Sphingolipids in Atherosclerosis: Chimeras in Structure and Function
Abstract
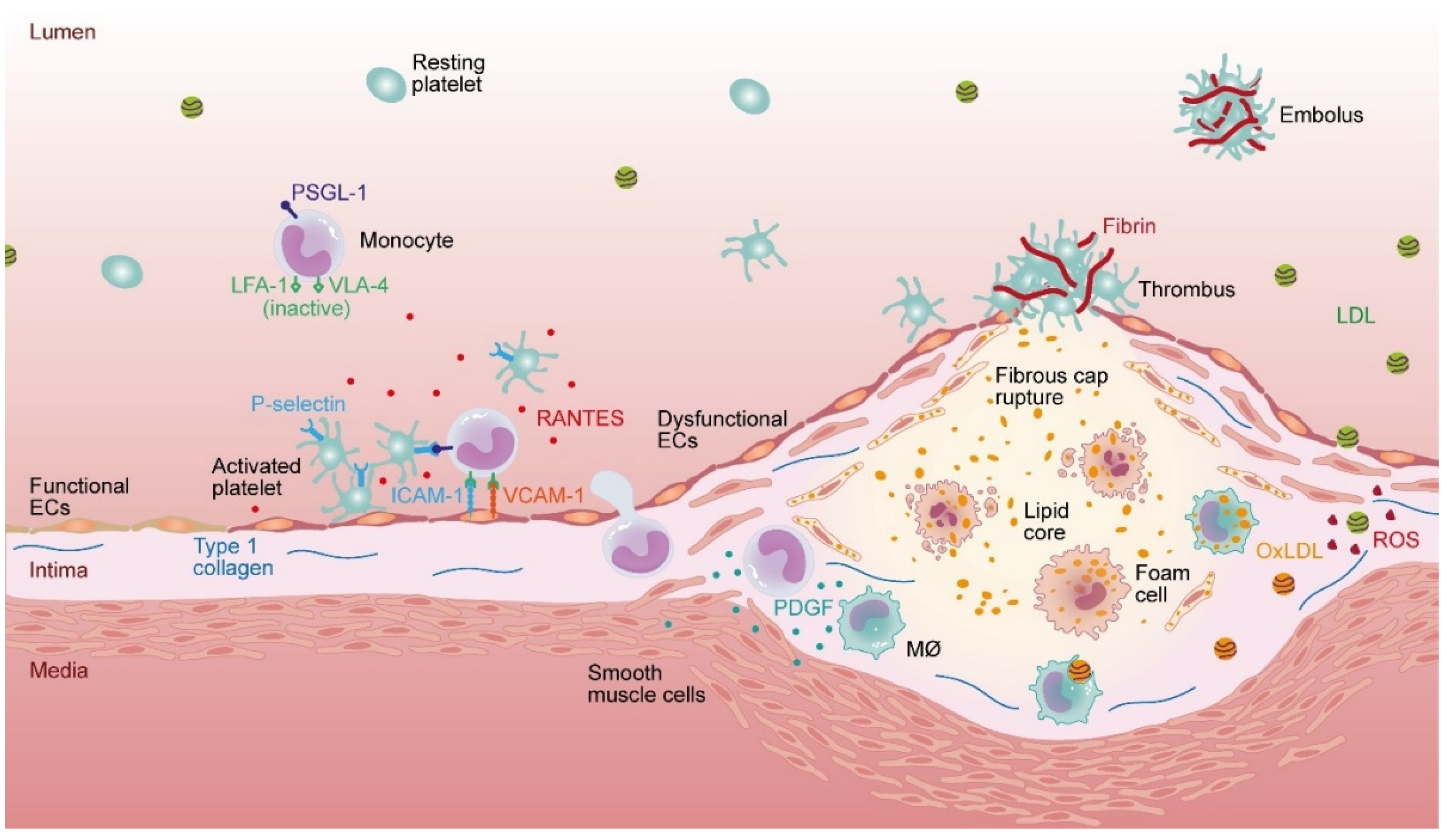
1. Introduction
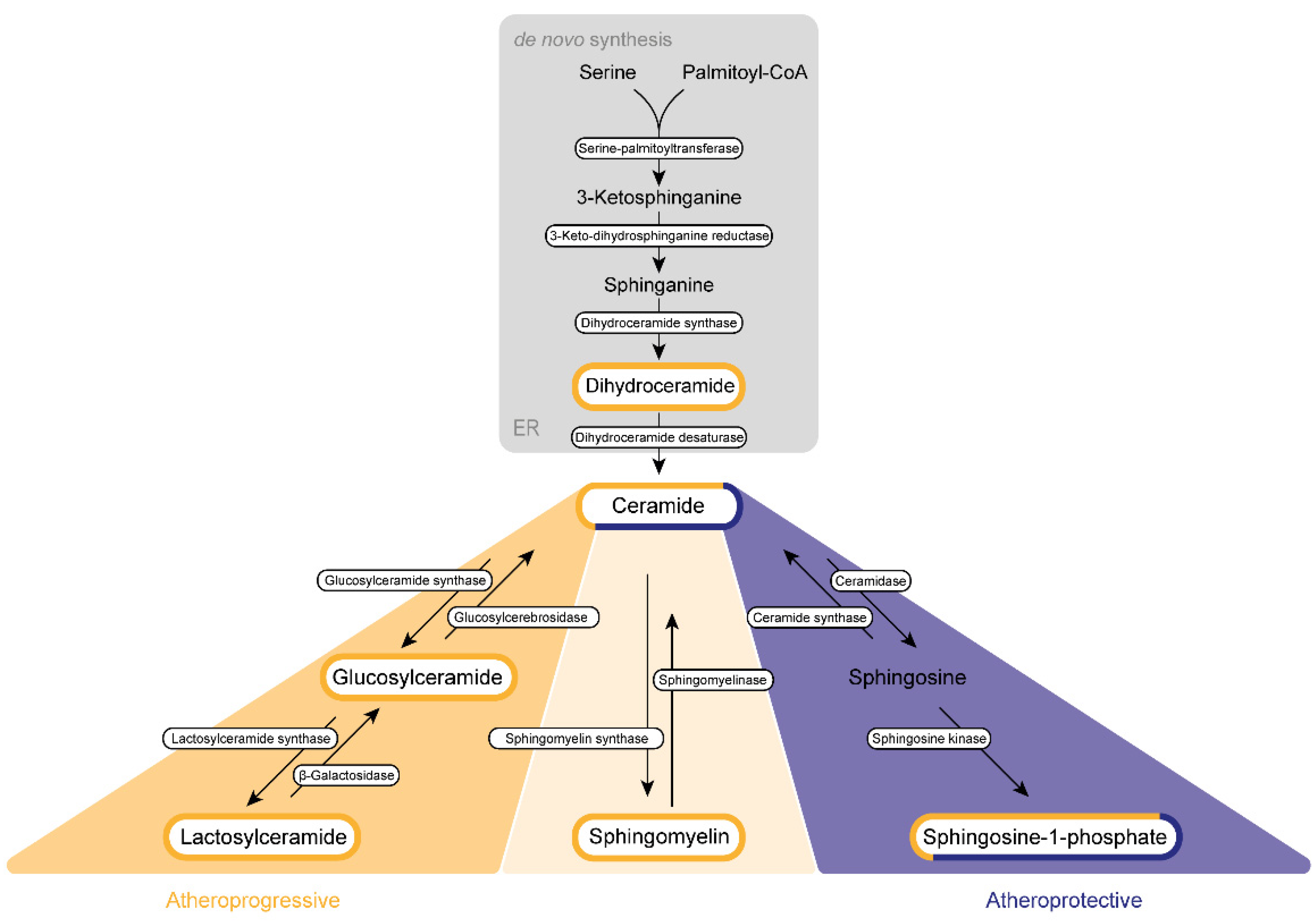
2. Dihydroceramide in Atherosclerosis Progression
2.1. Synthesis and Metabolism
2.2. Regulation of Inflammation
2.3. Regulation of Autophagy
3. Ceramide
3.1. Sphingomyelinases (SMases)
3.2. Regulation by Matrix Metalloproteinases (MMPs)
3.3. Regulation by Tumor Necrosis Factor Alpha (TNFα)
3.4. Regulation of NO Production
3.5. Regulation of LDL Aggregation
4. Sphingosine-1-Phosphate
5. Sphingomyelin (SM)
6. LacCer and GluCer—Sphingolipids with Non-Chimeric Functions?
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thudichum, J. A Treatise on the Chemical Constitution of the Brain Bailliere; Bailliere, Tindall, and Cox: London, UK, 1884. [Google Scholar]
- Murray, C.J.; Lopez, A.D. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997, 349, 1269–1276. [Google Scholar] [CrossRef]
- Kelly, B.B.; Fuster, V. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Reddick, R.L.; Burkey, B.; Maeda, N. Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein E gene disruption. J. Clin. Investig. 1994, 94, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Reddick, R.L.; Zhang, S.H.; Maeda, N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arter. Thromb. 1994, 14, 141–147. [Google Scholar] [CrossRef]
- Dutta, P.; Courties, G.; Wei, Y.; Leuschner, F.; Gorbatov, R.; Robbins, C.S.; Iwamoto, Y.; Thompson, B.; Carlson, A.L.; Heidt, T.; et al. Myocardial infarction accelerates atherosclerosis. Nature 2012, 487, 325–329. [Google Scholar] [CrossRef]
- Palasubramaniam, J.; Wang, X.; Peter, K. Myocardial Infarction-From Atherosclerosis to Thrombosis. Arter. Thromb. Vasc. Biol. 2019, 39, e176–e185. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, L.; Chen, Y.; Xie, Q.; Yan, Z.; Liu, Y.; Kang, J.; Li, S. Risk factors for ischemic stroke: Differences between cerebral small vessel and large artery atherosclerosis aetiologies. Folia Neuropathol. 2021, 59, 378–385. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- van Varik, B.J.; Rennenberg, R.J.; Reutelingsperger, C.P.; Kroon, A.A.; de Leeuw, P.W.; Schurgers, L.J. Mechanisms of arterial remodeling: Lessons from genetic diseases. Front. Genet. 2012, 3, 290. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927. [Google Scholar]
- Aronson, D.; Rayfield, E.J. How hyperglycemia promotes atherosclerosis: Molecular mechanisms. Cardiovasc. Diabetol. 2002, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Glasser, S.P.; Selwyn, A.P.; Ganz, P. Atherosclerosis: Risk factors and the vascular endothelium. Am. Heart J. 1996, 131, 379–384. [Google Scholar] [CrossRef]
- Landmesser, U.; Hornig, B.; Drexler, H. Endothelial function: A critical determinant in atherosclerosis? Circulation 2004, 109, II27–II33. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.; Goettsch, C.; Morawietz, H. Oxidative stress and endothelial dysfunction. Haemostaseologie 2007, 27, 5–1217. [Google Scholar] [CrossRef]
- Gryglewski, R.J.; Palmer, R.M.; Moncada, S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 1986, 320, 454–456. [Google Scholar] [CrossRef]
- Zou, M.H.; Cohen, R.; Ullrich, V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium 2004, 11, 89–97. [Google Scholar] [CrossRef]
- Vergnani, L.; Hatrik, S.; Ricci, F.; Passaro, A.; Manzoli, N.; Zuliani, G.; Brovkovych, V.; Fellin, R.; Malinski, T. Effect of native and oxidized low-density lipoprotein on endothelial nitric oxide and superoxide production: Key role of L-arginine availability. Circulation 2000, 101, 1261–1266. [Google Scholar] [CrossRef]
- Rueckschloss, U.; Galle, J.; Holtz, J.; Zerkowski, H.R.; Morawietz, H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: Antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation 2001, 104, 1767–1772. [Google Scholar] [CrossRef]
- Görlach, A.; Brandes, R.P.; Nguyen, K.; Amidi, M.; Dehghani, F.; Busse, R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ. Res. 2000, 87, 26–32. [Google Scholar] [CrossRef]
- Jones, S.A.; O’Donnell, V.B.; Wood, J.D.; Broughton, J.P.; Hughes, E.J.; Jones, O.T. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am. J. Physiol. 1996, 271, H1626–H1634. [Google Scholar] [CrossRef]
- Lüscher, T.F.; Barton, M. Biology of the endothelium. Clin. Cardiol. 1997, 20, II-3–II-10. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, S.; Heinecke, J.W. Oxidative stress and endothelial dysfunction in vascular disease. Curr. Diab. Rep. 2007, 7, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; Van Hinsbergh, V.W.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial permeability, LDL deposition, and cardiovascular risk factors—A review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Stemerman, M.B. Effects of moderate hypercholesterolemia on rabbit endothelium. Arterioscler. Off. J. Am. Heart Assoc. Inc. 1981, 1, 25–32. [Google Scholar] [CrossRef]
- De Caterina, R.; Libby, P. Endothelial Dysfunctions in Vascular Disease. 2008. Available online: https://books.google.co.jp/books?hl=zh-TW&lr=&id=et-Pl-xh2vQC&oi=fnd&pg=PP2&dq=Endothelial+Dysfunctions+in+Vascular+Disease&ots=C5lFSmT374&sig=-OnsuY2im7CVRTVSg8WeMkMUgV4&redir_esc=y#v=onepage&q=Endothelial%20Dysfunctions%20in%20Vascular%20Disease&f=false (accessed on 29 August 2022).
- Nording, H.; Baron, L.; Langer, H.F. Platelets as therapeutic targets to prevent atherosclerosis. Atherosclerosis 2020, 307, 97–108. [Google Scholar] [CrossRef]
- von Hundelshausen, P.; Weber, K.S.; Huo, Y.; Proudfoot, A.E.; Nelson, P.J.; Ley, K.; Weber, C. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation 2001, 103, 1772–1777. [Google Scholar] [CrossRef]
- OYu, P.; Peclo, M.; Gown, A. Various cell types in human atherosclerotic lesions express ICAM-1. Further immunocytochemical and immunochemical studies employing monoclonal antibody 10F3. Am. J. Pathol. 1992, 140, 889. [Google Scholar]
- Poston, R.; Haskard, D.; Coucher, J.; Gall, N.; Johnson-Tidey, R. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am. J. Pathol. 1992, 140, 665. [Google Scholar]
- Krieglstein, C.F.; Granger, D.N. Adhesion molecules and their role in vascular disease. Am. J. Hypertens. 2001, 14, 44S–54S. [Google Scholar] [CrossRef]
- Navab, M.; Imes, S.; Hama, S.; Hough, G.; Ross, L.; Bork, R.; Valente, A.; Berliner, J.; Drinkwater, D.; Laks, H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Investig. 1991, 88, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. IJBS 2008, 4, 89. [Google Scholar] [PubMed]
- Wolff, S.P.; Garner, A.; Dean, R.T. Free radicals, lipids and protein degradation. Trends Biochem. Sci. 1986, 11, 27–31. [Google Scholar] [CrossRef]
- Hurt-Camejo, E.; Camejo, G.; Rosengren, B.; Lopez, F.; Ahlström, C.; Fager, G.; Bondjers, G. Effect of arterial proteoglycans and glycosaminoglycans on low density lipoprotein oxidation and its uptake by human macrophages and arterial smooth muscle cells. Arterioscler. Thromb. A J. Vasc. Biol. 1992, 12, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.N.; Chen, M.; Jongstra-Bilen, J.; Cybulsky, M.I. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J. Exp. Med. 2009, 206, 2141–2149. [Google Scholar] [CrossRef]
- Subramanian, M.; Thorp, E.; Tabas, I. Identification of a non-growth factor role for GM-CSF in advanced atherosclerosis: Promotion of macrophage apoptosis and plaque necrosis through IL-23 signaling. Circ. Res. 2015, 116, e13–e24. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–60441. [Google Scholar] [CrossRef]
- Williams, H.J.; Fisher, E.A.; Greaves, D.R. Macrophage differentiation and function in atherosclerosis: Opportunities for therapeutic intervention? J. Innate Immun. 2012, 4, 498–508. [Google Scholar] [CrossRef]
- Steinberg, D. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 1997, 272, 20963–20966. [Google Scholar] [CrossRef]
- Febbraio, M.; Podrez, E.A.; Smith, J.D.; Hajjar, D.P.; Hazen, S.L.; Hoff, H.F.; Sharma, K.; Silverstein, R.L. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Investig. 2000, 105, 1049–1056. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Zingg, J.-M.; Azzi, A. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation 2000, 102, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Chen, M.; Fujiwara, H.; Masaki, T.; Sawamura, T. LOX-1 mediates lysophosphatidylcholine-induced oxidized LDL uptake in smooth muscle cells. FEBS Lett. 2000, 467, 217–220. [Google Scholar] [CrossRef]
- Ross, R. The pathogenesis of atherosclerosis—An update. N. Engl. J. Med. 1986, 314, 488–500. [Google Scholar] [CrossRef]
- Lao, K.H.; Zeng, L.; Xu, Q. Endothelial and smooth muscle cell transformation in atherosclerosis. Curr. Opin. Lipidol. 2015, 26, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Mietus-Snyder, M.; Gowri, M.S.; Pitas, R.E. Class A Scavenger Receptor Up-regulation in Smooth Muscle Cells by Oxidized Low Density Lipoprotein*: Enhancement by calcium flux and concurrent cyclooxygenase-2 up-regulation. J. Biol. Chem. 2000, 275, 17661–17670. [Google Scholar] [CrossRef]
- Jalvy, S.; Renault, M.-A.; Leen, L.L.S.; Belloc, I.; Bonnet, J.; Gadeau, A.-P.; Desgranges, C. Autocrine expression of osteopontin contributes to PDGF-mediated arterial smooth muscle cell migration. Cardiovasc. Res. 2007, 75, 738–747. [Google Scholar] [CrossRef][Green Version]
- Raines, E.W. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004, 15, 237–254. [Google Scholar] [CrossRef]
- Hegyi, L.; Skepper, J.N.; CARY, N.R.; Mitchinson, M.J. Foam cell apoptosis and the development of the lipid core of human atherosclerosis. J. Pathol. 1996, 180, 423–429. [Google Scholar] [CrossRef]
- Kalampogias, A.; Siasos, G.; Oikonomou, E.; Tsalamandris, S.; Mourouzis, K.; Tsigkou, V.; Vavuranakis, M.; Zografos, T.; Deftereos, S.; Stefanadis, C. Basic mechanisms in atherosclerosis: The role of calcium. Med. Chem. 2016, 12, 103–113. [Google Scholar] [CrossRef]
- Centelles, M.N.; Puy, C.; Lopez-Sagaseta, J.; Fukudome, K.; Montes, R.; Hermida, J. Blocking endothelial protein C receptor (EPCR) accelerates thrombus development in vivo. Thromb. Haemost. 2010, 103, 1239–1244. [Google Scholar] [CrossRef]
- Merlini, P.A.; Rossi, M.L.; Faioni, E.M.; Franchi, F.; Bramucci, E.; Lucreziotti, S.; Biguzzi, E.; Mannucci, P.M.; Ardissino, D. Expression of endothelial protein C receptor and thrombomodulin in human coronary atherosclerotic plaques. Ital. Heart J. 2004, 5, 42–47. [Google Scholar] [PubMed]
- Chen, P.-S.; Wang, K.-C.; Chao, T.-H.; Chung, H.-C.; Tseng, S.-Y.; Luo, C.-Y.; Shi, G.-Y.; Wu, H.-L.; Li, Y.-H. Recombinant thrombomodulin exerts anti-autophagic action in endothelial cells and provides anti-atherosclerosis effect in apolipoprotein E deficient mice. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stavik, B.; Holm, S.; Espada, S.; Iversen, N.; Sporsheim, B.; Bjerkeli, V.; Dahl, T.B.; Sandset, P.M.; Skjelland, M.; Espevik, T. Increased expression of TFPI in human carotid stenosis. Thromb. Res. 2017, 155, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Uszyński, M.; Żekanowska, E.; Uszyński, W.; Kuczyński, J. Tissue factor (TF) and tissue factor pathway inhibitor (TFPI) in amniotic fluid and blood plasma: Implications for the mechanism of amniotic fluid embolism. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 95, 163–166. [Google Scholar] [CrossRef]
- Smith, E.B. Intimal and medial lipids in human aortas. Lancet 1960, 1, 799–803. [Google Scholar] [CrossRef]
- Edsfeldt, A.; Dunér, P.; Ståhlman, M.; Mollet, I.G.; Asciutto, G.; Grufman, H.; Nitulescu, M.; Persson, A.F.; Fisher, R.M.; Melander, O. Sphingolipids contribute to human atherosclerotic plaque inflammation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1132–1140. [Google Scholar] [CrossRef]
- Hornemann, T.; Worgall, T.S. Sphingolipids and atherosclerosis. Atherosclerosis 2013, 226, 16–28. [Google Scholar] [CrossRef]
- Manicke, N.E.; Nefliu, M.; Wu, C.; Woods, J.W.; Reiser, V.; Hendrickson, R.C.; Cooks, R.G. Imaging of lipids in atheroma by desorption electrospray ionization mass spectrometry. Anal. Chem. 2009, 81, 8702–8707. [Google Scholar] [CrossRef]
- Portman, O.W.; Alexander, M. Metabolism of sphingolipids by normal and atherosclerotic aorta of squirrel monkeys. J. Lipid Res. 1970, 11, 23–30. [Google Scholar] [CrossRef]
- Zheng, W.; Kollmeyer, J.; Symolon, H.; Momin, A.; Munter, E.; Wang, E.; Kelly, S.; Allegood, J.C.; Liu, Y.; Peng, Q.; et al. Ceramides and other bioactive sphingolipid backbones in health and disease: Lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim. Biophys. Acta 2006, 1758, 1864–1884. [Google Scholar] [CrossRef]
- Gagliostro, V.; Casas, J.; Caretti, A.; Abad, J.L.; Tagliavacca, L.; Ghidoni, R.; Fabrias, G.; Signorelli, P. Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. Int. J. Biochem. Cell Biol. 2012, 44, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, P.; Munoz-Olaya, J.M.; Gagliostro, V.; Casas, J.; Ghidoni, R.; Fabriàs, G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009, 282, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Venant, H.; Rahmaniyan, M.; Jones, E.E.; Lu, P.; Lilly, M.B.; Garrett-Mayer, E.; Drake, R.R.; Kraveka, J.M.; Smith, C.D.; Voelkel-Johnson, C. The Sphingosine Kinase 2 Inhibitor ABC294640 Reduces the Growth of Prostate Cancer Cells and Results in Accumulation of Dihydroceramides In Vitro and In Vivo. Mol. Cancer 2015, 14, 2744–2752. [Google Scholar] [CrossRef]
- Breen, P.; Joseph, N.; Thompson, K.; Kraveka, J.M.; Gudz, T.I.; Li, L.; Rahmaniyan, M.; Bielawski, J.; Pierce, J.S.; van Buren, E.; et al. Dihydroceramide desaturase knockdown impacts sphingolipids and apoptosis after photodamage in human head and neck squamous carcinoma cells. Anticancer Res. 2013, 33, 77–84. [Google Scholar]
- Lachkar, F.; Ferré, P.; Foufelle, F.; Papaioannou, A. Dihydroceramides: Their emerging physiological roles and functions in cancer and metabolic diseases. Am. J. Physiol.-Endocrinol. Metab. 2021, 320, E122–E130. [Google Scholar] [CrossRef]
- Jiang, Q.; Rao, X.; Kim, C.Y.; Freiser, H.; Zhang, Q.; Jiang, Z.; Li, G. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int. J. Cancer 2012, 130, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Rahbarghazi, R.; Nouri, M.; Aghamohammadzadeh, N.; Safaei, N.; Ahmadi, M. Role of autophagy in atherosclerosis: Foe or friend? J. Inflamm. 2019, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stiban, J.; Fistere, D.; Colombini, M. Dihydroceramide hinders ceramide channel formation: Implications on apoptosis. Apoptosis 2006, 11, 773–780. [Google Scholar] [CrossRef]
- Magaye, R.R.; Savira, F.; Hua, Y.; Kelly, D.J.; Reid, C.; Flynn, B.; Liew, D.; Wang, B.H. The role of dihydrosphingolipids in disease. Cell. Mol. Life Sci. 2019, 76, 1107–1134. [Google Scholar] [CrossRef]
- Siddique, M.M.; Li, Y.; Chaurasia, B.; Kaddai, V.A.; Summers, S.A. Dihydroceramides: From bit players to lead actors. J. Biol. Chem. 2015, 290, 15371–15379. [Google Scholar] [CrossRef]
- Huwiler, A.; Kolter, T.; Pfeilschifter, J.; Sandhoff, K. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim. Biophys. Acta 2000, 1485, 63–99. [Google Scholar] [CrossRef]
- Augé, N.; Andrieu, N.; Nègre-Salvayre, A.; Thiers, J.-C.; Levade, T.; Salvayre, R. The sphingomyelin-ceramide signaling pathway is involved in oxidized low density lipoprotein-induced cell proliferation. J. Biol. Chem. 1996, 271, 19251–19255. [Google Scholar] [CrossRef] [PubMed]
- Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hauner, B.J.; Hopkins, P.N.; Hunt, S.C.; Holland, W.L.; Summers, S.A.; Playdon, M.C. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig. 2020, 130, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Jiang, Z.; Hong, X.; Long, H.; Hu, J. Ceramides induce apoptosis in HeLa cells and enhance cytochrome c-induced apoptosis in Xenopus egg extracts. Cell Mol. Life Sci. 2000, 57, 1117–1125. [Google Scholar] [CrossRef]
- Schissel, S.L.; Tweedie-Hardman, J.; Rapp, J.H.; Graham, G.; Williams, K.J.; Tabas, I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Investig. 1996, 98, 1455–1464. [Google Scholar] [CrossRef]
- Ichi, I.; Nakahara, K.; Miyashita, Y.; Hidaka, A.; Kutsukake, S.; Inoue, K.; Maruyama, T.; Miwa, Y.; Harada-Shiba, M.; Tsushima, M. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids 2006, 41, 859–863. [Google Scholar] [CrossRef]
- Tomiuk, S.; Zumbansen, M.; Stoffel, W. Characterization and subcellular localization of murine and human magnesium-dependent neutral sphingomyelinase. J. Biol. Chem. 2000, 275, 5710–5717. [Google Scholar] [CrossRef]
- Jung, S.Y.; Suh, J.H.; Park, H.J.; Jung, K.M.; Kim, M.Y.; Na, D.S.; Kim, D.K. Identification of Multiple Forms of Membrane-Associated Neutral Sphingomyelinase in Bovine Brain. J. Neurochem. 2000, 75, 1004–1014. [Google Scholar] [CrossRef]
- Casula, M.; Colpani, O.; Xie, S.; Catapano, A.L.; Baragetti, A. HDL in Atherosclerotic Cardiovascular Disease: In Search of a Role. Cells 2021, 10, 1869. [Google Scholar] [CrossRef]
- Elshourbagy, N.A.; Meyers, H.V.; Abdel-Meguid, S.S. Cholesterol: The good, the bad, and the ugly-therapeutic targets for the treatment of dyslipidemia. Med. Princ. Pract. 2014, 23, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Sharp, D.S.; Grove, J.S.; Bruce, C.; Yano, K.; Curb, J.D.; Tall, A.R. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J. Clin. Investig. 1996, 97, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Kolmakova, A.; Kwiterovich, P.; Virgil, D.; Alaupovic, P.; Knight-Gibson, C.; Martin, S.F.; Chatterjee, S. Apolipoprotein CI induces apoptosis in human aortic smooth muscle cells via recruiting neutral sphingomyelinase. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 264–269. [Google Scholar] [CrossRef]
- Devillard, R.; Galvani, S.; Thiers, J.-C.; Guenet, J.-L.; Hannun, Y.; Bielawski, J.; Nègre-Salvayre, A.; Salvayre, R.; Augé, N. Stress-induced sphingolipid signaling: Role of type-2 neutral sphingomyelinase in murine cell apoptosis and proliferation. PLoS ONE 2010, 5, e9826. [Google Scholar] [CrossRef] [PubMed]
- Zettler, M.E.; Prociuk, M.A.; Austria, J.A.; Massaeli, H.; Zhong, G.; Pierce, G.N. OxLDL stimulates cell proliferation through a general induction of cell cycle proteins. Am. J. Physiol.-Heart Circ. Physiol. 2003, 284, H644–H653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marathe, S.; Choi, Y.; Leventhal, A.R.; Tabas, I. Sphingomyelinase converts lipoproteins from apolipoprotein E knockout mice into potent inducers of macrophage foam cell formation. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2607–2613. [Google Scholar] [CrossRef]
- Devlin, C.M.; Leventhal, A.R.; Kuriakose, G.; Schuchman, E.H.; Williams, K.J.; Tabas, I. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1723–1730. [Google Scholar] [CrossRef]
- Pavoine, C.; Pecker, F. Sphingomyelinases: Their regulation and roles in cardiovascular pathophysiology. Cardiovasc. Res. 2009, 82, 175–183. [Google Scholar] [CrossRef]
- Goñi, F.M.; Alonso, A. Sphingomyelinases: Enzymology and membrane activity. FEBS Lett. 2002, 531, 38–46. [Google Scholar] [CrossRef]
- Marchesini, N.; Hannun, Y.A. Acid and neutral sphingomyelinases: Roles and mechanisms of regulation. Biochem. Cell Biol. 2004, 82, 27–44. [Google Scholar] [CrossRef]
- Grassme, H.; Jekle, A.; Riehle, A.; Schwarz, H.; Berger, J.; Sandhoff, K.; Kolesnick, R.; Gulbins, E. CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 2001, 276, 20589–20596. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yi, F.; Zhang, F.; Poklis, J.L.; Li, P.-L. Lysosomal targeting and trafficking of acid sphingomyelinase to lipid raft platforms in coronary endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2056–2062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Yi, F.; Jin, S.; Xia, M.; Chen, Q.Z.; Gulbins, E.; Li, P.L. Acid sphingomyelinase and its redox amplification in formation of lipid raft redox signaling platforms in endothelial cells. Antioxid. Redox Signal. 2007, 9, 817–828. [Google Scholar] [CrossRef]
- Jia, S.-J.; Jin, S.; Zhang, F.; Yi, F.; Dewey, W.L.; Li, P.-L. Formation and function of ceramide-enriched membrane platforms with CD38 during M1-receptor stimulation in bovine coronary arterial myocytes. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295, H1743–H1752. [Google Scholar] [CrossRef] [PubMed]
- Augé, N.; Maupas-Schwalm, F.; Elbaz, M.; Thiers, J.-C.; Waysbort, A.; Itohara, S.; Krell, H.-W.; Salvayre, R.; Nègre-Salvayre, A. Role for Matrix Metalloproteinase-2 in Oxidized Low-Density Lipoprotein–Induced Activation of the Sphingomyelin/Ceramide Pathway and Smooth Muscle Cell Proliferation. Circulation 2004, 110, 571–578. [Google Scholar] [CrossRef]
- Ikeda, U.; Shimada, K. Matrix metalloproteinases and coronary artery diseases. Clin. Cardiol. Int. Index. Peer-Rev. J. Adv. Treat. Cardiovasc. Dis. 2003, 26, 55–59. [Google Scholar] [CrossRef]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef]
- Xu, J.; Yeh, C.-H.; Chen, S.; He, L.; Sensi, S.L.; Canzoniero, L.M.; Choi, D.W.; Hsu, C.Y. Involvement of de NovoCeramide Biosynthesis in Tumor Necrosis Factor-α/Cycloheximide-induced Cerebral Endothelial Cell Death. J. Biol. Chem. 1998, 273, 16521–16526. [Google Scholar] [CrossRef]
- Modur, V.; Zimmerman, G.A.; Prescott, S.M.; McIntyre, T.M. Endothelial cell inflammatory responses to tumor necrosis factor α: Ceramide-dependent and-independent mitogen-activated protein kinase cascades. J. Biol. Chem. 1996, 271, 13094–13102. [Google Scholar] [CrossRef]
- Bhagat, K.; Vallance, P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation 1997, 96, 3042–3047. [Google Scholar] [CrossRef]
- Nakamura, M.; Yoshida, H.; Arakawa, N.; Saitoh, S.; Satoh, M.; Hiramori, K. Effects of tumor necrosis factor-α on basal and stimulated endothelium-dependent vasomotion in human resistance vessel. J. Cardiovasc. Pharmacol. 2000, 36, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ba, Z.F.; Chaudry, I.H. Administration of tumor necrosis factor-alpha in vivo depresses endothelium-dependent relaxation. Am. J. Physiol.-Heart Circ. Physiol. 1994, 266, H2535–H2541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.P.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-α in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef]
- Sawada, M.; Kiyono, T.; Nakashima, S.; Shinoda, J.; Naganawa, T.; Hara, S.; Iwama, T.; Sakai, N. Molecular mechanisms of TNF-α-induced ceramide formation in human glioma cells: p53-mediated oxidant stress-dependent and-independent pathways. Cell Death Differ. 2004, 11, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.X.; Zou, A.-P.; Li, P.-L. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am. J. Physiol.-Heart Circ. Physiol. 2003, 284, H605–H612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.X.; Fryer, R.M.; Hsu, A.K.; Zou, A.-P.; Gross, G.J.; Campbell, W.B.; Li, P.-L. Production and metabolism of ceramide in normal and ischemic-reperfused myocardium of rats. Basic Res. Cardiol. 2001, 96, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, M.; Kitabayashi, A.; Kuroki, J.; Miura, A.B. Induction of tissue factor production but not the upregulation of adhesion molecule expression by ceramide in human vascular endothelial cells. Tohoku J. Exp. Med. 2000, 191, 167–176. [Google Scholar] [CrossRef]
- Ito, H.; Koide, N.; Hassan, F.; Islam, S.; Tumurkhuu, G.; Mori, I.; Yoshida, T.; Kakumu, S.; Moriwaki, H.; Yokochi, T. Lethal endotoxic shock using α-galactosylceramide sensitization as a new experimental model of septic shock. Lab. Investig. 2006, 86, 254–261. [Google Scholar] [CrossRef]
- Hürlimann, D.; Forster, A.; Noll, G.; Enseleit, F.; Chenevard, R.; Distler, O.; Béchir, M.; Spieker, L.E.; Neidhart, M.; Michel, B.A. Anti–tumor necrosis factor-α treatment improves endothelial function in patients with rheumatoid arthritis. Circulation 2002, 106, 2184–2187. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Zou, A.-P. Homocysteine enhances TIMP-1 expression and cell proliferation associated with NADH oxidase in rat mesangial cells. Kidney Int. 2003, 63, 1012–1020. [Google Scholar] [CrossRef][Green Version]
- Yi, F.; Zhang, A.Y.; Janscha, J.L.; Li, P.-L.; Zou, A.-P. Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int. 2004, 66, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Barsacchi, R.; Rotiroti, D.; Borgese, N.; Clementi, E. Activation of the endothelial nitric-oxide synthase by tumor necrosis factor-α: A novel feedback mechanism regulating cell death. J. Biol. Chem. 2001, 276, 6529–6536. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, J.; Thatte, H.S.; Prabhakar, P.; Golan, D.E.; Michel, T. Calcium-independent activation of endothelial nitric oxide synthase by ceramide. Proc. Natl. Acad. Sci. USA 1999, 96, 12583–12588. [Google Scholar] [CrossRef]
- Li, H.; Junk, P.; Huwiler, A.; Burkhardt, C.; Wallerath, T.; Pfeilschifter, J.; Förstermann, U. Dual effect of ceramide on human endothelial cells: Induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation 2002, 106, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.K.; Han, H.; Snowden, A.; Chatterjee, S. Redox-regulated signaling by lactosylceramide in the proliferation of human aortic smooth muscle cells. J. Biol. Chem. 1997, 272, 15642–15649. [Google Scholar] [CrossRef] [PubMed]
- Harada-Shiba, M.; Kinoshita, M.; Kamido, H.; Shimokado, K. Oxidized low density lipoprotein induces apoptosis in cultured human umbilical vein endothelial cells by common and unique mechanisms. J. Biol. Chem. 1998, 273, 9681–9687. [Google Scholar] [CrossRef]
- Ruuth, M.; Nguyen, S.D.; Vihervaara, T.; Hilvo, M.; Laajala, T.D.; Kondadi, P.K.; Gisterå, A.; Lähteenmäki, H.; Kittilä, T.; Huusko, J. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur. Heart J. 2018, 39, 2562–2573. [Google Scholar] [CrossRef]
- Morita, S.-Y.; Kawabe, M.; Sakurai, A.; Okuhira, K.; Vertut-Doï, A.; Nakano, M.; Handa, T. Ceramide in lipid particles enhances heparan sulfate proteoglycan and low density lipoprotein receptor-related protein-mediated uptake by macrophages. J. Biol. Chem. 2004, 279, 24355–24361. [Google Scholar] [CrossRef]
- Morita, S.-Y.; Nakano, M.; Sakurai, A.; Deharu, Y.; Vertut-Doï, A.; Handa, T. Formation of ceramide-enriched domains in lipid particles enhances the binding of apolipoprotein E. FEBS Lett. 2005, 579, 1759–1764. [Google Scholar] [CrossRef]
- Öörni, K.; Hakala, J.K.; Annila, A.; Ala-Korpela, M.; Kovanen, P.T. Sphingomyelinase induces aggregation and fusion, but phospholipase A2 only aggregation, of low density lipoprotein (LDL) particles: Two distinct mechanisms leading to increased binding strength of LDL to human aortic proteoglycans. J. Biol. Chem. 1998, 273, 29127–29134. [Google Scholar] [CrossRef]
- Zelnik, I.D.; Ventura, A.E.; Kim, J.L.; Silva, L.C.; Futerman, A.H. The role of ceramide in regulating endoplasmic reticulum function. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158489. [Google Scholar] [CrossRef] [PubMed]
- Sneck, M.; Nguyen, S.D.; Pihlajamaa, T.; Yohannes, G.; Riekkola, M.-L.; Milne, R.; Kovanen, P.T.; Öörni, K. Conformational changes of apoB-100 in SMase-modified LDL mediate formation of large aggregates at acidic pH [S]. J. Lipid Res. 2012, 53, 1832–1839. [Google Scholar] [CrossRef]
- Benitez-Amaro, A.; Pallara, C.; Nasarre, L.; Rivas-Urbina, A.; Benitez, S.; Vea, A.; Bornachea, O.; de Gonzalo-Calvo, D.; Serra-Mir, G.; Villegas, S. Molecular basis for the protective effects of low-density lipoprotein receptor-related protein 1 (LRP1)-derived peptides against LDL aggregation. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Hojjati, M.R.; Li, Z.; Zhou, H.; Tang, S.; Huan, C.; Ooi, E.; Lu, S.; Jiang, X.-C. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem. 2005, 280, 10284–10289. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.; Lucks, J.; Fuchs, S.; Schiffmann, S.; Schreiber, Y.; Ferreirós, N.; Merkens, J.; Marschalek, R.; Geisslinger, G.; Grösch, S. Long chain ceramides and very long chain ceramides have opposite effects on human breast and colon cancer cell growth. Int. J. Biochem. Cell Biol. 2012, 44, 620–628. [Google Scholar] [CrossRef]
- Lallemand, T.; Rouahi, M.; Swiader, A.; Grazide, M.-H.; Geoffre, N.; Alayrac, P.; Recazens, E.; Coste, A.; Salvayre, R.; Nègre-Salvayre, A. nSMase2 (type 2-neutral sphingomyelinase) deficiency or inhibition by GW4869 reduces inflammation and atherosclerosis in Apoe−/−mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1479–1492. [Google Scholar] [CrossRef]
- Law, B.A.; Liao, X.; Moore, K.S.; Southard, A.; Roddy, P.; Ji, R.; Szulc, Z.; Bielawska, A.; Schulze, P.C.; Cowart, L.A. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 2018, 32, 1403–1416. [Google Scholar] [CrossRef]
- Stunff, H.L.; Milstien, S.; Spiegel, S. Generation and metabolism of bioactive sphingosine-1-phosphate. J. Cell. Biochem. 2004, 92, 882–899. [Google Scholar] [CrossRef]
- Yatomi, Y.; Ruan, F.; Hakomori, S.-I.; Igarashi, Y. Sphingosine-1-phosphate: A platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood 1995, 86, 193–202. [Google Scholar] [CrossRef]
- Pappu, R.; Schwab, S.R.; Cornelissen, I.; Pereira, J.P.; Regard, J.B.; Xu, Y.; Camerer, E.; Zheng, Y.-W.; Huang, Y.; Cyster, J.G. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 2007, 316, 295–298. [Google Scholar] [CrossRef]
- Venkataraman, K.; Lee, Y.-M.; Michaud, J.; Thangada, S.; Ai, Y.; Bonkovsky, H.L.; Parikh, N.S.; Habrukowich, C.; Hla, T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008, 102, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Simmons, S.; Kawamura, S.; Inoue, A.; Orba, Y.; Tokudome, T.; Sunden, Y.; Arai, Y.; Moriwaki, K.; Ishida, J.; et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Investig. 2012, 122, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.; Sasaki, N.; Umemoto, E.; Uchida, Y.; Fukuhara, S.; Kitazawa, Y.; Okudaira, M.; Inoue, A.; Tohya, K.; Aoi, K.; et al. High-endothelial cell-derived S1P regulates dendritic cell localization and vascular integrity in the lymph node. Elife 2019, 8, e41239. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.M.; Ishizu, A.N.; Foo, J.C.; Toh, X.R.; Zhang, F.; Whee, D.M.; Torta, F.; Cazenave-Gassiot, A.; Matsumura, T.; Kim, S.; et al. Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature 2017, 550, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Kawasaki-Nishi, S.; Otsuka, M.; Hisano, Y.; Yamaguchi, A.; Nishi, T. MFSD2B is a sphingosine 1-phosphate transporter in erythroid cells. Sci. Rep. 2018, 8, 4969. [Google Scholar] [CrossRef]
- Yanagida, K.; Hla, T. Vascular and Immunobiology of the Circulatory Sphingosine 1-Phosphate Gradient. Annu. Rev. Physiol. 2017, 79, 67–91. [Google Scholar] [CrossRef]
- Deutschman, D.H.; Carstens, J.S.; Klepper, R.L.; Smith, W.S.; Page, M.T.; Young, T.R.; Gleason, L.A.; Nakajima, N.; Sabbadini, R.A. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am. Heart J. 2003, 146, 62–68. [Google Scholar] [CrossRef]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010, 9, 883–897. [Google Scholar] [CrossRef]
- Keul, P.; Sattler, K.; Levkau, B. HDL and its sphingosine-1-phosphate content in cardioprotection. Heart Fail. Rev. 2007, 12, 301–306. [Google Scholar] [CrossRef]
- Nofer, J.R.; Bot, M.; Brodde, M.; Taylor, P.J.; Salm, P.; Brinkmann, V.; van Berkel, T.; Assmann, G.; Biessen, E.A. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2007, 115, 501–508. [Google Scholar] [CrossRef]
- Xu, N.; Dahlbäck, B. A novel human apolipoprotein (apoM). J. Biol. Chem. 1999, 274, 31286–31290. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, C.; Obinata, H.; Kumaraswamy, S.B.; Galvani, S.; Ahnström, J.; Sevvana, M.; Egerer-Sieber, C.; Muller, Y.A.; Hla, T.; Nielsen, L.B. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 2011, 108, 9613–9618. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Yatomi, Y.; Ohta, M.; Osada, M.; Kazama, F.; Satoh, K.; Nakahara, K.; Ozaki, Y. Sphingosine 1-phosphate-related metabolism in the blood vessel. J. Biochem. 2005, 138, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, R.; Nakamura, K.; Okubo, S.; Hosogaya, S.; Ozaki, Y.; Tozuka, M.; Osima, N.; Yokota, H.; Ikeda, H.; Yatomi, Y. Plasma sphingosine-1-phosphate measurement in healthy subjects: Close correlation with red blood cell parameters. Ann. Clin. Biochem. 2008, 45, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tomura, H.; Kuwabara, A.; Kimura, T.; Miura, S.; Noda, K.; Okajima, F.; Saku, K. Correlation of high density lipoprotein (HDL)-associated sphingosine 1-phosphate with serum levels of HDL-cholesterol and apolipoproteins. Atherosclerosis 2005, 178, 199–205. [Google Scholar] [CrossRef]
- Levkau, B. HDL-S1P: Cardiovascular functions, disease-associated alterations, and therapeutic applications. Front. Pharmacol. 2015, 6, 243. [Google Scholar] [CrossRef]
- Kurano, M.; Tsukamoto, K.; Hara, M.; Ohkawa, R.; Ikeda, H.; Yatomi, Y. LDL receptor and ApoE are involved in the clearance of ApoM-associated sphingosine 1-phosphate. J. Biol. Chem. 2015, 290, 2477–2488. [Google Scholar] [CrossRef]
- Kappelle, P.J.; Ahnström, J.; Dikkeschei, B.D.; de Vries, R.; Sluiter, W.J.; Wolffenbuttel, B.H.; van Tol, A.; Nielsen, L.B.; Dahlbäck, B.; Dullaart, R.P. Plasma apolipoprotein M responses to statin and fibrate administration in type 2 diabetes mellitus. Atherosclerosis 2010, 213, 247–250. [Google Scholar] [CrossRef]
- Galvani, S.; Sanson, M.; Blaho, V.A.; Swendeman, S.L.; Obinata, H.; Conger, H.; Dahlbäck, B.; Kono, M.; Proia, R.L.; Smith, J.D. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 2015, 8, ra79. [Google Scholar] [CrossRef]
- Kurano, M.; Yatomi, Y. Sphingosine 1-phosphate and atherosclerosis. J. Atheroscler. Thromb. 2017, 25, 16–26. [Google Scholar] [CrossRef]
- Argraves, K.M.; Gazzolo, P.J.; Groh, E.M.; Wilkerson, B.A.; Matsuura, B.S.; Twal, W.O.; Hammad, S.M.; Argraves, W.S. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J. Biol. Chem. 2008, 283, 25074–25081. [Google Scholar] [CrossRef] [PubMed]
- Filep, J.G.; Földes-Filep, É.; Sirois, P. Nitric oxide modulates vascular permeability in the rat coronary circulation. Br. J. Pharmacol. 1993, 108, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Nofer, J.-R.; Van Der Giet, M.; Tölle, M.; Wolinska, I.; von Wnuck Lipinski, K.; Baba, H.A.; Tietge, U.J.; Gödecke, A.; Ishii, I.; Kleuser, B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P 3. J. Clin. Investig. 2004, 113, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Tomura, H.; Mogi, C.; Kuwabara, A.; Damirin, A.; Ishizuka, T.; Sekiguchi, A.; Ishiwara, M.; Im, D.-S.; Sato, K. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J. Biol. Chem. 2006, 281, 37457–37467. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Frej, C.; Holmér, A.; Guo, L.J.; Tran, S.; Dahlbäck, B. High-density lipoprotein–associated apolipoprotein M limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 118–129. [Google Scholar] [CrossRef]
- Theilmeier, G.; Schmidt, C.; Herrmann, J.R.; Keul, P.; Schäfers, M.; Herrgott, I.; Mersmann, J.; Larmann, J.; Hermann, S.; Stypmann, J.R. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 2006, 114, 1403–1409. [Google Scholar] [CrossRef]
- Keul, P.; Lucke, S.; von Wnuck Lipinski, K.; Bode, C.; Gräler, M.; Heusch, G.; Levkau, B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ. Res. 2011, 108, 314–323. [Google Scholar] [CrossRef]
- Tamama, K.; Tomura, H.; Sato, K.; Malchinkhuu, E.; Damirin, A.; Kimura, T.; Kuwabara, A.; Murakami, M.; Okajima, F. High-density lipoprotein inhibits migration of vascular smooth muscle cells through its sphingosine 1-phosphate component. Atherosclerosis 2005, 178, 19–23. [Google Scholar] [CrossRef]
- Duenas, A.I.; Aceves, M.; Fernández-Pisonero, I.; Gómez, C.; Orduna, A.; Crespo, M.S.; García-Rodríguez, C. Selective attenuation of Toll-like receptor 2 signalling may explain the atheroprotective effect of sphingosine 1-phosphate. Cardiovasc. Res. 2008, 79, 537–544. [Google Scholar] [CrossRef]
- Skoura, A.; Michaud, J.; Im, D.-S.; Thangada, S.; Xiong, Y.; Smith, J.D.; Hla, T. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 81–85. [Google Scholar] [CrossRef]
- Sanchez, T.; Skoura, A.; Wu, M.T.; Casserly, B.; Harrington, E.O.; Hla, T. Induction of vascular permeability by the sphingosine-1-phosphate receptor–2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, A.J.; Carney, P.R.; Zhu, W.J.; Chaves, A.H.; Garcia, J.; Grimes, J.R.; Anderson, K.J.; Roper, S.N.; Lee, N. An essential role for the H218/AGR16/Edg-5/LPB2 sphingosine 1-phosphate receptor in neuronal excitability. Eur. J. Neurosci. 2001, 14, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Cattoretti, G.; Mandelbaum, J.; Lee, N.; Chaves, A.H.; Mahler, A.M.; Chadburn, A.; Dalla-Favera, R.; Pasqualucci, L.; MacLennan, A.J. Targeted disruption of the S1P2 sphingosine 1-phosphate receptor gene leads to diffuse large B-cell lymphoma formation. Cancer Res. 2009, 69, 8686–8692. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A.; Suzuki, K.; Cho, B.; Willison, L.D.; Palmer, D.; Allen, C.D.; Schmidt, T.H.; Xu, Y.; Proia, R.L.; Coughlin, S.R. The sphingosine 1-phosphate receptor S1P 2 maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat. Immunol. 2011, 12, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Proia, R.L.; Olivera, A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008, 8, 753–763. [Google Scholar] [CrossRef]
- Terai, K.; Soga, T.; Takahashi, M.; Kamohara, M.; Ohno, K.; Yatsugi, S.; Okada, M.; Yamaguchi, T. Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience 2003, 116, 1053–1062. [Google Scholar] [CrossRef]
- Wang, W.; Graeler, M.H.; Goetzl, E.J. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J. 2005, 19, 1731–1733. [Google Scholar] [CrossRef]
- Slotte, P.J. Molecular properties of various structurally defined sphingomyelins–correlation of structure with function. Prog. Lipid Res. 2013, 52, 206–219. [Google Scholar] [CrossRef]
- Patton, S. Correlative relationship of cholesterol and sphingomyelin in cell membranes. J. Theor. Biol. 1970, 29, 489–491. [Google Scholar] [CrossRef]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef]
- Jeong, T.; Schissel, S.L.; Tabas, I.; Pownall, H.J.; Tall, A.R.; Jiang, X.-C. Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. J. Clin. Investig. 1998, 101, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Bojic, L.A.; McLaren, D.G.; Shah, V.; Previs, S.F.; Johns, D.G.; Castro-Perez, J.M. Lipidome of atherosclerotic plaques from hypercholesterolemic rabbits. Int. J. Mol. Sci. 2014, 15, 23283–23293. [Google Scholar] [CrossRef] [PubMed]
- Camont, L.; Lhomme, M.; Rached, F.; Le Goff, W.; Nègre-Salvayre, A.; Salvayre, R.; Calzada, C.; Lagarde, M.; Chapman, M.J.; Kontush, A. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: Relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2715–2723. [Google Scholar] [CrossRef]
- Mäkinen, V.-P.; Tynkkynen, T.; Soininen, P.; Forsblom, C.; Peltola, T.; Kangas, A.J.; Groop, P.-H.; Ala-Korpela, M. Sphingomyelin is associated with kidney disease in type 1 diabetes (The FinnDiane Study). Metabolomics 2012, 8, 369–375. [Google Scholar] [CrossRef]
- Adachi, R.; Ogawa, K.; Matsumoto, S.-i.; Satou, T.; Tanaka, Y.; Sakamoto, J.; Nakahata, T.; Okamoto, R.; Kamaura, M.; Kawamoto, T. Discovery and characterization of selective human sphingomyelin synthase 2 inhibitors. Eur. J. Med. Chem. 2017, 136, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Peng, Q.; Huang, Y. Potential therapeutic targets for atherosclerosis in sphingolipid metabolism. Clin. Sci. 2019, 133, 763–776. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Liu, J.; Li, Y.; Huan, C.; Bui, H.H.; Kuo, M.S.; Park, T.S.; Cao, G.; Jiang, X.C. Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arter. Thromb. Vasc. Biol. 2012, 32, 1577–1584. [Google Scholar] [CrossRef]
- Liu, J.; Huan, C.; Chakraborty, M.; Zhang, H.; Lu, D.; Kuo, M.-S.; Cao, G.; Jiang, X.-C. Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ. Res. 2009, 105, 295–303. [Google Scholar] [CrossRef]
- Wang, X.; Dong, J.; Zhao, Y.; Li, Y.; Wu, M. Adenovirus-mediated sphingomyelin synthase 2 increases atherosclerotic lesions in ApoE KO mice. Lipids Health Dis. 2011, 10, 1–5. [Google Scholar] [CrossRef]
- Hailemariam, T.K.; Huan, C.; Liu, J.; Li, Z.; Roman, C.; Kalbfeisch, M.; Bui, H.H.; Peake, D.A.; Kuo, M.-S.; Cao, G. Sphingomyelin synthase 2 deficiency attenuates NFκB activation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1519–1526. [Google Scholar] [CrossRef]
- Dong, J.; Liu, J.; Lou, B.; Li, Z.; Ye, X.; Wu, M.; Jiang, X.-C. Adenovirus-mediated overexpression of sphingomyelin synthases 1 and 2 increases the atherogenic potential in mice. J. Lipid Res. 2006, 47, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.H.; Takemoto, M.; Watanabe, K.; Luo, H.; Nishimura, M.; Yano, M.; Tomimoto, H.; Okazaki, T.; Oike, Y.; Song, W.J. Deficiency of sphingomyelin synthase-1 but not sphingomyelin synthase-2 causes hearing impairments in mice. J. Physiol. 2012, 590, 4029–4044. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Watanabe, K.; Yamamoto, T.; Ikeda, K.; Senokuchi, T.; Lu, M.; Kadomatsu, T.; Tsukano, H.; Ikawa, M.; Okabe, M. Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J. Biol. Chem. 2011, 286, 3992–4002. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Watanabe, K.; Itoh, M.; Huan, C.-R.; Tong, X.-P.; Nakamura, T.; Miki, M.; Iwao, H.; Nakajima, A.; Sakai, T. CD4+ T-cell dysfunctions through the impaired lipid rafts ameliorate concanavalin A-induced hepatitis in sphingomyelin synthase 1-knockout mice. Int. Immunol. 2012, 24, 327–337. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ghosh, N. Oxidized low density lipoprotein stimulates aortic smooth muscle cell proliferation. Glycobiology 1996, 6, 303–311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chatterjee, S.; Bedja, D.; Mishra, S.; Amuzie, C.; Avolio, A.; Kass, D.A.; Berkowitz, D.; Renehan, M. Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in apolipoprotein E−/−mice and rabbits fed a high-fat and-cholesterol diet. Circulation 2014, 129, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Bhunia, A.K.; Chatterjee, S.; Bulkley, G.B. Lactosylceramide stimulates human neutrophils to upregulate Mac-1, adhere to endothelium, and generate reactive oxygen metabolites in vitro. Circ. Res. 1998, 82, 540–547. [Google Scholar] [CrossRef]
- Bhunia, A.K.; Han, H.; Snowden, A.; Chatterjee, S. Lactosylceramide Stimulates Ras-GTP Loading, Kinases (MEK, Raf), p44 Mitogen-activated Protein Kinase, and c-fos Expression in Human Aortic Smooth Muscle Cells (*). J. Biol. Chem. 1996, 271, 10660–10666. [Google Scholar] [CrossRef]
- Chatterjee, S.B.; Dey, S.; Shi, W.Y.; Thomas, K.; Hutchins, G.M. Accumulation of glycosphingolipids in human atherosclerotic plaque and unaffected aorta tissues. Glycobiology 1997, 7, 57–65. [Google Scholar] [CrossRef]
- Chatterjee, S. Lactosylceramide stimulates aortic smooth muscle cell proliferation. Biochem. Biophys. Res. Commun. 1991, 181, 554–561. [Google Scholar] [CrossRef]
- Chatterjee, S. Sphingolipids in atherosclerosis and vascular biology. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, M.; Yu, Z.-X.; Ferrans, V.J.; Irani, K.; Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 1995, 270, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Bietrix, F.; Lombardo, E.; van Roomen, C.P.; Ottenhoff, R.; Vos, M.; Rensen, P.C.; Verhoeven, A.J.; Aerts, J.M.; Groen, A.K. Inhibition of glycosphingolipid synthesis induces a profound reduction of plasma cholesterol and inhibits atherosclerosis development in APOE* 3 Leiden and low-density lipoprotein receptor−/−mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.K.; Arai, T.; Bulkley, G.; Chatterjee, S. Lactosylceramide mediates tumor necrosis factor-α-induced intercellular adhesion molecule-1 (ICAM-1) expression and the adhesion of neutrophil in human umbilical vein endothelial cells. J. Biol. Chem. 1998, 273, 34349–34357. [Google Scholar] [CrossRef] [PubMed]
| Sphingolipid | Associated Mechanism | Effect on Atherosclerosis | References |
|---|---|---|---|
| Dihydroceramide | ↑ Autophagy ↑ Oxidative stress ↑ Inflammatory cytokines ↑ Cell proliferation ↑ Plaque instability | Progressive | [59,63,64,65,66,67,69,71,72,73] |
| Cer Long-chain Very long-chain | ↑ Inflammation ↑ Proliferation ↑ LDL-Aggregation ↓ Cell proliferation ↑ Apoptosis ↑ Cell proliferation | Progressive Protective Progressive | [28,29,30,32,59,76,90,100,101,102,128,130] |
| S1P S1PR1 S1PR2 S1PR3 S1P/ApoM S1P/Albumin | ↑ Endothelial barrier function ↓ Apoptosis ↑ Chemotaxis of lymphocytes and NK cells ↓ ICAM1 and VCAM1 expression ↓ Barrier function ↑ Recruitment of inflam. macrophages ↑ Plaque and necrotic core formation ↓ SMC migration ↑ Endothelial barrier function ↑ Monocyte recruitment ↓ Thrombus formation ↓ Inflammation ↓ Apoptosis Not shown | Protective Progressive Progressive Protective Progressive Protective Protective Progressive Protective Protective + progressive | [59,139,141,143,145,149,150,152,153,156,157,158,160,161,162,163,164] |
| Sphingomyelin | ↑ Hypercholesterolemia ↑ Apoptosis ↑ Inflammatory cytokines ↑ Thrombus formation ↑ Plaque instability ↑ Atherosclerotic lesions ↑ Macrophage content in lesions | Progressive | [59,174,175,176,177,180,181,182,183,184] |
| Lactosylceramide | ↑ TNFα-induced NFκB expression ↑ ICAM-1 expression ↑ MAC1 expression ↑ Arterial stiffness ↑ Aortic wall thickening ↑ Presence of aortic Ca2+ deposits ↑ Apoptosis ↑ Inflammatory cytokines | Progressive | [59,118,189,190,191,192,193,197] |
| Glucosylceramide | ↑ Arterial stiffness ↑ Aortic wall thickening ↑ Presence of aortic Ca2+ deposits ↑ plaque development ↑ cholesterol level liver ↑ Apoptosis ↑ Inflammatory cytokines | Progressive | [1,2,5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, L.; Kuebler, W.M.; Simmons, S. Sphingolipids in Atherosclerosis: Chimeras in Structure and Function. Int. J. Mol. Sci. 2022, 23, 11948. https://doi.org/10.3390/ijms231911948
Peters L, Kuebler WM, Simmons S. Sphingolipids in Atherosclerosis: Chimeras in Structure and Function. International Journal of Molecular Sciences. 2022; 23(19):11948. https://doi.org/10.3390/ijms231911948
Chicago/Turabian StylePeters, Lisa, Wolfgang M. Kuebler, and Szandor Simmons. 2022. "Sphingolipids in Atherosclerosis: Chimeras in Structure and Function" International Journal of Molecular Sciences 23, no. 19: 11948. https://doi.org/10.3390/ijms231911948
APA StylePeters, L., Kuebler, W. M., & Simmons, S. (2022). Sphingolipids in Atherosclerosis: Chimeras in Structure and Function. International Journal of Molecular Sciences, 23(19), 11948. https://doi.org/10.3390/ijms231911948





