OsNAC15 Regulates Tolerance to Zinc Deficiency and Cadmium by Binding to OsZIP7 and OsZIP10 in Rice
Abstract
1. Introduction
2. Results
2.1. Sequence Analysis of OsNAC15
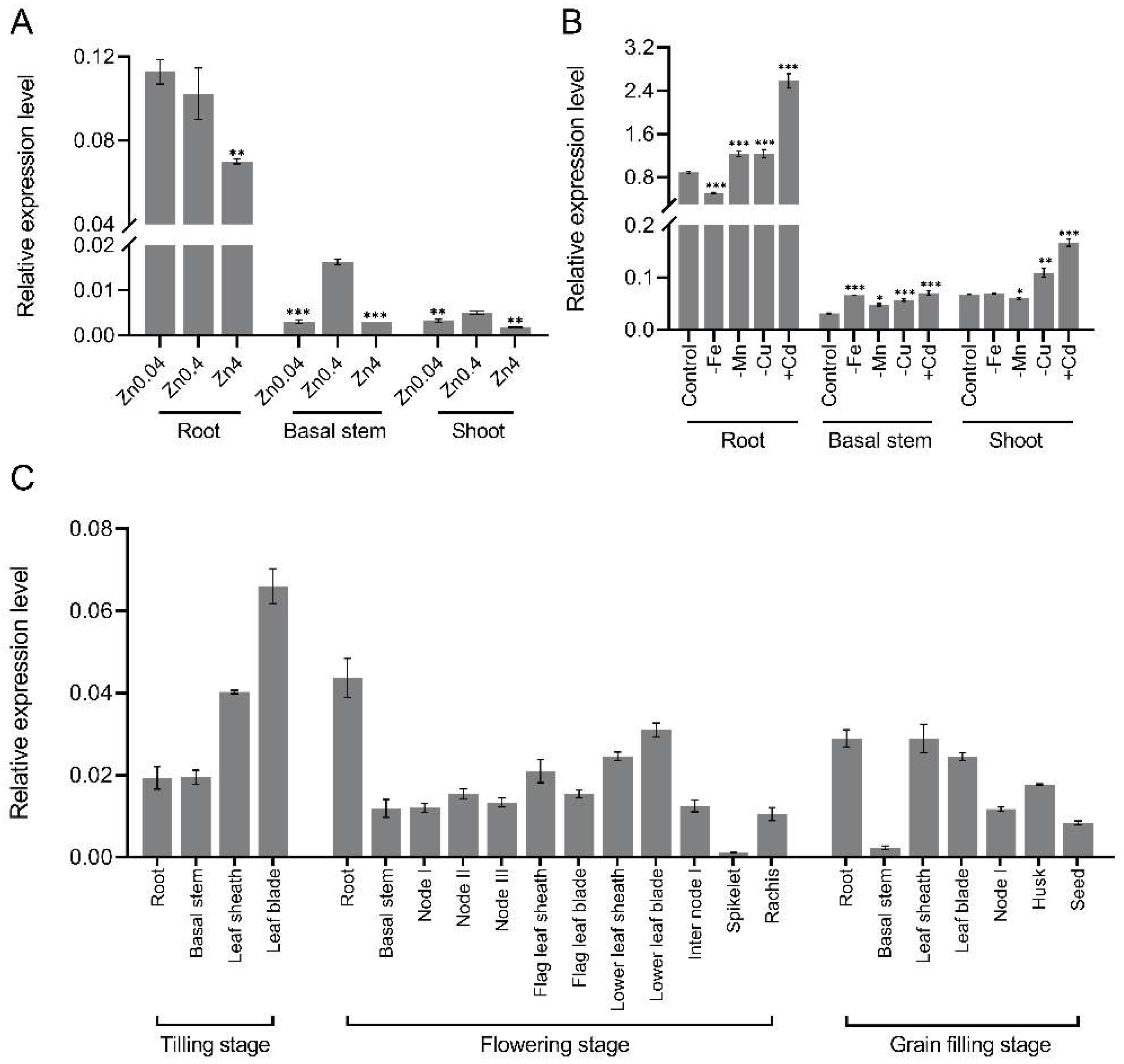
2.2. Expression Patterns of OsNAC15
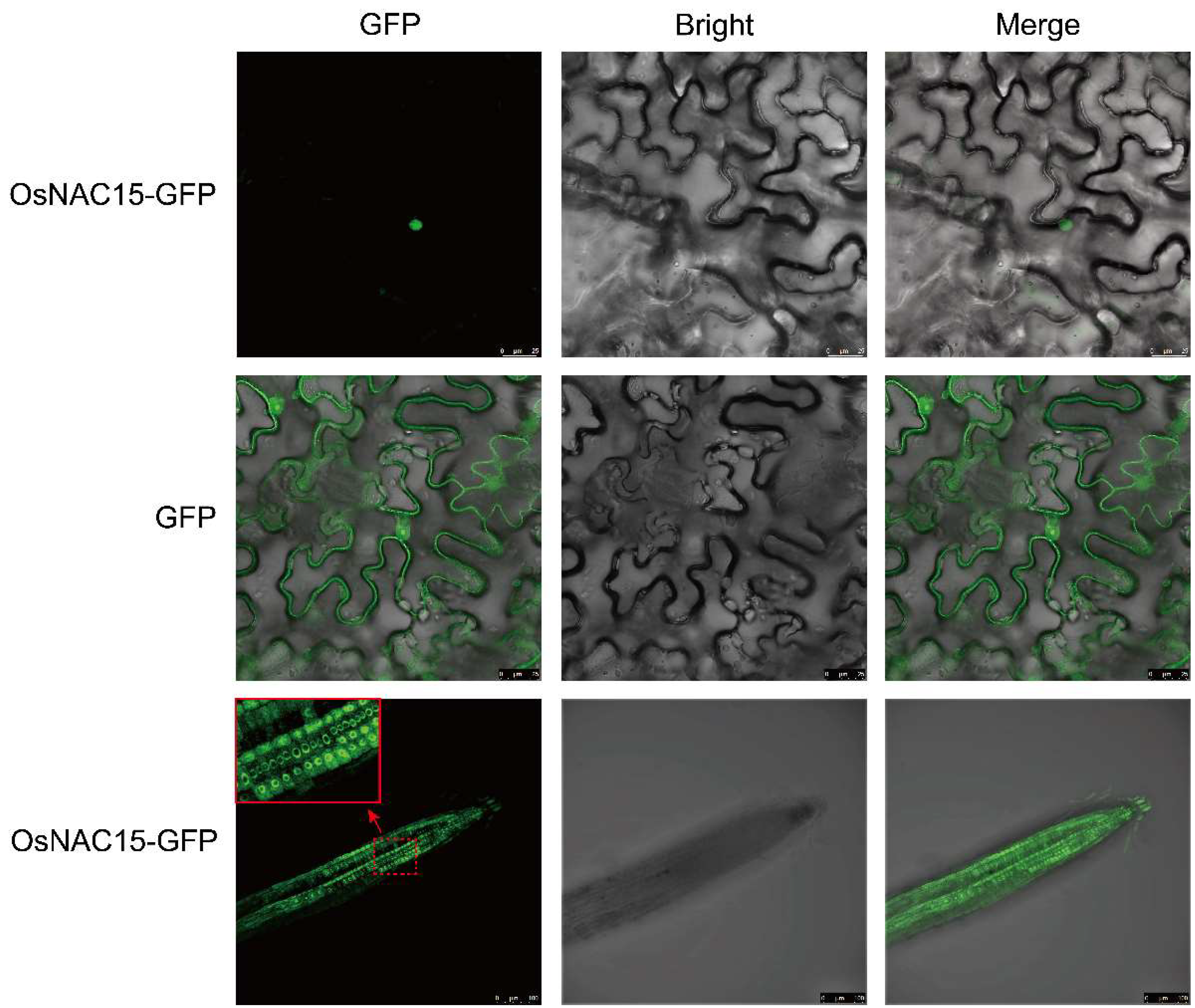
2.3. Subcellular Localization of OsNAC15
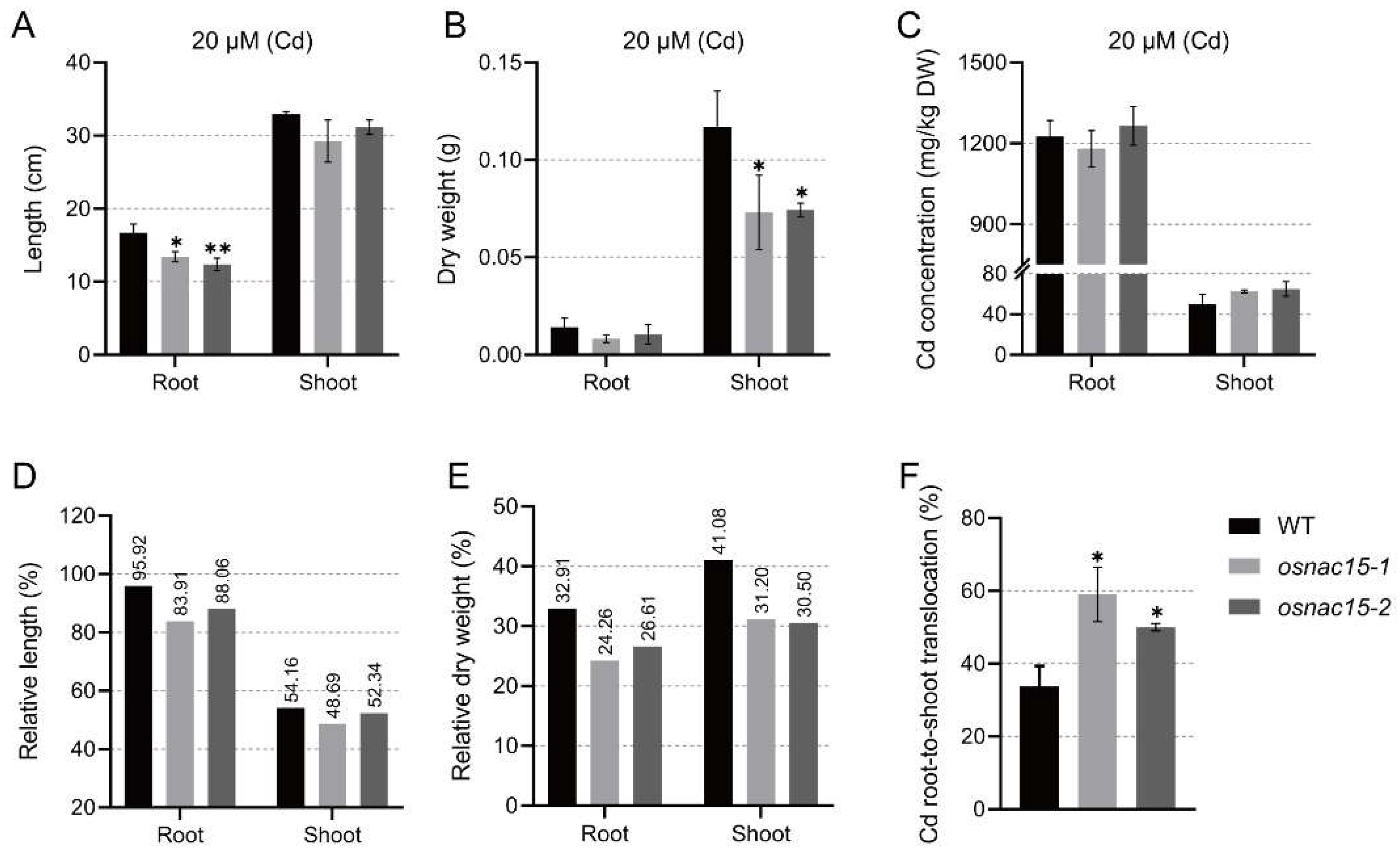
2.4. Knockout of OsNAC15 Reduces Zn and Cd Tolerance at the Vegetative Stage
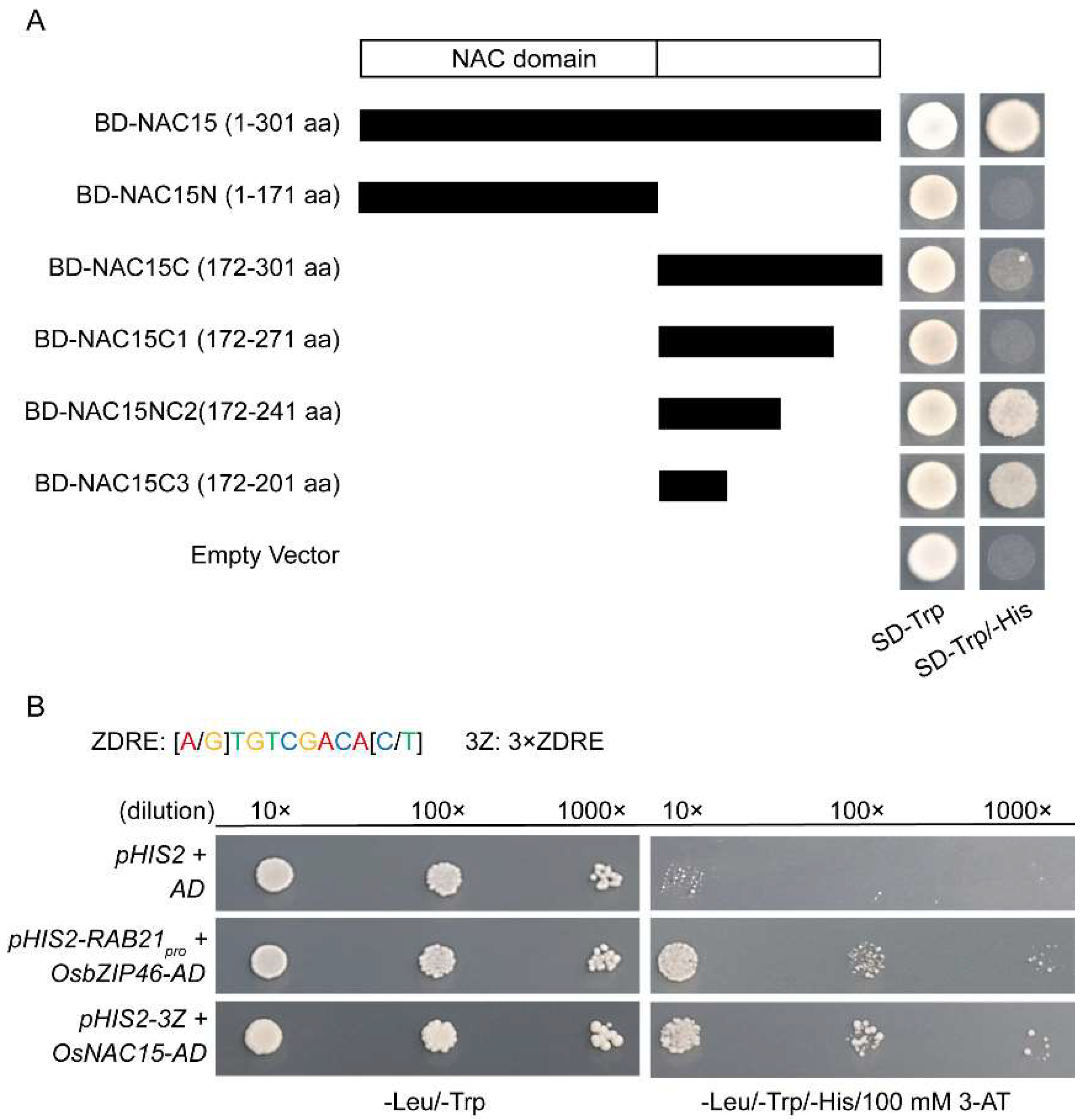
2.5. OsNAC15 Is a Functional Transcription Factor
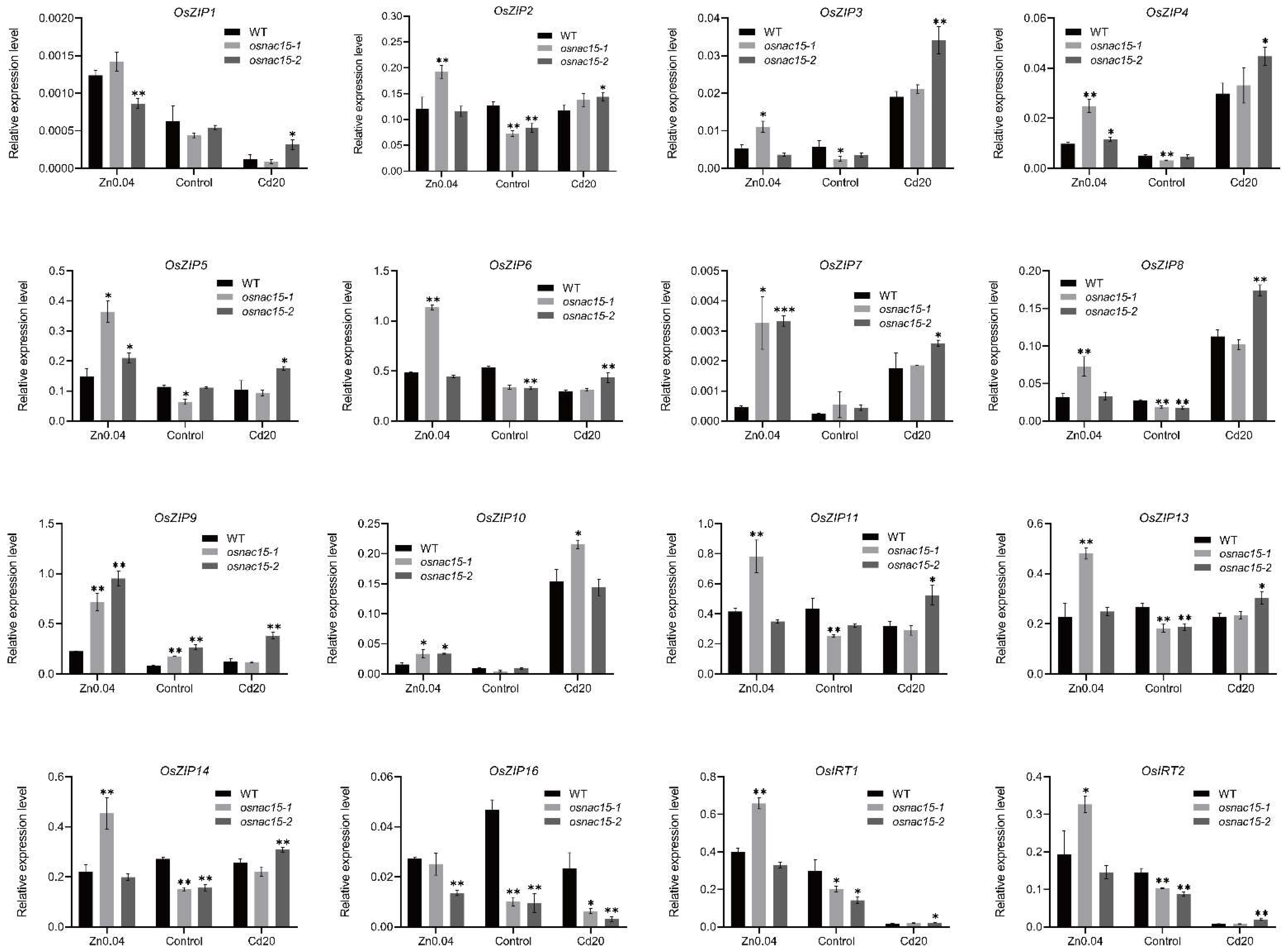
2.6. Knockout of OsNAC15 Changes Expression of Rice ZIP Family Genes
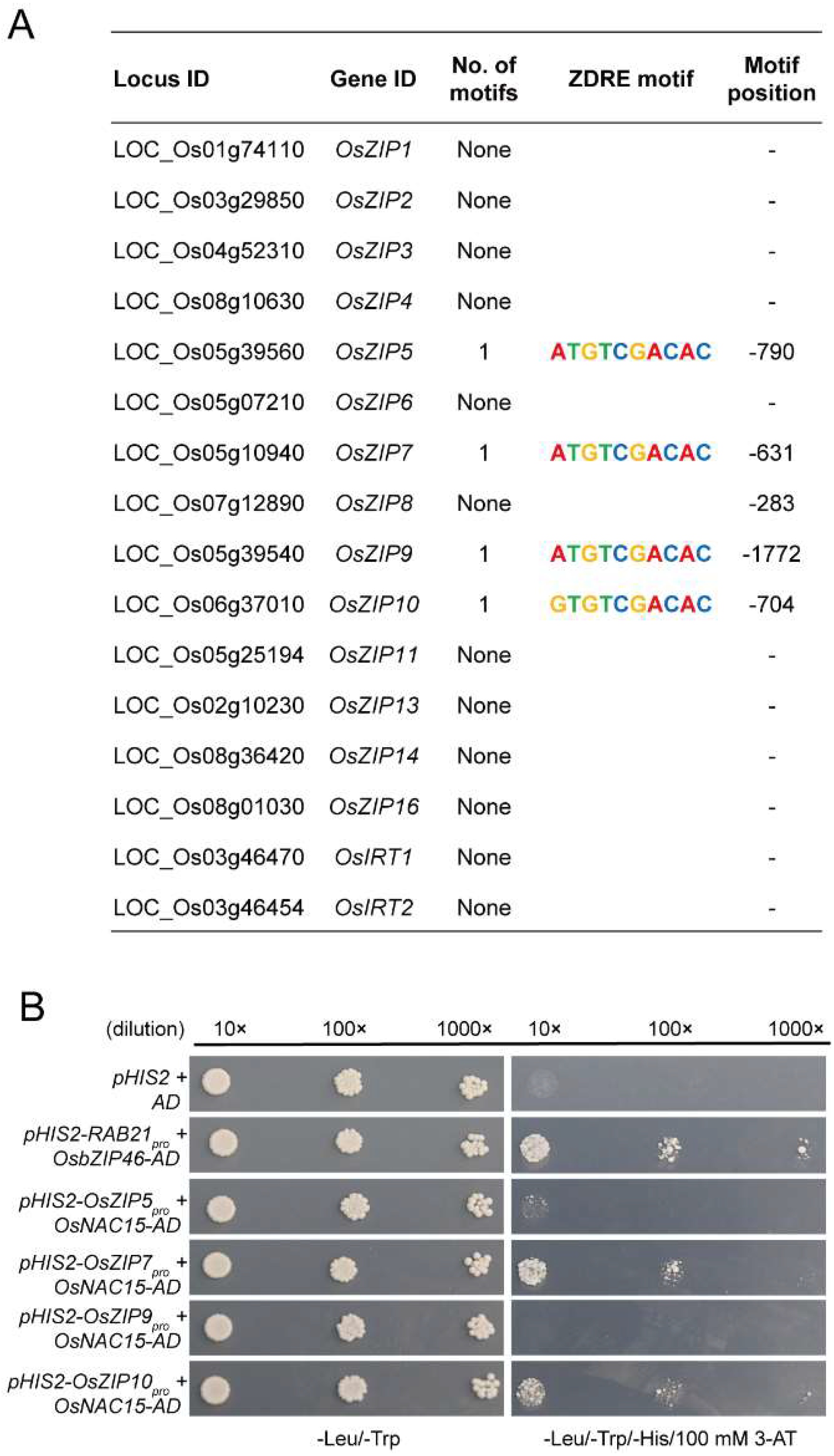
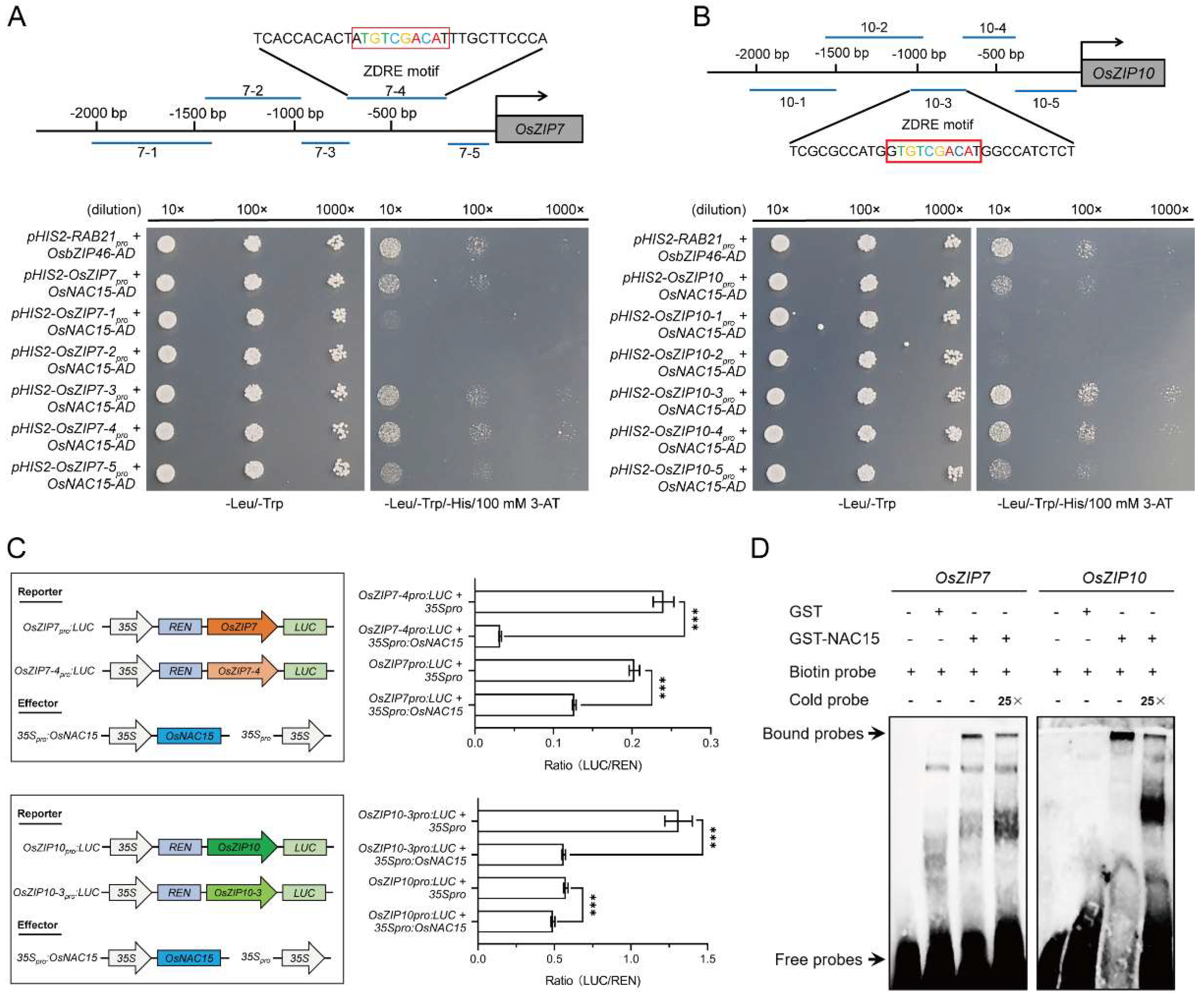
2.7. OsNAC15 Negatively Regulates OsZIP7 and OsZIP10 Transcriptions through Binding to the ZDRE Motif in Rice
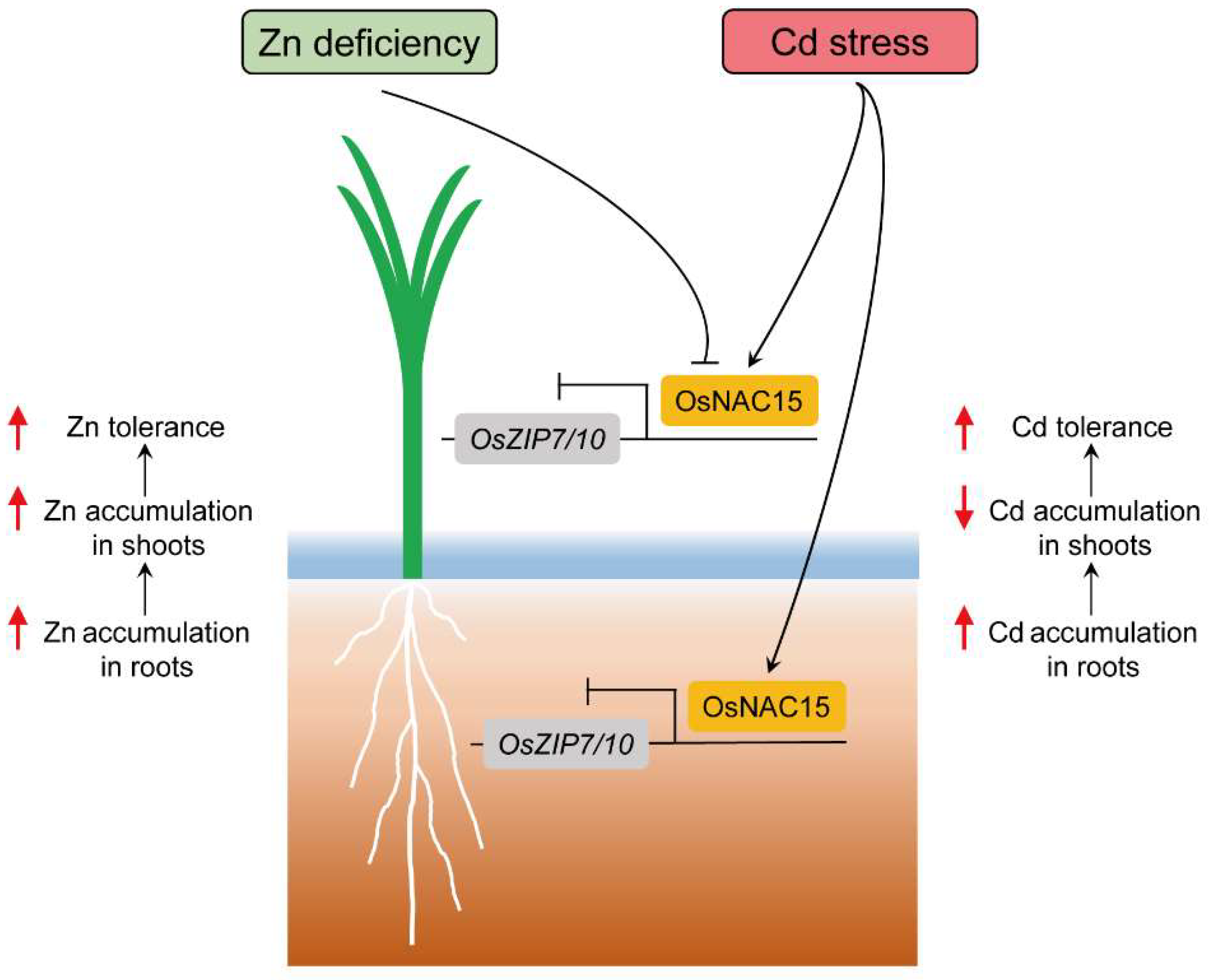
3. Discussion
4. Materials and Methods
4.1. Generation of osnac15 Mutants by CRISPR/Cas9 System
4.2. Plant Materials and Growth Conditions
4.3. Subcellular Localization of OsNAC15
4.4. Determination of Metal Concentrations in Plant
4.5. Transcription Activation Assay in Yeast
4.6. Yeast One-Hybrid Assays
4.7. Dual-LUC Assays of Transiently Transformed Tobacco Leaves
4.8. Electrophoretic Mobility Shift Assay
4.9. RNA Extraction and RT-qPCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vallee, B.L.; Auld, D.S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 1990, 29, 5647–5659. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, E.A. Marschner’s Mineral Nutrition of Higher Plants Third Edition Foreword. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Burchi, F.; Fanzo, J.; Frison, E. The Role of Food and Nutrition System Approaches in Tackling Hidden Hunger. Int. J. Environ. Res. Pub. Health 2011, 8, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar] [PubMed]
- Suzuki, M.; Bashir, K.; Inoue, H.; Takahashi, M.; Nakanishi, H.; Nishizawa, N.K. Accumulation of starch in Zn-deficient rice. Rice 2012, 5, 9. [Google Scholar] [CrossRef]
- Wessells, K.R.; Brown, K.H. Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Uraguchi, S.; Fujiwara, T. Cadmium transport and tolerance in rice: Perspectives for reducing grain cadmium accumulation. Rice 2012, 5, 5. [Google Scholar] [CrossRef]
- Tsukahara, T.; Ezaki, T.; Moriguchi, J.; Furuki, K.; Shimbo, S.; Matsuda-Inoguchi, N.; Ikeda, M. Rice as the most influential source of cadmium intake among general Japanese population. Sci. Total. Environ. 2003, 305, 41–51. [Google Scholar] [CrossRef]
- Cai, Y.M.; Xu, W.B.; Wang, M.E.; Chen, W.P.; Li, X.Z.; Li, Y.; Cai, Y.H. Mechanisms and uncertainties of Zn supply on regulating rice Cd uptake. Environ. Pollut. 2019, 253, 959–965. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Khan, F.; Wu, C.; Saud, S.; Hassan, S.; Ahmad, N.; Gang, D.; Ullah, A.; Huang, J.L. Effects of tire rubber ash and zinc sulfate on crop productivity and cadmium accumulation in five rice cultivars under field conditions. Environ. Sci. Pollut. Res. 2015, 22, 12439–12449. [Google Scholar] [CrossRef]
- Saifullah; Sarwar, N.; Bibi, S.; Ahmad, M.; Ok, Y.S. Effectiveness of zinc application to minimize cadmium toxicity and accumulation in wheat (Triticum aestivum L.). Environ. Earth Sci. 2014, 71, 1663–1672. [Google Scholar] [CrossRef]
- Sarwar, N.; Ishaq, W.; Farid, G.; Shaheen, M.R.; Imran, M.; Geng, M.J.; Hussain, S. Zinc-cadmium interactions: Impact on wheat physiology and mineral acquisition. Ecotox. Environ. Safe. 2015, 122, 528–536. [Google Scholar] [CrossRef]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef]
- Tan, L.T.; Zhu, Y.X.; Fan, T.; Peng, C.; Wang, J.R.; Sun, L.; Chen, C.Y. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Bioph. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef]
- Yan, J.L.; Wang, P.T.; Wang, P.; Yang, M.; Lian, X.M.; Tang, Z.; Huang, C.F.; Salt, D.E.; Zhao, F.J. A loss-of-function allele of OsHMA3 associated with high cadmium accumulation in shoots and grain of Japonica rice cultivars. Plant Cell Environ. 2016, 39, 1941–1954. [Google Scholar] [CrossRef]
- Liu, X.S.; Feng, S.J.; Zhang, B.Q.; Wang, M.Q.; Cao, H.W.; Rono, J.K.; Chen, X.; Yang, Z.M. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019, 19, 283. [Google Scholar] [CrossRef]
- Huang, S.; Sasaki, A.; Yamaji, N.; Okada, H.; Mitani-Ueno, N.; Ma, J.F. The ZIP Transporter Family Member OsZIP9 Contributes To Root Zinc Uptake in Rice under Zinc-Limited Conditions. Plant Physiol. 2020, 183, 1224–1234. [Google Scholar] [CrossRef]
- Tan, L.T.; Qu, M.M.; Zhu, Y.X.; Peng, C.; Wang, J.R.; Gao, D.Y.; Chen, C.Y. ZINC TRANSPORTER5 and ZINC TRANSPORTER9 Function Synergistically in Zinc/Cadmium. Plant Physiol. 2020, 183, 1235–1249. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.T.; Liu, Z.H.; Tian, J.J.; Liang, L.M.; Qiu, Y.; Wang, G.Y.; Du, Q.Q.; Cheng, D.; Cai, H.M.; et al. A high activity zinc transporter OsZIP9 mediates zinc uptake in rice. Plant J. 2020, 103, 1695–1709. [Google Scholar] [CrossRef]
- Cai, H.M.; Huang, S.; Che, J.; Yamaji, N.; Ma, J.F. The tonoplast-localized transporter OsHMA3 plays an important role in maintaining Zn homeostasis in rice. J. Exp. Bot. 2019, 70, 2717–2725. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Ma, J.F. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J. Exp. Bot. 2014, 65, 6013–6021. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Maurya, S.; Vishwakarma, A.K.; Dubey, M.; Shrivastava, P.; Shrivastava, R.; Chandel, G. Developing gene-tagged molecular marker for functional analysis of OsZIP10 metal transporter gene in rice. Indian J. Genet. Plant Breed. 2018, 78, 180–186. [Google Scholar] [CrossRef]
- Kumar, A. Genome-Wide association study and identification of candidate genes responsible for grain zinc content in rice (Oryza sativa L.). Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2021. [Google Scholar]
- Lee, S.; An, G. Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ. 2009, 32, 408–416. [Google Scholar] [CrossRef]
- Olsen, A.N.; Ernst, H.A.; Leggio, L.L.; Skriver, K. NAC transcription factors: Structurally distinct, functionally diverse. Trends Plant Sci. 2005, 10, 79–87. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Cai, J.T.; Bi, Y.; Li, D.Y.; Song, F.M. Rice NAC transcription factor ONAC066 functions as a positive regulator of drought and oxidative stress response. BMC Plant Biol. 2019, 19, 278. [Google Scholar] [CrossRef]
- Hu, H.H.; You, J.; Fang, Y.J.; Zhu, X.Y.; Qi, Z.Y.; Xiong, L.Z. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol. Biol. 2008, 67, 169–181. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Baek, K.H.; Jung, H.; Ha, S.H.; Do Choi, Y.; Kim, M.; Reuzeau, C.; Kim, J.K. Root-Specific Expression of OsNAC10 Improves Drought Tolerance and Grain Yield in Rice under Field Drought Conditions. Plant Physiol. 2010, 153, 185–197. [Google Scholar] [CrossRef]
- Mehrotra, S.; Verma, S.; Kumar, S.; Kumari, S.; Mishra, B.N. Transcriptional regulation and signalling of cold stress response in plants: An overview of current understanding. Environ. Exp. Bot. 2020, 180, 104243. [Google Scholar] [CrossRef]
- Redillas, M.C.F.R.; Jeong, J.S.; Kim, Y.S.; Jung, H.; Bang, S.W.; Choi, Y.D.; Ha, S.H.; Reuzeau, C.; Kim, J.K. The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol. J. 2012, 10, 792–805. [Google Scholar] [CrossRef]
- Shim, J.S.; Oh, N.; Chung, P.J.; Kim, Y.S.; Do Choi, Y.; Kim, J.K. Overexpression of OsNAC14 Improves Drought Tolerance in Rice. Front. Plant Sci. 2018, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Chen, Y.; Chen, J.; Dai, X.Y.; Zhang, W.H. Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta 2011, 234, 331–345. [Google Scholar] [CrossRef]
- Zhang, X.; Long, Y.; Huang, J.J.; Xia, J.X. OsNAC45 is Involved in ABA Response and Salt Tolerance in Rice. Rice 2020, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.N.; Chen, B.; Lu, G.J.; Han, B. Overexpression of a NAC transcription factor enhances rice drought and salt tolerance. Biochem. Biophys. Res. Commun. 2009, 379, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Huang, Z.Q.; Jiang, H.; Wang, Z.; Wu, F.S.; Xiong, Y.F.; Yao, J.L. A heat stress responsive NAC transcription factor heterodimer plays key roles in rice grain filling. J. Exp. Bot. 2021, 72, 2947–2964. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Lyu, Y.S.; Yang, W.P.; Yang, Z.T.; Lu, S.J.; Liu, J.X. A membrane-associated NAC transcription factor OsNTL3 is involved in thermotolerance in rice. Plant Biotechnol. J. 2020, 18, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Zhang, H.J.; Li, D.Y.; Huang, L.; Hong, Y.B.; Ding, X.S.; Nelson, R.S.; Zhou, X.P.; Song, F.M. Functions of rice NAC transcriptional factors, ONAC122 and ONAC131, in defense responses against Magnaporthe grisea. Plant Mol. Biol. 2013, 81, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Yokotani, N.; Tsuchida-Mayama, T.; Ichikawa, H.; Mitsuda, N.; Ohme-Takagi, M.; Kaku, H.; Minami, E.; Nishizawa, Y. OsNAC111, a Blast Disease-Responsive Transcription Factor in Rice, Positively Regulates the Expression of Defense-Related Genes. Mol. Plant Microbe. Interact. 2014, 27, 1027–1034. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, Y.F.; Xie, Z.Z.; Yu, B.; Sun, Y.; Huang, J.L. OsNAC016 regulates plant architecture and drought tolerance by interacting with the kinases GSK2 and SAPK8. Plant Physiol. 2022, 189, 1296–1313. [Google Scholar] [CrossRef]
- Wang, J.; Bao, J.L.; Zhou, B.B.; Li, M.; Li, X.Z.; Jin, J. The osa-miR164 target OsCUC1 functions redundantly with OsCUC3 in controlling rice meristem/organ boundary specification. New Phytol. 2021, 229, 1566–1581. [Google Scholar] [CrossRef]
- Jiang, D.G.; Chen, W.T.; Dong, J.F.; Li, J.; Yang, F.; Wu, Z.C.; Zhou, H.; Wang, W.S.; Zhuang, C.X. Overexpression of miR164b-resistant OsNAC2 improves plant architecture and grain yield in rice. J. Exp. Bot. 2018, 69, 1533–1543. [Google Scholar] [CrossRef]
- Mao, C.J.; Lu, S.C.; Lv, B.; Zhang, B.; Shen, J.B.; He, J.M.; Luo, L.Q.; Xi, D.D.; Chen, X.; Ming, F. A Rice NAC Transcription Factor Promotes Leaf Senescence via ABA Biosynthesis. Plant Physiol. 2017, 174, 1747–1763. [Google Scholar] [CrossRef]
- Xin, W.; Wang, J.G.; Li, J.; Zhao, H.W.; Liu, H.L.; Zheng, H.L.; Yang, L.M.; Wang, C.; Yang, F.; Chen, J.H.; et al. Candidate Gene Analysis for Nitrogen Absorption and Utilization in Japonica Rice at the Seedling Stage Based on a Genome-Wide Association Study. Front. Plant Sci. 2021, 12, 670861. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Pandit, E.; Pawar, S.; Pradhan, A.; Behera, L.; Das, S.R.; Pathak, H. Genetic regulation of homeostasis, uptake, bio-fortification and efficiency enhancement of iron in rice. Environ. Exp. Bot. 2020, 177, 104066. [Google Scholar] [CrossRef]
- Moreno-Alvarado, M.; Garcia-Morales, S.; Trejo-Tellez, L.I.; Hidalgo-Contreras, J.V.; Gomez-Merino, F.C. Aluminum Enhances Growth and Sugar Concentration, Alters Macronutrient Status and Regulates the Expression of NAC Transcription Factors in Rice. Front. Plant Sci. 2017, 8, 73. [Google Scholar] [CrossRef]
- Tan, M.P.; Cheng, D.; Yang, Y.N.; Zhang, G.Q.; Qin, M.J.; Chen, J.; Chen, Y.H.; Jiang, M.Y. Co-expression network analysis of the transcriptomes of rice roots exposed to various cadmium stresses reveals universal cadmium-responsive genes. BMC Plant Biol. 2017, 17, 194. [Google Scholar] [CrossRef]
- Hu, S.B.; Shinwari, K.I.; Song, Y.R.; Xia, J.X.; Xu, H.; Du, B.B.; Luo, L.; Zheng, L.Q. OsNAC300 Positively Regulates Cadmium Stress Responses and Tolerance in Rice Roots. Agronomy 2021, 11, 95. [Google Scholar] [CrossRef]
- Wang, B.X.; Tan, M.P.; Liu, Y.; Xu, D.Y.; Zheng, Q.S.; Zhao, H.Y.; Zhang, J. Application of rice transcription factor OsNAC3 in improving cadmium tolerance of plants. 2020, CN111041035A. (In Chinese). Available online: http://epub.cnipa.gov.cn/Dxb/IndexQuery (accessed on 25 July 2022).
- Lu, X.; Liu, S.; Zhi, S.; Chen, J.; Ye, G. Comparative transcriptome profile analysis of rice varieties with different tolerance to zinc deficiency. Plant Biol. 2021, 23, 375–390. [Google Scholar] [CrossRef]
- Hu, S.B.; Yu, Y.; Chen, Q.H.; Mu, G.M.; Shen, Z.G.; Zheng, L.Q. OsMYB45 plays an important role in rice resistance to cadmium stress. Plant Sci. 2017, 264, 1–8. [Google Scholar] [CrossRef]
- Shim, D.; Hwang, J.U.; Lee, J.; Lee, S.; Choi, Y.; An, G.; Martinoia, E.; Lee, Y. Orthologs of the Class A4 Heat Shock Transcription Factor HsfA4a Confer Cadmium Tolerance in Wheat and Rice. Plant Cell 2009, 21, 4031–4043. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Uraguchi, S.; Kajikawa, M.; Saito, A.; Ohmori, Y.; Fujiwara, T. A rice PHD-finger protein OsTITANIA, is a growth regulator that functions through elevating expression of transporter genes for multiple metals. Plant J. 2018, 96, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.F.; Gong, S.H.; Wang, Y.; Wang, F.J.; Bao, H.X.G.D.L.; Sun, J.W.; Cai, C.; Yi, K.K.; Chen, Z.X.; Zhu, C. MicroRNA166 Modulates Cadmium Tolerance and Accumulation in Rice. Plant Physiol. 2018, 177, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Calayugan, M.I.C.; Formantes, A.K.; Amparado, A.; Descalsota-Empleo, G.I.; Nha, C.T.; Inabangan-Asilo, M.A.; Swe, Z.M.; Hernandez, J.E.; Borromeo, T.H.; Lalusin, A.G.; et al. Genetic Analysis of Agronomic Traits and Grain Iron and Zinc Concentrations in a Doubled Haploid Population of Rice (Oryza sativa L.). Sci. Rep. 2020, 10, 2283. [Google Scholar] [CrossRef]
- Ji, L.; Hu, R.; Jiang, J.; Qi, G.; Yang, X.; Zhu, M.; Fu, C.; Zhou, G.; Yi, Z. Molecular cloning and expression analysis of 13 NAC transcription factors in Miscanthus lutarioriparius. Plant Cell Rep. 2014, 33, 2077–2092. [Google Scholar] [CrossRef]
- Assuncao, A.G.L.; Herrero, E.; Lin, Y.F.; Huettel, B.; Talukdar, S.; Smaczniak, C.; Immink, R.G.H.; van Eldik, M.; Fiers, M.; Schat, H.; et al. Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc. Natl. Acad. Sci. USA 2010, 107, 10296–10301. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Masuda, H.; Suzuki, M.; Bashir, K.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Overexpression of the OsZIP4 zinc transporter confers disarrangement of zinc distribution in rice plants. J. Exp. Bot. 2007, 58, 2909–2915. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, H.J.; Kim, S.A.; Lee, J.; Guerinot, M.L.; An, G. OsZIP5 is a plasma membrane zinc transporter in rice. Plant Mol. Biol. 2010, 73, 507–517. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.A.; Lee, J.; Guerinot, M.L.; An, G. Zinc Deficiency-Inducible OsZIP8 Encodes a Plasma Membrane-Localized Zinc Transporter in Rice. Mol. Cells 2010, 29, 551–558. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Mitani-Ueno, N.; Kashino, M.; Ma, J.F. A node-localized transporter OsZIP3 is responsible for the preferential distribution of Zn to developing tissues in rice. Plant J. 2015, 84, 374–384. [Google Scholar] [CrossRef]
- Fang, Y.J.; You, J.; Xie, K.B.; Xie, W.B.; Xiong, L.Z. Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Mol. Genet. Genom. 2008, 280, 547–563. [Google Scholar] [CrossRef]
- Lang, H.; He, Y.T.; Zeng, F.L.; Xu, F.; Zhao, M.H.; Ma, D.R. Comparative transcriptomic and physiological analyses of weedy rice and cultivated rice to identify vital differentially expressed genes and pathways regulating the ABA response. Sci. Rep. 2021, 11, 12881. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Manimekalai, R.; Sharoni, A.M.; Satoh, K.; Kondoh, H.; Ooka, H.; Kikuchi, S. Genome-wide analysis of NAC transcription factor family in rice. Gene 2010, 465, 30–44. [Google Scholar] [CrossRef]
- Tezuka, D.; Kawamata, A.; Kato, H.; Saburi, W.; Mori, H.; Imai, R. The rice ethylene response factor OsERF83 positively regulates disease resistance to Magnaporthe oryzae. Plant Physiol. Biochem. 2019, 135, 263–271. [Google Scholar] [CrossRef]
- Di, X.R.; Zheng, F.; Norton, G.J.; Beesley, L.; Zhang, Z.L.; Lin, H.; Zhi, S.L.; Liu, X.C.; Ding, Y.Z. Physiological responses and transcriptome analyses of upland rice following exposure to arsenite and arsenate. Environ. Exp. Bot. 2021, 183, 104366. [Google Scholar] [CrossRef]
- Mohanty, B. Promoter Architecture and Transcriptional Regulation of Genes Upregulated in Germination and Coleoptile Elongation of Diverse Rice Genotypes Tolerant to Submergence. Front. Genet. 2021, 12, 235. [Google Scholar] [CrossRef]
- Gindri, R.G.; Navarro, B.B.; da Cruz Dias, P.V.; Tarouco, C.P.; Nicoloso, F.T.; Brunetto, G.; Berghetti, A.L.P.; da Silva, L.O.S.; Fett, J.P.; Menguer, P.K.; et al. Physiological responses of rice (Oryza sativa L.) oszip7 loss-of-function plants exposed to varying Zn concentrations. Physiol. Mol. Biol. Plants 2020, 26, 1349–1359. [Google Scholar] [CrossRef]
- Lilay, G.H.; Castro, P.H.; Guedes, J.G.; Almeida, D.M.; Campilho, A.; Azevedo, H.; Aarts, M.G.M.; Saibo, N.J.M.; Assuncao, A.G.L. Rice F-bZIP transcription factors regulate the zinc deficiency response. J. Exp. Bot. 2020, 71, 3664–3677. [Google Scholar] [CrossRef]
- Evens, N.P.; Buchner, P.; Williams, L.E.; Hawkesford, M.J. The role of ZIP transporters and group F bZIP transcription factors in the Zn-deficiency response of wheat (Triticum aestivum). Plant J. 2017, 92, 291–304. [Google Scholar] [CrossRef]
- Martin, R.C.; Vining, K.; Dombrowski, J.E. Genome-wide (ChIP-seq) identification of target genes regulated by BdbZIP10 during paraquat-induced oxidative stress. BMC Plant Biol. 2018, 18, 58. [Google Scholar] [CrossRef]
- Nazri, A.Z.; Griffin, J.H.C.; Peaston, K.A.; Alexander-Webber, D.G.A.; Williams, L.E. F-group bZIPs in barley-a role in Zn deficiency. Plant Cell Environ. 2017, 40, 2754–2770. [Google Scholar] [CrossRef]
- Gao, S.P.; Xiao, Y.H.; Xu, F.; Gao, X.K.; Cao, S.Y.; Zhang, F.X.; Wang, G.D.; Sanders, D.; Chu, C.C. Cytokinin-dependent regulatory module underlies the maintenance of zinc nutrition in rice. New Phytol. 2019, 224, 202–215. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 Is a Major Transporter Responsible for Manganese and Cadmium Uptake in Rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, H.; Li, X.; Xiao, J.; Xiong, L. Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol. 2012, 158, 1755–1768. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, J.; Zou, W.; Li, S.; Tang, J.; Lu, X.; Meng, L.; Ye, G. OsNAC15 Regulates Tolerance to Zinc Deficiency and Cadmium by Binding to OsZIP7 and OsZIP10 in Rice. Int. J. Mol. Sci. 2022, 23, 11771. https://doi.org/10.3390/ijms231911771
Zhan J, Zou W, Li S, Tang J, Lu X, Meng L, Ye G. OsNAC15 Regulates Tolerance to Zinc Deficiency and Cadmium by Binding to OsZIP7 and OsZIP10 in Rice. International Journal of Molecular Sciences. 2022; 23(19):11771. https://doi.org/10.3390/ijms231911771
Chicago/Turabian StyleZhan, Junhui, Wenli Zou, Shuangyuyan Li, Jichun Tang, Xiang Lu, Lijun Meng, and Guoyou Ye. 2022. "OsNAC15 Regulates Tolerance to Zinc Deficiency and Cadmium by Binding to OsZIP7 and OsZIP10 in Rice" International Journal of Molecular Sciences 23, no. 19: 11771. https://doi.org/10.3390/ijms231911771
APA StyleZhan, J., Zou, W., Li, S., Tang, J., Lu, X., Meng, L., & Ye, G. (2022). OsNAC15 Regulates Tolerance to Zinc Deficiency and Cadmium by Binding to OsZIP7 and OsZIP10 in Rice. International Journal of Molecular Sciences, 23(19), 11771. https://doi.org/10.3390/ijms231911771







