Inhibition of Complex I of the Respiratory Chain, but Not Complex III, Attenuates Degranulation and Cytokine Secretion in Human Skin Mast Cells
Abstract
:1. Introduction
2. Results
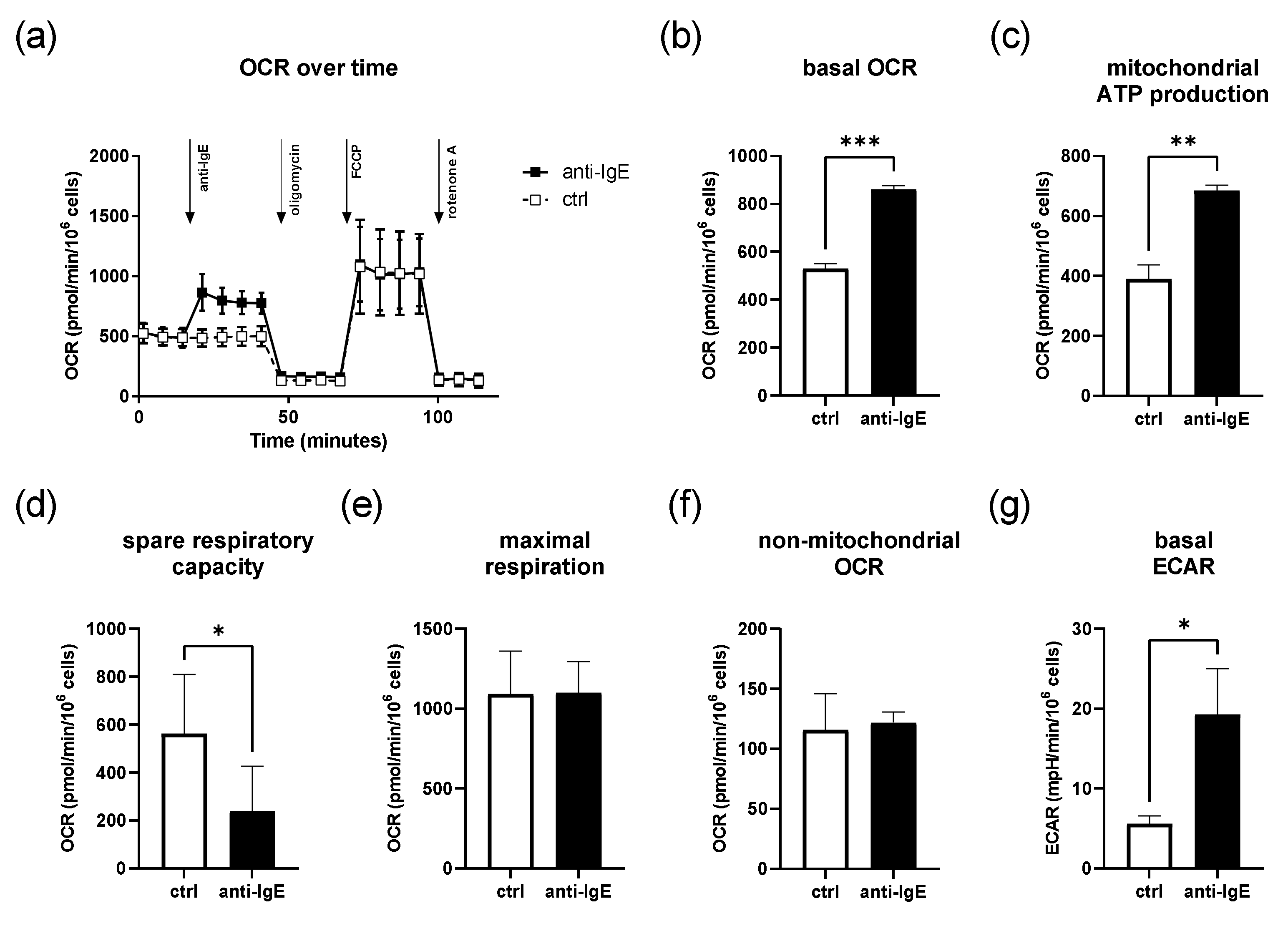
2.1. IgE-Mediated Activation of Human Skin MCs Enhances Mitochondrial Respiration and ATP Coupling Efficiency, Whereas Spare Respiratory Capacity Is Reduced
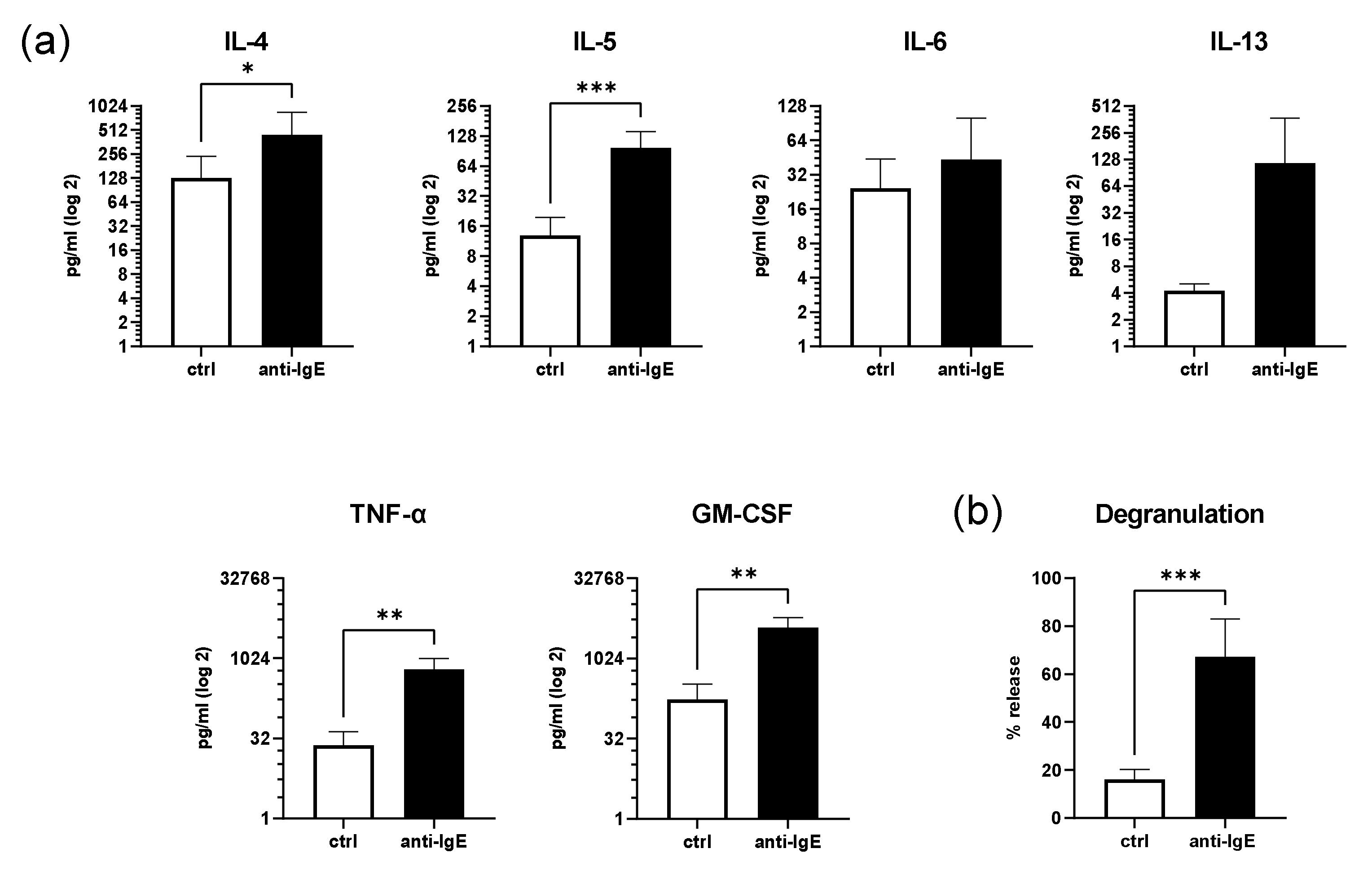
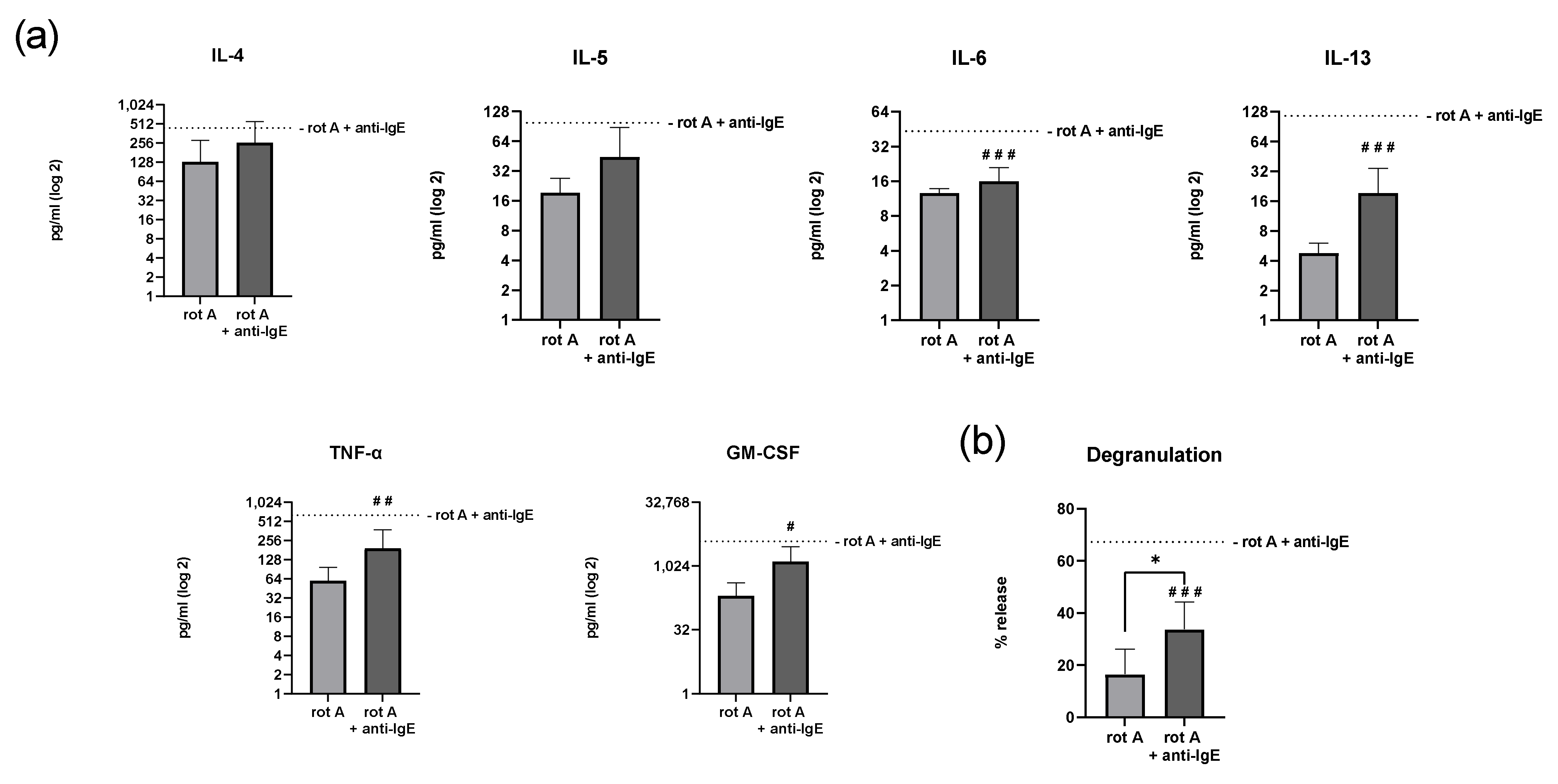
2.2. IgE-Mediated Activation of Human Skin MCs Enhances Cytokine and Growth Factor Secretion and Degranulation
2.3. Inhibition of Complex III of the Respiratory Chain in Human Skin MCs Reduces Mitochondrial Parameters but Not Viability
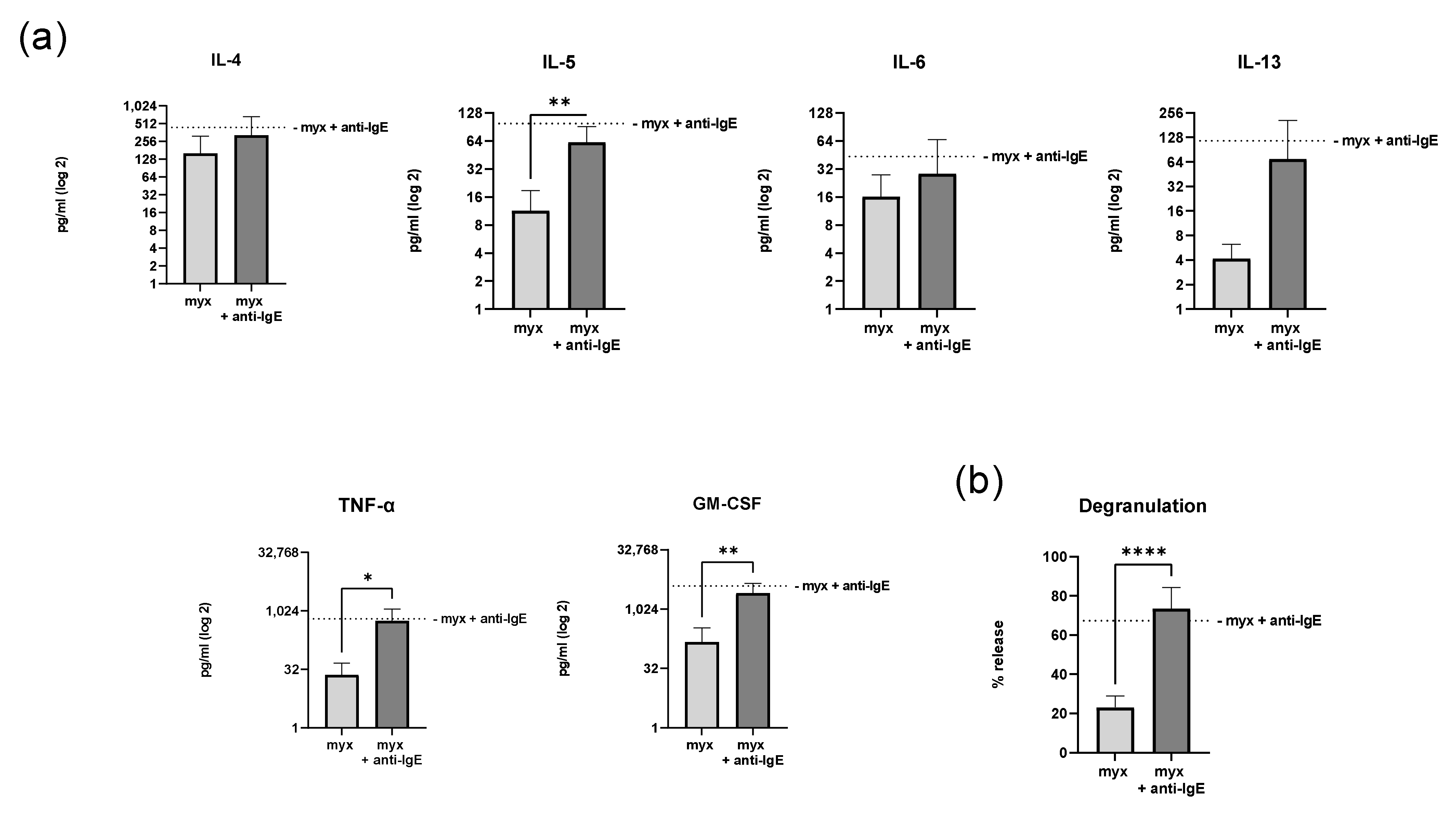
2.4. Inhibition of Complex III of the Respiratory Chain Does Not Affect Degranulation and Cytokine Secretion of Human Skin MCs
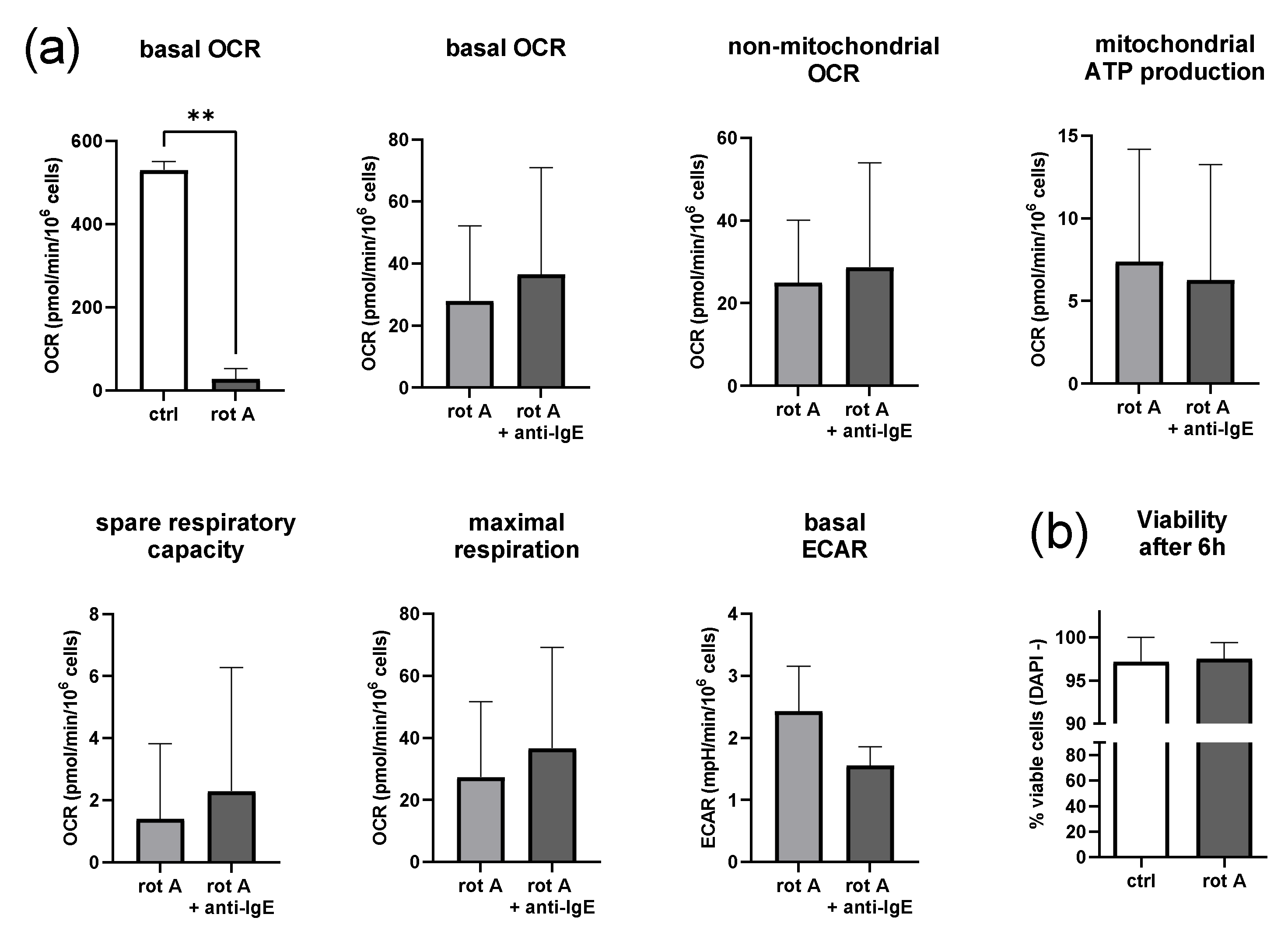
2.5. Inhibition of Complex I of the Respiratory Chain Decreases Mitochondrial Parameters but Not Viability
2.6. Inhibition of Complex I of the Respiratory Chain in Human Skin MCs Reduces Degranulation and Secretion of IL-6, IL-13, TNF-α and GM-CSF
3. Discussion
4. Materials and Methods
4.1. Preparation and Culture of Human Skin MCs
4.2. SeahorseTM Metabolic Flux Analysis
4.3. Viability Assay
4.4. β-Hexosaminidase Release Assay
4.5. Quantification of Cytokine Secretion
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metz, M.; Maurer, M. Mast cells–key effector cells in immune responses. Trends Immunol. 2007, 28, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Elieh-Ali-Komi, D.; Metz, M.; Siebenhaar, F.; Maurer, M. Understanding human mast cells: Lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 2022, 22, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Hakim-Rad, K.; Metz, M.; Maurer, M. Mast cells: Makers and breakers of allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Wohrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2019, 58, 342–365. [Google Scholar] [CrossRef]
- Maurer, M.; Köberle, M.; Metz, M.; Biedermann, T. Mast cells: Promoters of health and modulators of disease. J. Allergy Clin. Immunol. 2019, 144, S1–S3. [Google Scholar] [CrossRef]
- Ring, J.; Beyer, K.; Biedermann, T.; Bircher, A.; Fischer, M.; Heller, A.; Huttegger, I.; Jakob, T.; Klimek, L.; Kopp, M.V.; et al. Leitlinie zu Akuttherapie und Management der Anaphylaxie-Update 2021. Allergo J. 2021, 30, 20–49. [Google Scholar] [CrossRef]
- Wagner, N.; Staubach, P. Mastocytosis—pathogenesis, clinical manifestation and treatment. J. Dtsch. Dermatol. Ges 2018, 16, 42–57. [Google Scholar] [CrossRef]
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef]
- Church, M.K.; Kolkhir, P.; Metz, M.; Maurer, M. The role and relevance of mast cells in urticaria. Immunol. Rev. 2018, 282, 232–247. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C. Doctor, I Think I Am Suffering from MCAS: Differential Diagnosis and Separating Facts from Fiction. J. Allergy Clin. Immunol. Pract. 2019, 7, 1109–1114. [Google Scholar] [CrossRef]
- Afrin, L.B.; Ackerley, M.B.; Bluestein, L.S.; Brewer, J.H.; Brook, J.B.; Buchanan, A.D.; Cuni, J.R.; Davey, W.P.; Dempsey, T.T.; Dorff, S.R.; et al. Diagnosis of mast cell activation syndrome: A global “consensus-2”. Diagnosis 2021, 8, 137–152. [Google Scholar] [CrossRef]
- Buttgereit, T.; Gu, S.; Carneiro-Leão, L.; Gutsche, A.; Maurer, M.; Siebenhaar, F. Idiopathic mast cell activation syndrome is more often suspected than diagnosed—A prospective real-life study. Allergy 2022, 77, 2794–2802. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Erlich, T.H.; Yagil, Z.; Kay, G.; Peretz, A.; Migalovich-Sheikhet, H.; Tshori, S.; Nechushtan, H.; Levi-Schaffer, F.; Saada, A.; Razin, E. Mitochondrial STAT3 plays a major role in IgE-antigen-mediated mast cell exocytosis. J. Allergy Clin. Immunol. 2014, 134, 460–469. [Google Scholar] [CrossRef]
- Takei, M.; Endo, K. Histamine release and calcium concentrations in rat mast cells are dependent on intracellular ATP: Effects of prostaglandin D2. Prostaglandins Leukot. Essent. Fat. Acids 1994, 50, 357–362. [Google Scholar] [CrossRef]
- Johansen, T.; Chakravarty, N. The utilization of adenosine triphosphate in rat mast cells during histamine release induced by anaphylactic reaction and compound 48/80. Naunyn Schmiedebergs Arch. Pharmacol. 1975, 288, 243–260. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Bhavsar, P.K.; Mumby, S.; Xu, B.; Hui, C.K.M.; Chung, K.F.; Adcock, I.M. Role of Metabolic Reprogramming in Pulmonary Innate Immunity and Its Impact on Lung Diseases. J. Innate Immun. 2020, 12, 31–46. [Google Scholar] [CrossRef]
- Watt, I.N.; Montgomery, M.G.; Runswick, M.J.; Leslie, A.G.W.; Walker, J.E. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc. Natl. Acad. Sci. USA 2010, 107, 16823–16827. [Google Scholar] [CrossRef]
- Mendoza, R.P.; Fudge, D.H.; Brown, J.M. Cellular Energetics of Mast Cell Development and Activation. Cells 2021, 10, 524. [Google Scholar] [CrossRef]
- Chelombitko, M.A.; Chernyak, B.V.; Fedorov, A.V.; Zinovkin, R.A.; Razin, E.; Paruchuru, L.B. The Role Played by Mitochondria in FcεRI-Dependent Mast Cell Activation. Front. Immunol. 2020, 11, 584210. [Google Scholar] [CrossRef]
- Phong, B.; Avery, L.; Menk, A.V.; Delgoffe, G.M.; Kane, L.P. Cutting Edge: Murine Mast Cells Rapidly Modulate Metabolic Pathways Essential for Distinct Effector Functions. J. Immunol. 2017, 198, 640–644. [Google Scholar] [CrossRef]
- Sharkia, I.; Erlich, T.; Landolina, N.; Assayag, M.; Motzik, A.; Rachmin, I.; Kay, G.; Porat, Z.; Tshori, S.; Berkman, N.; et al. Pyruvate dehydrogenase has a major role in mast cell function, and its activity is regulated by mitochondrial microphthalmia transcription factor. J. Allergy Clin. Immunol. 2017, 140, 204–214.e8. [Google Scholar] [CrossRef]
- Möllerherm, H.; Branitzki-Heinemann, K.; Brogden, G.; Elamin, A.A.; Oehlmann, W.; Fuhrmann, H.; Singh, M.; Naim, H.Y.; Von Köckritz-Blickwede, M. Hypoxia Modulates the Response of Mast Cells to Staphylococcus aureus Infection. Front. Immunol. 2017, 8, 541. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Khomtchouk, K.; Maria, P.L.S. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2019, 58, 298–312. [Google Scholar] [CrossRef]
- Ramírez-Moreno, I.G.; Ibarra-Sánchez, A.; Castillo-Arellano, J.I.; Blank, U.; González-Espinosa, C. Mast Cells Localize in Hypoxic Zones of Tumors and Secrete CCL-2 under Hypoxia through Activation of L-Type Calcium Channels. J. Immunol. 2020, 204, 1056–1068. [Google Scholar] [CrossRef]
- Chakravarty, N. Respiration of rat peritoneal mast cells during histamine release induced by antigen-antibody reaction. Exp. Cell Res. 1968, 49, 160–168. [Google Scholar] [CrossRef]
- Caslin, H.L.; Taruselli, M.; Haque, T.; Pondicherry, N.; Baldwin, E.; Barnstein, B.O.; Ryan, J.J. Inhibiting Glycolysis and ATP Production Attenuates IL-33-Mediated Mast Cell Function and Peritonitis. Front. Immunol. 2018, 9, 3026. [Google Scholar] [CrossRef]
- Krauss, P.-L.; Pfeiffenberger, M.; Damerau, A.; Buttgereit, T.; Chen, Y.; Gaber, T.; Buttgereit, F. Production of IL-6 and Phagocytosis Are the Most Resilient Immune Functions in Metabolically Compromised Human Monocytes. Front. Immunol. 2021, 12, 730672. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, Y.; Krauß, P.-L.; Löwe, P.; Pfeiffenberger, M.; Damerau, A.; Ehlers, L.; Buttgereit, T.; Hoff, P.; Buttgereit, F.; et al. Age-related increase of mitochondrial content in human memory CD4+ T cells contributes to ROS-mediated increased expression of proinflammatory cytokines. Front. Immunol. 2022, 13, 911050. [Google Scholar] [CrossRef]
- Atiakshin, D.; Kostin, A.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Protease Profile of Tumor-Associated Mast Cells in Melanoma. Int. J. Mol. Sci. 2022, 23, 8930. [Google Scholar] [CrossRef]
- Kolkhir, P.; Pyatilova, P.; Ashry, T.; Jiao, Q.; Abad-Perez, A.T.; Altrichter, S.; Ayala, C.E.V.; Church, M.K.; He, J.; Lohse, K.; et al. Mast cells, cortistatin, and its receptor, MRGPRX2, are linked to the pathogenesis of chronic prurigo. J. Allergy Clin. Immunol. 2022, 149, 1998–2009.e5. [Google Scholar] [CrossRef] [PubMed]
- Thierbach, G.; Reichenbach, H. Myxothiazol, a new inhibitor of the cytochrome b-c1 segment of th respiratory chain. Biochim. Biophys. Acta 1981, 638, 282–289. [Google Scholar] [CrossRef]
- Davoudi, M.; Kallijärvi, J.; Marjavaara, S.; Kotarsky, H.; Hansson, E.; Levéen, P.; Fellman, V. A mouse model of mitochondrial complex III dysfunction induced by myxothiazol. Biochem. Biophys. Res. Commun. 2014, 446, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.A.; Fisher-Wellman, K.H.; Neufer, P.D. From OCR and ECAR to energy: Perspectives on the design and interpretation of bioenergetics studies. J. Biol. Chem. 2021, 297, 101140. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Sellge, G.; Lorentz, A.; Sebald, W.; Raab, R.; Manns, M.P. IL-4 enhances proliferation and mediator release in mature human mast cells. Proc. Natl. Acad. Sci. USA 1999, 96, 8080–8085. [Google Scholar] [CrossRef]
- McLeod, J.J.; Baker, B.; Ryan, J.J. Mast cell production and response to IL-4 and IL-13. Cytokine 2015, 75, 57–61. [Google Scholar] [CrossRef]
- Otani, I.M.; Anilkumar, A.A.; Newbury, R.O.; Bhagat, M.; Beppu, L.Y.; Dohil, R.; Broide, D.H.; Aceves, S.S. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J. Allergy Clin. Immunol. 2013, 131, 1576–1582. [Google Scholar] [CrossRef]
- Zinovkin, R.A.; Zamyatnin, A.A. Mitochondria-Targeted Drugs. Curr. Mol. Pharmacol. 2019, 12, 202–214. [Google Scholar] [CrossRef]
- Guhl, S.; Artuc, M.; Neou, A.; Babina, M.; Zuberbier, T. Long-term cultured human skin mast cells are suitable for pharmacological studies of anti-allergic drugs due to high responsiveness to FcεRI cross-linking. Biosci. Biotechnol. Biochem. 2011, 75, 382–384. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buttgereit, T.; Pfeiffenberger, M.; Frischbutter, S.; Krauß, P.-L.; Chen, Y.; Maurer, M.; Buttgereit, F.; Gaber, T. Inhibition of Complex I of the Respiratory Chain, but Not Complex III, Attenuates Degranulation and Cytokine Secretion in Human Skin Mast Cells. Int. J. Mol. Sci. 2022, 23, 11591. https://doi.org/10.3390/ijms231911591
Buttgereit T, Pfeiffenberger M, Frischbutter S, Krauß P-L, Chen Y, Maurer M, Buttgereit F, Gaber T. Inhibition of Complex I of the Respiratory Chain, but Not Complex III, Attenuates Degranulation and Cytokine Secretion in Human Skin Mast Cells. International Journal of Molecular Sciences. 2022; 23(19):11591. https://doi.org/10.3390/ijms231911591
Chicago/Turabian StyleButtgereit, Thomas, Moritz Pfeiffenberger, Stefan Frischbutter, Pierre-Louis Krauß, Yuling Chen, Marcus Maurer, Frank Buttgereit, and Timo Gaber. 2022. "Inhibition of Complex I of the Respiratory Chain, but Not Complex III, Attenuates Degranulation and Cytokine Secretion in Human Skin Mast Cells" International Journal of Molecular Sciences 23, no. 19: 11591. https://doi.org/10.3390/ijms231911591
APA StyleButtgereit, T., Pfeiffenberger, M., Frischbutter, S., Krauß, P.-L., Chen, Y., Maurer, M., Buttgereit, F., & Gaber, T. (2022). Inhibition of Complex I of the Respiratory Chain, but Not Complex III, Attenuates Degranulation and Cytokine Secretion in Human Skin Mast Cells. International Journal of Molecular Sciences, 23(19), 11591. https://doi.org/10.3390/ijms231911591





