Nonthermal Plasma Effects on Fungi: Applications, Fungal Responses, and Future Perspectives
Abstract
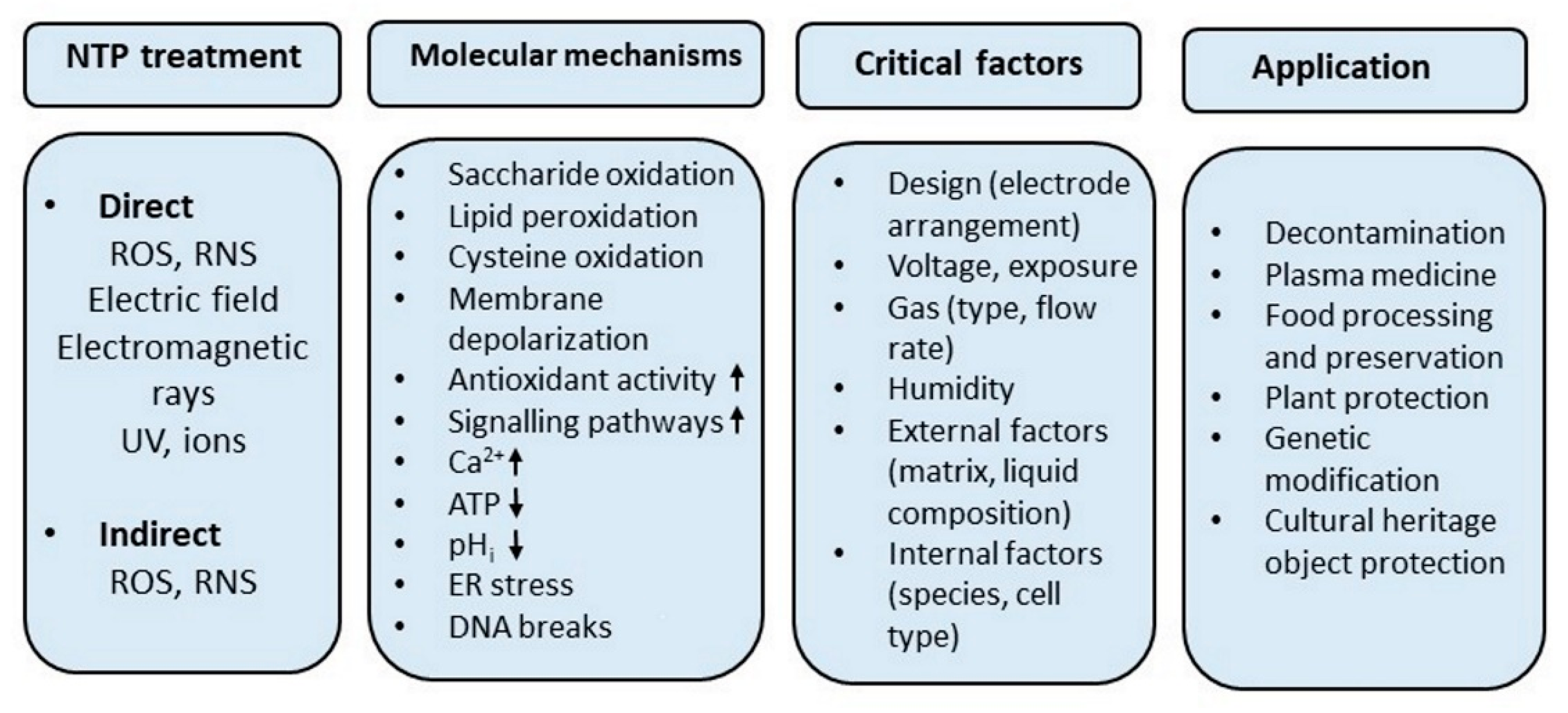
1. Introduction
2. NTP Devices, Plasma Composition, Biologically Active Agents, and Fungal Responses
2.1. Plasma Classification and Configurations of NTP Systems
2.1.1. Corona Discharge
2.1.2. Dielectric Barrier Discharge
2.1.3. Plasma Jet
2.2. Biologically Active Agents Generated by Plasma
2.3. Fungal Molecular Mechanisms in Response to NTP
3. NTP Technology in the Management of Fungal Contamination, Disease Control, Protection of Heritage Objects, and Strain Improvement
3.1. Plasma Medicine
3.2. Plasma Food Technology and Agriculture
3.3. Plasma and Cultural Heritage Objects
3.4. Plasma in Biotechnology
4. Summary and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veerana, M.; Yu, N.; Ketya, W.; Park, G. Application of non-thermal plasma to fungal resources. J. Fungi 2022, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Mravlje, J.; Regvar, M.; Vogel-Mikuš, K. Development of cold plasma technologies for surface decontamination of seed fungal pathogens: Present status and perspectives. J. Fungi 2021, 7, 650. [Google Scholar] [CrossRef] [PubMed]
- Polčic, P.; Machala, Z. Effects of non-thermal plasma on yeast Saccharomyces cerevisiae. Int. J. Mol. Sci. 2021, 22, 2247. [Google Scholar] [CrossRef]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal plasma inactivation of food-borne pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef]
- Bellan, P.M. Fundamentals of Plasma Physics; Cambridge University Press: Cambridge, UK, 2008; ISBN 9781139449731. [Google Scholar]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008; ISBN 9781139471732. [Google Scholar]
- Gershman, S. Pulsed Electrical Discharge in Gas Bubbles in Water. Ph.D. Thesis, Rutgers the State University of New Jersey-New Brunswick, New Brunswick, NJ, USA, 2008. [Google Scholar]
- Reedijk, J. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; von Woedtke, T.; Brandenburg, R.; von dem Hagen, T.; Weltmann, K.D. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2010, 44, 13002. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef]
- Gupta, T.T.; Ayan, H. Application of non-thermal plasma on biofilm: A review. Appl. Sci. 2019, 9, 3548. [Google Scholar] [CrossRef]
- Šrámková, P.; Zahoranová, A.; Kelar, J.; Kelar Tučeková, Z.; Stupavská, M.; Krumpolec, R.; Jurmanová, J.; Kováčik, D.; Černák, M. Cold atmospheric pressure plasma: Simple and efficient strategy for preparation of poly(2-oxazoline)-based coatings designed for biomedical applications. Sci. Rep. 2020, 10, 9478. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.N.; Chen, J.; Li, H.-P.; Ostrikov, K. Non-equilibrium synergistic effects in atmospheric pressure plasmas. Sci. Rep. 2018, 8, 4783. [Google Scholar] [CrossRef]
- Laroque, D.A.; Seó, S.T.; Valencia, G.A.; Laurindo, J.B.; Carciofi, B.A.M. Cold plasma in food processing: Design, mechanisms, and application. J. Food Eng. 2022, 312, 110748. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasm0061—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33 Pt 2, 1108–1119. [Google Scholar] [CrossRef]
- Ruangwong, K.; Rongsangchaicharean, T.; Thammaniphit, C.; Onwimol, D.; Srisonphan, S. Atmospheric corona discharge plasma for rice (Oryza sativa L.) seed surface modification, fungi decontamination, and shelf life extension. Plasma Med. 2020, 10, 191–201. [Google Scholar] [CrossRef]
- Porsev, E.G.; Druzhinina, N.S. Electric corona discharge as a basis for managing the quality of solid cereals. IOP Conf. Ser. Mater. Sci. Eng. 2019, 560, 12172. [Google Scholar] [CrossRef]
- Jose, J.; Ramanujam, S.; Philip, L. Applicability of pulsed corona discharge treatment for the degradation of chloroform. Chem. Eng. J. 2019, 360, 1341–1354. [Google Scholar] [CrossRef]
- Hassani, O.F.; Merbahi, N.; Oushabi, A.; Elfadili, M.H.; Kammouni, A.; Oueldna, N. Effects of corona discharge treatment on surface and mechanical properties of Aloe Vera fibers. Mater. Today Proc. 2020, 24, 46–51. [Google Scholar] [CrossRef]
- Singh, R.K.; Philip, L.; Ramanujam, S. Continuous flow pulse corona discharge reactor for the tertiary treatment of drinking water: Insights on disinfection and emerging contaminants removal. Chem. Eng. J. 2019, 355, 269–278. [Google Scholar] [CrossRef]
- KS Narayanan, S.S.; Wang, X.; Paul, J.; Paley, V.; Weng, Z.; Ye, L.; Zhong, Y. Disinfection and electrostatic recovery of N95 respirators by corona discharge for safe reuse. Environ. Sci. Technol. 2021, 55, 15351–15360. [Google Scholar] [CrossRef]
- Misra, N.N.; Yepez, X.; Xu, L.; Keener, K. In-package cold plasma technologies. J. Food Eng. 2019, 244, 21–31. [Google Scholar] [CrossRef]
- Wagner, H.E.; Brandenburg, R.; Kozlov, K.V.; Sonnenfeld, A.; Michel, P.; Behnke, J.F. The barrier discharge: Basic properties and applications to surface treatment. Vacuum 2003, 71, 417–436. [Google Scholar] [CrossRef]
- Pykönen, M.; Silvaani, H.; Preston, J.; Fardim, P.; Toivakka, M. Plasma activation induced changes in surface chemistry of pigment coating components. Colloids Surf. A Physicochem. Eng. Asp. 2009, 352, 103–112. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Bermudez-Aguirre, D. Advances in Cold Plasma Applications for Food Safety and Preservation; Elsevier Science: Amsterdam, The Netherlands, 2019; ISBN 9780128149225. [Google Scholar]
- Deng, X.; Shi, J.; Kong, M.G. Physical mechanisms of inactivation of Bacillus subtilis spores using cold atmospheric plasmas. IEEE Trans. Plasma Sci. 2006, 34, 1310–1316. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L.H. Low-temperature sterilization using gas plasmas: A review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Graves, D.B. Low temperature plasma biomedicine: A tutorial review. Phys. Plasmas 2014, 21, 80901. [Google Scholar] [CrossRef]
- Fridman, G.; Shereshevsky, A.; Jost, M.M.; Brooks, A.D.; Fridman, A.; Gutsol, A.; Vasilets, V.; Friedman, G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem. Plasma Process. 2007, 27, 163–176. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, Y.; Dusting, G.J. NADPH oxidase-mediated redox signaling: Roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 2011, 63, 218. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of atmospheric pressure plasmas on isolated and cellular DNA—A review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef]
- Misra, N.N.; Yadav, B.; Roopesh, M.S.; Jo, C. Cold plasma for effective fungal and mycotoxin control in foods: Mechanisms, inactivation effects, and applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.F.; Kang, J.G.; Lee, H.Y.; Uhm, H.S.; Moon, E.; Park, Y.H. Sterilization effect of atmospheric plasma on Escherichia coli and Bacillus subtilis endospores. Lett. Appl. Microbiol. 2009, 48, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Mendis, D.A.; Rosenberg, M. Plasma interaction with microbes. New J. Phys. 2003, 5, 41. [Google Scholar] [CrossRef]
- Bauer, G. Intercellular singlet oxygen-mediated bystander signaling triggered by long-lived species of cold atmospheric plasma and plasma-activated medium. Redox Biol. 2019, 26, 101301. [Google Scholar] [CrossRef] [PubMed]
- Machala, Z.; Tarabová, B.; Sersenová, D.; Janda, M.; Hensel, K. Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J. Phys. D Appl. Phys. 2018, 52, 34002. [Google Scholar] [CrossRef]
- Kondeti, V.S.S.K.; Phan, C.Q.; Wende, K.; Jablonowski, H.; Gangal, U.; Granick, J.L.; Hunter, R.C.; Bruggeman, P.J. Long-lived and short-lived reactive species produced by a cold atmospheric pressure plasma jet for the inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Free. Radic. Biol. Med. 2018, 124, 275–287. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Du, M.; Wang, Y.; Ju, S.; Ma, R.; Jiao, Z. Subcellular mechanism of microbial inactivation during water disinfection by cold atmospheric-pressure plasma. Water Res. 2021, 188, 116513. [Google Scholar] [CrossRef]
- Wheeler, K.A.; Hurdman, B.F.; Pitt, J.I. Influence of pH on the growth of some toxigenic species of Aspergillus, Penicillium and Fusarium. Int. J. Food Microbiol. 1991, 12, 141–149. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Li, B.; Chen, T.; Tian, S. Reactive oxygen species: A generalist in regulating development and pathogenicity of phytopathogenic fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3344–3349. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Šimončicová, J.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Lakatoš, B.; Kryštofová, S.; Hoppanová, L.; Palušková, V.; Hudecová, D.; Ďurina, P.; et al. Cold plasma treatment triggers antioxidative defense system and induces changes in hyphal surface and subcellular structures of Aspergillus flavus. Appl. Microbiol. Biotechnol. 2018, 102, 6647–6658. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Polčic, P.; Pakosová, L.; Chovančíková, P.; Machala, Z. Reactive cold plasma particles generate oxidative stress in yeast but do not trigger apoptosis. Can. J. Microbiol. 2018, 64, 367–375. [Google Scholar] [CrossRef]
- Hara, H.; Adachi, T. Molecular mechanisms of non-thermal atmospheric pressure plasma-induced cellular responses. Jpn. J. Appl. Phys. 2021, 60, 20501. [Google Scholar] [CrossRef]
- Itooka, K.; Takahashi, K.; Izawa, S. Fluorescence microscopic analysis of antifungal effects of cold atmospheric pressure plasma in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2016, 100, 9295–9304. [Google Scholar] [CrossRef]
- Spickett, C.M.; Pitt, A.R.; Morrice, N.; Kolch, W. Proteomic analysis of phosphorylation, oxidation and nitrosylation in signal transduction. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2006, 1764, 1823–1841. [Google Scholar] [CrossRef]
- Hoshi, T.; Heinemann, S.H. Regulation of cell function by methionine oxidation and reduction. J. Physiol. 2001, 531, 1–11. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Ni, Y.; Filipič, G.; Cvelbar, U.; Walsh, J.L. Effective fungal spore inactivation with an environmentally friendly approach based on atmospheric pressure air plasma. Environ. Sci. Technol. 2019, 53, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Hoppanová, L.; Dylíková, J.; Kováčik, D.; Medvecká, V.; Ďurina, P.; Kryštofová, S.; Zahoranová, A.; Kaliňáková, B. The effect of cold atmospheric pressure plasma on Aspergillus ochraceus and ochratoxin A production. Antonie Leeuwenhoek 2020, 113, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Hoppanová, L.; Dylíková, J.; Kováčik, D.; Medvecká, V.; Ďurina, P.; Kryštofová, S.; Hudecová, D.; Kaliňáková, B. Non-thermal plasma induces changes in aflatoxin production, devitalization, and surface chemistry of Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 2022, 106, 2107–2119. [Google Scholar] [CrossRef]
- Bogaerts, A.; Yusupov, M.; Razzokov, J.; Van der Paal, J. Plasma for cancer treatment: How can RONS penetrate through the cell membrane? Answers from computer modeling. Front. Chem. Sci. Eng. 2019, 13, 253–263. [Google Scholar] [CrossRef]
- Itooka, K.; Takahashi, K.; Kimata, Y.; Izawa, S. Cold atmospheric pressure plasma causes protein denaturation and endoplasmic reticulum stress in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2018, 102, 2279–2288. [Google Scholar] [CrossRef]
- Ma, R.N.; Feng, H.Q.; Liang, Y.D.; Zhang, Q.; Tian, Y.; Su, B.; Zhang, J.; Fang, J. An atmospheric-pressure cold plasma leads to apoptosis in Saccharomyces cerevisiae by accumulating intracellular reactive oxygen species and calcium. J. Phys. D Appl. Phys. 2013, 46, 285401. [Google Scholar] [CrossRef]
- Veerana, M.; Mitra, S.; Ki, S.-H.; Kim, S.-M.; Choi, E.-H.; Lee, T.; Park, G. Plasma-mediated enhancement of enzyme secretion in Aspergillus oryzae. Microb. Biotechnol. 2021, 14, 262–276. [Google Scholar] [CrossRef]
- Kurita, H.; Haruta, N.; Uchihashi, Y.; Seto, T.; Takashima, K. Strand breaks and chemical modification of intracellular DNA induced by cold atmospheric pressure plasma irradiation. PLoS ONE 2020, 15, e0232724. [Google Scholar] [CrossRef]
- Gaur, N.; Kurita, H.; Oh, J.-S.; Miyachika, S.; Ito, M.; Mizuno, A.; Cowin, A.J.; Allinson, S.; Short, R.D.; Szili, E.J. On cold atmospheric-pressure plasma jet induced DNA damage in cells. J. Phys. D Appl. Phys. 2020, 54, 35203. [Google Scholar] [CrossRef]
- Čtvrtečková, L.; Pichová, A.; Scholtz, V.; Khun, J.; Julák, J. Non-thermal plasma-induced apoptosis in yeast Saccharomyces cerevisiae. Contrib. Plasma Phys. 2019, 59, e201800064. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of cold atmospheric pressure plasma on maize seeds: Enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Akkermans, S.; Boehm, D.; Cullen, P.J.; Van Impe, J.; Bourke, P. Improving microbiological safety and quality characteristics of wheat and barley by high voltage atmospheric cold plasma closed processing. Food Res. Int. 2018, 106, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Soušková, H.; Scholtz, V.; Julák, J.; Kommová, L.; Savická, D.; Pazlarová, J. The survival of micromycetes and yeasts under the low-temperature plasma generated in electrical discharge. Folia Microbiol. 2011, 56, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Intanon, W.; Vichiansan, N.; Leksakul, K.; Boonyawan, D.; Kumla, J.; Suwannarach, N.; Lumyong, S. Inhibition of the aflatoxin-producing fungus Aspergillus flavus by a plasma jet system. J. Food Process. Preserv. 2021, 45, e15045. [Google Scholar] [CrossRef]
- Siadati, S.; Pet’ková, M.; Kenari, A.J.; Kyzek, S.; Gálová, E.; Zahoranová, A. Effect of a non-thermal atmospheric pressure plasma jet on four different yeasts. J. Phys. D Appl. Phys. 2020, 54, 25204. [Google Scholar] [CrossRef]
- Xu, H.; Ma, R.; Zhu, Y.; Du, M.; Zhang, H.; Jiao, Z. A systematic study of the antimicrobial mechanisms of cold atmospheric-pressure plasma for water disinfection. Sci. Total Environ. 2020, 703, 134965. [Google Scholar] [CrossRef]
- Ki, S.H.; Noh, H.; Ahn, G.R.; Kim, S.H.; Kaushik, N.K.; Choi, E.H.; Lee, G.J. Influence of nonthermal atmospheric plasma-activated water on the structural, optical, and biological properties of Aspergillus brasiliensis spores. Appl. Sci. 2020, 10, 6378. [Google Scholar] [CrossRef]
- Tyczkowska-Sieroń, E.; Kałużewski, T.; Grabiec, M.; Kałużewski, B.; Tyczkowski, J. Genotypic and phenotypic changes in Candida albicans as a result of cold plasma treatment. Int. J. Mol. Sci. 2020, 21, 8100. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Cui, D.; Du, M.; Wang, J.; Ma, R.; Jiao, Z. Evaluating the roles of OH radicals, H2O2, ORP and pH in the inactivation of yeast cells on a tissue model by surface micro-discharge plasma. J. Phys. D Appl. Phys. 2019, 52, 395201. [Google Scholar] [CrossRef]
- Fukuda, S.; Kawasaki, Y.; Izawa, S. Ferrous chloride and ferrous sulfate improve the fungicidal efficacy of cold atmospheric argon plasma on melanized Aureobasidium pullulans. J. Biosci. Bioeng. 2019, 128, 28–32. [Google Scholar] [CrossRef]
- Wu, M.; Liu, C.; Chiang, C.; Lin, Y.; Lin, Y.; Chang, Y.; Wu, J. Inactivation effect of Colletotrichum gloeosporioides by Long-lived chemical species using atmospheric-pressure corona plasma-activated water. IEEE Trans. Plasma Sci. 2019, 47, 1100–1104. [Google Scholar] [CrossRef]
- Noh, H.; Kim, J.E.; Kim, J.Y.; Kim, S.H.; Han, I.; Lim, J.S.; Ki, S.H.; Choi, E.H.; Lee, G.J. Spore viability and cell wall integrity of Cordyceps pruinosa treated with an electric shock-free, atmospheric-pressure air plasma jet. Appl. Sci. 2019, 9, 3921. [Google Scholar] [CrossRef]
- Julák, J.; Soušková, H.; Scholtz, V.; Kvasničková, E.; Savická, D.; Kříha, V. Comparison of fungicidal properties of non-thermal plasma produced by corona discharge and dielectric barrier discharge. Folia Microbiol. 2018, 63, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Nishime, T.M.C.; Borges, A.C.; Koga-Ito, C.Y.; Machida, M.; Hein, L.R.O.; Kostov, K.G. Non-thermal atmospheric pressure plasma jet applied to inactivation of different microorganisms. Surf. Coat. Technol. 2017, 312, 19–24. [Google Scholar] [CrossRef]
- Lee, G.J.; Park, G.; Choi, E.H. Optical and biological properties of plasma-treated Neurospora crassa spores as studied by absorption, circular dichroism, and Raman spectroscopy. J. Korean Phys. Soc. 2017, 71, 670–678. [Google Scholar] [CrossRef]
- Recek, N.; Zhou, R.; Zhou, R.; Te’o, V.S.J.; Speight, R.E.; Mozetič, M.; Vesel, A.; Cvelbar, U.; Bazaka, K.; Ostrikov, K. Improved fermentation efficiency of S. cerevisiae by changing glycolytic metabolic pathways with plasma agitation. Sci. Rep. 2018, 8, 8252. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen Patrick, J.; Bourke, P. Inactivation efficacies and mechanisms of gas plasma and plasma-activated water against Aspergillus flavus spores and biofilms: A comparative study. Appl. Environ. Microbiol. 2020, 86, e02619-19. [Google Scholar] [CrossRef]
- Borges, A.C.; Castaldelli Nishime, T.M.; Kostov, K.G.; de Morais Gouvêa Lima, G.; Lacerda Gontijo, A.V.; de Carvalho, J.N.M.M.; Yzumi Honda, R.; Yumi Koga-Ito, C. Cold atmospheric pressure plasma jet modulates Candida albicans virulence traits. Clin. Plasma Med. 2017, 7–8, 9–15. [Google Scholar] [CrossRef]
- He, M.; Duan, J.; Xu, J.; Ma, M.; Chai, B.; He, G.; Gan, L.; Zhang, S.; Duan, X.; Lu, X.; et al. Candida albicans biofilm inactivated by cold plasma treatment in vitro and in vivo. Plasma Process. Polym. 2020, 17, 1900068. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Kramer, A.; Metelmann, H.R.; Adler, F.; von Woedtke, T.; Niessner, F.; Weltmann, K.D.; Wende, K. High throughput image cytometry micronucleus assay to investigate the presence or absence of mutagenic effects of cold physical plasma. Environ. Mol. Mutagen. 2018, 59, 268–277. [Google Scholar] [CrossRef]
- Nam, S.H.; Ok, S.M.; Kim, G.C. Tooth bleaching with low-temperature plasma lowers surface roughness and Streptococcus mutans adhesion. Int. Endod. J. 2018, 51, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.-A.; Abdollahifar, M.-A.; Khoramgah, M.S.; Shokri, B. Comparison of direct and indirect cold atmospheric-pressure plasma methods in the B16F10 melanoma cancer cells treatment. Sci. Rep. 2018, 8, 7689. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xu, Y.; Cui, Q.; Liu, D.; Liu, Z.; Wang, X.; Yang, Y.; Feng, M.; Liang, R.; Chen, H.; et al. Cold atmospheric plasma as a potential tool for multiple myeloma treatment. Oncotarget 2018, 9, 18002–18017. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Da Silva, L.C.F.; de Almeida Freitas, R.; de Andrade, M.P., Jr.; Piva, M.R.; Martins-Filho, P.R.S.; de Santana Santos, T. Oral lesions in renal transplant. J. Craniofac. Surg. 2012, 23, e214–e218. [Google Scholar] [CrossRef]
- De la Rosa García, E.; de la Rosa-García, E.; Miramontes Zapata, M.; Miramontes-Zapata, M.; Sanchez-Vargas, L.-O.; Sánchez-Vargas, L.O.; Mondragón Padilla, A.; Mondragón-Padilla, A. Oral colonisation and infection by Candida sp. in diabetic and non-diabetic patients with chronic kidney disease on dialysis. Nefrología 2013, 33, 764–770. [Google Scholar] [CrossRef]
- Borges, A.C.; Lima, G.d.M.G.; Nishime, T.M.C.; Gontijo, A.V.L.; Kostov, K.G.; Koga-Ito, C.Y. Amplitude-modulated cold atmospheric pressure plasma jet for treatment of oral candidiasis: In Vivo study. PLoS ONE 2018, 13, e0199832. [Google Scholar] [CrossRef]
- Park, N.-S.; Yun, S.-E.; Lee, H.-Y.; Lee, H.J.; Choi, J.-H.; Kim, G.-C. No-ozone cold plasma can kill oral pathogenic microbes in H2O2-dependent and independent manner. Sci. Rep. 2022, 12, 7597. [Google Scholar] [CrossRef]
- Smith, M.B.; McGinnis, M.R. CHAPTER 82—Dermatophytosi. In Tropical Infectious Diseases: Principles, Pathogens and Practice, 3rd ed.; Guerrant, R.L., Walker, D.H., Weller, P.F., Eds.; W.B. Saunders: Edinburgh, UK, 2011; pp. 559–564. [Google Scholar]
- Ouf, S.A.; El-Adly, A.A.; Mohamed, A.-A.H. Inhibitory effect of silver nanoparticles mediated by atmospheric pressure air cold plasma jet against dermatophyte fungi. J. Med. Microbiol. 2015, 64, 1151–1161. [Google Scholar] [CrossRef][Green Version]
- Scholtz, V.; Soušková, H.; Švarcová, M.; Kříha, V.; Živná, H.; Julák, J. Inactivation of dermatophyte infection by nonthermal plasma on animal model. Med. Mycol. 2016, 55, 422–428. [Google Scholar] [CrossRef]
- Dubljanin, E.; Džamić, A.; Vujčić, I.; Grujičić, S.Š.; Arsenijević, V.A.; Mitrović, S.; Čalovski, I.Č. Epidemiology of onychomycosis in Serbia: A laboratory-based survey and risk factor identification. Mycoses 2017, 60, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Maraki, S.; Mavromanolaki, V.E. Epidemiology of onychomycosis in Crete, Greece: A 12-year study. Mycoses 2016, 59, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Clinical overview and diagnosis. J. Am. Acad. Dermatol. 2019, 80, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Bulson, J.M.; Liveris, D.; Derkatch, I.; Friedman, G.; Geliebter, J.; Park, S.; Singh, S.; Zemel, M.; Tiwari, R.K. Non-thermal atmospheric plasma treatment of onychomycosis in an in vitro human nail model. Mycoses 2020, 63, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Lux, J.; Dobiáš, R.; Kuklová, I.; Litvik, R.; Scholtz, V.; Soušková, H.; Khun, J.; Mrázek, J.; Kantorová, M.; Jaworská, P.; et al. Inactivation of dermatophytes causing onychomycosis and its therapy using non-thermal plasma. J. Fungi 2020, 6, 214. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Onyeaka, H.; Miri, T.; Obileke, K.; Anumudu, C.; Hart, A. A cold plasma technology for ensuring the microbiological safety and quality of foods. Food Eng. Rev. 2022, 14, 1–20. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U. Consequences of non-thermal cold plasma treatment on meat and dairy lipids—A review. Future Foods 2021, 4, 100095. [Google Scholar] [CrossRef]
- Shelar, A.; Singh, A.V.; Dietrich, P.; Maharjan, R.S.; Thissen, A.; Didwal, P.N.; Shinde, M.; Laux, P.; Luch, A.; Mathe, V.; et al. Emerging cold plasma treatment and machine learning prospects for seed priming: A step towards sustainable food production. RSC Adv. 2022, 12, 10467–10488. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S.-D. Application of cold oxygen plasma for the reduction of Cladosporium cladosporioides and Penicillium citrinum on the surface of dried filefish (Stephanolepis cirrhifer) fillets. Int. J. Food Sci. Technol. 2015, 50, 966–973. [Google Scholar] [CrossRef]
- Yong, H.I.; Lee, H.; Park, S.; Park, J.; Choe, W.; Jung, S.; Jo, C. Flexible thin-layer plasma inactivation of bacteria and mold survival in beef jerky packaging and its effects on the meat’s physicochemical properties. Meat Sci. 2017, 123, 151–156. [Google Scholar] [CrossRef]
- Royintarat, T.; Choi, E.H.; Boonyawan, D.; Seesuriyachan, P.; Wattanutchariya, W. Chemical-free and synergistic interaction of ultrasound combined with plasma-activated water (PAW) to enhance microbial inactivation in chicken meat and skin. Sci. Rep. 2020, 10, 1559. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, C.; Sunooj, K.V. 14—Cold plasma processing of fresh-cut fruits and vegetables. In Fresh-Cut Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 339–356. [Google Scholar]
- Misra, N.N.; Pankaj, S.K.; Segat, A.; Ishikawa, K. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; et al. Cold plasma processing of milk and dairy products. Trends Food Sci. Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Nierop Groot, M.; Abee, T.; van Bokhorst-van de Veen, H. Inactivation of conidia from three Penicillium spp. isolated from fruit juices by conventional and alternative mild preservation technologies and disinfection treatments. Food Microbiol. 2019, 81, 108–114. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Li, J.; Liu, S.; Zhang, H.; Bai, Y. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem. 2018, 254, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Starek, A.; Sagan, A.; Andrejko, D.; Chudzik, B.; Kobus, Z.; Kwiatkowski, M.; Terebun, P.; Pawłat, J. Possibility to extend the shelf life of NFC tomato juice using cold atmospheric pressure plasma. Sci. Rep. 2020, 10, 20959. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Liao, X.; Cullen, P.J.; Liu, D.; Xiang, Q.; Wang, J.; Chen, S.; Ye, X.; Ding, T. Effects of nonthermal plasma technology on functional food components. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1379–1394. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Sun, H.; Yang, X.; Cui, D.; Wang, Y.; Zhuang, J.; Wang, X.; Ma, R.; Jiao, Z. Potential use of atmospheric cold plasma for postharvest preservation of blueberries. Postharvest Biol. Technol. 2021, 179, 111564. [Google Scholar] [CrossRef]
- Puligundla, P.; Lee, T.; Mok, C. Effect of intermittent corona discharge plasma treatment for improving microbial quality and shelf life of kumquat (Citrus japonica) fruits. LWT 2018, 91, 8–13. [Google Scholar] [CrossRef]
- Go, S.-M.; Park, M.-R.; Kim, H.-S.; Choi, W.S.; Jeong, R.-D. Antifungal effect of non-thermal atmospheric plasma and its application for control of postharvest Fusarium oxysporum decay of paprika. Food Control 2019, 98, 245–252. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- Won, M.Y.; Lee, S.J.; Min, S.C. Mandarin preservation by microwave-powered cold plasma treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 25–32. [Google Scholar] [CrossRef]
- Liu, C.-M.; Nishida, Y.; Iwasaki, K.; Ting, K. Prolonged preservation and sterilization of fresh plants in controlled environments using high-field plasma. IEEE Trans. Plasma Sci. 2011, 39, 717–724. [Google Scholar] [CrossRef]
- Ambrico, P.F.; Šimek, M.; Rotolo, C.; Morano, M.; Minafra, A.; Ambrico, M.; Pollastro, S.; Gerin, D.; Faretra, F.; De Miccolis Angelini, R.M. Surface dielectric barrier discharge plasma: A suitable measure against fungal plant pathogens. Sci. Rep. 2020, 10, 3673. [Google Scholar] [CrossRef]
- Phan, K.T.K.; Phan, H.T.; Boonyawan, D.; Intipunya, P.; Brennan, C.S.; Regenstein, J.M.; Phimolsiripol, Y. Non-thermal plasma for elimination of pesticide residues in mango. Innov. Food Sci. Emerg. Technol. 2018, 48, 164–171. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, J.-H.; Sun, D.-W. Subcellular damages of Colletotrichum asianum and inhibition of mango anthracnose by dielectric barrier discharge plasma. Food Chem. 2022, 381, 132197. [Google Scholar] [CrossRef]
- Cui, D.; Hu, X.; Yin, Y.; Zhu, Y.; Zhuang, J.; Wang, X.; Ma, R.; Jiao, Z. Quality enhancement and microbial reduction of mung bean (Vigna radiata) sprouts by non-thermal plasma pretreatment of seeds. Plasma Sci. Technol. 2022, 24, 45504. [Google Scholar] [CrossRef]
- Choi, E.J.; Park, H.W.; Kim, S.B.; Ryu, S.; Lim, J.; Hong, E.J.; Byeon, Y.S.; Chun, H.H. Sequential application of plasma-activated water and mild heating improves microbiological quality of ready-to-use shredded salted kimchi cabbage (Brassica pekinensis L.). Food Control 2019, 98, 501–509. [Google Scholar] [CrossRef]
- Hosseini, S.I.; Farrokhi, N.; Shokri, K.; Khani, M.R.; Shokri, B. Cold low pressure O2 plasma treatment of Crocus sativus: An efficient way to eliminate toxicogenic fungi with minor effect on molecular and cellular properties of saffron. Food Chem. 2018, 257, 310–315. [Google Scholar] [CrossRef]
- Kim, J.E.; Oh, Y.J.; Won, M.Y.; Lee, K.-S.; Min, S.C. Microbial decontamination of onion powder using microwave-powered cold plasma treatments. Food Microbiol. 2017, 62, 112–123. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y. Application of a roller conveyor type plasma disinfection device with fungus-contaminated citrus fruits. AMB Express 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, A.; Hrycak, B.; Jasiński, M.; Rybak, K.; Kieliszek, M.; Kraśniewska, K.; Witrowa-Rajchert, D. Impact of atmospheric pressure microwave plasma treatment on quality of selected spices. Appl. Sci. 2020, 10, 6815. [Google Scholar] [CrossRef]
- Phan, K.T.K.; Phan, H.T.; Brennan, C.S.; Regenstein, J.M.; Jantanasakulwong, K.; Boonyawan, D.; Phimolsiripol, Y. Gliding arc discharge non-thermal plasma for retardation of mango anthracnose. LWT 2019, 105, 142–148. [Google Scholar] [CrossRef]
- Sen, Y.; Onal-Ulusoy, B.; Mutlu, M. Aspergillus decontamination in hazelnuts: Evaluation of atmospheric and low-pressure plasma technology. Innov. Food Sci. Emerg. Technol. 2019, 54, 235–242. [Google Scholar] [CrossRef]
- Dong, X.Y.; Yang, Y.L. A novel approach to enhance blueberry quality during storage using cold plasma at atmospheric air pressure. Food Bioprocess Technol. 2019, 12, 1409–1421. [Google Scholar] [CrossRef]
- Dasan, B.G.; Boyaci, I.H.; Mutlu, M. Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: Impact of process parameters and surveillance of the residual viability of spores. J. Food Eng. 2017, 196, 139–149. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Žigon, D.; Kovač, J.; Jurov, A.; Dickenson, A.; Walsh, J.L.; Cvelbar, U. Cold atmospheric pressure plasma-assisted removal of aflatoxin B1 from contaminated corn kernels. Plasma Process. Polym. 2021, 18, 2000163. [Google Scholar] [CrossRef]
- Makari, M.; Hojjati, M.; Shahbazi, S.; Askari, H. Elimination of Aspergillus flavus from pistachio nuts with dielectric barrier discharge (DBD) cold plasma and its impacts on biochemical indices. J. Food Qual. 2021, 2021, 9968711. [Google Scholar] [CrossRef]
- Puligundla, P.; Lee, T.; Mok, C. Effect of corona discharge plasma jet treatment on the degradation of aflatoxin B1 on glass slides and in spiked food commodities. LWT 2020, 124, 108333. [Google Scholar] [CrossRef]
- Sen, Y.; Onal-Ulusoy, B.; Mutlu, M. Detoxification of hazelnuts by different cold plasmas and gamma irradiation treatments. Innov. Food Sci. Emerg. Technol. 2019, 54, 252–259. [Google Scholar] [CrossRef]
- Jerushalmi, S.; Maymon, M.; Dombrovsky, A.; Freeman, S. Effects of cold plasma, gamma and e-beam irradiations on reduction of fungal colony forming unit levels in medical cannabis inflorescences. J. Cannabis Res. 2020, 2, 12. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, Y.Y.; Kim, Y.S.; Balaraju, K.; Mok, Y.S.; Yoo, S.J.; Jeon, Y. Enhancement of seed germination and microbial disinfection on ginseng by cold plasma treatment. J. Ginseng Res. 2021, 45, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Świecimska, M.; Tulik, M.; Šerá, B.; Golińska, P.; Tomeková, J.; Medvecká, V.; Bujdáková, H.; Oszako, T.; Zahoranová, A.; Šerý, M. Non-thermal plasma can be used in disinfection of scots pine (Pinus sylvestris L.) seeds infected with Fusarium oxysporum. Forests 2020, 11, 837. [Google Scholar] [CrossRef]
- Waskow, A.; Betschart, J.; Butscher, D.; Oberbossel, G.; Klöti, D.; Büttner-Mainik, A.; Adamcik, J.; von Rohr, P.R.; Schuppler, M. Characterization of efficiency and mechanisms of cold atmospheric pressure plasma decontamination of seeds for sprout production. Front. Microbiol. 2018, 9, 3164. [Google Scholar] [CrossRef] [PubMed]
- Pérez Pizá, M.C.; Prevosto, L.; Zilli, C.; Cejas, E.; Kelly, H.; Balestrasse, K. Effects of non–thermal plasmas on seed-borne Diaporthe/Phomopsis complex and germination parameters of soybean seeds. Innov. Food Sci. Emerg. Technol. 2018, 49, 82–91. [Google Scholar] [CrossRef]
- Štěpánová, V.; Slavíček, P.; Kelar, J.; Prášil, J.; Smékal, M.; Stupavská, M.; Jurmanová, J.; Černák, M. Atmospheric pressure plasma treatment of agricultural seeds of cucumber (Cucumis sativus L.) and pepper (Capsicum annuum L.) with effect on reduction of diseases and germination improvement. Plasma Process. Polym. 2018, 15, 1700076. [Google Scholar] [CrossRef]
- Ambrico, P.F.; Šimek, M.; Morano, M.; De Miccolis Angelini, R.M.; Minafra, A.; Trotti, P.; Ambrico, M.; Prukner, V.; Faretra, F. Reduction of microbial contamination and improvement of germination of sweet basil (Ocimum basilicum L.) seeds via surface dielectric barrier discharge. J. Phys. D Appl. Phys. 2017, 50, 305401. [Google Scholar] [CrossRef]
- Ochi, A.; Konishi, H.; Ando, S.; Sato, K.; Yokoyama, K.; Tsushima, S.; Yoshida, S.; Morikawa, T.; Kaneko, T.; Takahashi, H. Management of bakanae and bacterial seedling blight diseases in nurseries by irradiating rice seeds with atmospheric plasma. Plant Pathol. 2017, 66, 67–76. [Google Scholar] [CrossRef]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on fungal growth and aflatoxins production on groundnuts. Food Control 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Kim, J.-W.; Puligundla, P.; Mok, C. Effect of corona discharge plasma jet on surface-borne microorganisms and sprouting of broccoli seeds. J. Sci. Food Agric. 2017, 97, 128–134. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D. Food safety and increasing hazard of mycotoxin occurrence in foods and feeds. Crit. Rev. Food Sci. Nutr. 2013, 53, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Hojnik, N.; Modic, M.; Walsh, J.L.; Žigon, D.; Javornik, U.; Plavec, J.; Žegura, B.; Filipič, M.; Cvelbar, U. Unravelling the pathways of air plasma induced aflatoxin B1 degradation and detoxification. J. Hazard. Mater. 2021, 403, 123593. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, V.; Šerá, B.; Khun, J.; Šerý, M.; Julák, J. Effects of nonthermal plasma on wheat grains and products. J. Food Qual. 2019, 2019, 7917825. [Google Scholar] [CrossRef]
- Švubová, R.; Slováková, Ľ.; Holubová, Ľ.; Rovňanová, D.; Gálová, E.; Tomeková, J. Evaluation of the impact of cold atmospheric pressure plasma on soybean seed germination. Plants 2021, 10, 177. [Google Scholar] [CrossRef]
- Peťková, M.; Švubová, R.; Kyzek, S.; Medvecká, V.; Slováková, Ľ.; Ševčovičová, A.; Gálová, E. The effects of cold atmospheric pressure plasma on germination parameters, enzyme activities and induction of DNA damage in barley. Int. J. Mol. Sci. 2021, 22, 2833. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, Ľ.; Hensel, K. Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process. Polym. 2019, 16, 1800131. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Šerý, M.; Zahoranová, A.; Kerdík, A.; Šerá, B. Seed germination of black pine (Pinus nigra Arnold) after diffuse coplanar surface barrier discharge plasma treatment. IEEE Trans. Plasma Sci. 2020, 48, 939–945. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Hoppanová, L.; Medvecká, V.; Dylíková, J.; Hudecová, D.; Kaliňáková, B.; Kryštofová, S.; Zahoranová, A. Low-temperature plasma applications in chemical fungicide treatment reduction. Acta Chim. Slovaca 2020, 13, 26–33. [Google Scholar] [CrossRef]
- Todorova, Y.; Benova, E.; Marinova, P.; Yotinov, I.; Bogdanov, T.; Topalova, Y. Non-thermal atmospheric plasma for microbial decontamination and removal of hazardous chemicals: An overview in the circular economy context with data for test applications of microwave plasma torch. Processes 2022, 10, 554. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Rehakova, M.; Čeppan, M.; Mikula, M. Study of stabilization of documents containing iron gall inks by treatment of atmospheric DBD N2 plasma. Chem. Listy 2008, 102, 1061–1063. [Google Scholar]
- Vizárová, K.; Kaliňáková, B.; Tiňo, R.; Vajová, I.; Čížová, K. Microbial decontamination of lignocellulosic materials with low-temperature atmospheric plasma. J. Cult. Herit. 2021, 47, 28–33. [Google Scholar] [CrossRef]
- Huang, W.W.; Ge, X.Y.; Huang, Y.; Chai, X.T.; Zhang, L.; Zhang, Y.X.; Deng, L.N.; Liu, C.Q.; Xu, H.; Gao, J. High-yield strain of fusidic acid obtained by atmospheric and room temperature plasma mutagenesis and the transcriptional changes involved in improving its production in fungus Fusidium coccineum. J. Appl. Microbiol. 2021, 130, 405–415. [Google Scholar] [CrossRef]
- Hu, Z.-C.; Li, W.-J.; Zou, S.-P.; Niu, K.; Zheng, Y.-G. Mutagenesis of echinocandin B overproducing Aspergillus nidulans capable of using starch as main carbon source. Prep. Biochem. Biotechnol. 2020, 50, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, L.; Wu, D.; Dong, G.; Chen, W.; Zhang, H.; Yang, Y.; Wu, W. The structural characteristics and biological activities of intracellular polysaccharide derived from mutagenic Sanghuangporous sanghuang strain. Molecules 2020, 25, 3693. [Google Scholar] [CrossRef]
- Gu, L.-S.; Tan, M.-Z.; Li, S.-H.; Zhang, T.; Zhang, Q.-Q.; Li, C.-X.; Luo, X.-M.; Feng, J.-X.; Zhao, S. ARTP/EMS-combined multiple mutagenesis efficiently improved production of raw starch-degrading enzymes in Penicillium oxalicum and characterization of the enzyme-hyperproducing mutant. Biotechnol. Biofuels 2020, 13, 187. [Google Scholar] [CrossRef]
- Shu, L.; Si, X.; Yang, X.; Ma, W.; Sun, J.; Zhang, J.; Xue, X.; Wang, D.; Gao, Q. Enhancement of acid protease activity of Aspergillus oryzae using atmospheric and room temperature plasma. Front. Microbiol. 2020, 11, 1418. [Google Scholar] [CrossRef]
- Ma, X.-J.; Zhang, H.-M.; Lu, X.-F.; Han, J.; Zhu, H.-X.; Wang, H.; Yao, R.-S. Mutant breeding of Starmerella bombicola by atmospheric and room-temperature plasma (ARTP) for improved production of specific or total sophorolipids. Bioprocess Biosyst. Eng. 2020, 43, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Jiang, B.; Zhang, T.; Chen, J. Combined mutagenesis and metabolic regulation to enhance d-arabitol production from Candida parapsilosis. J. Ind. Microbiol. Biotechnol. 2020, 47, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qin, J.; Dai, Y.; Mu, W.; Zhang, T. Atmospheric and room temperature plasma (ARTP) mutagenesis enables xylitol over-production with yeast Candida tropicalis. J. Biotechnol. 2019, 296, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Liang, J.; Wang, B.; Chen, J. Improvement of kojic acid production in Aspergillus oryzae AR-47 mutant strain by combined mutagenesis. Bioprocess Biosyst. Eng. 2019, 42, 753–761. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, D.; Zhang, H.; Li, Q.; Zhang, Z.; Liu, Y.; Zhou, S.; Wang, W.; Li, Z.; Yang, Y. Effects of atmospheric and room temperature plasma (ARTP) mutagenesis on physicochemical characteristics and immune activity in vitro of Hericium erinaceus polysaccharides. Molecules 2019, 24, 262. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Q.; Zhang, Q.; He, H.; Chen, Z.; Zhao, Y.; Wei, D.; Kong, M.; Huang, Q. Improved production of polysaccharides in Ganoderma lingzhi mycelia by plasma mutagenesis and rapid screening of mutated strains through infrared spectroscopy. PLoS ONE 2018, 13, e0204266. [Google Scholar] [CrossRef]
- Zou, Z.; Zhao, Y.; Zhang, T.; Xu, J.; He, A.; Deng, Y. Efficient isolation and characterization of a cellulase hyperproducing mutant strain of Trichoderma reesei. J. Microbiol. Biotechnol. 2018, 28, 1473–1481. [Google Scholar] [CrossRef]
- Qi, F.; Zhao, X.; Kitahara, Y.; Li, T.; Ou, X.; Du, W.; Liu, D.; Huang, J. Integrative transcriptomic and proteomic analysis of the mutant lignocellulosic hydrolyzate-tolerant Rhodosporidium toruloides. Eng. Life Sci. 2017, 17, 249–261. [Google Scholar] [CrossRef]
- Luo, Z.; Zeng, W.; Du, G.; Liu, S.; Fang, F.; Zhou, J.; Chen, J. A high-throughput screening procedure for enhancing pyruvate production in Candida glabrata by random mutagenesis. Bioprocess Biosyst. Eng. 2017, 40, 693–701. [Google Scholar] [CrossRef]
- Zhu, X.; Arman, B.; Chu, J.; Wang, Y.; Zhuang, Y. Development of a method for efficient cost-effective screening of Aspergillus niger mutants having increased production of glucoamylase. Biotechnol. Lett. 2017, 39, 739–744. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Yang, Q.; Wang, P. Enhancing carotenoid production in Rhodotorula mucilaginosa KC8 by combining mutation and metabolic engineering. Ann. Microbiol. 2017, 67, 425–431. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Xu, J.; Xia, J.; Dai, B.; Xu, X.; Xu, J. Erythritol production by Yarrowia lipolytica mutant strain M53 generated through atmospheric and room temperature plasma mutagenesis. Food Sci. Biotechnol. 2017, 26, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.-F.; Li, H.-P.; Wang, L.-Y.; Zhang, C.; Xing, X.-H.; Bao, C.-Y. Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl. Microbiol. Biotechnol. 2014, 98, 5387–5396. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jia, H.; Zhang, L.; Wang, H.; Tang, H.; Zhang, L. Effects of GSH1 and GSH2 gene mutation on glutathione synthetases activity of Saccharomyces cerevisiae. Protein J. 2017, 36, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Farasat, M.; Arjmand, S.; Ranaei Siadat, S.O.; Sefidbakht, Y.; Ghomi, H. The effect of non-thermal atmospheric plasma on the production and activity of recombinant phytase enzyme. Sci. Rep. 2018, 8, 16647. [Google Scholar] [CrossRef]
- Veerana, M.; Lim, J.-S.; Choi, E.-H.; Park, G. Aspergillus oryzae spore germination is enhanced by non-thermal atmospheric pressure plasma. Sci. Rep. 2019, 9, 11184. [Google Scholar] [CrossRef]
- Veerana, M.; Choi, E.H.; Park, G. Influence of non-thermal atmospheric pressure plasma jet on extracellular activity of α-amylase in Aspergillus oryzae. Appl. Sci. 2021, 11, 691. [Google Scholar] [CrossRef]

| NTP Type | Process Gas | Time of Treatment | Fungus/Yeast | Effect | Ref. |
|---|---|---|---|---|---|
| RF plasma jet | A mixture of argon and oxygen | 1–10 min | Aspergillus flavus | 100% inhibition of growth after 10 min treatment | [69] |
| Plasma jet | Argon | 0–10 min | Candida parapsilosis Magnusiomyces magnusii Saccharomyces cerevisiae Schizosaccharomyces pombe | More than 90% inactivation of yeast cells after 10 min | [70] |
| Plasma microjet | A mixture of helium and oxygen | 0–5 min | Saccharomyces cerevisiae | The survival ratio of cells in water was significantly decreased from 40.2% to 1.5% after 5 min | [71] |
| PAW with the plasma jet | Air | 1–6 min water activation by plasma | Aspergillus brasiliensis | The spore viability dropped to 15% after 30 min in the PAW with a plasma activation time of 3 min | [72] |
| Linear micro discharge plasma jet | Helium | 1 min | Candida albicans | Changes in both the genotype and phenotype | [73] |
| DBSD plasma | Air | 0–480 s | Aspergillus flavus | A 5 log reduction of spore viability after 480 s under both the low and high power | [56] |
| Surface micro-discharge plasma | Helium | 0–10 min | Saccharomyces cerevisiae | The reduction in CFU was about 3.4 log after plasma treatment for 10 min | [74] |
| DBD plasma | Argon | 0–60 min | Aureobasidium pullulans | The non-melanized cells were efficiently inactivated, and more than 60% of melanized cells were still alive after the 60 min exposure | [75] |
| PAW with the CD plasma jet | Air or 99,99% oxygen | 0–30 min | Colletotrichum gloeosporioides | 96% inactivation after 30 min incubation in air-PAW; 55% inactivation after 30 min incubation in oxygen-PAW | [76] |
| Electric shock-free plasma jet | Air | 0–6 min | Cordyceps pruinosa | ~100% inactivation of spore viability after 6 min | [77] |
| CD plasma, DBD plasma | Air | 0–30 min | Alternaria sp. Aspergillus oryzae Byssochlamys nivea Cladosporium sphaerospermum | Spore inhibition after 10–40 min | [78] |
| Plasma jet | Helium | 0–180 s | Candida albicans | 20–30 mm2 inhibition zone area after 180 s | [79] |
| Plasma jet | Argon | 0–180 s | Neurospora crassa | Only ~5% spore viability after 3 min in water | [80] |
| NTP Type | Process Gas | Time of Treatment | Treated Sample | Fungus/Yeast | Effect | Ref. |
|---|---|---|---|---|---|---|
| DBD plasma | Air | 0–9 min | Mango | Colletotrichum asianum | The disease incidence and lesion diameter of mango treated for 9 min were decreased by 48.00% and 62.95%, respectively | [123] |
| Plasma jet | Argon, oxygen, nitrogen | 0–6 min | Mung bean | Natural fungal contamination | Reduction in natural fungal contamination ranging from 0.54 to 7.09 log at 96 h incubation | [124] |
| Gliding arc plasma | Nitrogen | 300–600 s | Tomato juice | Candida albicans Saccharomyces cerevisiae | 600 s treatment—reduction in fungal viability below the limit of quantification; extension of shelf life to 10 days | [113] |
| PAW | Air | 30–120 s | Kimchi cabbage | Natural fungal contamination | PAW treated with plasma for 120s caused a 1.8 log CFU/g reduction in fungal contamination | [125] |
| RF cold plasma | Oxygen | 0–15 min | Saffron | Aspergillus sp. Penicillium sp. Rhizopus sp. | Complete reduction in contamination after 15 min of treatment | [126] |
| Microwave plasma | Helium | 40 min | Onion powder | Aspergillus brasiliensis | 1.6 log spores/cm2 reduction | [127] |
| Flexible thin-layer plasma | Air | 10 min | Beef jerky packaged | Aspergillus flavus | 2–3 log CFU/g reduction in spore viability | [106] |
| DBD plasma | Air | 0–5 min | Citrus | Penicillium venetum Aspergillus brasiliensis | Significantly decreased to ~1.50 log CFU/mL at 2 min; significantly decreased viable count to ~1.62 log CFU/mL at 5 min | [128] |
| DBSD plasma | Air | 0–20 min | Blueberry | Botrytis cinerea | 15 and 20 min plasma treatment completely inhibited the mycelial growth | [115] |
| Microwave plasma | Air | 15–60 s | Allspice berry Black pepper Juniper berry | Aspergillus niger | Partial inactivation after 15 s treatment | [129] |
| Gliding arc plasma | Humid argon | 0–7 min | Mango | Colletotrichum gloeosporioides | Significantly lower mycelium growth rate constant, the maximum reduction in spores was 1 log spore/mL after 7 min of NTP treatment with 5 L/min gas flux | [130] |
| PAW with the plasma jet | Air | 0–30 min | Mung bean sprout | Natural yeast contamination | ~2.8 log CFU/g yeasts reduction after 30 min | [118] |
| Plasma jet | Air | 0–90 s | Paprika | Fusarium oxysporum | Complete inhibition of mycelial growth and spore germination after 90 s of treatment but only 50% inhibition of fungal growth on the paprika surface | [117] |
| AP plasma jet; LP RF plasma | Air, nitrogen, Oxygen | 0–30 min | Hazelnut | Aspergillus flavus Aspergillus parasiticus | Spore reductions of 4.7 and 5.6 log CFU/g after 30 min of LP air plasma treatment; spore reductions of 5.4 and 5.5 log CFU/g after 1.7 min of AP air plasma treatment; deformation of spores and loss of spore integrity after plasma treatments | [131] |
| DBD plasma | Air | 0–10 min | Blueberry | natural fungal contamination | The number of fungi decreased by 25.8%; the blueberry decay rates were reduced by 5.2% in the plasma treatment of 10 min after 20 days of storage | [132] |
| CD plasma jet | Air | 0–120 s | Kumquat | natural yeasts contamination | 0.77–1.57 log CFU/g reduction after 120 s treatment | [116] |
| Fluidized bed plasma | Air, nitrogen | 0–5 min | Hazelnuts | Aspergillus flavus Aspergillus parasiticus | ~4 log fungicidal effects after 5 min; the air plasma was more effective than nitrogen plasma | [133] |
| Surface barrier discharge | Air | 0–8 min | Corn kernels | - | Complete degradation of aflatoxin B1 after 6 min of treatment | [134] |
| DBD plasma | Air | 0–180 s | Pistachio nuts, glass slides | Aspergillus flavus | Decrease in spore population by 4 log after 180 s of the treatment; maximum reduction in AFB1 was observed after 180 s of the treatment, which was 64.63% for glass slides and 52.42% for pistachio nuts | [135] |
| CD plasma jet | Air | 0–30 min | Rice, Wheat | - | Initial AFB1 concentration on slides was decreased maximally by 95% in 30 min; in rice and wheat, the average levels of AFB1 degradation ranged between 45 and 56% following 30 min treatment | [136] |
| AP plasma jet; LP RF plasma | Air | 0–30 min | Hazelnuts | - | Both plasmas reduced 72–73% of AFB1 spiked on hazelnuts after plasma treatment | [137] |
| RF plasma | air with H2O2 (35%) | 0–10 min | Cannabis inflorescences | Botrytis cinerea | 5 log reduction in viable fungal spores after 10 min | [138] |
| DBD plasma | Argon or a mixture of 80% argon and 20% oxygen | 10 min | Ginseng seeds | natural fungal contamination from the surface of seeds | ~73% (Ar) and 60% (Ar/O2) inactivation of fungal spores | [139] |
| DBSD plasma | Air | 0–60 s | Scot pine seeds | Fusarium oxysporum | 100% disinfection efficiency of seeds after 30 s treatment | [140] |
| DCSBD plasma | Air | 0–10 min | Lentil seeds | Aspergillus niger Penicillium decumbens | Maximum logarithmic reduction of 1.6 log CFU/g for A. niger and 3.1 log CFU/g for P. decumbens after 10 min | [141] |
| DBD plasma | Nitrogen, Oxygen | 1–3 min | Soybean seeds | Diaporthe/Phomopsis complex | Reduction in infection by about 49–81% | [142] |
| DBD plasma | Air | 5, 20 min | Barley and wheat seeds | Penicillium verrucosum | Maximal reduction of 2.1 log CFU/g for barley and 2.5 log CFU/g for wheat | [67] |
| DCSBD plasma | Air | 0–50 s | Cucumber and pepper seeds | Cladosporium cucumerinum Didymella bryoniae Didymella licopersici | Total reduction in C. cucumerinum and 60–80% reduction in D. bryoniae on cucumber seeds after 20 s; 50–80% reduction in D. licopersici on pepper seeds | [143] |
| DCSBD plasma | Air | 0–300 s | Maize | Alternaria alternata Aspergillus flavus Fusarium culmorum | Reduction of 3.79 log CFU/g in F. culmorum after 60 s plasma treatment, 4.21 log CFU/g in A. flavus, and 3.22 log CFU/g in A. alternata after a 300 s plasma treatment | [66] |
| DBSD plasma | Air | 0–300 s | Sweet basil seeds | natural fungal contamination | ~30% reduction of natural fungal contamination after 300 s | [144] |
| Plasma jet | Humid air | 10 min | Rice seeds | Fusarium fujikuroi | Bakanae disease severity index and the percentage of plants with symptoms were reduced to 18.1% and 7.8% of nonirradiated control, respectively, after 10 min treatment of seeds in water | [145] |
| RF plane-type plasma | Air | 0–30 min | Groundnuts | Aspergillus flavus Aspergillus parasiticus | High percentage of inactivation, 99.9% and 99.5% of A. parasiticus and A. flavus, respectively | [146] |
| CD plasma jet | Air | 0–3 min | Broccoli seeds | natural fungal contamination | 1.5 log CFU/g reduction in natural fungal contamination after 3 min | [147] |
| NTP Type | Process Gas | Time of Treatment | Fungus/Yeast | Mutant | Ref. |
|---|---|---|---|---|---|
| ARTP | Helium | 0–180 s | Fusidium coccineum | ~60%increase in fusidic acid production | [163] |
| ARTP | Helium | 0–350 s | Aspergillus nidulans | 1.3× higher production of echinocandin B | [164] |
| ARTP | Helium | - | Sanghuangporous sanghuang | 1.2–1.5 fold increase in polysaccharides production | [165] |
| ARTP + etylmethanesulfonate | Helium | 0–550 s | Penicillium oxalicum | Enhanced raw starch-degrading enzyme production | [166] |
| ARTP | Helium | 30–240 s | Aspergillus oryzae | 54.7% increase in acid protease activity, 17.3% increase in neutral protease activity, 8.5% increase in total protease activity, 8.1% decrease in alkaline protease activity | [167] |
| ARTP | Helium | 0–360 s | Starmerella bombicola | 30% increase in lactonic, acidic, or total sophorolipid production | [168] |
| ARTP | Helium | 0–200 s | Candida parapsilosis | ~60% increase in D-arabitol production | [169] |
| ARTP | Helium | 0–150 s | Candida tropicalis | 22% increase in xylitol production | [170] |
| ARTP | Helium | 100–200 s | Aspergillus oryzae | ~292% increase in kojic acid production | [171] |
| ARTP | Helium | 30 s | Hericium erinaceum | 22% increase in yield of fruiting body, 16% increase in polysaccharide production | [172] |
| DBD plasma | Argon helium | 3–5 min | Ganoderma lingzhi | 25.6% increase in polysaccharides production | [173] |
| ARTP | Helium | Trichoderma reesei | Increase in cellulase production | [174] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoppanová, L.; Kryštofová, S. Nonthermal Plasma Effects on Fungi: Applications, Fungal Responses, and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 11592. https://doi.org/10.3390/ijms231911592
Hoppanová L, Kryštofová S. Nonthermal Plasma Effects on Fungi: Applications, Fungal Responses, and Future Perspectives. International Journal of Molecular Sciences. 2022; 23(19):11592. https://doi.org/10.3390/ijms231911592
Chicago/Turabian StyleHoppanová, Lucia, and Svetlana Kryštofová. 2022. "Nonthermal Plasma Effects on Fungi: Applications, Fungal Responses, and Future Perspectives" International Journal of Molecular Sciences 23, no. 19: 11592. https://doi.org/10.3390/ijms231911592
APA StyleHoppanová, L., & Kryštofová, S. (2022). Nonthermal Plasma Effects on Fungi: Applications, Fungal Responses, and Future Perspectives. International Journal of Molecular Sciences, 23(19), 11592. https://doi.org/10.3390/ijms231911592






