A Tripartite Efflux System Affects Flagellum Stability in Helicobacter pylori
Abstract
1. Introduction
2. Results
2.1. Identification of H. pylori Proteins That Are Found Preferentially in Helicobacter Species with Flagellar Sheaths
2.2. HP1486, HP1487 and HP1489 Are Required for Robust Motility of H. pylori
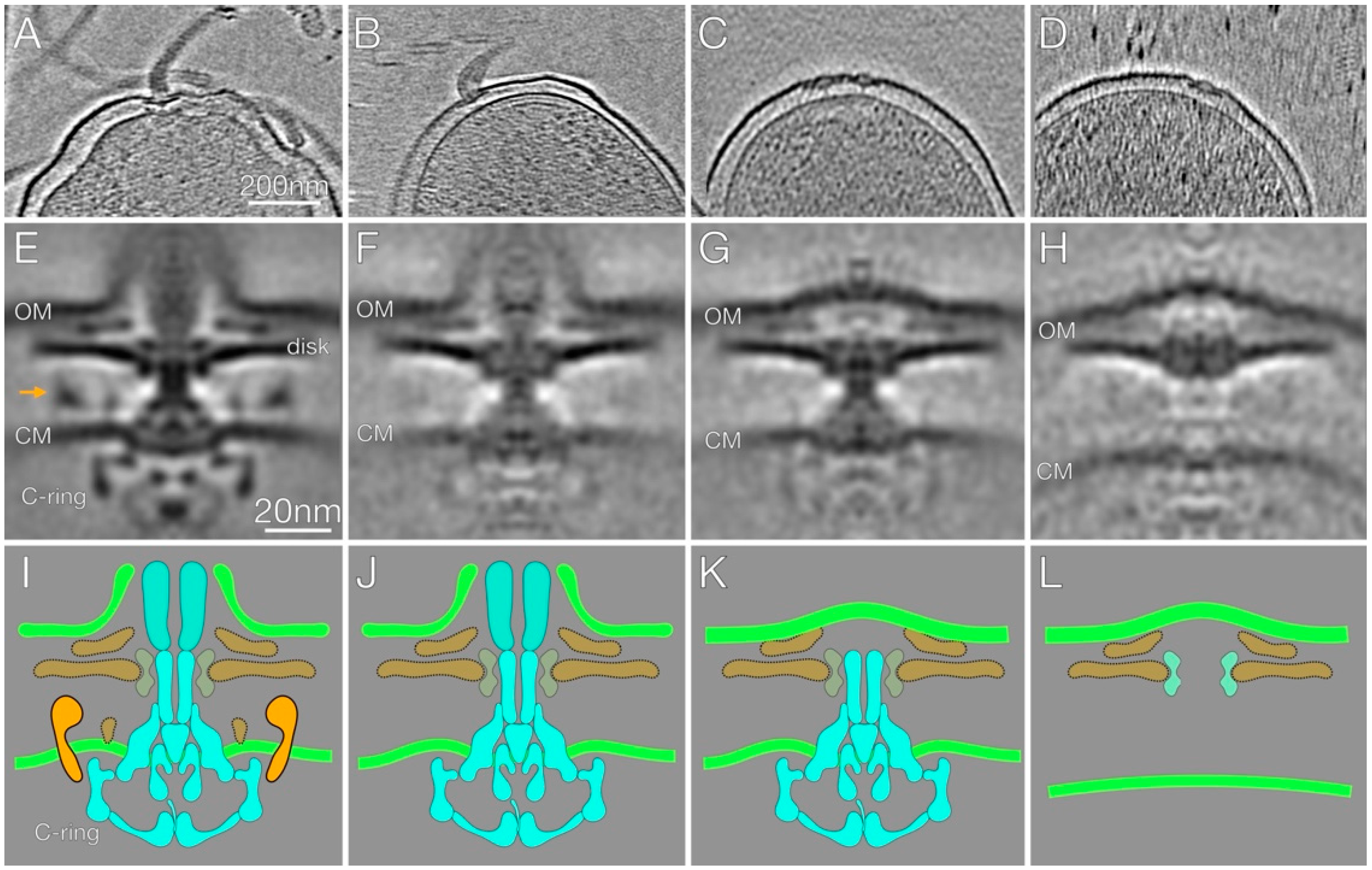
2.3. In-Situ Structures of Flagellar Motors in the Δhp1489 and Δhp1487/hp1486 Mutants Reveal Apparent Flagellum Disassembly Products
2.4. Isolation of Mutants That Suppress the Motility Defect in the Δhp1487/hp1486 Mutant
3. Discussion
3.1. The HP1489-HP1486 Tripartite Efflux System Has an Apparent Role in Stabilizing the H. pylori Flagellar Motor
3.2. Identification of Genes Potentially Involved in Sheath Formation
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Strain Construction
4.3. Motility Assay
4.4. Complementation of Δhp1489 Mutation
4.5. Isolation of Variants of ∆hp1487/hp1486 Mutant with Enhanced Motilities
4.6. Genome Sequencing and Analysis
4.7. Transmission Electron Microscopy
4.8. Sample Preparation for Cryo-EM Observation
4.9. Cryo-ET Data Collection and Image Processing
4.10. Sub-Tomogram Analysis with i3 Packages
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atherton, J.C.; Blaser, M.J. Coadaptation of Helicobacter pylori and humans: Ancient history, modern implications. J. Clinic. Investig. 2009, 119, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Eaton, K.A.; Morgan, D.R.; Krakowka, S. Motility as a factor in the colonisation of gnotobiotic piglets by Helicobacter pylori. J. Med. Microbiol. 1992, 37, 123–127. [Google Scholar] [CrossRef]
- Ottemann, K.M.; Lowenthal, A.C. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect. Immun. 2002, 70, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Chevance, F.F.; Hughes, K.T. Coordinating assembly of a bacterial macromolecular machine. Nat. Rev. Microbiol. 2008, 6, 455–465. [Google Scholar] [CrossRef]
- Macnab, R.M. How bacteria assemble flagella. Annu. Rev. Microbiol. 2003, 57, 77–100. [Google Scholar] [CrossRef]
- Manson, M.D.; Tedesco, P.; Berg, H.C.; Harold, F.M.; Van der Drift, C. A protonmotive force drives bacterial flagella. Proc. Natl. Acad. Sci. USA 1977, 74, 3060–3064. [Google Scholar] [CrossRef]
- Nakamura, S.; Minamino, T. Flagella-driven motility of bacteria. Biomolecules 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Lin, W.T.; Zhu, S.; Franco, A.T.; Liu, J. Imaging the motility and chemotaxis machineries in Helicobacter pylori by cryo-electron tomography. J. Bacteriol. 2017, 199, e00695-16. [Google Scholar] [CrossRef]
- Beeby, M.; Ribardo, D.A.; Brennan, C.A.; Ruby, E.G.; Jensen, G.J.; Hendrixson, D.R. Diverse high-torque bacterial flagellar motors assemble wider stator rings using a conserved protein scaffold. Proc. Natl. Acad. Sci. USA 2016, 113, E1917–E1926. [Google Scholar] [CrossRef]
- Chen, S.; Beeby, M.; Murphy, G.E.; Leadbetter, J.R.; Hendrixson, D.R.; Briegel, A.; Li, Z.; Shi, J.; Tocheva, E.I.; Muller, A.; et al. Structural diversity of bacterial flagellar motors. EMBO J. 2011, 30, 2972–2981. [Google Scholar] [CrossRef] [PubMed]
- Doig, P.; Trust, T.J. Identification of surface-exposed outer membrane antigens of Helicobacter pylori. Infect. Immun. 1994, 62, 4526–4533. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Logan, R.P.H.; Foynes, S.; Cackayne, A.; Wren, B.W.; Penn, C.W. A flagellar sheath protein of Helicobacter pylori is identical to HpaA, a putative N-acetylneuraminyllactose-binding hemagglutinin, but is not an adhesin for AGS cells. J. Bacteriol. 1997, 179, 5643–5647. [Google Scholar] [CrossRef] [PubMed]
- Luke, C.J.; Penn, C.W. Identification of a 29 kDa flagellar sheath protein in Helicobacter pylori using a murine monoclonal antibody. Microbiology 1995, 141 Pt 3, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, A.M.; Blom, K.; Sundaeus, V.; Bolin, I. HpaA shows variable surface localization but the gene expression is similar in different Helicobacter pylori strains. Microb. Pathog. 2001, 31, 243–253. [Google Scholar] [CrossRef]
- Radin, J.N.; Gaddy, J.A.; Gonzalez-Rivera, C.; Loh, J.T.; Algood, H.M.; Cover, T.L. Flagellar localization of a Helicobacter pylori autotransporter protein. mBio 2013, 4, e00613-12. [Google Scholar] [CrossRef]
- Chu, J.; Liu, J.; Hoover, T.R. Phylogenetic distribution, ultrastructure, and function of bacterial flagellar sheaths. Biomolecules 2020, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.S.; Mekalanos, J.J. Decreased potency of the Vibrio cholerae sheathed flagellum to trigger host innate immunity. Infect. Immun. 2008, 76, 1282–1288. [Google Scholar] [CrossRef]
- Lee, S.K.; Stack, A.; Katzowitsch, E.; Aizawa, S.I.; Suerbaum, S.; Josenhans, C. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect. 2003, 5, 1345–1356. [Google Scholar] [CrossRef]
- Smith, K.D.; Andersen-Nissen, E.; Hayashi, F.; Strobe, K.; Bergman, M.A.; Barrett, S.L.; Cookson, B.T.; Aderem, A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 2003, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, H.N.; Mills, D.C.; Jones, H.; Milioris, E.; Copland, A.; Dorrell, N.; Wren, B.W.; Crocker, P.R.; Escors, D.; Bajaj-Elliott, M. Pseudaminic acid on Campylobacter jejuni flagella modulates dendritic cell IL-10 expression via Siglec-10 receptor: A novel flagellin-host interaction. J. Infect. Dis. 2014, 210, 1487–1498. [Google Scholar] [CrossRef]
- Aschtgen, M.S.; Lynch, J.B.; Koch, E.; Schwartzman, J.; McFall-Ngai, M.; Ruby, E. Rotation of Vibrio fischeri flagella produces outer membrane vesicles that induce host development. J. Bacteriol. 2016, 198, 2156–2165. [Google Scholar] [CrossRef]
- Brennan, C.A.; Hunt, J.R.; Kremer, N.; Krasity, B.C.; Apicella, M.A.; McFall-Ngai, M.J.; Ruby, E.G. A model symbiosis reveals a role for sheathed-flagellum rotation in the release of immunogenic lipopolysaccharide. eLife 2014, 3, e01579. [Google Scholar] [CrossRef] [PubMed]
- Manning, A.J.; Kuehn, M.J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Zhao, Z.; Peng, D.; Zhou, X. Polar flagella rotation in Vibrio parahaemolyticus confers resistance to bacteriophage infection. Sci. Rep. 2016, 6, 26147. [Google Scholar] [CrossRef]
- Hinchliffe, P.; Symmons, M.F.; Hughes, C.; Koronakis, V. Structure and operation of bacterial tripartite pumps. Annu. Rev. Microbiol. 2013, 67, 221–242. [Google Scholar] [CrossRef]
- Pasqua, M.; Grossi, M.; Zennaro, A.; Fanelli, G.; Micheli, G.; Barras, F.; Colonna, B.; Prosseda, G. The varied role of efflux pumps of the MFS family in the interplay of bacteria with animal and plant cells. Microorganisms 2019, 7, 285. [Google Scholar] [CrossRef]
- Kaplan, M.; Subramanian, P.; Ghosal, D.; Oikonomou, C.M.; Pirbadian, S.; Starwalt-Lee, R.; Mageswaran, S.K.; Ortega, D.R.; Gralnick, J.A.; El-Naggar, M.Y.; et al. In situ imaging of the bacterial flagellar motor disassembly and assembly processes. EMBO J. 2019, 38, e100957. [Google Scholar] [CrossRef]
- Kaplan, M.; Sweredoski, M.J.; Rodrigues, J.; Tocheva, E.I.; Chang, Y.W.; Ortega, D.R.; Beeby, M.; Jensen, G.J. Bacterial flagellar motor PL-ring disassembly subcomplexes are widespread and ancient. Proc. Natl. Acad. Sci. USA 2020, 117, 8941–8947. [Google Scholar] [CrossRef]
- Tachiyama, S.; Chan, K.L.; Liu, X.; Hathroubi, S.; Peterson, B.; Khan, M.F.; Ottemann, K.M.; Liu, J.; Roujeinikova, A. The flagellar motor protein FliL forms a scaffold of circumferentially positioned rings required for stator activation. Proc. Natl. Acad. Sci. USA 2022, 119, e2118401119. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochber, Y. Controlling the false discovery rate: A practical and powerful approach to muliple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- McNally, D.J.; Hui, J.P.; Aubry, A.J.; Mui, K.K.; Guerry, P.; Brisson, J.R.; Logan, S.M.; Soo, E.C. Functional characterization of the flagellar glycosylation locus in Campylobacter jejuni 81-176 using a focused metabolomics approach. J. Biol. Chem. 2006, 281, 18489–18498. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Lara-Tejero, M.; Lefebre, M.; Goodman, A.L.; Galan, J.E. Novel components of the flagellar system in epsilonproteobacteria. mBio 2014, 5, e01349-14. [Google Scholar] [CrossRef] [PubMed]
- Appelmelk, B.J.; Martin, S.L.; Monteiro, M.A.; Clayton, C.A.; McColm, A.A.; Zheng, P.; Verboom, T.; Maaskant, J.J.; van den Eijnden, D.H.; Hokke, C.H.; et al. Phase variation in Helicobacter pylori lipopolysaccharide due to changes in the lengths of poly(C) tracts in alpha3-fucosyltransferase genes. Infect. Immun. 1999, 67, 5361–5366. [Google Scholar] [CrossRef] [PubMed]
- Stead, C.M.; Zhao, J.; Raetz, C.R.; Trent, M.S. Removal of the outer Kdo from Helicobacter pylori lipopolysaccharide and its impact on the bacterial surface. Mol. Microbiol. 2010, 78, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.W.; Giles, D.K.; Wolf, L.N.; Ecobichon, C.; Boneca, I.G.; Trent, M.S. Helicobacter pylori versus the host: Remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog. 2011, 7, e1002454. [Google Scholar] [CrossRef]
- Hug, I.; Couturier, M.R.; Rooker, M.M.; Taylor, D.E.; Stein, M.; Feldman, M.F. Helicobacter pylori lipopolysaccharide is synthesized via a novel pathway with an evolutionary connection to protein N-glycosylation. PLoS Pathog. 2010, 6, e1000819. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Parlow, M.H.; Schneider, J.; Burroughs, S.; Wickland, C.; Vakil, N.B.; Dunn, B.E.; Phadnis, S.H. Identification of a novel penicillin-binding protein from Helicobacter pylori. J. Bacteriol. 1999, 181, 5107–5110. [Google Scholar] [CrossRef]
- Mittl, P.R.; Luthy, L.; Hunziker, P.; Grutter, M.G. The cysteine-rich protein A from Helicobacter pylori is a beta-lactamase. J. Biol. Chem. 2000, 275, 17693–17699. [Google Scholar] [CrossRef]
- Alm, R.A.; Bina, J.; Andrews, B.M.; Doig, P.; Hancock, R.E.; Trust, T.J. Comparative genomics of Helicobacter pylori: Analysis of the outer membrane protein families. Infect. Immun. 2000, 68, 4155–4168. [Google Scholar] [CrossRef]
- Stahler, F.N.; Odenbreit, S.; Haas, R.; Wilrich, J.; Van Vliet, A.H.; Kusters, J.G.; Kist, M.; Bereswill, S. The novel Helicobacter pylori CznABC metal efflux pump is required for cadmium, zinc, and nickel resistance, urease modulation, and gastric colonization. Infect. Immun. 2006, 74, 3845–3852. [Google Scholar] [CrossRef]
- Barison, N.; Cendron, L.; Loconte, V.; Proctor, E.A.; Dokholyan, N.V.; Zanotti, G. Protein HP1028 from the human pathogen Helicobacter pylori belongs to the lipocalin family. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Homuth, G.; Domm, S.; Kleiner, D.; Schumann, W. Transcriptional analysis of major heat shock genes of Helicobacter pylori. J. Bacteriol. 2000, 182, 4257–4263. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.K.; Zhu, S.; Herrera, C.M.; Henderson, J.C.; Liu, J.; Trent, M.S.; Hoover, T.R. Loss of a cardiolipin synthase in Helicobacter pylori G27 blocks flagellum assembly. J. Bacteriol. 2019, 201, e00372-19. [Google Scholar] [CrossRef] [PubMed]
- Doig, P.; Austin, J.W.; Trust, T.J. The Helicobacter pylori 19.6-kilodalton protein is an iron-containing protein resembling ferritin. J. Bacteriol. 1993, 175, 557–560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perez-Gil, J.; Bergua, M.; Boronat, A.; Imperial, S. Cloning and functional characterization of an enzyme from Helicobacter pylori that catalyzes two steps of the methylerythritol phosphate pathway for isoprenoid biosynthesis. Biochim. Biophys. Acta 2010, 1800, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.M.; Hoffmann, S.; Darfeuille, F.; Reignier, J.; Findeiss, S.; Sittka, A.; Chabas, S.; Reiche, K.; Hackermuller, J.; Reinhardt, R.; et al. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature 2010, 464, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Copass, M.; Grandi, G.; Rappuoli, R. Introduction of unmarked mutations in the Helicobacter pylori vacA gene with a sucrose sensitivity marker. Infect. Immun. 1997, 65, 1949–1952. [Google Scholar] [CrossRef]
- van Amsterdam, K.; Bart, A.; van der Ende, A. A Helicobacter pylori TolC Efflux Pump Confers Resistance to Metronidazole. Antimicrob. Agents Chemother. 2005, 49, 1477–1482. [Google Scholar] [CrossRef]
- Berthenet, E.; Benejat, L.; Menard, A.; Varon, C.; Lacomme, S.; Gontier, E.; Raymond, J.; Boussaba, O.; Toulza, O.; Ducournau, A.; et al. Whole-genome sequencing and bioinformatics as pertinent tools to support Helicobacteracae taxonomy, based on three strains suspected to belong to novel Helicobacter species. Front. Microbiol. 2019, 10, 2820. [Google Scholar] [CrossRef]
- Huang, K.C.; Mukhopadhyay, R.; Wingreen, N.S. A curvature-mediated mechanism for localization of lipids to bacterial poles. PLoS Comput. Biol. 2006, 2, e151. [Google Scholar] [CrossRef]
- Renner, L.D.; Weibel, D.B. Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc. Natl. Acad. Sci. USA 2011, 108, 6264–6269. [Google Scholar] [CrossRef] [PubMed]
- Chu, J. Understanding the Role of Cardiolipin in Helicobacter pylori Flagellar Synthesis. Ph.D. Thesis, University of Georgia, Athens, Georgia, 2019. [Google Scholar]
- Birkholz, S.; Knipp, U.; Nietzki, C.; Adamek, R.J.; Opferkuch, W. Immunological activity of lipopolysaccharide of Helicobacter pylori on human peripheral mononuclear blood cells in comparison to lipopolysaccharides of other intestinal bacteria. FEMS Immunol. Med. Microbiol. 1993, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Muotiala, A.; Helander, I.M.; Pyhala, L.; Kosunen, T.U.; Moran, A.P. Low biological activity of Helicobacter pylori lipopolysaccharide. Infect. Immun. 1992, 60, 1714–1716. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, G.I.; Shepherd, V.L.; Morrow, J.D.; Blaser, M.J. Activation of human THP-1 cells and rat bone marrow-derived macrophages by Helicobacter pylori lipopolysaccharide. Infect. Immun. 1995, 63, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Mittl, P.R.; Schneider-Brachert, W. Sel1-like repeat proteins in signal transduction. Cell. Signal. 2007, 19, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Deml, L.; Aigner, M.; Decker, J.; Eckhardt, A.; Schutz, C.; Mittl, P.R.; Barabas, S.; Denk, S.; Knoll, G.; Lehn, N.; et al. Characterization of the Helicobacter pylori cysteine-rich protein A as a T-helper cell type 1 polarizing agent. Infect. Immun. 2005, 73, 4732–4742. [Google Scholar] [CrossRef] [PubMed]
- Israel, D.A.; Salama, N.; Arnold, C.N.; Moss, S.F.; Ando, T.; Wirth, H.P.; Tham, K.T.; Camorlinga, M.; Blaser, M.J.; Falkow, S.; et al. Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J. Clin. Investig. 2001, 107, 611–620. [Google Scholar] [CrossRef]
- Baltrus, D.A.; Amieva, M.R.; Covacci, A.; Lowe, T.M.; Merrell, D.S.; Ottemann, K.M.; Stein, M.; Salama, N.R.; Guillemin, K. The complete genome sequence of Helicobacter pylori strain G27. J. Bacteriol. 2009, 191, 447–448. [Google Scholar] [CrossRef]
- Heuermann, D.; Haas, R. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 1998, 257, 519–528. [Google Scholar] [CrossRef]
- Deatherage, D.E.; Barrick, J.E. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Meth. Mol. Biol. 2014, 1151, 165–188. [Google Scholar] [CrossRef]
- Zhu, S.; Qin, Z.; Wang, J.; Morado, D.R.; Liu, J. In situ structural analysis of the spirochetal flagellar motor by cryo-electron tomography. Meth. Mol. Biol. 2017, 1593, 229–242. [Google Scholar] [CrossRef]
- Agulleiro, J.I.; Fernandez, J.J. Tomo3D 2.0—Exploitation of advanced vector extensions (AVX) for 3D reconstruction. J. Struct. Biol. 2015, 189, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Winkler, H. 3D reconstruction and processing of volumetric data in cryo-electron tomography. J. Struct. Biol. 2007, 157, 126–137. [Google Scholar] [CrossRef]
- Winkler, H.; Zhu, P.; Liu, J.; Ye, F.; Roux, K.H.; Taylor, K.A. Tomographic subvolume alignment and subvolume classification applied to myosin V and SIV envelope spikes. J. Struct. Biol. 2009, 165, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Eppinger, M.; Baar, C.; Linz, B.; Raddatz, G.; Lanz, C.; Keller, H.; Morelli, G.; Gressmann, H.; Achtman, M.; Schuster, S.C. Who ate whom? Adaptive Helicobacter genomic changes that accompanied a host jump from early humans to large felines. PLoS Genet. 2006, 2, e120. [Google Scholar] [CrossRef] [PubMed]
- Joosten, M.; Linden, S.; Rossi, M.; Tay, A.C.; Skoog, E.; Padra, M.; Peters, F.; Perkins, T.; Vandamme, P.; Van Nieuwerburgh, F.; et al. Divergence between the highly virulent zoonotic pathogen Helicobacter heilmannii and its closest relative, the low-virulence “Helicobacter ailurogastricus” sp. nov. Infect. Immun 2016, 84, 293–306. [Google Scholar] [CrossRef]
- Patterson, M.M.; Schrenzel, M.D.; Feng, Y.; Xu, S.; Dewhirst, F.E.; Paster, B.J.; Thibodeau, S.A.; Versalovic, J.; Fox, J.G. Helicobacter aurati sp. nov., a urease-positive Helicobacter species cultured from gastrointestinal tissues of Syrian hamsters. J. Clin. Microbiol. 2000, 38, 3722–3728. [Google Scholar] [CrossRef]
- Baele, M.; Decostere, A.; Vandamme, P.; Van den Bulck, K.; Gruntar, I.; Mehle, J.; Mast, J.; Ducatelle, R.; Haesebrouck, F. Helicobacter baculiformis sp. nov., isolated from feline stomach mucosa. Int. J. Syst. Evol. Microbiol. 2008, 58, 357–364. [Google Scholar] [CrossRef]
- Fox, J.G.; Yan, L.L.; Dewhirst, F.E.; Paster, B.J.; Shames, B.; Murphy, J.C.; Hayward, A.; Belcher, J.C.; Mendes, E.N. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J. Clin. Microbiol. 1995, 33, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Schott, T.; Rossi, M.; Hanninen, M.L. Genome sequence of Helicobacter bizzozeronii strain CIII-1, an isolate from human gastric mucosa. J. Bacteriol. 2011, 193, 4565–4566. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Linton, D.; Burnens, A.P.; Dewhirst, F.E.; Owen, R.J.; Porter, A.; On, S.L.; Costas, M. Helicobacter canis sp. nov., a new species from dogs: An integrated study of phenotype and genotype. J. Gen. Microbiol. 1993, 139, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.G.; Feng, Y.; Xu, S.; Taylor, N.S.; Kinsel, M.; Dewhirst, F.E.; Paster, B.J.; Greenwell, M.; Levine, G.; Rogers, A.; et al. Helicobacter cetorum sp. nov., a urease-positive Helicobacter species isolated from dolphins and whales. J. Clin. Microbiol. 2002, 40, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Franklin, C.L.; Beckwith, C.S.; Livingston, R.S.; Riley, L.K.; Gibson, S.V.; Besch-Williford, C.L.; Hook, R.R., Jr. Isolation of a novel Helicobacter species, Helicobacter cholecystus sp. nov., from the gallbladders of Syrian hamsters with cholangiofibrosis and centrilobular pancreatitis. J. Clin. Microbiol. 1996, 34, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- Totten, P.A.; Fennell, C.L.; Tenover, F.C.; Wezenberg, J.M.; Perine, P.L.; Stamm, W.E.; Holmes, K.K. Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): Two new Campylobacter species associated with enteric disease in homosexual men. J. Infect. Dis. 1985, 151, 131–139. [Google Scholar] [CrossRef]
- Van den Bulck, K.; Decostere, A.; Baele, M.; Vandamme, P.; Mast, J.; Ducatelle, R.; Haesebrouck, F. Helicobacter cynogastricus sp. nov., isolated from the canine gastric mucosa. Int. J. Syst. Evol. Microbiol. 2006, 56, 1559–1564. [Google Scholar] [CrossRef]
- Moyaert, H.; Decostere, A.; Vandamme, P.; Debruyne, L.; Mast, J.; Baele, M.; Ceelen, L.; Ducatelle, R.; Haesebrouck, F. Helicobacter equorum sp. nov., a urease-negative Helicobacter species isolated from horse faeces. Int. J. Syst. Evol. Microbiol. 2007, 57, 213–218. [Google Scholar] [CrossRef]
- Arnold, I.C.; Zigova, Z.; Holden, M.; Lawley, T.D.; Rad, R.; Dougan, G.; Falkow, S.; Bentley, S.D.; Muller, A. Comparative whole genome sequence analysis of the carcinogenic bacterial model pathogen Helicobacter felis. Genome Biol. Evol. 2011, 3, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Smet, A.; Flahou, B.; D’Herde, K.; Vandamme, P.; Cleenwerck, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F. Helicobacter heilmannii sp. nov., isolated from feline gastric mucosa. Int. J. Syst. Evol. Microbiol. 2012, 62, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Suerbaum, S.; Josenhans, C.; Sterzenbach, T.; Drescher, B.; Brandt, P.; Bell, M.; Droge, M.; Fartmann, B.; Fischer, H.P.; Ge, Z.; et al. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc. Natl. Acad. Sci. USA 2003, 100, 7901–7906. [Google Scholar] [CrossRef]
- Hu, S.; Jin, D.; Lu, S.; Liu, S.; Zhang, J.; Wang, Y.; Bai, X.; Xiong, Y.; Huang, Y.; Xu, H.; et al. Helicobacter himalayensis sp. nov. isolated from gastric mucosa of Marmota himalayana. Int. J. Syst. Evol. Microbiol. 2015, 65, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Feng, Y.; Sheh, A.; Everitt, J.; Bertram, F.; Paster, B.J.; Fox, J.G. Isolation and characterization of a novel Helicobacter species, Helicobacter jaachi sp. nov., from common marmosets (Callithrix jaachus). J. Med. Microbiol. 2015, 64, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Feng, Y.; Muthupalani, S.; Sheh, A.; Cheaney, L.E.; Kaufman, C.A.; Gong, G.; Paster, B.J.; Fox, J.G. Novel Helicobacter species H. japonicum isolated from laboratory mice from Japan induces typhlocolitis and lower bowel carcinoma in C57BL/129 IL10-/- mice. Carcinogenesis 2016, 37, 1190–1198. [Google Scholar] [CrossRef]
- Fox, J.G.; Boutin, S.R.; Handt, L.K.; Taylor, N.S.; Xu, S.; Rickman, B.; Marini, R.P.; Dewhirst, F.E.; Paster, B.J.; Motzel, S.; et al. Isolation and characterization of a novel helicobacter species, “Helicobacter macacae”, from rhesus monkeys with and without chronic idiopathic colitis. J. Clin. Microbiol. 2007, 45, 4061–4063. [Google Scholar] [CrossRef] [PubMed]
- Traverso, F.R.; Bohr, U.R.; Oyarzabal, O.A.; Rohde, M.; Clarici, A.; Wex, T.; Kuester, D.; Malfertheiner, P.; Fox, J.G.; Backert, S. Morphologic, genetic, and biochemical characterization of Helicobacter magdeburgensis, a novel species isolated from the intestine of laboratory mice. Helicobacter 2010, 15, 403–415. [Google Scholar] [CrossRef]
- Fox, J.G.; Shen, Z.; Xu, S.; Feng, Y.; Dangler, C.A.; Dewhirst, F.E.; Paster, B.J.; Cullen, J.M. Helicobacter marmotae sp. nov. isolated from livers of woodchucks and intestines of cats. J. Clin. Microbiol. 2002, 40, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Phillips, M.W.; O’Rourke, J.L.; Paster, B.J.; Dewhirst, F.E.; Fraser, G.J.; Fox, J.G.; Sly, L.I.; Romaniuk, P.J.; Trust, T.J.; et al. Helicobacter muridarum sp. nov., a microaerophilic helical bacterium with a novel ultrastructure isolated from the intestinal mucosa of rodents. Int. J. Syst. Bacteriol. 1992, 42, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Cabot, E.B.; Taylor, N.S.; Laraway, R. Gastric colonization by Campylobacter pylori subsp. mustelae in ferrets. Infect. Immun. 1988, 56, 2994–2996. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Seymour, C.; Fraser, G.J.; Paster, B.J.; Fox, J.G. Phylogeny of Helicobacter isolates from bird and swine feces and description of Helicobacter pametensis sp. nov. Int. J. Syst. Bacteriol. 1994, 44, 553–560. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Fox, J.G.; Mendes, E.N.; Paster, B.J.; Gates, C.E.; Kirkbride, C.A.; Eaton, K.A. ‘Flexispira rappini’ strains represent at least 10 Helicobacter taxa. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 5, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Mannion, A.; Whary, M.T.; Muthupalani, S.; Sheh, A.; Feng, Y.; Gong, G.; Vandamme, P.; Holcombe, H.R.; Paster, B.J.; et al. Helicobacter saguini, a novel Helicobacter isolated from cotton-top tamarins with ulcerative colitis, has proinflammatory properties and induces typhlocolitis and dysplasia in gnotobiotic IL-10-/- mice. Infect. Immun. 2016, 84, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Jalava, K.; Kaartinen, M.; Utriainen, M.; Happonen, I.; Hanninen, M.L. Helicobacter salomonis sp. nov., a canine gastric Helicobacter sp. related to Helicobacter felis and Helicobacter bizzozeronii. Int. J. Syst. Bacteriol. 1997, 47, 975–982. [Google Scholar] [CrossRef]
- Baele, M.; Decostere, A.; Vandamme, P.; Ceelen, L.; Hellemans, A.; Mast, J.; Chiers, K.; Ducatelle, R.; Haesebrouck, F. Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int. J. Syst. Evol. Microbiol. 2008, 58, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Mendes, E.N.; Queiroz, D.M.; Dewhirst, F.E.; Paster, B.J.; Moura, S.B.; Fox, J.G. Helicobacter trogontum sp. nov., isolated from the rat intestine. Int. J. Syst. Bacteriol. 1996, 46, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Dingemanse, C.; Schmitz, A.M.; Vossen, R.H.; van Ommen, G.J.; den Dunnen, J.T.; Robanus-Maandag, E.C.; Anvar, S.Y. The complete genome sequence of the murine pathobiont Helicobacter typhlonius. Front. Microbiol. 2015, 6, 1549. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.J.; Dong, H.J.; Shin, J.H.; Kim, I.Y.; Ho, H.; Oh, S.H.; Yoon, Y.M.; Choi, Y.K.; Suh, J.G.; Nam, K.H.; et al. Helicobacter apodemus sp. nov., a new Helicobacter species identified from the gastrointestinal tract of striped field mice in Korea. J. Vet. Sci. 2015, 16, 475–481. [Google Scholar] [CrossRef]
- Loman, N.J.; Snyder, L.A.; Linton, J.D.; Langdon, R.; Lawson, A.J.; Weinstock, G.M.; Wren, B.W.; Pallen, M.J. Genome sequence of the emerging pathogen Helicobacter canadensis. J. Bacteriol. 2009, 191, 5566–5567. [Google Scholar] [CrossRef]
- Simmons, J.H.; Riley, L.K.; Besch-Williford, C.L.; Franklin, C.L. Helicobacter mesocricetorum sp. nov., a novel Helicobacter isolated from the feces of Syrian hamsters. J. Clin. Microbiol. 2000, 38, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Linton, D.; Burnens, A.P.; Dewhirst, F.E.; On, S.L.; Porter, A.; Owen, R.J.; Costas, M. Helicobacter pullorum sp. nov.-genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiology 1994, 140 Pt 12, 3441–3449. [Google Scholar] [CrossRef]
- Shen, Z.; Fox, J.G.; Dewhirst, F.E.; Paster, B.J.; Foltz, C.J.; Yan, L.; Shames, B.; Perry, L. Helicobacter rodentium sp. nov., a urease-negative Helicobacter species isolated from laboratory mice. Int. J. Syst. Bacteriol. 1997, 47, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Collado, L.; Jara, R.; Gonzalez, S. Description of Helicobacter valdiviensis sp. nov., an Epsilonproteobacteria isolated from wild bird faecal samples. Int. J. Syst. Evol. Microbiol. 2014, 64, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Melito, P.L.; Munro, C.; Chipman, P.R.; Woodward, D.L.; Booth, T.F.; Rodgers, F.G. Helicobacter winghamensis sp. nov., a novel Helicobacter sp. isolated from patients with gastroenteritis. J. Clin. Microbiol. 2001, 39, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
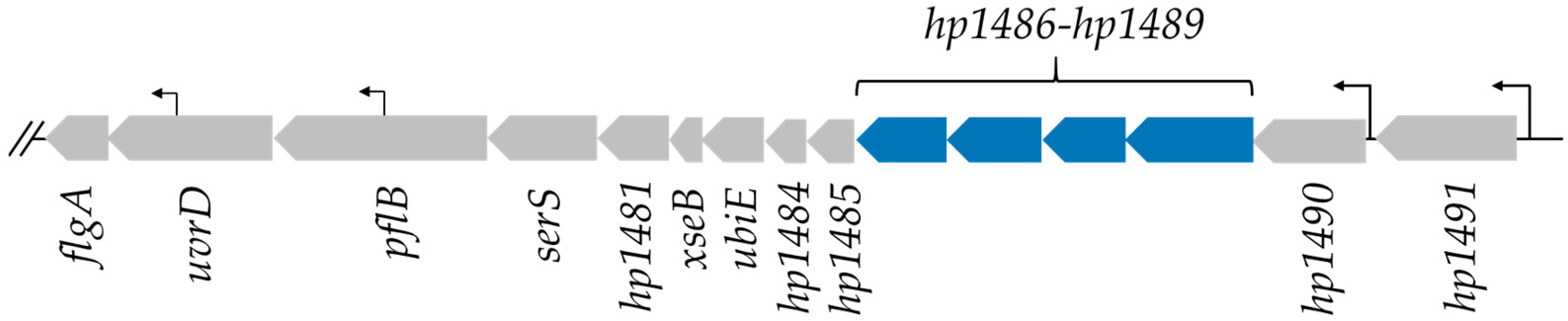



| NCBI Reference | Locus Tag | Gene | Known/Proposed Function | Reference |
|---|---|---|---|---|
| Flagellum function | ||||
| WP_000742695.1 | HP0327 | pseH | flagellin glycosylation | [31] |
| WP_000868000.1 | HPG27_395 | flgV | required for motility | [32] |
| Lipopolysaccharide biosynthesis | ||||
| WP_000487418.1 | HP0329 | futA | fucosylation of LPS | [33] |
| WP_000433778.1 | HP0580 | kdhA | Kdo-lipid A hydrolase | [34] |
| WP_000898474.1 | HP1580 | lpxF | lipid A 4′-phosphatase | [35] |
| WP_041201341.1 | HP1581 | wecA | O-antigen assembly | [36] |
| Sel1-like proteins | ||||
| WP_000597817.1 | HP0160 | hcpD | penicillin-binding protein | [37] |
| WP_000901623.1 | HP0211 | hcpA | penicillin-binding protein | [38] |
| WP_000111740.1 | HP0235 | hcpE | ||
| WP_000917816.1 | HP0628 | hcpF | ||
| WP_000892789.1 | HP1098 | hcpC | ||
| WP_000540103.1 | HP1117 | |||
| WP_000943858.1 | HPG27_1469 | hcpG | ||
| Outer membrane proteins | ||||
| WP_041201349.1 | HP0209 | hofA | [39] | |
| WP_000768629.1 | HP0486 | hofC | [39] | |
| WP_012552416.1 | HP0487 | hofD | [39] | |
| WP_000911466.1 | HP0782 | hofE | [39] | |
| WP_001108270.1 | HP0788 | hofF | [39] | |
| WP_041201373.1 | HP0914 | hofG | [39] | |
| WP_000797787.1 | HP1083 | hofB | [39] | |
| Transport proteins | ||||
| WP_000788001.1 | HP0839 | fatty acid transport protein | [39] | |
| WP_000816869.1 | HP0970 | cznB | metal efflux pump | [40] |
| WP_079990419.1 | HP0971 | cznC | metal efflux pump | [40] |
| WP_000780237.1 | HP1028 | lipocalin | [41] | |
| WP_001008850.1 | HP1486 | ABC-2 family transporter protein | this study | |
| WP_000489110.1 | HP1487 | ABC-2 family transporter protein | this study | |
| WP_012552579.1 | HP1488 | membrane fusion protein | this study | |
| WP_000754037.1 | HP1489 | outer membrane efflux protein | this study | |
| Miscellaneous functions | ||||
| WP_000820014.1 | HP0018 | predicted lipoprotein | ||
| WP_041201345.1 | HP0097 | predicted lipoprotein | ||
| WP_000233964.1 | HP0111 | hrcA | heat-inducible repressor | [42] |
| WP_000689112.1 | HP0190 | clsC | cardiolipin synthase | [43] |
| WP_001159179.1 | HP0199 | |||
| WP_000114771.1 | HP0468 | DUF5644 domain-containing protein | ||
| WP_000462324.1 | HP0640 | poly(A) polymerase | ||
| WP_000949206.1 | HP0653 | ftnA | bacterial non-heme ferritin | [44] |
| WP_000413451.1 | HP0664 | DUF2603 domain-containing protein | ||
| WP_001279170.1 | HP0700 | dgkA | diacylglycerol kinase | |
| WP_000790557.1 | HP0827 | RNA or ssDNA-binding protein | ||
| WP_001268507.1 | HP0838 | putative lipoprotein | ||
| WP_012552558.1 | HP1321 | conserved hypothetical ATP-binding protein | ||
| WP_000896338.1 | HP1440 | ispDF | isoprenoid biosynthesis | [45] |
| Class 1 | Class 2 | Class 3 | Class 4 | |
|---|---|---|---|---|
| Δhp1489 motor number | 40 | 4 | 18 | 9 |
| Δhp1487/hp1486 motor number | 91 | 17 | 15 | 6 |
| Isolate | Gene Description | Locus Tag | Sequence a | Impact b | Freq c |
|---|---|---|---|---|---|
| ESMV1 | arsS | CV725_RS0308 | (G)11→12 coding (1261/1281 nt) | P421fs | 84.9% |
| fliL | CV725_04935 | (A)9→8 coding (46/552 nt) | S16fs | 96.6% | |
| oipA | CV725_05780 | (AG)9→8 coding (51-52/958 nt) | S18sf | 93.3% | |
| glycosyltransferase family 25 protein | CV725_RS05870 | (G)13→14 coding (539/633 nt) | T180fs | 94.3% | |
| cag pathogenicity island protein | CV725_RS06350 | 2 bp→AG coding (2657-2658/5466 nt) | N886K | 96.6% | |
| ESMV2 | cation:proton antiporter | CV725_RS04480 | GCC→ACC | A249T | 98.9% |
| fliL | CV725_04935 | (A)9→8 coding (46/552 nt) | S16fs | 97.3% | |
| murJ | CV725_06605 | (A)9→8 coding (1174/1462 nt) | S392fs | 98.6% | |
| hypothetical protein | CV725_RS06835 | CTA→CGA | L4R | 100% | |
| hypothetical protein | CV725_RS06835 | TTC→TCC | F5S | 100% | |
| hypothetical protein | CV725_RS06835 | 2 bp→CT coding (17-18/2007 nt) | I6L | 100% | |
| outer membrane beta-barrel protein | CV725_RS07750 | (TC)9→8 pseudogene (14-15/1989 nt) | L5fs | 96.4% | |
| glycosyltransferase family 8 protein | CV725_RS03290 | (TC)9→8 pseudogene (95-96/1162 nt) | R28fs | 96.2% | |
| ESMV3 | fliL | CV725_04935 | (A)9→8 coding (46/552 nt) | S16fs | 99.7% |
| murJ | CV725_06605 | (A)9→8 coding (1174/1462 nt) | S392fs | 99.4% | |
| cation:proton antiporter | CV725_RS04480 | GCT→ACT | A201T | 99.1% |
| Strain | Relevant Genotype | Source |
| B128 | wild-type | [58] |
| G27 | wild-type | [59] |
| HP116 | H. pylori B128 hp1489:: kanR-PureA-sacB | this study |
| HP119 | H. pylori B128 ∆hp1489 | this study |
| HP174 | H. pylori B128 hp1487/hp148639:: kanR-PureA-sacB | this study |
| HP185 | H. pylori B128 ∆hp1487/hp1486 | this study |
| HP121 | H. pylori G27 hp1489:: kanR-PureA-sacB | this study |
| HP122 | H. pylori G27 ∆hp1489 | this study |
| HP128 | HP122 bearing pHel1489 | this study |
| Plasmid | Description | Source |
| pGEM-T Easy | TA cloning vector; ampR | Promega |
| pJC038 | pGEM-T Easy carrying kanR-PureA-sacB | [43] |
| pJC049 | pGEM-T Easy carrying flanking regions of hp1489 | this study |
| pJC051 | pJC049 with kanR-PureA-sacB insertion | this study |
| pJC076 | pGEM-T Easy carrying flanking regions of hp1487/hp1486 | this study |
| pJC080 | pJC076 with kanR-PureA-sacB insertion | this study |
| pJC083 | pGEM-T Easy carrying Php1491-hp1489 | this study |
| pHel3 | H. pylori shuttle vector; kanR | [60] |
| pHel1489 | pHel3 carrying hp1489 | this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibson, K.; Chu, J.K.; Zhu, S.; Nguyen, D.; Mrázek, J.; Liu, J.; Hoover, T.R. A Tripartite Efflux System Affects Flagellum Stability in Helicobacter pylori. Int. J. Mol. Sci. 2022, 23, 11609. https://doi.org/10.3390/ijms231911609
Gibson K, Chu JK, Zhu S, Nguyen D, Mrázek J, Liu J, Hoover TR. A Tripartite Efflux System Affects Flagellum Stability in Helicobacter pylori. International Journal of Molecular Sciences. 2022; 23(19):11609. https://doi.org/10.3390/ijms231911609
Chicago/Turabian StyleGibson, Katherine, Joshua K. Chu, Shiwei Zhu, Doreen Nguyen, Jan Mrázek, Jun Liu, and Timothy R. Hoover. 2022. "A Tripartite Efflux System Affects Flagellum Stability in Helicobacter pylori" International Journal of Molecular Sciences 23, no. 19: 11609. https://doi.org/10.3390/ijms231911609
APA StyleGibson, K., Chu, J. K., Zhu, S., Nguyen, D., Mrázek, J., Liu, J., & Hoover, T. R. (2022). A Tripartite Efflux System Affects Flagellum Stability in Helicobacter pylori. International Journal of Molecular Sciences, 23(19), 11609. https://doi.org/10.3390/ijms231911609





