Characterization in Potent Modulation on Voltage-Gated Na+ Current Exerted by Deltamethrin, a Pyrethroid Insecticide
Abstract
:1. Introduction
2. Results
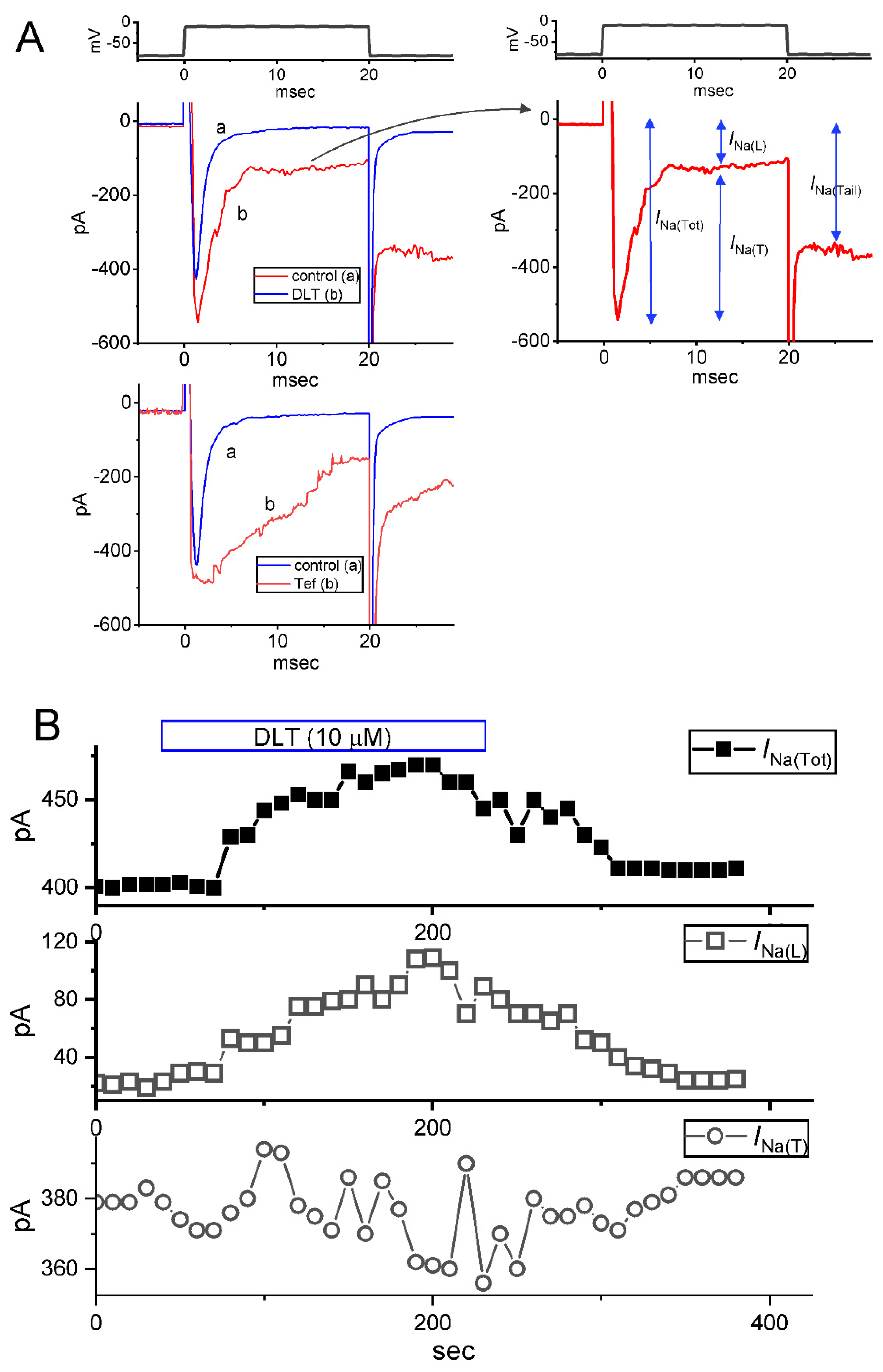
2.1. Modification by Deltamethrin (DLT) or Tefluthrin (Tef) on Voltage-Gated Na+ Current (INa) Measured from Pituitary GH3 Cells
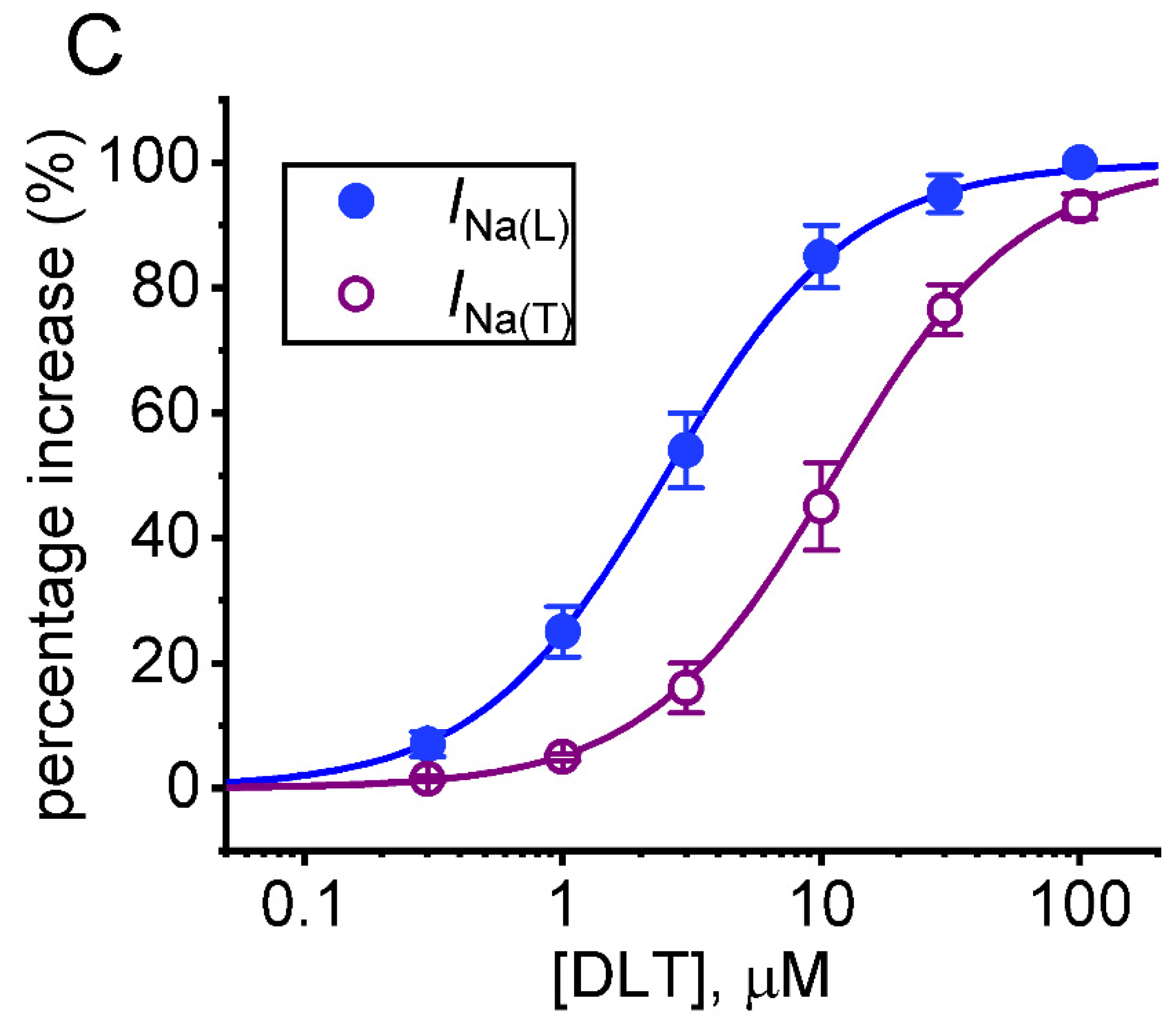
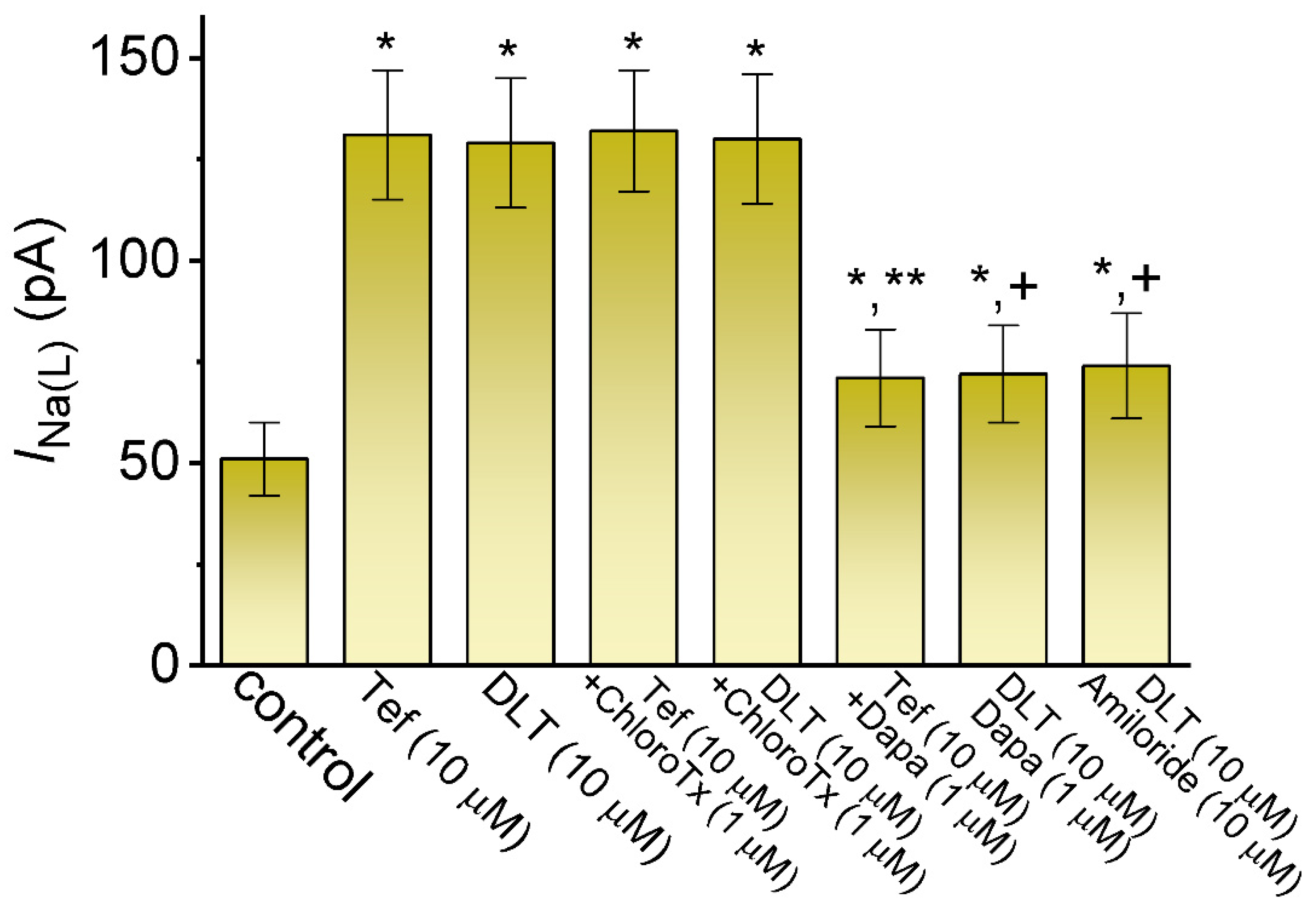
2.2. Comparison among Effects of Tef, DLT, Tef plus Chlorotoxin (ChloroTx), DLT plus ChloroTx, Tef plus Dapaglifozin (Dapa), DLT plus Dapa, and DLT plus Amiloride on INa(L) Amplitude Measured from GH3 Cells
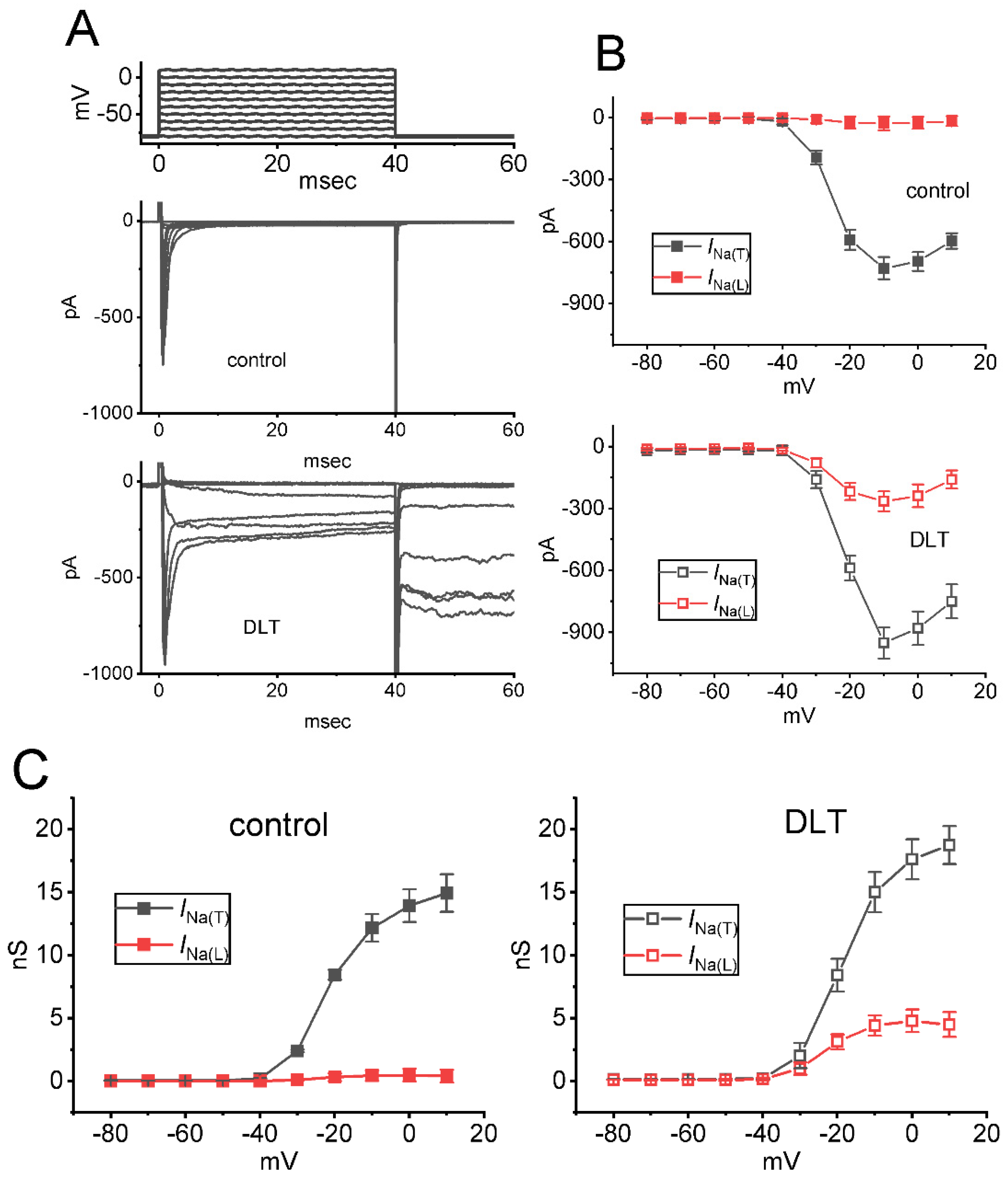
2.3. Effect of DLT on Mean Current Versus Voltage (I-V) Relationship of INa(T) and INa(L)
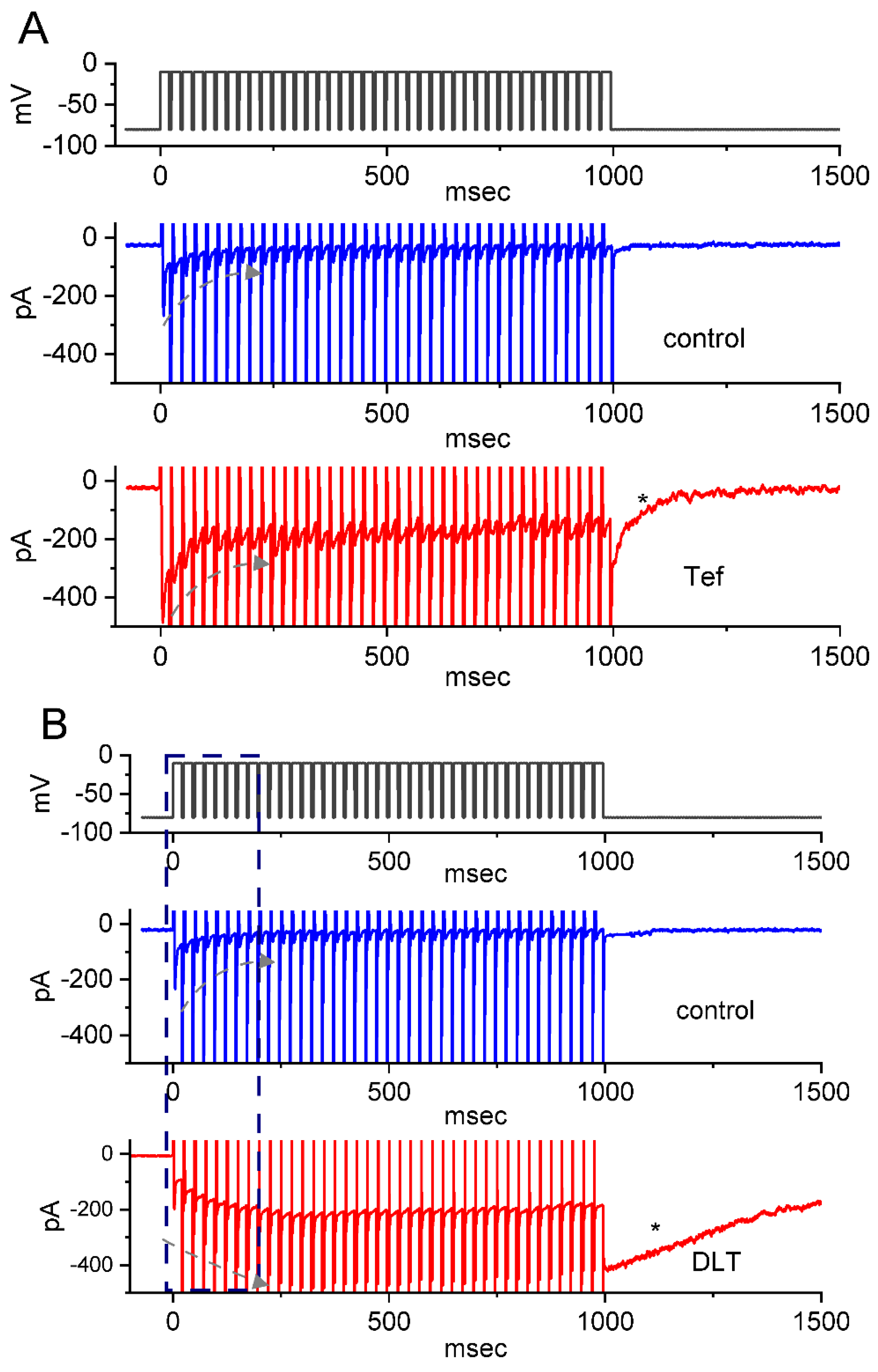
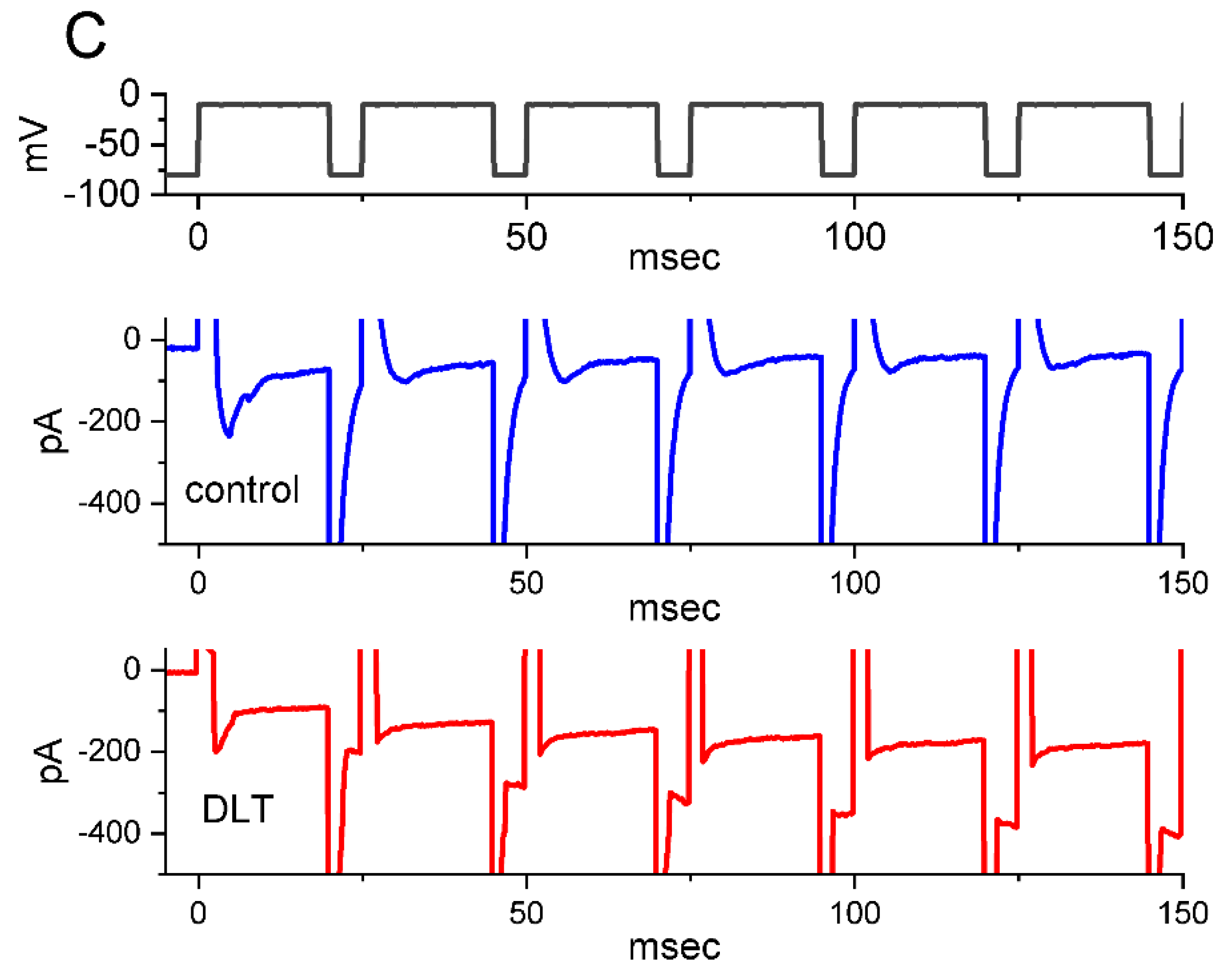
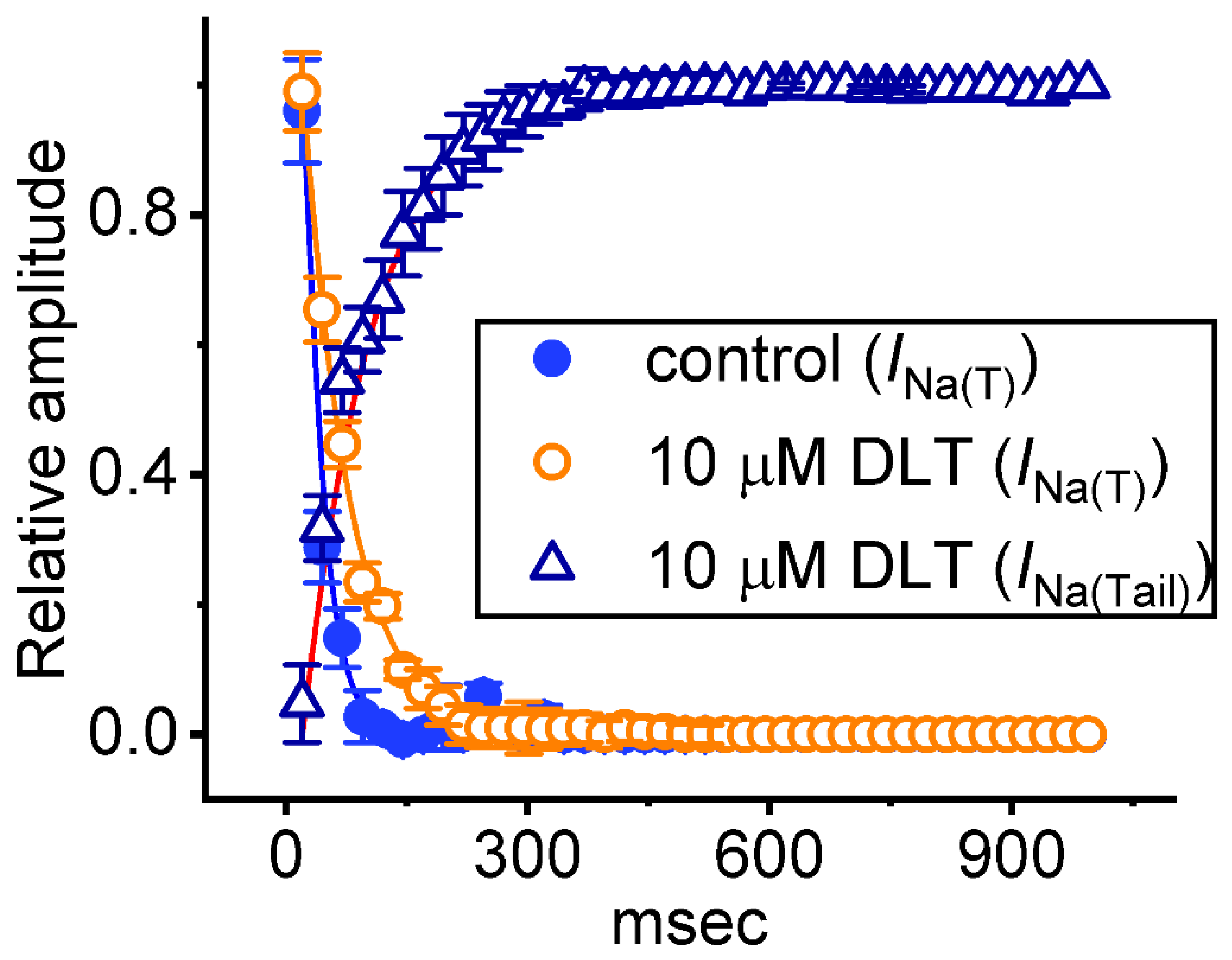
2.4. Tef- or DLT-Mediated Slowing in Cumulative Inhibition of INa(T) during Rapid Depolarizing Stimuli
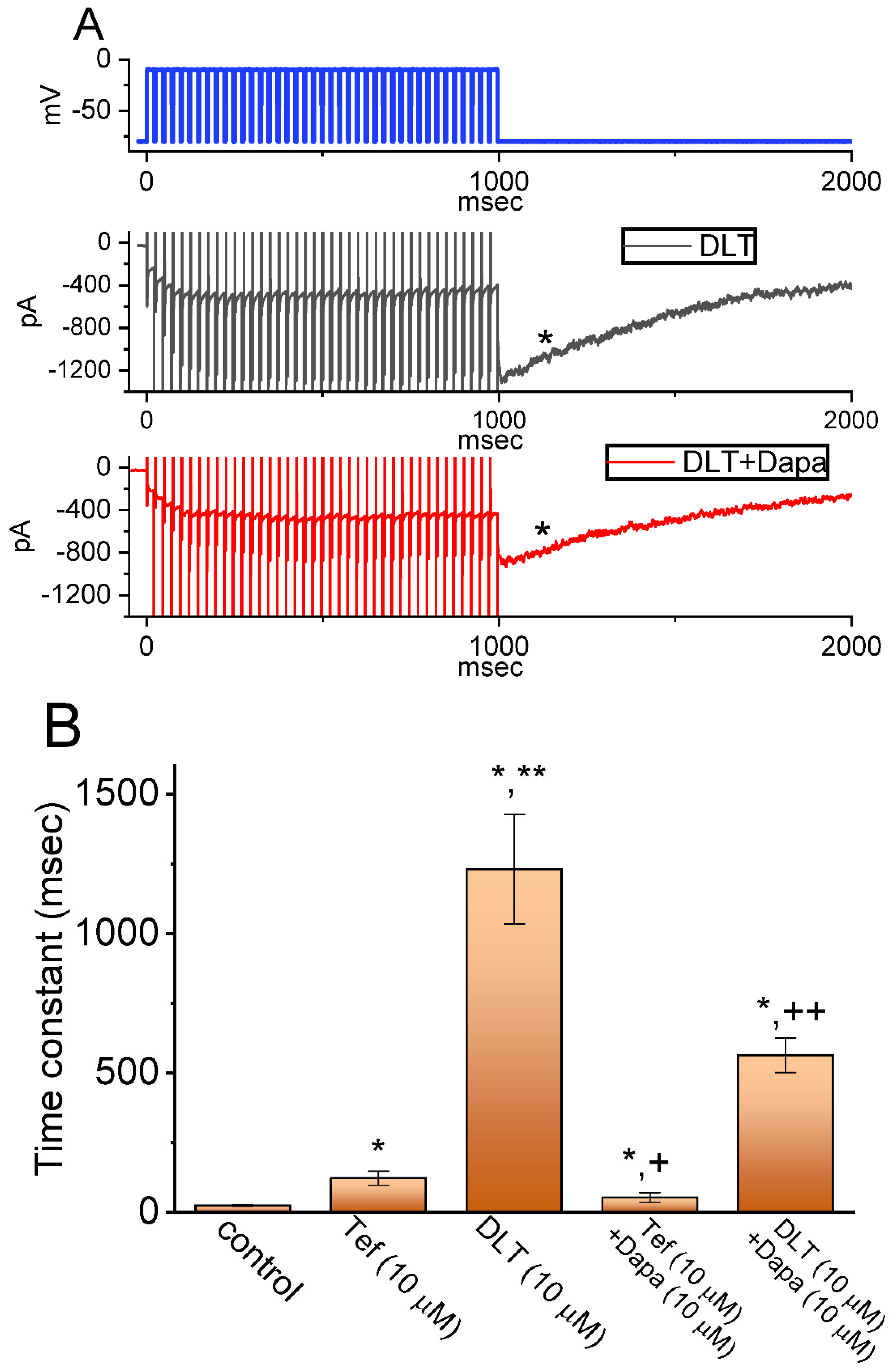
2.5. Effect of Tef or DLT on the Strength of Voltage-Dependent Hysteresis (Hys(V)) of Persistent INa (INa(P)) Elicited by an Upright Isosceles-Triangular Ramp Voltage (Vramp)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals, Drugs, Reagents, and Solution Used in This Work
5.2. Cell Preparation
5.3. Electrophysiological Measurements (Patch-Clamp Current Recordings)
5.4. Data Recordings and Processing
5.5. Data Analyses for Whole-Cell Ionic Currents
5.6. Curve-Fitting Approximations and Statistical Analyses Used in This Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DLT | deltamethrin |
| ChloroTx | chlorotoxin |
| Dapa | dapagliflozin |
| EC50 | concentration required for half-maximal stimulation |
| Hys(V) | voltage-dependent hysteresis |
| I-V relationship | current versus voltage relationship |
| INa | voltage-gated Na+ current |
| INa(L) | late Na+ current |
| INa(P) | persistent Na+ current |
| INa(Tot) | total Na+ current (i.e., INa(T) plus INa(L)) |
| INa(Tail) | tail Na+ current |
| NaV channel | voltage-gated Na+ channel |
| PT stimulation | pulse train stimulation |
| Tef | tefluthrin |
| Vramp | ramp voltage |
References
- Laskowski, D.A. Physical and chemical properties of pyrethroids. Rev. Environ. Contam. Toxicol. 2002, 174, 49–170. [Google Scholar] [PubMed]
- Bradberry, S.M.; Cage, S.A.; Proudfoot, A.T.; Vale, J.A. Poisoning due to pyrethroids. Toxicol. Rev. 2005, 24, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Yadav, K.; Acharya, B.N.; Nagar, D.P.; Rao Ghorpade, R. Deltamethrin Contact Exposure Mediated Toxicity and Histopathological Aberrations in Tissue Systems of Public Health Importance Cockroach Species Periplaneta americana and Blattella germanica. Front. Physiol. 2022, 13, 926267. [Google Scholar] [CrossRef]
- Eads, D.; Livieri, T.; Tretten, T.; Hughes, J.; Kaczor, N.; Halsell, E.; Grassel, S.; Dobesh, P.; Childers, E.; Lucas, D.; et al. Assembling a safe and effective toolbox for integrated flea control and plague mitigation: Fipronil experiments with prairie dogs. PLoS ONE 2022, 17, e0272419. [Google Scholar] [CrossRef]
- Ma, C.; Wei, D.; Liu, P.; Fan, K.; Nie, L.; Song, Y.; Wang, M.; Wang, L.; Xu, Q.; Wang, J.; et al. Pesticide Residues in Commonly Consumed Vegetables in Henan Province of China in 2020. Front. Public Health 2022, 10, 901485. [Google Scholar] [CrossRef]
- Tabarean, I.V.; Narahashi, T. Potent modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by the type II pyrethroid deltamethrin. J. Pharmacol. Exp. Ther. 1998, 284, 958–965. [Google Scholar]
- Tan, J.; Soderlund, D.M. Divergent actions of the pyrethroid insecticides S-bioallethrin, tefluthrin, and deltamethrin on rat Na(v)1.6 sodium channels. Toxicol. Appl. Pharmacol. 2010, 247, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCavera, S.J.; Soderlund, D.M. Differential state-dependent modification of inactivation-deficient Nav1.6 sodium channels by the pyrethroid insecticides S-bioallethrin, tefluthrin and deltamethrin. Neurotoxicology 2012, 33, 384–390. [Google Scholar] [CrossRef] [Green Version]
- James, T.F.; Nenov, M.N.; Tapia, C.M.; Lecchi, M.; Koshy, S.; Green, T.A.; Laezza, F. Consequences of acute Na(v)1.1 exposure to deltamethrin. Neurotoxicology 2017, 60, 150–160. [Google Scholar] [CrossRef] [Green Version]
- So, E.C.; Wu, S.N.; Lo, Y.C.; Su, K. Differential regulation of tefluthrin and telmisartan on the gating charges of I(Na) activation and inactivation as well as on resurgent and persistent INa in a pituitary cell line (GH3). Toxicol. Lett. 2018, 285, 104–112. [Google Scholar] [CrossRef]
- Breckenridge, C.B.; Holden, L.; Sturgess, N.; Weiner, M.; Sheets, L.; Sargent, D.; Soderlund, D.M.; Choi, J.S.; Symington, S.; Clark, J.M.; et al. Evidence for a separate mechanism of toxicity for the Type I and the Type II pyrethroid insecticides. Neurotoxicology 2009, 30 (Suppl. 1), S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.T.; Gutierrez, A.; Vorhees, C.V. Effects of Acute Deltamethrin Exposure in Adult and Developing Sprague Dawley Rats on Acoustic Startle Response in Relation to Deltamethrin Brain and Plasma Concentrations. Toxicol. Sci. 2019, 168, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Forshaw, P.J.; Lister, T.; Ray, D.E. Inhibition of a neuronal voltage-dependent chloride channel by the type II pyrethroid, deltamethrin. Neuropharmacology 1993, 32, 105–111. [Google Scholar] [CrossRef]
- Burr, S.A.; Ray, D.E. Structure-activity and interaction effects of 14 different pyrethroids on voltage-gated chloride ion channels. Toxicol. Sci. 2004, 77, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hołyńska-Iwan, I.; Bogusiewicz, J.; Chajdas, D.; Szewczyk-Golec, K.; Lampka, M.; Olszewska-Słonina, D. The immediate influence of deltamethrin on ion transport through rabbit skin. An in vitro study. Pestic. Biochem. Physiol. 2018, 148, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G. The neurotoxicity of organochlorine and pyrethroid pesticides. Handb. Clin. Neurol. 2015, 131, 135–148. [Google Scholar] [PubMed]
- Van Goor, F.; Zivadinovic, D.; Stojilkovic, S.S. Differential expression of ionic channels in rat anterior pituitary cells. Mol. Endocrinol. 2001, 15, 1222–1236. [Google Scholar] [CrossRef]
- Jukič, M.; Kikelj, D.; Anderluh, M. Isoform selective voltage-gated sodium channel modulators and the therapy of pain. Curr. Med. Chem. 2014, 21, 164–186. [Google Scholar] [CrossRef]
- Guérineau, N.C.; Monteil, A.; Lory, P. Sodium background currents in endocrine/neuroendocrine cells: Towards unraveling channel identity and contribution in hormone secretion. Front. Neuroendocrinol. 2021, 63, 100947. [Google Scholar] [CrossRef]
- Catterall, W.A. Forty Years of Sodium Channels: Structure, Function, Pharmacology, and Epilepsy. Neurochem. Res. 2017, 42, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Taddese, A.; Bean, B.P. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron 2002, 33, 587–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.W.; Hung, T.Y.; Wu, S.N. The inhibitory actions by lacosamide, a functionalized amino acid, on voltage-gated Na+ currents. Neuroscience 2015, 287, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.A.; Salari, A.; Lin, J.L.; Cowan, L.M.; Penington, N.J.; Milescu, M.; Milescu, L.S. Sodium channels implement a molecular leaky integrator that detects action potentials and regulates neuronal firing. Elife 2020, 9, e54940. [Google Scholar] [CrossRef]
- Wu, P.M.; Lai, P.C.; Cho, H.Y.; Chuang, T.H.; Wu, S.N.; Tu, Y.F. Effective Perturbations by Phenobarbital on I(Na), I(K(erg)), I(K(M)) and I(K(DR)) during Pulse Train Stimulation in Neuroblastoma Neuro-2a Cells. Biomedicines 2022, 10, 1968. [Google Scholar] [CrossRef]
- Wu, S.N.; Chen, B.S.; Hsu, T.I.; Peng, H.; Wu, Y.H.; Lo, Y.C. Analytical studies of rapidly inactivating and noninactivating sodium currents in differentiated NG108-15 neuronal cells. J. Theor. Biol. 2009, 259, 828–836. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Bjelobaba, I.; Zemkova, H. Ion Channels of Pituitary Gonadotrophs and Their Roles in Signaling and Secretion. Front. Endocrinol. 2017, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, X.; Eaton, M.; Wu, J.; Ma, Z.; Lai, S.; Park, A.; Ahmad, T.S.; Que, Z.; Lee, J.H.; et al. Severe deficiency of the voltage-gated sodium channel Na(V)1.2 elevates neuronal excitability in adult mice. Cell Rep. 2021, 36, 109495. [Google Scholar] [CrossRef]
- Hsu, C.L.; Zhao, X.; Milstein, A.D.; Spruston, N. Persistent Sodium Current Mediates the Steep Voltage Dependence of Spatial Coding in Hippocampal Pyramidal Neurons. Neuron 2018, 99, 147–162.e8. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, Y.; Tomé, H.V.V.; Guedes, R.N.C.; Oliveira, E.E. Deltamethrin toxicity and impaired swimming behavior of two backswimmer species. Environ. Toxicol. Chem. 2017, 36, 1235–1242. [Google Scholar] [CrossRef]
- Vega, A.V.; Espinosa, J.L.; López-Domínguez, A.M.; López-Santiago, L.F.; Navarrete, A.; Cota, G. L-type calcium channel activation up-regulates the mRNAs for two different sodium channel alpha subunits (Nav1.2 and Nav1.3) in rat pituitary GH3 cells. Brain Res. Mol. Brain Res. 2003, 116, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Philippaert, K.; Kalyaanamoorthy, S.; Fatehi, M.; Long, W.; Soni, S.; Byrne, N.J.; Barr, A.; Singh, J.; Wong, J.; Palechuk, T.; et al. Cardiac Late Sodium Channel Current Is a Molecular Target for the Sodium/Glucose Cotransporter 2 Inhibitor Empagliflozin. Circulation 2021, 143, 2188–2204. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Wu, C.L.; Cho, H.Y.; Chiang, C.W. Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents. Int. J. Mol. Sci. 2022, 23, 9453. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.; Morley, J.W.; Suaning, G.J.; Lovell, N.H. Frequency-dependent reduction of voltage-gated sodium current modulates retinal ganglion cell response rate to electrical stimulation. J. Neural Eng. 2011, 8, 066007. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, M.; Guo, T.; Shivdasani, M.N.; Tsai, D.; Fried, S.; Li, L.; Dokos, S.; Morley, J.W.; Lovell, N.H. Neural activity of functionally different retinal ganglion cells can be robustly modulated by high-rate electrical pulse trains. J. Neural Eng. 2020, 17, 045013. [Google Scholar] [CrossRef]
- Shiau, A.L.; Liao, C.S.; Tu, C.W.; Wu, S.N.; Cho, H.Y.; Yu, M.C. Characterization in Effective Stimulation on the Magnitude, Gating, Frequency Dependence, and Hysteresis of I(Na) Exerted by Picaridin (or Icaridin), a Known Insect Repellent. Int. J. Mol. Sci. 2022, 23, 9696. [Google Scholar] [CrossRef]
- Xue, N.; Li, F.; Hou, H.; Li, B. Occurrence of endocrine-disrupting pesticide residues in wetland sediments from Beijing, China. Environ. Toxicol. Chem. 2008, 27, 1055–1062. [Google Scholar] [CrossRef]
- Eni, G.; Ibor, O.R.; Andem, A.B.; Oku, E.E.; Chukwuka, A.V.; Adeogun, A.O.; Arukwe, A. Biochemical and endocrine-disrupting effects in Clarias gariepinus exposed to the synthetic pyrethroids, cypermethrin and deltamethrin. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 225, 108584. [Google Scholar] [CrossRef]
- Hołyńska-Iwan, I.; Szewczyk-Golec, K. Pyrethroids: How They Affect Human and Animal Health? Medicina 2020, 56, 582. [Google Scholar] [CrossRef]
- Knapke, E.T.; Magalhaes, D.P.; Dalvie, M.A.; Mandrioli, D.; Perry, M.J. Environmental and occupational pesticide exposure and human sperm parameters: A Navigation Guide review. Toxicology 2022, 465, 153017. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Shen, H.; Fan, H.; Liu, J.; Wu, N. Cis-bifenthrin inhibits cortisol and aldosterone biosynthesis in human adrenocortical H295R cells via cAMP signaling cascade. Environ. Toxicol. Pharmacol. 2022, 89, 103784. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Anand, S.S.; Kim, H.J.; White, C.A.; Fisher, J.W.; Tornero-Velez, R.; Bruckner, J.V. Age, dose, and time-dependency of plasma and tissue distribution of deltamethrin in immature rats. Toxicol Sci. 2010, 115, 354–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, C.C.; Chou, C.T.; Liang, W.Z.; Jan, C.R. Effect of the pesticide, deltamethrin, on Ca2+ signaling and apoptosis in OC2 human oral cancer cells. Drug. Chem. Toxicol. 2014, 37, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sasmal, D.; Sharma, N.; Bhaskar, A.; Chandra, S.; Mukhopadhyay, K.; Kumar, M. Deltamethrin, a pyrethroid insecticide, could be a promising candidate as an anticancer agent. Med. Hypotheses 2015, 85, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, L.; Sezgin, E.C.; Eckert, F.; Huber, S.M. Ion Channels in Brain Metastasis. Int. J. Mol. Sci. 2016, 17, 1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.H.; Chou, C.T.; Liang, W.Z.; Chen, W.C.; Wang, J.L.; Yeh, J.H.; Kuo, C.C.; Shieh, P.; Kuo, D.H.; Chen, F.A.; et al. Ca2+ Movement Induced by Deltamethrin in PC3 Human Prostate Cancer Cells. Chin. J. Physiol. 2016, 59, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Banerjee, S.; Mazumder, P.M. Evaluation of the mechanism of anticancer activity of deltamethrin in Jurkat-J6 cell line. Pestic. Biochem. Physiol. 2018, 149, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Lv, Z.; Zhang, X.; Lv, Y.; Li, S.; Wu, P.; Yang, Q.; Li, J.; Qu, B.; Zhang, Z. Deltamethrin induces liver fibrosis in quails via activation of the TGF-β1/Smad signaling pathway. Environ. Pollut. 2020, 259, 113870. [Google Scholar] [CrossRef]
- Sontheimer, H.; Black, J.A.; Waxman, S.G. Voltage-gated Na+ channels in glia: Properties and possible functions. Trends Neurosci. 1996, 19, 325–331. [Google Scholar] [CrossRef]
- Rizaner, N.; Onkal, R.; Fraser, S.P.; Pristerá, A.; Okuse, K.; Djamgoz, M.B. Intracellular calcium oscillations in strongly metastatic human breast and prostate cancer cells: Control by voltage-gated sodium channel activity. Eur. Biophys. J. 2016, 45, 735–748. [Google Scholar] [CrossRef]
- Gumushan Aktas, H.; Akgun, T. Naringenin inhibits prostate cancer metastasis by blocking voltage-gated sodium channels. Biomed. Pharmacother. 2018, 106, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Tzeng, R.C.; Huang, C.W.; Wu, S.N. The Novel Direct Modulatory Effects of Perampanel, an Antagonist of AMPA Receptors, on Voltage-Gated Sodium and M-type Potassium Currents. Biomolecules 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varró, P.; Kovács, M.; Világi, I. The insecticide esfenvalerate modulates neuronal excitability in mammalian central nervous system in vitro. Toxicol. Lett. 2017, 267, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.; Barrero, R.A.; Black, M.; Bellgard, M.I.; van Dalen, E.M.S.; Fourie, J.; Maritz-Olivier, C. Differentially expressed genes in response to amitraz treatment suggests a proposed model of resistance to amitraz in R. decoloratus ticks. Int. J. Parasitol. Drugs Drug. Resist. 2018, 8, 361–371. [Google Scholar] [CrossRef]
- Pitzer, E.M.; Sugimoto, C.; Gudelsky, G.A.; Huff Adams, C.L.; Williams, M.T.; Vorhees, C.V. Deltamethrin Exposure Daily From Postnatal Day 3-20 in Sprague-Dawley Rats Causes Long-term Cognitive and Behavioral Deficits. Toxicol. Sci. 2019, 169, 511–523. [Google Scholar] [CrossRef]
- Carpenter, J.M.; Brown, K.A.; Diaz, A.N.; Dockman, R.L.; Benbow, R.A.; Harn, D.A.; Norberg, T.; Wagner, J.J.; Filipov, N.M. Delayed treatment with the immunotherapeutic LNFPIII ameliorates multiple neurological deficits in a pesticide-nerve agent prophylactic mouse model of Gulf War Illness. Neurotoxicol. Teratol. 2021, 87, 107012. [Google Scholar] [CrossRef]
- Cassano, G.; Bellantuono, V.; Ardizzone, C.; Lippe, C. Pyrethroid stimulation of ion transport across frog skin. Environ. Toxicol. Chem. 2003, 22, 1330–1334. [Google Scholar] [CrossRef]
- Peng, C.-C.; Li, Y.-R. Parameters Identification of Nonlinear Lorenz Chaotic System and its Application to High Precision Model Reference Synchronization. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Cho, H.Y.; Chuang, T.H.; Wu, S.N. Effective Perturbations on the Amplitude and Hysteresis of Erg-Mediated Potassium Current Caused by 1-Octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6(undecyloxy)hexyl]amino]-octanoate (SM-102), a Cationic Lipid. Biomedicines 2021, 9, 1367. [Google Scholar] [CrossRef]

| Control | DLT (10 mM) | DLT (10 mM) Plus Dapa (10 mM) | DLT (10 mM) Plus Ami (10 mM) | Cell Number (n) | |
|---|---|---|---|---|---|
| Decaying time constant of INa(T) | 22.1 ± 2.8 ms | 56.4 ± 3.9 * ms | 29.6 ± 6.1 * ms | 30.9 ± 6.5 * ms | 8 |
| Rising time constant of INa(L) | (-) | 87.4 ± 4.6 ms | 19.1 ± 6.1 ** ms | 21.1 ± 6.5 ** ms | 8 |
| Recovery time constant of INa(Tail) | 25 ± 3 ms | 1.23 ± 0.19 + s | 0.56 ± 0.04 ** s | 0.58 ± 0.05 ** s | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-H.; Lin, J.-F.; Yu, M.-C.; Wu, S.-N.; Wu, C.-L.; Cho, H.-Y. Characterization in Potent Modulation on Voltage-Gated Na+ Current Exerted by Deltamethrin, a Pyrethroid Insecticide. Int. J. Mol. Sci. 2022, 23, 14733. https://doi.org/10.3390/ijms232314733
Lin M-H, Lin J-F, Yu M-C, Wu S-N, Wu C-L, Cho H-Y. Characterization in Potent Modulation on Voltage-Gated Na+ Current Exerted by Deltamethrin, a Pyrethroid Insecticide. International Journal of Molecular Sciences. 2022; 23(23):14733. https://doi.org/10.3390/ijms232314733
Chicago/Turabian StyleLin, Mao-Hsun, Jen-Feng Lin, Meng-Cheng Yu, Sheng-Nan Wu, Chao-Liang Wu, and Hsin-Yen Cho. 2022. "Characterization in Potent Modulation on Voltage-Gated Na+ Current Exerted by Deltamethrin, a Pyrethroid Insecticide" International Journal of Molecular Sciences 23, no. 23: 14733. https://doi.org/10.3390/ijms232314733
APA StyleLin, M.-H., Lin, J.-F., Yu, M.-C., Wu, S.-N., Wu, C.-L., & Cho, H.-Y. (2022). Characterization in Potent Modulation on Voltage-Gated Na+ Current Exerted by Deltamethrin, a Pyrethroid Insecticide. International Journal of Molecular Sciences, 23(23), 14733. https://doi.org/10.3390/ijms232314733







