Yeast-Fermented Rapeseed Meal Extract Is Able to Reduce Inflammation and Oxidative Stress Caused by Escherichia coli Lipopolysaccharides and to Replace ZnO in Caco-2/HTX29 Co-Culture Cells
Abstract
1. Introduction
2. Results
2.1. Effect of ZnO and FRSM Extract on Apoptosis in Caco-2/HT29MTX Co-Culture Challenged with LPS
2.2. Effect of ZnO and FRSM Extract on Oxidative Stress in Co-Cultured Caco-2/HT29MTX Challenged with LPS
2.2.1. ROS Production
2.2.2. Protein Oxidation
2.2.3. Lipid Peroxidation
2.3. Evaluation of the Effect of ZnO and FRSM Treatments on Global Protein Expression Profile upon LPS Exposure (Overview)
2.4. Effect of ZnO and FRSM Treatments on Protein Expression of Biomarkers Associated with Inflammation upon LPS Exposure
2.5. Effect of ZnO and FRSM Treatments on Metalloproteinase Activity upon LPS Exposure
2.6. Effect of ZnO and FRSM Treatments on Protein Expression of Biomarkers Linked to Signaling Pathway upon LPS Exposure
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fermented Rapeseed Meal Extract and ZnO Solutions
5.2. Cell Culture and Treatments
- (1)
- Untreated cells (control),
- (2)
- LPS = cells treated with 1 µg/mL LPS after 4 h of incubation until 24 h,
- (3)
- ZnO = cells treated with ZnO (50 µM) 24 h,
- (4)
- ZnO + LPS = cells pretreated with ZnO for 4 h and then challenge with LPS for another 24 h,
- (5)
- FRSM = cells treated with FRSM (1/50) 24 h,
- (6)
- FRSM + LPS = cells pretreated with FRSM for 4 h and then challenge with LPS for another 24 h.
5.3. Detection of Apoptosis
5.4. Detection of Oxidative Stress Markers
5.4.1. ROS Production
5.4.2. Lipid Peroxidation (Thiobarbituric-Acid-Reactive Substances—TBARS) Analysis
5.4.3. Protein Oxidation (Protein Carbonyl) Analysis
5.5. Detection of Inflammatory and Signaling Pathway Markers
Determination of Protein Expression (Protein Array)
5.6. Gelatine Zymography
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, X.; Santos, R.R.; Kettunen, H.; Vuorenmaa, J.; Molist, F. Effect of Resin Acid and Zinc Oxide on Immune Status of Weaned Piglets Challenged With, E. coli Lipopolysaccharide. Front. Vet. Sci. 2021, 8, 761742. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Eleonora, F.; Boglioni, M.; Giromini, C.; Rebucci, R.; Baldi, A. Effect of Zinc Oxide and Zinc Chloride on Human and Swine Intestinal Epithelial Cell Lines. Int. J. Health Anim. Sci. Food Saf. 2014, 1, 2. [Google Scholar]
- Peng, P.; Deng, D.; Chen, S.; Li, C.; Luo, J.; Romeo, A.; Li, T.; Tang, X.; Fang, R. The Effects of Dietary Porous Zinc Oxide Supplementation on Growth Performance, Inflammatory Cytokines and Tight Junction’s Gene Expression in Early-Weaned Piglets. J. Nutr. Sci. Vitaminol. 2020, 66, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J.; Miller, H.M.; Kerr, K.G.; Knapp, J.S. Effects of zinc oxide and Enterococcus faecium SF68 dietary supplementation on the performance, intestinal microbiota and immune status of weaned piglets. Res. Vet. Sci. 2006, 80, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Magnoli, A.P.; Parada, J.; de la Torre, F.C.; Watson, S.; Poloni, V.; Fochesato, A.; Martínez, M.P.; Coniglio, M.V.; Ortiz, M.E.; Cavaglieri, L. Respiratory tract clinometry, fat thickness, haematology and productive parameters associated with direct-fed microbials used as growth promoter antibiotic alternative in weaned piglets. Vet. Anim. Sci. 2022, 16, 100246. [Google Scholar] [CrossRef]
- Kiros, T.G.; Luise, D.; Derakhshani, H.; Petri, R.; Trevisi, P.; D’Inca, R.; Auclair, E.; van Kessel, A.G. Effect of live yeast Saccharomyces cerevisiae supplementation on the performance and cecum microbial profile of suckling piglets. PLoS ONE 2019, 14, e0219557. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: A review. Anim. Nutr. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Mukhopadhya, A.; O’Doherty, J.V.; Sweeney, T. A combination of yeast beta-glucan and milk hydrolysate is a suitable alternative to zinc oxide in the race to alleviate post-weaning diarrhoea in piglets. Sci. Rep. 2019, 9, 616. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, S.; Wang, Z.; Zhu, C.; Hu, S.; Zheng, C.; Chen, Z.; Hu, Y.; Wang, L.; Ma, X.; et al. Effects of different forms of yeast Saccharomyces cerevisiae on growth performance, intestinal development, and systemic immunity in early-weaned piglets. J. Anim. Sci. Biotechnol. 2015, 6, 47. [Google Scholar] [CrossRef]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal Absorption and Antioxidant Activity of Grape Pomace Polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Toschi, A.; Piva, A.; Grilli, E. Phenol-Rich Botanicals Modulate Oxidative Stress and Epithelial Integrity in Intestinal Epithelial Cells. Animals 2022, 12, 2188. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.R.; Li, Q.H.; Zhao, J.Q.; Li, H.F.; Wang, W.W.; Gao, J.J. Effects of grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livest. Sci. 2015, 178, 237–242. [Google Scholar] [CrossRef]
- Gessner, D.K.; Fiesel, A.; Most, E.; Dinges, J.; Wen, G.; Ringseis, R.; Eder, K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet. Scand. 2013, 55, 18. [Google Scholar] [CrossRef]
- La Marca, M.; Beffy, P.; Pugliese, A.; Longo, V. Fermented wheat powder induces the antioxidant and detoxifying system in primary rat hepatocytes. PLoS ONE 2013, 8, e83538. [Google Scholar] [CrossRef]
- Lionetti, L.; Cavaliere, G.; Bergamo, P.; Trinchese, G.; De Filippo, C.; Gifuni, G.; Gaita, M.; Pignalosa, A.; Donizzetti, I.; Putti, R.; et al. Diet supplementation with donkey milk upregulates liver mitochondrial uncoupling, reduces energy efficiency and improves antioxidant and antiinflammatory defences in rats. Mol. Nutr. Food Res. 2012, 56, 1596–1600. [Google Scholar] [CrossRef]
- Avramovic, N.; Dragutinovic, V.; Krstic, D.; Colovic, M.; Trbovic, A.; de Luka, S.; Milovanovic, I.; Popovic, T. The effects of omega 3 fatty acid supplementation on brain tissue oxidative status in aged wistar rats. Hippokratia 2012, 16, 241–245. [Google Scholar]
- Taranu, I.; Gras, M.; Pistol, G.C.; Motiu, M.; Marin, D.E.; Lefter, N.; Ropota, M.; Habeanu, M. ω-3 PUFA rich camelina oil by-products improve the systemic metabolism and spleen cell functions in fattening pigs. PLoS ONE 2014, 9, e110186. [Google Scholar] [CrossRef]
- Plaipetch, P.; Yakupitiyage, A. Effect of replacing soybean meal with yeast-fermented canola meal on growth and nutrient retention of Nile tilapia, Oreochromis niloticus (Linnaeus 1758). Aquac. Res. 2014, 45, 1744–1753. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Li, A.; Wang, Z.; Zhang, X.; Yun, T.; Qiu, L.; Yin, Y. Effects of fermented rapeseed meal on antioxidant functions, serum biochemical parameters and intestinal morphology in broilers. Food Agric. Immunol. 2015, 27, 1–12. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Ilhan, Z.E.; Garcia-Peña, E.I.; Krajmalnik-Brown, R. Insights into Butyrate Production in a Controlled Fermentation System via Gene Predictions. mSystems 2017, 2, e00051-17. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Olukomaiya, O.; Fernando, C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-state fermented plant protein sources in the diets of broiler chickens: A review. Anim. Nutr. 2019, 5, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Satessa, G.D.; Tamez-Hidalgo, P.; Hui, Y.; Cieplak, T.; Krych, L.; Kjærulff, S.; Brunsgaard, G.; Nielsen, D.S.; Nielsen, M.O. Impact of Dietary Supplementation of Lactic Acid Bacteria Fermented Rapeseed with or without Macroalgae on Performance and Health of Piglets Following Omission of Medicinal Zinc from Weaner Diets. Animals 2020, 10, 137. [Google Scholar] [CrossRef]
- Chiang, G.; Lu, W.Q.; Hu, J.K.; Gong, L.M.; Thacker, P.A. Effects of Feeding Solid-state Fermented Rapeseed Meal on Performance, Nutrient Digestibility, Intestinal Ecology and Intestinal Morphology of Broiler Chickens. Asian-Australas. J. Anim. Sci. 2010, 23, 263–271. [Google Scholar] [CrossRef]
- Plaipetch, P.; Yakupitiyage, A. Use of Yeast-Fermented Canola Meal to Replace Fishmeal in the Diet of Asian Sea Bass Lates Calcarifer (Bloch, 1790). J. Aquac. Res. Dev. 2011, 3, 2. [Google Scholar] [CrossRef]
- Pistol, G.C.; Marin, D.E.; Dragomir, C.; Taranu, I. Synbiotic combination of prebiotic grape pomace extract and probiotic Lactobacillus sp. reduced important intestinal inflammatory markers and in-depth signalling mediators in lipopolysaccharide-treated Caco-2 cells. Br. J. Nutr. 2019, 121, 291–305. [Google Scholar] [CrossRef]
- Jalota-Badhwar, A.; Bhatia, D.R.; Boreddy, S.; Joshi, A.; Venkatraman, M.; Desai, N.; Chaudhari, S.; Bose, J.; Kolla, L.S.; Deore, V.; et al. P7170: A Novel Molecule with Unique Profile of mTORC1/C2 and Activin Receptor-like Kinase 1 Inhibition Leading to Antitumor and Antiangiogenic Activity. Mol. Cancer Ther. 2015, 14, 1095–1106. [Google Scholar] [CrossRef]
- Kim, D.; Akcakanat, A.; Singh, G.; Sharma, C.; Meric-Bernstam, F. Regulation and localization of ribosomal protein S6 kinase 1 isoforms. Growth Factors 2009, 27, 12–21. [Google Scholar] [CrossRef]
- Liu, J.; Yang, M.; Jing, L.; Ren, L.; Wei, J.; Zhang, J.; Zhang, F.; Duan, J.; Zhou, X.; Sun, Z. Silica nanoparticle exposure inducing granulosa cell apoptosis and follicular atresia in female Balb/c mice. Environ. Sci. Pollut. Res. Int. 2018, 25, 3423–3434. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.R. The antioxidant properties of zinc. J. Nutr. 2000, 130, 1447s–1454s. [Google Scholar] [CrossRef] [PubMed]
- Pourahmad, J.; Shaki, F.; Tanbakosazan, F.; Ghalandari, R.; Ettehadi, H.A.; Dahaghin, E. Protective effects of fungal β-(1→3)-D-glucan against oxidative stress cytotoxicity induced by depleted uranium in isolated rat hepatocytes. Hum. Exp. Toxicol. 2011, 30, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Wu, F.; Long, L.N.; Li, T.J.; Xiong, X.; Liao, P.; Liu, H.N.; Yin, Y.L. Effects of yeast products on the intestinal morphology, barrier function, cytokine expression, and antioxidant system of weaned piglets. J. Zhejiang Univ. Sci. B 2016, 17, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Toklu, H.Z.; Sehirli, A.O.; Velioğlu-Oğünç, A.; Cetinel, S.; Sener, G. Acetaminophen-induced toxicity is prevented by beta-D-glucan treatment in mice. Eur. J. Pharmacol. 2006, 543, 133–140. [Google Scholar] [CrossRef]
- Slamenová, D.; Lábaj, J.; Krizková, L.; Kogan, G.; Sandula, J.; Bresgen, N.; Eckl, P. Protective effects of fungal (1-->3)-beta-D-glucan derivatives against oxidative DNA lesions in V79 hamster lung cells. Cancer Lett. 2003, 198, 153–160. [Google Scholar] [CrossRef]
- Canali, R.; Vignolini, F.; Nobili, F.; Mengheri, E. Reduction of oxidative stress and cytokine-induced neutrophil chemoattractant (CINC) expression by red wine polyphenols in zinc deficiency induced intestinal damage of rat. Free Radic. Biol. Med. 2000, 28, 1661–1670. [Google Scholar] [CrossRef]
- Braegger, C.P.; MacDonald, T.T. Immune mechanisms in chronic inflammatory bowel disease. Ann. Allergy 1994, 72, 135–141. [Google Scholar]
- Yagihashi, A.; Tsuruma, T.; Tarumi, K.; Kameshima, T.; Yajima, T.; Yanai, Y.; Watanabe, N.; Hirata, K. Prevention of small intestinal ischemia-reperfusion injury in rat by anti-cytokine-induced neutrophil chemoattractant monoclonal antibody. J. Surg. Res. 1998, 78, 92–96. [Google Scholar] [CrossRef]
- Barzegar, M.; Zaghari, M.; Zhandi, M.; Sadeghi, M. Effects of zinc dosage and particle size on gut morphology, tight junctions and TNF-α expression in broiler breeder hens. J. Anim. Physiol. Anim. Nutr. 2022, 106, 772–782. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, T.; Li, S.; Meng, Y.; Tan, Z.; Wu, M.; Yi, D.; Wang, L.; Zhao, D.; Hou, Y. Protective Effect of Zinc Oxide and Its Association with Neutrophil Degranulation in Piglets Infected with Porcine Epidemic Diarrhea Virus. Oxidative Med. Cell. Longev. 2021, 2021, 3055810. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.J.; Kim, I.H. Evaluation of coated zinc oxide in young pigs challenged with enterotoxigenic Escherichia coli K88. Anim. Feed Sci. Technol. 2020, 262, 114399. [Google Scholar] [CrossRef]
- Shen, J.; Chen, Y.; Wang, Z.; Zhou, A.; He, M.; Mao, L.; Zou, H.; Peng, Q.; Xue, B.; Wang, L.; et al. Coated zinc oxide improves intestinal immunity function and regulates microbiota composition in weaned piglets. Br. J. Nutr. 2014, 111, 2123–2134. [Google Scholar] [CrossRef]
- Senapati, V.A.; Kumar, A.; Gupta, G.S.; Pandey, A.K.; Dhawan, A. ZnO nanoparticles induced inflammatory response and genotoxicity in human blood cells: A mechanistic approach. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 85, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef]
- Che, L.; Xu, Q.; Wu, C.; Luo, Y.; Huang, X.; Zhang, B.; Auclair, E.; Kiros, T.; Fang, Z.; Lin, Y.; et al. Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Br. J. Nutr. 2017, 118, 949–958. [Google Scholar] [CrossRef]
- Taranu, I.; Marin, D.; Pistol, G.C.; Untea, A.; Vlassa, M.; Filip, M.; Gras, M.; Rotar, C.; Anghel, A.C. Assessment of the ability of dietary yeast-fermented rapeseed meal to modulate inflammatory and oxidative stress in piglets after weaning. J. Anim. Feed. Sci. 2022, 31, 109–122. [Google Scholar] [CrossRef]
- Hu, C.H.; Song, Z.H.; Xiao, K.; Song, J.; Jiao, L.F.; Ke, Y.L. Zinc oxide influences intestinal integrity, the expressions of genes associated with inflammation and TLR4-myeloid differentiation factor 88 signaling pathways in weanling pigs. Innate Immun. 2014, 20, 478–486. [Google Scholar] [CrossRef]
- Hoffmann, P.; Burmester, M.; Langeheine, M.; Brehm, R.; Empl, M.T.; Seeger, B.; Breves, G. Caco-2/HT29-MTX co-cultured cells as a model for studying physiological properties and toxin-induced effects on intestinal cells. PLoS ONE 2021, 16, e0257824. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Bulgaru, C.V.; Anghel, C.A.; Pistol, G.C.; Dore, M.I.; Palade, M.L.; Taranu, I. Grape Seed Waste Counteracts Aflatoxin B1 Toxicity in Piglet Mesenteric Lymph Nodes. Toxins 2020, 12, 800. [Google Scholar] [CrossRef] [PubMed]

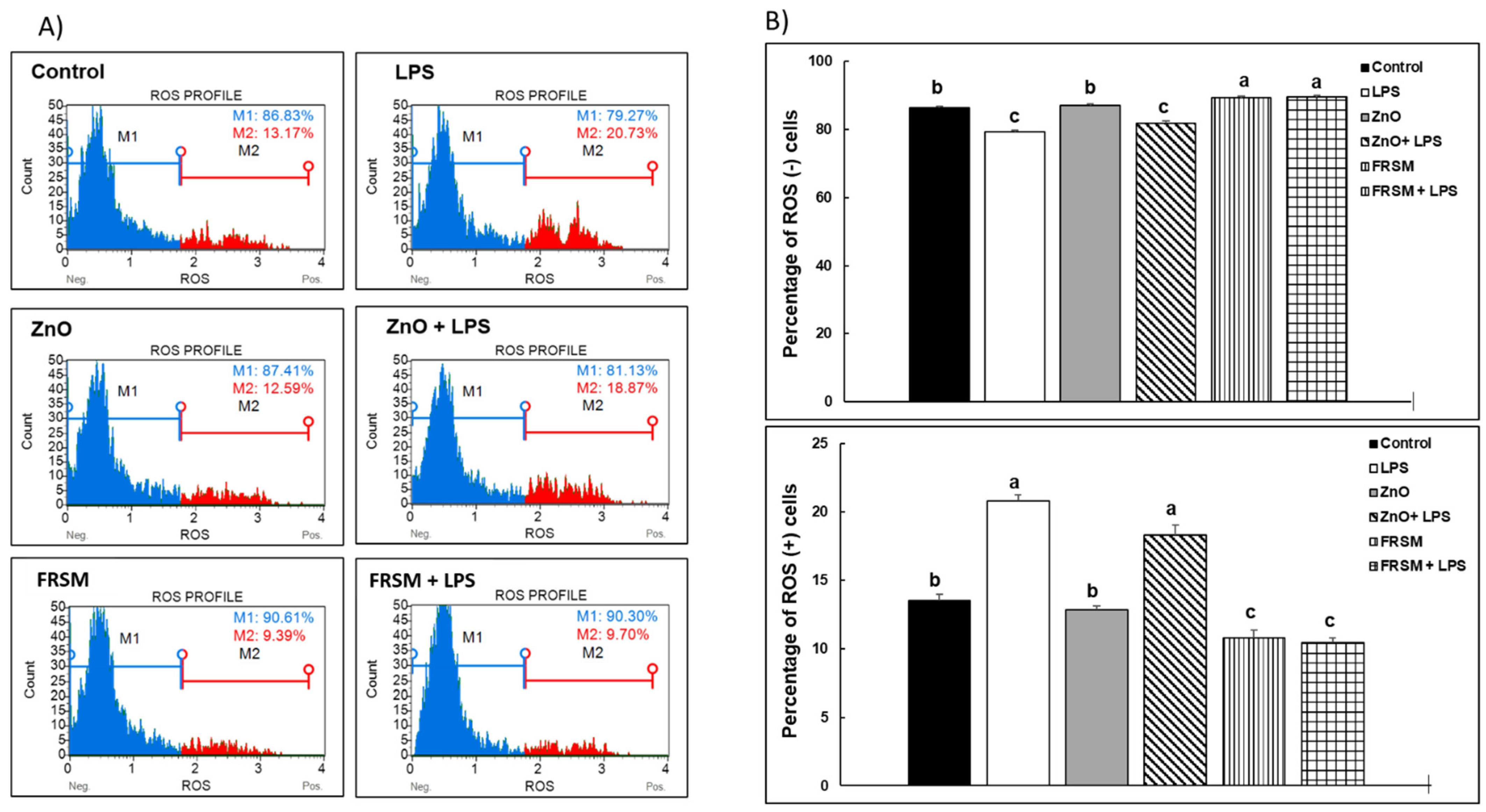

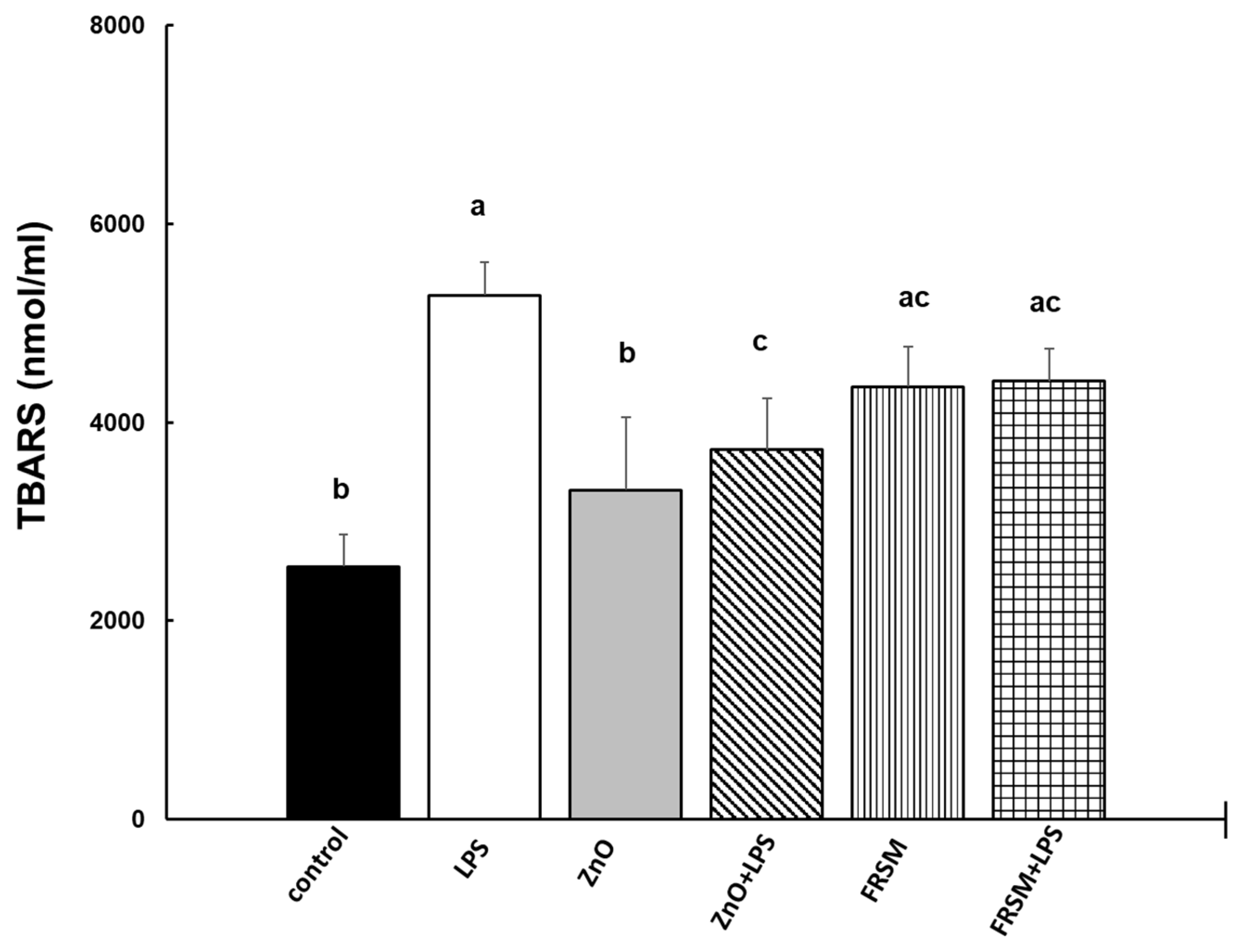
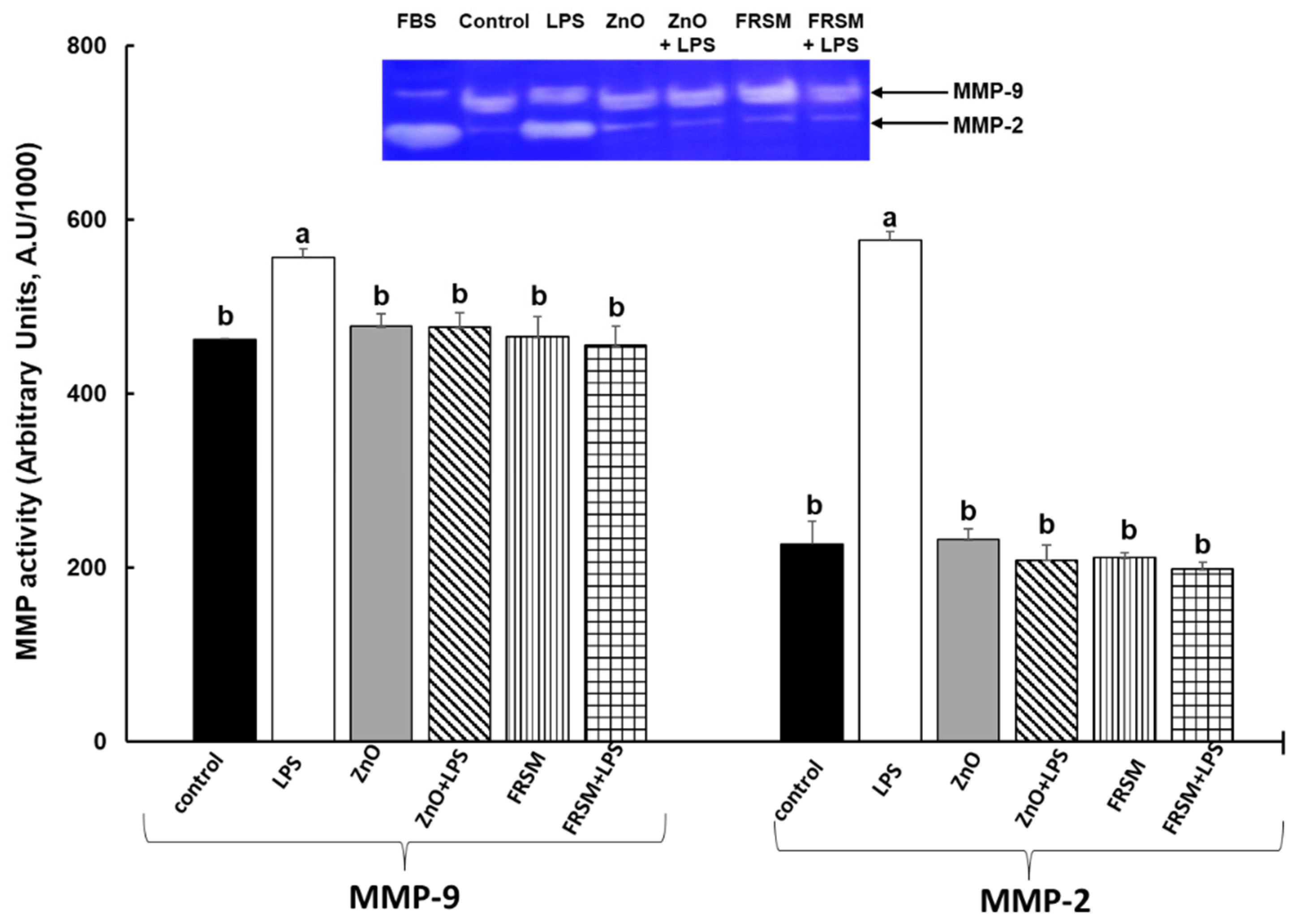
| Functional Classification | Experimental Treatments | ||||||
|---|---|---|---|---|---|---|---|
| Expression Level | Control (%) | LPS (%) | ZnO (%) | FRSM (%) | ZnO + LPS (%) | FRSM + LPS (%) | |
| Cytokines (19 proteins) | Overexpressed (%) | 0.00 a | 17.91 b ± 2.9 | 0.00 a | 0.00 a | 0.00 a | 1.49 c ± 0.9 |
| Suppressed (%) | 0.00 a | 0.00 a | 7.46 c | 5.97 d | 11.94 b | 5.97 d | |
| No effect (%) | 0.00 a | 10.46 d ± 5.1 | 20.89 b ± 4.2 | 22.39 b ± 3.9 | 16.42 c ± 3.7 | 20.90 b ± 1.9 | |
| Chemokines (12 proteins) | Overexpressed (%) | 0.00 a | 11.94 b ± 1.2 | 0.00 a | 0.00 a | 0.00 a | 0.0 a |
| Suppressed (%) | 0.00 a | 0.00 a | 1.49 c ± 0.4 | 1.49 c ± 0.7 | 4.48 b ± 1.0 | 1.49 c ± 0.2 | |
| No effect (%) | 0.00 a | 5.97 c ± 1.1 | 16.43 b ± 2.7 | 16.42 b ± 1.9 | 13.43 b ± 1.5 | 16.41 b ± 1.3 | |
| Signaling molecules (36 proteins) | Overexpressed (%) | 0.00 a | 35.82 b ± 3.9 | 19.40 c ± 2.0 | 11.94 c ± 1.7 | 10.45 c ± 1.6 | 8.96 c ± 1.3 |
| Suppressed (%) | 0.00 a | 1.49 f ± 0.8 | 4.48 d ± 0.8 | 8.95 c ± 1.2 | 2.98 e ± 0.3 | 13.44 b ± 2.2 | |
| No effect (%) | 0.00 a | 16.41 d ± 2.1 | 29.85 c ± 4.3 | 32.84 c ± 3.7 | 40.30 b ± 2.8 | 31.34 c ± 3.3 | |
| Experimental Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cytokines Involvement | Protein Name | Control (%) | LPS (%) | ZnO (%) | FRSM (%) | ZnO + LPS (%) | FRSM + LPS (%) | SEM |
| Inflammatory response | IL-1α | 100 a | 142.9 b ± 33.2 | 63.4 c ± 18.5 | 74.0 abc ± 12.3 | 62.6 c ± 9.3 | 126.3 ab ± 31.2 | 10.4 |
| IL-1β | 100 ab | 141.8 b ± 18.5 | 101.5 ab ± 25.6 | 91.8 a ± 21.0 | 82.2 a ± 20.0 | 82.3 a ± 10.2 | 9.6 | |
| IL-2 | 100 a | 112.1 a ± 21.8 | 49.6 b ± 10.9 | 57.8 b ± 9.2 | 55.9 b ± 17.8 | 59.1 b ± 10.5 | 2.7 | |
| IL-6 | 100 ab | 126.7 a ± 20.7 | 69.1 ab ± 10.5 | 111.8 ab ± 48.4 | 61.0 b ± 12.4 | 102.7 a ± 28.5 | 9.5 | |
| IL-12 p40/p70 | 100 a | 138.1 b ± 20.3 | 102.6 ab ± 40.3 | 104.7 ab ± 24.5 | 85.4 a ± 27.7 | 104.6 ab ± 15.8 | 6.3 | |
| IL-15 | 100 a | 141.6 b ± 17.0 | 99.6 ab ± 11.5 | 104.5 ab ± 21.5 | 78.4 ab ± 16.2 | 111.7 ab ± 19.8 | 8.2 | |
| TNF-α | 100 a | 165.0 b ± 20.9 | 114.9 ab ± 16.2 | 102.9 a ± 20.2 | 129.3 ab ± 11.4 | 114.9 ab ± 16.3 | 4.1 | |
| TNF-β | 100 a | 109.7 a ± 7.9 | 65.3 b ± 17.7 | 59.3 bc ± 14.6 | 40.1 c ± 6.41 | 47.9 bc ± 6.4 | 5.8 | |
| IFN-γ | 100 a | 141.7 b ± 15.9 | 116.3 ab ± 18.7 | 111.8 ab ± 5.7 | 107.1 a ± 14.5 | 106.1 a ± 8.2 | 1.8 | |
| MCSF | 100 a | 154.3 b ± 25.9 | 108.9 a ± 28.9 | 101.5 a ± 26.6 | 101.8 a ± 17.8 | 108.3 a ± 24.4 | 5.3 | |
| GCSF | 100 a | 193.1 b ± 13.9 | 79.2 a ± 19.7 | 88.1 a ± 18.1 | 89.3 a ± 15.4 | 152.5 b ± 12.3 | 2.7 | |
| OncostainM | 100 a | 148.3 b ± 19.4 | 95.5 a ± 15.6 | 113.3 ab ± 23.4 | 79.8 a ± 11.9 | 108.68 a ± 6.2 | 7.6 | |
| Anti-inflammatory response | IL-4 | 100 ab | 101.4 a ± 13.9 | 79.5 b ± 14.7 | 83.5 ab ± 24.9 | 59.4 c ± 12.7 | 92.5 ab ± 8.3 | 9.6 |
| IL-10 | 100 a | 95.1 a ± 16.7 | 85.2 a ± 18.9 | 75.1 ab ± 19.4 | 63.0 b ± 14.0 | 95.1 a ± 16.7 | 7.6 | |
| IL-13 | 100 a | 127.4 a ± 23.4 | 52.2 b ± 12.8 | 77.4 ab ± 12.9 | 65.6 b ± 11.8 | 96.0 a ± 15.6 | 10.3 | |
| Regulation of inflammatory response | IL-3 | 100 a | 161.9 b ± 34.1 | 88.0 a ± 21.03 | 85.9 a ± 16.1 | 81.1 a ± 15.1 | 91.1 a ± 17.5 | 3.9 |
| IL-7 | 100 a | 204.3 b ± 17.2 | 59.4 c ± 18.2 | 63.8 c ± 3.6 | 55.6 c ± 8.6 | 59.2 c ± 4.6 | 7.6 | |
| IL-5 | 100 a | 189.5 b ± 27.3 | 58.4 c ± 9.4 | 73.2 c ± 10.9 | 47.0 c ± 6.9 | 56.4 c ± 2.7 | 4.9 | |
| GM-CSF | 100 ab | 139.0 b ± 29.3 | 76.8 ab ± 18.3 | 84.2 b ± 17.4 | 74.6 b ± 7.6 | 112.6 ab ± 27.5 | 10.1 | |
| Experimental Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|
| Chemokine Type | Protein Name | Control (%) | LPS (%) | ZnO (%) | FRSM (%) | ZnO + LPS (%) | FRSM + LPS (%) | SEM |
| CXC | IL-8 | 100 a | 161.3 b ± 28.3 | 108.9 a ± 35.2 | 98.4 a ± 26.1 | 111.7 a ± 24.6 | 102.6 a ± 25.5 | 9.9 |
| MIG | 100 a | 132.2 b ± 36.9 | 110.7 a ± 32.0 | 102.6 a ± 28.2 | 114.4 a ± 37.3 | 115.8 a ± 28.8 | 9.8 | |
| ENA-78 | 100 ab | 128.6 a ± 10.1 | 98.3 ab ± 18.6 | 79.0 b ± 19.2 | 93.4 b ± 12.2 | 72.2 b ± 14.2 | 6.6 | |
| GRO-α | 100 a | 134.3 b ± 35.9 | 104.7 a ± 29.4 | 95.5 a ± 15.9 | 101.7 a ± 21.3 | 95.3 a ± 9.5 | 8.3 | |
| CCL | MCP-1 | 100 a | 152.8 b ± 18.9 | 107.1 a ± 11.1 | 103.1 a ± 3.3 | 119.0 ab ± 9.1 | 108.8 a ± 17.0 | 6.0 |
| MCP-2 | 100 a | 125.2 a ± 8.8 | 64.4 b ± 15.3 | 79.1 ab ± 20.2 | 57.7 b ± 13.2 | 74.8 ab ± 23.5 | 7.8 | |
| MCP-3 | 100 a | 133.8 b ± 19.0 | 74.1 ac ± 20.2 | 72.8 ac ± 30.3 | 55.7 c ± 15.2 | 75.6 ac ± 27.1 | 10.3 | |
| MIP-1δ | 100 a | 130.7 b ± 20.6 | 98.3 a ± 11.7 | 94.9 a ± 5.7 | 117.6 ab ± 5.4 | 89.2 a ± 7.8 | 5.5 | |
| RANTES | 100 a | 147.1 b ± 18.4 | 113.3 a ± 21.6 | 93.5 a ± 9.2 | 88.2 a ± 19.7 | 77.5 a ± 26.5 | 8.4 | |
| I-309 | 100 ab | 120.2 a ± 25.5 | 75.4 b ± 27.3 | 83.5 b ± 20.2 | 49.3 c ± 4.4 | 85.2 b ± 18.9 | 10.5 | |
| MDC | 100 a | 134.3 b ± 15.6 | 94.9 a ± 14.9 | 64.5 c ± 23.1 | 73.1 a ± 10.5 | 64.0 c ± 17.7 | 8.4 | |
| TARC | 100 a | 121.5 a ± 31.7 | 81.4 ab ± 14.1 | 85.8 ab ± 18.1 | 58.5 b ± 10.7 | 79.7 ab ± 11.9 | 8.4 | |
| (A) | ||||||||
| Experimental Treatments | ||||||||
| Pathway Involvement | Protein Name | Control (%) | LPS (%) | ZnO (%) | FRSM (%) | ZnO + LPS (%) | FRSM + LPS (%) | SEM |
| Inflammation/ oxidative stress/apoptosis | MAPK-p38-α | 100 a | 226.2 b ± 29.1 | 105.5 a ± 16.8 | 139.1 a ± 20.6 | 122.3 a ± 25.0 | 167.9 b ± 23.7 | 19.6 |
| JNK1/2/3 | 100 a | 158.4 b ± 31.7 | 93.4 a ± 14.8 | 107.5 a ± 10.8 | 106.6 a ± 21.2 | 99.6 a ± 11.2 | 13.8 | |
| MSK1/2 | 100 ac | 130.5 b ± 19.4 | 100.9 a ± 14.1 | 103.4 a ± 16.9 | 83.62 a ± 25.1 | 120.8 a ± 19.6 | 16.2 | |
| GSK-3β | 100 a | 78.6 b ± 5.81 | 82.2 ab ± 14.9 | 77.3 ab ± 20.6 | 74.2 ab ± 17.2 | 65.6 b ± 9.8 | 4.7 | |
| HSP27 | 100 a | 145.5 b ± 25.3 | 96.3 a ± 11.7 | 116.3 a ± 35.3 | 82.67 a ± 10.9 | 116.1 a ± 22.3 | 8.6 | |
| Src | 100 a | 147.5 b ± 25.9 | 116.4 a ± 22.7 | 122.7 a ± 30.0 | 106.2 a ± 22.7 | 123.3 a ± 18.7 | 8.9 | |
| Lck | 100 a | 67.6 b ± 11.1 | 75.7 ab ± 14.5 | 112.0 a ± 14.9 | 97.9 a ± 15.3 | 84.3 ab ± 16.7 | 7.2 | |
| CREB | 100 a | 193.8 b ± 34.7 | 111.4 a ± 13.7 | 103.0 a ± 11..2 | 102.5 b ± 13.0 | 103.9 b ± 26.8 | 18.4 | |
| c-Jun | 100 a | 275.7 b ± 36.4 | 85.4 ac ± 9.7 | 93.4 a ± 12.8 | 77.8 c ± 11.6 | 75.6 d ± 16.1 | 16.5 | |
| Transcription factors | STAT1 | 100 a | 112.9 a ± 24.7 | 154.6 b ± 15.7 | 85.3 a ± 11.5 | 93.4 a ± 8.9 | 94.8 a ± 18.6 | 17.1 |
| STAT2 | 100 ac | 203.8 b ± 33.6 | 115.6 a ± 21.9 | 86.3 c ± 10.0 | 126.1 a ± 21.7 | 127.0 a ± 31.7 | 20.7 | |
| STAT3 | 100 a | 241.3 b ± 23.9 | 202.6 b ± 20.1 | 127.0 a ± 13.6 | 115.2 a ± 12.4 | 106.0 a ± 22.1 | 13 | |
| STAT5 α/β | 100 a | 110.7 a ± 30.6 | 99.9 a ± 31.7 | 83.9 a ± 14.0 | 81.5 a ± 10.5 | 97.5 a ± 14.5 | 19.8 | |
| STAT6 | 100 a | 131.3 b ± 28.2 | 127.7 b ± 26.1 | 102.9 a ± 33.7 | 100.4 a ± 16.0 | 67.6 c ± 11.0 | 13.5 | |
| beta-catenin | 100 a | 118.3 a ± 11.7 | 116.3 a ± 10.3 | 109.1 a ± 24.2 | 84.3 a ± 15.2 | 86.4 a ± 14.6 | 17.5 | |
| Cellular migration/ chemotaxis | PYK2 | 100 a | 144.7 b ± 31.1 | 145.6 b ± 10.1 | 89.3 ac ± 10.9 | 93.9 a ± 10.3 | 71.9 c ± 13.9 | 16.9 |
| PDGF Rβ | 100 a | 89.4 a ± 12.6 | 103.1 a ± 20.6 | 102.6 a ± 15.6 | 83.71 a ± 22.0 | 104.6 a ± 11.0 | 4.71 | |
| PLC-γ1 | 100 a | 150.5 b ± 21.9 | 105.3 a ± 25.5 | 103.9 a ± 27.7 | 79.5 a ± 19.4 | 108.7 a ± 14.4 | 9 | |
| Fgr | 100 a | 96.5 a ± 10.7 | 102.2 a ± 24.6 | 91.3 a ± 13.7 | 80.2 a ± 17.3 | 96.6 a ± 14.8 | 3.5 | |
| (B) | ||||||||
| Pathway Involvement | Protein Name | Control (%) | LPS (%) | ZnO (%) | FRSM (%) | ZnO + LPS (%) | FRSM + LPS (%) | SEM |
| Cell proliferation/cell cycle/ differentiation | RSK1/2 | 100 a | 142.0 b ± 21.9 | 136.4 b ± 16.6 | 67.5 c ± 10.3 | 73.8 c ± 12.1 | 56.2 d ± 13.2 | 18.2 |
| RSK1/2/3 | 100 a | 147.9 b ± 27.1 | 139.1 b ± 16.3 | 59.0 c ± 7.5 | 117.9 a ± 26.0 | 66.1 c ± 14.4 | 17.7 | |
| ERK1/2 | 100 a | 114.9 ab ± 28.9 | 90.0 ab ± 21.9 | 80.3 b ± 17.6 | 53.8 c ± 9.3 | 71.8 b ± 19.8 | 21.6 | |
| Chk-2 | 100 a | 174.3 b ± 35.4 | 129.3 a ± 21.9 | 139.0 c ± 25.9 | 93.3 a ± 10.9 | 83.0 c ± 12.1 | 20.8 | |
| p53 (S15) | 100 a | 156.4 b ± 33.7 | 139.4 c ± 16.5 | 83.9 a ± 15.9 | 84.6 a ± 17.5 | 83.3 a ± 18.8 | 16.5 | |
| p53 (S392) | 100 a | 169.4 b ± 36.5 | 143.8 bc ± 16.1 | 124.7 ac ± 30.4 | 150.3 b ± 27.1 | 135.2 c ± 23.6 | 12.1 | |
| p53 (S46) | 100 a | 99.7 a ± 7.2 | 141.6 b ± 28.3 | 54.8 d ± 10.4 | 110.8 a ± 22.7 | 66.9 c ± 16.4 | 15.6 | |
| EGF R | 100 a | 90.4 a ± 27.8 | 64.3 b ± 12.5 | 98.8 a ± 25.1 | 84.3 a ± 15.9 | 83.2 a ± 12.5 | 13.2 | |
| p70S6k (T389) | 100 a | 174.3 b ± 20.5 | 154.9 b ± 22.0 | 94.2 a ± 29.4 | 99.9 a ± 20.7 | 70.1 c ± 19.5 | 18.2 | |
| p70S6k (T421/S42) | 100 a | 146.8 b ± 23.9 | 134.1 b ± 30.0 | 77.6 c ± 16.8 | 76.1 c ± 10.6 | 70.3 c ± 10.1 | 15.2 | |
| Akt1/2/3 (S473) | 100 a | 171.7 b ± 9.2 | 114.0 a ± 29.9 | 69.7 c ± 20.6 | 86.9 a ± 20.7 | 73.0 c ± 13.1 | 15.7 | |
| Akt1/2/3 (T308) | 100 ac | 171.6 b ± 16.0 | 117.1 a ± 25.9 | 63.0 d ± 9.5 | 85.9 ac ± 14.7 | 79.0 c ± 14.3 | 16.4 | |
| PRAS40 | 100 a | 121.8 b ± 26.3 | 135.9 b ± 33.0 | 87.0 ac ± 26.7 | 105.7 a ± 22.5 | 66.2 d ± 11.4 | 15.1 | |
| Other kinases | eNOS | 100 a | 214.4 b ± 38.6 | 152.3 c ± 30.2 | 116.9 a ± 18.0 | 99.3 a ± 19.3 | 99.4 a ± 11.9 | 15.6 |
| Yes | 100 a | 133.4 c ± 17.9 | 97.9 a ± 11.4 | 199.7 b ± 31.7 | 89.4 a ± 16.0 | 98.2 a ± 18.9 | 10.3 | |
| GSK3α/β | 100 a | 89.5 b ± 12.5 | 86.8 b ± 24.1 | 101.2 a ± 11.4 | 90.1 ab ± 18.0 | 91.5 c ± 11.5 | 8.9 | |
| Lyn | 100 a | 107.3 a ± 21.9 | 109.8 a ± 20.9 | 78.0 c ± 20.2 | 118.6 a ± 27.7 | 65.4 d ± 7.6 | 6.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taranu, I.; Pistol, G.C.; Anghel, A.C.; Marin, D.; Bulgaru, C. Yeast-Fermented Rapeseed Meal Extract Is Able to Reduce Inflammation and Oxidative Stress Caused by Escherichia coli Lipopolysaccharides and to Replace ZnO in Caco-2/HTX29 Co-Culture Cells. Int. J. Mol. Sci. 2022, 23, 11640. https://doi.org/10.3390/ijms231911640
Taranu I, Pistol GC, Anghel AC, Marin D, Bulgaru C. Yeast-Fermented Rapeseed Meal Extract Is Able to Reduce Inflammation and Oxidative Stress Caused by Escherichia coli Lipopolysaccharides and to Replace ZnO in Caco-2/HTX29 Co-Culture Cells. International Journal of Molecular Sciences. 2022; 23(19):11640. https://doi.org/10.3390/ijms231911640
Chicago/Turabian StyleTaranu, Ionelia, Gina Cecilia Pistol, Andrei Cristian Anghel, Daniela Marin, and Cristina Bulgaru. 2022. "Yeast-Fermented Rapeseed Meal Extract Is Able to Reduce Inflammation and Oxidative Stress Caused by Escherichia coli Lipopolysaccharides and to Replace ZnO in Caco-2/HTX29 Co-Culture Cells" International Journal of Molecular Sciences 23, no. 19: 11640. https://doi.org/10.3390/ijms231911640
APA StyleTaranu, I., Pistol, G. C., Anghel, A. C., Marin, D., & Bulgaru, C. (2022). Yeast-Fermented Rapeseed Meal Extract Is Able to Reduce Inflammation and Oxidative Stress Caused by Escherichia coli Lipopolysaccharides and to Replace ZnO in Caco-2/HTX29 Co-Culture Cells. International Journal of Molecular Sciences, 23(19), 11640. https://doi.org/10.3390/ijms231911640










