Hepatocyte-Derived Prostaglandin E2-Modulated Macrophage M1-Type Polarization via mTOR-NPC1 Axis-Regulated Cholesterol Transport from Lysosomes to the Endoplasmic Reticulum in Hepatitis B Virus x Protein-Related Nonalcoholic Steatohepatitis
Abstract
1. Introduction
2. Results
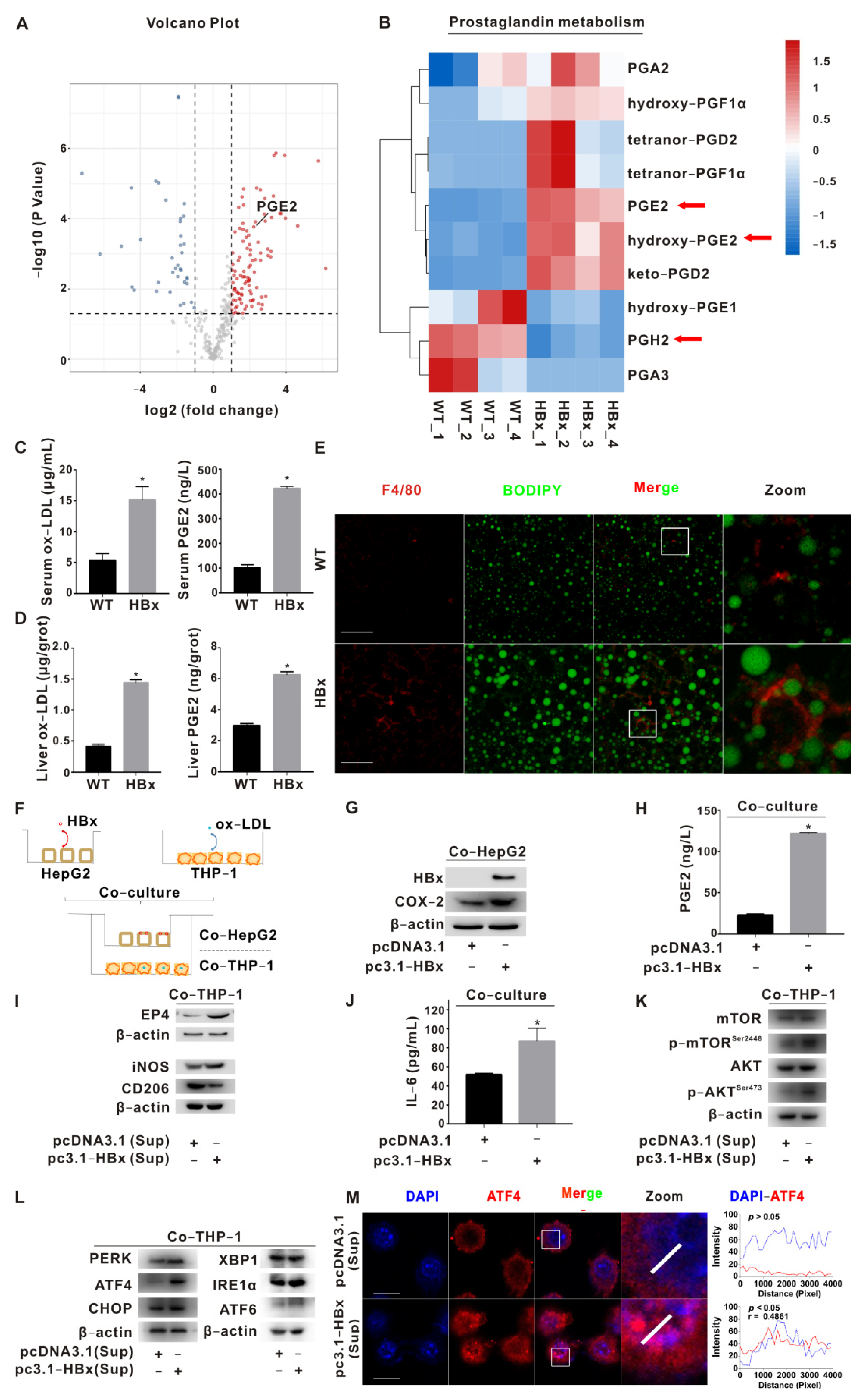
2.1. PGE2-EP4 Induced ER Stress-Mediated Macrophage Polarization Imbalance via mTOR Signaling
2.2. NPC1 Mediated Lyso-ER Cholesterol Transport to Regulate ER Redox and ER Stress Homeostasis
2.3. Regulation of Macrophage Polarization by mTOR-NPC1-ERO1α Pathway Was Verified in HBx-Tg Mouse Ex Vivo Assay
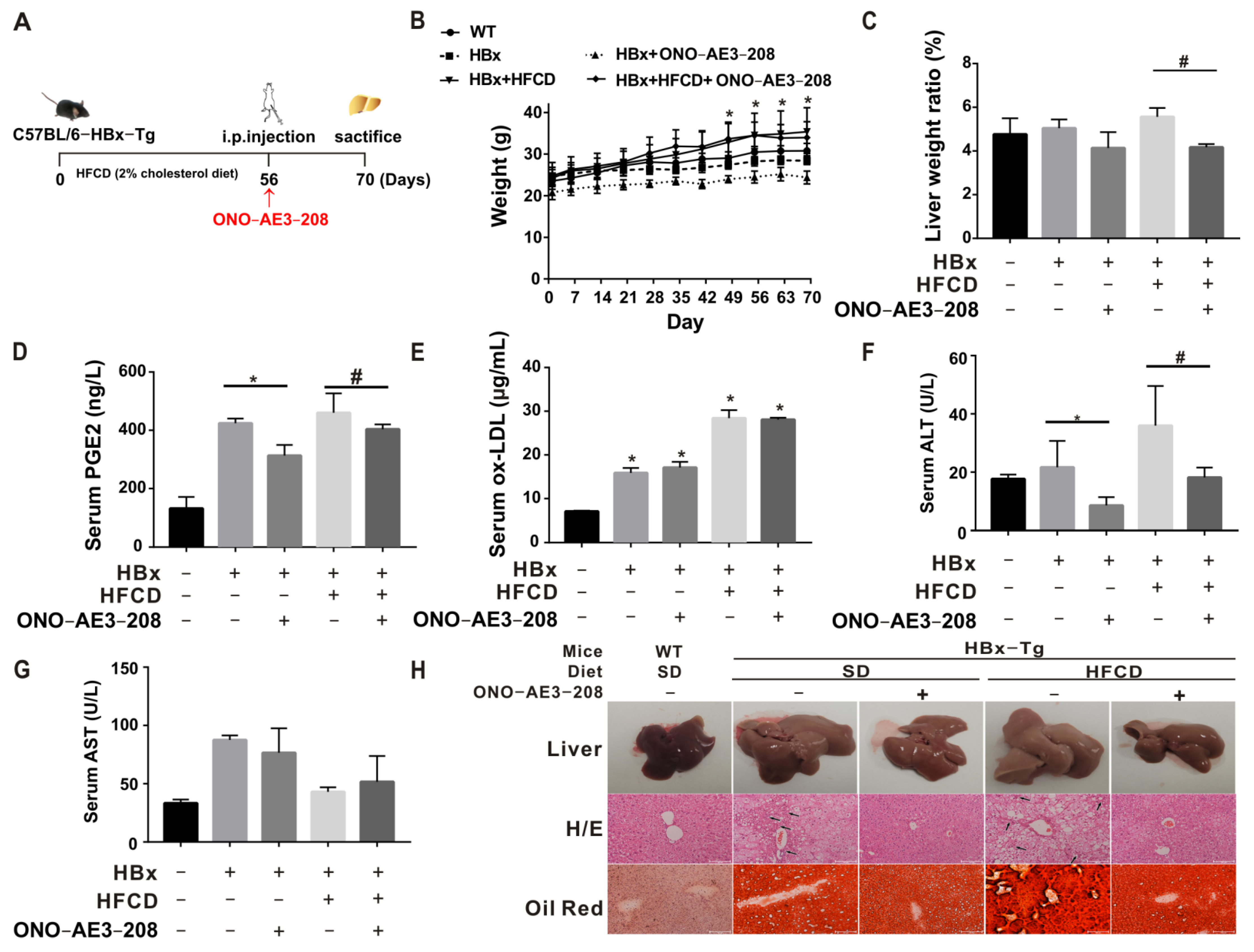
2.4. EP4 Intervention Reduced the HBx-Related Lipotoxicity and NASH In Vivo
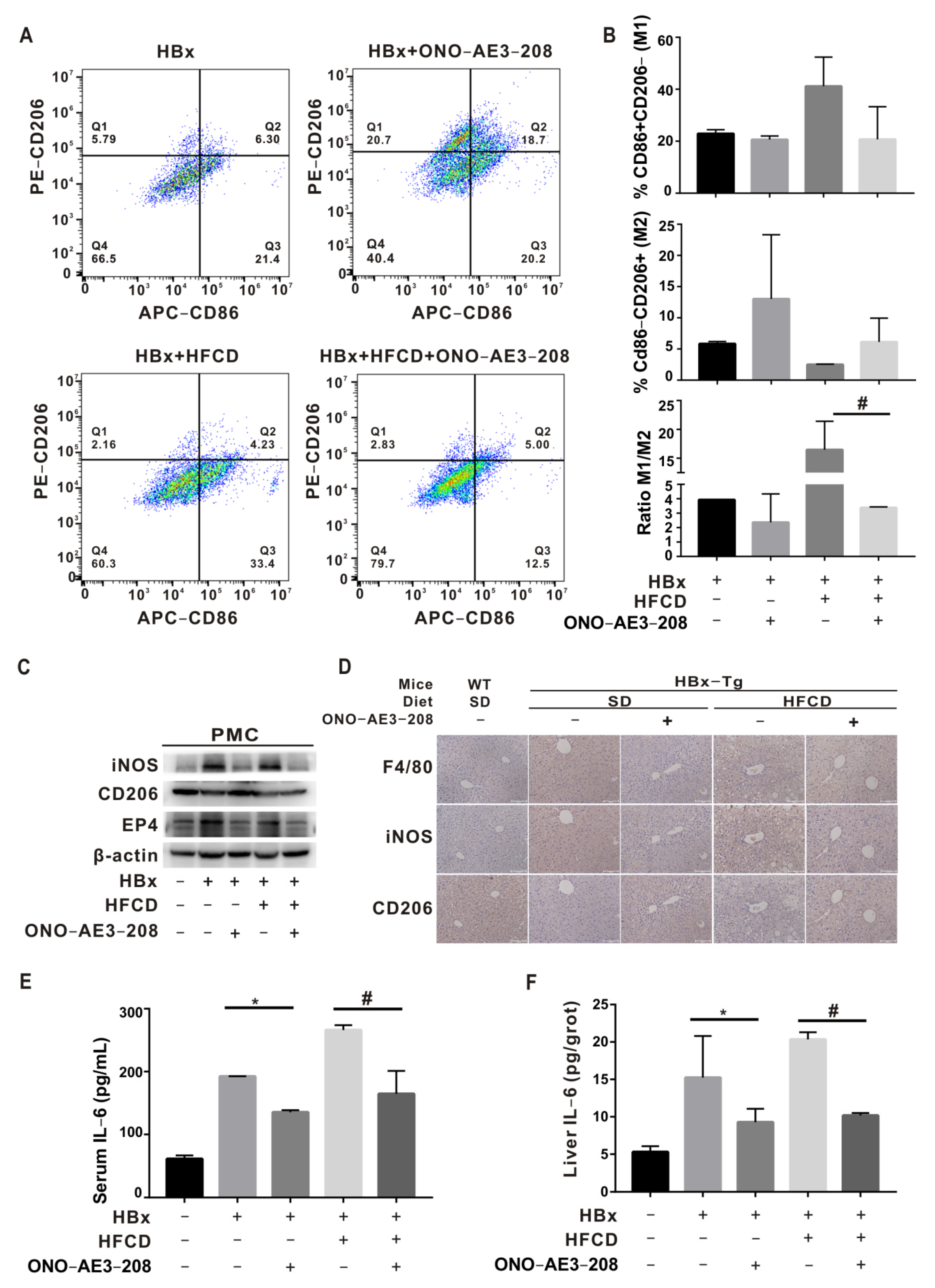
2.5. EP4 Intervention Rescued Hepatic Macrophage Polarization Imbalance In Vivo
3. Discussion
4. Materials and Methods
4.1. Bioinformatics Analysis
4.2. Reagents and Antibodies
4.3. Cell Grouping and Processing
4.4. Western Blotting (WB)
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Immunofluorescence (IF)
4.7. Flow Cytometry (FCM)
4.8. Transmission Electron Microscopy (TEM)
4.9. Total Glutathione–Oxidized Glutathione Test Kit
4.10. Isolation of Hepatic Macrophages by Mouse Hepatic Perfusion
4.11. Co-Culture of Mouse PHCs and PMCs
4.12. Animal Model and Diets
4.13. Serum Index Quantification
4.14. Liver Homogenate Index Quantification
4.15. FCM Detection and Analysis of the Polarization Ratio of Mononuclear-Derived KC
4.16. Histopathological Observation of Liver
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef]
- Hoogerland, J.A.; Staels, B.; Dombrowicz, D. Immune-metabolic interactions in homeostasis and the progression to NASH. Trends Endocrinol. Metab. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, H.; Bu, Q.; Wei, S.; Li, L.; Zhou, J.; Zhou, S.; Su, W.; Liu, M.; Liu, Z.; et al. Role of XBP1 in regulating the progression of non-alcoholic steatohepatitis. J. Hepatol. 2022, 77, 312–325. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Jiang, D.; Li, M.; Chen, Y.; Li, J.; Fan, J. Chronic hepatitis B and non-alcoholic fatty liver disease: Conspirators or competitors? Liver Int. 2020, 40, 496–508. [Google Scholar] [CrossRef]
- Wu, Y.L.; Peng, X.E.; Zhu, Y.B.; Yan, X.L.; Chen, W.N.; Lin, X. Hepatitis B Virus X Protein Induces Hepatic Steatosis by Enhancing the Expression of Liver Fatty Acid Binding Protein. J. Virol. 2016, 90, 1729–1740. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lin, Y.; Han, P.Y.; Jiang, S.; Che, L.; He, C.Y.; Lin, Y.C.; Lin, Z.N. HBx combined with AFB1 triggers hepatic steatosis via COX-2-mediated necrosome formation and mitochondrial dynamics disorder. J. Cell Mol. Med. 2019, 23, 5920–5933. [Google Scholar] [CrossRef]
- Mahesh, G.; Anil Kumar, K.; Reddanna, P. Overview on the Discovery and Development of Anti-Inflammatory Drugs: Should the Focus Be on Synthesis or Degradation of PGE2? J. Inflamm. Res. 2021, 14, 253–263. [Google Scholar] [CrossRef]
- Su, W.; Liu, G.; Mohajer, B.; Wang, J.; Shen, A.; Zhang, W.; Liu, B.; Guermazi, A.; Gao, P.; Cao, X.; et al. Senescent preosteoclast secretome promotes metabolic syndrome associated osteoarthritis through cyclooxygenase 2. Elife 2022, 11, e79773. [Google Scholar] [CrossRef]
- Martinez-Colon, G.J.; Moore, B.B. Prostaglandin E2 as a Regulator of Immunity to Pathogens. Pharmacol. Ther. 2018, 185, 135–146. [Google Scholar] [CrossRef]
- Govaere, O.; Petersen, S.K.; Martinez-Lopez, N.; Wouters, J.; Van Haele, M.; Mancina, R.M.; Jamialahmadi, O.; Bilkei-Gorzo, O.; Lassen, P.B.; Darlay, R.; et al. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog. Lipid Res. 2013, 52, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, P.; Velagapudi, S.; Yalcinkaya, M.; Rohrer, L.; von Eckardstein, A. Endocytosis of lipoproteins. Atherosclerosis 2018, 275, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, W.; Bai, F.; Xu, Y.; Liang, X.; Ma, C.; Gao, L. Hepatic Macrophage as a Key Player in Fatty Liver Disease. Front. Immunol. 2021, 12, 708978. [Google Scholar] [CrossRef]
- Bieghs, V.; Wouters, K.; van Gorp, P.J.; Gijbels, M.J.; de Winther, M.P.; Binder, C.J.; Lutjohann, D.; Febbraio, M.; Moore, K.J.; van Bilsen, M.; et al. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology 2010, 138, 2477–2486. [Google Scholar] [CrossRef]
- Sukhorukov, V.N.; Khotina, V.A.; Chegodaev, Y.S.; Ivanova, E.; Sobenin, I.A.; Orekhov, A.N. Lipid Metabolism in Macrophages: Focus on Atherosclerosis. Biomedicines 2020, 8, 262. [Google Scholar] [CrossRef]
- Sukhorukov, V.N.; Khotina, V.A.; Bagheri Ekta, M.; Ivanova, E.A.; Sobenin, I.A.; Orekhov, A.N. Endoplasmic Reticulum Stress in Macrophages: The Vicious Circle of Lipid Accumulation and Pro-Inflammatory Response. Biomedicines 2020, 8, 210. [Google Scholar] [CrossRef]
- He, C.; Jiang, S.; Yao, H.; Zhang, L.; Yang, C.; Zhan, D.; Lin, G.; Zeng, Y.; Xia, Y.; Lin, Z.; et al. Endoplasmic reticulum stress mediates inflammatory response triggered by ultra-small superparamagnetic iron oxide nanoparticles in hepatocytes. Nanotoxicology 2018, 12, 1198–1214. [Google Scholar] [CrossRef]
- He, C.; Qiu, Y.; Han, P.; Chen, Y.; Zhang, L.; Yuan, Q.; Zhang, T.; Cheng, T.; Yuan, L.; Huang, C.; et al. ER stress regulating protein phosphatase 2A-B56gamma, targeted by hepatitis B virus X protein, induces cell cycle arrest and apoptosis of hepatocytes. Cell Death Dis. 2018, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Zhan, D.L.; Chen, Y.Y.; Wang, W.H.; He, C.Y.; Lin, Y.; Lin, Y.C.; Lin, Z.N. Aflatoxin B1 enhances pyroptosis of hepatocytes and activation of Kupffer cells to promote liver inflammatory injury via dephosphorylation of cyclooxygenase-2: An in vitro, ex vivo and in vivo study. Arch. Toxicol. 2019, 93, 3305–3320. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Manik, M.K.; Im, Y.J. Structural basis of sterol recognition and nonvesicular transport by lipid transfer proteins anchored at membrane contact sites. Proc. Natl. Acad. Sci. USA 2018, 115, E856–E865. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Meng, T.; Huang, H.; Zhuang, H.; He, Z.; Yang, H.; Feng, D. Molecular machineries and physiological relevance of ER-mediated membrane contacts. Theranostics 2021, 11, 974–995. [Google Scholar] [CrossRef] [PubMed]
- Hoglinger, D.; Burgoyne, T.; Sanchez-Heras, E.; Hartwig, P.; Colaco, A.; Newton, J.; Futter, C.E.; Spiegel, S.; Platt, F.M.; Eden, E.R. NPC1 regulates ER contacts with endocytic organelles to mediate cholesterol egress. Nat. Commun. 2019, 10, 4276. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Kumar, J.; Ferguson, C.; Schulz, T.A.; Ong, Y.S.; Hong, W.; Prinz, W.A.; Parton, R.G.; Brown, A.J.; Yang, H. A role for oxysterol-binding protein-related protein 5 in endosomal cholesterol trafficking. J. Cell Biol. 2011, 192, 121–135. [Google Scholar] [CrossRef]
- Wilhelm, L.P.; Wendling, C.; Vedie, B.; Kobayashi, T.; Chenard, M.P.; Tomasetto, C.; Drin, G.; Alpy, F. STARD3 mediates endoplasmic reticulum-to-endosome cholesterol transport at membrane contact sites. EMBO J. 2017, 36, 1412–1433. [Google Scholar] [CrossRef]
- Castellano, B.M.; Thelen, A.M.; Moldavski, O.; Feltes, M.; van der Welle, R.E.; Mydock-McGrane, L.; Jiang, X.; van Eijkeren, R.J.; Davis, O.B.; Louie, S.M.; et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science 2017, 355, 1306–1311. [Google Scholar] [CrossRef]
- Xu, J.; Dang, Y.; Ren, Y.R.; Liu, J.O. Cholesterol trafficking is required for mTOR activation in endothelial cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4764–4769. [Google Scholar] [CrossRef]
- Che, L.; Yao, H.; Yang, C.L.; Guo, N.J.; Huang, J.; Wu, Z.L.; Zhang, L.Y.; Chen, Y.Y.; Liu, G.; Lin, Z.N.; et al. Cyclooxygenase-2 modulates ER-mitochondria crosstalk to mediate superparamagnetic iron oxide nanoparticles induced hepatotoxicity: An in vitro and in vivo study. Nanotoxicology 2020, 14, 162–180. [Google Scholar] [CrossRef]
- Henkel, J.; Coleman, C.D.; Schraplau, A.; Johrens, K.; Weiss, T.S.; Jonas, W.; Schurmann, A.; Puschel, G.P. Augmented liver inflammation in a microsomal prostaglandin E synthase 1 (mPGES-1)-deficient diet-induced mouse NASH model. Sci. Rep. 2018, 8, 16127. [Google Scholar] [CrossRef] [PubMed]
- Shergalis, A.G.; Hu, S.; Bankhead, A., 3rd; Neamati, N. Role of the ERO1-PDI interaction in oxidative protein folding and disease. Pharmacol. Ther. 2020, 210, 107525. [Google Scholar] [CrossRef] [PubMed]
- Lebeaupin, C.; Vallee, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Song, M.J.; Malhi, H. The unfolded protein response and hepatic lipid metabolism in non alcoholic fatty liver disease. Pharmacol. Ther. 2019, 203, 107401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shen, H.; Li, X.; Wang, H. Endoplasmic reticulum stress in innate immune cells—A significant contribution to non-alcoholic fatty liver disease. Front. Immunol. 2022, 13, 951406. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, A.; Halquist, M.S.; Yuan, X.; Henderson, S.C.; Dewey, W.L.; Li, P.L.; Li, N.; Zhang, F. Simvastatin promotes NPC1-mediated free cholesterol efflux from lysosomes through CYP7A1/LXRalpha signalling pathway in oxLDL-loaded macrophages. J. Cell Mol. Med. 2017, 21, 364–374. [Google Scholar] [CrossRef]
- Borthwick, F.; Allen, A.M.; Taylor, J.M.; Graham, A. Overexpression of STARD3 in human monocyte/macrophages induces an anti-atherogenic lipid phenotype. Clin. Sci. 2010, 119, 265–272. [Google Scholar] [CrossRef]
- Yan, D.; Jauhiainen, M.; Hildebrand, R.B.; Willems van Dijk, K.; Van Berkel, T.J.; Ehnholm, C.; Van Eck, M.; Olkkonen, V.M. Expression of human OSBP-related protein 1L in macrophages enhances atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1618–1624. [Google Scholar] [CrossRef]
- Hendrikx, T.; Walenbergh, S.M.; Hofker, M.H.; Shiri-Sverdlov, R. Lysosomal cholesterol accumulation: Driver on the road to inflammation during atherosclerosis and non-alcoholic steatohepatitis. Obes. Rev. 2014, 15, 424–433. [Google Scholar] [CrossRef]
- Head, S.A.; Shi, W.Q.; Yang, E.J.; Nacev, B.A.; Hong, S.Y.; Pasunooti, K.K.; Li, R.J.; Shim, J.S.; Liu, J.O. Simultaneous Targeting of NPC1 and VDAC1 by Itraconazole Leads to Synergistic Inhibition of mTOR Signaling and Angiogenesis. ACS Chem. Biol. 2017, 12, 174–182. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, X.; Guo, J. Role of 2series prostaglandins in the pathogenesis of type 2 diabetes mellitus and nonalcoholic fatty liver disease (Review). Int. J. Mol. Med. 2021, 47, 114. [Google Scholar] [CrossRef] [PubMed]
- Luan, B.; Yoon, Y.S.; Le Lay, J.; Kaestner, K.H.; Hedrick, S.; Montminy, M. CREB pathway links PGE2 signaling with macrophage polarization. Proc. Natl. Acad. Sci. USA 2015, 112, 15642–15647. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, N.; Ito, Y.; Eshima, K.; Ohkubo, H.; Kojo, K.; Inoue, T.; Raouf, J.; Jakobsson, P.J.; Uematsu, S.; Akira, S.; et al. Inhibition of microsomal prostaglandin E synthase-1 facilitates liver repair after hepatic injury in mice. J. Hepatol. 2018, 69, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Iwatsubo, K.; Umemura, M.; Fujita, T.; Ishikawa, Y. The prostanoid EP4 receptor and its signaling pathway. Pharmacol. Rev. 2013, 65, 1010–1052. [Google Scholar] [CrossRef]
- Wang, D.; Fu, L.; Sun, H.; Guo, L.; DuBois, R.N. Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology 2015, 149, 1884–1895.e4. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Peng, S.; Han, Z.; Shen, J.; Cai, M. Association of Low High-Density Lipoprotein Cholesterol Levels with Poor Outcomes in Hepatitis B-Associated Decompensated Cirrhosis Patients. Biomed. Res. Int. 2021, 2021, 9927330. [Google Scholar] [CrossRef]
- Wu, Q.; Mao, W. New prognostic factor for hepatitis B virus-related decompensated cirrhosis: Ratio of monocytes to HDL-cholesterol. J. Clin. Lab. Anal. 2021, 35, e24007. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Duan, X.; Yang, C.; Xu, M.; Chen, L. Macrophage Phenotypes and Hepatitis B Virus Infection. J. Clin. Transl. Hepatol. 2020, 8, 424–431. [Google Scholar] [CrossRef]
- Che, L.; Du, Z.B.; Wang, W.H.; Wu, J.S.; Han, T.; Chen, Y.Y.; Han, P.Y.; Lei, Z.; Chen, X.X.; He, Y.; et al. Intracellular antibody targeting HBx suppresses invasion and metastasis in hepatitis B virus-related hepatocarcinogenesis via protein phosphatase 2A-B56gamma-mediated dephosphorylation of protein kinase B. Cell Prolif. 2022, e13304. [Google Scholar] [CrossRef]
- Che, L.; Wu, J.S.; Du, Z.B.; He, Y.Q.; Yang, L.; Lin, J.X.; Lei, Z.; Chen, X.X.; Guo, D.B.; Li, W.G.; et al. Targeting Mitochondrial COX-2 Enhances Chemosensitivity via Drp1-Dependent Remodeling of Mitochondrial Dynamics in Hepatocellular Carcinoma. Cancers 2022, 14, 821. [Google Scholar] [CrossRef]
- Che, L.; Wu, J.S.; Xu, C.Y.; Cai, Y.X.; Lin, J.X.; Du, Z.B.; Shi, J.Z.; Han, T.; He, Y.Q.; Lin, Y.C.; et al. Protein phosphatase 2A-B56gamma-Drp1-Rab7 signaling axis regulates mitochondria-lysosome crosstalk to sensitize the anti-cancer therapy of hepatocellular carcinoma. Biochem. Pharmacol. 2022, 202, 115132. [Google Scholar] [CrossRef] [PubMed]
- Santhekadur, P.K.; Kumar, D.P.; Sanyal, A.J. Preclinical models of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 230–237. [Google Scholar] [CrossRef] [PubMed]



| Mice | WT | HBx-Tg | |||
|---|---|---|---|---|---|
| Diet | SD | SD | HFCD | ||
| ONO-AE3-208 | − | − | + | − | + |
| Steatosis | 0 | 2 | 1 | 3 | 1 |
| Lobular inflammation | 0 | 2 | 0 | 3 | 0 |
| Hepatocyte ballooning | 0 | 2 | 0 | 2 | 1 |
| NAFLD activity score | 0 | 6 | 1 | 8 | 2 |
| Diagnostic classification | N | NASH | N | NASH | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.; Qian, B.; Huang, H.-Y.; Wang, P.; Li, T.; Yuan, Q.; Zhang, H.-Y.; Lin, Y.-C.; Lin, Z.-N. Hepatocyte-Derived Prostaglandin E2-Modulated Macrophage M1-Type Polarization via mTOR-NPC1 Axis-Regulated Cholesterol Transport from Lysosomes to the Endoplasmic Reticulum in Hepatitis B Virus x Protein-Related Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2022, 23, 11660. https://doi.org/10.3390/ijms231911660
Lan Y, Qian B, Huang H-Y, Wang P, Li T, Yuan Q, Zhang H-Y, Lin Y-C, Lin Z-N. Hepatocyte-Derived Prostaglandin E2-Modulated Macrophage M1-Type Polarization via mTOR-NPC1 Axis-Regulated Cholesterol Transport from Lysosomes to the Endoplasmic Reticulum in Hepatitis B Virus x Protein-Related Nonalcoholic Steatohepatitis. International Journal of Molecular Sciences. 2022; 23(19):11660. https://doi.org/10.3390/ijms231911660
Chicago/Turabian StyleLan, You, Bo Qian, Hai-Yan Huang, Pan Wang, Ting Li, Qi Yuan, Han-Yu Zhang, Yu-Chun Lin, and Zhong-Ning Lin. 2022. "Hepatocyte-Derived Prostaglandin E2-Modulated Macrophage M1-Type Polarization via mTOR-NPC1 Axis-Regulated Cholesterol Transport from Lysosomes to the Endoplasmic Reticulum in Hepatitis B Virus x Protein-Related Nonalcoholic Steatohepatitis" International Journal of Molecular Sciences 23, no. 19: 11660. https://doi.org/10.3390/ijms231911660
APA StyleLan, Y., Qian, B., Huang, H.-Y., Wang, P., Li, T., Yuan, Q., Zhang, H.-Y., Lin, Y.-C., & Lin, Z.-N. (2022). Hepatocyte-Derived Prostaglandin E2-Modulated Macrophage M1-Type Polarization via mTOR-NPC1 Axis-Regulated Cholesterol Transport from Lysosomes to the Endoplasmic Reticulum in Hepatitis B Virus x Protein-Related Nonalcoholic Steatohepatitis. International Journal of Molecular Sciences, 23(19), 11660. https://doi.org/10.3390/ijms231911660




