Chromone-Containing Allylmorpholines Influence Ion Channels in Lipid Membranes via Dipole Potential and Packing Stress
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effects of Chromone-Containing Allylmorpholines on the Transmembrane Distribution of Electrical Potential
2.1.1. Membrane Boundary Potential
2.1.2. Membrane Surface Potential
2.1.3. Membrane Dipole Potential
2.1.4. Gramicidin A channels
2.2. The Effect of Chromone-Containing Allylmorpholines on Lipid Packing
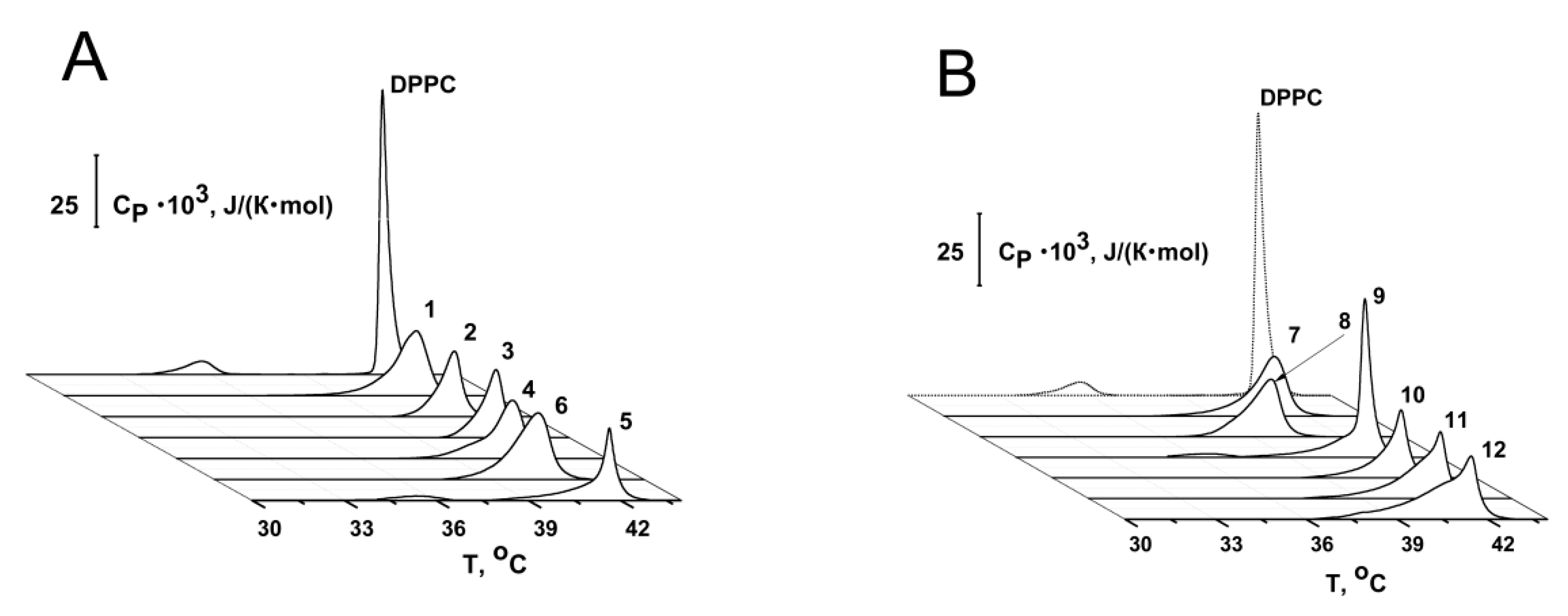
2.2.1. Thermotropic Behavior of Membrane Lipids
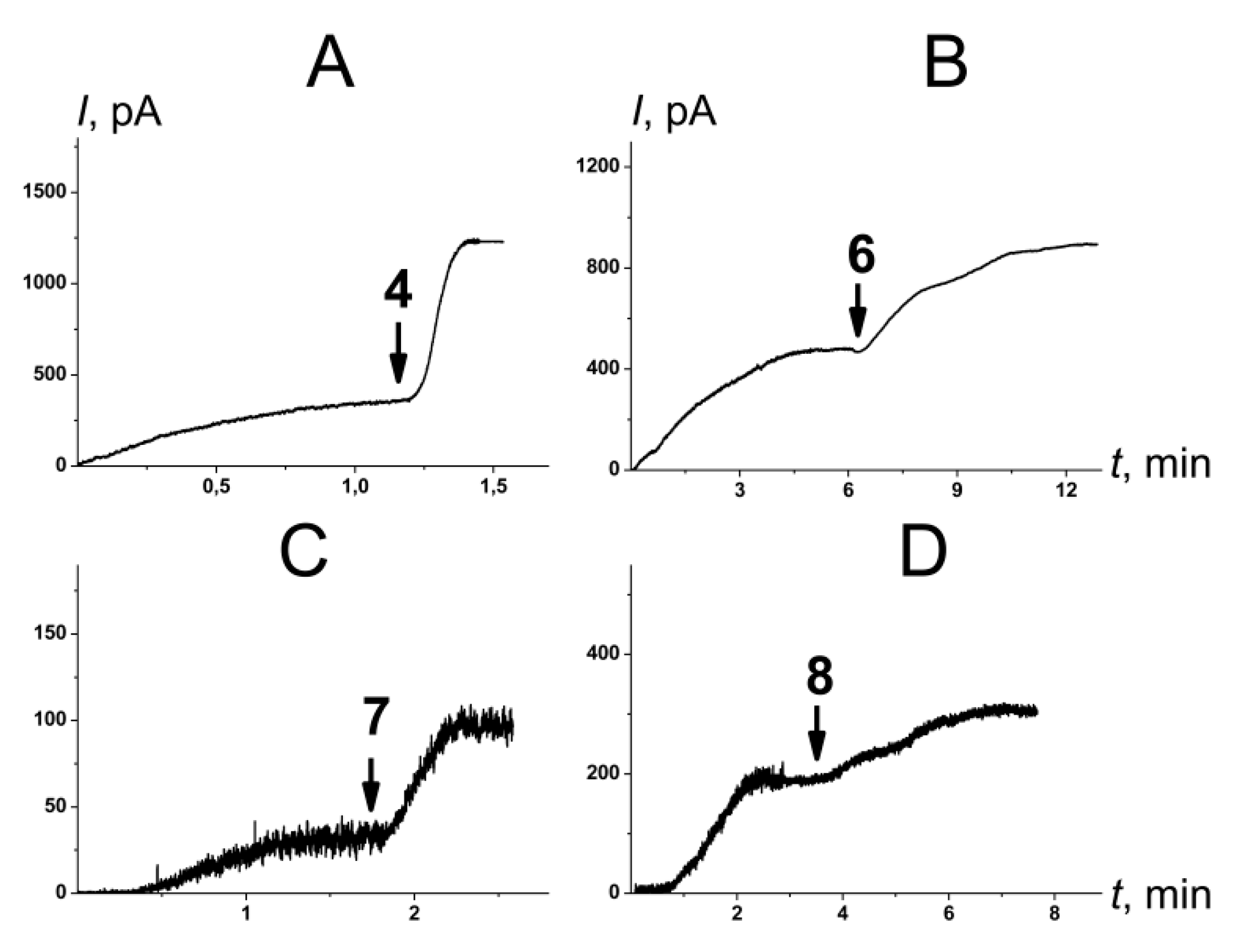
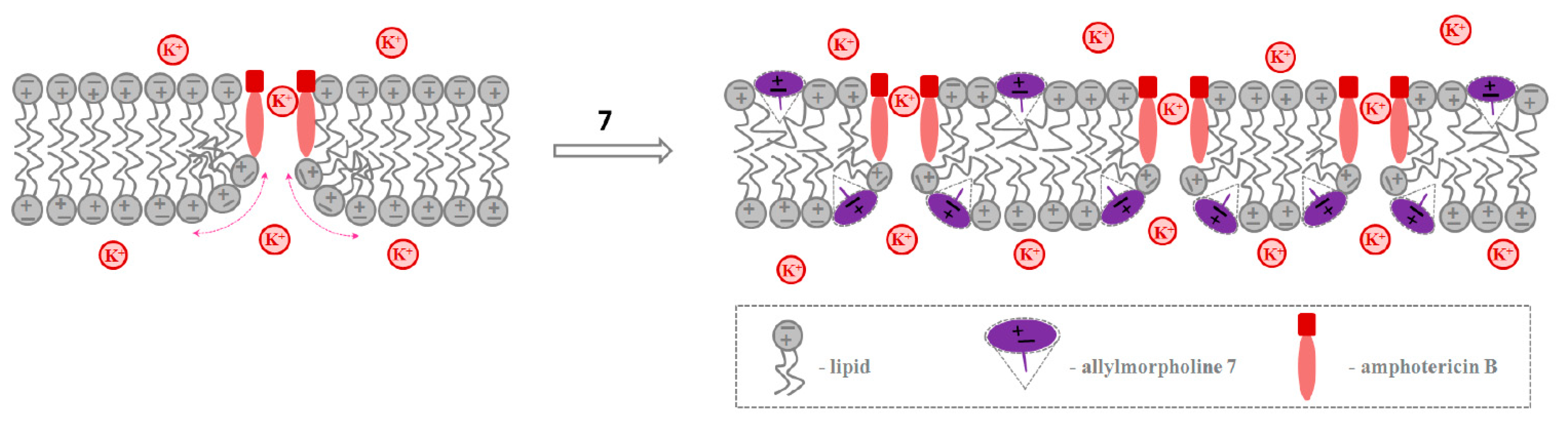
2.2.2. Amphotericin B Channels
3. Materials and Methods
3.1. Chemical Reagents
3.2. Electrophysiological Method for Measuring the Membrane Boundary Potential
3.3. Dynamic Light Scattering of Liposome Suspensions
3.4. Fluorimetry of Membrane Dipole Potential
3.5. Electrophysiological Method for Reconstitution of Ion Channels into Planar Lipid Bilayers
3.6. Differential Scanning Microcalorimetry of Liposomal Suspensions
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hughes, R.E.; Nikolic, K.; Ramsay, R.R. One for all hitting multiple Alzheimer’s disease targets with one drug. Front. Neurosci. 2016, 10, 177. [Google Scholar] [CrossRef]
- Manera, C.; Martinelli, A.; Nencetti, S.; Romagnoli, F.; Rossello, A.; Giannaccini, G.; Scatizzi, R.; Cozzini, P.; Domiano, P. X-ray analysis, theoretical studies and aadrenergic biopharmacological properties of 1-(2,5 dimethoxyphenyl)-2- aminoethanol and its morpholine analogue. Eur. J. Med. Chem. 1994, 29, 519–525. [Google Scholar] [CrossRef]
- Repsold, B.P.; Malan, S.F.; Joubert, J.; Oliver, D.W. Multi-targeted directed ligands for Alzheimer’s disease: Design of novel lead coumarin conjugates. SAR QSAR Environ. Res. 2018, 29, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Bertron, J.L.; Cho, H.P.; Garcia-Barrantes, P.M.; Panarese, J.D.; Salovich, J.M.; Nance, K.D.; Engers, D.W.; Rook, J.M.; Blobaum, A.L.; Niswender, C.M.; et al. The discovery of VU0486846: Steep SAR from a series of M1 PAMs based on a novel benzomorpholine core. Bioorg. Med. Chem. Lett. 2018, 28, 2175–2179. [Google Scholar] [CrossRef] [PubMed]
- Rook, J.M.; Bertron, J.L.; Cho, H.P.; Garcia-Barrantes, P.M.; Moran, S.P.; Maksymetz, J.T.; Nance, K.D.; Dickerson, J.W.; Remke, D.H.; Chang, S.; et al. A novel M1 PAM VU0486846 exerts efficacy in cognition models without displaying agonist activity or cholinergic toxicity. ACS Chem. Neurosci. 2018, 9, 2274–2285. [Google Scholar] [CrossRef] [PubMed]
- Colestock, T.; Wallach, J.; Mansi, M.; Filemban, N.; Morris, H.; Elliott, S.P.; Westphal, F.; Brandt, S.D.; Adejare, A. Syntheses, analytical and pharmacological characterizations of the ‘legal high’ 4-[1-(3-methoxyphenyl)cyclohexyl] morpholine (3-MeO-PCMo) and analogues. Drug Test Anal. 2018, 10, 272–283. [Google Scholar] [CrossRef]
- Chernov, N.; Shutov, R.; Barygin, O.; Dron, M.; Starova, G.; Kuz’mich, N.; Yakovlev, I. Synthesis of chromone-containing allylmorpholines through a Morita-Baylis-Hillman-type reaction. Eur. J. Org. Chem. 2018, 2018, 6304–6313. [Google Scholar] [CrossRef]
- Degorce, S.L.; Bodnarchuk, M.S.; Cumming, I.A.; Scott, J.S. Lowering lipophilicity by adding carbon: One-carbon bridges of morpholines and piperazines. J. Med. Chem. 2018, 61, 8934–8943. [Google Scholar] [CrossRef]
- Lenci, E.; Innocenti, R.; Menchi, G.; Trabocchi, A. Diversity-oriented synthesis and chemoinformatic analysis of the molecular diversity of sp3-rich morpholine peptidomimetics. Front. Chem. 2018, 6, 522. [Google Scholar] [CrossRef]
- Frenkel, E.J.; Roelofsen, B.; Brodbeck, U.; van Deenen, L.L.; Ott, P. Lipid-protein interactions in human erythrocyte-membrane acetylcholinesterase. Modulation of enzyme activity by lipids. Eur. J. Biochem. 1980, 109, 377–382. [Google Scholar] [CrossRef]
- Mazzanti, L.; Pastuszko, A.; Lenaz, G. Effects of ketamine anesthesia on rat-brain membranes: Fluidity changes and kinetics of acetylcholinesterase. Biochim. Biophys. Acta 1986, 861, 105–110. [Google Scholar] [CrossRef]
- Curatola, G.; Mazzanti, L.; Lenaz, G.; Pastuszko, A. General anesthetics inhibit erythrocyte acetylcholinesterase only when membrane-bound. Bull. Mel. Biol. Med. 1979, 4, 139146. [Google Scholar]
- Casado, M.; Ascher, P. Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: Common features with mechanosensitivity. J. Physiol. 1998, 513, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Gawrisch, K.; Ruston, D.; Zimmerberg, J.; Parsegian, V.A.; Rand, R.P.; Fuller, N. Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys. J. 1992, 61, 1213–1223. [Google Scholar] [CrossRef]
- Ermakov, Y.A.; Sokolov, V.S. Boundary potentials of bilayer lipid membranes: Methods and interpretations. Membr. Sci. Technol. 2003, 7, 109–141. [Google Scholar]
- Ermakov, Y.A.; Nesterenko, A.M. Boundary potential of lipid bilayers: Methods and interpretations. In Journal of Physics: Conference Series, Proceedings of the INERA Workshop 2016: Membrane and Liquid Crystal Nanostructures (MELINA 2016), Varna, Bulgaria, 3–6 September 2016; IOP Publishing: Bristol, UK, 2017; Volume 780, p. 012002. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Yesylevskyy, S.O. Nanoscopic description of biomembrane electrostatics: Results of molecular dynamics simulations and fluorescence probing. Chem. Phys. Lipids. 2009, 160, 63–84. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Efimova, S.S.; Malev, V.V. Modifiers of membrane dipole potentials as tools for investigating ion channel formation and functioning. Int. Rev. Cell Mol. Biol. 2015, 315, 245–297. [Google Scholar] [CrossRef]
- Rokitskaya, T.I.; Antonenko, Y.N.; Kotova, E.A. Effect of the dipole potential of a bilayer lipid membrane on gramicidin channel dissociation kinetics. Biophys. J. 1997, 73, 850–854. [Google Scholar] [CrossRef]
- Busath, D.D.; Thulin, C.D.; Hendershot, R.W.; Phillips, L.R.; Maughan, P.; Cole, C.D.; Bingham, N.C.; Morrison, S.; Baird, L.C.; Hendershot, R.J.; et al. Noncontact dipole effects on channel permeation. I. Experiments with (5F-indole)Trp13 gramicidin A channels. Biophys. J. 1998, 75, 2830–2844. [Google Scholar] [CrossRef]
- Duffin, R.L.; Garrett, M.P.; Flake, K.B.; Durrant, J.D.; Busath, D.D. Modulation of lipid bilayer interfacial dipole potential by phloretin, RH 421, and 6-ketocholestanol as probed by gramicidin channel conductance. Langmuir 2003, 19, 1439–1442. [Google Scholar] [CrossRef]
- Hwang, T.C.; Koeppe, R.E.; Andersen, O.S. Genistein can modulate channel function by a phosphorylation-independent mechanism: Importance of hydrophobic mismatch and bilayer mechanics. Biochemistry 2003, 42, 13646–13658. [Google Scholar] [CrossRef] [PubMed]
- Efimova, S.S.; Zakharova, A.A.; Ostroumova, O.S. Alkaloids modulate the functioning of ion channels produced by antimicrobial agents via an influence on the lipid host. Front. Cell Dev. Biol. 2020, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Efimova, S.S.; Ostroumova, O.S. Is the membrane lipid matrix a key target for action of pharmacologically active plant saponins? Int. J. Mol. Sci. 2021, 22, 3167. [Google Scholar] [CrossRef]
- Zakharova, A.A.; Efimova, S.S.; Ostroumova, O.S. Phosphodiesterase Type 5 Inhibitors Greatly Affect Physicochemical Properties of Model Lipid Membranes. Membranes 2021, 11, 893. [Google Scholar] [CrossRef]
- Latorre, R.; Donovan, J.J. Modulation of alamethicin-induced conductance by membrane composition. Acta Physiol. Scand. Suppl. 1980, 481, 37–45. [Google Scholar] [PubMed]
- Luchian, T.; Mereuta, L. Phlorizin- and 6-ketocholestanol-mediated antagonistic modulation of alamethicin activity in phospholipid planar membranes. Langmuir 2006, 22, 8452–8457. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, A.; Mereuta, L.; Luchian, T. The RH 421 styryl dye induced, pore model-dependent modulation of antimicrobial peptides activity in reconstituted planar membranes. Biochim. Biophys. Acta 2009, 1790, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Ostroumova, O.S.; Kaulin, Y.A.; Gurnev, P.A.; Schagina, L.V. Effect of agents modifying the membrane dipole potential on properties of syringomycin E channels. Langmuir 2007, 23, 6889–6892. [Google Scholar] [CrossRef] [PubMed]
- Ostroumova, O.S.; Malev, V.V.; Bessonov, A.N.; Takemoto, J.Y.; Schagina, L.V. Altering the activity of syringomycin E via the membrane dipole potential. Langmuir 2008, 24, 2987–2991. [Google Scholar] [CrossRef]
- Efimova, S.S.; Zakharova, A.A.; Schagina, L.V.; Ostroumova, O.S. Two types of syringomycin E channels in sphingomyelin-containing bilayers. Eur. Biophys. J. 2016, 45, 91–98. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Malev, V.V.; Ilin, M.G.; Schagina, L.V. Surfactin activity depends on the membrane dipole potential. Langmuir 2010, 26, 15092–15097. [Google Scholar] [CrossRef] [PubMed]
- Efimova, S.S.; Schagina, L.V.; Ostroumova, O.S. Channel-forming activity of cecropins in lipid bilayers: Effect of agents modifying the membrane dipole potential. Langmuir 2014, 30, 7884–7892. [Google Scholar] [CrossRef] [PubMed]
- Mereuta, L.; Luchian, T.; Park, Y.; Hahm, K.S. Single-molecule investigation of the interactions between reconstituted planar lipid membranes and an analogue of the HP(2-20) antimicrobial peptide. Biochem. Biophys. Res. Commun. 2008, 373, 467–472. [Google Scholar] [CrossRef]
- Asandei, A.; Mereuta, L.; Luchian, T. Influence of membrane potentials upon reversible protonation of acidic residues from the OmpF eyelet. Biophys. Chem. 2008, 135, 32–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pearlstein, R.A.; Dickson, C.J.; Hornak, V. Contributions of the membrane dipole potential to the function of voltage-gated cation channels and modulation by small molecule potentiators. Biochim. Biophys. Acta Biomembr. 2017, 1859, 177–194. [Google Scholar] [CrossRef]
- Finkelstein, A.; Andersen, O.S. The gramicidin A channel: A review of its permeability characteristics with special reference to the single-file aspect of transport. J. Membr. Biol. 1981, 59, 155–171. [Google Scholar] [CrossRef]
- Kelkar, D.A.; Chattopadhyay, A. The gramicidin ion channel: A model membrane protein. Biochim. Biophys. Acta 2007, 1768, 2011–2025. [Google Scholar] [CrossRef]
- Andersen, O.S.; Finkelstein, A.; Katz, I.; Cass, A. Effect of phloretin on the permeability of thin lipid membranes. J. Gen. Physiol. 1976, 67, 749–771. [Google Scholar] [CrossRef]
- Jordan, P.C. Electrostatic modeling of ion pores. II. Effects attributable to the membrane dipole potential. Biophys. J. 1983, 41, 189–195. [Google Scholar] [CrossRef]
- Bezrukov, S.M. Functional consequences of lipid packing stress. Curr. Opin. Colloid Interface Sci. 2000, 5, 237–243. [Google Scholar] [CrossRef]
- Lundbaek, J.A.; Andersen, O.S. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 1994, 104, 645–673. [Google Scholar] [CrossRef] [PubMed]
- Lundbaek, J.A.; Maer, A.M.; Andersen, O.S. Lipid bilayer electrostatic energy, curvature stress, and assembly of gramicidin channels. Biochemistry 1997, 36, 5695–5701. [Google Scholar] [CrossRef]
- Lundbaek, J.A.; Andersen, O.S. Spring constants for channel-induced lipid bilayer deformations. Estimates using gramicidin channels. Biophys. J. 1999, 76, 889–895. [Google Scholar] [CrossRef]
- Riske, K.A.; Barroso, R.P.; Vequi-Suplicy, C.C.; Germano, R.; Henriques, V.B.; Lamy, M.T. Lipid bilayer pre-transition as the beginning of the melting process. Biochim. Biophys. Acta 2009, 1788, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A.; Dea, P.K. Differential Scanning Calorimetry Studies of Phospholipid Membranes: The Interdigitated Gel Phase. In Applications of Calorimetry in a Wide Context—Differential Scanning Calorimetry, Isothermal Titration Calorimetry and Microcalorimetry; Elkordy, A.A., Ed.; Intechopen: London, UK, 2013; pp. 407–444. [Google Scholar] [CrossRef]
- Lewis, R.N.; Mannock, D.A.; McElhaney, R.N. Differential scanning calorimetry in the study of lipid phase transitions in model and biological membranes: Practical considerations. Methods Mol. Biol. 2007, 400, 171–195. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M. Diacylglycerols, lysolecithin, or hydrocarbons markedly alter the bilayer to hexagonal phase transition temperature of phosphatidylethanolamines. Biochemistry 1985, 24, 7092–7095. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F.; Lancaster, C.R. Modulation of the bilayer to hexagonal phase transition of phosphatidylethanolamines by acylglycerols. Biochim. Biophys. Acta 1988, 945, 161–166. [Google Scholar] [CrossRef]
- St Vincent, M.R.; Colpitts, C.C.; Ustinov, A.V.; Muqadas, M.; Joyce, M.A.; Barsby, N.L.; Epand, R.F.; Epand, R.M.; Khramyshev, S.A.; Valueva, O.A.; et al. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 17339–17344. [Google Scholar] [CrossRef]
- Kim, Y.A.; Tarahovsky, Y.S.; Yagolnik, E.A.; Kuznetsova, S.M.; Muzafarov, E.N. Lipophilicity of flavonoid complexes with iron(II) and their interaction with liposomes. Biochem. Biophys. Res. Commun. 2013, 431, 680–685. [Google Scholar] [CrossRef]
- Kim, Y.A.; Tarahovsky, Y.S.; Yagolnik, E.A.; Kuznetsova, S.M.; Muzafarov, E.N. Integration of quercetin-iron complexes into phosphatidylcholine or phosphatidylethanolamine liposomes. Appl. Biochem. Biotechnol. 2015, 176, 1904–1913. [Google Scholar] [CrossRef]
- Nakazawa, K.; Hishida, M.; Nagatomo, S.; Yamamura, Y.; Saito, K. Photoinduced Bilayer-to-Nonbilayer Phase Transition of POPE by Photoisomerization of Added Stilbene Molecules. Langmuir 2016, 32, 7647–7653. [Google Scholar] [CrossRef] [PubMed]
- Sobko, A.A.; Kotova, E.A.; Antonenko, Y.N.; Zakharov, S.D.; Cramer, W.A. Effect of lipids with different spontaneous curvature on the channel activity of colicin E1: Evidence in favor of a toroidal pore. FEBS Lett. 2004, 576, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Pfeffermann, J.; Eicher, B.; Boytsov, D.; Hannesschlaeger, C.; Galimzyanov, T.R.; Glasnov, T.N.; Pabst, G.; Akimov, S.A.; Pohl, P. Photoswitching of model ion channels in lipid bilayers. J. Photochem. Photobiol. B. 2021, 224, 112320. [Google Scholar] [CrossRef] [PubMed]
- Markin, V.S.; Sachs, F. Thermodynamics of mechanosensitivity. Phys. Biol. 2004, 1, 110–124. [Google Scholar] [CrossRef]
- Lee, K.J. Effects of hydrophobic mismatch and spontaneous curvature on ion channel gating with a hinge. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2005, 72, 031917. [Google Scholar] [CrossRef]
- Malev, V.V.; Schagina, L.V.; Gurnev, P.A.; Takemoto, J.Y.; Nestorovich, E.M.; Bezrukov, S.M. Syringomycin E channel: A lipidic pore stabilized by lipopeptide? Biophys. J. 2002, 82, 1985–1994. [Google Scholar] [CrossRef]
- Umegawa, Y.; Yamamoto, T.; Dixit, M.; Funahashi, K.; Seo, S.; Nakagawa, Y.; Suzuki, T.; Matsuoka, S.; Tsuchikawa, H.; Hanashima, S.; et al. Amphotericin B assembles into seven-molecule ion channels: An NMR and molecular dynamics study. Sci. Adv. 2022, 8, eabo2658. [Google Scholar] [CrossRef]
- Kasumov, K.M.; Karakozov, S.D. Effect of amphotericin B added to one side of a membrane. Biofizika 1985, 30, 281–284. [Google Scholar]
- Kleinberg, M.E.; Finkelstein, A. Single-length and double-length channels formed by nystatin in lipid bilayer membranes. J. Membr. Biol. 1984, 80, 257–269. [Google Scholar] [CrossRef]
- Bolard, J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim. Biophys. Acta 1986, 864, 257–304. [Google Scholar] [CrossRef]
- Chulkov, E.G.; Schagina, L.V.; Ostroumova, O.S. Membrane dipole modifiers modulate single-length nystatin channels via reducing elastic stress in the vicinity of the lipid mouth of a pore. Biochim. Biophys. Acta Biomembr. 2015, 1848, 192–199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Montal, M.; Muller, P. Formation of bimolecular membranes from lipid monolayers and study of their electrical properties. Proc. Natl. Acad. Sci. USA 1972, 65, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.; Bedlack, R.S.; Loew, L.M. Dual-wavelength ratiometric fluorescence measurement of the membrane dipole potential. Biophys. J. 1994, 67, 208–216. [Google Scholar] [CrossRef]
- Starke-Peterkovic, T.; Turner, N.; Else, P.L.; Clarke, R.J. Electric field strength of membrane lipids from vertebrate species: Membrane lipid composition and Na+-K+-ATPase molecular activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R663–R670. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.J.; Kane, D.J. Optical detection of membrane dipole potential: Avoidance of fluidity and dye-induced effects. Biochim. Biophys. Acta 1997, 1323, 223–239. [Google Scholar] [CrossRef]
- Clarke, R.J. The dipole potential of phospholipid membranes and methods for its detection. Adv. Colloid. Interface Sci. 2001, 89–90, 263–281. [Google Scholar] [CrossRef]



| Agent | 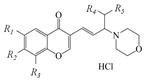 | –Δφb, mV | –ξ, mV | –Δφd, mV | gsc, pS | NPop | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | ||||||
| 1 | -Br | -H | -H | -CH3 | -CH3 | 105 ± 10 | 0.1 ± 0.3 | 106 ± 23 | 24.9 ± 1.1 | 2.5 ± 1.3 |
| 2 | -H | -H | -H | -CH3 | -CH3 | 26 ± 9 | 3.4 ± 4.1 | – | 23.6 ± 1.0 | 0.3 ± 0.1 |
| 3 | -F | -H | -H | -CH3 | -CH3 | 58 ± 10 | 2.5 ± 0.4 | 77 ± 25 | 24.8 ± 0.7 | 1.1 ± 0.2 |
| 4 | -CH3 | -H | -Br | -CH3 | -CH3 | −25 ± 7 | 0.2 ± 0.9 | – | 22.8 ± 0.9 | 0.6 ± 0.3 |
| 5 | -CH3 | -H | -Br | -(CH2)5− | -(CH2)5− | −20 ± 5 | 0.1 ± 0.3 | – | 22.7 ± 1.2 | 0.2 ± 0.1 |
| 6 | -NO2 | -H | -H | -CH3 | -CH3 | 103 ± 11 | 3.2 ± 0.2 | 79 ± 25 | 27.1 ± 1.1 | 3.1 ± 0.4 |
| 7 | -Cl | -H | -H | -CH3 | -CH3 | 110± 14 | 3.4 ± 0.3 | 87 ± 23 | 26.1 ± 1.1 | 4.1 ± 2.4 |
| 8 | -Cl | -H | -H | -H | -CH3 | 96 ± 13 | 0.1 ± 0.2 | 117 ± 26 | 29.1 ± 1.1 | 5.9 ± 3.7 |
| 9 | -Cl | -H | -H | -H | -n-C8H17 | 26 ± 12 | 3.9 ± 0.7 | – | 22.8 ± 1.1 | 0.3 ± 0.1 |
| 10 | -Cl | -H | -H | -(CH2)5− | -(CH2)5− | 49 ± 9 | −0.1 ± 0.1 | 61 ± 25 | 24.7 ± 1.7 | 1.5 ± 0.4 |
| 11 | -Cl | -H | -H | -H | -n-C4H9 | 69 ± 13 | 0.7 ± 1.3 | 78 ± 24 | 24.2 ± 1.1 | 3.9 ± 1.1 |
| 12 | -Cl | -H | -H | -C2H5 | -C2H5 | 54 ± 12 | 3.5 ± 0.3 | 60 ± 20 | 24.3 ± 0.7 | 1.8 ± 0.2 |
| Agent | ΔTp, °C | ΔTm_1, °C | ΔΔTb, °C | CUScontrol/CUSagent | I∞agent/I∞control |
|---|---|---|---|---|---|
| 1 | no * | 0 | 2.2 ± 0.2 | 2.2 ± 0.1 | 1.1 ± 0.1 |
| 2 | no * | 0 | 2.1 ± 0.5 | 1.9 ± 0.2 | 1.1 ± 0.1 |
| 3 | no * | 0 | 1.1 ± 0.1 | 1.6 ± 0.1 | 0.8 ± 0.1 |
| 4 | no * | −0.6 | 3.9 ± 1.4 | 3.2 ± 1.3 | 3.4 ± 1.8 |
| 5 | 0.8 ± 0.1 | 0 | 2.4 ± 0.6 | 2.4 ± 1.4 | 1.0 ± 0.1 |
| 6 | no * | −1.0 | 1.6 ± 0.3 | 2.0 ± 0.2 | 1.9 ± 0.3 |
| 7 | no * | −0.5 | 3.1 ± 0.2 | 2.5 ± 0.4 | 2.6 ± 1.6 |
| 8 | no * | −1.9 | 2.1 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0.4 |
| 9 | 0.5 ± 0.1 | 0 | 2.4 ± 0.2 | 1.2 ± 0.2 | 0.8 ± 0.1 |
| 10 | no * | 0 | 2.1 ± 0.2 | 1.1 ± 0.1 | 0.9 ± 0.1 |
| 11 | no * | 0 | 2.7 ± 0.5 | 1.2 ± 0.1 | 1.1 ± 0.2 |
| 12 | no * | 0 | 1.8 ± 0.8 | 1.2 ± 0.2 | 0.9 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimova, S.S.; Martynyuk, V.A.; Zakharova, A.A.; Yudintceva, N.M.; Chernov, N.M.; Yakovlev, I.P.; Ostroumova, O.S. Chromone-Containing Allylmorpholines Influence Ion Channels in Lipid Membranes via Dipole Potential and Packing Stress. Int. J. Mol. Sci. 2022, 23, 11554. https://doi.org/10.3390/ijms231911554
Efimova SS, Martynyuk VA, Zakharova AA, Yudintceva NM, Chernov NM, Yakovlev IP, Ostroumova OS. Chromone-Containing Allylmorpholines Influence Ion Channels in Lipid Membranes via Dipole Potential and Packing Stress. International Journal of Molecular Sciences. 2022; 23(19):11554. https://doi.org/10.3390/ijms231911554
Chicago/Turabian StyleEfimova, Svetlana S., Vera A. Martynyuk, Anastasiia A. Zakharova, Natalia M. Yudintceva, Nikita M. Chernov, Igor P. Yakovlev, and Olga S. Ostroumova. 2022. "Chromone-Containing Allylmorpholines Influence Ion Channels in Lipid Membranes via Dipole Potential and Packing Stress" International Journal of Molecular Sciences 23, no. 19: 11554. https://doi.org/10.3390/ijms231911554
APA StyleEfimova, S. S., Martynyuk, V. A., Zakharova, A. A., Yudintceva, N. M., Chernov, N. M., Yakovlev, I. P., & Ostroumova, O. S. (2022). Chromone-Containing Allylmorpholines Influence Ion Channels in Lipid Membranes via Dipole Potential and Packing Stress. International Journal of Molecular Sciences, 23(19), 11554. https://doi.org/10.3390/ijms231911554






