Selective Calpain Inhibition Improves Functional and Histopathological Outcomes in a Canine Spinal Cord Injury Model
Abstract
1. Introduction
2. Results
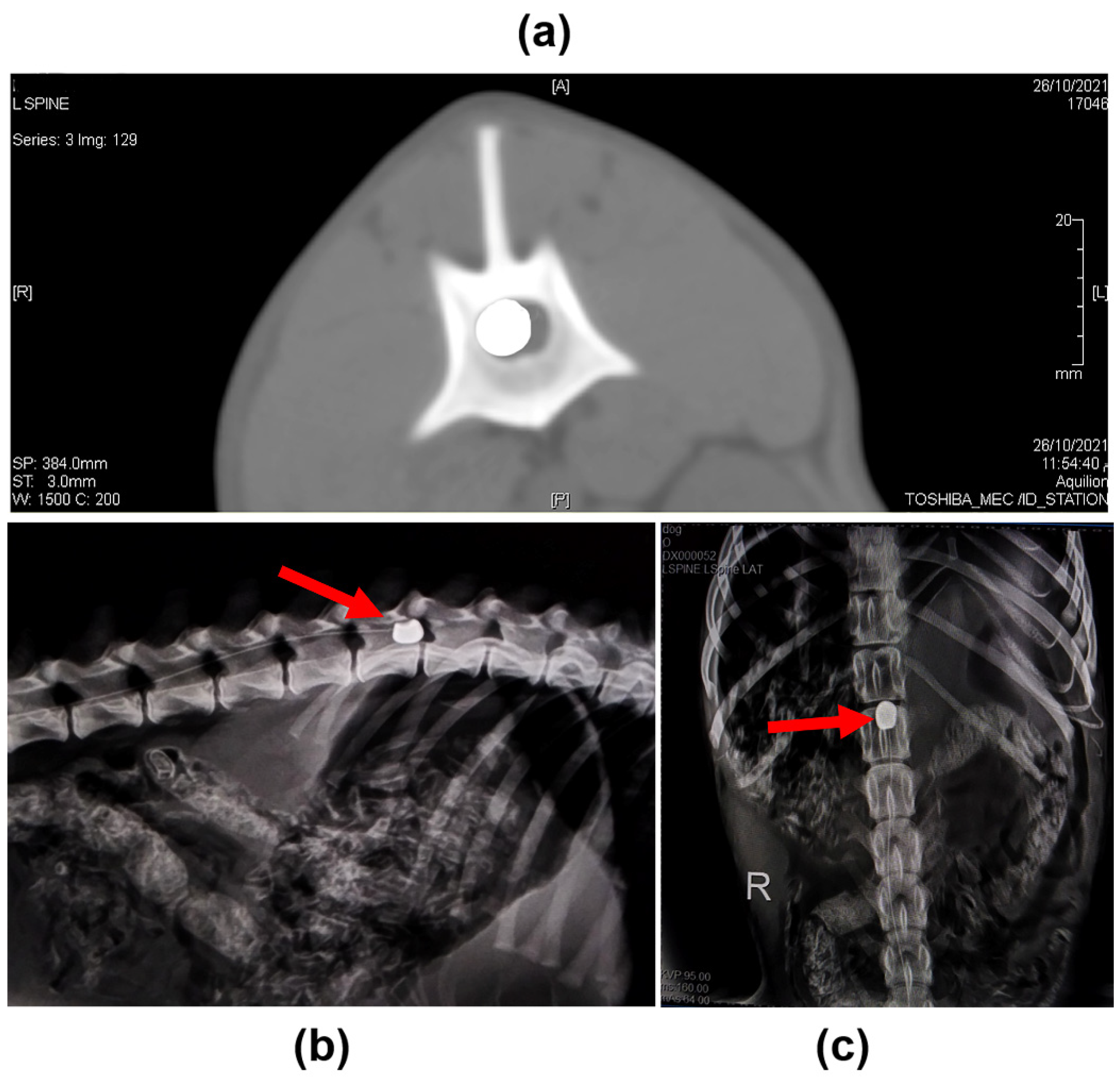
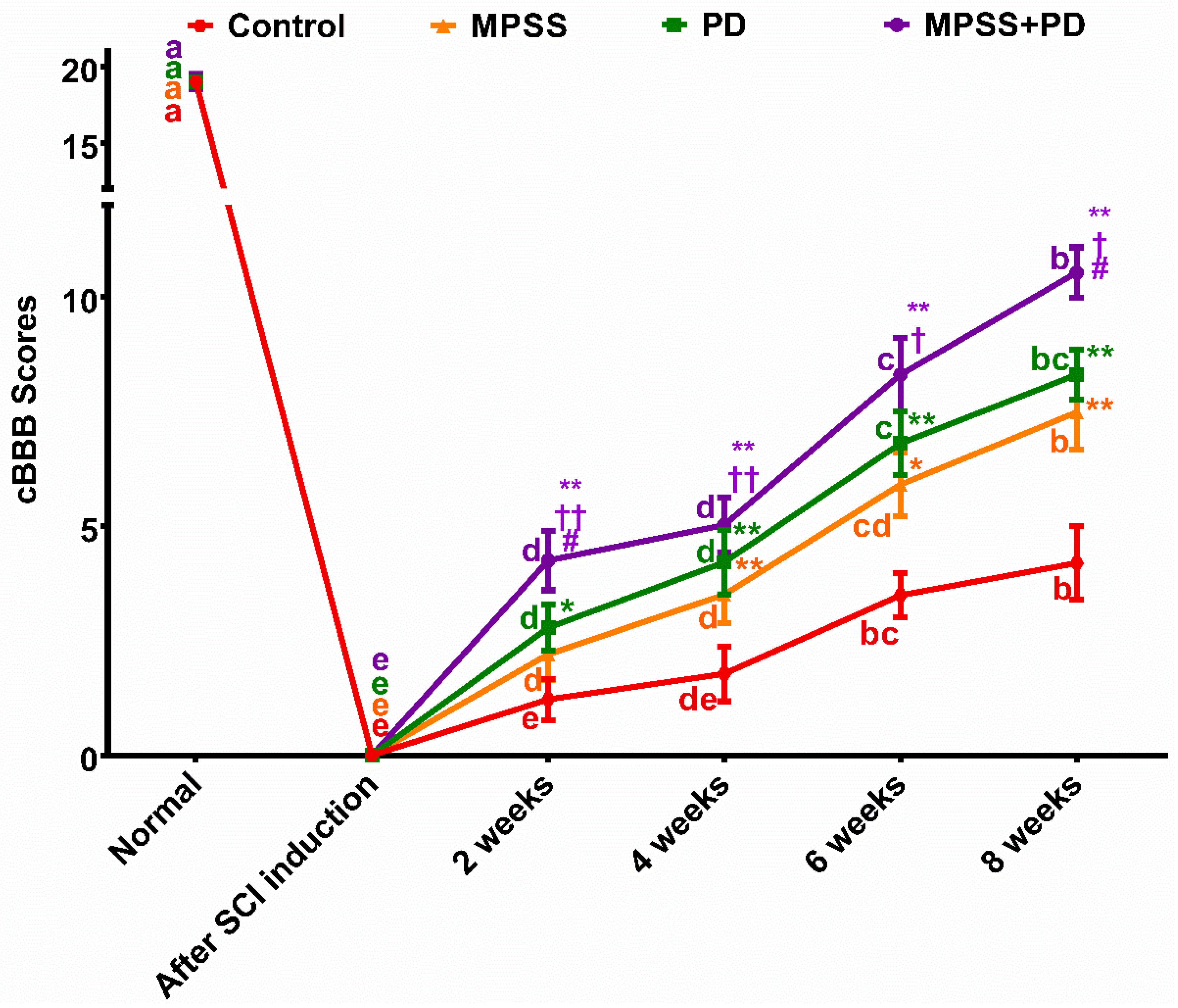
2.1. Clinical Evaluation
2.2. Serum Biochemical Analysis
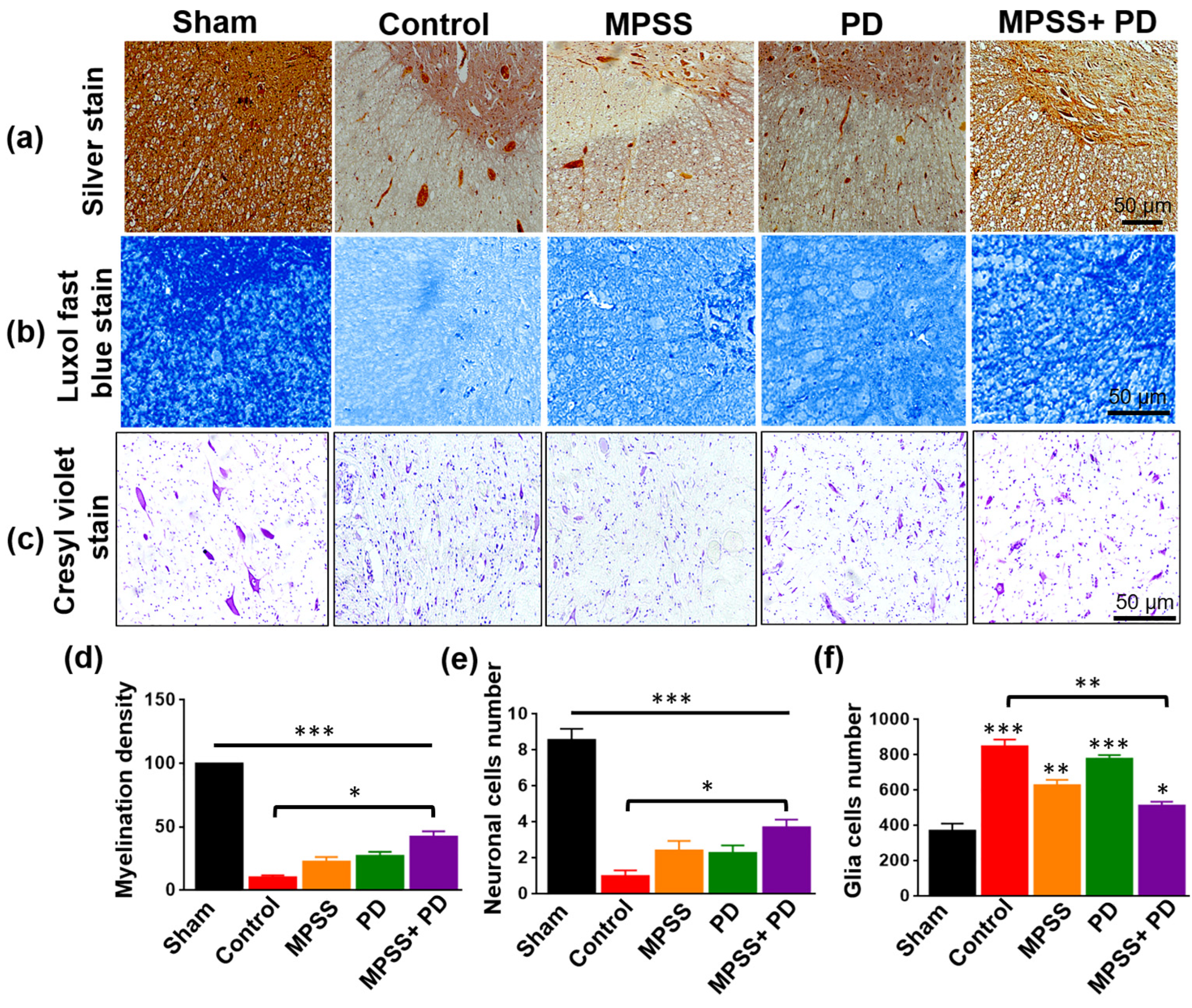
2.3. Histopathological Evaluation of the Therapeutic Efficacy of MPSS and the Calpain Inhibitor PD150606 following SCI
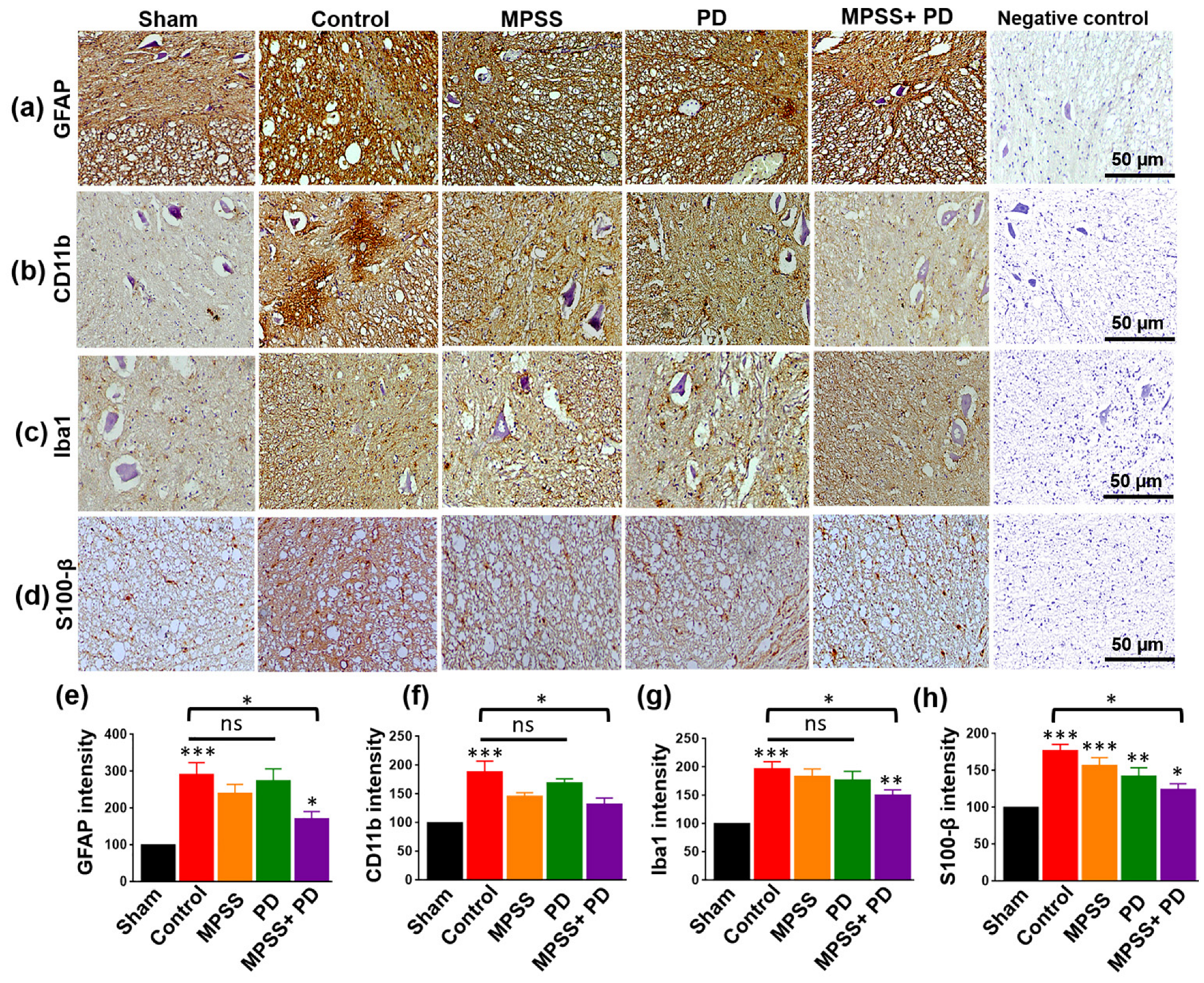
2.4. Assessment of Glial Inflammatory Markers and Calcium Binding Proteins
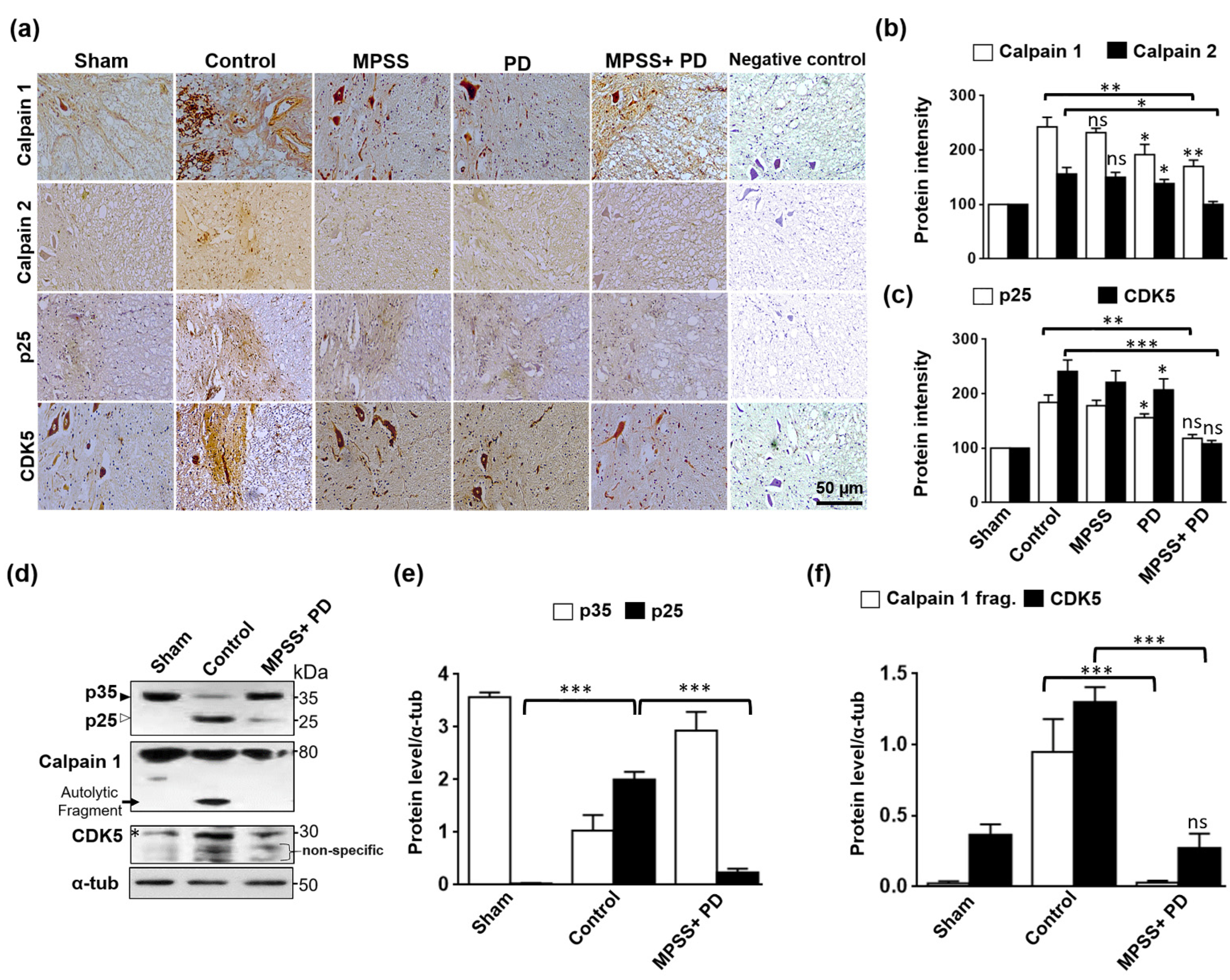
2.5. Inhibition of Calpain Abolishes the Generation of p25 and, Consequently, the CDK5 Activation in SCI
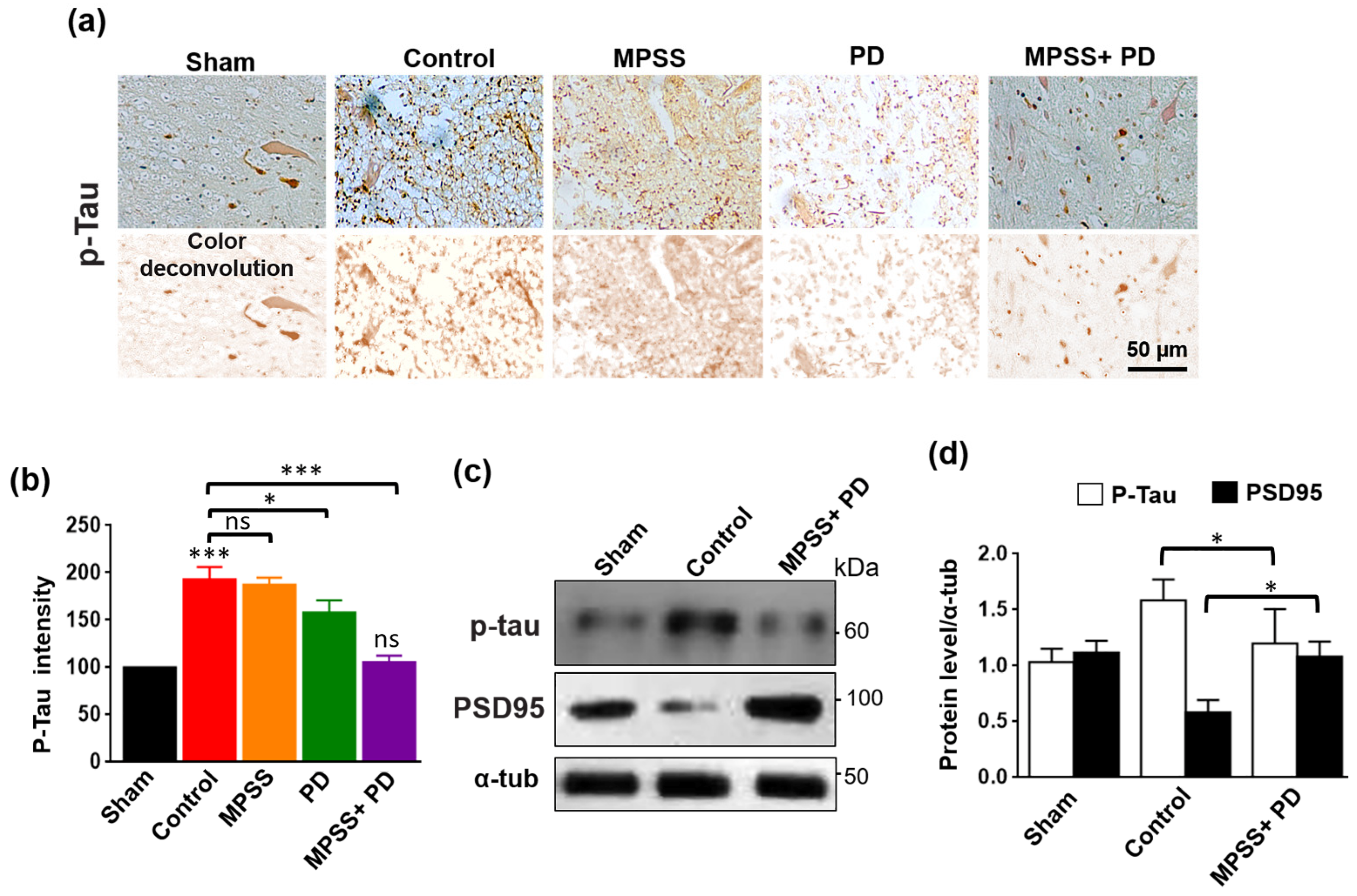
2.6. Inhibition of Calpains Abolishes the Increase in Tau Hyperphosphorylation after SCI
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Induction of SCI
4.3. Postoperative Management and Administration of MPSS and Calpain Inhibitor
4.4. Postoperative Clinical Follow-Up
4.5. Serum Biochemical Analysis
4.6. Somatosensory Evoked Potential (SSEP)
4.7. Histopathological and Immunohistochemical (IHC) Analysis
4.8. Western Blotting Analysis
4.9. Imaging
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Min, J.; Kim, J.Y.; Seo, C.H.; Jeon, S.R.; Choi, K.H.; Jeong, J.H. Changes of the Electrophysiological Study in Dogs with Acute Spinal Cord Injury. Korean J. Neurotrauma 2014, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H. Neuroplasticity of spinal cord injury and repair. In Handbook of Clinical Neurology; Quartarone, A., Ghilardi, M., Boller, F., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2022; Volume 2, pp. 317–330. [Google Scholar] [CrossRef]
- Park, S.S.; Lee, Y.J.; Lee, S.H.; Lee, D.; Choi, K.; Kim, W.H.; Kweon, O.K.; Han, H.J. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy 2012, 14, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Murgoci, A.N.; Cubinkova, V.; Humenik, F.; Mojzisova, Z.; Maloveska, M.; Cizek, M.; Fournier, I.; Salzet, M. Spinal Cord Injury: Animal Models, Imaging Tools and the Treatment Strategies. Neurochem. Res. 2020, 45, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Nakae, A.; Nakai, K.; Yano, K.; Hosokawa, K.; Shibata, M.; Mashimo, T. The animal model of spinal cord injury as an experimental pain model. J. Biomed. Biotechnol. 2011, 2011, 939023. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef] [PubMed]
- Van Sandt, R.L.; Welsh, C.J.; Jeffery, N.D.; Young, C.R.; McCreedy, D.A.; Wright, G.A.; Boudreau, C.E.; Levine, G.J.; Levine, J.M. Circulating neutrophil activation in dogs with naturally occurring spinal cord injury secondary to intervertebral disk herniation. Am. J. Vet. Res. 2022, 83, 324–330. [Google Scholar] [CrossRef]
- Nakamoto, Y.; Tsujimoto, G.; Ikemoto, A.; Omori, K.; Nakamura, T. Pathological changes within two weeks following spinal cord injury in a canine model. Eur. Spine J. 2021, 30, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Yoon, Y.; Kim, A.; Jo, K.R.; Choi, K.U.; Jung, T.; Kim, N.; Son, Y.; Kim, W.H.; Kweon, O.K. Improved Healing after the Co-Transplantation of HO-1 and BDNF Overexpressed Mesenchymal Stem Cells in the Subacute Spinal Cord Injury of Dogs. Cell Transplant 2018, 27, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; He, T.; Tang, W.; Li, H.; Wang, C.; Zheng, M.; Hu, J.; Song, X.; Ding, Y.; Chen, Y.Y.; et al. Local application of MDL28170-loaded PCL film improves functional recovery by preserving survival of motor neurons after traumatic spinal cord injury. Neurosci. Lett. 2019, 694, 161–167. [Google Scholar] [CrossRef]
- Kim, W.K.; Kim, W.H.; Kweon, O.K.; Kang, B.J. Heat-Shock Proteins Can Potentiate the Therapeutic Ability of Cryopreserved Mesenchymal Stem Cells for the Treatment of Acute Spinal Cord Injury in Dogs. Stem Cell Rev. 2022, 18, 1461–1477. [Google Scholar] [CrossRef] [PubMed]
- Song, R.B.; Basso, D.M.; da Costa, R.C.; Fisher, L.C.; Mo, X.; Moore, S.A. Adaptation of the Basso-Beattie-Bresnahan locomotor rating scale for use in a clinical model of spinal cord injury in dogs. J. Neurosci. Methods 2016, 268, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhuang, X.; Chen, Y.; Niu, Z.; Xia, H. Plasma Erythropoietin, IL-17A, and IFNγ as Potential Biomarkers of Motor Function Recovery in a Canine Model of Spinal Cord Injury. J. Mol. Neurosci. 2020, 70, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Laverty, P.H.; Leskovar, A.; Breur, G.J.; Coates, J.R.; Bergman, R.L.; Widmer, W.R.; Toombs, J.P.; Shapiro, S.; Borgens, R.B. A preliminary study of intravenous surfactants in paraplegic dogs: Polymer therapy in canine clinical SCI. J. Neurotrauma 2004, 21, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, L. Comparison of minipig, dog, monkey and human drug metabolism and disposition. J. Pharmacol. Toxicol. Methods 2015, 74, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jo, S.H.; Kim, W.H.; Kweon, O.K. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res. Ther. 2015, 6, 229. [Google Scholar] [CrossRef]
- Metwally, E.; Zhao, G.; Zhang, Y.Q. The calcium-dependent protease calpain in neuronal remodeling and neurodegeneration. Trends Neurosci. 2021, 44, 741–752. [Google Scholar] [CrossRef]
- Ono, Y.; Saido, T.C.; Sorimachi, H. Calpain research for drug discovery: Challenges and potential. Nat. Rev. Drug Discov. 2016, 15, 854–876. [Google Scholar] [CrossRef]
- Yu, C.G.; Bondada, V.; Joshi, A.; Reneer, D.V.; Telling, G.C.; Saatman, K.E.; Geddes, J.W. Calpastatin Overexpression Protects against Excitotoxic Hippocampal Injury and Traumatic Spinal Cord Injury. J. Neurotrauma 2020, 37, 2268–2276. [Google Scholar] [CrossRef]
- Li, Y.; Gong, Z.H.; Sheng, L.; Gong, Y.T.; Tan, X.Y.; Li, W.M.; Dong, D.L.; Yang, B.F.; Fu, S.B.; Xue, H.J. Anti-apoptotic effects of a calpain inhibitor on cardiomyocytes in a canine rapid atrial fibrillation model. Cardiovasc. Drugs Ther. 2009, 23, 361–368. [Google Scholar] [CrossRef]
- Kimura, T.; Ishiguro, K.; Hisanaga, S. Physiological and pathological phosphorylation of tau by Cdk5. Front. Mol. Neurosci. 2014, 7, 65. [Google Scholar] [CrossRef]
- Hung, K.S.; Hwang, S.L.; Liang, C.L.; Chen, Y.J.; Lee, T.H.; Liu, J.K.; Howng, S.L.; Wang, C.H. Calpain inhibitor inhibits p35-p25-Cdk5 activation, decreases tau hyperphosphorylation, and improves neurological function after spinal cord hemisection in rats. J. Neuropathol. Exp. Neurol. 2005, 64, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Baltin, M.E.; Sabirova, D.E.; Kiseleva, E.I.; Kamalov, M.I.; Abdullin, T.I.; Petrova, N.V.; Ahmetov, N.F.; Sachenkov, O.A.; Baltina, T.V.; Lavrov, I.A. Comparison of systemic and localized carrier-mediated delivery of methylprednisolone succinate for treatment of acute spinal cord injury. Exp. Brain Res. 2021, 239, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Burks, S.S.; Al-Khayat, H.; Levi, A.D. The effect of steroids on the incidence of gastrointestinal hemorrhage after spinal cord injury: A case-controlled study. Spinal Cord. 2014, 52, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Hurlbert, R.J. Methylprednisolone for the treatment of acute spinal cord injury: Point. Neurosurgery 2014, 61 (Suppl. 1), 32–35. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Liu, S.; Yi, Z.; Liu, H.; Li, J.; Shi, J.; Ji, L.; Xu, B.; Zhang, X.; Zhang, W. S100-β aggravates spinal cord injury via activation of M1 macrophage phenotype. J. Musculoskelet. Neuronal Interact. 2021, 21, 401–412. [Google Scholar] [PubMed]
- Mandwie, M.; Piper, J.A.; Gorrie, C.A.; Keay, K.A.; Musumeci, G.; Al-Badri, G.; Castorina, A. Rapid GFAP and Iba1 expression changes in the female rat brain following spinal cord injury. Neural Regen. Res. 2022, 17, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.M.; Kim, W.; Chung, J.Y.; Im, W.; Yoo, D.Y.; Jung, H.Y.; Won, M.H.; Choi, J.H.; Hwang, I.K. Neuroprotective Effects of Adipose-Derived Stem Cells Are Maintained for 3 Weeks against Ischemic Damage in the Rabbit Spinal Cord. BioMed Res. Int. 2014, 2014, 539051. [Google Scholar] [CrossRef]
- Sribnick, E.A.; Matzelle, D.D.; Banik, N.L.; Ray, S.K. Direct evidence for calpain involvement in apoptotic death of neurons in spinal cord injury in rats and neuroprotection with calpain inhibitor. Neurochem. Res. 2007, 32, 2210–2216. [Google Scholar] [CrossRef]
- Lee, M.S.; Kwon, Y.T.; Li, M.; Peng, J.; Friedlander, R.M.; Tsai, L.H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 2000, 405, 360–364. [Google Scholar] [CrossRef]
- Kurbatskaya, K.; Phillips, E.C.; Croft, C.L.; Dentoni, G.; Hughes, M.M.; Wade, M.A.; Al-Sarraj, S.; Troakes, C.; O’Neill, M.J.; Perez-Nievas, B.G.; et al. Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer’s disease brain. Acta Neuropathol. Commun. 2016, 4, 34. [Google Scholar] [CrossRef]
- Caprelli, M.T.; Mothe, A.J.; Tator, C.H. Hyperphosphorylated Tau as a Novel Biomarker for Traumatic Axonal Injury in the Spinal Cord. J. Neurotrauma. 2018, 35, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Tanaka, H.; Kitamura, M.; Inaba, T.; Nakayama, M. Methylprednisolone sodium succinate reduces spinal cord swelling but does not affect recovery of dogs with surgically treated thoracolumbar intervertebral disk herniation. Jpn. J. Vet. Res. 2016, 64, 191–196. [Google Scholar] [PubMed]
- Yang, J.W.; Jeong, S.M.; Seo, K.M.; Nam, T.C. Effects of corticosteroid and electroacupuncture on experimental spinal cord injury in dogs. J. Vet. Sci. 2003, 4, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Cheriyan, T.; Ryan, D.J.; Weinreb, J.H.; Cheriyan, J.; Paul, J.C.; Lafage, V.; Kirsch, T.; Errico, T.J. Spinal cord injury models: A review. Spinal Cord. 2014, 52, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, C.B.; Chung, D.J.; Kang, E.H.; Chang, H.S.; Hwang, S.H.; Han, H.; Choe, B.Y.; Sur, J.H.; Lee, S.Y.; et al. Development of an improved canine model of percutaneous spinal cord compression injury by balloon catheter. J. Neurosci. Methods 2008, 167, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Nakamura, T.; Kishigami, Y.; Endo, K.; Azuma, T.; Fujikawa, T.; Tsutsumi, S.; Shimizu, Y. New canine spinal cord injury model free from laminectomy. Brain Res. Brain Res. Protoc. 2005, 14, 171–180. [Google Scholar] [CrossRef]
- Ryu, H.H.; Lim, J.H.; Byeon, Y.E.; Park, J.R.; Seo, M.S.; Lee, Y.W.; Kim, W.H.; Kang, K.S.; Kweon, O.K. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J. Vet. Sci. 2009, 10, 273–284. [Google Scholar] [CrossRef]
- Purdy, P.D.; Duong, R.T.; White, C.L., III; Baer, D.L.; Reichard, R.R.; Pride, G.L., Jr.; Adams, C.; Miller, S.; Hladik, C.L.; Yetkin, Z. Percutaneous translumbar spinal cord compression injury in a dog model that uses angioplasty balloons: MR imaging and histopathologic findings. Am. J. Neuroradiol. 2003, 24, 177–184. [Google Scholar]
- Purdy, P.D.; White, C.L., III; Baer, D.L.; Frawley, W.H.; Reichard, R.R.; Pride, G.L., Jr.; Adams, C.; Miller, S.; Hladik, C.L.; Yetkin, Z. Percutaneous translumbar spinal cord compression injury in dogs from an angioplasty balloon: MR and histopathologic changes with balloon sizes and compression times. Am. J. Neuroradiol. 2004, 25, 1435–1442. [Google Scholar]
- Haldipur, N.; Tan, P.; Katory, M.; Singh, S. A safe method of retrograde passage of fogarty embolectomy catheter through difficult iliac arteries. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 559–561. [Google Scholar] [CrossRef][Green Version]
- Molano Mdel, R.; Broton, J.G.; Bean, J.A.; Calancie, B. Complications associated with the prophylactic use of methylprednisolone during surgical stabilization after spinal cord injury. J. Neurosurg. 2002, 96 (Suppl. 3), 267–272. [Google Scholar] [CrossRef] [PubMed]
- Plantier, V.; Sanchez-Brualla, I.; Dingu, N.; Brocard, C.; Liabeuf, S.; Gackière, F.; Brocard, F. Calpain fosters the hyperexcitability of motoneurons after spinal cord injury and leads to spasticity. eLife 2019, 8, e51404. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Hogan, E.L.; Banik, N.L. Calpain in the pathophysiology of spinal cord injury: Neuroprotection with calpain inhibitors. Brain Res. Brain Res. Rev. 2003, 42, 169–185. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Wang, J.A.; Bosken, J.M.; Singh, I.N. Lipid peroxidation in brain or spinal cord mitochondria after injury. J. Bioenerg. Biomembr. 2016, 48, 169–174. [Google Scholar] [CrossRef]
- Dimitrijevic, M.R.; Danner, S.M.; Mayr, W. Neurocontrol of Movement in Humans With Spinal Cord Injury. Artif. Organs. 2015, 39, 823–833. [Google Scholar] [CrossRef]
- Park, E.H.; White, G.A.; Tieber, L.M. Mechanisms of injury and emergency care of acute spinal cord injury in dogs and cats. J. Vet. Emerg. Crit. Care 2012, 22, 160–178. [Google Scholar] [CrossRef]
- Banik, N.L.; Matzelle, D.; Terry, E.; Hogan, E.L. A new mechanism of methylprednisolone and other corticosteroids action demonstrated in vitro: Inhibition of a proteinase (calpain) prevents myelin and cytoskeletal protein degradation. Brain Res. 1997, 748, 205–210. [Google Scholar] [CrossRef]
- Ray, S.K.; Wilford, G.G.; Matzelle, D.C.; Hogan, E.L.; Banik, N.L. Calpeptin and methylprednisolone inhibit apoptosis in rat spinal cord injury. Ann. N. Y. Acad. Sci. 1999, 890, 261–269. [Google Scholar] [CrossRef]
- Mills, C.D.; Xu, G.Y.; McAdoo, D.J.; Hulsebosch, C.E. Involvement of metabotropic glutamate receptors in excitatory amino acid and GABA release following spinal cord injury in rat. J Neurochem. 2001, 79, 835–848. [Google Scholar] [CrossRef]
- Wang, C.; Shi, D.; Song, X.; Chen, Y.; Wang, L.; Zhang, X. Calpain inhibitor attenuates ER stress-induced apoptosis in injured spinal cord after bone mesenchymal stem cells transplantation. Neurochem. Int. 2016, 97, 15–25. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Z.; Dai, H.; Wei, L.; Sun, S.; Gao, F. Therapeutic Efficacy of E-64-d, a Selective Calpain Inhibitor, in Experimental Acute Spinal Cord Injury. Biomed. Res. Int. 2015, 2015, 134242. [Google Scholar] [CrossRef][Green Version]
- Wang, K.K.; Nath, R.; Posner, A.; Raser, K.J.; Buroker-Kilgore, M.; Hajimohammadreza, I.; Probert, A.W.; Marcoux, F.W.; Ye, Q.; Takano, E.; et al. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc. Natl. Acad. Sci. USA 1996, 93, 6687–6692. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell. 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Vidal, P.M.; Lemmens, E.; Geboes, L.; Vangansewinkel, T.; Nelissen, S.; Hendrix, S. Late blocking of peripheral TNF-α is ineffective after spinal cord injury in mice. Immunobiology 2013, 218, 281–284. [Google Scholar] [CrossRef]
- Milatovic, D.; Zaja-Milatovic, S.; Brockett, M.M.; Breyer, R.M.; Aschner, M.; Montine, T.J. Neuroinflammation and oxidative injury in developmental neurotoxicity. In Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2022; pp. 1129–1140. [Google Scholar] [CrossRef]
- Yu, L.; Qian, J. Dihydrotanshinone I Alleviates Spinal Cord Injury via Suppressing Inflammatory Response, Oxidative Stress and Apoptosis in Rats. Med. Sci. Monit. 2020, 26, e920738. [Google Scholar] [CrossRef] [PubMed]
- Spitzbarth, I.; Bock, P.; Haist, V.; Stein, V.M.; Tipold, A.; Wewetzer, K.; Baumgärtner, W.; Beineke, A. Prominent microglial activation in the early proinflammatory immune response in naturally occurring canine spinal cord injury. J. Neuropathol. Exp. Neurol. 2011, 70, 703–714. [Google Scholar] [CrossRef]
- Pusterla, N.; Wilson, W.D.; Conrad, P.A.; Mapes, S.; Leutenegger, C.M. Comparative analysis of cytokine gene expression in cerebrospinal fluid of horses without neurologic signs or with selected neurologic disorders. Am. J. Vet. Res. 2006, 67, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Shohami, E.; Beit-Yannai, E.; Horowitz, M.; Kohen, R. Oxidative stress in closed-head injury: Brain antioxidant capacity as an indicator of functional outcome. J. Cereb. Blood Flow Metab. 1997, 17, 1007–1019. [Google Scholar] [CrossRef]
- Naik, A.K.; Tandan, S.K.; Dudhgaonkar, S.P.; Jadhav, S.H.; Kataria, M.; Prakash, V.R.; Kumar, D. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur. J. Pain. 2006, 10, 573–579. [Google Scholar] [CrossRef]
- Morsy, M.D.; Mostafa, O.A.; Hassan, W.N. A potential protective effect of alpha-tocopherol on vascular complication in spinal cord reperfusion injury in rats. J. Biomed. Sci. 2010, 17, 55. [Google Scholar] [CrossRef]
- Marquis, A.; Packer, R.A.; Borgens, R.B.; Duerstock, B.S. Increase in oxidative stress biomarkers in dogs with ascending-descending myelomalacia following spinal cord injury. J. Neurol. Sci. 2015, 353, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Liu, H.; Li, S.; Chen, L.; Yao, J. Antioxidative and Antiapoptotic Effects of Delta-Opioid Peptide [D-Ala(2), D-Leu(5)] Enkephalin on Spinal Cord Ischemia-Reperfusion Injury in Rabbits. Front. Neurosci. 2017, 11, 603. [Google Scholar] [CrossRef]
- Zirak, A.; Soleimani, M.; Jameie, S.B.; Abdollahifar, M.A.; Fadaei Fathabadi, F.; Hassanzadeh, S.; Esmaeilzadeh, E.; Farjoo, M.H.; Norouzian, M. Related Fluoxetine and Methylprednisolone Changes of TNF-α and IL-6 Expression in The Hypothyroidism Rat Model of Spinal Cord Injury. Cell J. 2021, 23, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Can, M.; Gul, S.; Bektas, S.; Hanci, V.; Acikgoz, S. Effects of dexmedetomidine or methylprednisolone on inflammatory responses in spinal cord injury. Acta Anaesthesiol. Scand. 2009, 53, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Tseng, F.Y.; Tan, C.T.; Lin-Shiau, S.Y.; Hsu, C.J. Effects of methylprednisolone on nitric oxide formation and survival of facial motor neurons after axotomy. Brain Res. 2008, 1197, 23–31. [Google Scholar] [CrossRef]
- Cavus, G.; Altas, M.; Aras, M.; Ozgür, T.; Serarslan, Y.; Yilmaz, N.; Sefil, F.; Ulutas, K.T. Effects of montelukast and methylprednisolone on experimental spinal cord injury in rats. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1770–1777. [Google Scholar] [PubMed]
- Keles, I.; Bozkurt, M.F.; Aglamis, E.; Fidan, A.F.; Ceylan, C.; Karalar, M.; Coban, S.; Denk, B.; Buyukokuroglu, M.E. Protective effects of dantrolene and methylprednisolone against spinal cord injury-induced early oxidative damage in rabbit bladder: A comparative experimental study. Adv. Clin. Exp. Med. 2019, 28, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yan, Z.; Minshall, R.D.; Schwartz, D.E.; Chen, Y.; Hu, G. Activation of calpains mediates early lung neutrophilic inflammation in ventilator-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L370–L379. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Zheng, D.; Xiong, S.; Hill, D.J.; Sun, T.; Gardiner, R.B.; Fan, G.C.; Lu, Y.; Abel, E.D.; Greer, P.A.; et al. Mitochondrial Calpain-1 Disrupts ATP Synthase and Induces Superoxide Generation in Type 1 Diabetic Hearts: A Novel Mechanism Contributing to Diabetic Cardiomyopathy. Diabetes 2016, 65, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Wang, S.K.; Yao, P.W.; Liao, G.J.; Na, X.D.; Li, Y.Y.; Zeng, W.A.; Liu, X.G.; Zang, Y. Early CALP2 expression and microglial activation are potential inducers of spinal IL-6 up-regulation and bilateral pain following motor nerve injury. J. Neurochem. 2018, 145, 154–169. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Dogs and Cats; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Clarke, K.W.; Trim, C.M.; Hall, L.W. Anaesthesia of the dog. In Veterinary Anaesthesia, 11th ed.; Clarke, K.W., Trim, C.M., Hall, L.W., Eds.; W.B. Saunders: Oxford, UK, 2014; pp. 405–498. [Google Scholar]
- Lee, S.H.; Kim, Y.; Rhew, D.; Kim, A.; Jo, K.R.; Yoon, Y.; Choi, K.U.; Jung, T.; Kim, W.H.; Kweon, O.K. Effect of canine mesenchymal stromal cells overexpressing heme oxygenase-1 in spinal cord injury. J. Vet. Sci. 2017, 18, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, S.C.; Levine, J.M.; Mankin, J.M. Thoracolumbar Vertebral Column. In Veterinary Surgery: Small Animal, 2nd ed.; Johnston, S.A., Tobias, K.M., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018; Volume 2, p. 510. [Google Scholar]
- David, S.; López-Vales, R.; Wee Yong, V. Harmful and beneficial effects of inflammation after spinal cord injury: Potential therapeutic implications. In Handbook of Clinical Neurology; Verhaagen, J., McDonald, J.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 109, pp. 485–502. [Google Scholar]
- Freeman, L.M.; Rush, J.E.; Milbury, P.E.; Blumberg, J.B. Antioxidant status and biomarkers of oxidative stress in dogs with congestive heart failure. J. Vet. Intern. Med. 2005, 19, 537–541. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Zhang, X.H.; Pei, G.X.; Wei, K.H.; Zhou, X.J. Dynamic changes of SOD and MDA in canine limbs with gunshot wound in hot and humid environment: An experimental study. Di Yi Jun Yi Da Xue Xue Bao 2002, 22, 331–334. [Google Scholar]
- Merbl, Y.; Sommer, A.; Chai, O.; Aroch, I.; Zimmerman, G.; Friedman, A.; Soreq, H.; Shamir, M.H. Tumor necrosis factor-α and interleukin-6 concentrations in cerebrospinal fluid of dogs after seizures. J. Vet. Intern. Med. 2014, 28, 1775–1781. [Google Scholar] [CrossRef]
- Poncelet, L.; Michaux, C.; Balligand, M. Somatosensory potentials in dogs with naturally acquired thoracolumbar spinal cord disease. Am. J. Vet. Res. 1993, 54, 1935–1941. [Google Scholar] [PubMed]
- Close, B.; Banister, K.; Baumans, V.; Bernoth, E.M.; Bromage, N.; Bunyan, J.; Erhardt, W.; Flecknell, P.; Gregory, N.; Hackbarth, H. Recommendations for euthanasia of experimental animals: Part 2. Lab. Anim. 1997, 31, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Layton, C.; Bancroft, J.D.; Suvarna, S.K. Fixation of tissues. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 40–63. [Google Scholar]
- Deng, W.S.; Liu, X.Y.; Ma, K.; Liang, B.; Liu, Y.F.; Wang, R.J.; Chen, X.Y.; Zhang, S. Recovery of motor function in rats with complete spinal cord injury following implantation of collagen/silk fibroin scaffold combined with human umbilical cord-mesenchymal stem cells. Rev. Da Assoc. Médica Bras. 2021, 67, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Campos, A.; Alaminos, M.; Raimondo, S.; Geuna, S. Staining Methods for Normal and Regenerative Myelin in the Nervous System. Methods Mol. Biol. 2017, 1560, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Buchwalow, I.B.; Böcker, W. Background Staining, Autofluorescence and Blocking Steps. In Immunohistochemistry: Basics and Methods; Springer: Berlin/Heidelberg, Germany, 2010; pp. 41–46. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metwally, E.; Al-Abbadi, H.A.; Hashem, M.A.; Mahmoud, Y.K.; Ahmed, E.A.; Maaty, A.I.; Helal, I.E.; Ahmed, M.F. Selective Calpain Inhibition Improves Functional and Histopathological Outcomes in a Canine Spinal Cord Injury Model. Int. J. Mol. Sci. 2022, 23, 11772. https://doi.org/10.3390/ijms231911772
Metwally E, Al-Abbadi HA, Hashem MA, Mahmoud YK, Ahmed EA, Maaty AI, Helal IE, Ahmed MF. Selective Calpain Inhibition Improves Functional and Histopathological Outcomes in a Canine Spinal Cord Injury Model. International Journal of Molecular Sciences. 2022; 23(19):11772. https://doi.org/10.3390/ijms231911772
Chicago/Turabian StyleMetwally, Elsayed, Hatim A. Al-Abbadi, Mohamed A. Hashem, Yasmina K. Mahmoud, Eman A. Ahmed, Ahmed I. Maaty, Ibrahim E. Helal, and Mahmoud F. Ahmed. 2022. "Selective Calpain Inhibition Improves Functional and Histopathological Outcomes in a Canine Spinal Cord Injury Model" International Journal of Molecular Sciences 23, no. 19: 11772. https://doi.org/10.3390/ijms231911772
APA StyleMetwally, E., Al-Abbadi, H. A., Hashem, M. A., Mahmoud, Y. K., Ahmed, E. A., Maaty, A. I., Helal, I. E., & Ahmed, M. F. (2022). Selective Calpain Inhibition Improves Functional and Histopathological Outcomes in a Canine Spinal Cord Injury Model. International Journal of Molecular Sciences, 23(19), 11772. https://doi.org/10.3390/ijms231911772





