Prevalence of Bacterial Coinfections with Vibrio harveyi in the Industrialized Flow-through Aquaculture Systems in Hainan Province: A Neglected High-Risk Lethal Causative Agent to Hybrid Grouper
Abstract
1. Introduction
2. Results
2.1. Predominant of Vibrio spp. in the Liver of Diseased Hybrid Groupers
2.2. Higher Mortality Rate in Coinfections with V. harveyi GDH11385 Than Monoinfection
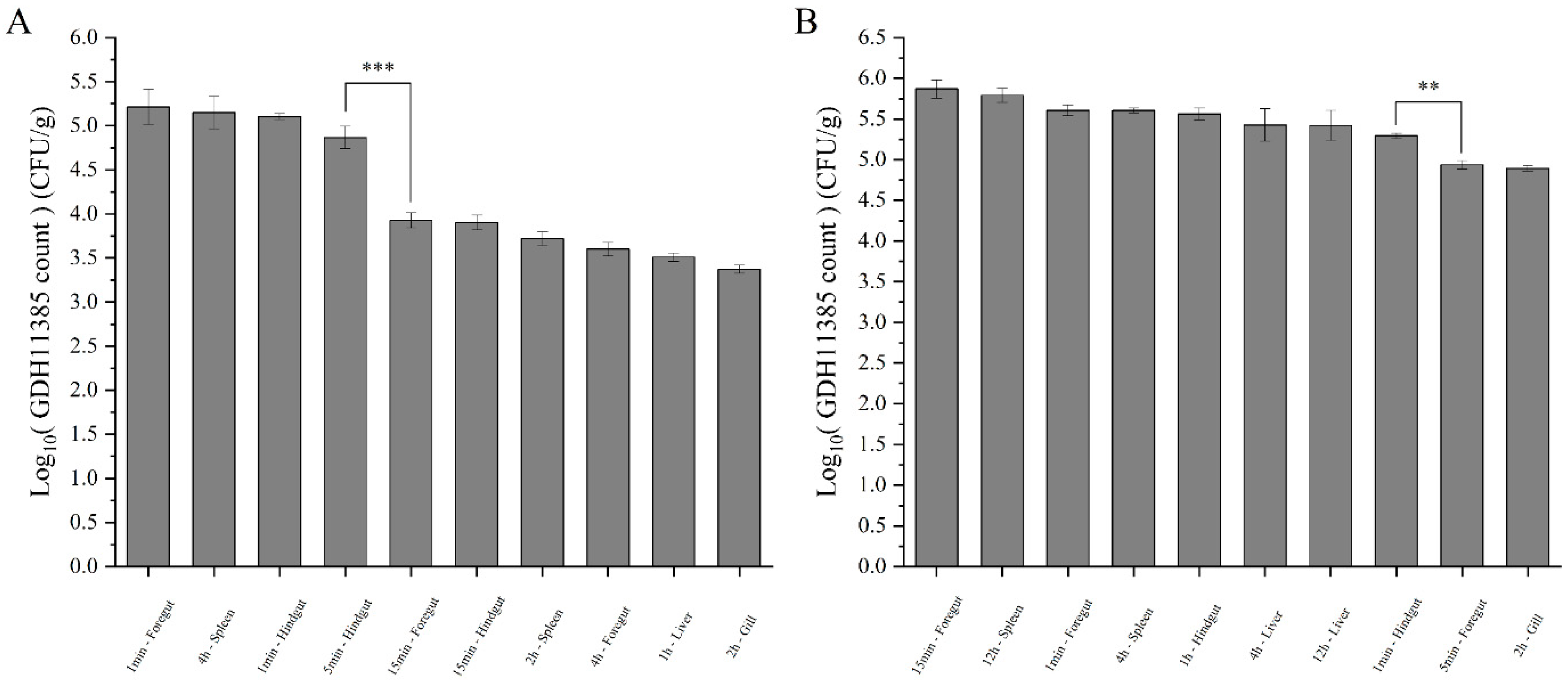
2.3. The Duration of the Coinfection State Depends on the Infection Concentration of Strain GDH11385
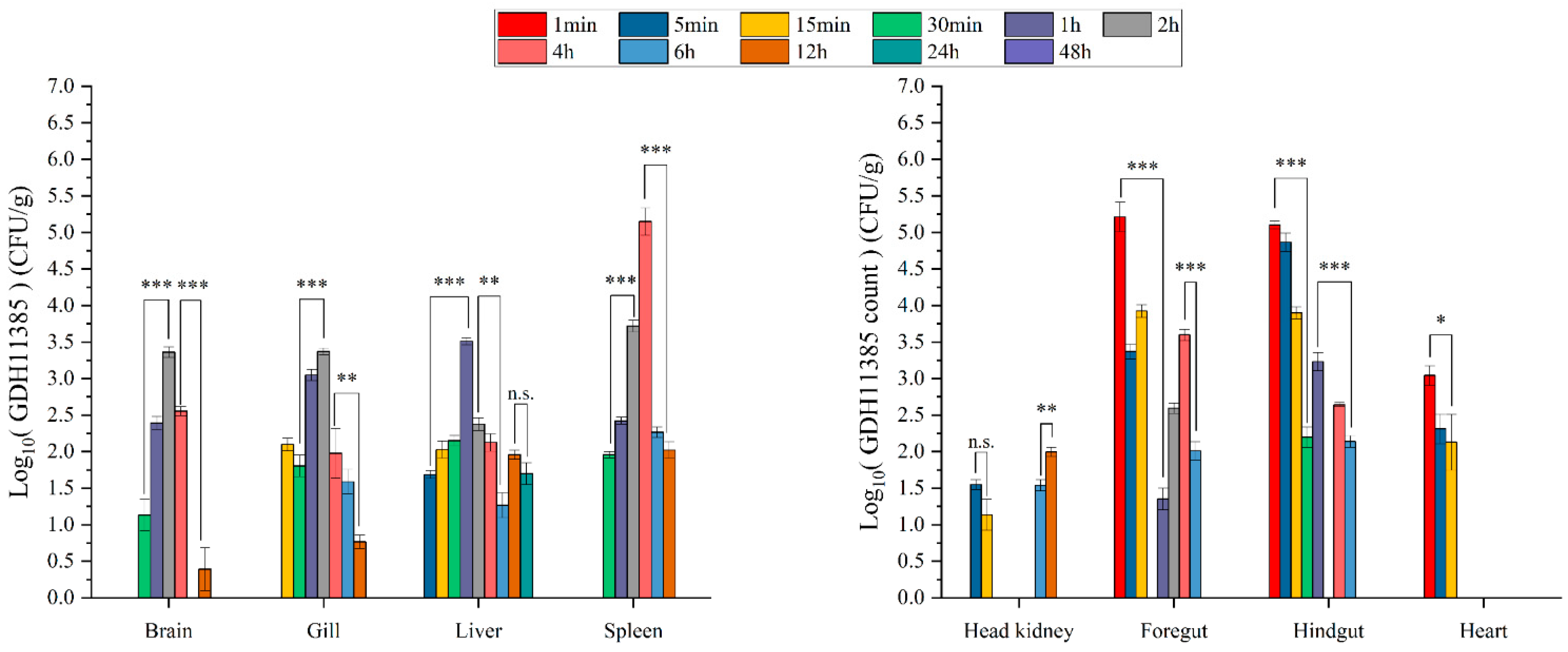
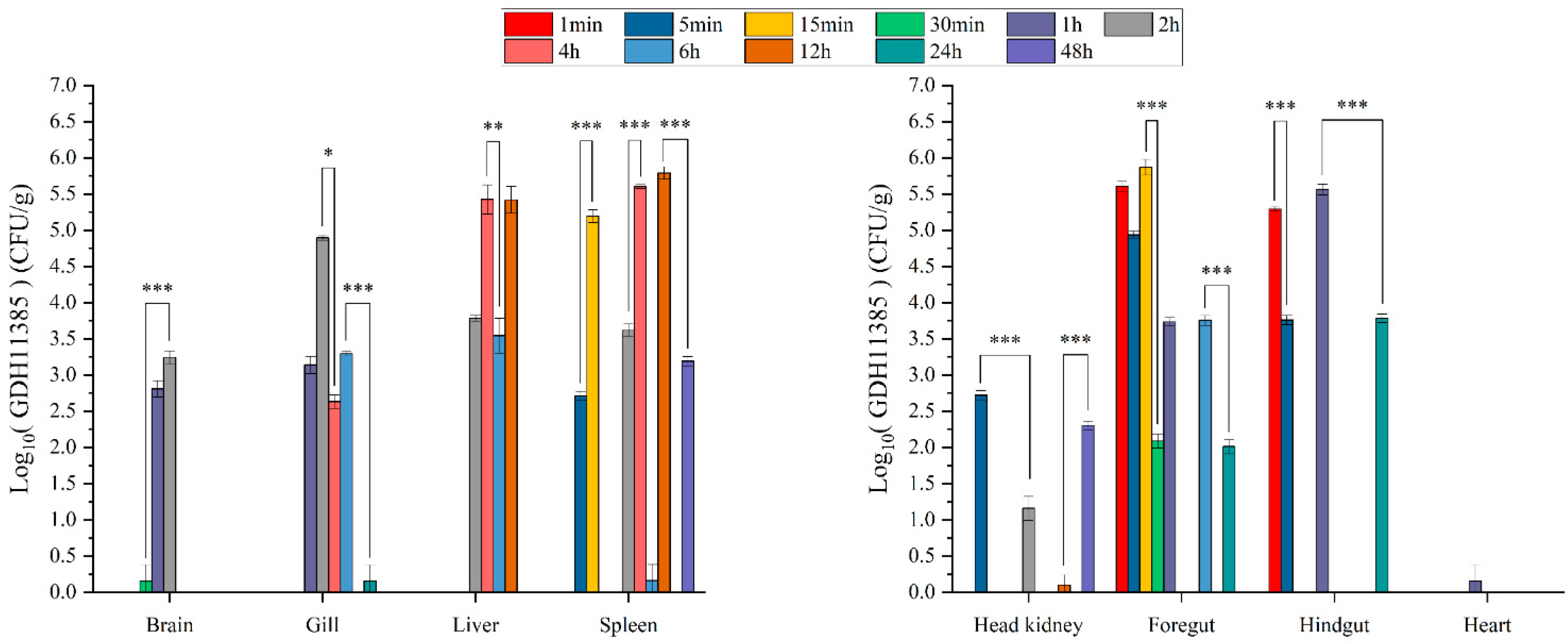
2.4. Intestine, Liver and Spleen Were Target Organs of GDH11385 in Coinfections and Monoinfection
3. Discussion
4. Materials and Methods
4.1. Isolation and Identification of Bacterial Pathogens from Diseased Hybrid Groupers
4.2. Preparation of Bacterial Cultures
4.3. Artificial Infection
4.4. Total Bacterial Colony Counting
4.5. Colony Identification of Strain GDH11385
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eichelberger, K.R.; Cassat, J.E. Metabolic Adaptations During Staphylococcus aureus and Candida albicans Co-Infection. Front. Immunol. 2021, 12, 797550. [Google Scholar] [CrossRef]
- Brogden, K.; Guthmiller, J.; Taylor, C. Human polymicrobial infections. Lancet 2005, 365, 253–255. [Google Scholar] [CrossRef]
- Massey, R.C.; Buckling, A.; ffrench-Constant, R. Interference competition and parasite virulence. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, 785–788. [Google Scholar] [CrossRef]
- Susi, H.; Barres, B.; Vale, P.F.; Laine, A.L. Co-infection alters population dynamics of infectious disease. Nat. Commun. 2015, 6, 5975. [Google Scholar] [CrossRef]
- Alizon, S.; de Roode, J.C.; Michalakis, Y. Multiple infections and the evolution of virulence. Ecol. Lett. 2013, 16, 556–567. [Google Scholar] [CrossRef]
- Zhang, X.H.; He, X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar] [CrossRef]
- Brown, S.P.; Cornforth, D.M.; Mideo, N. Evolution of virulence in opportunistic pathogens: Generalism, plasticity, and control. Trends Microbiol. 2012, 20, 336–342. [Google Scholar] [CrossRef]
- Leggett, H.C.; Buckling, A.; Long, G.H.; Boots, M. Generalism and the evolution of parasite virulence. Trends Ecol. Evol. 2013, 28, 592–596. [Google Scholar] [CrossRef]
- Sundberg, L.R.; Ketola, T.; Laanto, E.; Kinnula, H.; Bamford, J.K.; Penttinen, R.; Mappes, J. Intensive aquaculture selects for increased virulence and interference competition in bacteria. Proc. R. Soc. Lond. Ser. B Biol. 2016, 283, 20153069. [Google Scholar] [CrossRef]
- Jiang, S.; Wu, X.; Luo, Y.; Wu, M.; Lu, S.; Jin, Z.; Yao, W. Optimal dietary protein level and protein to energy ratio for hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) juveniles. Aquaculture 2016, 465, 28–36. [Google Scholar] [CrossRef]
- Xu, X.; Liu, K.; Wang, S.; Guo, W.; Xie, Z.; Zhou, Y. Identification of pathogenicity, investigation of virulent gene distribution and development of a virulent strain-specific detection PCR method for Vibrio harveyi isolated from Hainan Province and Guangdong Province, China. Aquaculture 2017, 468, 226–234. [Google Scholar] [CrossRef]
- Kraxberger-Beatty, T.; Mcgarey, D.J.; Grier, H.J.; Lim, D.V. Vibrio harveyi, an opportunistic pathogen of common snook, Centropomus undecimalis (Bloch), held in captivity. J. Fish Dis. 2010, 13, 557–560. [Google Scholar] [CrossRef]
- Ishimaru, K.; Muroga, K. Taxonomical Re-examination of Two Pathogenic Vibrio Species Isolated from Milkfish and Swimming Crab. Fish Pathol. 1997, 32, 59–64. [Google Scholar] [CrossRef]
- Gauger, E.; Smolowitz, R.; Uhlinger, K.; Casey, J.; Gómez-Chiarri, M. Vibrio harveyi and other bacterial pathogens in cultured summer flounder, Paralichthys dentatus. Aquaculture 2006, 260, 10–20. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Defoirdt, T.; Ina-Salwany, M.Y.; Yusoff, F.M.; Shariff, M.; Ismail, S.I.; Natrah, I. Vibrio parahaemolyticus and Vibrio harveyi causing Acute Hepatopancreatic Necrosis Disease (AHPND) in Penaeus vannamei (Boone, 1931) isolated from Malaysian shrimp ponds—ScienceDirect. Aquaculture 2019, 511, 734227. [Google Scholar] [CrossRef]
- Becker, P.; Gillan, D.; Lanterbecq, D.; Jangoux, M.; Rasolofonirina, R.; Rakotovao, J.; Eeckhaut, I. The skin ulceration disease in cultivated juveniles of Holothuria scabra (Holothuroidea, Echinodermata). Aquaculture 2004, 242, 13–30. [Google Scholar] [CrossRef]
- Wilkins, S.; Millar, M.; Hemsworth, S.; Johnson, G.; Warwick, S.; Pizer, B. Vibrio harveyi sepsis in a child with cancer. Pediatric Blood Cancer 2010, 50, 891–892. [Google Scholar] [CrossRef]
- Kang, C.H.; Kim, Y.; Oh, S.J.; Mok, J.S.; Cho, M.H.; So, J.S. Antibiotic resistance of Vibrio harveyi isolated from seawater in Korea. Mar. Pollut. Bull. 2014, 86, 261–265. [Google Scholar] [CrossRef]
- Brehm, T.T.; Berneking, L.; Rohde, H.; Chistner, M.; Schlickewei, C.; Sena Martins, M.; Schmiedel, S. Wound infection with Vibrio harveyi following a traumatic leg amputation after a motorboat propeller injury in Mallorca, Spain: A case report and review of literature. BMC Infect. Dis. 2020, 20, 104. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Z.; Qu, M.; Ding, S.; Zheng, L. Genomic organization, promoter characterization and expression analysis of the leukocyte cell-derived chemotaxin-2 gene in Epinephelus akaraa. Fish Shellfish Immunol. 2012, 32, 1041–1050. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, T.; Luo, P.; Huang, W.; Huo, D.; Yun, L.; Hu, C.; Cheng, C. A fibrinogen-related protein, LvFREP2, from Litopenaeus vannamei facilitates the clearance of Vibrio harveyi. Fish Shellfish Immunol. 2018, 78, 364–371. [Google Scholar] [CrossRef]
- Hispano, C.; Nebra, Y.; Blanch, A.R. Isolation of Vibrio harveyi from an ocular lesion in the short sunfish (Mola mola). Bull. Eur. Assoc. Fish Pathol. 1997, 17, 104–107. [Google Scholar]
- Peng, H.; Yang, B.; Li, B.; Cai, Z.; Cui, Q.; Chen, M.; Liu, X.; Yang, X.; Jiang, C. Comparative transcriptomic analysis reveals the gene expression profiles in the liver and spleen of Japanese pufferfish (Takifugu rubripes) in response to Vibrio harveyi infection. Fish Shellfish Immunol. 2019, 90, 308–316. [Google Scholar] [CrossRef]
- Mao, X.; Tian, Y.; Wen, H.; Liu, Y.; Sun, Y.; Yanglang, A.; Li, Y. Effects of Vibrio harveyi infection on serum biochemical parameters and expression profiles of interleukin-17 (IL-17)/interleukin-17 receptor (IL-17R) genes in spotted sea bass. Dev. Comp. Immunol. 2020, 110, 103731. [Google Scholar] [CrossRef]
- Lee, K.K.; Liu, P.C.; Chuang, W.H. Pathogenesis of gastroenteritis caused by Vibrio carchariae in cultured marine fish. Mar. Biotechnol. 2002, 4, 267–277. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Chen, H.; Xu, L.; Wang, Q.; Feng, J. Gut-Liver Immune Response and Gut Microbiota Profiling Reveal the Pathogenic Mechanisms of Vibrio harveyi in Pearl Gentian Grouper (Epinephelus lanceolatus male symbol × E. fuscoguttatus female symbol). Front. Immunol. 2020, 11, 607754. [Google Scholar] [CrossRef]
- Ransangan, J.; Mustafa, S. Identification of Vibrio harveyi isolated from diseased Asian seabass Lates calcarifer by use of 16S ribosomal DNA sequencing. J. Aquat. Anim. Health 2009, 21, 150–155. [Google Scholar] [CrossRef]
- Montanchez, I.; Kaberdin, V.R. Vibrio harveyi: A brief survey of general characteristics and recent epidemiological traits associated with climate change. Mar. Environ. Res. 2020, 154, 104850. [Google Scholar] [CrossRef]
- Peeralil, S.; Joseph, T.C.; Murugadas, V.; Akhilnath, P.G.; Sreejith, V.N.; Lalitha, K.V. Vibrio harveyi virulence gene expression in vitro and in vivo during infection in black tiger shrimp Penaeus monodon. Dis. Aquat. Organ. 2020, 139, 153–160. [Google Scholar] [CrossRef]
- Defoirdt, T. Virulence mechanisms of bacterial aquaculture pathogens and antivirulence therapy for aquaculture. Rev. Aquac. 2014, 6, 100–114. [Google Scholar] [CrossRef]
- Mohamad, N.; Mohd Roseli, F.A.; Azmai, M.N.A.; Saad, M.Z.; Md Yasin, I.S.; Zulkiply, N.A.; Nasruddin, N.S. Natural Concurrent Infection of Vibrio harveyi and V. alginolyticus in Cultured Hybrid Groupers in Malaysia. J. Aquat. Anim. Health 2019, 31, 88–96. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Dyess, D.L.; Garrison, R.N.; Fry, D.E. Candida sepsis. Implications of polymicrobial blood-borne infection. Arch. Surg. 1985, 120, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Rolston, K.V.; Bodey, G.P.; Safdar, A. Polymicrobial infection in patients with cancer: An underappreciated and underreported entity. Clin. Infect. Dis. 2007, 45, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Carolus, H.; Van Dyck, K.; Van Dijck, P. Candida albicans and Staphylococcus Species: A Threatening Twosome. Front. Microbiol. 2019, 10, 2162. [Google Scholar] [CrossRef]
- Diard, M.; Hardt, W.D. Evolution of bacterial virulence. FEMS Microbiol. Rev. 2017, 41, 679–697. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M. Virulence Factors in Candida species. Curr. Protein Pept. Sci. 2020, 21, 313–323. [Google Scholar] [CrossRef]
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.R.H.; Sun, H.; Zhang, C. Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection. Front. Cell. Infect. Microbiol. 2021, 11, 716299. [Google Scholar] [CrossRef]
- Lemos, M.L.; Balado, M. Iron uptake mechanisms as key virulence factors in bacterial fish pathogens. J. Appl. Microbiol. 2020, 129, 104–115. [Google Scholar] [CrossRef]
- Yii, K.C.; Yang, T.I.; Lee, K.K. Isolation and Characterization of Vibrio carchariae, a Causative Agent of Gastroenteritis in the Groupers, Epinephelus coioides. Curr. Microbiol. 1997, 35, 109–115. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, C.; Fu, M.; Bao, W.; Qiu, L. Molecular cloning, characterization and expression analysis of Toll-like receptor 5M gene in Japanese sea perch (Lateolabrax japonicas) after bacterial infection. Fish Shellfish Immunol. 2016, 56, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Kim, A.; Lee, W.; Kim, S.; Lee, S.; Yoon, D.; Bae, J.S.; Park, C.I.; Kim, S. Vibrio harveyi Infection Significantly Alters Amino Acid and Carbohydrate Metabolism in Whiteleg Shrimp, Litopenaeus vannamei. Metabolites 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, L.; Liu, W.; Xu, X.; Su, Y.; Qin, Y.; Yan, Q. Dual RNA-Seq Unveils Pseudomonas plecoglossicida htpG Gene Functions During Host-Pathogen Interactions with Epinephelus coioides. Front. Immunol. 2019, 10, 984. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Sun, Y.; Huang, L.; Su, Y.; Zhao, L.; Qin, Y.; Xu, X.; Yan, Q. Time-resolved dual RNA-seq of tissue uncovers Pseudomonas plecoglossicida key virulence genes in host-pathogen interaction with Epinephelus coioides. Environ. Microbiol. 2020, 22, 677–693. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Cristiani, M.; Flores, M.J.; Brandi, R.J.; Tedeschi, F.A.; Zalazar, F.E.; Labas, M.D. ERIC-PCR technique applied to monitoring and quantification of DNA damage during water disinfection process. J. Photochem. Photobiol. B Biol. 2020, 202, 111699. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
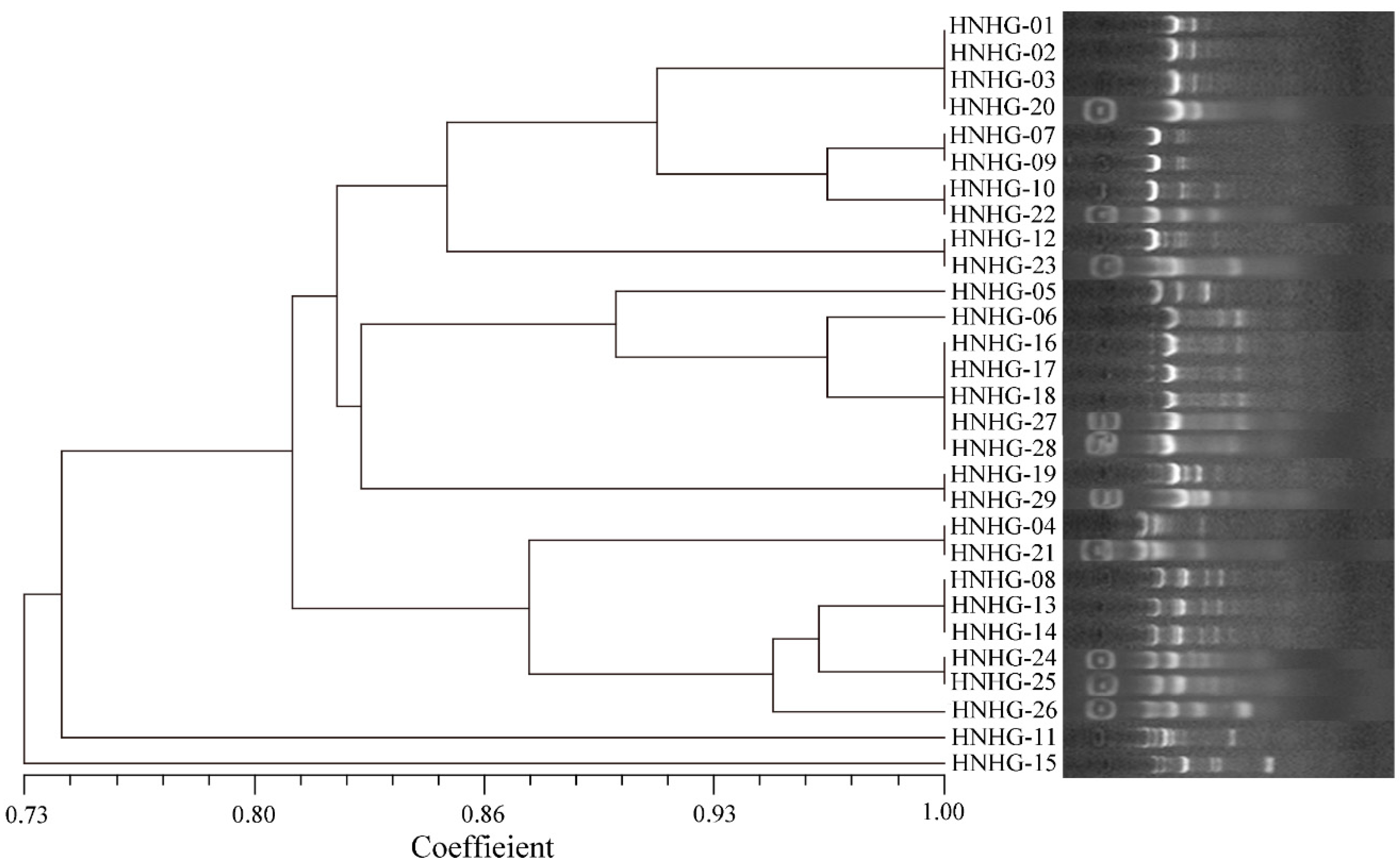

| Isolates | Top-Hit Taxon and Strain | Similarity (%) |
|---|---|---|
| HNHG-01 | Vibrio proteolyticus NBRC 13287 | 99.22 |
| HNHG-02 | Vibrio proteolyticus NBRC 13287 | 99.26 |
| HNHG-03 | Vibrio proteolyticus NBRC 13287 | 99.27 |
| HNHG-04 | Vibrio fortis LMG 21557 | 99.86 |
| HNHG-05 | Vibrio harveyi NBRC 15634 | 99.79 |
| HNHG-06 | Shewanella corallii fav-2-10-05 | 99.07 |
| HNHG-07 | Vibrio owensii LMG 25443 | 99.86 |
| HNHG-08 | Vibrio ponticus CECT 5869 | 99.72 |
| HNHG-09 | Vibrio owensii LMG 25443 | 99.86 |
| HNHG-10 | Vibrio hyugaensis 090810a | 99.78 |
| HNHG-11 | Vibrio parahaemolyticus NBRC 12711 | 99.43 |
| HNHG-12 | Vibrio taketomensis C4III282 | 99.93 |
| HNHG-13 | Vibrio owensii LMG 25443 | 99.86 |
| HNHG-14 | Vibrio ponticus CECT 5869 | 99.86 |
| HNHG-15 | Photobacterium damselae subsp. piscicida NCIMB 2058 | 99.16 |
| HNHG-16 | Shewanella corallii fav-2-10-05 | 99.14 |
| HNHG-17 | Shewanella corallii fav-2-10-05 | 98.81 |
| HNHG-18 | Shewanella corallii fav-2-10-05 | 99.00 |
| HNHG-19 | Providencia rettgeri DSM 4542 | 99.64 |
| HNHG-20 | Vibrio proteolyticus NBRC 13287 | 99.20 |
| HNHG-21 | Vibrio fortis LMG 21557 | 99.78 |
| HNHG-22 | Vibrio hyugaensis 090810a | 99.33 |
| HNHG-23 | Vibrio taketomensis C4III282 | 99.86 |
| HNHG-24 | Vibrio rhodolitus G98 | 99.86 |
| HNHG-25 | Vibrio ponticus CECT 5869 | 99.92 |
| HNHG-26 | Vibrio ponticus CECT 5869 | 99.86 |
| HNHG-27 | Shewanella corallii fav-2-10-05 | 99.85 |
| HNHG-28 | Shewanella corallii fav-2-10-05 | 99.48 |
| HNHG-29 | Providencia rettgeri DSM 4542 | 99.49 |
| Group | V. harveyi GDH11385 | Mixture of Isolated Pathogens | Number of Fish | 7-Day Average Mortality |
|---|---|---|---|---|
| A | 0 (1 × PBS) | 1 × 105 CFU/g | 10 | 0% |
| B | 1 × 103 CFU/g | 1 × 105 CFU/g | 10 | 3.33% |
| C | 1 × 104 CFU/g | 1 × 105 CFU/g | 10 | 13.33% |
| D | 1 × 105 CFU/g | 1 × 105 CFU/g | 10 | 46.67% |
| E | 1 × 105 CFU/g | 0 (1 × PBS) | 10 | 33.33% |
| CK | 0 (1 × PBS) | 0 (1 × PBS) | 10 | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Zeng, Y.-H.; Yin, W.-L.; Lu, H.-B.; Gong, X.-X.; Zhang, N.; Zhang, X.; Long, H.; Ren, W.; Cai, X.-N.; et al. Prevalence of Bacterial Coinfections with Vibrio harveyi in the Industrialized Flow-through Aquaculture Systems in Hainan Province: A Neglected High-Risk Lethal Causative Agent to Hybrid Grouper. Int. J. Mol. Sci. 2022, 23, 11628. https://doi.org/10.3390/ijms231911628
Xu H, Zeng Y-H, Yin W-L, Lu H-B, Gong X-X, Zhang N, Zhang X, Long H, Ren W, Cai X-N, et al. Prevalence of Bacterial Coinfections with Vibrio harveyi in the Industrialized Flow-through Aquaculture Systems in Hainan Province: A Neglected High-Risk Lethal Causative Agent to Hybrid Grouper. International Journal of Molecular Sciences. 2022; 23(19):11628. https://doi.org/10.3390/ijms231911628
Chicago/Turabian StyleXu, He, Yan-Hua Zeng, Wen-Liang Yin, Hong-Bin Lu, Xiao-Xiao Gong, Na Zhang, Xiang Zhang, Hao Long, Wei Ren, Xiao-Ni Cai, and et al. 2022. "Prevalence of Bacterial Coinfections with Vibrio harveyi in the Industrialized Flow-through Aquaculture Systems in Hainan Province: A Neglected High-Risk Lethal Causative Agent to Hybrid Grouper" International Journal of Molecular Sciences 23, no. 19: 11628. https://doi.org/10.3390/ijms231911628
APA StyleXu, H., Zeng, Y.-H., Yin, W.-L., Lu, H.-B., Gong, X.-X., Zhang, N., Zhang, X., Long, H., Ren, W., Cai, X.-N., Huang, A.-Y., & Xie, Z.-Y. (2022). Prevalence of Bacterial Coinfections with Vibrio harveyi in the Industrialized Flow-through Aquaculture Systems in Hainan Province: A Neglected High-Risk Lethal Causative Agent to Hybrid Grouper. International Journal of Molecular Sciences, 23(19), 11628. https://doi.org/10.3390/ijms231911628







