Eosinophilic Infiltration of the Sino-Atrial Node in Sudden Cardiac Death Caused by Long QT Syndrome
Abstract
1. Introduction
2. Cases Presentation
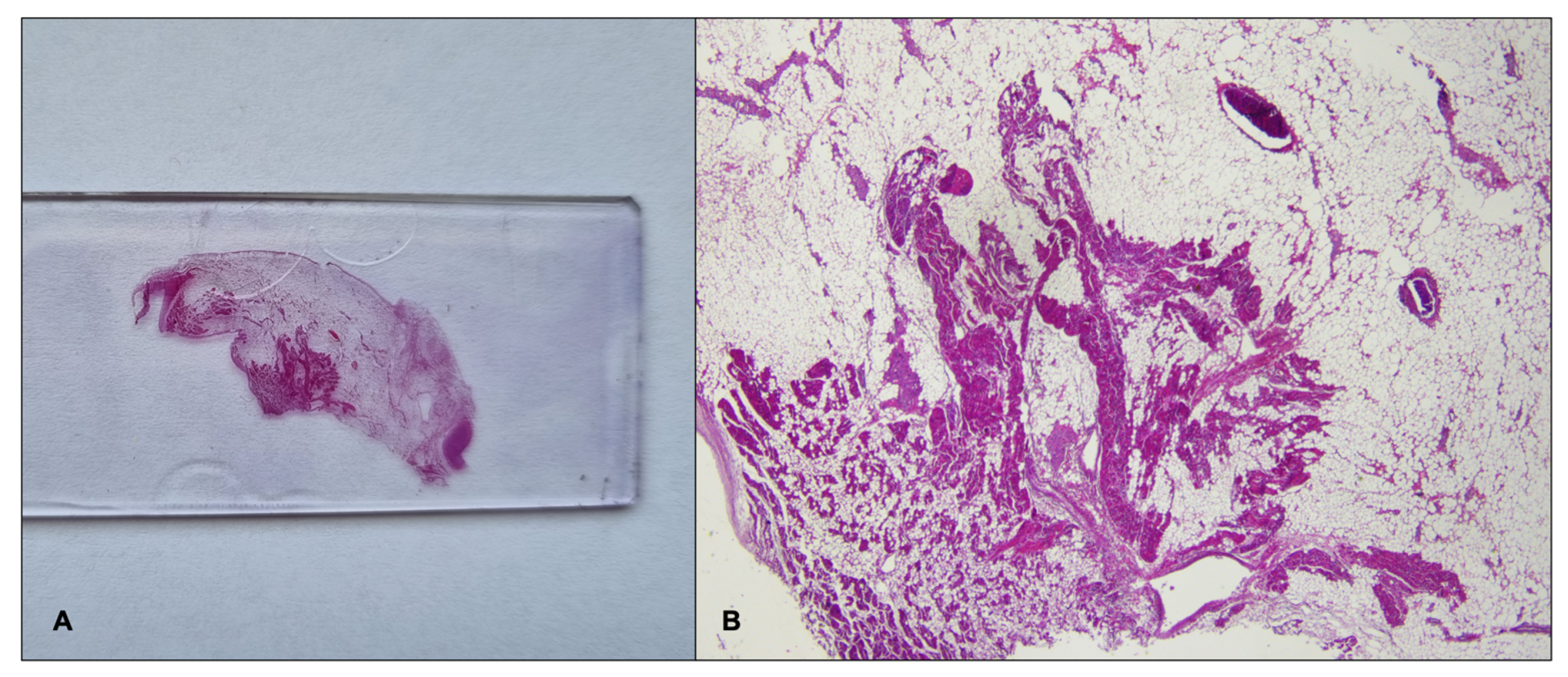
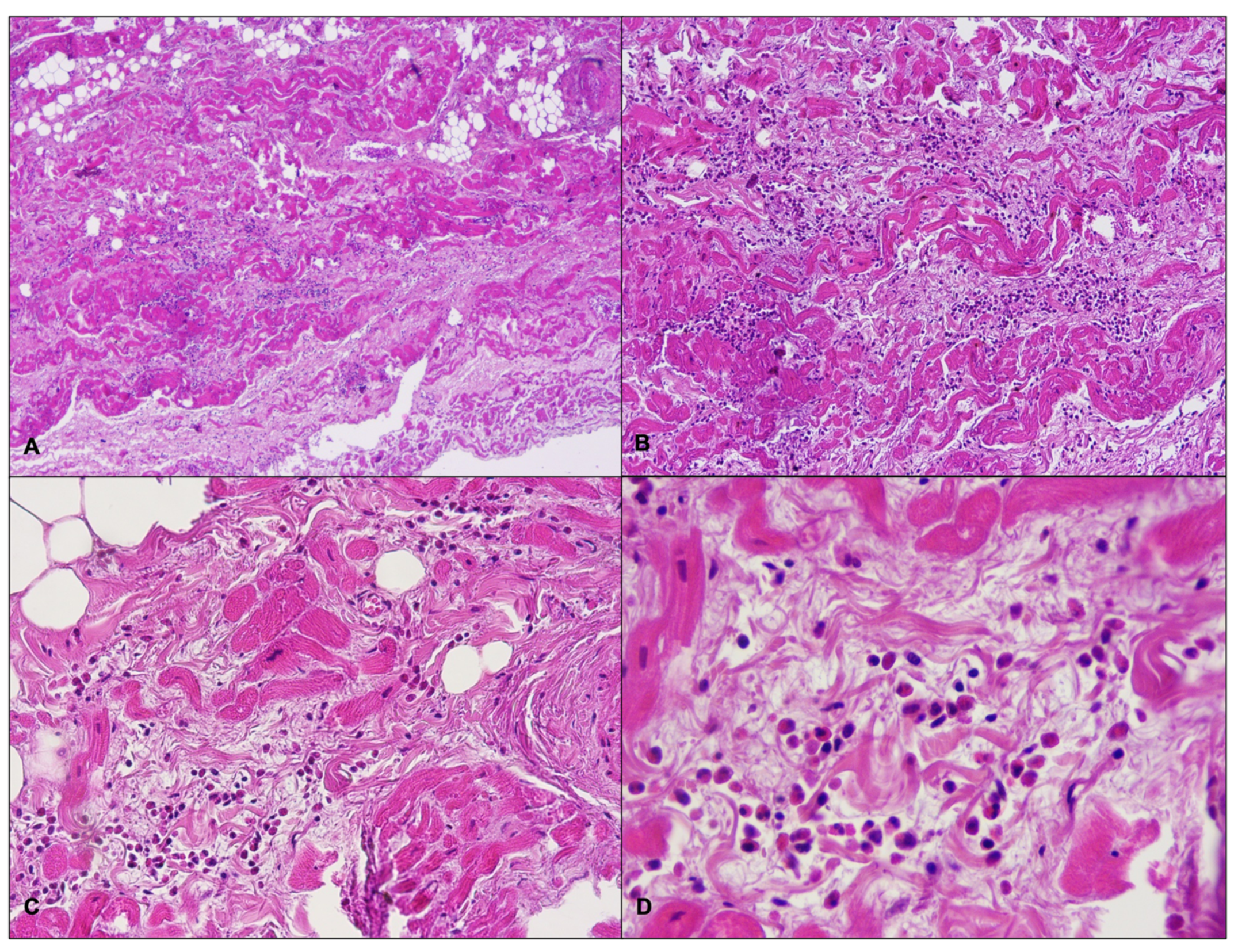
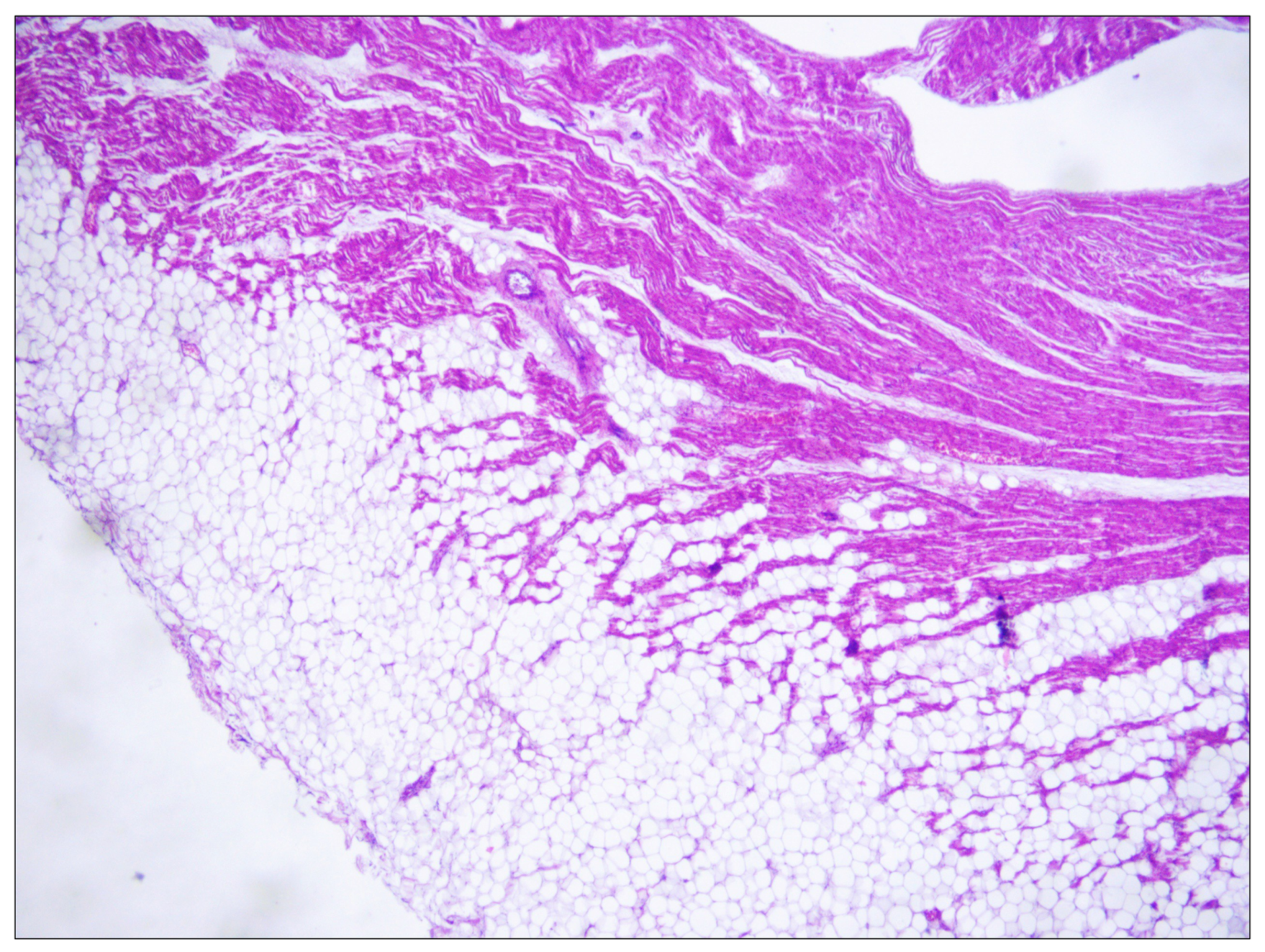
2.1. Case Report
2.2. Genetic Testing
2.3. Genetic Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grassi, S.; Vidal, M.C.; Campuzano, O.; Arena, V.; Alfonsetti, A.; Rossi, S.S.; Scarnicci, F.; Iglesias, A.; Brugada, R.; Oliva, A. Sudden Death without a Clear Cause after Comprehensive Investigation: An Example of Forensic Approach to Atypical/Uncertain Findings. Diagnostics 2021, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Shimizu, W.; Albert, C.M. The Spectrum of Epidemiology Underlying Sudden Cardiac Death. Circ. Res. 2015, 116, 1887–1906. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Falgueras, A.; Sarquella-Brugada, G.; Brugada, J.; Brugada, R.; Campuzano, O. Cardiac Channelopathies and Sudden Death: Recent Clinical and Genetic Advances. Biology 2017, 6, 7. [Google Scholar] [CrossRef]
- Couper, K.; Putt, O.; Field, R.; Poole, K.; Bradlow, W.; Clarke, A.; Perkins, G.D.; Royle, P.; Yeung, J.; Taylor-Phillips, S. Incidence of Sudden Cardiac Death in the Young: A Systematic Review. BMJ Open 2020, 10, e040815. [Google Scholar] [CrossRef]
- Teodorovich, N.; Mikhaylov, E.N.; Mitrofanova, L.B.; Swissa, M. Sudden Cardiac Death, Not What You Thought. Cardiology 2022, 147, 335–336. [Google Scholar] [CrossRef]
- Grassi, S.; Campuzano, O.; Coll, M.; Brión, M.; Arena, V.; Iglesias, A.; Carracedo, Á.; Brugada, R.; Oliva, A. Genetic Variants of Uncertain Significance: How to Match Scientific Rigour and Standard of Proof in Sudden Cardiac Death? Leg. Med. 2020, 45, 101712. [Google Scholar] [CrossRef]
- Eckart, R.E.; Shry, E.A.; Burke, A.P.; McNear, J.A.; Appel, D.A.; Castillo-Rojas, L.M.; Avedissian, L.; Pearse, L.A.; Potter, R.N.; Tremaine, L.; et al. Sudden Death in Young Adults. J. Am. Coll. Cardiol. 2011, 58, 1254–1261. [Google Scholar] [CrossRef]
- Campuzano, O.; Sarquella-Brugada, G.; Fernandez-Falgueras, A.; Coll, M.; Iglesias, A.; Ferrer-Costa, C.; Cesar, S.; Arbelo, E.; García-Álvarez, A.; Jordà, P.; et al. Reanalysis and Reclassification of Rare Genetic Variants Associated with Inherited Arrhythmogenic Syndromes. EBioMedicine 2020, 54, 102732. [Google Scholar] [CrossRef]
- Vallverdú-Prats, M.; Alcalde, M.; Sarquella-Brugada, G.; Cesar, S.; Arbelo, E.; Fernandez-Falgueras, A.; Coll, M.; Pérez-Serra, A.; Puigmulé, M.; Iglesias, A.; et al. Rare Variants Associated with Arrhythmogenic Cardiomyopathy: Reclassification Five Years Later. J. Pers. Med. 2021, 11, 162. [Google Scholar] [CrossRef]
- Sarquella-Brugada, G.; Fernandez-Falgueras, A.; Cesar, S.; Arbelo, E.; Coll, M.; Perez-Serra, A.; Puigmulé, M.; Iglesias, A.; Alcalde, M.; Vallverdú-Prats, M.; et al. Clinical Impact of Rare Variants Associated with Inherited Channelopathies: A 5-Year Update. Hum. Genet. 2022, 141, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Papadakis, M.; Dhutia, H.; Cole, D.; Behr, E.R.; Tome, M.; Sharma, S.; Sheppard, M.N. Obesity and Sudden Cardiac Death in the Young: Clinical and Pathological Insights from a Large National Registry. Eur. J. Prev. Cardiol. 2018, 25, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Margolis, G.; Elbaz-Greener, G.; Ruskin, J.N.; Roguin, A.; Amir, O.; Rozen, G. The Impact of Obesity on Sudden Cardiac Death Risk. Curr. Cardiol. Rep. 2022, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Schlesinger, S.; Norat, T.; Riboli, E. Body Mass Index, Abdominal Fatness, and the Risk of Sudden Cardiac Death: A Systematic Review and Dose–Response Meta-Analysis of Prospective Studies. Eur. J. Epidemiol. 2018, 33, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Mahajan, R.; Lau, D.H.; Sanders, P. The Implications of Obesity for Cardiac Arrhythmia Mechanisms and Management. Can. J. Cardiol. 2015, 31, 203–210. [Google Scholar] [CrossRef]
- Semsarian, C.; Ingles, J. Molecular Autopsy in Victims of Inherited Arrhythmias. J. Arrhythm. 2016, 32, 359–365. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Eur. Heart J. 2022, ehac262. [Google Scholar] [CrossRef]
- Stiles, M.K.; Wilde, A.A.M.; Abrams, D.J.; Ackerman, M.J.; Albert, C.M.; Behr, E.R.; Chugh, S.S.; Cornel, M.C.; Gardner, K.; Ingles, J.; et al. 2020 APHRS/HRS Expert Consensus Statement on the Investigation of Decedents with Sudden Unexplained Death and Patients with Sudden Cardiac Arrest, and of Their Families. Heart Rhythm. 2021, 18, e1–e50. [Google Scholar] [CrossRef]
- Ottaviani, G.; Buja, L.M. Anatomopathological Changes of the Cardiac Conduction System in Sudden Cardiac Death, Particularly in Infants: Advances over the Last 25 Years. Cardiovasc. Pathol. 2016, 25, 489–499. [Google Scholar] [CrossRef]
- The Royal College of Pathologists Guidelines on Autopsy Practice: Sudden Death with Likely Cardiac Pathology. Available online: https://www.rcpath.org/uploads/assets/3d973908-c2af-46ea-8190b2dc57fa3ba6/G145-GAP-CardiacDeath_Dec16.pdf (accessed on 20 September 2022).
- Kersey, P.J.; Staines, D.M.; Lawson, D.; Kulesha, E.; Derwent, P.; Humphrey, J.C.; Hughes, D.S.T.; Keenan, S.; Kerhornou, A.; Koscielny, G.; et al. Ensembl Genomes: An Integrative Resource for Genome-Scale Data from Non-Vertebrate Species. Nucleic Acids Res. 2012, 40, D91–D97. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and Accurate Long-Read Alignment with Burrows–Wheeler Transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A Method and Server for Predicting Damaging Missense Mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the Effects of Coding Non-Synonymous Variants on Protein Function Using the SIFT Algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef]
- Choi, Y.; Sims, G.E.; Murphy, S.; Miller, J.R.; Chan, A.P. Predicting the Functional Effect of Amino Acid Substitutions and Indels. PLoS ONE 2012, 7, e46688. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation Prediction for the Deep-Sequencing Age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef]
- Yeo, G.; Burge, C.B. Maximum Entropy Modeling of Short Sequence Motifs with Applications to RNA Splicing Signals. J. Comput. Biol. 2004, 11, 377–394. [Google Scholar] [CrossRef]
- Pertea, M. GeneSplicer: A New Computational Method for Splice Site Prediction. Nucleic Acids Res. 2001, 29, 1185–1190. [Google Scholar] [CrossRef]
- Reese, M.G.; Eeckman, F.H.; Kulp, D.; Haussler, D. Improved Splice Site Detection in Genie. J. Comput. Biol. 1997, 4, 311–323. [Google Scholar] [CrossRef]
- Richards, C.S.; Bale, S.; Bellissimo, D.B.; Das, S.; Grody, W.W.; Hegde, M.R.; Lyon, E.; Ward, B.E. ACMG Recommendations for Standards for Interpretation and Reporting of Sequence Variations: Revisions 2007. Genet. Med. 2008, 10, 294–300. [Google Scholar] [CrossRef]
- Crehalet, H.; Millat, G.; Albuisson, J.; Bonnet, V.; Rouvet, I.; Rousson, R.; Bozon, D. Combined Use of In Silico and In Vitro Splicing Assays for Interpretation of Genomic Variants of Unknown Significance in Cardiomyopathies and Channelopathies. Cardiogenetics 2012, 2, e6. [Google Scholar] [CrossRef]
- Moss, A.J.; Shimizu, W.; Wilde, A.A.M.; Towbin, J.A.; Zareba, W.; Robinson, J.L.; Qi, M.; Vincent, G.M.; Ackerman, M.J.; Kaufman, E.S.; et al. Clinical Aspects of Type-1 Long-QT Syndrome by Location, Coding Type, and Biophysical Function of Mutations Involving the KCNQ1 Gene. Circulation 2007, 115, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Kapa, S.; Tester, D.J.; Salisbury, B.A.; Harris-Kerr, C.; Pungliya, M.S.; Alders, M.; Wilde, A.A.M.; Ackerman, M.J. Genetic Testing for Long-QT Syndrome: Distinguishing Pathogenic Mutations from Benign Variants. Circulation 2009, 120, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Lieve, K.V.; Williams, L.; Daly, A.; Richard, G.; Bale, S.; Macaya, D.; Chung, W.K. Results of Genetic Testing in 855 Consecutive Unrelated Patients Referred for Long QT Syndrome in a Clinical Laboratory. Genet. Test. Mol. Biomark. 2013, 17, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassnan, Z.N.; Al-Fayyadh, M.; Al-Ghamdi, B.; Shafquat, A.; Mallawi, Y.; Al-Hadeq, F.; Tulbah, S.; Shinwari, Z.M.A.; Almesned, A.; Alakhfash, A.; et al. Clinical Profile and Mutation Spectrum of Long QT Syndrome in Saudi Arabia: The Impact of Consanguinity. Heart Rhythm. 2017, 14, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Koponen, M.; Havulinna, A.S.; Marjamaa, A.; Tuiskula, A.M.; Salomaa, V.; Laitinen-Forsblom, P.J.; Piippo, K.; Toivonen, L.; Kontula, K.; Viitasalo, M.; et al. Clinical and Molecular Genetic Risk Determinants in Adult Long QT Syndrome Type 1 and 2 Patients: Koponen et al. Follow-up of Adult LQTS Patients. BMC Med. Genet. 2018, 19, 56. [Google Scholar] [CrossRef]
- Barsheshet, A.; Goldenberg, I.; O-Uchi, J.; Moss, A.J.; Jons, C.; Shimizu, W.; Wilde, A.A.; McNitt, S.; Peterson, D.R.; Zareba, W.; et al. Mutations in Cytoplasmic Loops of the KCNQ1 Channel and the Risk of Life-Threatening Events. Circulation 2012, 125, 1988–1996. [Google Scholar] [CrossRef]
- Ruwald, M.H.; Xu Parks, X.; Moss, A.J.; Zareba, W.; Baman, J.; McNitt, S.; Kanters, J.K.; Shimizu, W.; Wilde, A.A.; Jons, C.; et al. Stop-Codon and C-Terminal Nonsense Mutations Are Associated with a Lower Risk of Cardiac Events in Patients with Long QT Syndrome Type 1. Heart Rhythm. 2016, 13, 122–131. [Google Scholar] [CrossRef]
- Huttunen, H.; Hero, M.; Lääperi, M.; Känsäkoski, J.; Swan, H.; Hirsch, J.A.; Miettinen, P.J.; Raivio, T. The Role of KCNQ1 Mutations and Maternal Beta Blocker Use during Pregnancy in the Growth of Children with Long QT Syndrome. Front. Endocrinol. 2018, 9, 194. [Google Scholar] [CrossRef]
- Wang, C.; Lu, Y.; Cheng, J.; Zhang, L.; Liu, W.; Peng, W.; Zhang, D.; Duan, H.; Han, D.; Yuan, H. Identification of KCNQ1 Compound Heterozygous Mutations in Three Chinese Families with Jervell and Lange-Nielsen Syndrome. Acta Otolaryngol. 2017, 137, 522–528. [Google Scholar] [CrossRef]
- Cohle, S.D.; Suarez-Mier, M.P.; Aguilera, B. Sudden Death Resulting from Lesions of the Cardiac Conduction System. Am. J. Forensic Med. Pathol. 2002, 23, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bharati, S.; Lev, M. Sudden Death in Athletes—Conduction System: Practical Approach to Dissection and Pertinent Pathology. Cardiovasc. Pathol. 1994, 3, 117–127. [Google Scholar] [CrossRef]
- Bharati, S.; Lev, M. Conduction System Findings in Sudden Death in Young Adults with a History of Bronchial Asthma. J. Am. Coll. Cardiol. 1994, 23, 741–746. [Google Scholar] [CrossRef]
- Bharati, S.; Lev, M. Cardiac Conduction System Involvement in Sudden Death of Obese Young People. Am. Heart J. 1995, 129, 273–281. [Google Scholar] [CrossRef]
- Csepe, T.A.; Kalyanasundaram, A.; Hansen, B.J.; Zhao, J.; Fedorov, V.v. Fibrosis: A Structural Modulator of Sinoatrial Node Physiology and Dysfunction. Front. Physiol. 2015, 6, 37. [Google Scholar] [CrossRef]
- Hurlé, A.; Climent, V.; Sánchez-Quintana, D. Sinus Node Structural Changes in Patients with Long-Standing Chronic Atrial Fibrillation. J. Thorac. Cardiovasc. Surg. 2006, 131, 1394–1395. [Google Scholar] [CrossRef]
- Yeh, Y.-H.; Burstein, B.; Qi, X.Y.; Sakabe, M.; Chartier, D.; Comtois, P.; Wang, Z.; Kuo, C.-T.; Nattel, S. Funny Current Downregulation and Sinus Node Dysfunction Associated with Atrial Tachyarrhythmia. Circulation 2009, 119, 1576–1585. [Google Scholar] [CrossRef]
- Wu, Y.; Anderson, M.E. CaMKII in Sinoatrial Node Physiology and Dysfunction. Front. Pharmacol. 2014, 5, 48. [Google Scholar] [CrossRef]
- Ottaviani, G.; Alfonsi, G.; Ramos, S.G.; Buja, L.M. Sudden Unexpected Death Associated with Arrhythmogenic Cardiomyopathy: Study of the Cardiac Conduction System. Diagnostics 2021, 11, 1323. [Google Scholar] [CrossRef]
- Grassi, S.; Campuzano, O.; Coll, M.; Cazzato, F.; Sarquella-Brugada, G.; Rossi, R.; Arena, V.; Brugada, J.; Brugada, R.; Oliva, A. Update on the Diagnostic Pitfalls of Autopsy and Post-Mortem Genetic Testing in Cardiomyopathies. Int. J. Mol. Sci. 2021, 22, 4124. [Google Scholar] [CrossRef]
- de la Grandmaison, G.L.; Clairand, I.; Durigon, M. Organ Weight in 684 Adult Autopsies: New Tables for a Caucasoid Population. Forensic Sci. Int. 2001, 119, 149–154. [Google Scholar] [CrossRef]
- Messerli, F.H. Overweight and Sudden Death. Increased Ventricular Ectopy in Cardiopathy of Obesity. Arch. Intern. Med. 1987, 147, 1725–1728. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Plehn, J.F.; Cupples, L.A. Cardiac Failure and Sudden Death in the Framingham Study. Am. Heart J. 1988, 115, 869–875. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef]
- Brenyo, A.; Pietrasik, G.; Barsheshet, A.; Huang, D.T.; Polonsky, B.; McNitt, S.; Moss, A.J.; Zareba, W. QRS Fragmentation and the Risk of Sudden Cardiac Death in MADIT II. J. Cardiovasc. Electrophysiol. 2012, 23, 1343–1348. [Google Scholar] [CrossRef]
- Narayanan, K.; Zhang, L.; Kim, C.; Uy-Evanado, A.; Teodorescu, C.; Reinier, K.; Zheng, Z.; Gunson, K.; Jui, J.; Chugh, S.S. QRS Fragmentation and Sudden Cardiac Death in the Obese and Overweight. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef]
- Konno, T.; Hayashi, K.; Fujino, N.; Oka, R.; Nomura, A.; Nagata, Y.; Hodatsu, A.; Sakata, K.; Furusho, H.; Takamura, M.; et al. Electrocardiographic QRS Fragmentation as a Marker for Myocardial Fibrosis in Hypertrophic Cardiomyopathy. J. Cardiovasc. Electrophysiol. 2015, 26, 1081–1087. [Google Scholar] [CrossRef]
- Chi, P.-C.; Chang, S.-C.; Yun, C.-H.; Kuo, J.-Y.; Hung, C.-L.; Hou, C.J.-Y.; Liu, C.-Y.; Yang, F.-S.; Wu, T.-H.; Bezerra, H.G.; et al. The Associations between Various Ectopic Visceral Adiposity and Body Surface Electrocardiographic Alterations: Potential Differences between Local and Remote Systemic Effects. PLoS ONE 2016, 11, e0158300. [Google Scholar] [CrossRef]
- Wu, C.-K.; Tsai, H.-Y.; Su, M.-Y.M.; Wu, Y.-F.; Hwang, J.-J.; Tseng, W.-Y.; Lin, J.-L.; Lin, L.-Y. Pericardial Fat Is Associated with Ventricular Tachyarrhythmia and Mortality in Patients with Systolic Heart Failure. Atherosclerosis 2015, 241, 607–614. [Google Scholar] [CrossRef]
- Plourde, B.; Sarrazin, J.-F.; Nault, I.; Poirier, P. Sudden Cardiac Death and Obesity. Expert Rev. Cardiovasc. Ther. 2014, 12, 1099–1110. [Google Scholar] [CrossRef]
- Esposito, K.; Nicoletti, G.; Marzano, S.; Gualdiero, P.; Carusone, C.; Marfella, R.; Beneduce, F.; Giugliano, D. Autonomic Dysfunction Associates with Prolongation of QT Intervals and Blunted Night BP in Obese Women with Visceral Obesity. J. Endocrinol. Investig. 2002, 25, RC32–RC35. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, F.; Parrillo, J.E. Eosinophilic Myocarditis. Heart Fail. Clin. 2005, 1, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Kubo, N.; Hiramitsu, S.; Uemura, A.; Ohtsuki, M.; Kato, S.; Kato, Y.; Sugiura, A.; Miyagishima, K.; Mori, N.; et al. Changes in the Peripheral Eosinophil Count in Patients with Acute Eosinophilic Myocarditis. Heart Vessel. 2003, 18, 193–196. [Google Scholar] [CrossRef]
- Ameratunga, R.; Woon, S.-T.; Sheppard, M.N.; Garland, J.; Ondruschka, B.; Wong, C.X.; Stewart, R.A.H.; Tatley, M.; Stables, S.R.; Tse, R.D. First Identified Case of Fatal Fulminant Necrotizing Eosinophilic Myocarditis Following the Initial Dose of the Pfizer-BioNTech MRNA COVID-19 Vaccine (BNT162b2, Comirnaty): An Extremely Rare Idiosyncratic Hypersensitivity Reaction. J. Clin. Immunol. 2022, 42, 441–447. [Google Scholar] [CrossRef]
- Coll, M.; Oliva, A.; Grassi, S.; Brugada, R.; Campuzano, O. Update on the Genetic Basis of Sudden Unexpected Death in Epilepsy. Int. J. Mol. Sci. 2019, 20, 1979. [Google Scholar] [CrossRef] [PubMed]
- Gurrieri, F.; Zollino, M.; Oliva, A.; Pascali, V.; Orteschi, D.; Pietrobono, R.; Camporeale, A.; Coll Vidal, M.; Partemi, S.; Brugada, R.; et al. Mild Beckwith-Wiedemann and Severe Long-QT Syndrome Due to Deletion of the Imprinting Center 2 on Chromosome 11p. Eur. J. Hum. Genet. 2013, 21, 965–969. [Google Scholar] [CrossRef]
- Fellmann, F.; van El, C.G.; Charron, P.; Michaud, K.; Howard, H.C.; Boers, S.N.; Clarke, A.J.; Duguet, A.-M.; Forzano, F.; Kauferstein, S.; et al. European Recommendations Integrating Genetic Testing into Multidisciplinary Management of Sudden Cardiac Death. Eur. J. Hum. Genet. 2019, 27, 1763–1773. [Google Scholar] [CrossRef]
- Mates, J.; Mademont-Soler, I.; Fernandez-Falgueras, A.; Sarquella-Brugada, G.; Cesar, S.; Arbelo, E.; García-Álvarez, A.; Jordà, P.; Toro, R.; Coll, M.; et al. Sudden Cardiac Death and Copy Number Variants: What Do We Know after 10 Years of Genetic Analysis? Forensic Sci. Int. Genet. 2020, 47, 102281. [Google Scholar] [CrossRef]
- Lacaze, P.; Sebra, R.; Riaz, M.; Ingles, J.; Tiller, J.; Thompson, B.A.; James, P.A.; Fatkin, D.; Semsarian, C.; Reid, C.M.; et al. Genetic Variants Associated with Inherited Cardiovascular Disorders among 13,131 Asymptomatic Older Adults of European Descent. NPJ Genom. Med. 2021, 6, 51. [Google Scholar] [CrossRef]
- Westphal, D.S.; Burkard, T.; Moscu-Gregor, A.; Gebauer, R.; Hessling, G.; Wolf, C.M. Reclassification of Genetic Variants in Children with Long QT Syndrome. Mol. Genet. Genom. Med. 2020, 8, e1300. [Google Scholar] [CrossRef]
- Marschall, C.; Moscu-Gregor, A.; Klein, H.-G. Variant Panorama in 1385 Index Patients and Sensitivity of Expanded next-Generation Sequencing Panels in Arrhythmogenic Disorders. Cardiovasc. Diagn. Ther. 2019, 9, S292–S298. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Barhanin, J.; Lesage, F.; Guillemare, E.; Fink, M.; Lazdunski, M.; Romey, G. KvLQT1 and IsK (MinK) Proteins Associate to Form the IKS Cardiac Potassium Current. Nature 1996, 384, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, N.; Richard, P.; Vignier, N.; Donger, C.; Denjoy, I.; Demay, L.; Shkolnikova, M.; Pesce, R.; Chevalier, P.; Hainque, B.; et al. Genomic Organization of the KCNQ1 K+ Channel Gene and Identification of C-Terminal Mutations in the Long-QT Syndrome. Circ. Res. 1999, 84, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Johnson, J.N.; Bos, J.M.; Phillips, B.L.; Eidem, B.W.; Ackerman, M.J. Subclinical Cardiomyopathy and Long QT Syndrome: An Echocardiographic Observation. Congenit. Heart Dis. 2013, 8, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Leren, I.S.; Hasselberg, N.E.; Saberniak, J.; Håland, T.F.; Kongsgård, E.; Smiseth, O.A.; Edvardsen, T.; Haugaa, K.H. Cardiac Mechanical Alterations and Genotype Specific Differences in Subjects with Long QT Syndrome. JACC Cardiovasc. Imaging 2015, 8, 501–510. [Google Scholar] [CrossRef]
- Bos, I.; Johannisson, R.; Djonlagic, H. Morphologic Alterations in the Long Q-T Syndrome. Pathol. Res. Pract. 1985, 180, 691–696. [Google Scholar] [CrossRef]
- Rogers, A.; Taylor, R.; Poulik, J.; Shehata, B.M. Histopathology of the Conduction System in Long QT Syndrome. Fetal Pediatr. Pathol. 2021, 10, 2562–2585. [Google Scholar] [CrossRef]

| Gene | KCNQ1 |
| Isoform | NM_000218 |
| Protein Variant | NA * |
| cDNA Variant | c.683 + 5G > A (rs397508122) |
| gnomAD Database | 0.0002980% |
| ClinVar Database | 2 Likely Pathogenic, 1 Pathogenic, 1 VUS |
| HGMD Database | CS072218 |
| MaxEntScan (In Silico) | 0 |
| FSPLICE (In Silico) | 1 |
| GeneSplicer (In Silico) | 0 |
| ACMG KeyWords | PM2_Supp + PS4_Moderate + PS3_Strong |
| Classification | Likely Pathogenic in a LQTS context |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, S.; Campuzano, O.; Coll, M.; Cazzato, F.; Iglesias, A.; Ausania, F.; Scarnicci, F.; Sarquella-Brugada, G.; Brugada, J.; Arena, V.; et al. Eosinophilic Infiltration of the Sino-Atrial Node in Sudden Cardiac Death Caused by Long QT Syndrome. Int. J. Mol. Sci. 2022, 23, 11666. https://doi.org/10.3390/ijms231911666
Grassi S, Campuzano O, Coll M, Cazzato F, Iglesias A, Ausania F, Scarnicci F, Sarquella-Brugada G, Brugada J, Arena V, et al. Eosinophilic Infiltration of the Sino-Atrial Node in Sudden Cardiac Death Caused by Long QT Syndrome. International Journal of Molecular Sciences. 2022; 23(19):11666. https://doi.org/10.3390/ijms231911666
Chicago/Turabian StyleGrassi, Simone, Oscar Campuzano, Mònica Coll, Francesca Cazzato, Anna Iglesias, Francesco Ausania, Francesca Scarnicci, Georgia Sarquella-Brugada, Josep Brugada, Vincenzo Arena, and et al. 2022. "Eosinophilic Infiltration of the Sino-Atrial Node in Sudden Cardiac Death Caused by Long QT Syndrome" International Journal of Molecular Sciences 23, no. 19: 11666. https://doi.org/10.3390/ijms231911666
APA StyleGrassi, S., Campuzano, O., Coll, M., Cazzato, F., Iglesias, A., Ausania, F., Scarnicci, F., Sarquella-Brugada, G., Brugada, J., Arena, V., Oliva, A., & Brugada, R. (2022). Eosinophilic Infiltration of the Sino-Atrial Node in Sudden Cardiac Death Caused by Long QT Syndrome. International Journal of Molecular Sciences, 23(19), 11666. https://doi.org/10.3390/ijms231911666









