A Hormetic Spatiotemporal Photosystem II Response Mechanism of Salvia to Excess Zinc Exposure
Abstract
1. Introduction
2. Results
2.1. Zinc and Nutrient Concentrations before and after Zinc Exposure
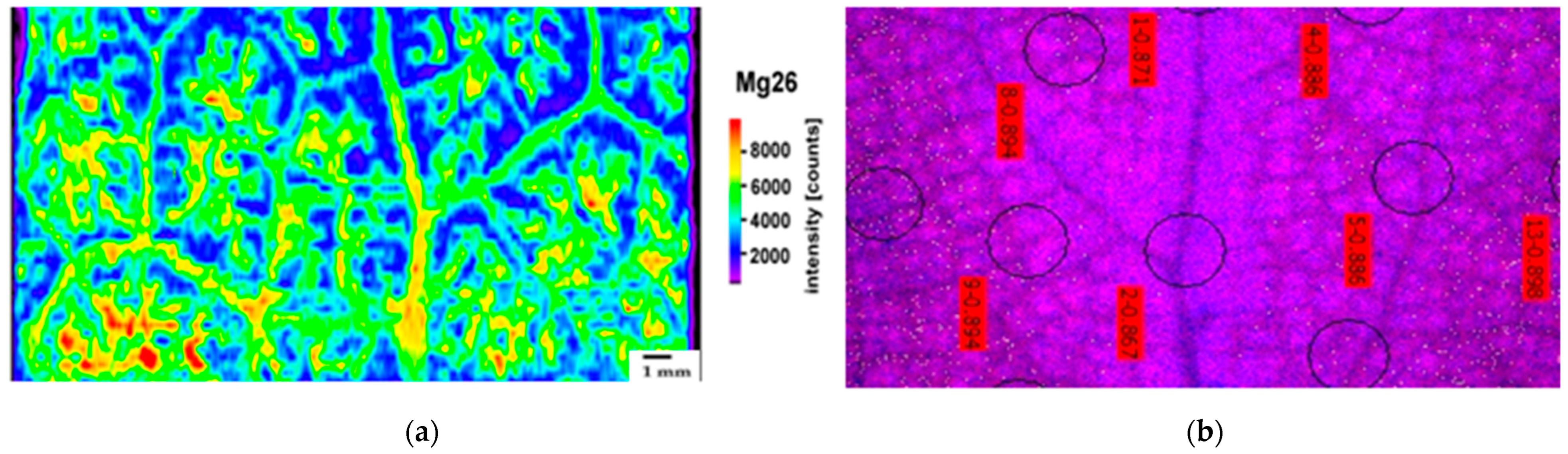
2.2. Leaf Distribution Pattern of Zn, Ca, and Mg
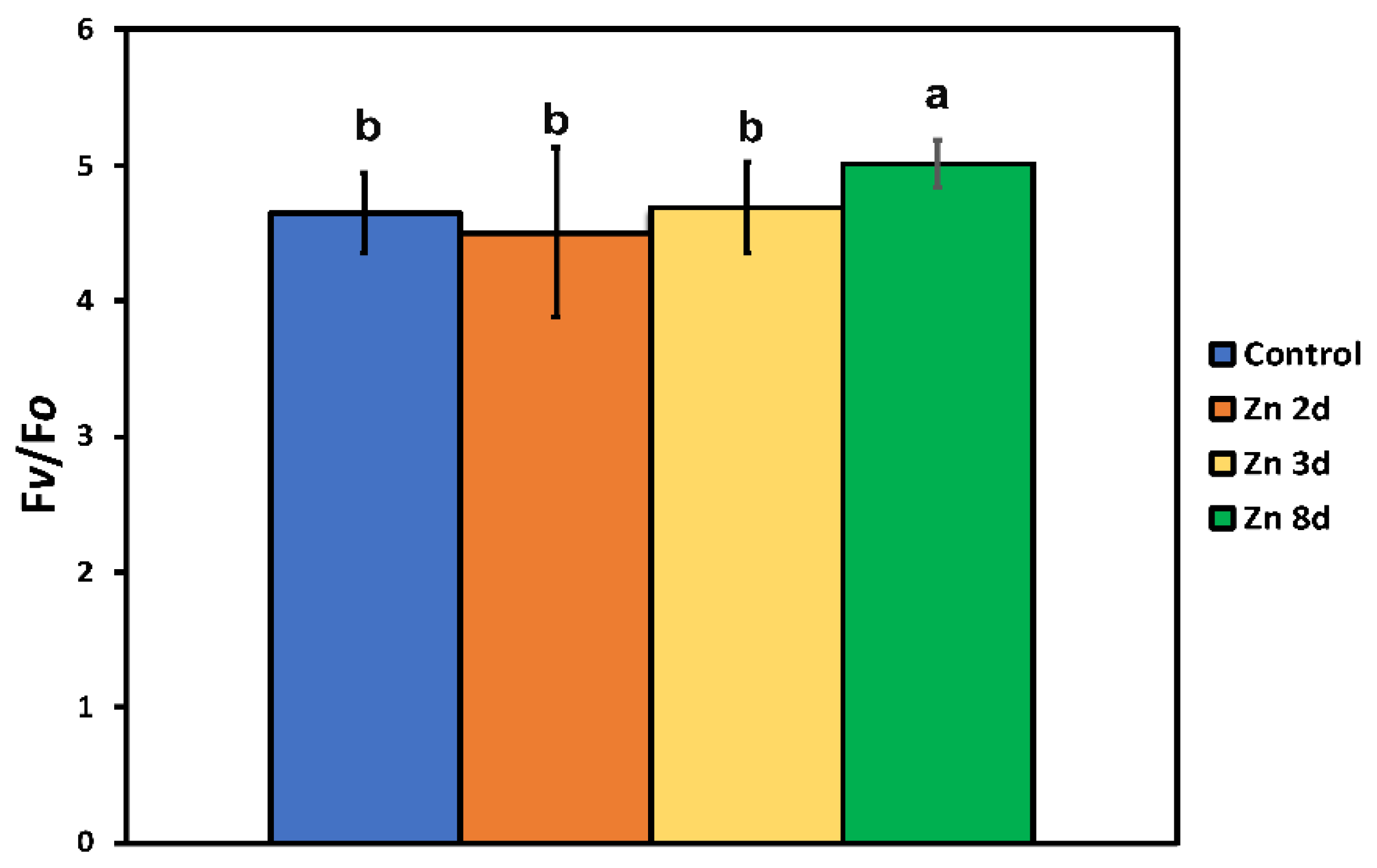
2.3. Maximum Efficiency of Photosystem II Photochemistry and Light-Energy Utilization in PSII before and after Zinc Exposure
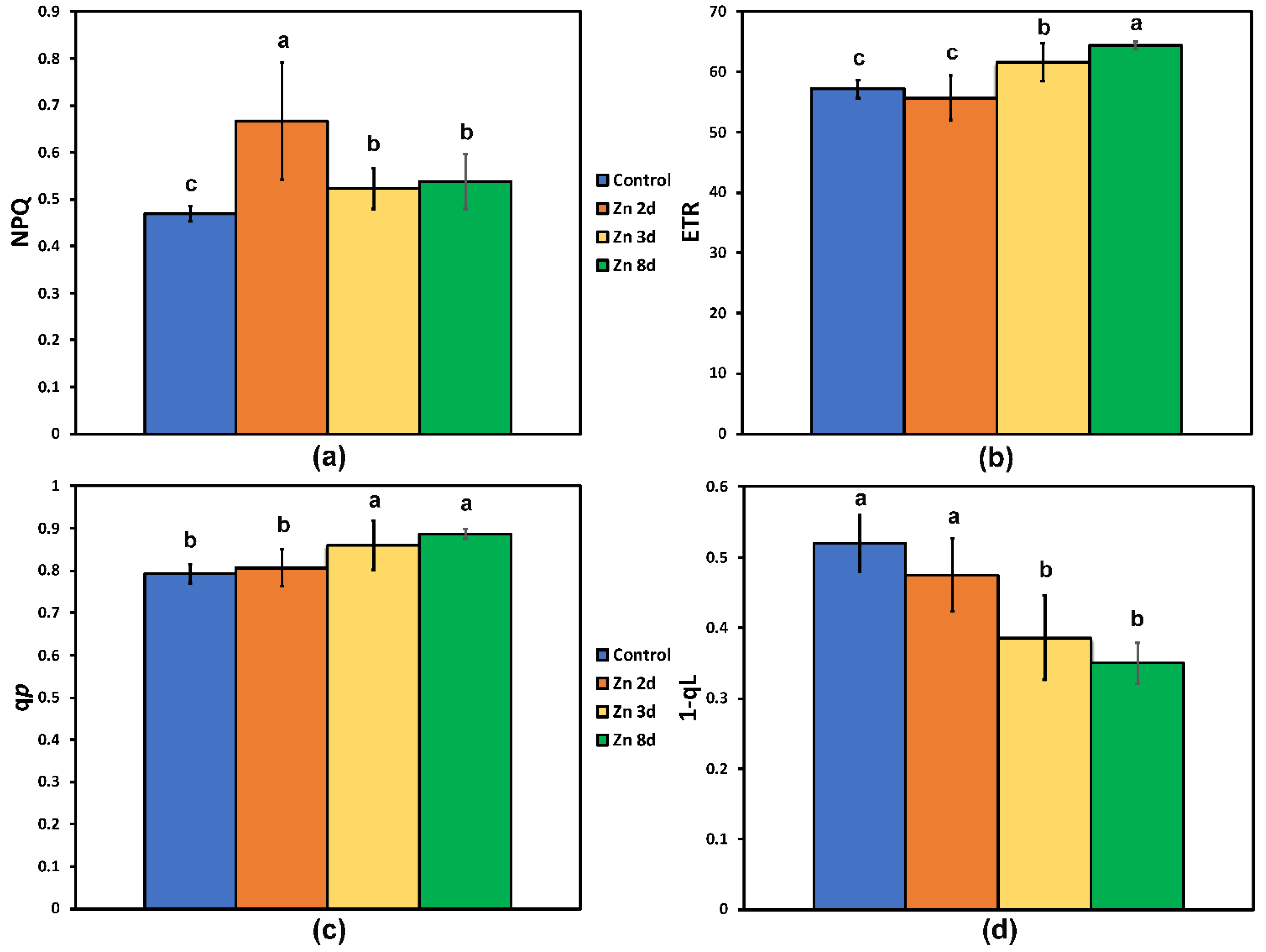
2.4. Changes in the Photoprotective Heat Dissipation and the Redox State of the Plastoquinone Pool after Zinc Exposure
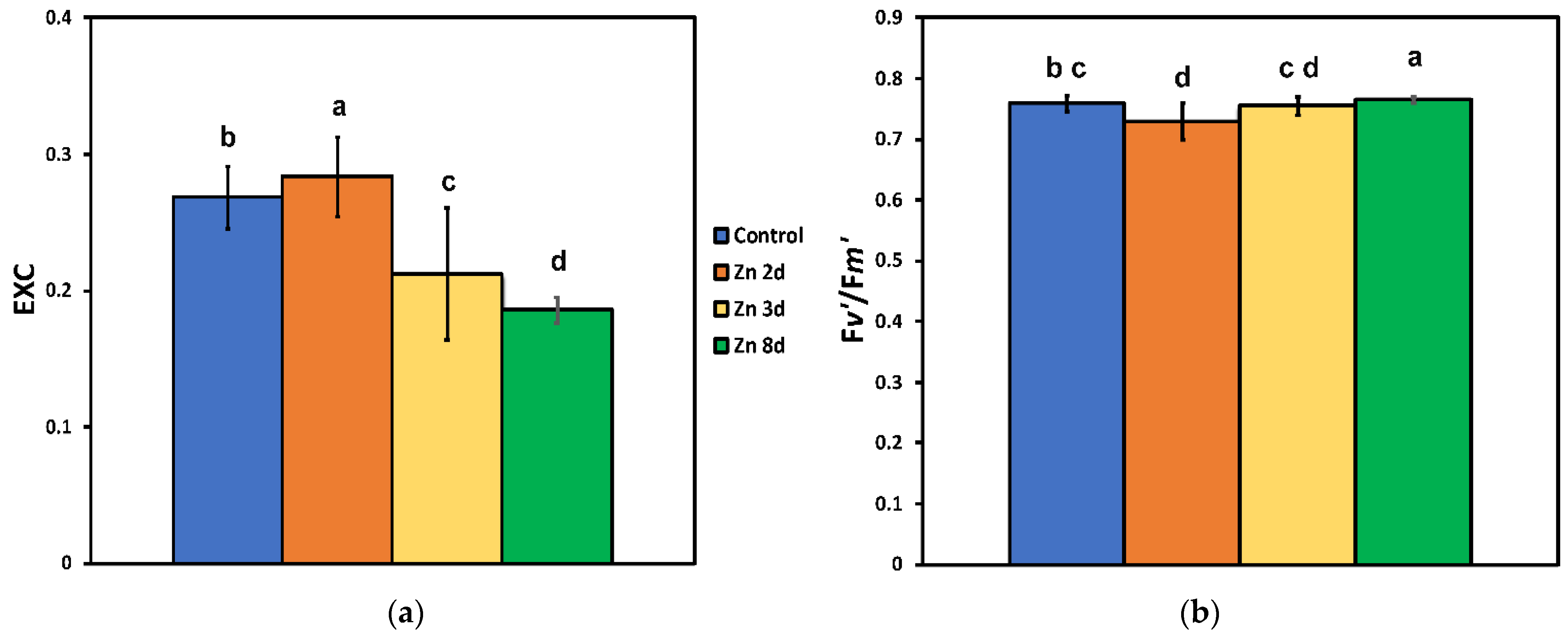
2.5. Changes in the Excess Excitation Energy in Photosystem II and the Efficiency of Open PSII Reaction Centers after Zinc Exposure
2.6. The Efficiency of the Oxygen Evolving Complex before and after Zinc Exposure
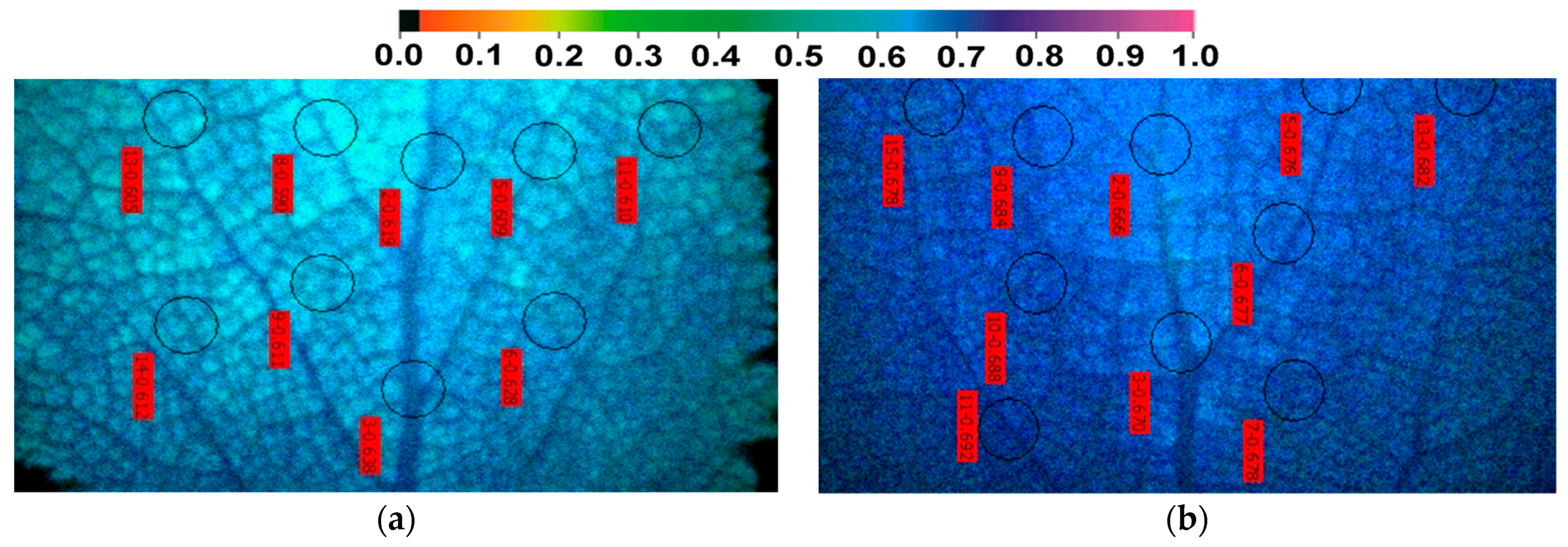
2.7. The Spatial Pattern of the Quantum Yield of PSII Photochemistry (ΦPSΙΙ) before and after Zinc Exposure
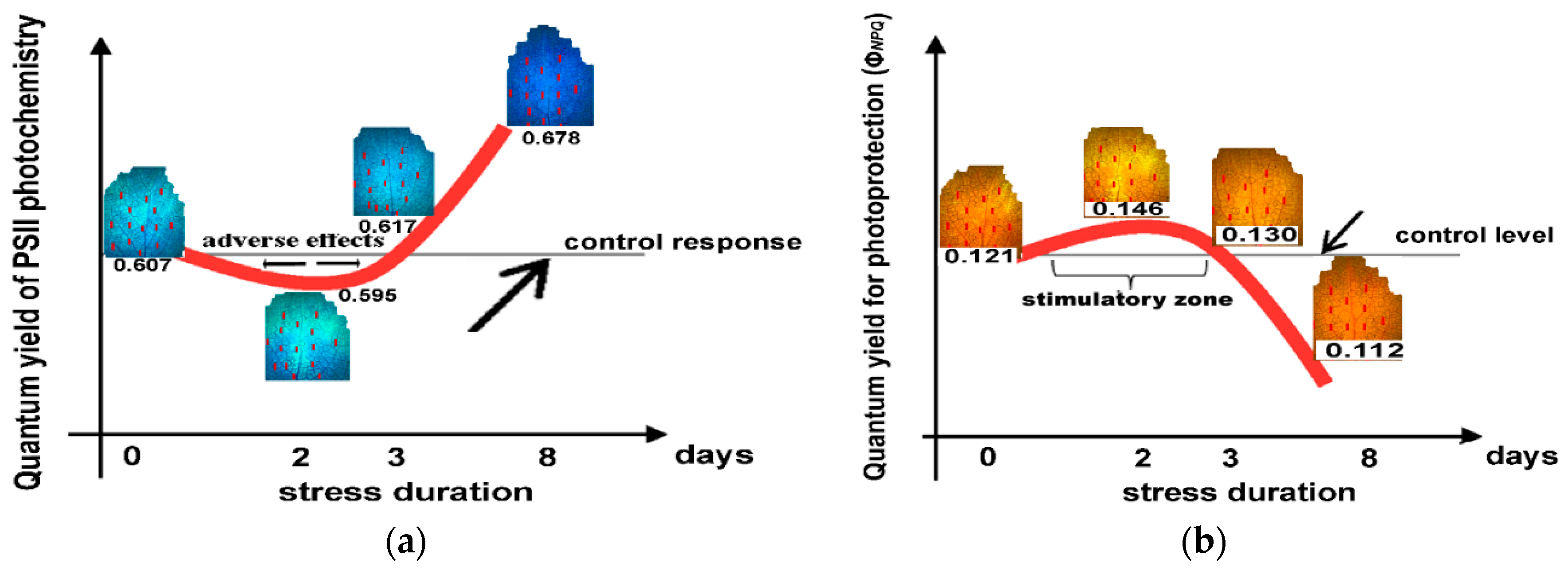
2.8. The Hormetic Response Curves of the Quantum Yield of PSII Photochemistry (ΦPSII) and the Quantum Yield of Regulated Non-photochemical Energy Loss in PSII (ΦNPQ) in Response to Excess Zinc
2.9. Leaf Heterogeneity in Phenolic Accumulation
2.10. Heterogeneity in Chlorophyll Fluorescence Parameters between Paraveinal Areas and Intercostal Fields
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Zinc Treatments
4.3. Zinc and Nutrient Cation Determination by Inductively Coupled Plasma–Mass Spectrometry (ICP–MS)
4.4. Laser Ablation–Inductively Coupled Plasma–Mass Spectrometry
4.5. Chlorophyll Fluorescence Imaging Analysis
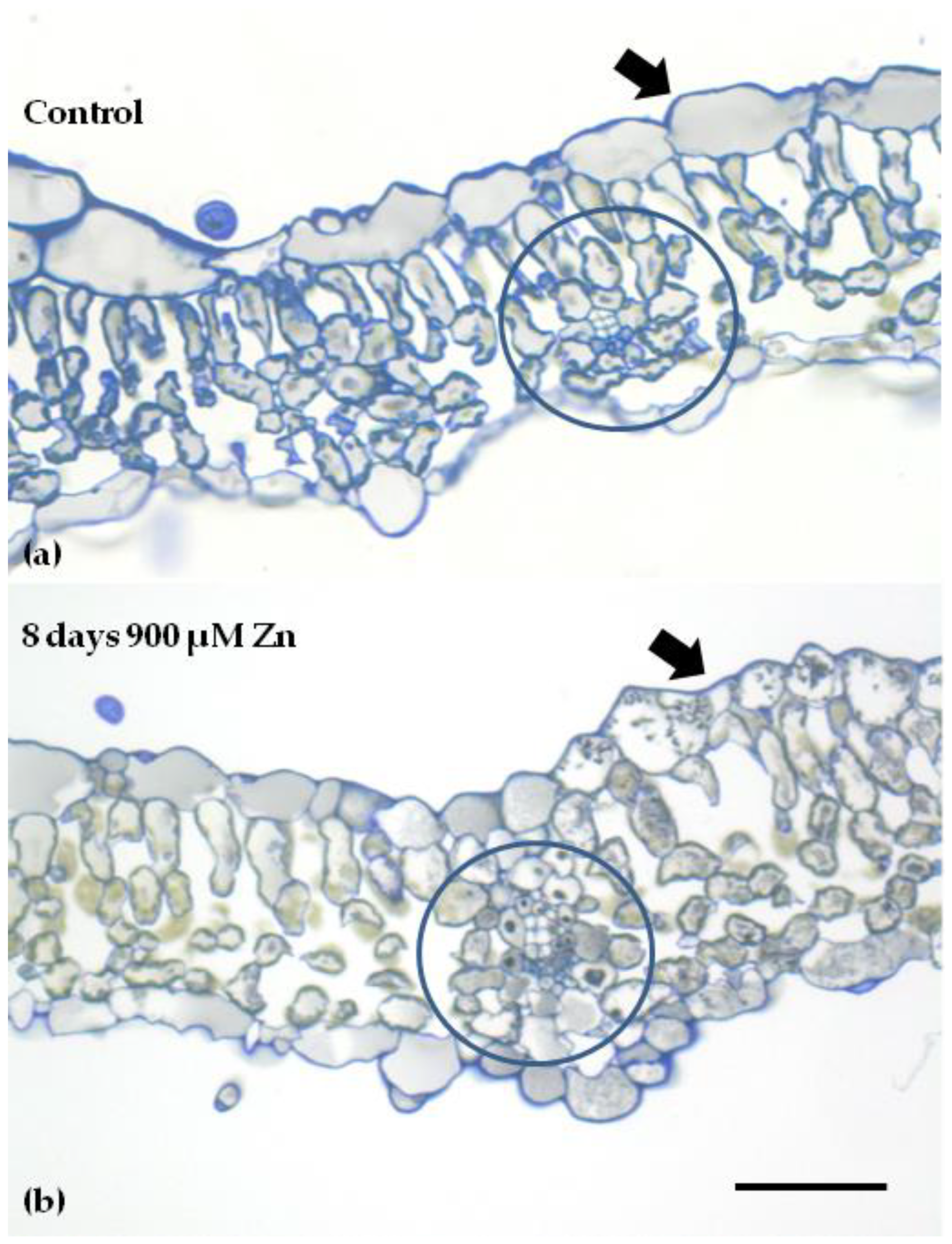
4.6. Light Microscopy
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Cheah, B.H.; Chen, Y.L.; Lo, J.C.; Tang, I.C.; Yeh, K.C.; Lin, Y.F. Divalent nutrient cations: Friend and foe during zinc stress in rice. Plant Cell Environ. 2021, 44, 3358–3375. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Adamakis, I.D.S.; Dobrikova, A.; Apostolova, E.; Hanć, A.; Moustakas, M. Excess zinc supply reduces cadmium uptake and mitigates cadmium toxicity effects on chloroplast structure, oxidative stress, and photosystem II photochemical efficiency in Salvia sclarea plants. Toxics 2022, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, S.; Stoyanova, Z.; Velikova, V. The influence of succinate on zinc toxicity of pea plant. J. Plant Nutr. 2001, 24, 789–804. [Google Scholar] [CrossRef]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc hyperaccumulation in plants: A review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]
- Dobrikova, A.; Apostolova, E.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis, I.D.S.; Moustakas, M. Tolerance mechanisms of the aromatic and medicinal plant Salvia sclarea L. to excess zinc. Plants 2021, 10, 194. [Google Scholar] [CrossRef]
- Tsonev, T.; Lidon, F.J.C. Zinc in plants—An overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Caldelas, C.; Weiss, D.J. Zinc homeostasis and isotopic fractionation in plants: A review. Plant Soil 2017, 411, 17–46. [Google Scholar] [CrossRef]
- Jan, A.U.; Hadi, F.; Ditta, A.; Suleman, M.; Ullah, M. Zinc-induced anti-oxidative defense and osmotic adjustments to enhance drought stress tolerance in sunflower (Helianthus annuus L.). Environ. Exp. Bot. 2022, 193, 104682. [Google Scholar] [CrossRef]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef]
- Moustakas, M. The role of metal ions in biology, biochemistry and medicine. Materials 2021, 14, 549. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Bayçu, G.; Moustaka, J.; Gevrek-Kürüm, N.; Moustakas, M. Chlorophyll fluorescence imaging analysis for elucidating the mechanism of photosystem II acclimation to cadmium exposure in the hyperaccumulating plant Noccaea caerulescens. Materials 2018, 11, 2580. [Google Scholar] [CrossRef] [PubMed]
- Van de Mortel, J.E.; Almar Villanueva, L.; Schat, H.; Kwekkeboom, J.; Coughlan, S.; Moerland, P.D.; Ver Loren van Themaat, E.; Koornneef, M.; Aarts, M.G. Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2006, 142, 1127–1147. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Photoprotective mechanism of the non-target organism Arabidopsis thaliana to paraquat exposure. Pest. Biochem. Physiol. 2014, 111, 1–6. [Google Scholar] [CrossRef]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age dependent photoprotective and antioxidative mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Takagi, D.; Takumi, S.; Hashiguchi, M.; Sejima, T.; Miyake, C. Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant. Physiol. 2016, 171, 1626–1634. [Google Scholar] [CrossRef]
- Sperdouli, I.; Mellidou, I.; Moustakas, M. Harnessing chlorophyll fluorescence for phenotyping analysis of wild and cultivated tomato for high photochemical efficiency under water deficit for climate change resilience. Climate 2021, 9, 154. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Andreadis, S.; Moustaka, J.; Panteris, E.; Tsaballa, A.; Moustakas, M. Changes in light energy utilization in photosystem II and reactive oxygen species generation in potato leaves by the pinworm Tuta absoluta. Molecules 2021, 26, 2984. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef]
- De Bianchi, S.; Betterle, N.; Kouril, R.; Cazzaniga, S.; Boekema, E.; Bassi, R.; Dall’Osto, L. Arabidopsis mutants deleted in the light-harvesting protein Lhcb4 have a disrupted photosystem II macrostructure and are defective in photoprotection. Plant Cell 2011, 23, 2659–2679. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Moustaka, J.; Meyling, N.V.; Hauser, T.P. Induction of a compensatory photosynthetic response mechanism in tomato leaves upon short time feeding by the chewing insect Spodoptera exigua. Insects 2021, 12, 562. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustaka, J.; Ouzounidou, G.; Moustakas, M. Leaf age-dependent photosystem II photochemistry and oxidative stress responses to drought stress in Arabidopsis thaliana are modulated by flavonoid accumulation. Molecules 2021, 26, 4157. [Google Scholar] [CrossRef]
- Bayçu, G.; Gevrek-Kürüm, N.; Moustaka, J.; Csatári, I.; Rognes, S.E.; Moustakas, M. Cadmium-zinc accumulation and photosystem II responses of Noccaea caerulescens to Cd and Zn exposure. Environ. Sci. Pollut. Res. Int. 2017, 24, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Bayçu, G.; Gevrek-Kürüm, N.; Moustaka, J.; Csatári, I.; Rognes, S.E. Spatiotemporal heterogeneity of photosystem II function during acclimation to zinc exposure and mineral nutrition changes in the hyperaccumulator Noccaea caerulescens. Environ. Sci. Pollut. Res. Int. 2019, 26, 6613–6624. [Google Scholar] [CrossRef]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Goltsev, V. Effects of different metals on photosynthesis: Cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef] [PubMed]
- Szopiński, M.; Sitko, K.; Gieroń, Ż.; Rusinowski, S.; Corso, M.; Hermans, C.; Verbruggen, N.; Małkowski, E. Toxic Effects of Cd and Zn on the photosynthetic apparatus of the Arabidopsis halleri and Arabidopsis arenosa pseudo-metallophytes. Front. Plant Sci. 2019, 10, 748. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustaka, J.; Antonoglou, O.; Adamakis, I.-D.S.; Dendrinou-Samara, C.; Moustakas, M. Leaf age-dependent effects of foliar-sprayed CuZn nanoparticles on photosynthetic efficiency and ROS generation in Arabidopsis thaliana. Materials 2019, 12, 2498. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.E.A.; Castro, P.R.C.; Azevedo, R.A. Hormesis in plants under Cd exposure: From toxic to beneficial element? J. Hazard. Mater. 2020, 384, 121434. [Google Scholar] [CrossRef] [PubMed]
- Malea, P.; Charitonidou, K.; Sperdouli, I.; Mylona, Z.; Moustakas, M. Zinc uptake, photosynthetic efficiency and oxidative stress in the seagrass Cymodocea nodosa exposed to ZnO nanoparticles. Materials 2019, 12, 2101. [Google Scholar] [CrossRef]
- Adamakis, I.-D.S.; Sperdouli, I.; Hanć, A.; Dobrikova, A.; Apostolova, E.; Moustakas, M. Rapid hormetic responses of photosystem II photochemistry of clary sage to cadmium exposure. Int. J. Mol. Sci. 2021, 22, 41. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Harayama, H. On the non-monotonic, hormetic photoprotective response of plants to stress. Dose-Response 2019, 17, 1559325819838420. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: Highly generalizable and beyond laboratory. Trends Plant Sci. 2020, 25, 1076–1086. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis: The dose response for the 21st Century: The future has arrived. Toxicology 2019, 425, 152249. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E. The rise and fall of photosynthesis: Hormetic dose response in plants. J. For. Res. 2021, 32, 889–898. [Google Scholar] [CrossRef]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic approach. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol. Sci. 2001, 22, 285–291. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef]
- Calabrese, E.J. Evidence that hormesis represents an ‘‘overcompensation’’ response to a disruption in homeostasis. Ecotoxicol. Environ. Saf. 1999, 42, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A compelling platform for sophisticated plant science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef]
- Małkowski, E.; Sitko, K.; Szopiński, M.; Gieroń, Z.; Pogrzeba, M.; Kalaji, H.M.; Zieléznik-Rusinowska, P. Hormesis in plants: The role of oxidative stress, auxins and photosynthesis in corn treated with Cd or Pb. Int. J. Mol. Sci. 2020, 21, 2099. [Google Scholar] [CrossRef]
- Barbagallo, R.P.; Oxborough, K.; Pallett, K.E.; Baker, N.R. Rapid, noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol. 2003, 132, 485–493. [Google Scholar] [CrossRef]
- Moustaka, J.; Meyling, V.N.; Hauser, T.P. Root-associated entomopathogenic fungi modulate host plant’ s photosystem II photochemistry and its response to herbivorous insects. Molecules 2022, 27, 207. [Google Scholar] [CrossRef]
- Stamelou, M.L.; Sperdouli, I.; Pyrri, I.; Adamakis, I.D.S.; Moustakas, M. Hormetic responses of photosystem II in tomato to Botrytis cinerea. Plants 2021, 10, 521. [Google Scholar] [CrossRef] [PubMed]
- Oláh, V.; Hepp, A.; Irfan, M.; Mészáros, I. Chlorophyll fluorescence imaging-based duckweed phenotyping to assess acute phytotoxic effects. Plants 2021, 10, 2763. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Calatayud, A.; Guidi, L. Chlorophyll fluorescence imaging analysis in biotic and abiotic stress. Front. Plant Sci. 2021, 12, 658500. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.D.S.; Malea, P.; Sperdouli, I.; Panteris, E.; Kokkinidi, D.; Moustakas, M. Evaluation of the spatiotemporal effects of bisphenol A on the leaves of the seagrass Cymodocea nodosa. J. Hazard. Mater. 2021, 404, 124001. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Hanć, A.; Dobrikova, A.; Sperdouli, I.; Adamakis, I.D.S.; Apostolova, E. Spatial heterogeneity of cadmium effects on Salvia sclarea leaves revealed by chlorophyll fluorescence imaging analysis and laser ablation inductively coupled plasma mass spectrometry. Materials 2019, 12, 2953. [Google Scholar] [CrossRef]
- Doble, P.A.; de Vega, R.G.; Bishop, D.P.; Hare, D.J.; Clases, D. Laser ablation-inductively coupled plasma-mass spectrometry imaging in biology. Chem. Rev. 2021, 121, 11769–11822. [Google Scholar] [CrossRef]
- Karalija, E.; Dahija, S.; Tarkowski, P.; Zeljković, S.Ć. Influence of climate-related environmental stresses on economically important essential oils of mediterranean Salvia sp. Front. Plant Sci. 2022, 13, 864807. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Cvetković, M.T.; Stanković Jeremić, J.M.; Pezo, L.L.; Varga, A.O.; Čabarkapa, I.S.; Kiprovski, B. Biological activity and profiling of Salvia sclarea essential oil obtained by steam and hydrodistillation extraction methods via chemometrics tools. Flavour Fragr. J. 2022, 37, 20–32. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Iapichino, G.; Licata, M.; Virga, G.; Leto, C.; La Bella, S. Agronomic evaluation and chemical characterization of Sicilian Salvia sclarea L. accessions. Agronomy 2020, 10, 1114. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef]
- Kuźma, L.; Kalemba, D.; Rózalski, M.; Rózalska, B.; Wieckowska-Szakiel, M.; Krajewska, U.; Wysokińska, H. Chemical composition and biological activities of essential oil from Salvia sclarea plants regenerated in vitro. Molecules 2009, 14, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Pitarokili, D.; Couladis, M.; Petsikos-Panayotarou, N.; Tzakou, O. Composition and antifungal activity on soil-borne pathogens of the essential oil of Salvia sclarea from Greece. J. Agric. Food Chem. 2002, 50, 6688–6691. [Google Scholar] [CrossRef]
- Dobrikova, A.; Apostolova, E.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis, I.D.S.; Moustakas, M. Cadmium toxicity in Salvia sclarea L.: An integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotoxicol. Environ. Saf. 2021, 209, 111851. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Jiao, Q.; Agathokleous, E.; Liu, H.; Li, G.; Zhang, J.; Fahad, S.; Jiang, Y. Hormetic effects of zinc on growth and antioxidant defense system of wheat plants. Sci. Total Environ. 2022, 807, 150992. [Google Scholar] [CrossRef] [PubMed]
- Lasat, M.M.; Baker, A.J.; Kochian, L.V. Altered Zn compartmentation in the root symplasm and stimulated Zn absorption into the leaf as mechanisms involved in Zn hyperaccumulation in Thlaspi caerulescens. Plant Physiol. 1998, 118, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Li, C.; Rengel, Z.; Liu, H.G.; Zhao, P. Excess Zn-induced changes in physiological parameters and expression levels of TaZips in two wheat genotypes. Environ. Exp. Bot. 2020, 177, 104133. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Moustaka, J.; Ouzounidou, G.; Sperdouli, I.; Moustakas, M. Photosystem II is more sensitive than photosystem I to Al3+ induced phytotoxicity. Materials 2018, 11, 1772. [Google Scholar] [CrossRef]
- Guidi, L.; Calatayud, A. Non-invasive tools to estimate stress-induced changes in photosynthetic performance in plants inhabiting Mediterranean areas. Environ. Exp. Bot. 2014, 103, 42–52. [Google Scholar] [CrossRef]
- Moustakas, M.; Bayçu, G.; Sperdouli, I.; Eroğlu, H.; Eleftheriou, E.P. Arbuscular mycorrhizal symbiosis enhances photosynthesis in the medicinal herb Salvia fruticosa by improving photosystem II photochemistry. Plants 2020, 9, 962. [Google Scholar] [CrossRef]
- Von Bremen-Kühne, M.; Ahmadi, H.; Sperling, M.; Krämer, U.; Karst, U. Elemental bioimaging of Zn and Cd in leaves of hyperaccumulator Arabidopsis halleri using laser ablation-inductively coupled plasma-mass spectrometry and referencing strategies. Chemosphere 2022, 305, 135267. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gao, Y.; Wang, X.; Li, S.; Zheng, H.; Chen, Z.; Wu, F.; Du, X.; Sui, N. Exogenous calcium application enhances salt tolerance of sweet sorghum seedling. J. Agron. Crop Sci. 2022, 208, 441–453. [Google Scholar] [CrossRef]
- Sitko, K.; Rusinowski, S.; Kalaji, H.M.; Szopiński, M.; Małkowski, E. Photosynthetic efficiency as bioindicator of environmental pressure in A. halleri. Plant Physiol. 2017, 175, 290–302. [Google Scholar] [CrossRef]
- Szopiński, M.; Sitko, K.; Rusinowski, S.; Zieleźnik-Rusinowska, P.; Corso, M.; Rostański, A.; Rojek-Jelonek, M.; Verbruggen, N.; Małkowski, E. Different strategies of Cd tolerance and accumulation in Arabidopsis halleri and Arabidopsis arenosa. Plant Cell Environ. 2020, 43, 3002–3019. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.H.; Nkrumah, P.N.; Erskine, P.D.; van der Ent, A. Polymetallic (zinc and cadmium) hyperaccumulation in the Australian legume Crotalaria novae-hollandiae compared to Crotalaria cunninghamii. Plant Soil 2022, 1–18. [Google Scholar] [CrossRef]
- Merlot, S.; de la Torre, V.S.G.; Hanikenne, M. Physiology and molecular biology of trace element hyperaccumulation. In Agromining: Farming for Metals; Springer: Berlin, Germany, 2018; pp. 93–116. [Google Scholar]
- Cosio, C.; DeSantis, L.; Frey, B.; Diallo, S.; Keller, C. Distribution of cadmium in leaves of Thlaspi caerulescens. J. Exp. Bot. 2005, 56, 765–775. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Moustakas, M.; Symeonidis, L.; Karataglis, S. Response of wheat seedlings to Ni stress: Effects of supplemental calcium. Arch. Environ. Contam. Toxicol. 2006, 50, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
- Hankamer, B.; Barber, J.; Boekema, E.J. Structure and membrane organization of photosystem II in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 641–671. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Díaz, M.; Alberdi, M.; Ivanov, A.G.; Krol, M.; Hüner, N.P. Excess manganese differentially inhibits photosystem I versus II in Arabidopsis thaliana. J. Exp. Bot. 2013, 64, 343–354. [Google Scholar] [CrossRef]
- Sinha, S.; Mukherji, S.; Dutta, J. Effect of manganese toxicity on pigment content, Hill activity and photosynthetic rate of Vigna radiata L. Wilczek seedlings. J. Environ. Biol. 2002, 23, 253–257. [Google Scholar]
- González, A.; Steffen, K.L.; Lynch, J.P. Light and excess manganese. Implication for oxidative stress in common bean. Plant Physiol. 1998, 18, 493–504. [Google Scholar]
- Mora, M.; Rosas, A.; Ribera, A.; Rengel, R. Differential tolerance to Mn toxicity in perennial ryegrass genotypes: Involvement of antioxidative enzymes and root exudation of carboxylates. Plant Soil 2009, 320, 79–89. [Google Scholar] [CrossRef]
- Lei, Y.; Korpelainen, H.; Li, C. Physiological and biochemical responses to high Mn concentrations in two contrasting Populus cathayana populations. Chemosphere 2007, 68, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Smith, S. Mineral nutrition. In Plant physiology and development, 6th ed.; Taiz, L., Zeiger, E., Møller, I.M., Murphy, A., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 107–130. [Google Scholar]
- Bezerra-Neto, E.; Coelho, J.B.M.; Jarma-Orozco, A.; Rodríguez-Páez, L.A.; Pompelli, M.F. Modulation of photosynthesis under salinity and the role of mineral nutrients in Jatropha curcas L. J. Agro. Crop Sci. 2022, 208, 314–334. [Google Scholar] [CrossRef]
- Beale, S.I. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 1999, 60, 43–73. [Google Scholar] [CrossRef]
- Liu, J.-P.; Deng, Q.-J.; Shang, Y.-J.; Yao, X.-W.; Wang, H.-K.; Tang, Y.-J.; Peng, F.-R.; Tan, P.-P. Effects of zinc application on the growth and photosynthetic characteristics of pecan at the seedling stage. Plant Biol. 2021, 23, 1149–1156. [Google Scholar] [CrossRef]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef]
- Ort, D.R.; Zhu, X.; Melis, A. Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol. 2011, 155, 79–85. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Moustaka, J.; İşgören, S.; Şaş, B. Harnessing the role of foliar applied salicylic acid in decreasing chlorophyll content to reassess photosystem II photoprotection in crop plants. Int. J. Mol. Sci. 2022, 23, 7038. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Magnesium: A Forgotten Element in Crop Production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant. Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Niyogi, K.K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Light harvesting control in plants. FEBS Lett. 2018, 592, 3030–3039. [Google Scholar] [CrossRef]
- Wang, G.; Zeng, F.; Song, P.; Sun, B.; Wang, Q.; Wang, J. Effects of reduced chlorophyll content on photosystem functions and photosynthetic electron transport rate in rice leaves. J. Plant Physiol. 2022, 272, 153669. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. A better energy allocation of absorbed light in photosystem II and less photooxidative damage contribute to acclimation of Arabidopsis thaliana young leaves to water deficit. J. Plant Physiol. 2014, 171, 587–593. [Google Scholar] [CrossRef]
- Serôdio, J.; Campbell, D.A. Photoinhibition in optically thick samples: Effects of light attenuation on chlorophyll fluorescence-based parameters. J. Theor. Biol. 2021, 513, 110580. [Google Scholar] [CrossRef]
- Raven, J.A. The cost of photoinhibition. Physiol. Plant. 2011, 142, 87–104. [Google Scholar] [CrossRef]
- Campbell, D.A.; Tyystjärvi, E. Parameterization of photosystem II photoinactivation and repair. Biochim. Biophys. Acta 2012, 1817, 258–265. [Google Scholar] [CrossRef]
- Kasajima, I.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H. Estimation of the relative sizes of rate constants for chlorophyll de-excitation processes through comparison of inverse fluorescence intensities. Plant Cell Physiol. 2009, 50, 1600–1616. [Google Scholar] [CrossRef]
- Wilson, K.E.; Ivanov, A.G.; Öquist, G.; Grodzinski, B.; Sarhan, F.; Huner, N.P.A. Energy balance, organellar redox status, and acclimation to environmental stress. Can. J. Bot. 2006, 84, 1355–1370. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Gawroński, P.; Witoń, D.; Vashutina, K.; Bederska, M.; Betliński, B.; Rusaczonek, A.; Karpiński, S. Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol. Plant 2014, 7, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Complementary PSII quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Adamakis, I.D.S.; Sperdouli, I.; Eleftheriou, E.P.; Moustakas, M. Hydrogen peroxide production by the spot-like mode action of bisphenol A. Front. Plant Sci. 2020, 11, 1196. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Yang, S.; Zeng, X.; Li, T.; Liu, M.; Zhang, S.; Gao, S.; Wang, Y.; Peng, C.; Li, L.; Yang, C. AtACDO1, an ABC1-like kinase gene, is involved in chlorophyll degradation and the response to photooxidative stress in Arabidopsis. J. Exp. Bot. 2012, 63, 3959–3973. [Google Scholar] [CrossRef]
- Szabò, I.; Spetea, C. Impact of the ion transportome of chloroplasts on the optimization of photosynthesis. J. Exp. Bot. 2017, 68, 3115–3128. [Google Scholar] [CrossRef]
- Poudyal, R.S.; Nath, K.; Zulfugarov, I.S.; Lee, C.H. Production of superoxide from photosystem II-light harvesting complex II supercomplex in STN8 kinase knock-out rice mutants under photoinhibitory illumination. J. Photochem. Photobiol. B. 2016, 162, 240–247. [Google Scholar] [CrossRef]
- Agathokleous, E. Environmental hormesis, a fundamental non-monotonic biological phenomenon with implications in ecotoxicology and environmental safety. Ecotoxicol. Environ. Saf. 2018, 148, 1042–1053. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The dose-response revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Erofeeva, E.A. Environmental hormesis: From cell to ecosystem. Curr. Opin. Environ. Sci. Health 2022, 29, 100378. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Editorial overview: Hormesis and dose-response. Curr. Opin. Toxicol. 2022, 30, 100343. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M. Plant Photochemistry, Reactive Oxygen Species, and Photoprotection. Photochem 2022, 2, 5–8. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Van Breusegem, F. Improving oxidative stress resilience in plants. Plant J. 2022, 109, 359–372. [Google Scholar] [CrossRef]
- Huertas, R.; Karpinska, B.; Ngala, S.; Mkandawire, B.; Maling’a, J.; Wajenkeche, E.; Kimani, P.M.; Boesch, C.; Stewart, D.; Hancock, R.D.; et al. Biofortification of common bean (Phaseolus vulgaris L.) with iron and zinc: Achievements and challenges. Food Energy Secur. 2022, e406. [Google Scholar] [CrossRef]
- Kong, D.; Khan, S.A.; Wu, H.; Liu, Y.; Ling, H.-Q. Biofortification of iron and zinc in rice and wheat. J. Integr. Plant Biol. 2022, 64, 1157–1167. [Google Scholar] [CrossRef]
- Zhao, T.; Dai, A. The magnitude and causes of global drought changes in the twenty-first century under a low–severe emissions scenario. J. Clim. 2015, 28, 4490–4512. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Ogaya, R.; Fernández-Martínez, M.; Pugh, T.A.M.; Peñuelas, J. Increasing climatic sensitivity of global grassland vegetation biomass and species diversity correlates with water availability. New Phytol. 2021, 230, 1761–1771. [Google Scholar] [CrossRef]
- Sattar, A.; Wang, X.; Ul-Allah, S.; Sher, A.; Ijaz, M.; Irfan, M.; Abbas, T.; Hussain, S.; Nawaz, F.; Al-Hashimi, A.; et al. Foliar application of zinc improves morpho-physiological and antioxidant defense mechanisms, and agronomic grain biofortification of wheat (Triticum aestivum L.) under water stress. Saudi J. Biol. Sci. 2022, 29, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Pavia, I.; Roque, J.; Rocha, L.; Ferreira, H.; Castro, C.; Carvalho, A.; Silva, E.; Brito, C.; Gonçalves, A.; Lima-Brito, J.; et al. Zinc priming and foliar application enhances photoprotection mechanisms in drought-stressed wheat plants during anthesis. Plant Physiol. Biochem. 2019, 140, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, F.; Zhou, H.; Xia, Y.; Wang, X. Photoprotection conferring plant tolerance to freezing stress through rescuing photosystem in evergreen Rhododendron. Plant Cell Environ. 2022, 29, 100378. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Reis, A.R.D. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Accumulator plants and hormesis. Environ. Pollut. 2021, 274, 116526. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Spatio-temporal heterogeneity in Arabidopsis thaliana leaves under drought stress. Plant Biol. 2012, 14, 118–128. [Google Scholar] [CrossRef]
- Bresson, J.; Vasseur, F.; Dauzat, M.; Koch, G.; Granier, C.; Vile, D. Quantifying spatial heterogeneity of chlorophyll fluorescence during plant growth and in response to water stress. Plant Methods 2015, 11, 23. [Google Scholar] [CrossRef]
- Hanć, A.; Małecka, A.; Kutrowska, A.; Bagniewska-Zadworna, A.; Tomaszewska, B.; Barałkiewicz, D. Direct analysis of elemental biodistribution in pea seedlings by LA-ICP-MS, EDX and confocal microscopy: Imaging and quantification. Microchem. J. 2016, 128, 305–311. [Google Scholar] [CrossRef]
- López-Fernandez, H.; Pessôa, G.S.; Arruda, M.A.Z.; Capelo-Martínez, J.L.; Fdez-Riverola, F.; Glez-Peña, D.; Reboiro-Jato, M. LA-iMageS: A software for elemental distribution bioimaging using LA-ICP-MS data. J. Cheminform. 2016, 8, 1758–2946. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—Calculation of qP and Fv’/Fm’ without measuring Fo’. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Sperdouli, I.; Andreadis, S.S.; Adamakis, I.-D.S.; Moustaka, J.; Koutsogeorgiou, E.I.; Moustakas, M. Reactive oxygen species initiate defence responses of potato photosystem II to sap-sucking insect feeding. Insects 2022, 13, 409. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Plant tissues | Zn | Mn | Ca | Fe | Cu | Mg |
|---|---|---|---|---|---|---|---|
| Control | aboveground | 88.1 ± 2.6 | 37.2 ± 1.1 | 8531 ± 252 | 77.2 ± 2.3 | 11.6 ± 0.3 | 4377 ± 131 * |
| belowground | 89.3 ± 2.7 | 40.8 ± 1.2 | 6214 ± 186 * | 561 ± 17 | 20.8 ± 0.6 | 3273 ± 107 * | |
| Zn 8d | aboveground | 175.9 ± 5.3 * | 72.6 ± 2.2 * | 10,394 ± 301 * | 106.5 ± 3.2 * | 13.1 ± 0.4 | 3982 ± 119 |
| belowground | 40,060 ± 1202 * | 37.5 ± 1.1 | 5874 ± 176 | 2643 ± 78 * | 264.5 ± 7.9 * | 1835 ± 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustakas, M.; Dobrikova, A.; Sperdouli, I.; Hanć, A.; Adamakis, I.-D.S.; Moustaka, J.; Apostolova, E. A Hormetic Spatiotemporal Photosystem II Response Mechanism of Salvia to Excess Zinc Exposure. Int. J. Mol. Sci. 2022, 23, 11232. https://doi.org/10.3390/ijms231911232
Moustakas M, Dobrikova A, Sperdouli I, Hanć A, Adamakis I-DS, Moustaka J, Apostolova E. A Hormetic Spatiotemporal Photosystem II Response Mechanism of Salvia to Excess Zinc Exposure. International Journal of Molecular Sciences. 2022; 23(19):11232. https://doi.org/10.3390/ijms231911232
Chicago/Turabian StyleMoustakas, Michael, Anelia Dobrikova, Ilektra Sperdouli, Anetta Hanć, Ioannis-Dimosthenis S. Adamakis, Julietta Moustaka, and Emilia Apostolova. 2022. "A Hormetic Spatiotemporal Photosystem II Response Mechanism of Salvia to Excess Zinc Exposure" International Journal of Molecular Sciences 23, no. 19: 11232. https://doi.org/10.3390/ijms231911232
APA StyleMoustakas, M., Dobrikova, A., Sperdouli, I., Hanć, A., Adamakis, I.-D. S., Moustaka, J., & Apostolova, E. (2022). A Hormetic Spatiotemporal Photosystem II Response Mechanism of Salvia to Excess Zinc Exposure. International Journal of Molecular Sciences, 23(19), 11232. https://doi.org/10.3390/ijms231911232











