An Analysis of the Effects of In Vitro Photodynamic Therapy on Prostate Cancer Tissue by Histopathological Examination and Magnetic Resonance Imaging
Abstract
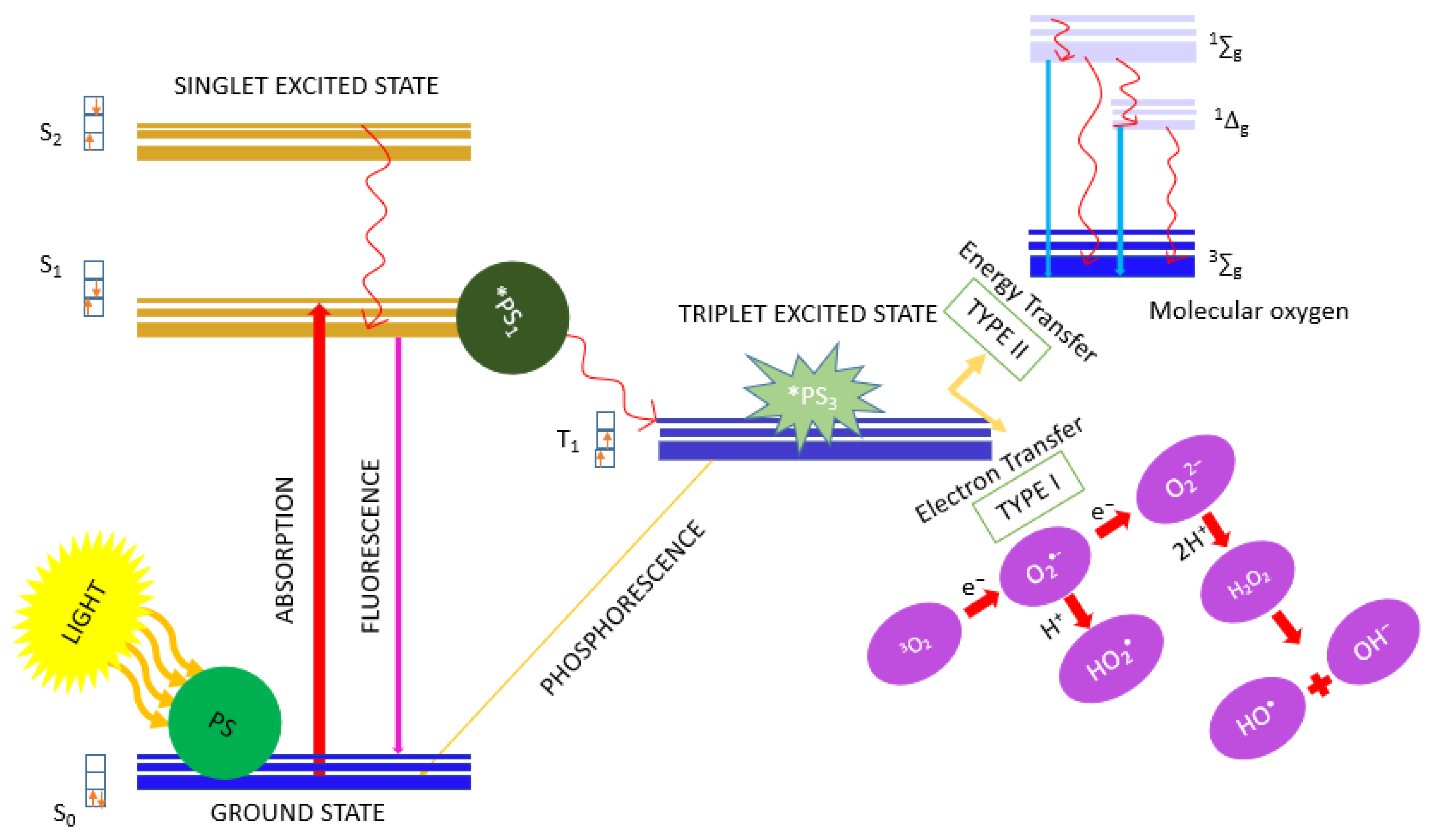
1. Introduction
2. Results
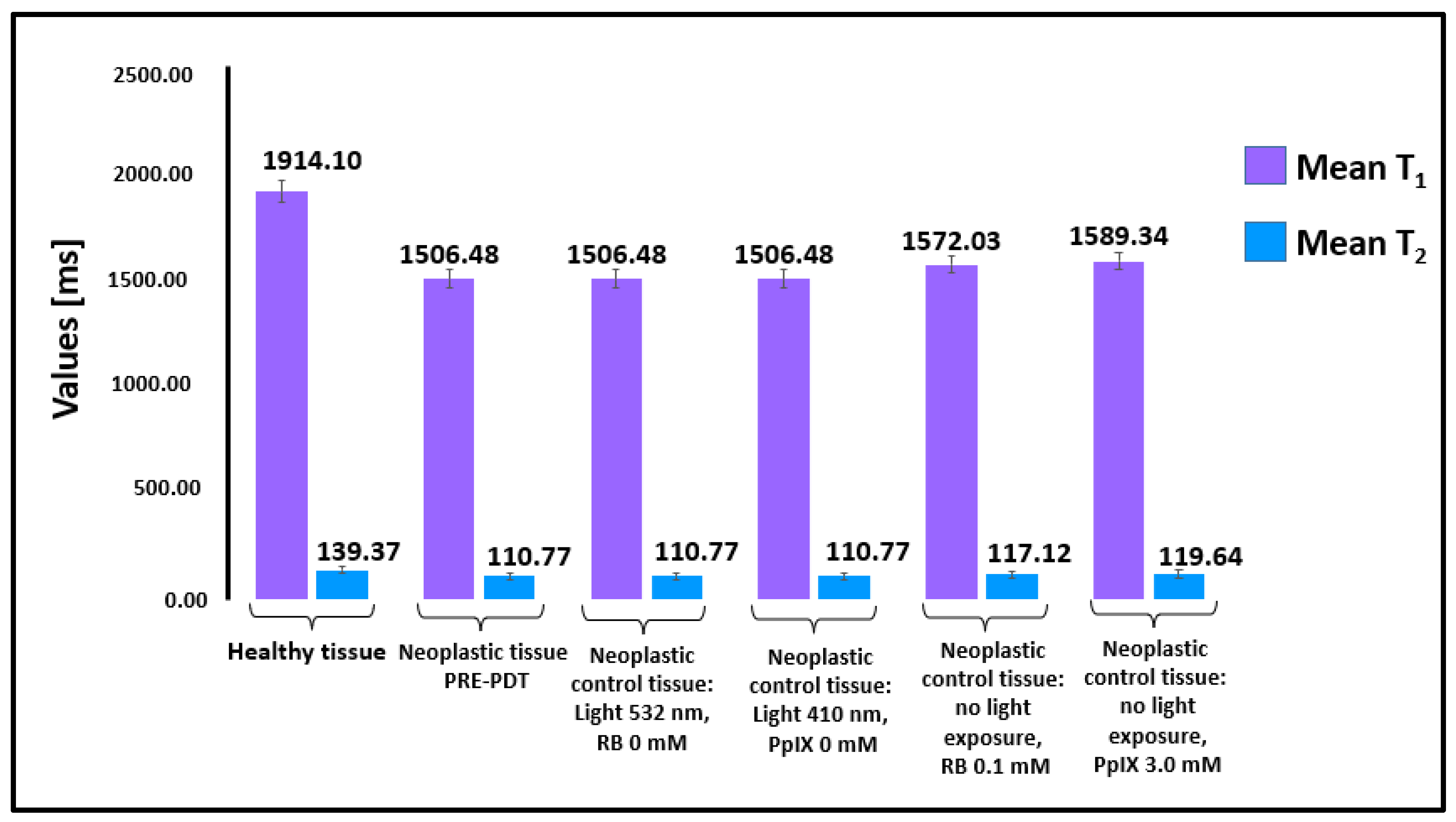
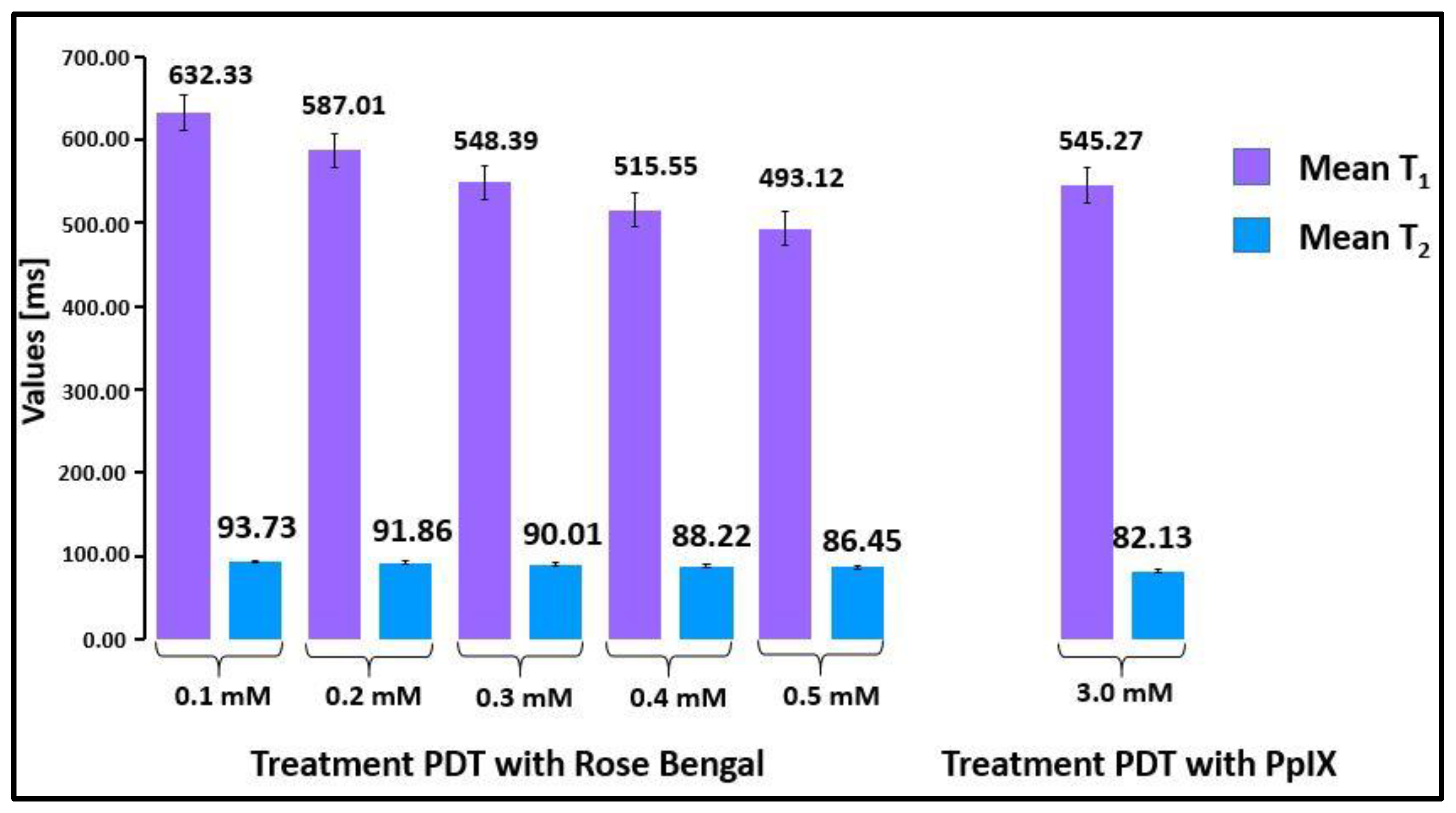
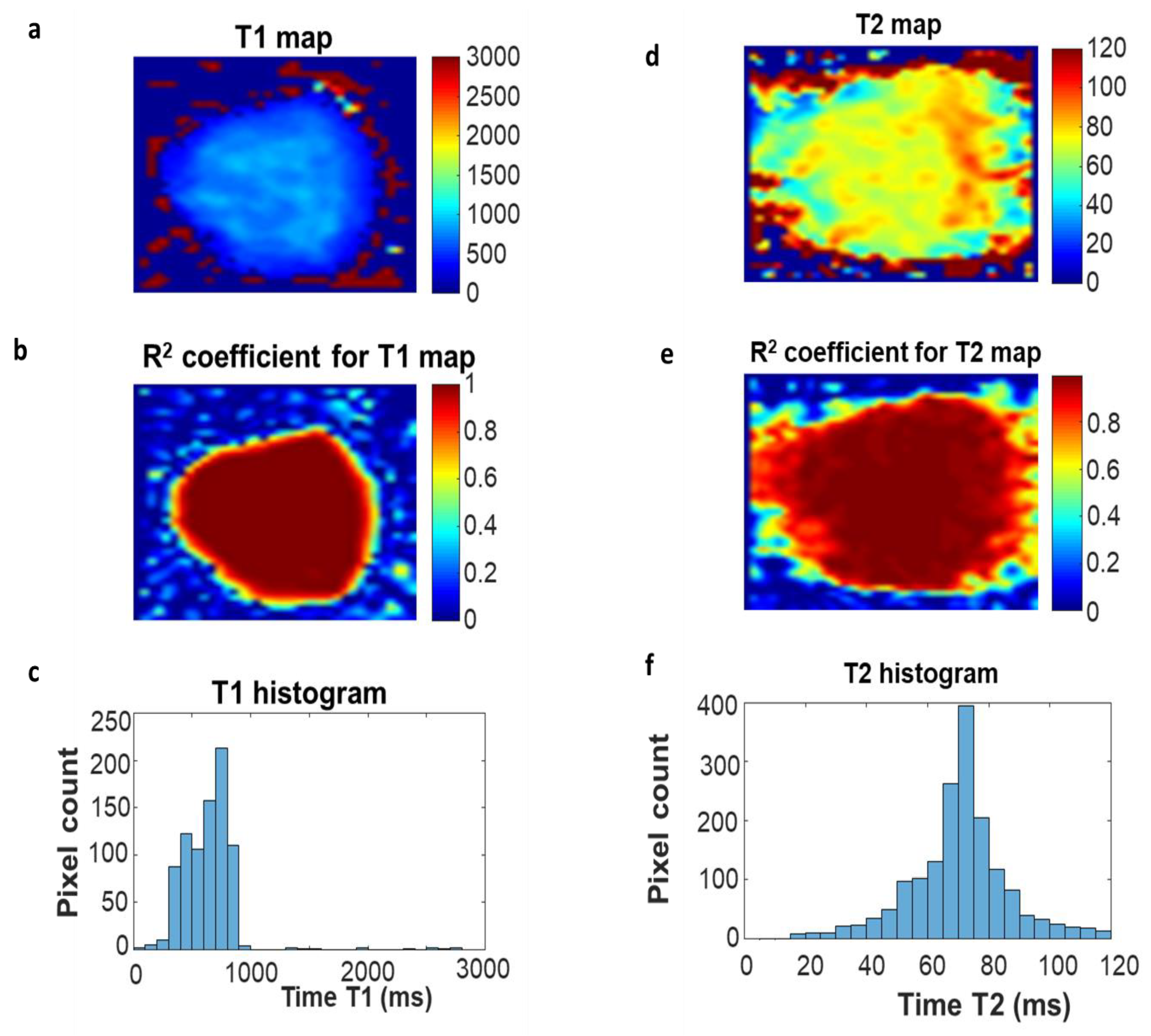
2.1. Relaxation Time Measurements
2.2. Histopathological Analysis
3. Discussion
4. Materials and Methods
4.1. Prostate Tissue Samples
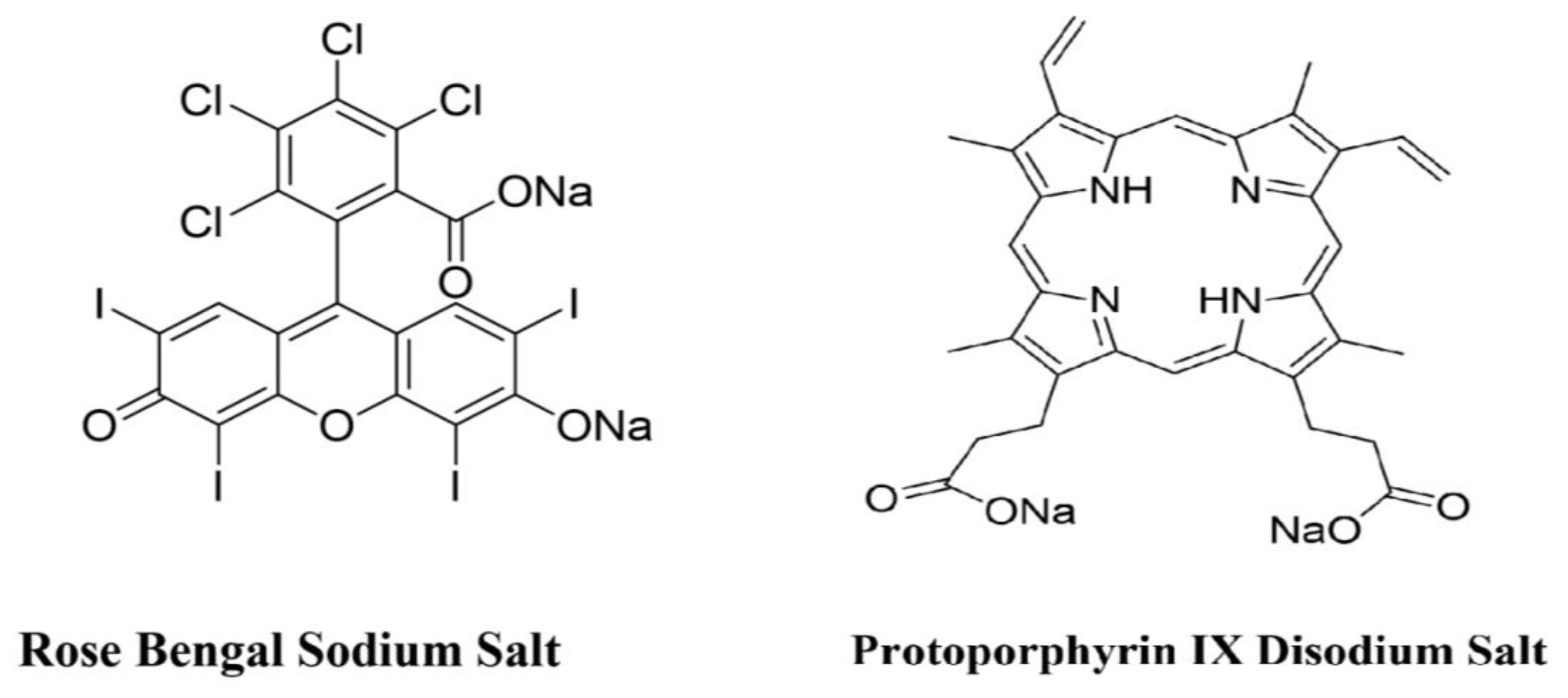
4.2. Photosensitizers
4.3. The PDT Procedure
4.4. Analysis of Prostate Tissue Samples by MRI
4.5. Statistical Significance
4.6. Histopathological Preparations
4.7. Microscopic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ni Raghallaigh, H.; Eeles, R. Genetic predisposition to prostate cancer: An update. Fam. Cancer 2022, 21, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Cereda, V.; Falbo, P.T.; Manna, G.; Iannace, A.; Menghi, A.; Corona, M.; Semenova, D.; Calò, L.; Carnevale, R.; Frati, G.; et al. Hormonal prostate cancer therapies and cardiovascular disease: A systematic review. Heart Fail. Rev. 2022, 27, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Toprak, T.; Suarez-Ibarrola, R.; Sigle, A.; Gratzke, C.; Miernik, A. Incidental prostate cancer after holmium laser enucleation of the prostate—A narrative review. Andrologia 2022, 54, e14332. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kwak, C. Immunotherapy for prostate cancer: Requirements for a successful regime transfer. Investig. Clin. Urol. 2022, 63, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kamal, O.; Foster, B.R.; Young, D.J.; Hansel, D.E.; Coakley, F.V. MRI appearance of BRCA-associated prostate cancer. Clin. Imaging 2022, 84, 135–139. [Google Scholar]
- Prashar, J.; Schartau, P.; Murray, E. Supportive care needs of men with prostate cancer: A systematic review update. Eur. J. Cancer Care 2022, 31, e13541. [Google Scholar] [CrossRef]
- Osuchowski, M.; Bartusik-Aebisher, D.; Osuchowski, F.; Aebisher, D. Photodynamic therapy for prostate cancer—A narrative review. Photodiagn. Photodyn. Ther. 2021, 33, 102158. [Google Scholar] [CrossRef]
- World Health Organization. 2020. Available online: https://gco.iarc.fr/today/online-analysis-multi-bars?v=2020&mode=cancer&mode_population=countries&population=900&populations=900&key=cum_risk&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=0&include_nmsc_other=1&type_multiple=%257B%2522inc%2522%253Atrue%252C%2522mort%2522%253Afalse%252C%2522prev%2522%253Afalse%257D&orientation=horizontal&type_sort=0&type_nb_items=%257B%2522top%2522%253Atrue%252C%2522bottom%2522%253Afalse%257D (accessed on 25 February 2021).
- Merkens, L.; Sailer, V.; Lessel, D.; Janzen, E.; Greimeier, S.; Kirfel, J.; Perner, S.; Pantel, K.; Werner, S.; von Amsberg, G. Aggressive variants of prostate cancer: Underlying mechanisms of neuroendocrine transdifferentiation. J. Exp. Clin. Cancer Res. 2022, 41, 46. [Google Scholar] [CrossRef]
- Nugent, T.S.; Low, E.Z.; Fahy, M.R.; Donlon, N.E.; McCormick, P.H.; Mehigan, B.J.; Cunningham, M.; Gillham, C.; Kavanagh, D.O.; Kelly, M.E.; et al. Prostate radiotherapy and the risk of secondary rectal cancer-a meta-analysis. Int. J. Colorectal Dis. 2022, 37, 437–447. [Google Scholar] [CrossRef]
- Achard, V.; Putora, P.M.; Omlin, A.; Zilli, T.; Fischer, S. Metastatic Prostate Cancer: Treatment Options. Oncology 2022, 100, 48–59. [Google Scholar] [CrossRef]
- Ryman-Tubb, T.; Lothion-Roy, J.H.; Metzler, V.M.; Harris, A.E.; Robinson, B.D.; Rizvanov, A.A.; Jeyapalan, J.N.; James, V.H.; England, G.; Rutland, C.S.; et al. Comparative pathology of dog and human prostate cancer. Vet. Med. Sci. 2022, 8, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Marcus, S.L. Photodynamic therapy. Eur. J. Cancer 1992, 28A, 1734–1742. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Lui, H.; Anderson, R.R. Photodynamic therapy in dermatology. Shedding a different light on skin disease. Arch. Dermatol. 1992, 128, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Jori, G. Photodynamic therapy of microbial infections: State of the art and perspectives. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- De Rosa, F.S.; Bentley, M.V. Photodynamic therapy of skin cancers: Sensitizers, clinical studies and future directives. Pharm. Res. 2000, 17, 1447–1455. [Google Scholar] [CrossRef]
- Korbelik, M. PDT-associated host response and its role in the therapy outcome. Lasers Surg. Med. 2006, 38, 500–508. [Google Scholar] [CrossRef]
- Lou, P.J.; Jäger, H.R.; Jones, L.; Theodossy, T.; Bown, S.G.; Hopper, C. Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br. J. Cancer 2004, 91, 441–446. [Google Scholar] [CrossRef]
- Bown, S.G.; Rogowska, A.Z.; Whitelaw, D.E.; Lees, W.R.; Lovat, L.B.; Ripley, P.; Jones, L.; Wyld, P.; Gillams, A.; Hatfield, A.W. Photodynamic therapy for cancer of the pancreas. Gut 2002, 50, 549–557. [Google Scholar] [CrossRef]
- Marberger, M.; Carroll, P.R.; Zelefsky, M.J.; Coleman, J.A.; Hricak, H.; Scardino, P.T.; Abenhaim, L.L. New treatments for localized prostate cancer. Urology 2008, 72, S36–S43. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, A.R.; Lebdai, S.; Benzaghou, F.; Stief, C. Vascular-targeted photodynamic therapy with TOOKAD® Soluble in localized prostate cancer: Standardization of the procedure. World J. Urol. 2015, 33, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Yano, T.; Wang, K.K. Photodynamic Therapy for Gastrointestinal Cancer. Photochem. Photobiol. 2020, 96, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, G.; Wu, D.; Li, X.; Hu, N.; Chen, J.; Chen, G.; Wu, Y. Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy. Biosens. Bioelectron. 2019, 137, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, W.; Zhang, T.; Jiang, X.; Hu, Y. Hybrid nanoparticle composites applied to photodynamic therapy: Strategies and applications. J. Mater. Chem. B 2020, 8, 4726–4737. [Google Scholar] [CrossRef] [PubMed]
- Khoo, V.S.; Joon, D.L. New developments in MRI for target volume determination in radiotherapy. Fr. J. Radiol. 2006, 79, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Colafati, G.S.; Voicu, I.P.; Carducci, C.; Miele, E.; Carai, A.; Di Loreto, S.; Marrazzo, A.; Cacchione, A.; Cecinati, V.; Tornesello, A.; et al. MRI features as a helpful tool to predict the molecular subgroups of medulloblastoma: State of the art. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418775375. [Google Scholar] [CrossRef]
- Fei, B.; Wang, H.; Meyers, J.D.; Feyes, D.K.; Oleinick, N.L.; Duerk, J.L. High-field magnetic resonance imaging of the response of human prostate cancer to Pc 4-based photodynamic therapy in an animal model. Lasers Surg. Med. 2007, 39, 723–730. [Google Scholar] [CrossRef]
- Sandgren, K.; Nilsson, E.; Keeratijarut Lindberg, A.; Strandberg, S.; Blomqvist, L.; Bergh, A.; Frederick, B.; Axelsson, J.; Ögren, M.; Ögren, M.; et al. Registration of Histopathology for Magnetic Resonance Imaging of Prostate Cancer. Phys. Imaging Radiat. Oncol. 2021, 18, 19–25. [Google Scholar] [CrossRef]
- Popita, C.; Popita, A.R.; Sitar-Taut, A.; Petrut, B.; Fetica, B.; Coman, I. 1.5-Tesla Multiparamet-ric-Magnetic Resonance Imaging for the detection of clinically significant prostate cancer. Clujul. Med. 2017, 90, 40–48. [Google Scholar] [PubMed]
- Fütterer, J.J.; Briganti, A.; De Visschere, P.; Emberton, M.; Giannarini, G.; Kirkham, A.; Taneja, S.S.; Thoeny, H.; Villeirs, G.; Villers, A. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur. Urol. 2015, 68, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Haaf, P.; Garg, P.; Messroghli, D.; Broadbent, D.; Greenwood, J.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in Clinical Practice: A Comprehensive Review. J. Cardiovasc. Magn. Reson. 2016, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.K.; Hong, Y.J.; Im, D.J.; Suh, Y.J.; Park, C.H.; Kim, J.Y.; Chang, S.; Lee, H.J.; Hur, J.; Kim, Y.J.; et al. Myocardial T1 and T2 Mapping: Techniques and Clinical Applications. Korean J. Radiol. 2017, 18, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Newman, E.L. On the mechanism of the tumour-localising effect in photodynamic therapy. J. Photochem. Photobiol. B 1994, 23, 3–8. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The epr effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.W.; Dolphin, D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 1996, 64, 469–485. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Babu, A.; Kim, S.J.; Murugesan, R.; Jeyasubramanian, K. Enhanced photodynamic efficacy and efficient delivery of Rose Bengal using nanostructured poly(amidoamine) dendrimers: Potential application in photodynamic therapy of cancer. Cancer Nanotechnol. 2011, 2, 95–103. [Google Scholar] [CrossRef]
- Xu, H.; Sun, Y.; Zhang, Y.; Wang, W.; Dan, J.; Yao, J.; Chen, H.; Tian, F.; Sun, X.; Guo, S.; et al. Protoporphyrin IX Induces a Necrotic Cell Death in Human THP-1 Macrophages through Activation of Reactive Oxygen Species/c-Jun N-Terminal Protein Kinase Pathway and Opening of Mitochondrial Permeability Transition Pore. Cell. Physiol. Biochem. 2014, 34, 1835–1848. [Google Scholar] [CrossRef]
- Panzarini, E.; Inguscio, V.; Dini, L. Overview of Cell Death Mechanisms Induced by Rose Bengal Acetate-Photodynamic Therapy. Int. J. Photoenergy 2011, 2011, 713726. [Google Scholar] [CrossRef]
- Chung, J.; Chen, C.; Paw, B.H. Heme metabolism and erythropoiesis. Curr. Opin. Hematol. 2012, 19, 156–162. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Zhao, P.; Zhu, S.; Wang, X.; Liu, Q. Apoptosis induced by sonodynamic treatment by protoporphyrin ix on mda-mb-231 cells. Ultrasonics 2012, 52, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Sander, W.J.; Fourie, C.; Sabiu, S.; O’Neill, F.H.; Pohl, C.H.; O’Neill, H.G. Reactive oxygen species as potential antiviral targets. Rev. Med. Virol. 2022, 32, e2240. [Google Scholar] [CrossRef] [PubMed]
- Sugita, N.; Iwase, Y.; Yumita, N.; Ikeda, T.; Umemura, S. Sonodynamically induced cell damage using rose bengal derivative. Anticancer Res. 2010, 30, 3361–3366. [Google Scholar] [PubMed]
- Sugita, N.; Kawabata, K.; Sasaki, K.; Sakata, I.; Umemura, S. Synthesis of amphiphilic derivatives of rose bengal and their tumor accumulation. Bioconjug. Chem. 2007, 18, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Eggener, S.E.; Coleman, J.A. Focal treatment of prostate cancer with vascular-targeted photodynamic therapy. ScientificWorldJournal 2008, 8, 963–973. [Google Scholar] [CrossRef]
- Kato, H.; Ono, J.; Konaka, C.; Kawate, N.; Yoneyama, K.; Kinoshita, K.; Nishimiya, K.; Sakai, H.; Noguchi, M.; Tomono, T.; et al. Clinical measurement of tumor fluorescence using a new diagnostic system with hematoporphyrin derivative, laser photoradiation, and a spectroscope. Lasers Surg. Med. 1984, 4, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.S.; Ahn, J.C.; Lee, S.J.; Peijie, H.E.; Moon, J.H. Effect of Photodynamic Therapy in Melanoma Skin Cancer Cell Line A375: In vivo Study Crossref. Med. Laser 2014, 3, 27–30. [Google Scholar] [CrossRef]
- Qiu, H.; Kim, M.M.; Penjweini, R.; Finlay, J.C.; Busch, T.M.; Wang, T.; Guo, W.; Cengel, K.A.; Simone, C.B.; Glatstein, E.; et al. A Comparison of Dose Metrics to Predict Local Tumor Control for Photofrin-mediated Photodynamic Therapy. Photochem. Photobiol. 2017, 93, 1115–1122. [Google Scholar] [CrossRef]
- Huang, Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.E.; Liu, Y.; Lee, T.H.; Lee, W.K.; Yoon, I.; Kim, K. Tumor Size-Dependent Anticancer Efficacy of Chlorin Derivatives for Photodynamic Therapy. Int. J. Mol. Sci. 2018, 19, 1596. [Google Scholar] [CrossRef] [PubMed]
- Pigula, M.; Huang, H.C.; Mallidi, S.; Anbil, S.; Liu, J.; Mai, Z.; Hasan, T. Size-dependent Tumor Response to Photodynamic Therapy and Irinotecan Monotherapies Revealed by Longitudinal Ultrasound Monitoring in an Orthotopic Pancreatic Cancer Model. Photochem. Photobiol. 2019, 95, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Zaak, D.; Sroka, R.; Höppner, M.; Khoder, W.; Reich, O.; Tritschler, S.; Muschter, R.; Knüchel, R.; Hofstetter, A. Photodynamic therapy by means of 5-ALA induced PPIX in human prostate—Preliminary results. Med. Laser Appl. 2003, 18, 91–95. [Google Scholar] [CrossRef]
- Wang, X.; Tsui, B.; Ramamurthy, G.; Zhang, P.; Meyers, J.; Kenney, M.E.; Kiechle, J.; Ponsky, L.; Basilion, J.P. Theranostic Agents for Photodynamic Therapy of Prostate Cancer by Targeting Prostate-Specific Membrane Antigen. Mol. Cancer Ther. 2016, 15, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Liu, H.; Chen, H.; Wang, G.; Teng, H.; Chang, Y. Polyphotosensitizer nanogels for GSH-responsive histone deacetylase inhibitors delivery and enhanced cancer photodynamic therapy. Colloids Surf. B Biointerfaces 2020, 188, 110753. [Google Scholar] [CrossRef] [PubMed]
- Zaak, D.; Sroka, R.; Stocker, S.; Bise, K.; Lein, M.; Höppner, M.; Frimberger, D.; Schneede, P.; Reich, O.; Kriegmair, M.; et al. Photodynamic therapy of prostate cancer by means of 5-aminolevulinic acid-induced protoporphyrin IX—In vivo experiments on the dunning rat tumor model. Urol. Int. 2004, 72, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fei, B. Diffusion-weighted MRI for monitoring tumor response to photodynamic therapy. J. Magn. Reason. Imaging 2010, 32, 409–417. [Google Scholar] [CrossRef]
- Sooriakumaran, P.; Dev, H.S.; Skarecky, D.; Ahlering, T. The importance of surgical margins in prostate cancer. J. Surg. Oncol. 2016, 113, 310–315. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with curative intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Hubel, A.; Spindler, R.; Skubitz, A.P. Storage of human biospecimens: Selection of the optimal storage temperature. Biopreserv. Biobank. 2014, 12, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Esteva-Socias, M.; Artiga, M.-J.; Bahamonde, O.; Belar, O.; Bermudo, R.; Castro, E.; Escámez, T.; Fraga, M.; Jauregui-Mosquera, L.; Novoa, I.; et al. In search of an evidence-based strategy for quality assessment of human tissue samples: Report of the tissue Biospecimen Research Working Group of the Spanish Biobank Network. J. Transl. Med. 2019, 17, 370. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Ferreira, A.R.; Neves, M.d.G.P.M.S.; Ribeiro, D.; Fardilha, M.; Faustino, M.A.F. Photodynamic therapy of prostate cancer using porphyrinic formulations. J. Photochem. Photobiol. B Biol. 2021, 223, 112301. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Pinillos Bayona, A.; Woodhams, J.H.; Pye, H.; Hamoudi, R.A.; Moore, C.M.; MacRobert, A.J. Efficacy of photochemical internalisation using disulfonated chlorin and porphyrin photosensitisers: An in vitro study in 2D and 3D prostate cancer models. Cancer Lett. 2017, 393, 68–75. [Google Scholar] [CrossRef] [PubMed]


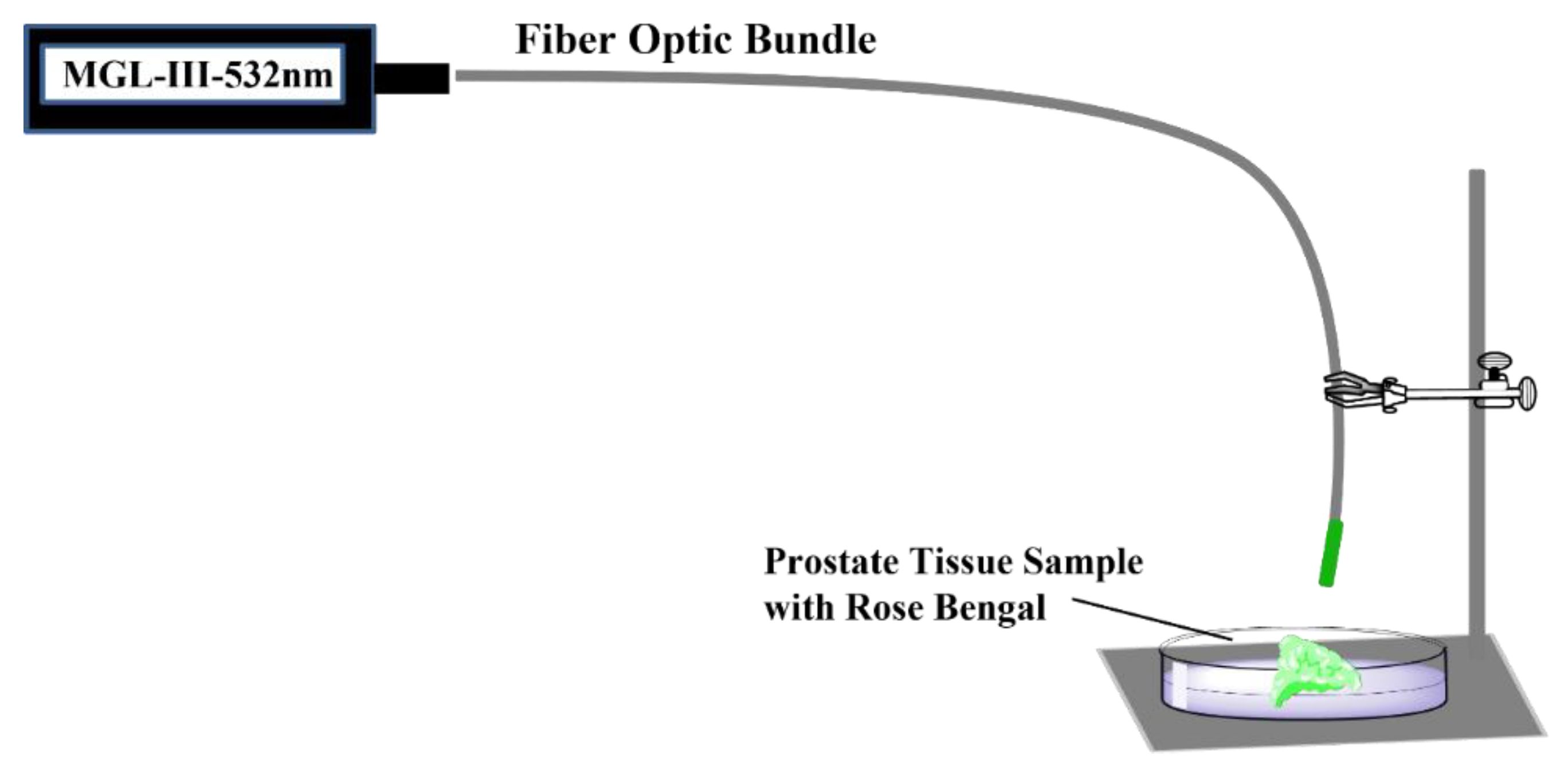
| Prostate Tissue Sample Photosensitizer Concentrations | |||
|---|---|---|---|
| Photosensitizer | Concentration [mM] | Wavelength of Light [nm] | Number of Samples |
| 0.1 | control, no light exposure | 5 | |
| Rose Bengal (RB) | control, 0 mM RB | 532 | 5 |
| 0.1 | 532 | 6 | |
| 0.2 | 532 | 6 | |
| 0.3 | 532 | 6 | |
| 0.4 | 532 | 6 | |
| 0.5 | 532 | 6 | |
| 3 | control, no light exposure | 5 | |
| Protoporphyrin IX disodium salt (PpIX) | control, 0 mM PpIX | 410 | 5 |
| 3 | 410 | 15 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aebisher, D.; Osuchowski, M.; Bartusik-Aebisher, D.; Krupka-Olek, M.; Dynarowicz, K.; Kawczyk-Krupka, A. An Analysis of the Effects of In Vitro Photodynamic Therapy on Prostate Cancer Tissue by Histopathological Examination and Magnetic Resonance Imaging. Int. J. Mol. Sci. 2022, 23, 11354. https://doi.org/10.3390/ijms231911354
Aebisher D, Osuchowski M, Bartusik-Aebisher D, Krupka-Olek M, Dynarowicz K, Kawczyk-Krupka A. An Analysis of the Effects of In Vitro Photodynamic Therapy on Prostate Cancer Tissue by Histopathological Examination and Magnetic Resonance Imaging. International Journal of Molecular Sciences. 2022; 23(19):11354. https://doi.org/10.3390/ijms231911354
Chicago/Turabian StyleAebisher, David, Michał Osuchowski, Dorota Bartusik-Aebisher, Magdalena Krupka-Olek, Klaudia Dynarowicz, and Aleksandra Kawczyk-Krupka. 2022. "An Analysis of the Effects of In Vitro Photodynamic Therapy on Prostate Cancer Tissue by Histopathological Examination and Magnetic Resonance Imaging" International Journal of Molecular Sciences 23, no. 19: 11354. https://doi.org/10.3390/ijms231911354
APA StyleAebisher, D., Osuchowski, M., Bartusik-Aebisher, D., Krupka-Olek, M., Dynarowicz, K., & Kawczyk-Krupka, A. (2022). An Analysis of the Effects of In Vitro Photodynamic Therapy on Prostate Cancer Tissue by Histopathological Examination and Magnetic Resonance Imaging. International Journal of Molecular Sciences, 23(19), 11354. https://doi.org/10.3390/ijms231911354








