosphorylate TabZIP60 transcription factor from wheat (Triticum aestivum L.). Plant Sci. 2022, 321, 111304. [Google Scholar] [CrossRef]
- Jameel, A.; Ketehouli, T.; Wang, Y.; Wang, F.; Li, X.; Li, H. Detection and validation of cis-regulatory motifs in osmotic stress-inducible synthetic gene switches via computational and experimental approaches. Funct. Plant Biol. 2022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).