Abstract
Picaridin (icaridin), a member of the piperidine chemical family, is a broad-spectrum arthropod repellent. Its actions have been largely thought to be due to its interaction with odorant receptor proteins. However, to our knowledge, to what extent the presence of picaridin can modify the magnitude, gating, and/or the strength of voltage-dependent hysteresis (Hys(V)) of plasmalemmal ionic currents, such as, voltage-gated Na+ current [INa], has not been entirely explored. In GH3 pituitary tumor cells, we demonstrated that with exposure to picaridin the transient (INa(T)) and late (INa(L)) components of voltage-gated Na+ current (INa) were differentially stimulated with effective EC50’s of 32.7 and 2.8 μM, respectively. Upon cell exposure to it, the steady-state current versus voltage relationship INa(T) was shifted to more hyperpolarized potentials. Moreover, its presence caused a rightward shift in the midpoint for the steady-state inactivate curve of the current. The cumulative inhibition of INa(T) induced during repetitive stimuli became retarded during its exposure. The recovery time course from the INa block elicited, following the conditioning pulse stimulation, was satisfactorily fitted by two exponential processes. Moreover, the fast and slow time constants of recovery from the INa block by the same conditioning protocol were noticeably increased in the presence of picaridin. However, the fraction in fast or slow component of recovery time course was, respectively, increased or decreased with an increase in picaridin concentrations. The Hys(V)’s strength of persistent INa (INa(P)), responding to triangular ramp voltage, was also enhanced during cell exposure to picaridin. The magnitude of resurgent INa (INa(R)) was raised in its presence. Picaritin-induced increases of INa(P) or INa(R) intrinsically in GH3 cells could be attenuated by further addition of ranolazine. The predictions of molecular docking also disclosed that there are possible interactions of the picaridin molecule with the hNaV1.7 channel. Taken literally, the stimulation of INa exerted by the exposure to picaridin is expected to exert impacts on the functional activities residing in electrically excitable cells.
1. Introduction
Picaridin (icaridine, 1-piperidinecarboxylic acid 2-(2-hydroxyethyl)-1-methylpropylester), a cyclic amine and a member of the piperidine chemical family, is viewed as a synthetic, broad-spectrum arthropod repellent [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. The repellent and deterrent activities of picaridin have been previously demonstrated to involve olfactory sensing in mosquitoes and ticks, via their interactions with odorant receptor proteins residing in neurons [17,18,19,20,21,22]. However, to the best of our knowledge, the questions of whether and how picaridin, or other relevant compounds, act to cause any modifications on other types of transmembrane ionic currents have not yet been answered.
It has been established that nine isoforms (i.e., NaV1.1–1.9 [or SCN1A-SCN5A and SCN8A-SCN11A] of voltage-gated Na+ (NaV) channels are distributed in mammalian excitable tissues which are present in the central or peripheral nervous system, as well as in the neuroendocrine system [23,24,25]. Once activated, the increased activity of these channels can quickly depolarize the cell membrane and, in turn, elicit the upstroke of the action potential, thereby intrinsically governing the amplitude, frequency, and/or pattern of firing action potentials in an array of excitable cells [23,24,25,26,27]. Of additional note, some inhibitors of NaV channels (e.g., KMUP-1 and ranolazine) were demonstrated to increase the inactivation rate of INa [28,29,30], while some activations of NaV channels (e.g., tefluthrin) could preferentially retard the inactivation rate, as well as enhance the transient (INa(T)) and late (INa(L)) components of INa [31,32]. KMUP-1 was viewed as a xanthine and piperazine derivative [30], and ranolazine as a piperazine derivative, which is structurally similar to trimetazidine and known to be a blocker of INa(L) [28,29,33,34], while tefluthrin has been reported to be a synthetic type-I pyrethroid used to kill a wide range of insects [35,36]. However, whether and how picaridin can affect the magnitude, gating kinetics, and/or voltage-dependent hysteresis (Hys(V)) of INa remains mostly obscure, although it is an insect repellent which belongs to the piperidine chemical family.
Due to the reasons elaborated above, the overall objective of the current investigation was to explore if picaridin, and other relevant compounds, could exert any adjustments on the magnitude, gating, use dependence, and/or Hys(V) behavior of plasmalemmal ionic currents, particularly voltage-gated Na+ current (INa). The biophysical and pharmacological properties of INa, including transient INa (INa(T)), late INa (INa(L)), persistent INa (INa(P)) and resurgent INa (INa(R)), were extensively studied in pituitary GH3 lactotrophs. Findings from the present studies provide the notion that picaridin is capable of perturbing the magnitude, gating, and/or Hys(V) strength of INa(T) in concentration-, time-, frequency- and Hys(V)-dependent manners.
2. Results
2.1. Stimulatory Effect of Picaridin on Voltage-Gated Na+ Current (INa) Experimentally Recorded from Pituitary GH3 Cells
In the start of experiments, we attempted to explore if cell exposure to picaridin produced any modifications on the magnitude of INa identified in these cells. To verify the appearance of INa, we used Ca2+-free Tyrode’s solution as a bathing medium in which 10 mM tetraethylammonium chloride (TEA) and 0.5 mM CdCl2 were contained, and the recording electrode was then filled up with a Cs+-containing solution. As illustrated in Figure 1A, one minute after cells were exposed to picaridin (3 or 10 μM), the INa magnitude (with a rapidly activating and inactivating property) elicited by 30-ms depolarizing step from −100 to −10 mV became progressively increased. For example, one minute after cell exposure to 3 or 10 μM, the peak (or transient, INa(T)) component of INa was respectively raised to 828 ± 44 pA (n = 8, p < 0.05) or 900 ± 50 pA (n = 8, p < 0.05) from a control value of 712 ± 39 pA (n = 8, p < 0.05). The observed picaridin-mediated increase of INa(T) was also concurrently accompanied by a striking retardation in the inactivation time course of the current, as evidenced by a striking increase in the value of the slow component of the inactivation time constant (τinact(S)) from 12 ± 2 to 19 ± 4 ms (n = 8, p < 0.05). After washout of picaridin, current magnitude returned to 719 ± 41 pA (n = 8, p < 0.05). Moreover, picaridin-mediated stimulation of INa was also counteracted by further addition of tetrodotoxin (TTX, 1 μM) or ranolazine (Ran, 10 μM). TTX or Ran was reported to suppress INa in pituitary lactotrophs [26,28,37].
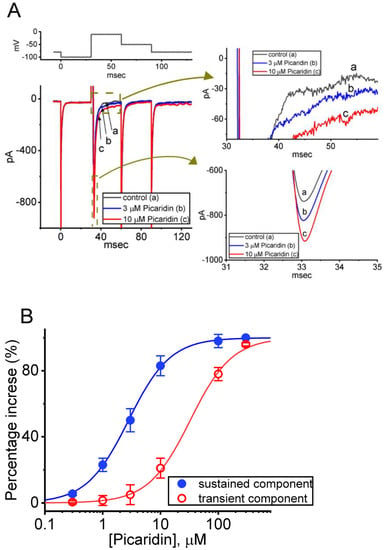
Figure 1.
Stimulatory effect of picaridin on voltage-gated Na+ current (INa) measured from pituitary GH3 cells. In this set of measurements, we placed cells in Ca2+-free Tyrode’s solution containing 0.5 mM CdCl2 and 10 mM tetraethylammonium chloride (TEA), and the recording pipette was filled up with a Cs+-enriched solution. (A) Exemplar current traces evoked by a 30-ms depolarizing pulse from −100 to −10 mV (indicated in the uppermost part (A)). a: control (i.e., picaridin was not present); b: 3 μM picaridin; and c: 10 μM picaridin. The right side shows the expanded records with curve arrows from the brown dashed boxes in the left side. (B) Concentration-dependent stimulation of transient (peak, INa(T), red open circles) or sustained (late, INa(L), blue filled circles) caused by picaridin. The amplitude of INa(T) or INa(L) was taken at the start- or end-pulse of the depolarizing command voltage from −100 to −10 mV for a duration of 30 ms. Each point represents the mean ± SEM (n = 8). The sigmoidal curves, on which the data points are overlaid, were reliably fitted to a modified Hill equation, as detailed under Materials and Methods.
The relationship between the picaridin concentration and the percentage increase of INa(T) or INa(L) was further constructed (Figure 1B). Addition of picaridin was noticed to increase the INa(T) or INa(L) amplitude in a concentration-dependent fashion. When the experimental data became least-squares fitted to a Hill function, as detailed in Materials and Methods, the half-maximal concentration (i.e., EC50) required for the stimulatory effect of picaridin on INa(T) or INa(L) observed in these cells was yielded as 32.7 or 2.8 μM, respectively. Overall, the data from these experiments allowed us to ascertain that the presence of picaridin caused a stimulatory but differential effect on INa(T) and INa(L) in a concentration-dependent manner.
2.2. Effect of Picaridin on Mean Current versus Voltage (I–V) Relationship of INa(T)
We next examined the perturbations of this compound on INa(T) measured at various membrane potentials. As depicted in Figure 2, a steady-state I–V relationship of INa(T) with or without the picaridin presence was constructed in these cells. The application of 10 μM picaridin resulted in a striking increase in the amplitude of INa(T) elicited by depolarizing steps. For example, as the examined cells were rapidly depolarized from −80 to −20 mV, the addition of picaridin (10 μM) noticeably raised the INa(T) magnitude from 744 ± 49 to 940 ± 56 pA (n = 7, p < 0.05). Moreover, the steady-state I–V relationship of INa(T) was found to be shifted to more negative potentials during GH3-cell exposure to 10 μM picaridin.
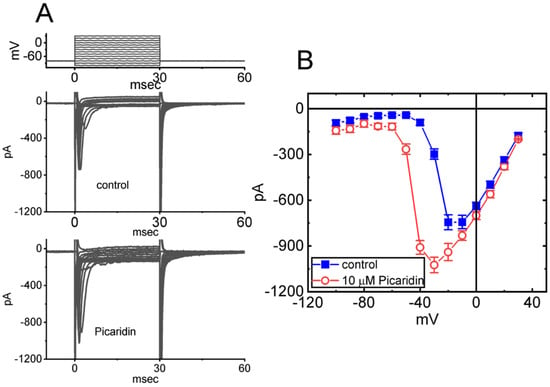
Figure 2.
Mean current versus voltage (I–V) relationship of INa(T) caused by picaridin. Current amplitude (i.e., INa(T)) was taken at the start of each abrupt depolarization. (A) Exemplar current traces achieved in the control period (upper) and during cell exposure to 10 μM picaridin (lower). The voltage-clamp protocol imposed is shown in the uppermost part. (B) Steady-state I–V relationship of INa(T) without (blue filled squares) and with (red open circles) cell exposure to 10 μM picaridin (mean ± SEM; n = 8 for each point). Note that cell exposure to picaridin depressed the amplitude of INa(T); moreover, the overall steady-state I–V relationship of the current was shifted to more negative potentials in its presence.
2.3. Characterization of Picaridin-Induced Modifications on the Steady-State Inactivation Curve of INa(T)
To further characterize the stimulatory effect of picaridin on INa(T) in response to an abrupt depolarization pulse, we continued to examine the steady-state inactivation curve of the current by using a two-step voltage protocol. Figure 3 shows the steady-state inactivation curve of INa(T) acquired with or without exposure to picaridin (10 μM). In this series of experiments, a 30 ms conditioning pulse was given to various membrane potentials to precede the test pulse (30 ms in duration) to −10 mV from a holding potential of −80 mV. The interval between two sets of voltage pulses applied was about 1 min to allow complete recovery of INa(T). The relationship between the conditioning potentials and the normalized amplitudes of INa(T) with or without the picaridin addition were plotted and, then, appropriately fitted to a Boltzmann function, detailed under Materials and Methods. In control, V1/2 = −48 ± 2 mV, q = 3.5 ± 0.6 e (n = 7), whereas in the presence of 10 μM picaridin, V1/2 −43 ± 2 mV, q = 3.6 ± 0.6 e (n = 7). It meant, therefore, that the presence of this compound not only elevated the maximal magnitude of INa(T), but it also shifted the steady-state inactivation curve of INa(T) to more depolarized potential (i.e., in the rightward direction) by approximately 5 mV. However, we found no appreciable adjustment in the q (apparent gating charge) value of the inactivation curve in its presence. These results reflected that the presence of picaridin could increase the INa(T) magnitude in a voltage-dependent fashion in GH3 cells.
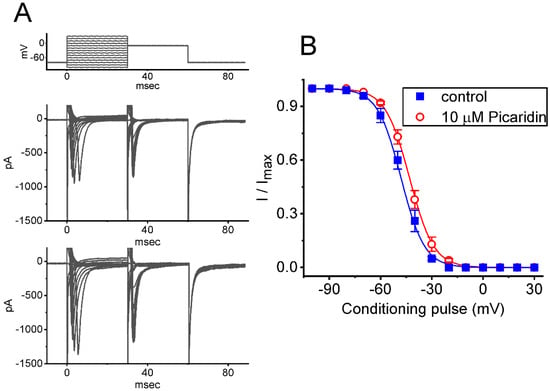
Figure 3.
Modification by picaridin on steady-state inactivation curve of INa(T) identified from GH3 cells. This set of experiments was undertaken in situations where conditioning voltage pulses with a duration of 30 ms to various potentials, ranging between −100 and +30 mV, were applied from a holding potential of −80 mV. Following each conditioning pulse, a test pulse to −10 mV for a duration of 30 ms was, subsequently, applied to evoke INa(T). An example of current traces obtained by a two-pulse protocol in the control period (upper) and during the exposure to 10 μM picaridin (lower) is illustrated in (A). The uppermost part is the voltage protocol given. (B) Quasi-steady-state inactivation curves of INa(T) obtained with the absence (blue filled squares) and presence (red open circles) of 10 μM picaridine (mean ± SEM; n = 7 for each point). The normalized amplitude of INa(T) (i.e., I/Imax) was constructed against the different levels of conditioning potential, and the sigmoidal curves (blue and red lines) were fitted to the Boltzmann equation (see Materials and Methods).
2.4. Picaridin-Induced Retardation in Cumulative Inhibition of INa(T) Inactivation during Rapid Depolarizing Stimuli
It has been previously demonstrated that, prior to being activated during repetitieve short pulses, the inactivation of INa(T) can accumulate [27,38,39,40,41]. Therefore, in subsequent experiments, we proceeded to explore if the presence of picaridin could adjust the inactivation process of INa(T) during a train of membrane depolarizations. The tested cell was held at −80 mV, and the stimulus protocol, which was designed to consist of repetitive depolarization to −10 mV (20 ms in each pulse with a rate of 40 Hz for 1 s), was then imposed on it. In accordance with previous observations [38,40,41,42], as depicted in Figure 4A–C, during the control period (i.e., picaridin was not present), the process of INa(T) inactivation detected in GH3 cells was robustly evoked by a 1 s repetitive depolarization from −80 to −10 mV, and an evolving inactivation time constant of 26.5 ± 2.4 ms (n = 7) was yielded. That is, there was a sudden current decay with a single-exponential process. It is also noteworthy that during cell exposure of picaridin at a concentration of 3 or 10 μM, the time constant of INa(T) decay evoked by the same train of depolarizing voltage pulses was, respectively, raised to 48.1 ± 2.6 ms (n = 7, p < 0.05) or 63.5 ± 2.8 ms (n = 7, p < 0.05), in addition to a considerable increase in INa(T) amplitude. Moreover, upon continued presence of 10 μM picaridin, a further application of brivaracetam (BRV, 10 μM) noticeably attenuated the picaridin-prolonged time constant of INa(T) inactivation during repetitive depolarizations to 41.1 ± 2.6 ms (n = 7, p < 0.05). BRV was recently reported to suppress INa(T) amplitude [43]. Overall, these results meant that, apart from the increase in INa(T) amplitude, with cell exposure to picaridin the decrease in the decaying of INa(T) in response to a 1-s train of depolarizing pulses (i.e., accumulative inactivation of INa(T)) could become retarded in these cells.
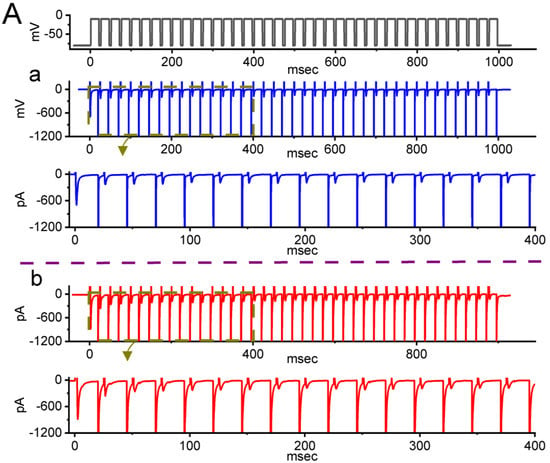
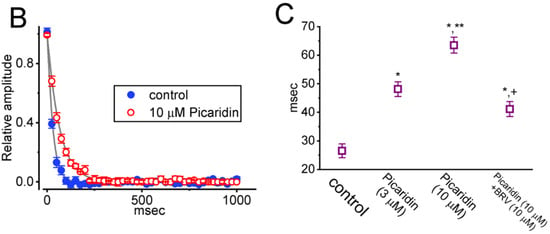
Figure 4.
Effect of picaridin on INa(T) activated by a train of depolarizing pulses in GH3 cells. The voltage protocol employed consisted of 40 20-ms pulses (stepped to −10 mV) separated by 5 ms intervals at −80 mV for a total duration of 1 s. (A) Representative current traces taken during either the control period (a, absence of picaridin) or cell exposure to 10 μM picaridin (b). The voltage-clamp protocol delivered is illustrated atop the current traces. To provide single INa trace, the lower side in panels (a,b) denotes the expanded record from the brown broken box (with curve arrows) of the upper side in each panel. (B) The relationship of INa(T) versus the pulse train duration in the absence (blue filled circles) and presence (red open circles) of 10 μM picaridin (mean ± SEM; n = 7 for each point). The gray continuous lines on which the data points are overlaid was accurately fitted by a single exponential. Note that with cell exposure to picaridin, the time course of INa(T) inactivation during a train of depolarizing pulses slowed, together with an increase in time constant of the current inactivation. (C) Summary graph demonstrating the effect of picaridin (3 or 10 mM) and picaridin plus ranolazine (Ran) on the time constant of current decay in response to a train of depolarizing command voltage from −80 to −10 mV (mean ± SEM; n = 7 for each point). Current amplitude was measured at the beginning of each depolarizing pulse during depolarizing stimuli. * Signficantly different from control (p < 0.05), ** Significantly different from picaridin (3 μM) alone group (p < 0.05), and + Significantly different from picaridin (10 μM) alone group (p < 0.05).
2.5. Effect of Picaridin on the Recovery Time Course of INa(T) Inactivation after the Conditioning Train of Depolarizing Stimuli
The earlier investigations demonstrated a distinguishing type of recovery process from INa(T) inactivation, as elicited by a train of conditioning depolarizing stimuli ([38,41,44]. In light of these previous studies, we next sought to investigate if such a recovery process as that emerging in INa(T) could be modified by adding picaridin. In this separate set of experiments, the preceding condition train given was composed of 40 20-ms pulses separated by 5 ms intervals at −80 mV for a duration of 1 s. Following such conditioning train, a two-step voltage protocol, where the recovery process was measured, was further imposed on the tested cells to evoke INa(T). That is, the INa(T) was initially induced by a 30 ms step from −80 to −10 mV, voltage was thereafter returned to −80 mV for a variable length of time in a geometric progression with a common ratio of 2 (Figure 5A). In keeping with previous investigations, the resulting recovery time course, which slowed inactivation of INa(T) in response to the conditioning depolarizing stimuli, was noticed to emerge largely in a biphasic manner (i.e., fast and slowly recovering pools), while the recovery time course of current inactivation with no preceding conditioning train pulse was fitted in a satisfactory way by a single exponential. However, when the conditioning train pulse stimulation was applied (Figure 5B), during the control period, the experimental data were optimally fit with a sum of two exponential functions, which was characterized by fast (τfast) and slow time constants (τslow). Additionally, with cell exposure to picaridin, the evolving values of both τfast and τslow were noted to rise, while the fraction in the fast component increased and that in the slow component decreased. Table 1 summarizes the values of numerical parameters used for estimation of nonlinear recovery time course in the absence and presence of picaridin (3 or 10 μM). The results allowed us to propose that, in GH3 cells, the INa activated by a conditioning train of pulse could cause a large fraction of NaV channels to shift toward the slowly recovering pool, as demonstrated in previous studies [38,44]. Moreover, of interest, during exposure to picaridin, the fraction of the slowly recovering pool of NaV channels during the conditioning train pulse became smaller, while that of the fast recovery pool of the channels tended to be greater; however, both τfast and τslow values of recovery time course rose in its presence.
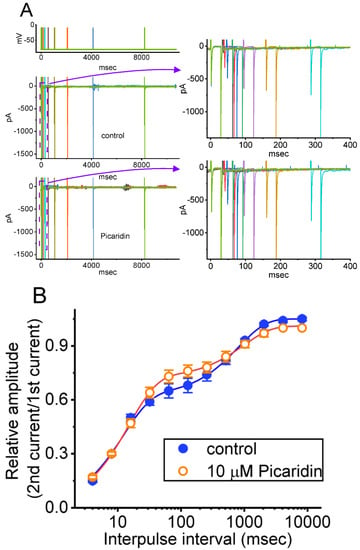
Figure 5.
Modification by picaridin on the recovery of INa(T) inactivation evoked by varying inter-pulse intervals following the conditioning train of depolarizing pulses. In these measurements, we kept GH3ˇcells bathed in Ca2+-free Tyrode’s solution, while the recording pipette was filled with a solution enriched with Cs+. The tested cells were depolarized from −80 to −10 mV for a duration of 30 ms, and subsequently variable inter-pulse durations with a geometric progression (indicated in the uppermost part) were imposed on them. (A) In our voltage-clamp protocol, the different colors indicated in current traces (lower) correspond with those in potential traces (upper). Exemplar current traces acquired in the absence (upper) and presence (lower) of 10 μM picaridin. Current traces in the right side show the expanded records from the purple dashed boxes with purple curve arrows in the left side for better illustrations, while the uppermost part is the voltage-clamp protocol applied. Brief inward deflection with a progressive rise with increasing inter-pulse interval showed the occurrence of INa(T) activated by short depolarizing pulse. (B) The relationships of the relative amplitude of INa(T) versus the inter-pulse interval acquired from the absence (blue filled circles) and presence (red open circles) of 10 μM picaridin (mean ± SEM; n = 8 for each point). The smooth curve obtained with or without the presence of picaridin was least-squares fitted with a two-exponential function, as described in Materials and Methods. Of note, the x-axis is given in a logarithmic scale.

Table 1.
Summary of results showing the parameter values for effects of picaridin on the recovery of the INa block during the preceding train pulse. These parameters are elaborated in detail in Materials and Methods. All values are mean ± SEM. * Significantly different from controls (p < 0.05).
2.6. Effect of Picaridin on the Strength of Voltage-Dependent Hysteresis (Hys(V)) of Persistent INa (INa(P)) Elicited by an Upright Isosceles-Triangular Vramp
The Hys(V) behavior of INa(P) has been recently demonstrated with a figure-of-eight (i.e., ∞-shaped) configuration as current trace was induced by an upright isosceles-triangular Vramp [33,37,45,46]. Any modifications on INa(P) were expected to have a high impact on membrane excitability [47,48,49,50,51]. In this regard, we continued to study if the picaridin existence could modify the Hys(V) strength in response to the upright isosceles-triangular Vramp. The measurements were undertaken in cells bathed in Ca2+-free Tyrode’s solution, and we filled up the electrodes with a solution containing Cs+. The tested cells were held at −80 mV and an upright isosceles-triangular Vramp, ranging between −100 and +50 mV for a duration of 1.6 s (i.e., ramp speed of ±0.25 mV/ms), was imposed on them. As demonstrated in Figure 6A,B in the control period, current traces in response to such triangular Vramp evolved to exhibit two types of hysteretic loops (i.e., low- and high-threshold loops). The peak amplitude at low- and high-threshold loop appeared at around −80 and −20 mV, respectively, while current trajectory at low- and high-threshold loop occurred in a clockwise and anti-clockwise direction, respectively. As shown in Figure 6B, upon cell exposure to picaridin, the strength of INa(P)’s Hys(V) became overly increased. For example, upon cell exposure to this compound at a concentration of 10 μM, the INa(P) amplitudes at either high-threshold loop (i.e., at the level of −20 mV) or low-threshold loop (i.e., at the level of −80 mV), respectively, increased from 35 ± 6 to 65 ± 8 pA (n = 7, p < 0.05) or from 12 ± 2 to 27 ± 4 pA (n = 7, p < 0.05). Moreover, with continued exposure to 10 μM picaridin, subsequent addition of ranolazine (Ran, 10 μM) markedly attenuated INa(P) magnitude measured at −20 or −80 mV to 42 ± 7 (n = 7, p < 0.05) or 17 ± 3 pA (n = 7, p < 0.05), respectively. As such, it was reasonable to assume that the strength of INa(P)’s Hys(V) in response to long-lasting triangular Vramp was susceptible to being enhanced in the presence of picaridin.
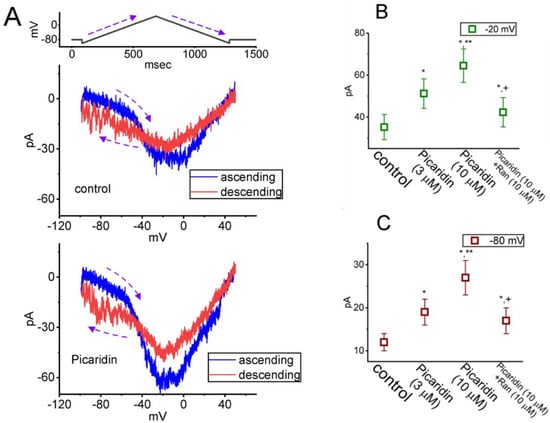
Figure 6.
Modification by picaridin on voltage-dependent hysteresis (Hys(V)) of persistent INa (IN(P)) elicited by an upright isosceles-triangular ramp voltage (Vramp). The experiments were undertaken when the tested cell was voltage-clamped at −80 mV, and the Vramp from −100 to +50 mV for a duration of 1.2 s (i.e., ramp speed of ±0.25 mV/ms) was applied to it. (A) Exemplar current traces acquired during the control period (upper part) and in the presence of 10 μM picaridin (lower part). The trajectory shown in blue or red color indicates current trace evoked at the ascending (up-sloping) or descending (down-sloping) limb of the upright triangular Vramp, respectively. The voltage protocol is illustrated atop current traces. The dashed curve arrows in each panel represent the direction of potential or current trajectory by which time goes. Summary graph showing the effects of picaridin (3 or 10 μM) and picaridin plus Ran on INa(P) amplitude activated by the up-sloping (ascending), (B) or down-sloping (descending), (C) limb of 1.2-s triangular Vramp is, respectively, illustrated. The INa(P) amplitude at the up-sloping or down-sloping end of Vramp was measured at −20 or −80 mV, respectively. Each point indicates the mean ± SEM (n = 8). * Significantly different from control (p < 0.05), ** Significantly different from picaridin (3 μM) alone group (p < 0.05), and + Significantly different from picaridin (10 μM) alone group (p < 0.05).
2.7. Picaridin-Mediated Increase of Resurgent INa (INa(R)) Measured from GH3 Cells
INa(R) has been increasingly demonstrated to be responsible for burst generation and membrane excitability present in different excitable cells [32,41,51,52,53,54,55,56,57,58]. In this regard, we, thus, next wanted to study if the existence of picaridin produced any effects on INa(R) identified from these cells. The experiments used to measure INa(R) were, then, undertaken in situations where the examined cell was maintained at −80 mV and a brief depolarizing step to +30 mV for a duration of 30 ms was given to activate INa(T). Following the abrupt depolarizing step, a series of down-sloping Vramp’s from +30 to −80 mV, with varying durations in a geometric progression (with a common ratio of 2), was subsequently given to evoke INa(R) (Figure 7) [54]. The INa(R) amplitude evoked by such down-sloping Vramp became progressively increased as cells were exposed to picaridin. For example, as GH3 cells were exposed to picaridin at a concentration of 3 or 10 μM, the INa(R) amplitude at the level of −50 mV evoked by an 8.2-s down-sloping Vramp was respectively raised to 49.8 ± 3.3 pA (n = 7, p < 0.05) or 53.3 ± 3.5 pA (n = 7, p < 0.05) from a control value of 46.1 ± 3.1 pA (n = 7). Moreover, under continued exposure to picaridin, subsequent addition of Ran (10 μM) noticeably attenuated INa(R) amplitude at the same level of membrane potential to 46.6 ± 3.2 pA (n = 7).
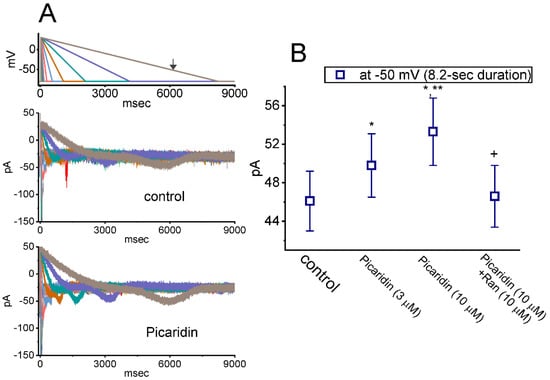
Figure 7.
Augmentation by picaridin on resurgent INa (INa(R)) evoked by the descending Vramp with varying durations. The examined cell was held at the level of −80 mV, and a 30 ms depolarizing pulse to +30 mV was imposed. Following brief step depolarization, the down-sloping Vramp from +30 to −80 mV, with varying duration in a geometric progression, was delivered to the cells. (A) In our voltage-clamp protocol, the different colors indicated in current traces (lower) correspond with those in potential traces (upper). Representative current traces obtained without (upper) or with (lower) the exposure to 10 μM picaridin. The uppermost part is the voltage-clamp protocol given, and the arrow indicates the level of −50 mV at which INa(R) amplitude evoked by Vramp with a duration of 8.2 s was measured. (B) Summary graph demonstrating effects of picaridin (3 or 10 μM) and picaridin plus Ran on INa(R) amplitude (mean ± SEM; n = 7 for each point). Current amplitude was measured at the level of −50 mV during the 8.2-s descending Vramp. * This result is significantly different from control (p < 0.05), ** Significantly different from picaridin (3 μM) alone group (p < 0.05), and + Significantly different from picaridin (10 μM) alone group (p < 0.05).
2.8. Molecular Docking on Interaction between NaV1.7 Channel and Picaridin
In this study, we also continued to explore how the protein of the hNaV1.7 channel was optimally docked with the picaridin molecule, by using PyRx software. The protein structure of hNaV1.7 was taken from RCSB PDB (PDB ID: 5EK0) [59]. The predicted docking sites of the picaridin molecule, with which the amino-acid residues can interact, are presented in Figure 8A,B. The picaridin molecule was noted to form hydrophobic contacts with certain amino-acid residues, such as Val1501(D), Phe1506(D), Ile 1510(D), Glu1550(D), Asp1565(D), Arg1611(D), and Ala1615(D). The atoms in the picaridin molecule also have several hydrogen bonds with residue Thr1507(D) at a distance of 3.13 Å, Arg 1554(D) at 3.01 Å, Ser1568(D) at 3.16 Å, and Arg1620(D) at 3.25 Å, respectively (Figure 8A). The results of molecular docking hinted at the possibility that, apart from the ability of this compound to be active in targeting the odorant binding protein 1 (OBP1) [20,22], the picaridin molecule could potentially dock to the transmembrane region (position: 1597–1613) of hNa1.7 channel (PDB: 5EK0) with a binding affinity of −5.2 kcal/mol.
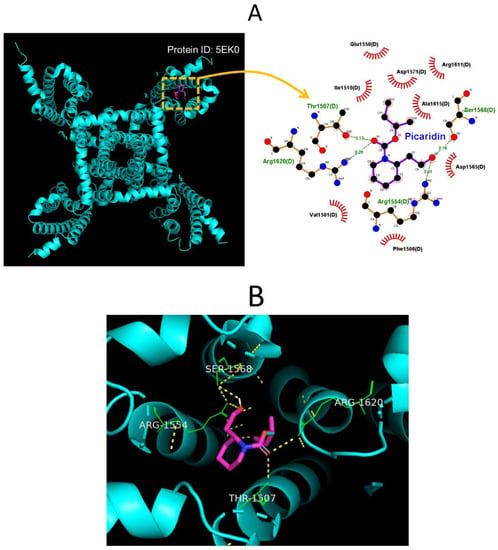
Figure 8.
Results on molecular docking between the hNaV1.7 channel and the picaridin molecule. The protein structure of hNaV1.7 was obtained from RCSB (Research Collaboratory for Structural Bioinformatics) PDB (PDB ID: 5EK0), while the picaridin molecule was from PubChem (Compound CID: 125098 [3D conformer]). The structure about the hNaV1.7 channel was docked by the picaridin molecule with PyRx software (http://pyrx.sourceforge.io/) (accessed on 18 August 2022). (A) Diagram of the interaction between the hNaV1.7 channel and the picaridin molecule created from the LigPlot+ program (http://www.ebi.ac.uk/thornton-srv/software/LIGPLOT/) (accessed on 18 August 2022). Of notice, the red arcs on which small bars radiated toward the ligand (i.e., picaridin) represent hydrophobic interactions, while the green dotted lines residing in amino-acid residue (i.e., Thr1507(D), Arg1554(D), Ser1568(D), and Arg1820(D)) represent formation of hydrogen bonds. (B) Expanded graph for two-dimensional interaction sketch illustrating the predicted interactions of picaridin with residues (green color) in which hydrogen bonds are formed (indicated in yellow dotted line). The center in the diagram indicates the picaridin molecule.
3. Discussion
In the present work, we provided evidence to show that the presence of picaridin, known to exert a repellent effect on insects, was able to exert stimulatory action on INa(T) and INa(L) seen in pituitary GH3 cells. The INa(L) in response to brief step depolarization was stimulated to a greater extent than the INa(T). Consequently, effective EC50 values needed for picaridin-stimulated INa(T) and INa(L) in these cells were yielded as 32.7 and 2.8 μM, respectively. The overall I–V relationship of INa(T) was shifted to a hyperpolarized potential by approximately 20 mV during exposure to picaridin, whereas the quasi-steady-state inactivation curve of INa(T) was shifted to a more depolarized potential by about 5 mV, being devoid of changes in the gating charge of the curve in its presence. The decaying time course of INa(T) activated during pulse train stimulation became noticeably slowed in the presence of this compound. Moreover, further addition of brivaracetam (BRV) attenuated picaridin-induced increase of INa(T)’s decaying time constant responding to rapid membrane depolarizations. The recovery time course of INa(T) during the preceding conditioning pulse train was also strikingly altered upon cell exposure to this agent. When cells were exposed to this agent, the Hys(V) magnitude in response to the upright isosceles-triangular Vramp became strikingly augmented. The INa(R) magnitude in response to the descending Vramp following initial brief depolarization was greatly enhanced by adding picaridin. Together, the activity of NaV channels inherent in excitable cells (e.g., GH3 cells) may confer susceptibility to perturbations by picaridin or other structurally similar compounds; although, unlike other piperidine derivatives [28,30,32], this agent was observed to induce an excitatory action on INa. The differential stimulation by picaridin of INa(T) and INa(L) is important and it may, thus, be responsible for its modulation of the electrical behaviors of excitable cells [3,60,61], presuming that the findings appear in vivo.
In the present study, the time-dependent decline of INa(T) activated during a 40 Hz train of depolarizing voltage steps, the protocol of which consisted of 20 ms pulses delivered from −80 to −10 mV at a rate of 40 Hz for a total duration of 1 s, was robustly detected in an exponential fashion, strongly indicating that there is marked use dependence of INa(T) elicited by repetitive depolarizations, as stated previously [39,40,41,42]. Moreover, such exponential decrease in INa(T) activated during pulse train stimulation was noted to become overly blunted with cell exposure to picaridin. Therefore, it is reasonable to assume that the existence of picaridin results in a gain-of-function change perturbed by the slowed time course of INa(T) inactivation, and that picaridin-induced increase of measured INa(T) is concurrently associated with conceivable use-dependent attenuation of INa(T), elicited as either high-frequency firing of action potentials or rapid repetitive stimuli occurring [38,42,62,63,64,65,66].
In this work, we also revealed that GH3-cell exposure to picaridin could cause the modifications on the recovery of INa(T) inactivation evoked by a train of conditioning depolarizing stimuli. Of note, under our experimental observations, the inactivation recovery process of measured INa(T), following the conditioning membrane depolarizations, was found to consist of two different fractions of current recovery, i.e., fast and slow recovering pool of INa(T). In other words, in agreement with earlier observations [38,41,44,67], the INa(T) activated by a conditioning train of pulses can lead to a certain fraction of NaV channels to shift toward the slowly recovering pool during the current recovery process. As a result, there appears to be two distinct fractions of recovering pool (i.e., fast and slow) following the conditioning rapid membrane depolarization [38,68]. Furthermore, as summarized in Table 1, upon continued exposure to picaridin, the fraction of the fast recovery pool of NaV channels observed under these experimental conditions tended to become greater, while that in the slow recovery pool of the channel was smaller.
Work in our laboratory explicitly demonstrated the non-equilibrium Hys(V) behavior of INa(P) activated by the upright isosceles-triangular Vramp, strongly indicating the striking voltage dependence of double Vramp-evoked INa(P) [49]. The experimental results also revealed two distinct types of measured Hys(V) loops, which is reminiscent of the dynamics of the Lorenz-like system (i.e., figure-of-eight configuration) [69]. In other words, the Hys(V) motion of the current as a function of time was noted to move in both counterclockwise and clockwise directions. One is a high-threshold counterclockwise loop with a peak of −20 mV, while the other is a low-threshold clockwise loop with a peak falling at around −80 mV. Furthermore, the exposure to picaridin was noted to accentuate Hys(V)’s strength of INa(P) responding to triangular Vramp. In continued exposure to picaridin, further addition of ranolazine markedly resulted in an attenuation of picaridin-mediated increase of Hys(V) strength seen in GH3 cells. Ranolazine has been previously demonstrated to be an inhibitor of INa(L) [28,29,33,34]. Findings from the current observations, therefore, unveiled that the double Vramp-induced INa(P) resulted in striking Hys(V) changes in voltage dependence and that such strength can be evidently enhanced by adding picaridin.
In our study, the INa(R) in response to varying durations of repolarizing voltage was robustly detectable [32,41]. Of additional note, when cells were continually exposed to picaridin, the magnitude of INa(R) became overly increased. Moreover, the subsequent addition of ranolazine, still during continued exposure to picaridin, was able to attenuate the INa(R) magnitude, especially at the level of −50 mV. In accordance with previous reports showing that the INa(R) magnitude may alter the INa(T) inactivation during high-frequency stimulation [32,51,52,53,55,56,57,58,63,68], the current investigations allowed us to indicate that the existence of picaridin may perturb the electrical behaviors (e.g., aberrant and rapid firing of action potentials) residing in varying excitable cells.
4. Materials and Methods
4.1. Chemicals, Drugs, REAGENTS and Solutions Used in This Work
Zingerone Picaridin (icaridin, KBR 3023, BayrepelTM, saltidin®, 1-piperidinecarboxylic acid 2-(2-hydroxyethyl)-1-methylpropylester, hydroxyethyl isobutyl piperidine carboxylate [INCI], butan-2-yl 2-(2-hydroxyethyl)piperidine-1-carboxylate [IUPAC], C12H23NO3) were acquired from MedChemExpress (MCE®; Everything Biotech, New Taipei City, Taiwan). Ranolazine (Ran), tefluthrin, tetraethylammonium chloride (TEA), and tetrodotoxin (TTX) were obtained from Sigma-Aldrich (Genechain, Kaohsiung, Taiwan), while brivaracetam (BRV) was kindly provided by UCB (Union Chimique Belge) Pharm (PRA Health Sciences, Taipei, Taiwan). To protect picaridin from being degraded by light, stock solution containing this compound was wrapped with aluminum foil, and it was then kept under −20 °C for long-term storage. Unless specified otherwise, fetal calf serum, horse serum, culture media (e.g., Ham’s F-12 medium), L-glutamine, and trypsin/EDTA were mostly acquired from HyCloneTM (Genechain), whereas other chemicals or reagents were acquired from Sigma-Aldrich or Merck (Genchain) and were of laboratory grade.
The standard external solution (i.e., normal Tyrode’s solution) had the composition (in mM): NaCl 136.5, CaCl2 1.8, KCl 5.4, MgCl2 0.53, glucose 5.5, HEPES 5.5, and the pH was adjusted to 7.4 by adding NaOH. To record ionic flowing through K+ currents, the electrode was filled up with the internal pipette solution containing (in mM): K-aspartate 130, KCl 20, KH2PO4 1, MgCl2 1, EGTA 0.1, Na2ATP3, Na2GTP 0.1, and HEPES 5, and the pH was adjusted to 7.2 by adding KOH. However, to measure INa, K+ ions in the pipette solution were replaced with equimolar Cs+ ions, and the pH was adjusted to 7.2 with CsOH. The twice-distilled water used for the current experiments was deionized through Millipore-Q® system (Merck, Tainan, Taiwan).
4.2. Cell Preparations
Clonal pituitary (GH3) somatolactotrophs, originally acquired from the Bioresources Collection and Research Center ([BCRC-60015, http://catalog.bcrc.firdi.org.tw/BcrcContent?bid=60015] (accessed on 18 August 2022), Hsinchu, Taiwan), were cultured in Ham’s F-12 medium, which was supplemented with 15% heat-inactivated horse serum (v/v), 2.5% fetal calf serum (v/v), and 2 mM L-glutamine. They were grown at 37 °C in monolayer cultures in 50-mL plastic culture flasks in a humidified environment of 5% CO2/95% air. We performed electrical recordings 5 or 6 days after GH3 cells underwent subculture (60–70% confluence).
4.3. Electrophysiological Measurements
Shortly before the measurements, we carefully dispersed cells by using 1% trypsin-EDTA solution, and quickly put a small portion of the suspension containing clumps of cells into a recording cubicle fixed on the stage of an inverted microscope (DM-IL; Leica; Major Instruments, Tainan, Taiwan). We maintained cells at room temperature (20–25 °C) in HEPES-buffered normal Tyrode’s solution, the ionic compositions of which are stated above. The patch electrodes used to record were constructed from melting point capillaries made of Kimax®-34500-99 glass with 1.5–1.8 mm outer diameter (Sigma-Aldrich, Genechain, Kaohsiung, Taiwan) by using a PP-83 vertical puller (Narishige, Major Instruments). When filled with different internal solutions described above, the recording pipettes had tip resistances of 3–5 MΩ. The electrodes were held in place by micromanipulators. We recorded ionic currents in the whole-cell configuration by making some small modifications to the original patch-clamp technique with an RK-400 patch amplifier (Bio-Logic, Claix, France), as described elsewhere [31,32,70,71]. The GΩ-seals, which were made in an all-or-nothing fashion, led to a significant improvement in the signal-to-noise ratio. The liquid junction potential, which occurred due to different compositions between external and internal solutions, became nulled shortly before the GΩ-seal formation was made, and the whole-cell data were then corrected.
4.4. Data Recordings and Processing
The signal output (i.e., potential and current traces) was monitored and digitized online at 10 kHz or more in an ASUS ExpertBook laptop computer (Yuan-Dai, Tainan, Taiwan). For analog-to-digital (A/D) and digital-to-analog (D/A) conversion, a Digidata® 1440 A device interfaced to the computer was controlled by pCLAMPTM 10.6 program run under Microsoft Windows 7 (Redmond, WA, USA) [70,71]. We low-pass filtered current signals at 2 kHz by using a FL-4 four-pole Bessel filter (Dagan, Minneapolis, MN, USA). The voltage-clamp protocols, consisting of manifold rectangular or ramp waveforms, were specifically designed, and they were then delivered to the tested cells through D/A conversion.
4.5. Data Analyses for Whole-CELL Ionic Currents
To determine concentration-dependent stimulation of picaridin on the amplitude of INa(T) or INa(L), we placed GH3 cells into Ca2+-free Tyrode’s solution. During the measurements, we maintained each cell at −100 mV, and a short depolarizing pulse to −10 mV for 30 ms was delivered to evoke INa. The INa(T) or INa(L) magnitudes were then, respectively, measured at the beginning- or end-pulse of 30-ms depolarizing step when cumulative application of different picaridin concentrations was given. The amplitude of INa(T) obtained in the presence of picaridin at a concentration of 100 μM was taken to be 100% and current magnitudes during exposure to varying concentrations of this compound were subsequently compared. The concentration-dependent stimulation by picaridin of INa(T) or INa(L) residing in GH3 cells was determined by fitting experimental data set to a modified Hill function [31,32]. That is:
where [picaridin] = the picaridin concentration given; nH = the Hill coefficient (i.e., coefficient for cooperativity); EC50 = the concentration required for a 50% stimulation of INa(T) or INa(L) amplitude activated by abrupt depolarizing step; and Emax = maximal stimulation of INa(T) or INa(L) caused by the picaridin presence.
To evaluate picaridin-mediated adjustments on the quasi-steady-state inactivation of INa(T), the relationship of the normalized amplitude of INa(T) versus the conditioning potential obtained with or without the presence of 10 μM picaridin was established, and the data set was then fitted optimally to a Boltzmann function on the following form:
where Imax = the maximal INa(T) magnitude acquired in the absence or presence of picaridin (10 μM) at the conditioning membrane potential of −100 mV; V = the conditioning potential; V1/2 = the potential required for half-maximal activation (i.e., midpoint potential); q = the apparent gating charge needed; F = Faraday’s constant; R = the universal gas constant; T = the absolute temperature; and RT/F = 25.2 mV.
By use of a two-step voltage protocol with variable interpule intervals in a geometric progression, the recovery time course of INa from block activated in response to the 1-s conditioning pulse train was optimally constructed, and the results acquired with or without the addition of picaridin were thereafter drawn by plotting the INa(T) amplitude (normalized with respect to the steady-state amplitude activated at 0.1 Hz). A basic assumption of the analyses was that the recovery time course of the current measured under these experimental conditions could be described in a satisfactory manner by exponential function [38,67,72]. Due to the recovery time course in GH3 cells exhibiting a rapidly rising recovery phase, followed by a late slow phase, there should be at least two underlying exponential terms. Therefore, the data points showing recovery time course with or without exposure to picaridin were least-squares fitted to the exponential function with biexponential process. That is:
where y is the relative INa(T) amplitude at time t, A or B is the relative amplitude of each exponential component, and τfast or τslow is the fast or slow time constant in the recovery of INa block, respectively. The experimental results were optimally analyzed and they are summarized in Table 1.
4.6. Curve-Fitting Approximations and Statistical Analyse
Curve-fitting (linear or nonlinear) to the experimental data set applied was fitted by use of manifold analytical tools, that included the Microsoft “Solver” embedded into Excel® 2022 (Microsoft) and OriginPro® 2022b program (OriginLab®; Microcal; Scientific Formosa, Kaohsiung, Taiwan) [40,41]. The electrophysiological data are presented as the mean ± standard error of the mean (SEM). The size of independent observations (n) is indicated in cell numbers collected during the experiments. The data distribution obtained was found to satisfy the tests for normality. For comparisons between the two different groups, we performed Student’s t-tests (for paired or unpaired samples). However, for more than two different groups, analysis of variance (for one- or two-way ANOVA) with or without repeated measure was followed by a post-hoc Fisher’s least-significance difference test, which was applied where appropriate to evaluate individual differences among multiple groups of data. A statistical significance (indicated with *, ** or + in the figures) was considered when p < 0.05.
5. Conclusions
The emerging data reveal that picaridin, known to be an insect and acarid repellent in the piperidine chemical family, leads to profound stimulation in INa in concentration-, time-, voltage-, frequency-, and Hys(V)-dependent manners. The precise ionic mechanism of picaridin actions on INa needs to be further clarified. The GH3 cell line used was demonstrated to express the α-subunits of NaV1.1, NaV1.2, NaV1.3, and NaV1.6, as well as the β1 and β3-subunits of NaV channel [24,73]. Moreover, the results of molecular docking also prompt us to predict the effectiveness of picaridin in binding to certain amino-acid residues intrinsically in the hNaV1.7 channel, by forming either hydrophobic contacts or hydrogen bonds. The present data obtained from GH3 cells, thus, sheds light on the evidence demonstrating the effectiveness of picaridin in adjusting specific ionic currents (e.g., INa(T), INa(L), INa(P) and INa(R)). These results may be linked to the additional, but important, actions of picaridin, or other structurally similar compounds, which are potentially useful in pharmacological or toxicological applications in different excitable cells occurring in vivo.
Author Contributions
Conceptualization, S.-N.W., A.-L.S., C.-S.L., H.-Y.C., C.-W.T. and M.-C.Y.; methodology, S.-N.W.; software, S.-N.W.; validation, A.-L.S., C.-S.L., H.-Y.C., M.-C.Y., C.-W.T. and S.-N.W.; formal analysis, S.-N.W.; investigation, H.-Y.C., M.-C.Y. and S.-N.W.; resources, S.-N.W.; data curation, S.-N.W.; writing—original draft preparation, S.-N.W.; writing—review and editing, A.-L.S., C.-S.L., H.-Y.C., M.-C.Y., C.-W.T. and S.-N.W.; visualization, H.-Y.C., M.-C.Y. and S.-N.W.; supervision, S.-N.W., A.-L.S., C.-S.L. and C.-W.T.; project administration, A.-L.S., C.-S.L., C.-W.T. and S.-N.W.; funding acquisition, A.-L.S., C.-S.L. and S.-N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly aided by the grants from Ministry of Science and Technology (MOST-110-2320-B-006-028 and MOST-111-2320-B-006-028), Taiwan and Ditmanson Medical Foundation Chia-Yi Christian Hospital Research Program (R111-09).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data is available upon reasonable request to the corresponding author.
Acknowledgments
The authors gratefully acknowledge Hsin-Yen Cho and Meng-Cheng Yu for their contributions to the earlier experiments.
Conflicts of Interest
No conflicts of interest, financial or otherwise, are declared by the author(s). The content and writing of this paper are solely the responsibility of the authors.
Abbreviations
| BRV | brivaracetam |
| EC50 | concentration required for half-maximal stimulation |
| Hys(V) | voltage-dependent hysteresis |
| INa(L) | late Na+ current |
| INa(T) | transient (peak) Na+ current |
| NaV channel | voltage-gated Na+ channel |
| Ran | ranolazine |
| SEM | standard error of mean |
| TEA | tetraethylammonium chloride |
| τinact(S) | slow component in inactivation time constant |
| Vramp | ramp voltage |
| TTX | tetrodotoxin |
| Vramp | ramp voltage |
References
- Katz, T.M.; Miller, J.H.; Hebert, A.A. Insect repellents: Historical perspectives and new developments. J. Am. Acad. Dermatol. 2008, 58, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Pages, F.; Dautel, H.; Duvallet, G.; Kahl, O.; de Gentile, L.; Boulanger, N. Tick repellents for human use: Prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014, 14, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.H. Chemical and Plant-Based Insect Repellents: Efficacy, Safety, and Toxicity. Wilderness Environ. Med. 2016, 27, 153–163. [Google Scholar] [CrossRef]
- Heng, S.; Sluydts, V.; Durnez, L.; Mean, V.; Polo, K.; Tho, S.; Coosemans, M.; van Griensven, J. Safety of a topical insect repellent (picaridin) during community mass use for malaria control in rural Cambodia. PLoS ONE 2017, 12, e0172566. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Zaman, K.; Duarah, S.; Raju, P.S.; Chattopadhyay, P. Mosquito repellents: An insight into the chronological perspectives and novel discoveries. Acta Trop. 2017, 167, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Nentwig, G.; Frohberger, S.; Sonneck, R. Evaluation of Clove Oil, Icaridin, and Transfluthrin for Spatial Repellent Effects in Three Tests Systems Against the Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2017, 54, 150–158. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef]
- Devillers, J. 2D and 3D structure-activity modelling of mosquito repellents: A review ($). SAR QSAR Environ. Res. 2018, 29, 693–723. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Vu, M.N.; Hebert, A.A. Insect Repellents: An Updated Review for the Clinician. J. Am. Acad Dermatol. 2018. [Google Scholar] [CrossRef]
- Tavares, M.; da Silva, M.R.M.; de Oliveira de Siqueira, L.B.; Rodrigues, R.A.S.; Bodjolle-d’Almeida, L.; Dos Santos, E.P.; Ricci-Júnior, E. Trends in insect repellent formulations: A review. Int J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef]
- Chauhan, K.R.; McPhatter, L.P.; O’Dell, K.; Syed, Z.; Wheeler, A.; Debboun, M. Evaluation of a Novel User-Friendly Arthropod Repellent Gel, Verdegen. J. Med. Entomol. 2021, 58, 2479–2483. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.C.; Janich, A.J.; Sanchez-Vargas, I.; Markle, E.D.; Gray, M.; Foster, J.R.; Black Iv, W.C.; Foy, B.D.; Olson, K.E. Nootkatone Is an Effective Repellent against Aedes aegypti and Aedes albopictus. Insects 2021, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Germano-Costa, T.; Bilesky-José, N.; Guilger-Casagrande, M.; Pasquoto-Stigliani, T.; Rogério, C.B.; Abrantes, D.C.; Maruyama, C.R.; Oliveira, J.L.; Fraceto, L.F.; Lima, R. Use of 2D and co-culture cell models to assess the toxicity of zein nanoparticles loading insect repellents icaridin and geraniol. Colloids Surf. B Biointerfaces 2022, 216, 112564. [Google Scholar] [CrossRef]
- Kopsco, H.L.; Mather, T.N. Tick-Borne Disease Prevention Behaviors Among Participants in a Tick Surveillance System Compared with a Sample Of Master Gardeners. J. Community Health 2022, 47, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, C.B.; Carvalho Abrantes, D.; de Oliveira, J.L.; Ribeiro de Araújo, D.; Germano da Costa, T.; de Lima, R.; Fernandes Fraceto, L. Cellulose Hydrogels Containing Geraniol and Icaridin Encapsulated in Zein Nanoparticles for Arbovirus Control. ACS Appl. Bio Mater. 2022, 5, 1273–1283. [Google Scholar] [CrossRef]
- Zeng, W.; Ren, H.; Zhu, H.; Shi, W.; Xu, L. Icaridin-Loading Nitrocellulose As a New Repellent Against Aquatic Leech with Waterproof and Long-Acting Properties. Vector Borne Zoonotic Dis. 2022, 22, 114–119. [Google Scholar] [CrossRef]
- Bohbot, J.D.; Dickens, J.C. Insect repellents: Modulators of mosquito odorant receptor activity. PLoS ONE 2010, 5, e12138. [Google Scholar] [CrossRef]
- Xu, P.; Choo, Y.M.; De La Rosa, A.; Leal, W.S. Mosquito odorant receptor for DEET and methyl jasmonate. Proc. Natl. Acad. Sci. USA 2014, 111, 16592–16597. [Google Scholar] [CrossRef]
- Sparks, J.T.; Dickens, J.C. Bitter-sensitive gustatory receptor neuron responds to chemically diverse insect repellents in the common malaria mosquito Anopheles quadrimaculatus. Naturwissenschaften 2016, 103, 39. [Google Scholar] [CrossRef]
- Drakou, C.E.; Tsitsanou, K.E.; Potamitis, C.; Fessas, D.; Zervou, M.; Zographos, S.E. The crystal structure of the AgamOBP1 Icaridin complex reveals alternative binding modes and stereo-selective repellent recognition. Cell Mol. Life Sci. 2017, 74, 319–338. [Google Scholar] [CrossRef]
- Afify, A.; Betz, J.F.; Riabinina, O.; Lahondère, C.; Potter, C.J. Commonly Used Insect Repellents Hide Human Odors from Anopheles Mosquitoes. Curr. Biol. 2019, 29, 3669–3680.e3665. [Google Scholar] [CrossRef] [PubMed]
- Kritsi, E.; Liggri, P.G.V.; Stamati, E.C.V.; Tsitsanou, K.E.; Zographos, S.E.; Michaelakis, A.; Papachristos, D.; Zoumpoulakis, P. A Combined Computational Methodology for the Discovery of Hit Compounds with Putative Insect Repellency Properties. ChemMedChem 2022, 17, e202200271. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Forty Years of Sodium Channels: Structure, Function, Pharmacology, and Epilepsy. Neurochem. Res. 2017, 42, 2495–2504. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Bjelobaba, I.; Zemkova, H. Ion Channels of Pituitary Gonadotrophs and Their Roles in Signaling and Secretion. Front. Endocrinol. 2017, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Chen, B.S.; Hsu, T.I.; Peng, H.; Wu, Y.H.; Lo, Y.C. Analytical studies of rapidly inactivating and noninactivating sodium currents in differentiated NG108-15 neuronal cells. J. Theor. Biol. 2009, 259, 828–836. [Google Scholar] [CrossRef]
- Huang, C.W.; Hung, T.Y.; Wu, S.N. The inhibitory actions by lacosamide, a functionalized amino acid, on voltage-gated Na+ currents. Neuroscience 2015, 287, 125–136. [Google Scholar] [CrossRef]
- Chen, B.S.; Lo, Y.C.; Peng, H.; Hsu, T.I.; Wu, S.N. Effects of ranolazine, a novel anti-anginal drug, on ion currents and membrane potential in pituitary tumor GH(3) cells and NG108-15 neuronal cells. J. Pharmacol. Sci. 2009, 110, 295–305. [Google Scholar] [CrossRef]
- Maier, L.S. A novel mechanism for the treatment of angina, arrhythmias, and diastolic dysfunction: Inhibition of late I(Na) using ranolazine. J. Cardiovasc. Pharmacol. 2009, 54, 279–286. [Google Scholar] [CrossRef]
- Lo, Y.C.; Tseng, Y.T.; Liu, C.M.; Wu, B.N.; Wu, S.N. Actions of KMUP-1, a xanthine and piperazine derivative, on voltage-gated Na+ and Ca2+ -activated K+ currents in GH3 pituitary tumour cells. Br. J. Pharmacol. 2015, 172, 5110–5122. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.N.; Wu, Y.H.; Chen, B.S.; Lo, Y.C.; Liu, Y.C. Unnderlying mechanism of actions of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology 2009, 258, 70–77. [Google Scholar] [CrossRef] [PubMed]
- So, E.C.; Wu, S.N.; Lo, Y.C.; Su, K. Differential regulation of tefluthrin and telmisartan on the gating charges of I(Na) activation and inactivation as well as on resurgent and persistent I(Na) in a pituitary cell line (GH(3)). Toxicol. Lett. 2018, 285, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.H.; Cho, H.Y.; Wu, S.N. Effective Accentuation of Voltage-Gated Sodium Current Caused by Apocynin (4’-Hydroxy-3’-methoxyacetophenone), a Known NADPH-Oxidase Inhibitor. Biomedicines 2021, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Charles, I.; James, A.F.; Abdala, A.P.; Hancox, J.C. Delayed Ventricular Repolarization and Sodium Channel Current Modification in a Mouse Model of Rett Syndrome. Int. J. Mol. Sci. 2022, 23, 5735. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhou, L.; Li, D.; Lai, Q.; Shi, H.; Wang, M. Ecotoxicological effects of the pyrethroid insecticide tefluthrin to the earthworm Eisenia fetida: A chiral view. Environ. Res. 2020, 190, 109991. [Google Scholar] [CrossRef]
- Wu, G.; Li, Q.; Liu, X.; Li-Byarlay, H.; He, B. Differential state-dependent effects of deltamethrin and tefluthrin on sodium channels in central neurons of Helicoverpa armigera. Pestic. Biochem. Physiol. 2021, 175, 104836. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Effectiveness of Columbianadin, a Bioactive Coumarin Derivative, in Perturbing Transient and Persistent I(Na). Int. J. Mol. Sci. 2021, 22, 621. [Google Scholar] [CrossRef]
- Taddese, A.; Bean, B.P. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron 2002, 33, 587–600. [Google Scholar] [CrossRef]
- Tsai, D.; Morley, J.W.; Suaning, G.J.; Lovell, N.H. Frequency-dependent reduction of voltage-gated sodium current modulates retinal ganglion cell response rate to electrical stimulation. J. Neural Eng. 2011, 8, 066007. [Google Scholar] [CrossRef]
- Wu, P.M.; Cho, H.Y.; Chiang, C.W.; Chuang, T.H.; Wu, S.N.; Tu, Y.F. Characterization in Inhibitory Effectiveness of Carbamazepine in Voltage-Gated Na+ and Erg-Mediated K+ Currents in a Mouse Neural Crest-Derived (Neuro-2a) Cell Line. Int. J. Mol. Sci. 2022, 23, 7892. [Google Scholar] [CrossRef]
- Wu, C.L.; Chuang, C.W.; Cho, H.Y.; Chuang, T.H.; Wu, S.N. The Evidence for Effective Inhibition of I(Na) Produced by Mirogabalin ((1R,5S,6S)-6-(aminomethyl)-3-ethyl-bicyclo [3.2.0] hept-3-ene-6-acetic acid), a Known Blocker of Ca(V) Channels. Int. J. Mol. Sci. 2022, 23, 3845. [Google Scholar] [CrossRef]
- Carter, B.C.; Bean, B.P. Incomplete inactivation and rapid recovery of voltage-dependent sodium channels during high-frequency firing in cerebellar Purkinje neurons. J. Neurophysiol. 2011, 105, 860–871. [Google Scholar] [CrossRef]
- Hung, T.Y.; Wu, S.N.; Huang, C.W. The Integrated Effects of Brivaracetam, a Selective Analog of Levetiracetam, on Ionic Currents and Neuronal Excitability. Biomedicines 2021, 9, 369. [Google Scholar] [CrossRef]
- Wang, D.W.; Mistry, A.M.; Kahlig, K.M.; Kearney, J.A.; Xiang, J.; George, A.L., Jr. Propranolol blocks cardiac and neuronal voltage-gated sodium channels. Front. Pharmacol. 2010, 1, 144. [Google Scholar] [CrossRef]
- Powers, R.K.; Binder, M.D. Persistent sodium and calcium currents in rat hypoglossal motoneurons. J. Neurophysiol. 2003, 89, 615–624. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Characterization of Direct Perturbations on Voltage-Gated Sodium Current by Esaxerenone, a Nonsteroidal Mineralocorticoid Receptor Blocker. Biomedicines 2021, 9, 549. [Google Scholar] [CrossRef]
- Stafstrom, C.E. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007, 7, 15–22. [Google Scholar] [CrossRef]
- Boeri, J.; Le Corronc, H.; Lejeune, F.X.; Le Bras, B.; Mouffle, C.; Angelim, M.; Mangin, J.M.; Branchereau, P.; Legendre, P.; Czarnecki, A. Persistent Sodium Current Drives Excitability of Immature Renshaw Cells in Early Embryonic Spinal Networks. J. Neurosci. 2018, 38, 7667–7682. [Google Scholar] [CrossRef]
- Hsu, C.L.; Zhao, X.; Milstein, A.D.; Spruston, N. Persistent Sodium Current Mediates the Steep Voltage Dependence of Spatial Coding in Hippocampal Pyramidal Neurons. Neuron 2018, 99, 147–162.e148. [Google Scholar] [CrossRef]
- Müller, P.; Draguhn, A.; Egorov, A.V. Persistent sodium current modulates axonal excitability in CA1 pyramidal neurons. J. Neurochem. 2018, 146, 446–458. [Google Scholar] [CrossRef] [Green Version]
- Meredith, F.L.; Rennie, K.J. Persistent and resurgent Na+ currents in vestibular calyx afferents. J. Neurophysiol. 2020, 124, 510–524. [Google Scholar] [CrossRef]
- Enomoto, A.; Han, J.M.; Hsiao, C.F.; Wu, N.; Chandler, S.H. Participation of sodium currents in burst generation and control of membrane excitability in mesencephalic trigeminal neurons. J. Neurosci. 2006, 26, 3412–3422. [Google Scholar] [CrossRef]
- Hong, H.; Lu, T.; Wang, X.; Wang, Y.; Sanchez, J.T. Resurgent sodium current promotes action potential firing in the avian auditory brainstem. J. Physiol. 2018, 596, 423–443. [Google Scholar] [CrossRef]
- Kuo, P.C.; Kao, Z.H.; Lee, S.W.; Wu, S.N. Effects of Sesamin, the Major Furofuran Lignan of Sesame Oil, on the Amplitude and Gating of Voltage-Gated Na+ and K+ Currents. Molecules 2020, 25, 3062. [Google Scholar] [CrossRef]
- Tidball, A.M.; Lopez-Santiago, L.F.; Yuan, Y.; Glenn, T.W.; Margolis, J.L.; Clayton Walker, J.; Kilbane, E.G.; Miller, C.A.; Martina Bebin, E.; Scott Perry, M.; et al. Variant-specific changes in persistent or resurgent sodium current in SCN8A-related epilepsy patient-derived neurons. Brain 2020, 143, 3025–3040. [Google Scholar] [CrossRef]
- Quattrocolo, G.; Dunville, K.; Nigro, M.J. Resurgent Sodium Current in Neurons of the Cerebral Cortex. Front. Cell Neurosci. 2021, 15, 760610. [Google Scholar] [CrossRef]
- Zemel, B.M.; Nevue, A.A.; Dagostin, A.; Lovell, P.V.; Mello, C.V.; von Gersdorff, H. Resurgent Na+ currents promote ultrafast spiking in projection neurons that drive fine motor control. Nat. Commun. 2021, 12, 6762. [Google Scholar] [CrossRef]
- Ransdell, J.L.; Moreno, J.D.; Bhagavan, D.; Silva, J.R.; Nerbonne, J.M. Intrinsic mechanisms in the gating of resurgent Na+ currents. Elife 2022, 11, e70173. [Google Scholar] [CrossRef]
- Ahuja, S.; Mukund, S.; Deng, L.; Khakh, K.; Chang, E.; Ho, H.; Shriver, S.; Young, C.; Lin, S.; Johnson, J.P., Jr.; et al. Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science 2015, 350, aac5464. [Google Scholar] [CrossRef]
- Charlton, N.P.; Murphy, L.T.; Parker Cote, J.L.; Vakkalanka, J.P. The toxicity of picaridin containing insect repellent reported to the National Poison Data System. Clin. Toxicol. 2016, 54, 655–658. [Google Scholar] [CrossRef]
- Spirhanzlova, P.; Fini, J.B.; Demeneix, B.; Lardy-Fontan, S.; Vaslin-Reimann, S.; Lalere, B.; Guma, N.; Tindall, A.; Krief, S. Composition and endocrine effects of water collected in the Kibale national park in Uganda. Environ. Pollut. 2019, 251, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Magariños-Ascone, C.; Pazo, J.H.; Macadar, O.; Buño, W. High-frequency stimulation of the subthalamic nucleus silences subthalamic neurons: A possible cellular mechanism in Parkinson’s disease. Neuroscience 2002, 115, 1109–1117. [Google Scholar] [CrossRef]
- Khaliq, Z.M.; Gouwens, N.W.; Raman, I.M. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: An experimental and modeling study. J. Neurosci. 2003, 23, 4899–4912. [Google Scholar] [CrossRef] [PubMed]
- Avendano-Coy, J.; Serrano-Munoz, D.; Taylor, J.; Goicoechea-Garcia, C.; Gomez-Soriano, J. Peripheral Nerve Conduction Block by High-Frequency Alternating Currents: A Systematic Review. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.A.; Salari, A.; Lin, J.L.; Cowan, L.M.; Penington, N.J.; Milescu, M.; Milescu, L.S. Sodium channels implement a molecular leaky integrator that detects action potentials and regulates neuronal firing. Elife 2020, 9, e54940. [Google Scholar] [CrossRef]
- Zhu, T.; Wei, S.; Wang, Y. Post-Inhibitory Rebound Firing of Dorsal Root Ganglia Neurons. J. Pain Res. 2022, 15, 2029–2040. [Google Scholar] [CrossRef]
- Leão, R.M.; Kushmerick, C.; Pinaud, R.; Renden, R.; Li, G.L.; Taschenberger, H.; Spirou, G.; Levinson, S.R.; von Gersdorff, H. Presynaptic Na+ channels: Locus, development, and recovery from inactivation at a high-fidelity synapse. J. Neurosci. 2005, 25, 3724–3738. [Google Scholar] [CrossRef] [PubMed]
- Osteen, J.D.; Sampson, K.; Iyer, V.; Julius, D.; Bosmans, F. Pharmacology of the Na(v)1.1 domain IV voltage sensor reveals coupling between inactivation gating processes. Proc. Natl. Acad. Sci. USA 2017, 114, 6836–6841. [Google Scholar] [CrossRef]
- Durey, M. Bifurcations and chaos in a Lorenz-like pilot-wave system. Chaos 2020, 30, 103115. [Google Scholar] [CrossRef]
- Wu, S.N.; Chern, J.H.; Shen, S.; Chen, H.H.; Hsu, Y.T.; Lee, C.C.; Chan, M.H.; Lai, M.C.; Shie, F.S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell Physiol. 2017, 232, 3409–3421. [Google Scholar] [CrossRef]
- Chang, W.T.; Liu, P.Y.; Gao, Z.H.; Lee, S.W.; Lee, W.K.; Wu, S.N. Evidence for the Effectiveness of Remdesivir (GS-5734), a Nucleoside-Analog Antiviral Drug in the Inhibition of I (K(M)) or I (K(DR)) and in the Stimulation of I (MEP). Front. Pharmacol. 2020, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Weiser, T.; Qu, Y.; Catterall, W.A.; Scheuer, T. Differential interaction of R-mexiletine with the local anesthetic receptor site on brain and heart sodium channel alpha-subunits. Mol. Pharmacol. 1999, 56, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.V.; Espinosa, J.L.; López-Domínguez, A.M.; López-Santiago, L.F.; Navarrete, A.; Cota, G. L-type calcium channel activation up-regulates the mRNAs for two different sodium channel alpha subunits (Nav1.2 and Nav1.3) in rat pituitary GH3 cells. Brain Res. Mol. Brain Res. 2003, 116, 115–125. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).