The Application of Cellulose Acetate Membranes for Separation of Fermentation Broths by the Reverse Osmosis: A Feasibility Study
Abstract
1. Introduction
2. Results and Discussion
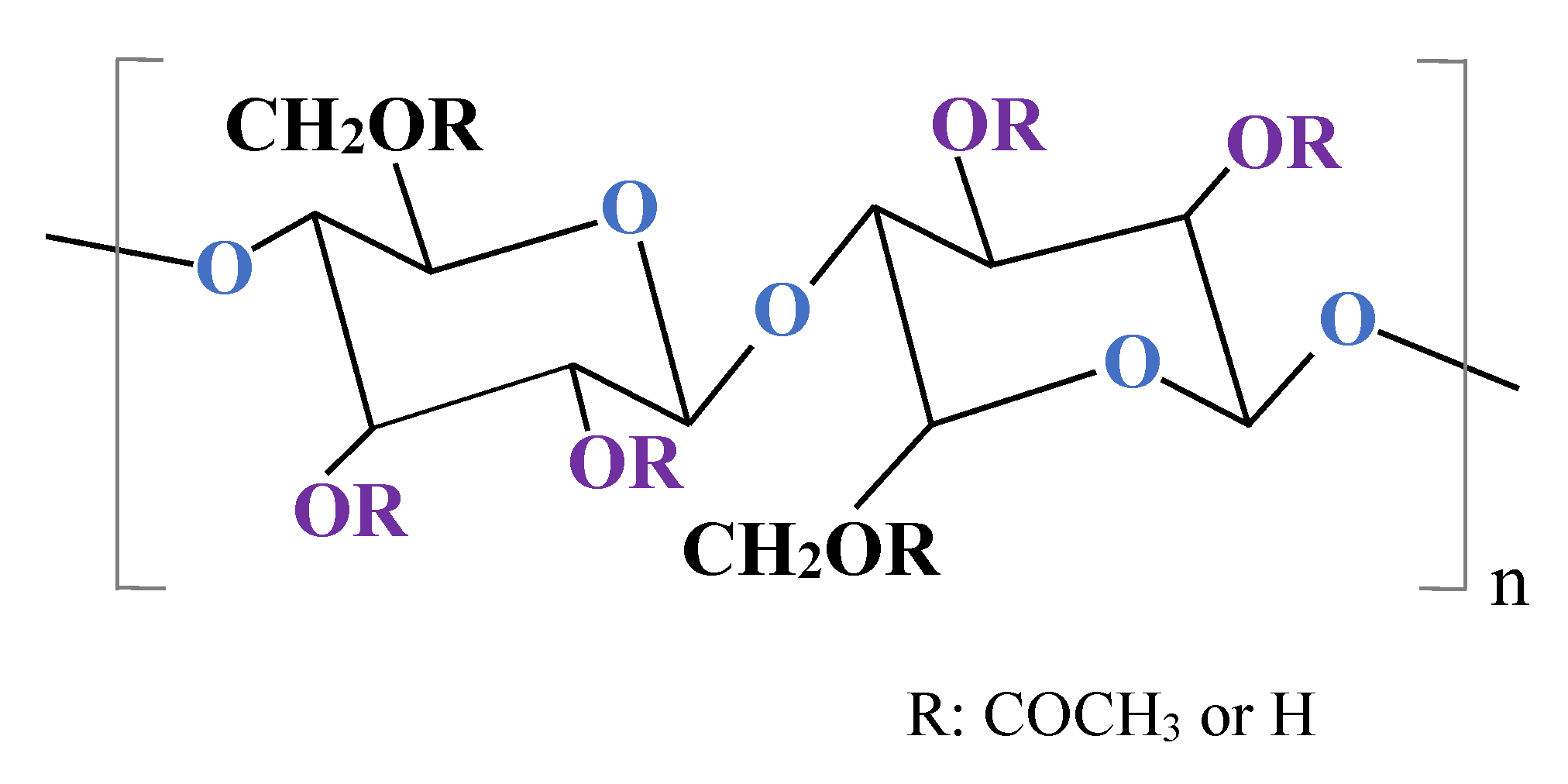
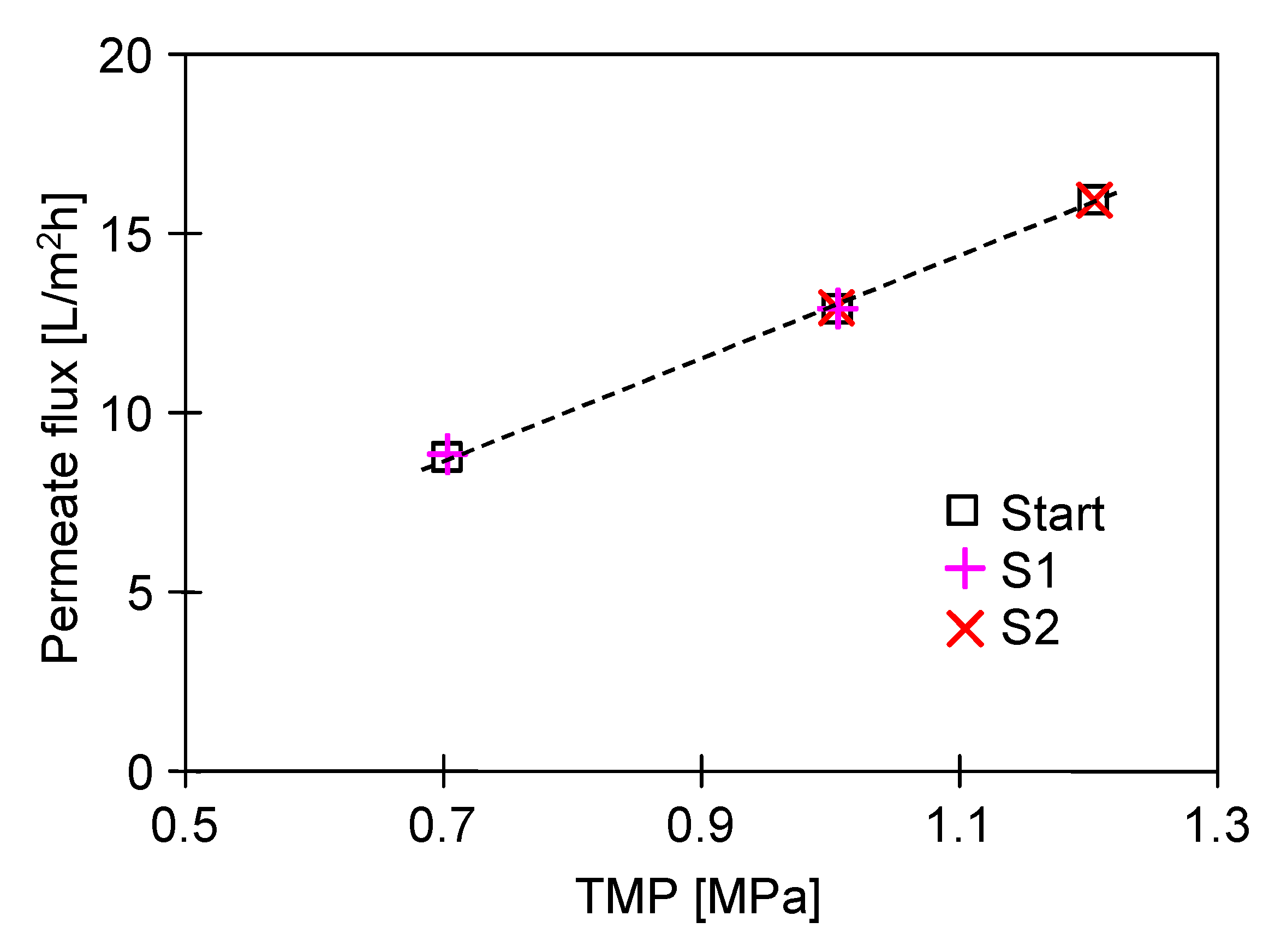
2.1. Membrane Performance
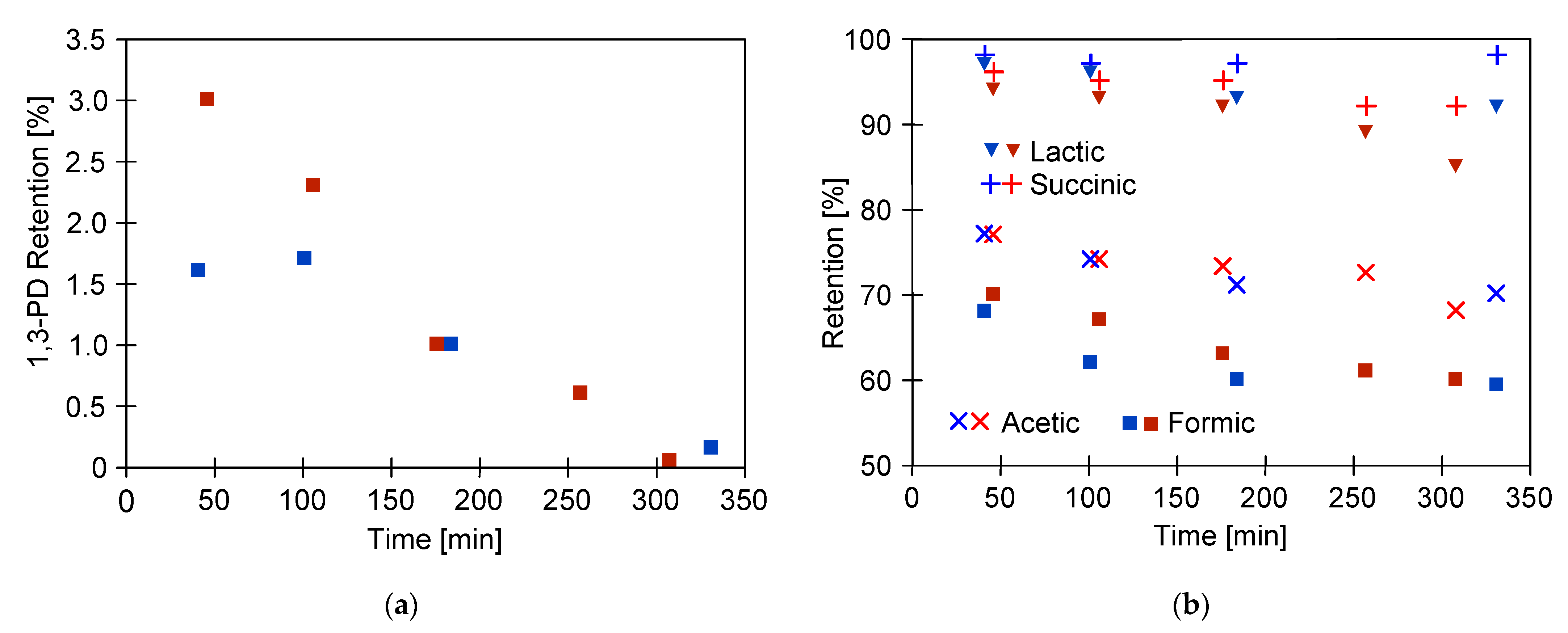
2.2. Separation of 1,3-Propanediol and Carboxylic Acids
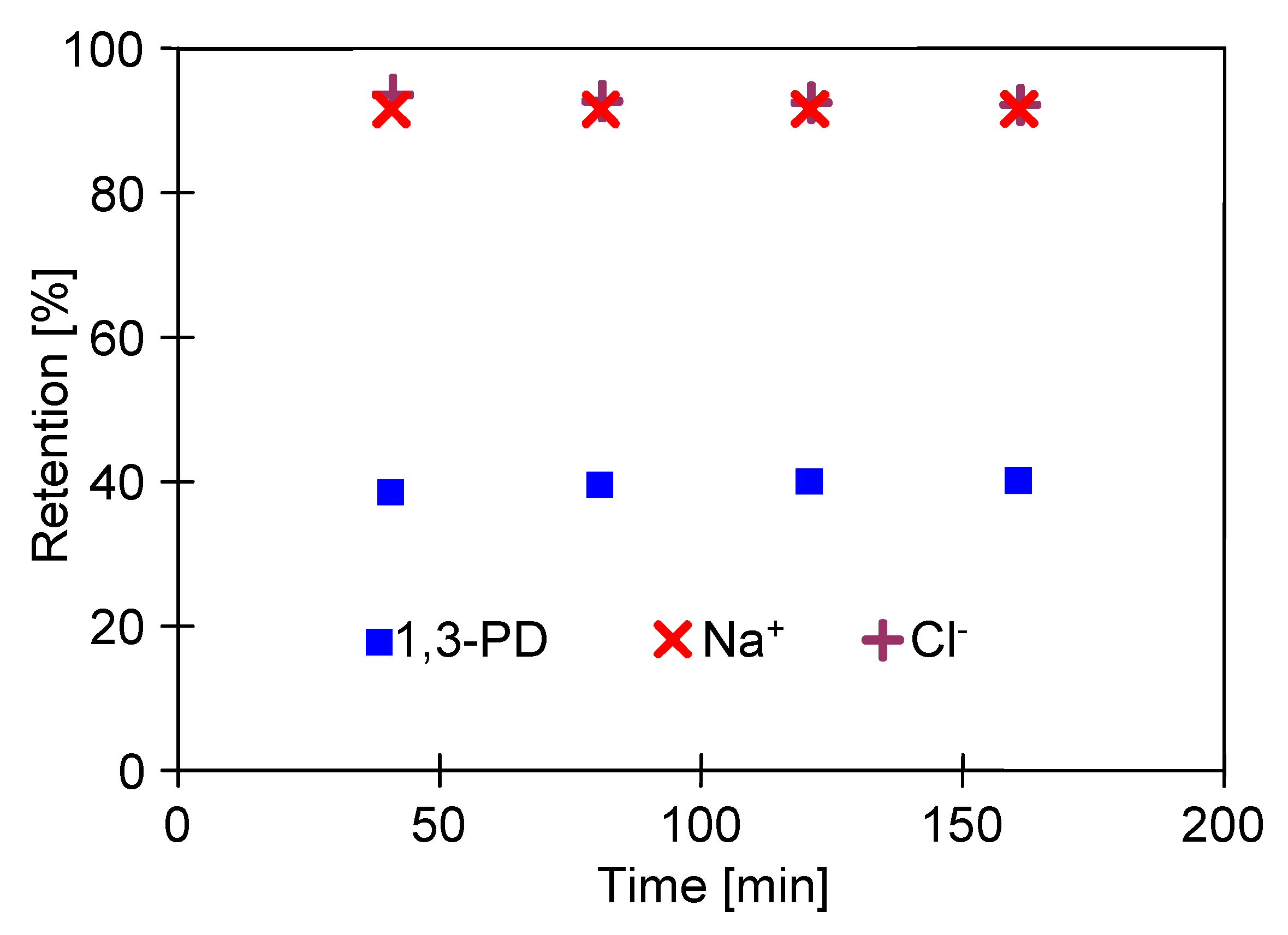
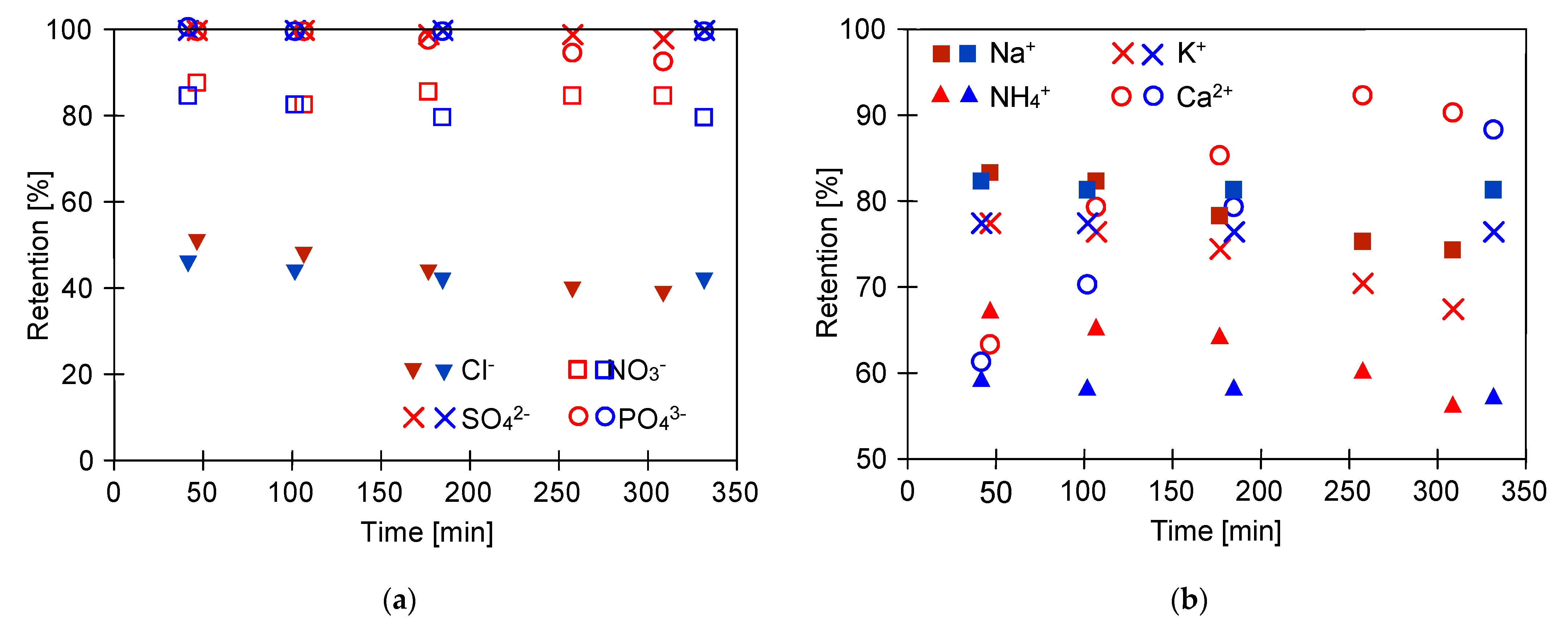
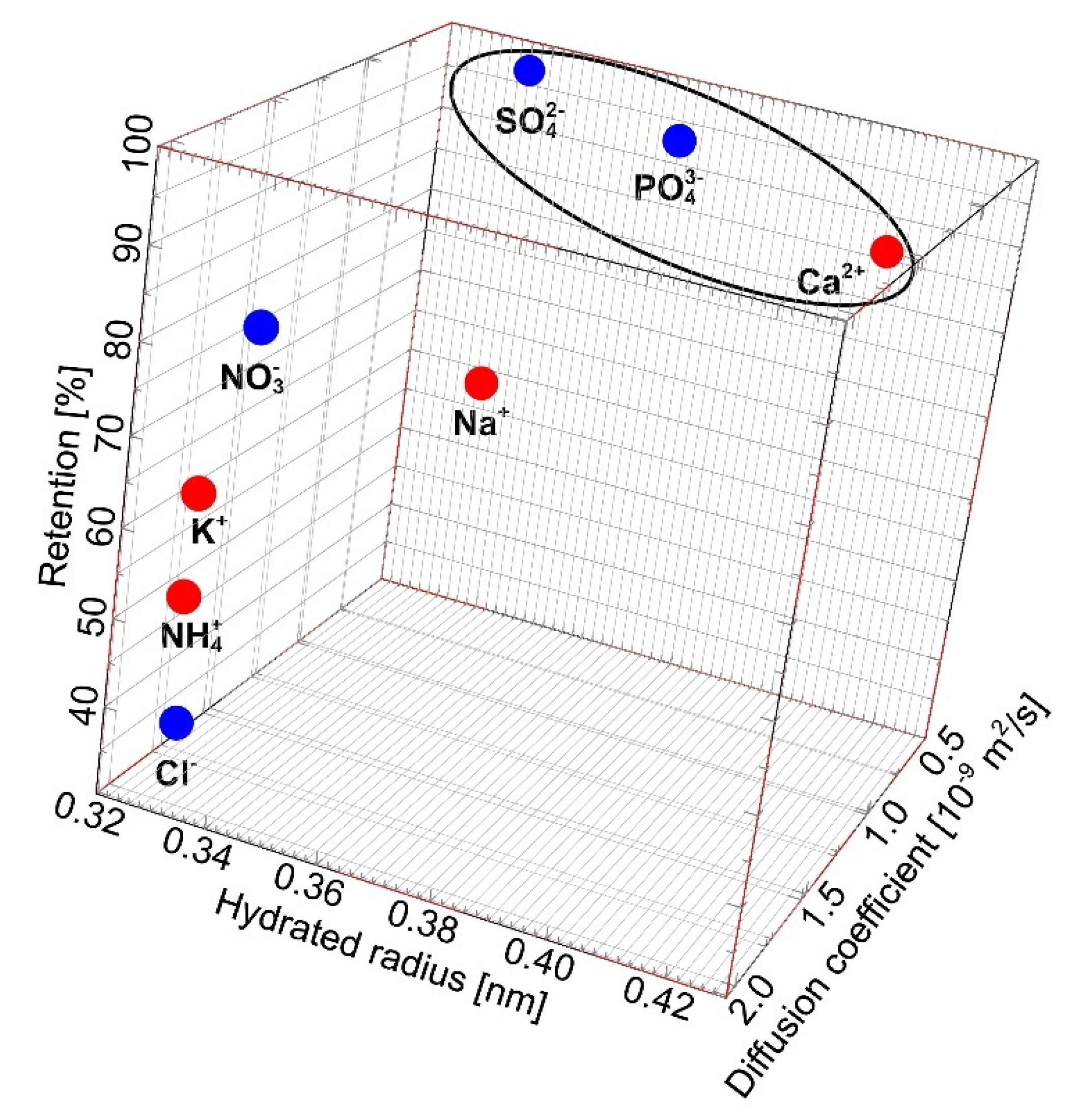
2.3. Separation of Salts Anions and Cations
3. Materials and Methods
3.1. Glycerol Fermentation
3.2. RO Set-Up
3.3. Analytical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damasceno, A.P.K.; Rossi, D.M.; Ayub, M.A.Z. Biosynthesis of 1,3-propanodiol and 2,3-butanodiol from Residual Glycerol in Continuous Cell-immobilized Klebsiella pneumoniae Bioreactors. Biotechnol. Prog. 2022, 38, e3265. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, Y.-H. Fermentation Redox Potential Control on the 1,3-Propanediol Production by Lactobacillus panis PM1. Process Biochem. 2022, 114, 139–146. [Google Scholar] [CrossRef]
- Fokum, E.; Zabed, H.M.; Ravikumar, Y.; Elshobary, M.E.; Chandankere, R.; Zhang, Y.; Yun, J.; Qi, X. Co-Fermentation of Glycerol and Sugars by Clostridium beijerinckii: Enhancing the Biosynthesis of 1,3-Propanediol. Food Biosci. 2021, 41, 101028. [Google Scholar] [CrossRef]
- Kongjan, P.; Jariyaboon, R.; Reungsang, A.; Sittijunda, S. Co-Fermentation of 1,3-Propanediol and 2,3-Butanediol from Crude Glycerol Derived from the Biodiesel Production Process by Newly Isolated Enterobacter Sp.: Optimization Factors Affecting. Bioresour. Technol. Rep. 2021, 13, 100616. [Google Scholar] [CrossRef]
- Morita, T.; Kiyoshi, K.; Nonaka, D.; Seta, K.; Suzuki, T.; Nakajima-Kambe, T. Conversion of Glycerol from Biodiesel Fuels to 1,3-Propanediol by Citrobacter braakii TB-96. New Biotechnol. 2016, 33, S93. [Google Scholar] [CrossRef]
- Antczak, J.; Szczygielda, M.; Prochaska, K. An Environment-Friendly Multi-Step Membrane-Based System to Succinic Acid Recovery from the Fermentation Broth. Desalination Water Treat. 2018, 128, 51–60. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Clarification of 1,3-Propanediol Fermentation Broths by Using a Ceramic Fine UF Membrane. Membranes 2020, 10, 319. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, H.D.; Park, H.B. Integrated Membrane Processes for Separation and Purification of Organic Acid from a Biomass Fermentation Process. Ind. Eng. Chem. Res. 2012, 51, 10207–10219. [Google Scholar] [CrossRef]
- Westbrook, A.W.; Miscevic, D.; Kilpatrick, S.; Bruder, M.R.; Moo-Young, M.; Chou, C.P. Strain Engineering for Microbial Production of Value-Added Chemicals and Fuels from Glycerol. Biotechnol. Adv. 2019, 37, 538–568. [Google Scholar] [CrossRef]
- Lima, P.J.M.; da Silva, R.M.; Neto, C.A.C.G.; Gomes e Silva, N.C.; da Souza, J.E.S.; Nunes, Y.L.; Sousa dos Santos, J.C. An Overview on the Conversion of Glycerol to Value-added Industrial Products via Chemical and Biochemical Routes. Biotech. App. Biochem. 2021. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Kurihara, M.; Wang, R. Seawater Desalination by Reverse Osmosis: Current Development and Future Challenges in Membrane Fabrication—A Review. J. Membr. Sci. 2021, 629, 119292. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse Osmosis Desalination: A State-of-the-Art Review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Hailemariam, R.H.; Woo, Y.C.; Damtie, M.M.; Kim, B.C.; Park, K.-D.; Choi, J.-S. Reverse Osmosis Membrane Fabrication and Modification Technologies and Future Trends: A Review. Adv. Colloid Interface Sci. 2020, 276, 102100. [Google Scholar] [CrossRef]
- Wei, Q.J.; Tucker, C.I.; Wu, P.J.; Trueworthy, A.M.; Tow, E.W.; Lienhard, J.H. Impact of Salt Retention on True Batch Reverse Osmosis Energy Consumption: Experiments and Model Validation. Desalination 2020, 479, 114177. [Google Scholar] [CrossRef]
- Pérez-González, A.; Urtiaga, A.M.; Ibáñez, R.; Ortiz, I. State of the Art and Review on the Treatment Technologies of Water Reverse Osmosis Concentrates. Water Res. 2012, 46, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Košutić, K.; Dolar, D.; Ašperger, D.; Kunst, B. Removal of Antibiotics from a Model Wastewater by RO/NF Membranes. Sep. Purif. Technol. 2007, 53, 244–249. [Google Scholar] [CrossRef]
- Davey, C.J.; Havill, A.; Leak, D.; Patterson, D.A. Nanofiltration and Reverse Osmosis Membranes for Purification and Concentration of a 2,3-Butanediol Producing Gas Fermentation Broth. J. Membr. Sci. 2016, 518, 150–158. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Porada, S.; Elimelech, M.; Dykstra, J.E. Tutorial Review of Reverse Osmosis and Electrodialysis. J. Membr. Sci. 2022, 647, 120221. [Google Scholar] [CrossRef]
- Pervez, M.N.; Mahboubi, A.; Uwineza, C.; Zarra, T.; Belgiorno, V.; Naddeo, V.; Taherzadeh, M.J. Factors Influencing Pressure-Driven Membrane-Assisted Volatile Fatty Acids Recovery and Purification-A Review. Sci. Total Environ. 2022, 817, 152993. [Google Scholar] [CrossRef]
- Asadollahi, M.; Bastani, D.; Musavi, S.A. Enhancement of Surface Properties and Performance of Reverse Osmosis Membranes after Surface Modification: A Review. Desalination 2017, 420, 330–383. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Membrane-Based Separation of Potential Emerging Pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Li, H. Rejection of Organic Compounds by Ultra-Low Pressure Reverse Osmosis Membrane. Water Res. 2002, 36, 123–130. [Google Scholar] [CrossRef]
- Sadare, O.O.; Ejekwu, O.; Moshokoa, M.F.; Jimoh, M.O.; Daramola, M.O. Membrane Purification Techniques for Recovery of Succinic Acid Obtained from Fermentation Broth during Bioconversion of Lignocellulosic Biomass: Current Advances and Future Perspectives. Sustainability 2021, 13, 6794. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Yang, B.; Xiao, K.; Zhao, H. Separation, Anti-Fouling, and Chlorine Resistance of the Polyamide Reverse Osmosis Membrane: From Mechanisms to Mitigation Strategies. Water Res. 2021, 195, 116976. [Google Scholar] [CrossRef]
- Li, S.; Zhuang, J.; Zhi, T.; Chen, H.; Zhang, L. Combination of Complex Extraction with Reverse Osmosis for the Treatment of Fumaric Acid Industrial Wastewater. Desalination 2008, 234, 362–369. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, C.; Wei, J. Separation of Acetic Acid from Monosaccharides by NF and RO Membranes: Performance Comparison. J. Membr. Sci. 2013, 429, 243–251. [Google Scholar] [CrossRef]
- Li, D.; Yan, Y.; Wang, H. Recent Advances in Polymer and Polymer Composite Membranes for Reverse and Forward Osmosis Processes. Prog. Polym. Sci. 2016, 61, 104–155. [Google Scholar] [CrossRef]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental Water and Salt Transport Properties of Polymeric Materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Kim, D.H. A Review of Desalting Process Techniques and Economic Analysis of the Recovery of Salts from Retentates. Desalination 2011, 270, 1–8. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse Osmosis Pretreatment Technologies and Future Trends: A Comprehensive Review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Tabatabai, S.A.A.; Anderson, D.M.; Amy, G.L.; Schippers, J.C.; Kennedy, M.D. Seawater Reverse Osmosis Desalination and (Harmful) Algal Blooms. Desalination 2015, 360, 61–80. [Google Scholar] [CrossRef]
- Pearson, J.L.; Michael, P.R.; Ghaffour, N.; Missimer, T.M. Economics and Energy Consumption of Brackish Water Reverse Osmosis Desalination: Innovations and Impacts of Feedwater Quality. Membranes 2021, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiao, K.; Huang, X. Characterizing the Roles of Organic and Inorganic Foulants in RO Membrane Fouling Development: The Case of Coal Chemical Wastewater Treatment. Sep. Purif. Technol. 2019, 210, 1008–1016. [Google Scholar] [CrossRef]
- Schmidt, S.-A.; Gukelberger, E.; Hermann, M.; Fiedler, F.; Großmann, B.; Hoinkis, J.; Ghosh, A.; Chatterjee, D.; Bundschuh, J. Pilot Study on Arsenic Removal from Groundwater Using a Small-Scale Reverse Osmosis System—Towards Sustainable Drinking Water Production. J. Hazard. Mater. 2016, 318, 671–678. [Google Scholar] [CrossRef]
- Timmer, J.M.K.; Kromkamp, J.; Robbertsen, T. Lactic Acid Separation from Fermentation Broths by Reverse Osmosis and Nanofiltration. J. Membr. Sci. 1994, 92, 185–197. [Google Scholar] [CrossRef]
- Schlicher, L.R.; Cheryan, M. Reverse Osmosis of Lactic Acid Fermentation Broths. J. Chem. Technol. Biotechnol. 1990, 49, 129–140. [Google Scholar] [CrossRef]
- Phanthumchinda, N.; Rampai, T.; Prasirtsak, B.; Thitiprasert, S.; Tanasupawat, S.; Assabumrungrat, S.; Thongchul, N. Alternative Reverse Osmosis to Purify Lactic Acid from a Fermentation Broth. CICEQ 2018, 24, 179–190. [Google Scholar] [CrossRef]
- Priya, A.; Dureja, P.; Rathi, R.; Lal, B. Comparative Assessment of Separation Techniques for Downstream Processing of 2,3-Butanediol. Fuel 2021, 292, 120351. [Google Scholar] [CrossRef]
- Omwene, P.I.; Sarihan, Z.B.O.; Karagunduz, A.; Keskinler, B. Bio-Based Succinic Acid Recovery by Ion Exchange Resins Integrated with Nanofiltration/Reverse Osmosis Preceded Crystallization. Food Bioprod. Process. 2021, 129, 1–9. [Google Scholar] [CrossRef]
- Khunnonkwao, P.; Jantama, K.; Kanchanatawee, S.; Galier, S.; Roux-de Balmann, H. A Two Steps Membrane Process for the Recovery of Succinic Acid from Fermentation Broth. Sep. Purif. Technol. 2018, 207, 451–460. [Google Scholar] [CrossRef]
- Sagne, C.; Fargues, C.; Lewandowski, R.; Lameloise, M.-L.; Decloux, M. Screening of Reverse Osmosis Membranes for the Treatment and Reuse of Distillery Condensates into Alcoholic Fermentation. Desalination 2008, 219, 335–347. [Google Scholar] [CrossRef]
- Diltz, R.A.; Marolla, T.V.; Henley, M.V.; Li, L. Reverse Osmosis Processing of Organic Model Compounds and Fermentation Broths. Bioresour. Technol. 2007, 98, 686–695. [Google Scholar] [CrossRef]
- Reid, C.E.; Breton, E.J. Water and Ion Flow across Cellulosic Membranes. J. Appl. Polym. Sci. 1959, 1, 133–143. [Google Scholar] [CrossRef]
- Paul, D. Reformulation of the Solution-Diffusion Theory of Reverse Osmosis. J. Membr. Sci. 2004, 241, 371–386. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef]
- Li, D.; Wang, H. Recent Developments in Reverse Osmosis Desalination Membranes. J. Mater. Chem. 2010, 20, 4551. [Google Scholar] [CrossRef]
- Vatanpour, V.; Pasaoglu, M.E.; Barzegar, H.; Teber, O.O.; Kaya, R.; Bastug, M.; Khataee, A.; Koyuncu, I. Cellulose Acetate in Fabrication of Polymeric Membranes: A Review. Chemosphere 2022, 295, 133914. [Google Scholar] [CrossRef]
- De Guzman, M.R.; Andra, C.K.A.; Ang, M.B.M.Y.; Dizon, G.V.C.; Caparanga, A.R.; Huang, S.-H.; Lee, K.-R. Increased Performance and Antifouling of Mixed-Matrix Membranes of Cellulose Acetate with Hydrophilic Nanoparticles of Polydopamine-Sulfobetaine Methacrylate for Oil-Water Separation. J. Membr. Sci. 2021, 620, 118881. [Google Scholar] [CrossRef]
- Andrade, P.F.; de Faria, A.F.; Quites, F.J.; Oliveira, S.R.; Alves, O.L.; Arruda, M.A.Z.; Gonçalves, M.d.C. Inhibition of Bacterial Adhesion on Cellulose Acetate Membranes Containing Silver Nanoparticles. Cellulose 2015, 22, 3895–3906. [Google Scholar] [CrossRef]
- Worthley, C.H.; Constantopoulos, K.T.; Ginic-Markovic, M.; Pillar, R.J.; Matisons, J.G.; Clarke, S. Surface Modification of Commercial Cellulose Acetate Membranes Using Surface-Initiated Polymerization of 2-Hydroxyethyl Methacrylate to Improve Membrane Surface Biofouling Resistance. J. Membr. Sci. 2011, 385–386, 30–39. [Google Scholar] [CrossRef]
- Idress, H.; Zaidi, S.Z.J.; Sabir, A.; Shafiq, M.; Khan, R.U.; Harito, C.; Hassan, S.; Walsh, F.C. Cellulose Acetate Based Complexation-NF Membranes for the Removal of Pb(II) from Waste Water. Sci. Rep. 2021, 11, 1806. [Google Scholar] [CrossRef]
- El-Din, L.A.N.; El-Gendi, A.; Ismail, N.; Abed, K.A.; Ahmed, A.I. Evaluation of Cellulose Acetate Membrane with Carbon Nanotubes Additives. J. Ind. Eng. Chem. 2015, 26, 259–264. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, F. Hydrophilicity and Antifouling Property of Membrane Materials from Cellulose Acetate/Polyethersulfone in DMAc. Int. J. Biol. Macromol. 2016, 91, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-S.; Liu, Z.-T.; Liu, Z.-W.; Lu, J. Cellulose Acetate Membrane Synthesis from Biomass of Ramie. J. Appl. Polym. Sci. 2010, 117, 588–595. [Google Scholar] [CrossRef]
- McCray, S.B.; Vilker, V.L.; Nobe, K. Reverse Osmosis Cellulose Acetate Membranes. I. Rate of Hydrolysis. J. Membr. Sci. 1991, 59, 305–316. [Google Scholar] [CrossRef]
- Goosen, M.F.A.; Sablani, S.S.; Al-Maskari, S.S.; Al-Belushi, R.H.; Wilf, M. Effect of Feed Temperature on Permeate Flux and Mass Transfer Coefficient in Spiral-Wound Reverse Osmosis Systems. Desalination 2002, 144, 367–372. [Google Scholar] [CrossRef]
- Nguyen, N.; Fargues, C.; Guiga, W.; Lameloise, M.-L. Assessing Nanofiltration and Reverse Osmosis for the Detoxification of Lignocellulosic Hydrolysates. J. Membr. Sci. 2015, 487, 40–50. [Google Scholar] [CrossRef]
- Jin, X.; Jawor, A.; Kim, S.; Hoek, E.M.V. Effects of Feed Water Temperature on Separation Performance and Organic Fouling of Brackish Water RO Membranes. Desalination 2009, 239, 346–359. [Google Scholar] [CrossRef]
- Bódalo, A.; Gómez, J.-L.; Gómez, E.; León, G.; Tejera, M. Ammonium Removal from Aqueous Solutions by Reverse Osmosis Using Cellulose Acetate Membranes. Desalination 2005, 184, 149–155. [Google Scholar] [CrossRef]
- Guu, Y.-K.; Chiu, C.-H.; Young, J.-K. Processing of Soybean Soaking Water with a NF−RO Membrane System and Lactic Acid Fermentation of Retained Solutes. J. Agric. Food Chem. 1997, 45, 4096–4100. [Google Scholar] [CrossRef]
- Amiri, M.C.; Samiei, M. Enhancing Permeate Flux in a RO Plant by Controlling Membrane Fouling. Desalination 2007, 207, 361–369. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, L.; Du, H.; Xu, S.; Du, Y. Study on the Concentration of Acrylic Acid and Acetic Acid by Reverse Osmosis. Membranes 2020, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Najid, N.; Hakizimana, J.N.; Kouzbour, S.; Gourich, B.; Ruiz-García, A.; Vial, C.; Stiriba, Y.; Semiat, R. Fouling Control and Modeling in Reverse Osmosis for Seawater Desalination: A Review. Comput. Chem. Eng. 2022, 162, 107794. [Google Scholar] [CrossRef]
- Matin, A.; Laoui, T.; Falath, W.; Farooque, M. Fouling Control in Reverse Osmosis for Water Desalination & Reuse: Current Practices & Emerging Environment-Friendly Technologies. Sci. Total Environ. 2021, 765, 142721. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, N. A Short Review on Reverse Osmosis Membranes: Fouling and Control. OAJWX 2019, 2, 1–10. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chong, T.H.; Fane, A.G. Colloidal Interactions and Fouling of NF and RO Membranes: A Review. Adv. Colloid Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, A.; Melián-Martel, N.; Nuez, I. Short Review on Predicting Fouling in RO Desalination. Membranes 2017, 7, 62. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Nuez, I. Performance Assessment of SWRO Spiral-Wound Membrane Modules with Different Feed Spacer Dimensions. Processes 2020, 8, 692. [Google Scholar] [CrossRef]
- Ruiz-García, A.; de la Nuez Pestana, I. Feed Spacer Geometries and Permeability Coefficients. Effect on the Performance in BWRO Spriral-Wound Membrane Modules. Water 2019, 11, 152. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Voutchkov, N.; Weinrich, L.; Jacangelo, J.G. Selection of Pretreatment Technologies for Seawater Reverse Osmosis Plants: A Review. Desalination 2019, 449, 78–91. [Google Scholar] [CrossRef]
- Gryta, M.; Waszak, M.; Tomaszewska, M. Studies of Polypropylene Membrane Fouling during Microfiltration of Broth with Citrobacter freundii Bacteria. Pol. J. Chem. Technol. 2015, 17, 56–64. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Cross-Flow Microfiltration of Glycerol Fermentation Broths with Citrobacter freundii. Membranes 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Gryta, M. Comparison of Polypropylene and Ceramic Microfiltration Membranes Applied for Separation of 1,3-PD Fermentation Broths and Saccharomyces Cerevisiae Yeast Suspensions. Membranes 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.Q.; Pham, T.T. Refinery of Gamma- Aminobutyric Acid (GABA) from the Fermentation of Rice Bran Extract by Ultrafiltration. Chem. Eng. Trans. 2020, 78, 463–468. [Google Scholar] [CrossRef]
- Maguire, N.A.P.; Ebrahimi, M.; Fan, R.; Gießelmann, S.; Ehlen, F.; Schütz, S.; Czermak, P. Influence of Ceramic Membrane Surface Characteristics on the Flux Behavior of a Complex Fermentation Broth. Membranes 2021, 11, 402. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. The Application of Ultrafiltration for Separation of Glycerol Solution Fermented by Bacteria. Pol. J. Chem. Technol. 2013, 15, 115–120. [Google Scholar] [CrossRef]
- Li, F.; Fei, P.; Cheng, B.; Meng, J.; Liao, L. Synthesis, Characterization and Excellent Antibacterial Property of Cellulose Acetate Reverse Osmosis Membrane via a Two-Step Reaction. Carbohydr. Polym. 2019, 216, 312–321. [Google Scholar] [CrossRef]
- Jafari, M.; Vanoppen, M.; van Agtmaal, J.M.C.; Cornelissen, E.R.; Vrouwenvelder, J.S.; Verliefde, A.; van Loosdrecht, M.C.M.; Picioreanu, C. Cost of Fouling in Full-Scale Reverse Osmosis and Nanofiltration Installations in the Netherlands. Desalination 2021, 500, 114865. [Google Scholar] [CrossRef]
- Boussouga, Y.A.; Lhassani, A. Study of Mass Transfer Mechanisms for Reverse Osmosis and Nanofiltration Membranes Intended for Desalination. JMES 2017, 8, 1266–1276. [Google Scholar]
- Lonsdale, H.K.; Merten, U.; Riley, R.L. Transport Properties of Cellulose Acetate Osmotic Membranes. J. Appl. Polym. Sci. 1965, 9, 1341–1362. [Google Scholar] [CrossRef]
- Irvine, G.J.; Rajesh, S.; Georgiadis, M.; Phillip, W.A. Ion Selective Permeation Through Cellulose Acetate Membranes in Forward Osmosis. Environ. Sci. Technol. 2013, 47, 13745–13753. [Google Scholar] [CrossRef] [PubMed]
- Epsztein, R.; Cheng, W.; Shaulsky, E.; Dizge, N.; Elimelech, M. Elucidating the Mechanisms Underlying the Difference between Chloride and Nitrate Rejection in Nanofiltration. J. Membr. Sci. 2018, 548, 694–701. [Google Scholar] [CrossRef]
- Hausmanns, S.; Laufenberg, G.; Kunz, B. Rejection of Acetic Acid and Its Improvement by Combination with Organic Acids in Dilute Solutions Using Reverse Osmosis. Desalination 1996, 104, 95–98. [Google Scholar] [CrossRef]
- Wang, L.; Cao, T.; Dykstra, J.E.; Porada, S.; Biesheuvel, P.M.; Elimelech, M. Salt and Water Transport in Reverse Osmosis Membranes: Beyond the Solution-Diffusion Model. Environ. Sci. Technol. 2021, 55, 16665–16675. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; SenGupta, A.K. Ion Exchange Selectivity as a Surrogate Indicator of Relative Permeability of Ions in Reverse Osmosis Processes. Environ. Sci. Technol. 2003, 37, 1432–1440. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Samson, E.; Marchand, J.; Snyder, K.A. Calculation of Ionic Diffusion Coefficients on the Basis of Migration Test Results. Mat. Struct. 2003, 36, 156–165. [Google Scholar] [CrossRef]
- Kiriukhin, M.Y.; Collins, K.D. Dynamic Hydration Numbers for Biologically Important Ions. Biophys. Chem. 2002, 99, 155–168. [Google Scholar] [CrossRef]
- Waszak, M.; Gryta, M. The Ultrafiltration Ceramic Membrane Used for Broth Separation in Membrane Bioreactor. Chem. Eng. J. 2016, 305, 129–135. [Google Scholar] [CrossRef]
- Gryta, M.; Markowska-Szczupak, A.; Grzechulska-Damszel, J.; Bastrzyk, J.; Waszak, M. The Study of Glycerol-Based Fermentation and Broth Downstream by Nanofiltration. Pol. J. Chem. Technol. 2014, 16, 117–122. [Google Scholar] [CrossRef]


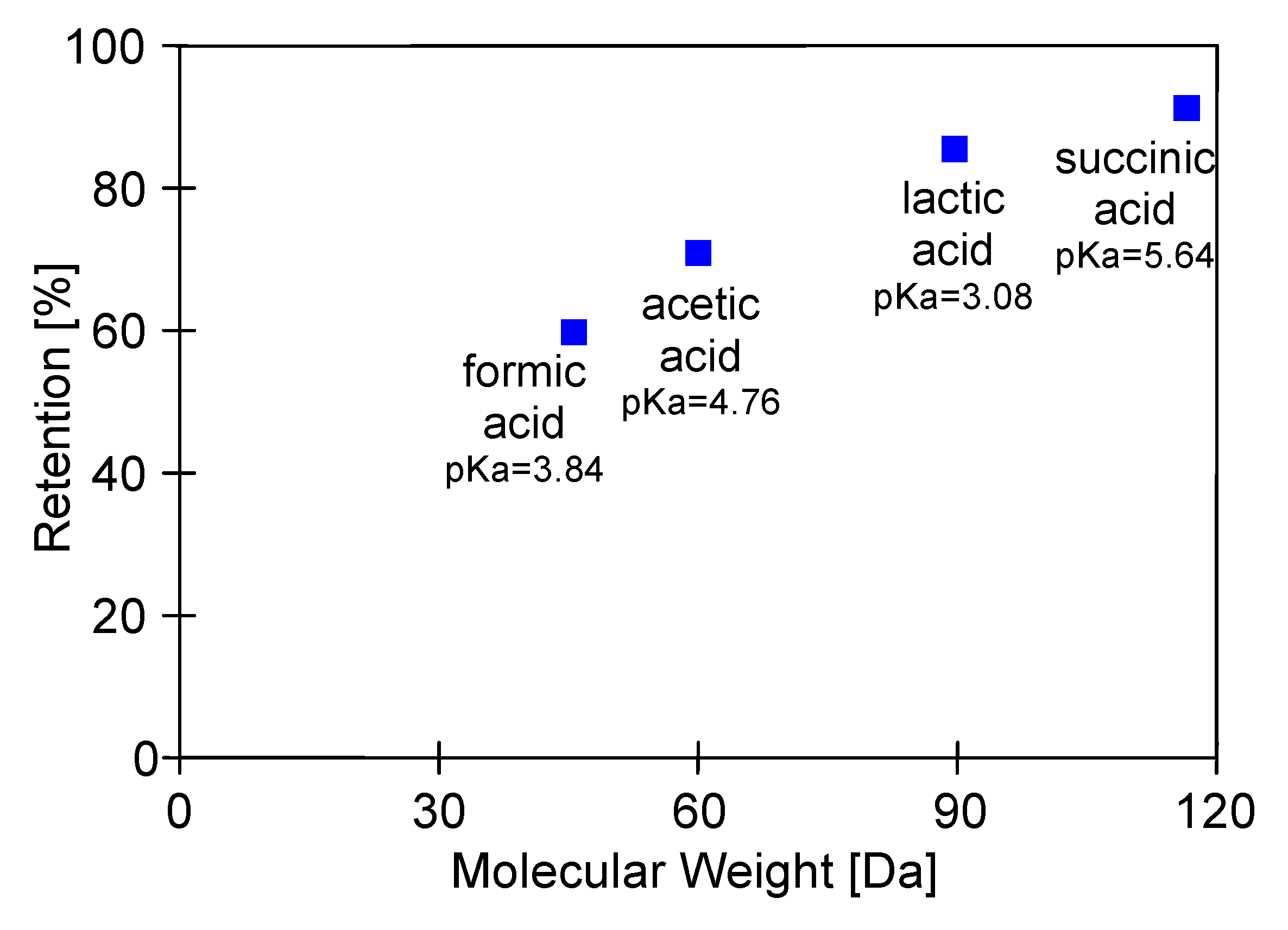

| Component | Molecular Formula | Molecular Weight [Da] | Dissociation Constant pKa | Concentration [g/L] | |
|---|---|---|---|---|---|
| Series 1 | Series 2 | ||||
| 1,3-propanediol | C3H8O2 | 76.09 | 14.46 | 12.65 | 11.52 |
| acetic acid | C2H4O2 | 60.05 | 4.76 | 2.98 | 3.13 |
| succinic acid | C4H6O4 | 118.08 | 4.21 and 5.64 | 1.35 | 1.45 |
| lactic acid | C3H6O3 | 90.08 | 3.08 | 0.78 | 0.63 |
| formic acid | CH2O2 | 46.05 | 3.84 | 0.68 | 0.56 |
| glycerol | C3H8O3 | 92.09 | 14.40 | 0.08 | 0.62 |
| Ion | Hydrated Radius [nm] 1 | Diffusion Coefficient [m2/s] 1 | Concentration [g/L] | |
|---|---|---|---|---|
| Series 1 | Series 2 | |||
| Cl− | 0.332 | 2.03 | 1.34 | 1.92 |
| PO43− | 0.339 | 0.61 | 2.56 | 2.67 |
| SO42− | 0.379 | 1.07 | 1.86 | 1.58 |
| NH4+ | 0.331 | 1.98 | 0.62 | 0.65 |
| K+ | 0.331 | 1.96 | 1.82 | 1.94 |
| Na+ | 0.358 | 1.33 | 3.85 | 3.94 |
| Ca2+ | 0.412 | 0.79 | 0.05 | 0.06 |
| Mg2+ | 0.428 | 0.71 | 0.01 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W.; Gryta, M. The Application of Cellulose Acetate Membranes for Separation of Fermentation Broths by the Reverse Osmosis: A Feasibility Study. Int. J. Mol. Sci. 2022, 23, 11738. https://doi.org/10.3390/ijms231911738
Tomczak W, Gryta M. The Application of Cellulose Acetate Membranes for Separation of Fermentation Broths by the Reverse Osmosis: A Feasibility Study. International Journal of Molecular Sciences. 2022; 23(19):11738. https://doi.org/10.3390/ijms231911738
Chicago/Turabian StyleTomczak, Wirginia, and Marek Gryta. 2022. "The Application of Cellulose Acetate Membranes for Separation of Fermentation Broths by the Reverse Osmosis: A Feasibility Study" International Journal of Molecular Sciences 23, no. 19: 11738. https://doi.org/10.3390/ijms231911738
APA StyleTomczak, W., & Gryta, M. (2022). The Application of Cellulose Acetate Membranes for Separation of Fermentation Broths by the Reverse Osmosis: A Feasibility Study. International Journal of Molecular Sciences, 23(19), 11738. https://doi.org/10.3390/ijms231911738







