Exercise Normalized the Hippocampal Renin-Angiotensin System and Restored Spatial Memory Function, Neurogenesis, and Blood-Brain Barrier Permeability in the 2K1C-Hypertensive Mouse
Abstract
1. Introduction
2. Results
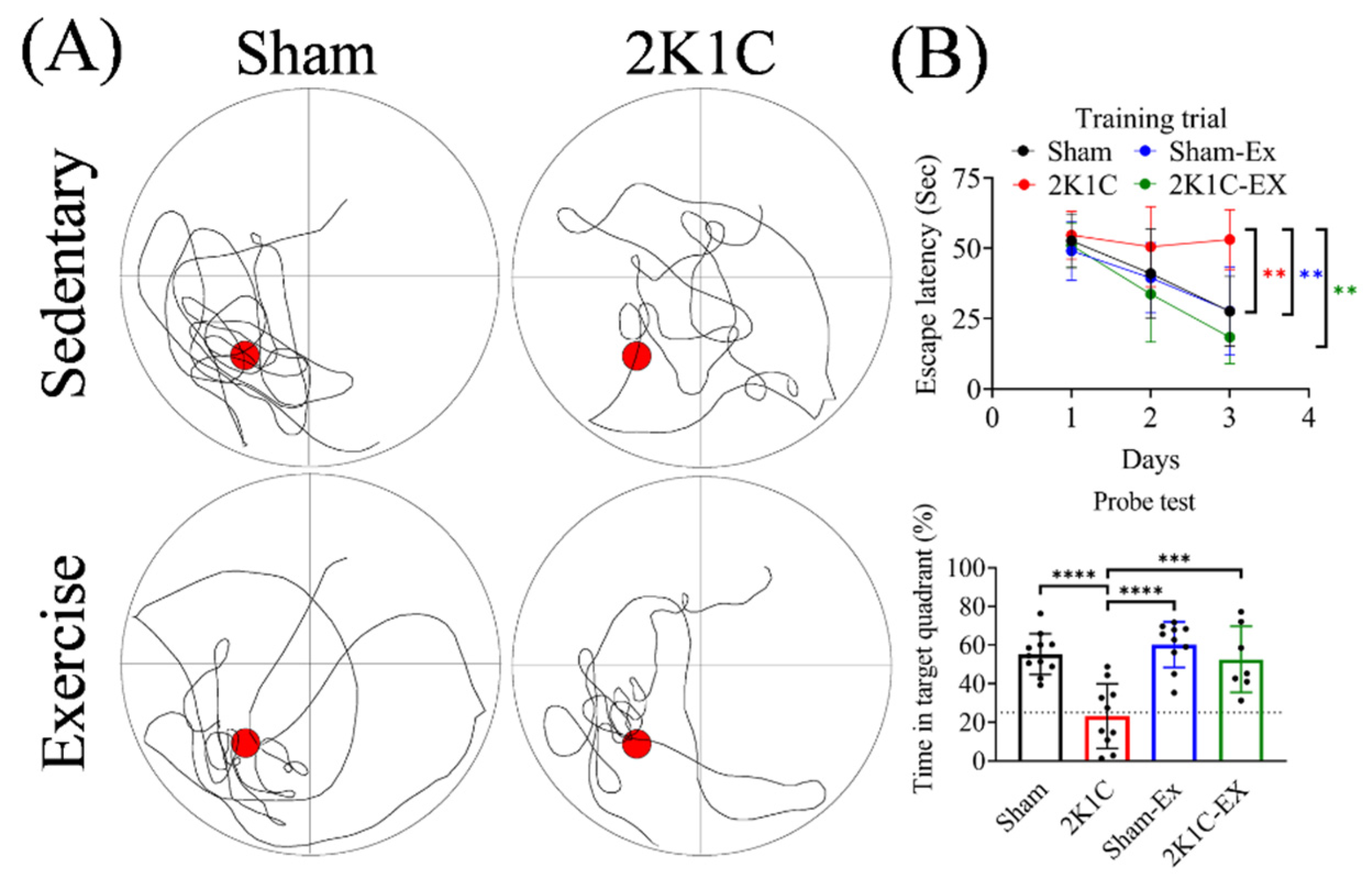
2.1. Treadmill Exercise Improved the Hypertension-Induced Spatial Memory Impairment
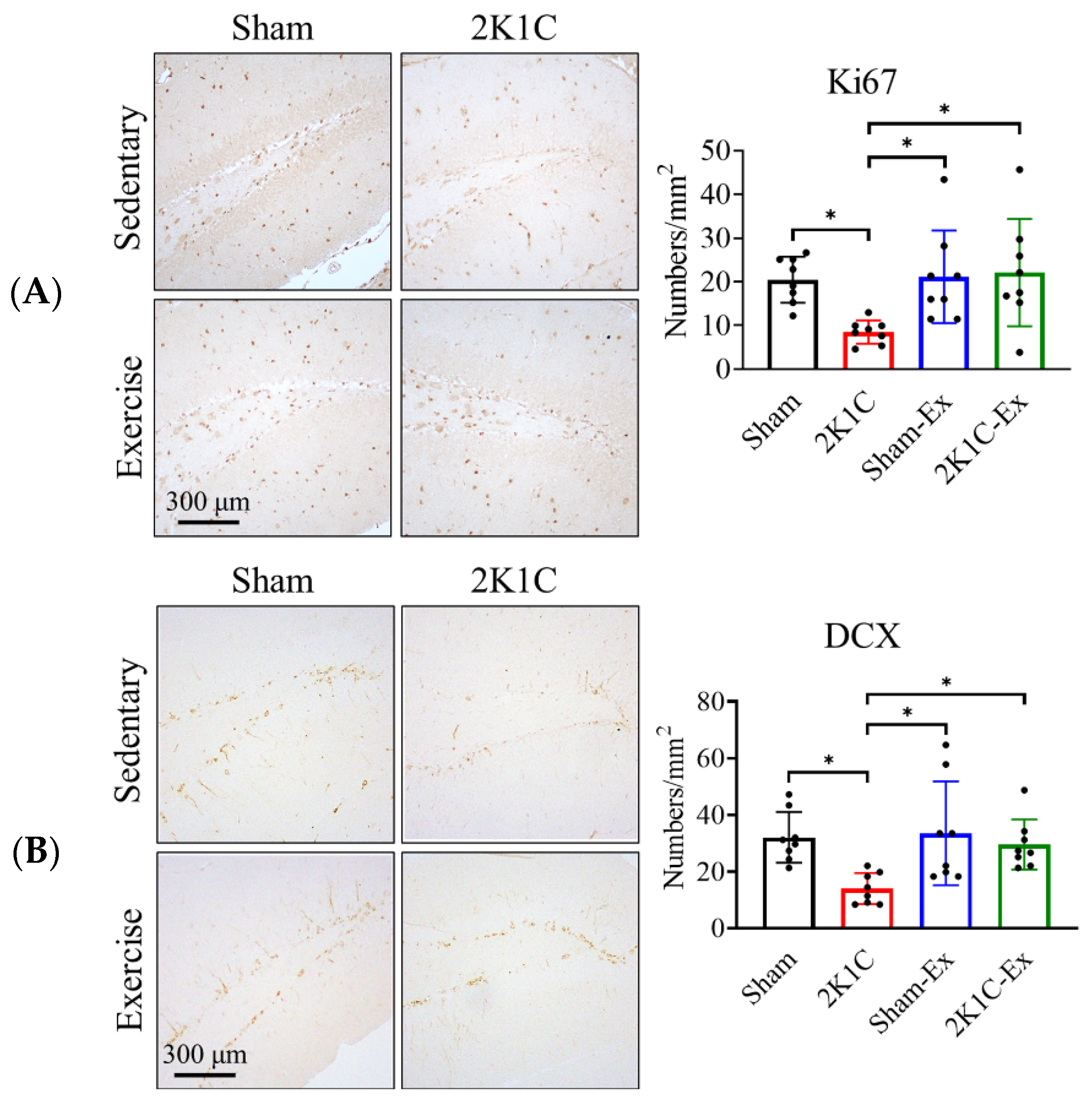
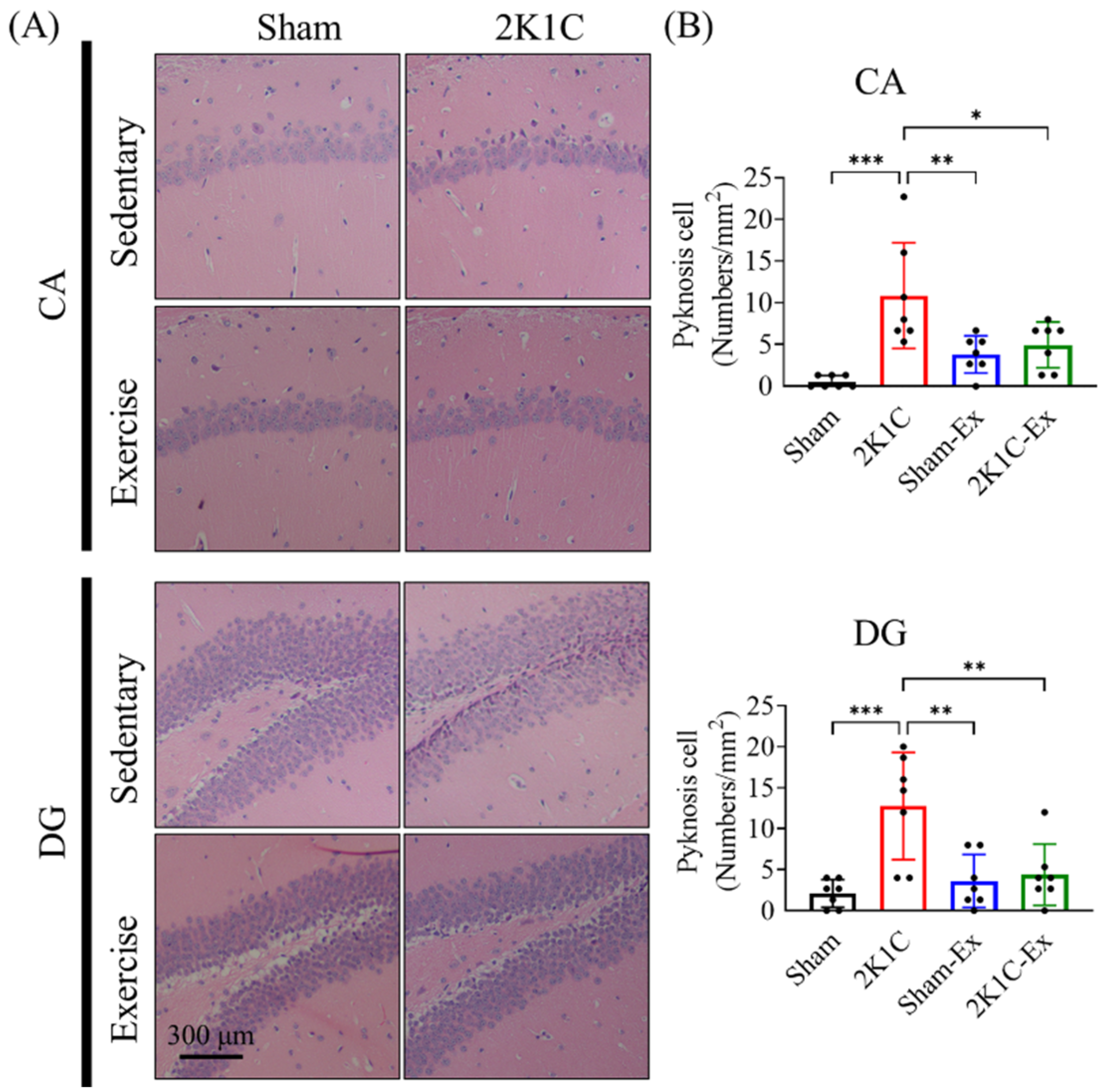
2.2. Treadmill Exercise Prevents the 2K1C-Induced Adult Hippocampal Neurogenesis Impairment
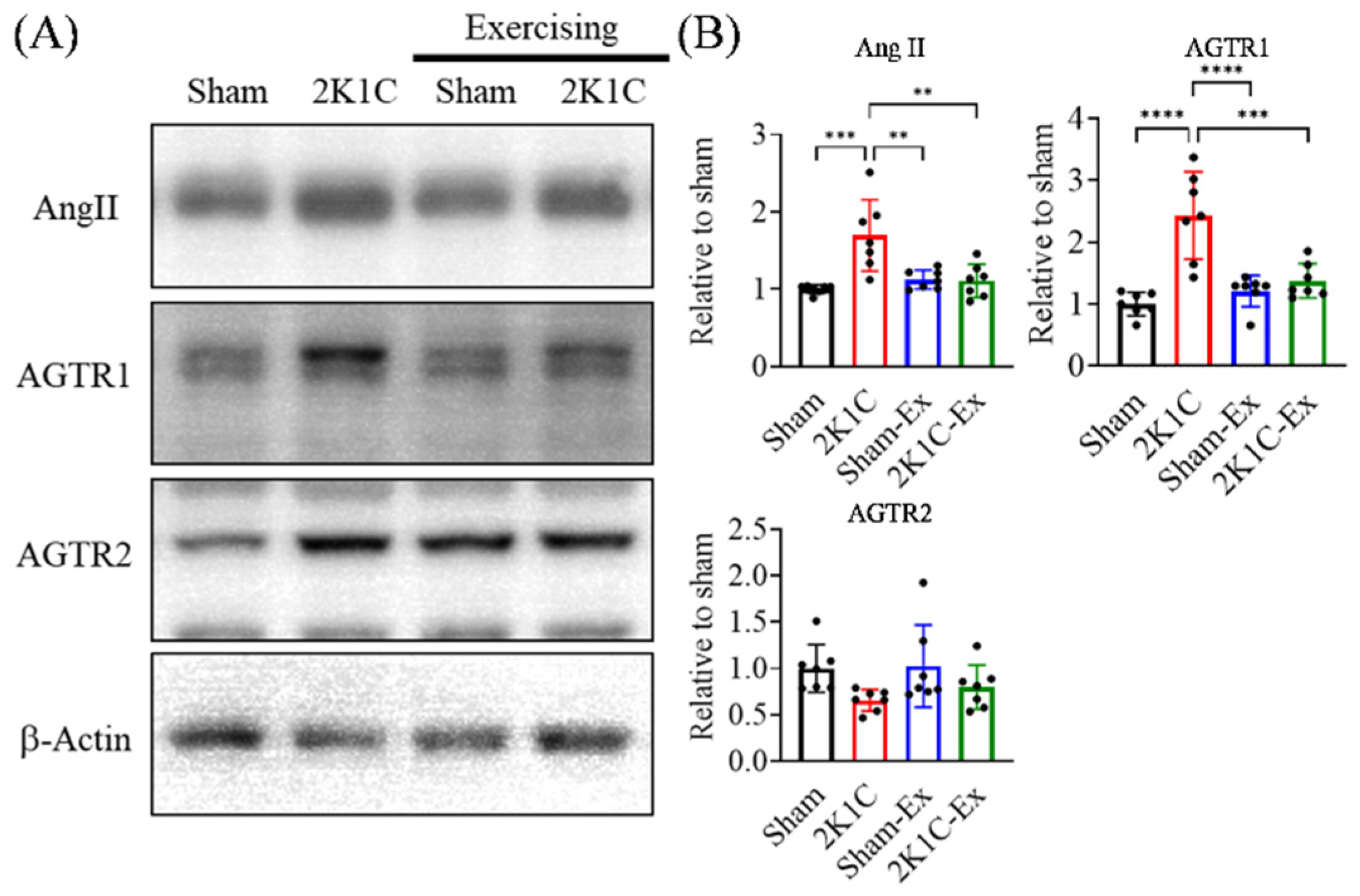
2.3. Treadmill Exercise Restores the Angiotensin II Level without Improving the Elevated Blood Pressure
2.4. Treadmill Exercise Decreases the Angiotensin II and Angiotensin II Receptor Type I Level of the Hippocampus in the Brains of Hypertension Mice
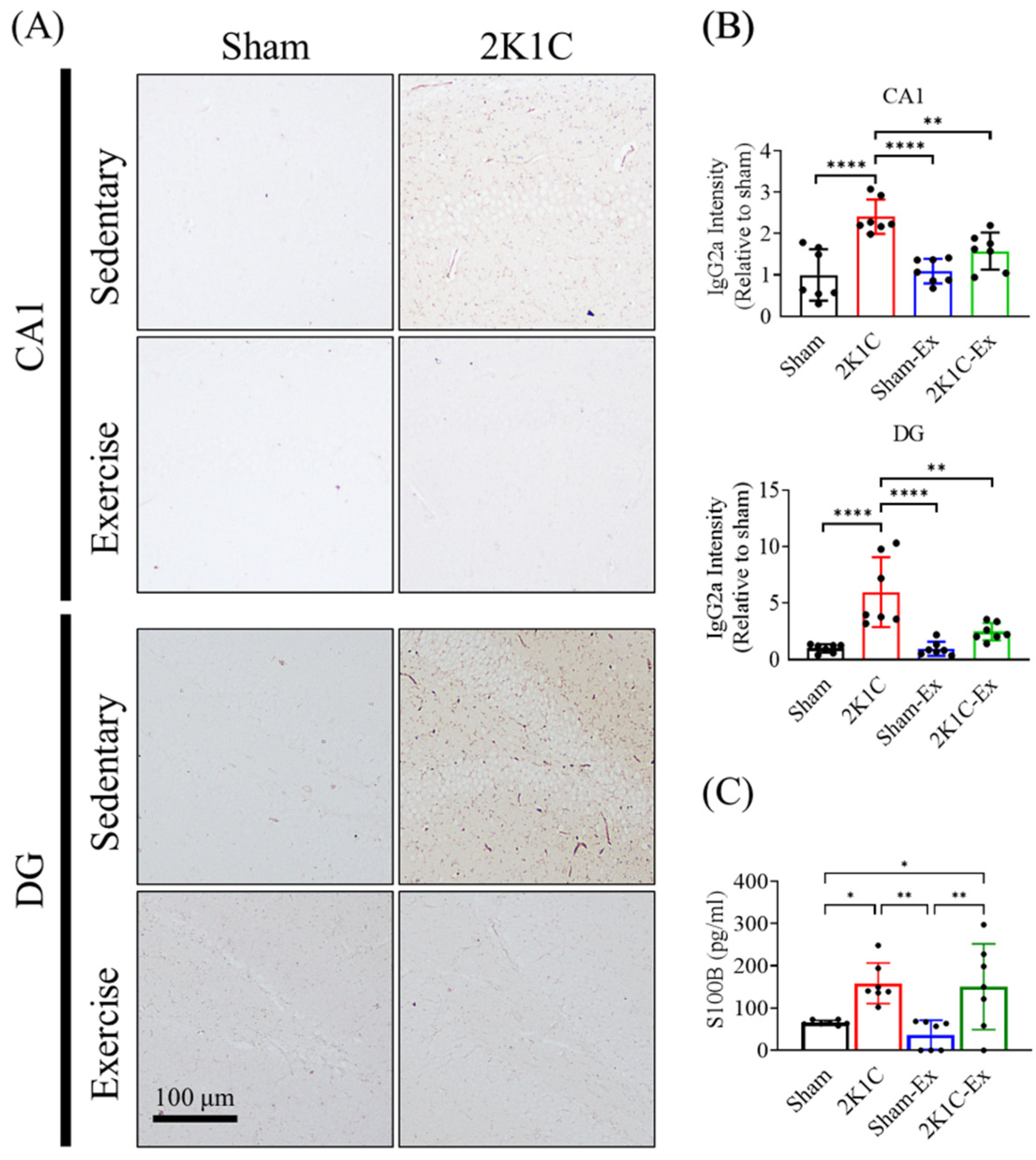
2.5. Treadmill Exercise Prevents the Blood-Brain Barrier Leakage of the Hippocampus in the Brains of Hypertension Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Hypertension Animal Model
4.3. Blood Pressure Measurement
4.4. Treadmill Exercise
4.5. Morris Water Maze
4.6. Tissue Collection
4.7. Immunohistochemistry (IHC)
4.8. Immunoblotting
4.9. ELISA
4.10. Sholl Analysis
4.11. TUNEL Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Duchemin, S.; Belanger, E.; Wu, R.; Ferland, G.; Girouard, H. Chronic perfusion of angiotensin II causes cognitive dysfunctions and anxiety in mice. Physiol. Behav. 2013, 109, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, M.; Strocchi, P.; Vitaioli, L.; Amenta, F. The hippocampus in spontaneously hypertensive rats: A quantitative microanatomical study. Neuroscience 2000, 100, 251–258. [Google Scholar] [CrossRef]
- Sánchez, F.; Gómez-Villalobos, M.D.; Juarez, I.; Quevedo, L.; Flores, G. Dendritic morphology of neurons in medial prefrontal cortex, hippocampus, and nucleus accumbens in adult SH rats. Synapse 2011, 65, 198–206. [Google Scholar] [CrossRef]
- Swan, G.E.; DeCarli, C.; Miller, B.L.; Reed, T.; Wolf, P.A.; Jack, L.M.; Carmelli, D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology 1998, 51, 986–993. [Google Scholar] [CrossRef]
- Kivipelto, M.; Helkala, E.-L.; Hanninen, T.; Laakso, M.P.; Hallikainen, M.; Alhainen, K.; Soininen, H.; Tuomilehto, J.; Nissinen, A. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology 2001, 56, 1683–1689. [Google Scholar] [CrossRef]
- Goldstein, F.C.; Levey, A.I.; Steenland, N.K. Steenland, High blood pressure and cognitive decline in mild cognitive impairment. J. Am. Geriatr. Soc. 2013, 61, 67–73. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Schneider, A.L.; Albert, M.; Alonso, A.; Bandeen-Roche, K.; Coker, L.; Coresh, J.; Knopman, D.; Power, M.C.; Rawlings, A.; et al. Midlife hypertension and 20-year cognitive change: The atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014, 71, 1218–1227. [Google Scholar] [CrossRef]
- Westhoff, T.H.; Schubert, F.; Wirth, C.; Joppke, M.; Klär, A.A.; Zidek, W.; Gallinat, J. The impact of blood pressure on hippocampal glutamate and mnestic function. J. Hum. Hypertens. 2011, 25, 256–261. [Google Scholar] [CrossRef][Green Version]
- Gelber, R.P.; Ross, G.W.; Petrovitch, H.; Masaki, K.H.; Launer, L.J.; White, L.R. Antihypertensive medication use and risk of cognitive impairment: The Honolulu-Asia Aging Study. Neurology 2013, 81, 888–895. [Google Scholar] [CrossRef]
- Sink, K.M.; Leng, X.; Williamson, J.; Kritchevsky, S.B.; Yaffe, K.; Kuller, L.; Yasar, S.; Atkinson, H.; Robbins, M.; Psaty, B.; et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2009, 169, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Amenta, F.; Mignini, F.; Rabbia, F.; Tomassoni, D.; Veglio, F. Protective effect of anti-hypertensive treatment on cognitive function in essential hypertension: Analysis of published clinical data. J. Neurol. Sci. 2002, 203–204, 147–151. [Google Scholar] [CrossRef]
- Ryu, J.R.; Hong, C.J.; Kim, J.Y.; Kim, E.-K.; Sun, W.; Yu, S.-W. Control of adult neurogenesis by programmed cell death in the mammalian brain. Mol. Brain 2016, 9, 43. [Google Scholar] [CrossRef]
- Ming, G.-L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Akers, K.G.; Martinez-Canabal, A.; Restivo, L.; Yiu, A.P.; De Cristofaro, A.; Hsiang, H.-L.; Wheeler, A.L.; Guskjolen, A.; Niibori, Y.; Shoji, H.; et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 2014, 344, 598–602. [Google Scholar] [CrossRef]
- Kitamura, T.; Saitoh, Y.; Takashima, N.; Murayama, A.; Niibori, Y.; Ageta, H.; Sekiguchi, M.; Sugiyama, H.; Inokuchi, K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 2009, 139, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Olsen, R.H.; Sun, J.; Ming, G.L.; Song, H. Neuronal Circuitry Mechanisms Regulating Adult Mammalian Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018937. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- Dupret, D.; Fabre, A.; Dobrossy, M.; Panatier, A.; Rodríguez, J.J.; Lamarque, S.; Lemaire, V.; Oliet, S.H.R.; Piazza, P.-V.; Abrous, D.N. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007, 5, e214. [Google Scholar] [CrossRef]
- Pietranera, L.; Saravia, F.E.; Roig, P.; Lima, A.; De Nicola, A.F. Protective effects of estradiol in the brain of rats with genetic or mineralocorticoid-induced hypertension. Psychoneuroendocrinology 2008, 33, 270–281. [Google Scholar] [CrossRef]
- Pietranera, L.; Lima, A.; Roig, P.; De Nicola, A.F. Involvement of brain-derived neurotrophic factor and neurogenesis in oestradiol neuroprotection of the hippocampus of hypertensive rats. J. Neuroendocrinol. 2010, 22, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.K.; Yoon, Y.S.; Choi, J.H.; Yoo, K.Y.; Yi, S.S.; Chung, D.W.; Kim, H.J.; Kim, C.S.; Seong, J.K.; Lee, I.S.; et al. Doublecortin-immunoreactive neuronal precursors in the dentate gyrus of spontaneously hypertensive rats at various age stages: Comparison with Sprague-Dawley rats. J. Vet. Med. Sci. 2008, 70, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Tsai, S.-F.; Huang, S.-H.; Chiang, Y.-T.; Hughes, M.; Wu, S.-Y.; Lee, C.-W.; Yang, T.-T.; Kuo, Y.-M. Hypertension impairs hippocampus-related adult neurogenesis, CA1 neuron dendritic arborization and long-term memory. Neuroscience 2016, 322, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Chaddock, L.; Erickson, K.I.; Prakash, R.S.; Kim, J.S.; Voss, M.W.; Vanpatter, M.; Pontifex, M.B.; Raine, L.B.; Konkel, A.; Hillman, C.H.; et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010, 1358, 172–183. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef]
- Clark, P.J.; Brzezinska, W.J.; Puchalski, E.K.; Krone, D.A.; Rhodes, J.S. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus 2009, 19, 937–950. [Google Scholar] [CrossRef]
- Van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef]
- Lin, T.-W.; Shih, Y.-H.; Chen, S.-J.; Lien, C.-H.; Chang, C.-Y.; Huang, T.-Y.; Chen, S.-H.; Jen, C.J.; Kuo, Y.-M. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 2015, 118, 189–197. [Google Scholar]
- Yang, T.-T.; Lo, C.-P.; Tsai, P.-S.; Wu, S.-Y.; Wang, T.-F.; Chen, Y.-W.; Jiang-Shieh, Y.-F.; Kuo, Y.-M. Aging and Exercise Affect Hippocampal Neurogenesis via Different Mechanisms. PLoS ONE 2015, 10, e0132152. [Google Scholar]
- Wiesel, P.; Mazzolai, L.; Nussberger, J.; Pedrazzini, T. Pedrazzini, Two-kidney, one clip and one-kidney, one clip hypertension in mice. Hypertension 1997, 29, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Alhusban, A.; Kozak, A.; Ergul, A.; Fagan, S.C. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J. Pharmacol. Exp. Ther. 2013, 344, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Ping, G.; Qian, W.; Song, G.; Zhaochun, S. Valsartan reverses depressive/anxiety-like behavior and induces hippocampal neurogenesis and expression of BDNF protein in unpredictable chronic mild stress mice. Pharmacol. Biochem. Behav. 2014, 124, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, W.; Zhu, M.; Zhu, Y.; Xu, P.; Miao, C. Angiotensin II-induced mouse hippocampal neuronal HT22 cell apoptosis was inhibited by propofol: Role of neuronal nitric oxide synthase and metallothinonein-3. Neuroscience 2015, 305, 117–127. [Google Scholar] [CrossRef]
- El-Sherbiny, M.; Shaibah, H.S.; Elsify, A.-E.K.; Medhat, T.M.; Rezk, H.M. Histopathological and immunohistochemical study of the protective effect of triptorelin on the neurocytes of the hippocampus and the cerebral cortex of male albino rats after short-term exposure to cyclophosphamide. J. Microsc. Ultrastruct. 2016, 4, 123–132. [Google Scholar] [CrossRef]
- Lorenz, J.N.; Lasko, V.M.; Nieman, M.L.; Damhoff, T.; Prasad, V.; Beierwaltes, W.H.; Lingrel, J.B. Renovascular hypertension using a modified two-kidney, one-clip approach in mice is not dependent on the α1 or α2 Na-K-ATPase ouabain-binding site. Am. J. Physiol. Renal. Physiol. 2011, 301, F615–F621. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Ahn, S.J.; Lane, D.; Faraco, G.; Garcia-Bonilla, L.; Racchumi, G.; Poon, C.; Schaeffer, S.; Segarra, S.G.; Körbelin, J.; et al. Endothelium-Macrophage Crosstalk Mediates Blood-Brain Barrier Dysfunction in Hypertension. Hypertension 2020, 76, 795–807. [Google Scholar] [CrossRef]
- Woodward, N.C.; Haghani, A.; Johnson, R.G.; Hsu, T.M.; Saffari, A.; Sioutas, C.; Kanoski, S.E.; Finch, C.E.; Morgan, T.E. Prenatal and early life exposure to air pollution induced hippocampal vascular leakage and impaired neurogenesis in association with behavioral deficits. Transl. Psychiatry 2018, 8, 261. [Google Scholar] [CrossRef]
- Mokhtarzade, M.; Motl, R.; Negaresh, R.; Zimmer, P.; Khodadoost, M.; Baker, J.S.; Patel, D.; Majdinasab, N.; Ranjbar, R. Exercise-induced changes in neurotrophic factors and markers of blood-brain barrier permeability are moderated by weight status in multiple sclerosis. Neuropeptides 2018, 70, 93–100. [Google Scholar] [CrossRef]
- Souza, P.S.; Gonçalves, E.D.; Pedroso, G.S.; Farias, H.R.; Junqueira, S.C.; Marcon, R.; Tuon, T.; Cola, M.; Silveira, P.C.L.; Santos, A.; et al. Physical Exercise Attenuates Experimental Autoimmune Encephalomyelitis by Inhibiting Peripheral Immune Response and Blood-Brain Barrier Disruption. Mol. Neurobiol. 2017, 54, 4723–4737. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Wu, S.-Y.; Yu, M.; Huang, S.-H.; Lee, C.-W.; Jiang, M.-J.; Lin, P.-Y.; Yang, T.-T.; Kuo, Y.-M. Hypertension Accelerates Alzheimer’s Disease-Related Pathologies in Pigs and 3xTg Mice. Front. Aging Neurosci. 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.; Rasmussen, P.; Kapural, M.; Fazio, V.; Kight, K.; Mayberg, M.R.; Kanner, A.; Ayumar, B.; Albensi, B.; Cavaglia, M.; et al. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor. Neurol. Neurosci. 2003, 21, 109–121. [Google Scholar] [PubMed]
- Agarwal, D.; Welsch, M.A.; Keller, J.N.; Francis, J. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res. Cardiol. 2011, 106, 1069–1085. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Gao, L.; Zucker, I.H. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J. Appl. Physiol. 2010, 108, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Kazama, K.; Anrather, J.; Zhou, P.; Girouard, H.; Frys, K.; Milner, T.A.; Iadecola, C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ. Res. 2004, 95, 1019–1026. [Google Scholar] [CrossRef]
- Wei, Y.; Whaley-Connell, A.; Chen, K.; Habibi, J.; Uptergrove, G.M.-E.; Clark, S.E.; Stump, C.S.; Ferrario, C.M.; Sowers, J.R. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension 2007, 50, 384–391. [Google Scholar] [CrossRef]
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.; Lavandero, S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef]
- Liu, G.; Hosomi, N.; Hitomi, H.; Pelisch, N.; Fu, H.; Masugata, H.; Murao, K.; Ueno, M.; Matsumoto, M.; Nishiyama, A. Angiotensin II induces human astrocyte senescence through reactive oxygen species production. Hypertens. Res. 2011, 34, 479–483. [Google Scholar] [CrossRef]
- DiMeo, F.; Pagonas, N.; Seibert, F.; Arndt, R.; Zidek, W.; Westhoff, T.H. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension 2012, 60, 653–658. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Fagard, R.H. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 2005, 46, 667–675. [Google Scholar] [CrossRef]
- Zheng, H.; Sharma, N.; Liu, X.; Patel, K.P. Exercise training normalizes enhanced sympathetic activation from the paraventricular nucleus in chronic heart failure: Role of angiotensin II. Am. J. Physiol. Integr. Comp. Physiol. 2012, 303, R387–R394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mousa, T.M.; Liu, D.; Cornish, K.G.; Zucker, I.H. Exercise training enhances baroreflex sensitivity by an angiotensin II-dependent mechanism in chronic heart failure. J. Appl. Physiol. 2008, 104, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, D.M.; Nunes-Silva, A.; Rocha, G.C.; Vaz, L.N.; de Faria, M.H.; Vieira, E.L.; Rocha, N.P.; e Silva, A.C. Two protocols of aerobic exercise modulate the counter-regulatory axis of the renin-angiotensin system. Heliyon 2020, 6, e03208. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.M.; Martell, N.; Yunis, C.; Flack, J.M.; Chappell, M.C.; Brosnihan, K.B.; Dean, R.H.; Fernandez, A.; Novikov, S.V.; Pinillas, C.; et al. Characterization of angiotensin-(1-7) in the urine of normal and essential hypertensive subjects. Am. J. Hypertens. 1998, 11, 137–146. [Google Scholar] [CrossRef]
- E Silva, A.C.; Diniz, J.S.; Filho, A.R.; Santos, R.A. The renin angiotensin system in childhood hypertension: Selective increase of angiotensin-(1-7) in essential hypertension. J. Pediatr. 2004, 145, 93–98. [Google Scholar] [CrossRef]
- Nunes-Silva, A.; Rocha, G.C.; Magalhaes, D.M.; Vaz, L.N.; De Faria, M.H.S.; E Silva, A.C.S. Physical Exercise and ACE2-Angiotensin-(1-7)-Mas Receptor Axis of the Renin Angiotensin System. Protein Pept. Lett. 2017, 24, 809–816. [Google Scholar] [CrossRef]
- Shim, C.Y.; Ha, J.-W.; Park, S.; Choi, E.-Y.; Choi, D.; Rim, S.-J.; Chung, N. Exaggerated blood pressure response to exercise is associated with augmented rise of angiotensin II during exercise. J. Am. Coll. Cardiol. 2008, 52, 287–292. [Google Scholar] [CrossRef]
- Kim, C.-H.; Park, Y.; Chun, M.Y.; Kim, Y.-J. Exercise-induced hypertension is associated with angiotensin II activity and total nitric oxide. Medicine 2020, 99, e20943. [Google Scholar] [CrossRef]
- Ito, H.; Takemori, K.; Kawai, J.; Suzuki, T. AT1 receptor antagonist prevents brain edema without lowering blood pressure. Acta Neurochir. 2000, 76, 141–145. [Google Scholar]
- Buttler, L.; Jordão, M.T.; Fragas, M.; Ruggeri, A.; Ceroni, A.; Michelini, L.C. Maintenance of Blood-Brain Barrier Integrity in Hypertension: A Novel Benefit of Exercise Training for Autonomic Control. Front. Physiol. 2017, 8, 1048. [Google Scholar] [CrossRef]
- Savić, B.; Murphy, D.; Japundžić-Žigon, N. The Paraventricular Nucleus of the Hypothalamus in Control of Blood Pressure and Blood Pressure Variability. Front. Physiol. 2022, 13, 858941. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.; Yang, M.; Enikolopov, G.; Iacovitti, L. Circumventricular organs: A novel site of neural stem cells in the adult brain. Mol. Cell. Neurosci. 2009, 41, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Frahm, K.A.; Tobet, S.A. Development of the blood-brain barrier within the paraventricular nucleus of the hypothalamus: Influence of fetal glucocorticoid excess. Brain Struct. Funct. 2015, 220, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Ganong, W.F. Circumventricular organs: Definition and role in the regulation of endocrine and autonomic function. Clin. Exp. Pharmacol. Physiol. 2000, 27, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Leenen, F.H.H.; Blaustein, M.P.; Hamlyn, J.M. Update on angiotensin II: New endocrine connections between the brain, adrenal glands and the cardiovascular system. Endocr. Connect. 2017, 6, R131–R145. [Google Scholar] [CrossRef] [PubMed]
- Fragas, M.G.; Cândido, V.B.; Davanzo, G.G.; Rocha-Santos, C.; Ceroni, A.; Michelini, L.C. Transcytosis within PVN capillaries: A mechanism determining both hypertension-induced blood-brain barrier dysfunction and exercise-induced correction. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2021, 321, R732–R741. [Google Scholar] [CrossRef] [PubMed]
- Van Craenenbroeck, E.M.; Hoymans, V.Y.; Beckers, P.J.; Possemiers, N.M.; Wuyts, K.; Paelinck, B.P.; Vrints, C.J.; Conraads, V.M. Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res. Cardiol. 2010, 105, 665–676. [Google Scholar] [CrossRef]
- Anderson, R.E.; Hansson, L.O.; Nilsson, O.; Dijlai-Merzoug, R.; Settergren, G. High serum S100B levels for trauma patients without head injuries. Neurosurgery 2001, 48, 1255–1258. [Google Scholar]
- Koh, S.X.T.; Lee, J.K.W. S100B as a marker for brain damage and blood-brain barrier disruption following exercise. Sports Med. 2014, 44, 369–385. [Google Scholar] [CrossRef]
- Kleindienst, A.; Schmidt, C.; Parsch, H.; Emtmann, I.; Xu, Y.; Buchfelder, M. The Passage of S100B from Brain to Blood Is Not Specifically Related to the Blood-Brain Barrier Integrity. Cardiovasc. Psychiatry Neurol. 2010, 2010, 801295. [Google Scholar] [CrossRef]
- Shih, Y.H.; Tu, L.H.; Chang, T.Y.; Ganesan, K.; Chang, W.W.; Chang, P.S.; Fang, Y.S.; Lin, Y.T.; Jin, L.W.; Chen, Y.R. TDP-43 interacts with amyloid-β, inhibits fibrillization, and worsens pathology in a model of Alzheimer’s disease. Nat. Commun. 2020, 11, 5950. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-S.; Lin, C.-L.; Lee, C.-W.; Lin, H.-C.; Wu, Y.-T.; Shih, Y.-H. Exercise Normalized the Hippocampal Renin-Angiotensin System and Restored Spatial Memory Function, Neurogenesis, and Blood-Brain Barrier Permeability in the 2K1C-Hypertensive Mouse. Int. J. Mol. Sci. 2022, 23, 5531. https://doi.org/10.3390/ijms23105531
Chang Y-S, Lin C-L, Lee C-W, Lin H-C, Wu Y-T, Shih Y-H. Exercise Normalized the Hippocampal Renin-Angiotensin System and Restored Spatial Memory Function, Neurogenesis, and Blood-Brain Barrier Permeability in the 2K1C-Hypertensive Mouse. International Journal of Molecular Sciences. 2022; 23(10):5531. https://doi.org/10.3390/ijms23105531
Chicago/Turabian StyleChang, Ying-Shuang, Chih-Lung Lin, Chu-Wan Lee, Han-Chen Lin, Yi-Ting Wu, and Yao-Hsiang Shih. 2022. "Exercise Normalized the Hippocampal Renin-Angiotensin System and Restored Spatial Memory Function, Neurogenesis, and Blood-Brain Barrier Permeability in the 2K1C-Hypertensive Mouse" International Journal of Molecular Sciences 23, no. 10: 5531. https://doi.org/10.3390/ijms23105531
APA StyleChang, Y.-S., Lin, C.-L., Lee, C.-W., Lin, H.-C., Wu, Y.-T., & Shih, Y.-H. (2022). Exercise Normalized the Hippocampal Renin-Angiotensin System and Restored Spatial Memory Function, Neurogenesis, and Blood-Brain Barrier Permeability in the 2K1C-Hypertensive Mouse. International Journal of Molecular Sciences, 23(10), 5531. https://doi.org/10.3390/ijms23105531





