Recent Advances in Prion Inactivation by Plasma Sterilizer
Abstract
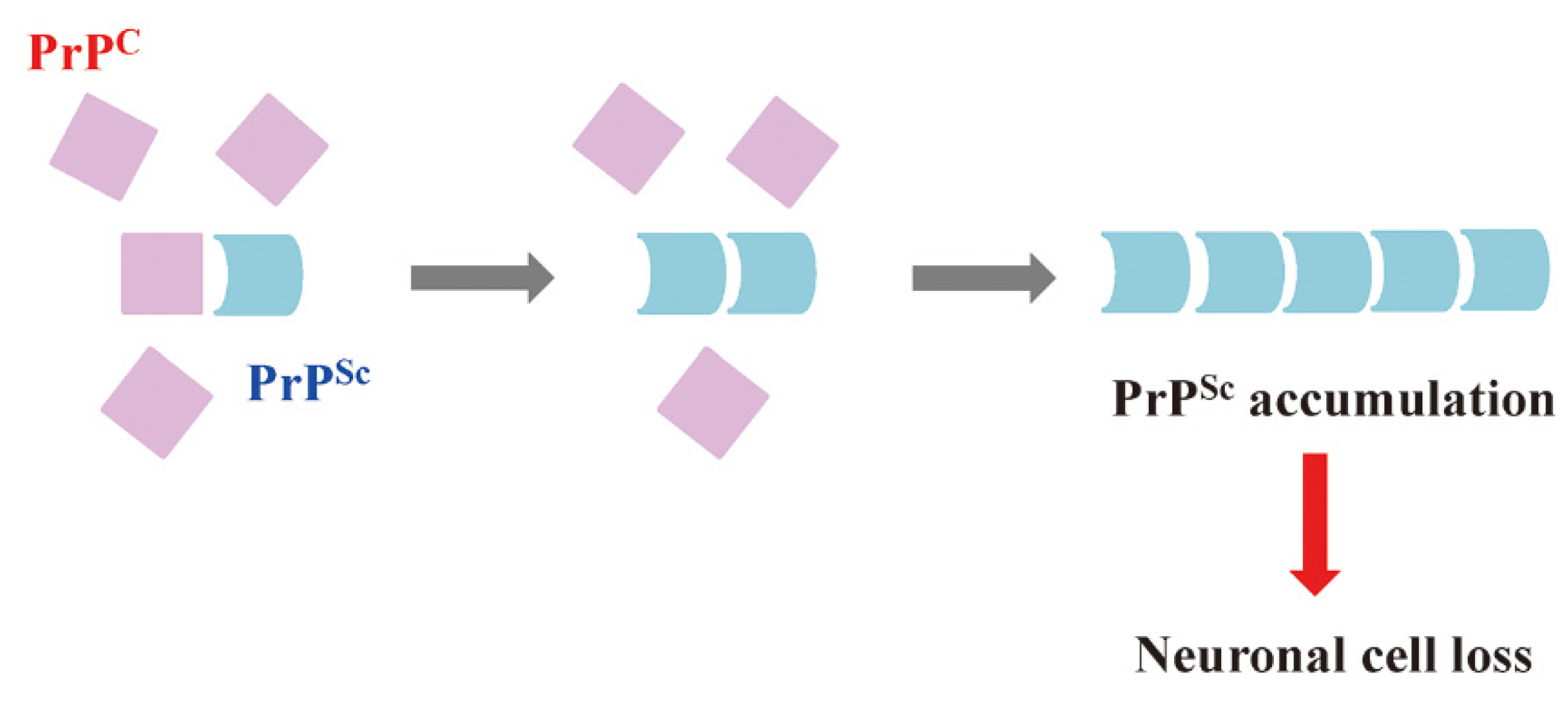
1. Prions and Hierarchy of Resistance
2. Conventional Methods for Prion Inactivation
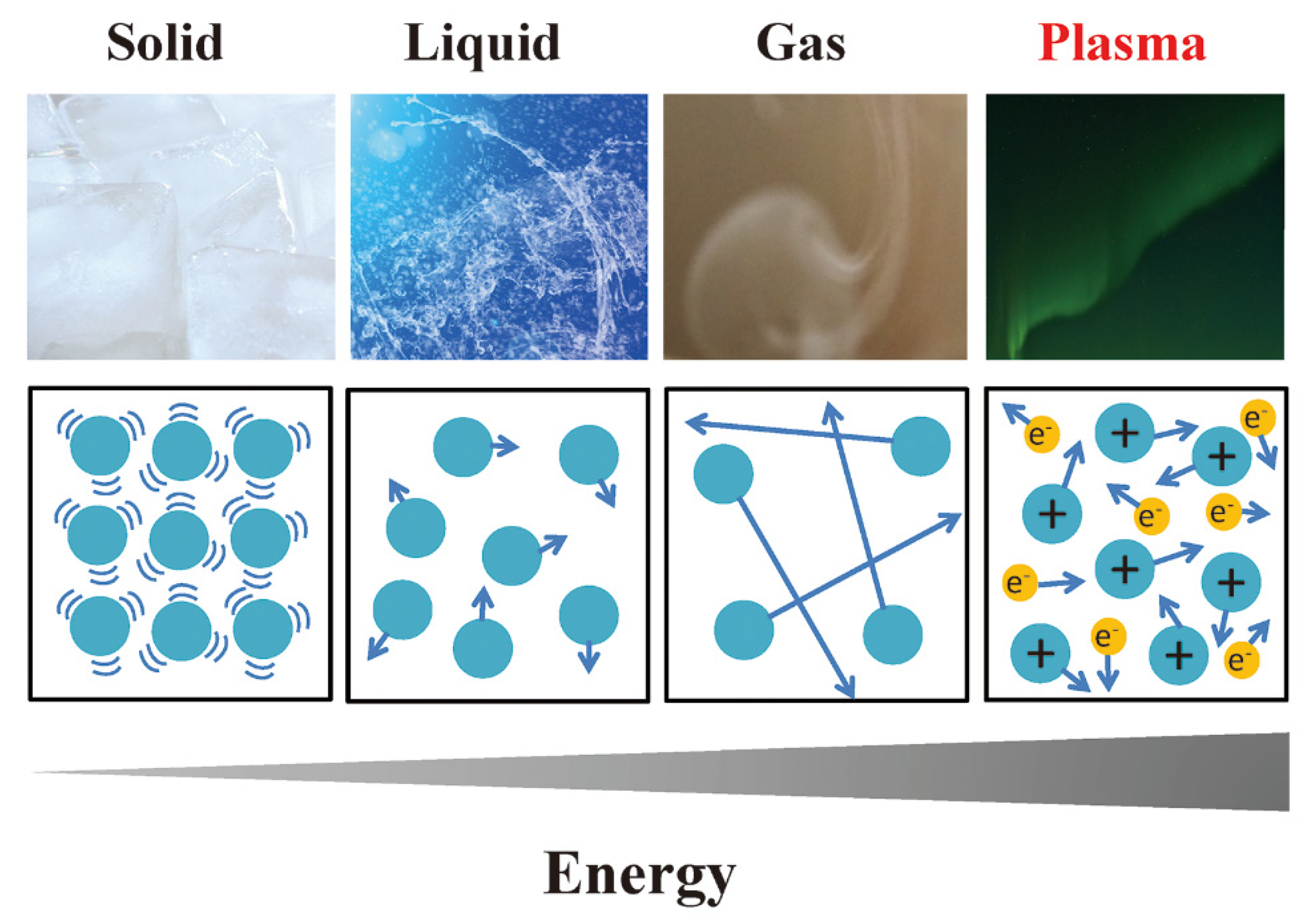
3. Fundamentals of Plasma
4. Sterilization/Disinfection/Inactivation by Plasma
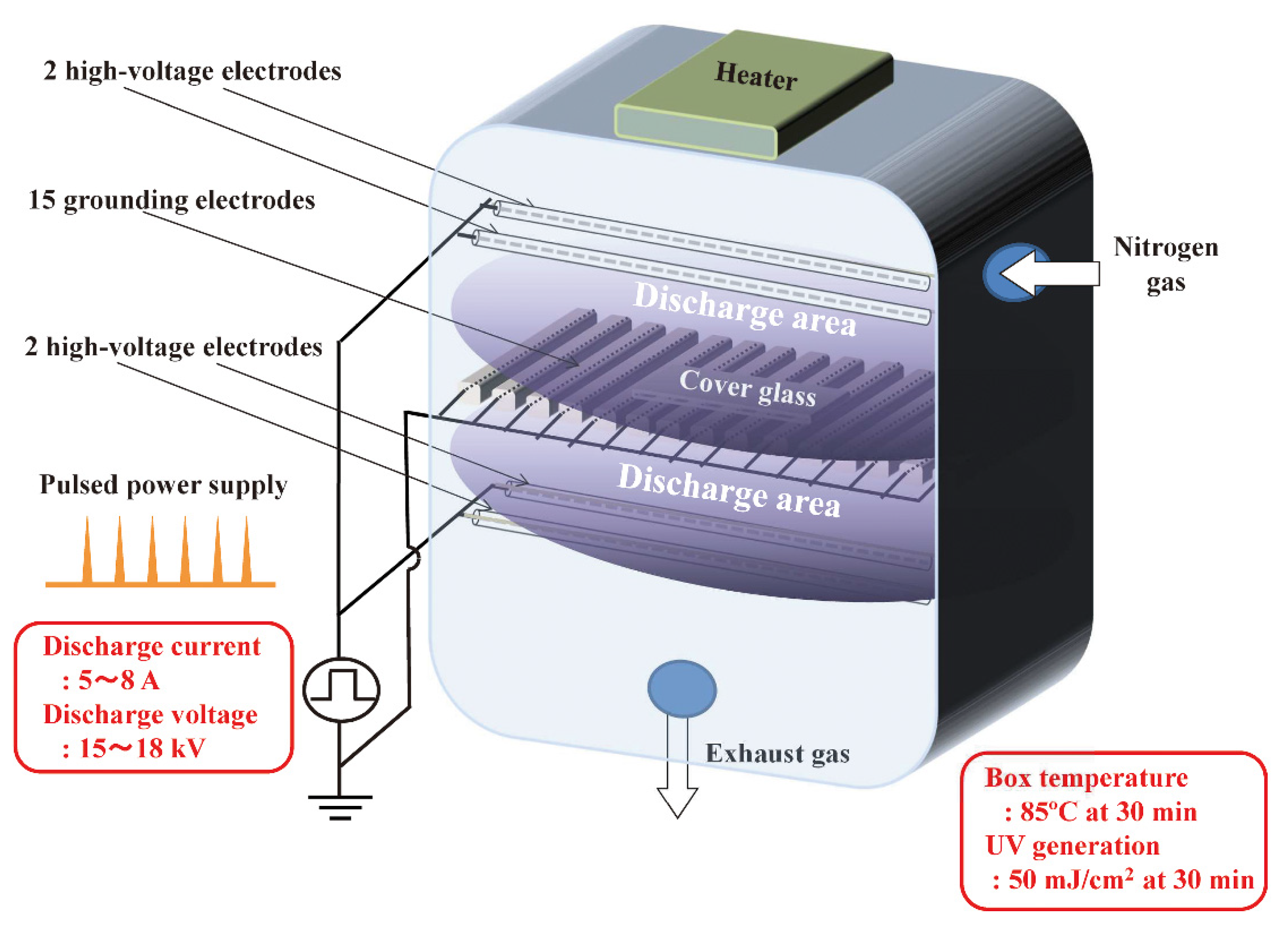
5. Prion Inactivation Using Hydrogen Peroxide Gas Plasma
| Prions | Instruments | Plasma Types (Total Process Time for the Treatment) | Sample Types | Main Results of Inactivation | Reference |
|---|---|---|---|---|---|
| Scrapie (263 K) | Hydrogen peroxide gas plasma (STERRAD®) | RF plasma (for degradation of residual hydrogen peroxide) | Prion-contaminated steel wire | KOH-based detergent—70 °C for 10 min + STERRAD® 100S GMP (59% hydrogen peroxide gas, 50 °C, 16 min, two cycles). Survival rates of animals were 100% (hamster), while those of untreated controls was 0% (incubation time: >397 days vs. control 81 days) | [62] |
| Scrapie (263 K) | Hydrogen peroxide gas plasma (STERRAD®) | RF plasma (for degradation of residual hydrogen peroxide) | Prion-contaminated steel wire | STERRAD® NX (90% hydrogen peroxide gas, 53 °C, 7 min). More than 5 log reduction of prion infectivity. Survival rates of animals were 100% (incubation time: 528 days vs. control 85 days) | [63] |
| Scrapie (263 K) | Hydrogen peroxide gas plasma (STERRAD®) | RF plasma (for degradation of residual hydrogen peroxide) | Prion-contaminated steel wire | STERRAD® NX (90% hydrogen peroxide gas, 53 °C, 7 min). More than 5 log reduction of prion infectivity. Survival rates of animals were 100% (incubation time: 570 days vs. control 78 days) | [64] |
| Scrapie (Chandler) | Hydrogen peroxide gas plasma (RENO-S130) | DBD plasma (for sterilization) and corona plasma (for degradation of residual hydrogen peroxide) | Prion-contaminated cover glass | RENO-S130 (50% hydrogen peroxide, below 60 °C; non-lumen mode: 28 min; Eco mode: 45 min). Survival rates of animals were 83% (both non-lumen and Eco mode) after injection with plasma-treated prions. Survival of untreated controls was 0% | [65] |
6. Inactivation of Prions Using Plasma Derived from Air, Nitrogen, Oxygen, Ar, and Their Mixtures
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonnell, G.E. Antisepsis, Disinfection, and Sterilization; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, J.D.F.; Collinge, J. Molecular pathology of human prion disease. Acta Neuropathol. 2011, 121, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Mead, S. Prion disease genetics. Eur. J. Hum. Genet. 2006, 14, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Sanjo, N. Review of basic knowledge, surveillance and infectious control of prion disease. Rinsho Shinkeigaku 2013, 53, 1243–1245. [Google Scholar] [CrossRef][Green Version]
- Mackenzie, G.; Will, R. Creutzfeldt-Jakob disease: Recent developments. F1000Research 2017, 6, 2053. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Manjila, S.; Mehndiratta, P.; Khan, F.; Miller, B.R.; Onwuzulike, K.; Puoti, G.; Cohen, M.L.; Schonberger, L.B.; Cali, I. Human prion diseases: Surgical lessons learned from iatrogenic prion transmission. Neurosurg. Focus 2016, 41, E10. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D.L.; Peden, A.H.; Barria, M.A. Variant CJD: Reflections a quarter of a century on. Pathogens 2021, 10, 1413. [Google Scholar] [CrossRef]
- Geschwing, M.D. Prion diseases. Continuum (Minneap. Minn.) 2016, 21, 1612–1638. [Google Scholar]
- Sakudo, A. Inactivation Methods for Prions. Curr. Issues Mol. Biol. 2020, 36, 23–32. [Google Scholar] [CrossRef]
- Department of Health and Social Care (DHSC) UK Government. Guidance: Minimise transmission risk of CJD and vCJD in healthcare settings, Prevention of CJD and vCJD by the Advisory Committee on Dangerous Pathogens’ Transmissible Spongiform Encephalopathy (ACDP TSE) Subgroup. Available online: https://www.gov.uk/government/publications/guidance-from-the-acdp-tse-risk-management-subgroup-formerly-tse-working-group (accessed on 23 August 2022).
- World Health Organization (WHO). Tables on Tissue Infectivity Distribution in Transmissible Spongiform Encephalopathies; WHO: Geneva, Switzerland, 2010.
- World Health Organization (WHO). Infection Control Guidelines for Transmissible Spongiform Encephalopathies; WHO/Center for the Study of Religion: Geneva, Switzerland, 2000.
- Rutala, W.A.; Weber, D.J.; Society for Healthcare Epidemiology of America. Guideline for disinfection and sterilization of prion-contaminated medical instruments. Infect. Control Hosp. Epidemiol. 2010, 31, 107–117. [Google Scholar] [CrossRef]
- The Research Committee on Surveillance and Infection Control of Prion Diseases. Research on Measures for Intractable Dis-eases Health and Labour Sciences Research Grants, The Ministry of Health, Labour and Welfare, Japan, Japanese Society of Neurology. Guideline for Infection Control of Prion Diseases 2020. 2020. Available online: https://minds.jcqhc.or.jp/n/med/4/med0425/G0001176 (accessed on 12 July 2022).
- Vassey, M.; Budge, C.; Poolman, T.; Jones, P.; Perrett, D.; Nayuni, N.; Bennett, P.; Groves, P.; Smith, A.; Fulford, M.; et al. A quantitative assessment of residual protein levels on dental instruments reprocessed by manual, ultrasonic and automated cleaning methods. Br. Dent. J. 2011, 210, E14. [Google Scholar] [CrossRef]
- Hashoul, J.; Saliba, W.; Bloch, I.; Jabaly-Habib, H. Heidenhain variant of Creutzfeldt-Jakob disease in a patient who had bovine bioprosthetic valve implantation. Indian J. Ophthalmol. 2016, 64, 767–769. [Google Scholar] [CrossRef]
- Kampf, G.; Jung, M.; Suchomel, M.; Saliou, P.; Griffiths, H.; Vos, M.C. Prion disease and recommended procedure for flexible en-doscope processing—A review of policies worldwide and proposal for a simplified approach. J. Hosp. Infect. 2020, 104, 92–110. [Google Scholar] [CrossRef]
- McDonnell, G.; Dehen, C.; Perrin, A.; Thomas, V.; Igel-Egalon, A.; Burke, P.A.; Deslys, J.P.; Comoy, E. Cleaning, disinfection and sterilization of surface prion contamination. J. Hosp. Infect. 2013, 85, 268–273. [Google Scholar] [CrossRef]
- Liste des Produits Inactivants et Format de Dossier Pour la Revendication de Performances D’inactivation (Mis à Jour le 01/06/2021), Liste Des Produits Conformes au PSP v2018. 2021. Available online: https://ansm.sante.fr/vos-demarches/industriel/liste-des-produits-inactivants-et-format-de-dossier-pour-la-revendication-de-performances-dinactivation (accessed on 12 July 2022).
- ISO 17664-1:2021; Processing of Health Care Products—Information to Be Provided by the Medical Device Manufacturer for the Processing of Medical Devices—Part 1: Critical and Semi-Critical Medical Devices. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/81720.html (accessed on 12 July 2022).
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization using plasma technology: Fundamentals and Future Perspec-tives for Biological Applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef]
- Sakudo, A.; Shintani, H.; Medical, N. Sterilization and Disinfection by Plasma: Sterilization Mechanisms, Biological and Medical Applications (Medical Devices and Equipment); Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Krebs, M.C.; Bécasse, P.; Verjat, D.; Darbord, J.C. Gas-plasma sterilization: Relative efficacy of the hydrogen peroxide phase compared with that of the plasma phase. Int. J. Pharm. 1998, 160, 75–81. [Google Scholar] [CrossRef]
- Shintani, H. Is SteradR from J&J is Truly Plasma Gas Sterilizer? Pharmaceut. Reg. Aff. 2013, 3, e124. [Google Scholar]
- Eveland, R.W. Disinfection and sterilization with hydrogen peroxide. In Block’s Disinfection, Sterilization, and Preservation, 6th ed.; McDonnell, G., Hansen, J., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2020; pp. 671–683. [Google Scholar]
- Sakudo, A.; Imanishi, Y.; Hirata, A.; Koga, Y.; Shintani, H. Effect of nitrogen gas plasma generated by a fast-pulsed power supply using a static induction thyristor on scrapie prion. Pathogens 2020, 9, 819. [Google Scholar] [CrossRef]
- Julák, J.; Janoušková, O.; Scholtz, V.; Holada, K. Inactivation of prions using electrical DC discharges at atmospheric pressure and ambient temperature. Plasma Process Polym. 2011, 8, 316–323. [Google Scholar] [CrossRef]
- Baxter, H.C.; Campbell, G.A.; Whittaker, A.G.; Jones, A.C.; Aitken, A.; Simpson, A.H.; Casey, M.; Bountiff, L.; Gibbard, L.; Baxter, R.L. Elimination of transmissible spongiform encephalopathy infectivity and decontamination of surgical instruments by using radio-frequency gas-plasma treatment. J. Gen. Virol. 2005, 86, 2393–2399. [Google Scholar] [CrossRef]
- von Keudell, A.; Awakowicz, P.; Benedikt, J.; Raballand, V.; Yanguas-Gil, A.; Opretzka, J.; Flötgen, C.; Reuter, R.; Byelykh, L.; Halfmann, H.; et al. Inactivation of bacteria and biomolecules by low-pressure plasma discharges. Plasma Process Polym. 2010, 7, 327–352. [Google Scholar] [CrossRef]
- Langmuir, I. Oscillations in ionized gases. Proc. Natl. Acad. Sci. USA 1928, 14, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Goldston, R.J.; Rutherford, P.H. Introduction to Plasma Physics; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Shintani, H.; Sakudo, A. Gas Plasma Sterilization in Microbiology: Theory, Applications, Pitfalls and New Perspectives; Caister Academic Press: London, UK, 2016. [Google Scholar]
- Alfven, H.O.G. Cosmology in the plasma universe: An introductory exposition. IEEE Trans. Plasma Sci. 1990, 18, 5–10. [Google Scholar] [CrossRef]
- Sandholt, P.E.; Deehr, C.S.; Egeland, A.; Lybekk, B.; Viereck, R.; Romick, G.J. Signatures in the dayside aurora of plasma transfer from the magnetosheath. J. Geophys. Res. 1986, 91, 10063–10079. [Google Scholar] [CrossRef]
- Waymouth, J.F.; Bitter, F. Analysis of the plasma of fluorescent lamps. J. Appl. Phys. 1956, 27, 122–131. [Google Scholar] [CrossRef]
- Trunec, D.; Brablec, A.; Buchta, J. Atmospheric pressure glow discharge in neon. J. Phys. D Appl. Phys. 2001, 34, 1697–1699. [Google Scholar] [CrossRef]
- Ichimaru, S. Nuclear fusion in dense plasmas. Rev. Mod. Phys. 1993, 65, 255–299. [Google Scholar] [CrossRef]
- Coburn, J.W.; Winters, H.F. Plasma etching—A discussion of mechanisms. J. Vac. Sci. Technol. 1979, 16, 391–403. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold atmospheric plasma: A powerful tool for modern medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Strnádel, J.; Matáková, T.; Halašová, E.; Škovierová, H. Effect of cold atmospheric plasma on epigenetic changes, DNA damage, and possibilities for its use in synergistic cancer therapy. Int. J. Mol. Sci. 2021, 22, 12252. [Google Scholar] [CrossRef]
- Itarashiki, T.; Ohshiro, S.; Sakudo, A.; Hayashi, N. Current trend of medical sterilization and disinfection methods using plas-mas. J. Plasma Fusion Res. 2015, 91, 505–513. [Google Scholar]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Roth, S.; Feichtinger, J.; Hertel, C. Characterization of Bacillus subtilis spore inactivation in low-pressure, low-temperature gas plasma sterilization processes. J. Appl. Microbiol. 2010, 108, 521–531. [Google Scholar] [CrossRef]
- Sakudo, A.; Misawa, T.; Shimizu, N.; Imanishi, Y. N2 gas plasma inactivates influenza virus mediated by oxidative stress. Front. Biosci. 2014, 6, 69–79. [Google Scholar] [CrossRef]
- Shintani, H.; Shimizu, N.; Imanishi, Y.; Sekiya, T.; Tamazawa, K.; Taniguchi, A.; Kido, N. Inactivation of microorganisms and en-dotoxins by low temperature nitrogen gas plasma exposure. Biocontrol Sci. 2007, 12, 131–143. [Google Scholar] [CrossRef][Green Version]
- Sakudo, A.; Toyokawa, Y.; Nakamura, T.; Yagyu, Y.; Imanishi, Y. Nitrogen gas plasma treatment of bacterial spores induces oxidative stress that damages the genomic DNA. Mol. Med. Rep. 2017, 15, 396–402. [Google Scholar] [CrossRef]
- Sakudo, A.; Toyokawa, Y.; Imanishi, Y.; Murakami, T. Crucial roles of reactive chemical species in modification of respiratory syncytial virus by nitrogen gas plasma. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 131–136. [Google Scholar] [CrossRef]
- Sakudo, A.; Toyokawa, Y.; Imanishi, Y. Nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply inactivates adenovirus. PLoS ONE 2016, 11, e0157922. [Google Scholar] [CrossRef]
- Sakudo, A.; Shimizu, N.; Imanishi, Y.; Ikuta, K. N2 gas plasma inactivates influenza virus by inducing changes in viral surface morphology, protein, and genomic RNA. BioMed Res. Int. 2013, 2013, 694269. [Google Scholar] [CrossRef]
- Yamashiro, R.; Misawa, T.; Sakudo, A. Key role of singlet oxygen and peroxynitrite in viral RNA damage during virucidal effect of plasma torch on feline calicivirus. Sci Rep. 2018, 8, 17947. [Google Scholar] [CrossRef] [PubMed]
- Shintani, H.; Sakudo, A.; Burke, P.; McDonnell, G. Gas plasma sterilization of microorganisms and mechanisms of action. Exp. Ther. Med. 2010, 1, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Sakudo, A.; Shintani, H.; Yagyu, Y. Plasma sterilization. In Block’s Disinfection, Sterilization, and Preservation, 6th ed.; McDonnell, G., Hansen, J., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2021; pp. 702–725. [Google Scholar]
- Jacobs, P.T.; Lin, S.M. Hydrogen Peroxide Plasma Sterilization System. U.S. Patent 4,756,882, 27 January 1987. [Google Scholar]
- Advanced Sterilization Products (APS). Terminal Sterilization. Available online: https://www.asp.com/en-us/products/terminal-sterilization (accessed on 1 September 2022).
- Moulton, K.A.; Campbell, B.A.; Caputo, R.A. Plasma Sterilizing Process with Pulsed Antimicrobial Agent. U.S. Patent 5,084,239, 31 August 1990. [Google Scholar]
- Finnegan, M.; Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Mode of action of hydrogen peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010, 65, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Matthees, S.; UBM Americas; UBM plc Company. Lessons Learned from the AbTox Ruling. Available online: https://www.mddionline.com/lessons-learned-abtox-ruling (accessed on 12 July 2022).
- Furuhata, S.; Nishimura, C.; Furuhashi, N.; Miyakawa, J.; Ozawa, M.; Usami, K.; Misawa, A.; Karasawa, H. Effectiveness test of low temperature plasma sterilization method using peracetic acid and hydrogen peroxide. Jpn. J. Assoc. Op. Med. 2000, 21, 140–144. (In Japanese) [Google Scholar]
- Yan, Z.X.; Stitz, L.; Heeg, P.; Pfaff, E.; Roth, K. Infectivity of prion protein bound to stainless steel wires: A model for testing de-contamination procedures for transmissible spongiform encephalopathies. Infect. Control Hosp. Epidemiol. 2004, 25, 280–283. [Google Scholar] [CrossRef]
- Yan, Z.X.; Stitz, L.; Heeg, P.; Roth, K.; Mauz, P.-S. Low-temperature inactivation of prion-protein on surgical steel surfaces with hydrogen peroxide gas plasma sterilization. Zentr. Steril. 2008, 16, 26–34. [Google Scholar]
- Rogez-Kreuz, C.; Yousfi, R.; Soufflet, C.; Quadrio, I.; Yan, Z.X.; Huyot, V.; Aubenque, C.; Destrez, P.; Roth, K.; Roberts, C.; et al. In-activation of animal and human prions by hydrogen peroxide gas plasma sterilization. Infect. Control Hosp. Epidemiol. 2009, 30, 769–777. [Google Scholar] [CrossRef]
- Sakudo, A.; Tsuji, Y. Hydrogen peroxide gas plasma sterilizer combined with dielectric barrier discharge and corona discharge inactivates prions. Appl. Sci. 2021, 11, 9777. [Google Scholar] [CrossRef]
- Reno, S. RENO-S130. Available online: https://www.media-inc.co.jp/icbu/product/reno/products_s130.html (accessed on 11 July 2022).
- Hara, H.; Chida, J.; Pasiana, A.D.; Uchiyama, K.; Kikuchi, Y.; Naito, T.; Takahashi, Y.; Yamamura, J.; Kuromatsu, H.; Sakaguchi, S. Vaporized hydrogen peroxide and ozone gas synergistically reduce prion infectivity on stainless steel wire. Int. J. Mol. Sci. 2021, 22, 3268. [Google Scholar] [CrossRef]
- Sakudo, A.; Higa, M.; Maeda, K.; Shimizu, N.; Imanishi, Y.; Shintani, H. Sterilization mechanism of nitrogen gas plasma: Induc-tion of secondary structural change in protein. Microbiol. Immunol. 2013, 57, 536–542. [Google Scholar]
- Shintani, H. Inactivation of bacterial spore, endotoxin, lipid A, normal prion and abnormal prion by exposures to several sorts of gases plasma. Biocontrol Sci. 2016, 21, 1–12. [Google Scholar] [CrossRef]
- Zobeley, E.; Flechsig, E.; Cozzio, A.; Enari, M.; Weissmann, C. Infectivity of scrapie prions bound to a stainless steel surface. Mol. Med. 1999, 5, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Flechsig, E.; Hegyi, I.; Enari, M.; Schwarz, P.; Collinge, J.; Weissmann, C. Transmission of scrapie by steel-surface-bound prions. Mol. Med. 2001, 7, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Merritt, K.; Hitchins, V.M.; Brown, S.A. Safety and cleaning of medical materials and devices. J. Biomed. Mater. Res. 2000, 53, 131–136. [Google Scholar] [CrossRef]
- Peretz, D.; Supattapone, S.; Giles, K.; Vergara, J.; Freyman, Y.; Lessard, P.; Safar, J.G.; Glidden, D.V.; McCulloch, C.; Nguyen, H.O.; et al. Inactivation of prions by acidic sodium dodecyl sulfate. J. Virol. 2006, 80, 322–331. [Google Scholar] [CrossRef]
- Giles, K.; Glidden, D.V.; Beckwith, R.; Seoanes, R.; Peretz, D.; DeArmond, S.J.; Prusiner, S.B. Resistance of bovine spongiform encephalopathy (BSE) prions to inactivation. PLOS Pathog. 2008, 4, e1000206. [Google Scholar] [CrossRef] [PubMed]
- Head, M.W.; Ironside, J.W. Review: Creutzfeldt-Jakob disease: Prion protein type, disease phenotype and agent strain. Neuro-pathol. Appl. Neurobiol. 2012, 38, 296–310. [Google Scholar] [CrossRef]
- Parchi, P.; Saverioni, D. Molecular pathology, classification, and diagnosis of sporadic human prion disease variants. Folia Neuropathol. 2012, 50, 20–45. [Google Scholar]
- Parchi, P.; Cescatti, M.; Notari, S.; Schulz-Schaeffer, W.J.; Capellari, S.; Giese, A.; Zou, W.Q.; Kretzschmar, H.; Ghetti, B.; Brown, P. Agent strain variation in human prion disease: Insights from a molecular and pathological review of the National Institutes of Health series of experimentally transmitted disease. Brain 2010, 133, 3030–3042. [Google Scholar] [CrossRef]
- Bishop, M.T.; Will, R.G.; Manson, J.C. Defining sporadic Creutzfeldt-Jakob disease strains and their transmission properties. Proc. Natl. Acad. Sci. USA 2010, 107, 12005–12010. [Google Scholar] [CrossRef]
- Moda, F.; Suardi, S.; Di Fede, G.; Indaco, A.; Limido, L.; Vimercati, C.; Ruggerone, M.; Campagnani, I.; Langeveld, J.; Terruzzi, A.; et al. MM2-thalamic Creutzfeldt-Jakob disease: Neuropathological, biochemical and transmission studies identify a dis-tinctive prion strain. Brain Pathol. 2012, 22, 662–669. [Google Scholar] [CrossRef]
- Traba, C.; Chen, L.; Liang, J.F. Low power gas discharge plasma mediated inactivation and removal of biofilms formed on bi-omaterials. Curr. Appl. Phys. 2013, 13, S12–S18. [Google Scholar] [CrossRef][Green Version]
- Kylián, O.; Sasaki, T.; Rossi, F. Plasma sterilization of Geobacillus stearothermophilus by O2:N2 RF inductively coupled plasma. Eur. Phys. J. Appl. Phys. 2006, 34, 139–142. [Google Scholar] [CrossRef]
- Rossi, F.; Kylián, O.; Hasiwa, M. Decontamination of surfaces by low pressure plasma discharges. Plasma Process Polym. 2006, 3, 431–442. [Google Scholar] [CrossRef]
- Monari, L.; Chen, S.G.; Brown, P.; Parchi, P.; Petersen, R.B.; Mikol, J.; Gray, F.; Cortelli, P.; Montagna, P.; Ghetti, B. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: Different prion proteins determined by a DNA polymorphism. Proc. Natl. Acad. Sci. USA 1994, 91, 2839–2842. [Google Scholar] [CrossRef]
- Kocisko, D.A.; Come, J.H.; Priola, S.A.; Chesebro, B.; Raymond, G.J.; Lansbury, P.T.; Caughey, B. Cell-free formation of protease-resistant prion protein. Nature 1994, 370, 471–474. [Google Scholar] [CrossRef]
- Capellari, S.; Zaidi, S.I.A.; Urig, C.B.; Perry, G.; Smith, M.A.; Petersen, R.B. Prion protein glycosylation is sensitive to redox change. J. Biol. Chem. 1999, 274, 34846–34850. [Google Scholar] [CrossRef]
- Prusiner, S.B. Cell biology. A unifying role for prions in neurodegenerative diseases. Science 2012, 336, 1511–1513. [Google Scholar] [CrossRef]
- Kovacs, G.G. Molecular pathology of neurodegenerative diseases: Principles and practice. J. Clin. Pathol. 2019, 72, 725–735. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Brandner, S. Invited Review: The role of prion-like mechanisms in neurodegenerative diseases. Neuropathol. Appl. Neurobiol. 2020, 46, 522–545. [Google Scholar] [CrossRef]
- Aguzzi, A.; Lakkaraju, A.K.K. Cell biology of prions and prionoids: A status report. Trends Cell Biol. 2016, 26, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Ayers, J.I.; Paras, N.A.; Prusiner, S.B. Expanding spectrum of prion diseases. Emerg. Top. Life Sci. 2020, 4, 155–167. [Google Scholar] [CrossRef] [PubMed]


| Treatment | N/N0 1 |
|---|---|
| Untreated | 6/6 |
| Non-lumen mode | 1/6 |
| Eco mode | 1/6 |
| Treatment | Incubation Times |
|---|---|
| Untreated | 191.0 ± 9.0 days |
| Non-lumen mode | >576 days (401 days) 1 |
| Eco mode | >576 days (181 days) 1 |
| Prions | Instruments | Plasma Types (Total Process Time for the Treatment) | Sample Types | Main Results of Inactivation | Reference |
|---|---|---|---|---|---|
| Scrapie (Chandler) | Nitrogen gas plasma (BLP-TES) | Plasma generated by applying a short high-voltage pulse for a short time period using an IES circuit with an SI thyristor to nitrogen (30 min) | Prion-contaminated cover glass | Mean incubation time of animals prolonged to 251 days (plasma-treated) from 218 days (untreated) | [27] |
| Scrapie (RML5) | Air plasma (derived from HT2103 source) | Corona plasma (20 min) | Drops of prion-infected brain homogenate in well of microplate | Density of prion-staining in cell infectivity assay was reduced to less than 1/8 (1% prion-infected brain homogenate) and 1/30 (0.1% prion-infected brain homogenate) compared to untreated counterparts. | [28] |
| Scrapie (263 K) | Ar/O2 plasma | RF plasma (60 min) | Prion-contaminated steel wire | Survival rates of animals were 100% (mouse) after injection with Ar/O2 plasma-treated prions (steel wire). Survival of untreated control was 0%. | [29] |
| Scrapie (263K) and mouse-adapted BSE (6PB1) | Ar, Ar/O2, Ar/N2, Ar/N2/O2 -plasma (BIODECON ICP reactor) | Microwave-derived ICP (10 min) | Prion- contaminated steel wire or silk suture | Survival rates of animals were 50% (hamster) and 100% (mouse) after injection with Ar/O2 plasma-treated prions (steel wire). Survival of untreated control was 0%. | [30] |
| Nitrogen Gas Plasma Treatment Time | Mean Incubation Time ± SEM 1 |
|---|---|
| 0 min | 218.8 ± 3.2 days |
| 30 min | 251.3 ± 9.4 days 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakudo, A.; Yamashiro, R.; Onodera, T. Recent Advances in Prion Inactivation by Plasma Sterilizer. Int. J. Mol. Sci. 2022, 23, 10241. https://doi.org/10.3390/ijms231810241
Sakudo A, Yamashiro R, Onodera T. Recent Advances in Prion Inactivation by Plasma Sterilizer. International Journal of Molecular Sciences. 2022; 23(18):10241. https://doi.org/10.3390/ijms231810241
Chicago/Turabian StyleSakudo, Akikazu, Risa Yamashiro, and Takashi Onodera. 2022. "Recent Advances in Prion Inactivation by Plasma Sterilizer" International Journal of Molecular Sciences 23, no. 18: 10241. https://doi.org/10.3390/ijms231810241
APA StyleSakudo, A., Yamashiro, R., & Onodera, T. (2022). Recent Advances in Prion Inactivation by Plasma Sterilizer. International Journal of Molecular Sciences, 23(18), 10241. https://doi.org/10.3390/ijms231810241







