Metallophenolomics: A Novel Integrated Approach to Study Complexation of Plant Phenolics with Metal/Metalloid Ions
Abstract
1. Introduction
2. Metallomics as a Scientific Approach
2.1. Basic Concepts of Metallomics
2.2. Differentiation of Metallomic Studies
2.3. Potential Role of Phenolic Chelators in Plant Metallomic Studies
3. Plant Phenolics as Ligands for Metal(loid)s
3.1. Complexing In Vitro
- (1)
- Evaluation of the complexation of individual PCs with Men+, based on the features of the ligands, which are modified due to chelation;
- (2)
- Assessment of metal chelating ability toward PCs and plant extracts based on the alterations in the absorption of metallochromic indicators.
3.1.1. Individual Phenolic Compounds
- (1)
- How are metal binding properties manifested for the natural compounds from different PC subclasses, which are formed in the process of plant phenolic metabolism?
- (2)
- Which structural fragments of PCs are crucial for the complexation?
- (3)
- Can PCs be considered as universal ligands for multiple Men+?
3.1.2. Metal Chelating Ability
3.2. Chelating Effects In Vivo
3.3. Properties of Phenolic Chelators
- 1.
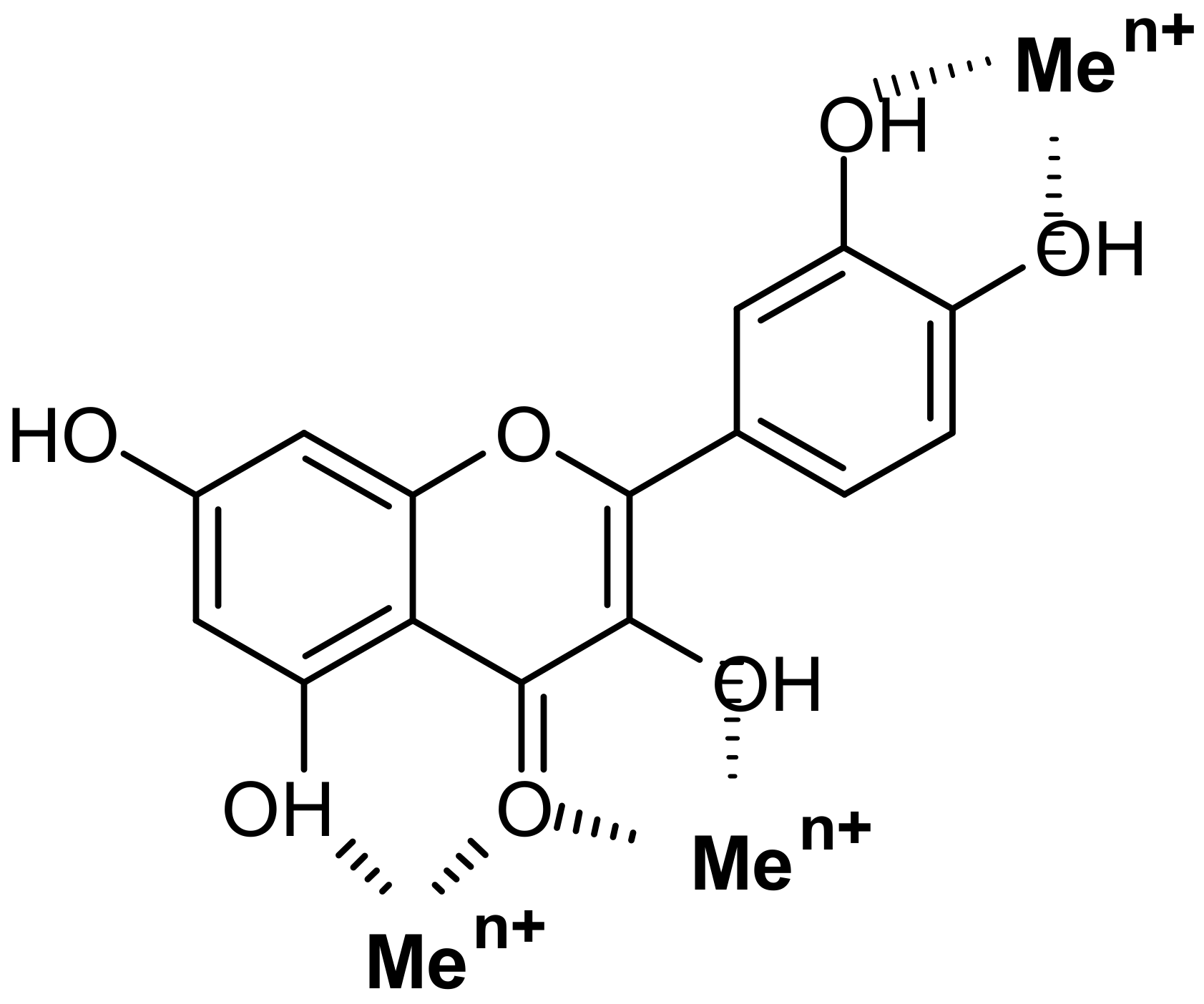
- Presence of Men+ binding sites with O atoms of carbonyl, hydroxyl, or carboxylate groups: Depending on the phenolic subgroup and a number of OH- groups as substituents, different chelation variants are possible. Thus, for quercetin, Men+ chelation can take place at three binding sites (Figure 3). For ACNs, Men+ binding occurs due to two or three hydroxyl substitutions in the B ring [13]. The formation of chelate structures with an unsaturated cycle with two or three coordinated O atoms defines the stability of such metallocomplexes. However, the coordination of Men+ with two O atoms of the carboxylate groups of PCs is also possible, e.g., in p-coumaric acid [207];
- 2.
- Universal affinity of PCs in relation to different Men+ in both cationic and anionic forms: During the systematizing of the experimental and literature data, we revealed that PC ligands from various subgroups form complexes with 69 Men+ ions (63 chemical elements; Table 1, Figure 1). Binding to PCs is a characteristic of/universal for the elements differing in their roles in plants:
- Essential macronutrients (Ca, Mg, K) and essential micronutrients (Fe, Mn, Zn, Ni, Cu, B, Mo), which are necessary for the plant life cycle, cannot be substituted by other elements and are directly involved in plant metabolism [208];
- Non-essential metal(loid)s that are not involved in primary plant metabolism;
- Rare earth elements (REEs), which include the lanthanide group with 15 elements (Figure 2).
The complexation of PC ligands with 11 elements essential for human life (Na, Mg, K, Ca, Mn, Fe, Co, Cu, Zn, Mo, Se) has also been confirmed [211]. Such universalism of PC ligands is due to the multi-elemental composition of the plant metallome. Thus, according to Watanabe et al. [212], in the leaves of species from different families of terrestrial plants, 42 chemical elements have been found. An additional argument for a close PC–Men+ interrelation is the correlation between the plant accumulation of essential, beneficial, and non-essential elements with the content of total phenolics and total flavonoids [213]; - 3.
- 4.
- The phenolic ligands’ capacity to form complexes with multiple different Men+. The examples of such chelates are the heterobimetallic complexes of quercetin–Cu–Sn2 and quercetin–Zn–Sn2 [214];
- 5.
- The capability of certain PCs to form mixed ligand complexes and multiligand metal–phenolic assemblies. Thus, mixed ligand complexes have been obtained for Pt(II)–naringin–caffeic acid, Pt(II)–naringin–sinaptic acid, and complexes of V(V) with those ligands [106]. Porkodi and Raman [215] synthesized mixed complexes of curcumin and quercetin derivatives with Co(II), Ni(II), Cu(II), and Zn(II). This capability was employed for the fabrication of hybrid functional materials using metal–phenolic networks based on the polyphenol components of green tea infusions [216];
- 6.
- Complexation of PC ligands with different metal(loid)s’ species (ions, oxides, isotopes, nanoparticles): In addition to Men+, solid-phase chelation of flavonoids has been confirmed for Al2O3, SiO2, and TiO2 [217,218,219]. Radiolabelled complexes have been synthesized for 99mTc with curcumin, rutin, and luteolin [139,220,221], and 68Ga with curcumin [139]. The chelating capacities of PCs are used in the “green chemistry” synthesis of biocompatible nanomaterials [222];
- 7.
- The formation of metabolites with chelating capacity at all stages of the phenolic biosynthetic pathway: Such a property is manifested regardless of the features of a particular biosynthetic pathway, which are determined by the complexity of the interrelated and consequent transformations of metabolites from various subgroups and the specificity of the dominant metabolite accumulated in a particular plant species. According to the systematization of Men+ complexation reactions undertaken, we identified 18 subgroups of PCs capable of metal(loid) binding (Table 1). This enables multivariant scenarios of Men+ binding in plants, thus confirming the universal nature of phenolic chelators;
- 8.
- Metal ions’ binding has been demonstrated for PCs localized in plant tissues with various functions and for endogenous metabolites secreted by plants into the rhizosphere. Thus, binding with Men+ has been confirmed for PCs in roots [11,180,196,199], hypocotyls [197,198], stems [179], leaves [200], flowers [192], berries [202], and the rhizosphere [203,204,205];
- 9.
- The chelating capacity of PCs is manifested in different physiological processes of plants. Phenolic metallocomplexes are engaged in photoreception and photoprotection [12], plant–pollinator interactions [12], antioxidant and prooxidant mechanisms [188], metal detoxifying in plant tissues and the rhizosphere [11,196,197,198,199,200,203], vacuolar sequestration [12], the mobilization and phytoavailability of deficient elements [204,205];
- 10.
- The phenomenon of pH-dependent dynamic equilibrium between different structural forms of ACNs: Both unbound and chelated ACN forms could be present simultaneously in plant tissue, thus creating its colour variation: red–purple–blue [13];
- Reversible nature of ACN binding with metal ions in plant tissue; the unbound ACN form can be regenerated by varying the pH: Such a peculiarity has been established for the binding of Pb2+ with Cy-3-glu in maize roots [13]. This feature is based on the pH-dependent transformation of the ACN structure;
- ACNs and copigmentation: According to Trouillas et al. [191], copigmentation is defined as the formation (in the presence or absence of metal ions) of non-covalent complexes involving anthocyanin or anthocyanin-derived pigment(s) and a copigment(s), with the resulting changes in the optical properties of the pigment complex. Major natural copigments are the phenolic metabolites: hydrolysable tannins, flavonols, flavones, dihydroflavonols, flavanones, phenolic acids, and derivatives thereof [191]. ACNs themselves can act as copigments due to the self-association of the two molecules [191].
- (i)
- The ACN chromophore within the metallocomplex absorbs light in the UV and visible region, which provides the UV protection of plant tissue and attracts pollinators to flower petals. Binding with Men+ increases can resistance to solar radiation;
- (ii)
- ACN copigmentation occurs with the participation of both PCs and chelators from other groups (organic acids, amino acids). Moreover, some ACNs and phenolic copigments contain malonyl, succinyl, and quinic fragments in their molecules [192]. Such structural features of ACNs and copigments increase the number of binding sites and modify the chelation activity;
- (iii)
- The ACNs’ capacity to biotransform biogenic (nutrient) elements and engage them in the processes of photoprotection and plant–pollinator interactions, which was formed during natural evolution, can be used by plants for the other functional role—detoxication of abiogenic metals as pollutants resulting from man-induced activities.
4. Concept of Metallophenolomics
4.1. Definitions
4.2. Research Subjects
- (1)
- Quantitative distribution and imaging analysis of elements in plant tissues. The use of the chelating effect of PCs is exemplified by the determination of Al localization in plant tissues by complexing to morin as the fluorochrome with subsequent detection by confocal laser microscopy [230,231]. This technique complements histochemical assays, where chelating dyes are used, and metal localization is examined by light microscopy [232] or visible reflectance and tristimulus colorimetry [233]. Tannic acid can be employed as a natural chelator for labile iron imaging in the prevention and treatment of iron-associated cancer or other iron-overload disorders [234];
- (2)
- Speciation of elements in plants: To establish the chemical forms of metals in which they are bound to plant chelators, the general analytical approaches of metallomics can be employed, along with a set of specific methods based on the structural peculiarities of these bioligands.General approaches include universal techniques for analysing the metal-containing biomolecules, i.e., hyphenated techniques (e.g., HPLC-ICPMS/ESI-MS) [235]. Hydrangea blue complexes composed of 3-O-glycosyldelphinidin, Al3+, and 5-O-acilquinic acid were investigated by electrospray-ionization mass spectrometry [236]. However, some supplementary non-destructive methods for phenol bioligands’ investigation are available due to their following specific features. Firstly, some of those metabolites (e.g., anthocyanins) are in most cases localized in surface plant tissues. Secondly, the chromophore system in some phenolic metal chelating molecules defines selective light absorption in the visible region, thus enabling the use of non-destructive methods (e.g., reflectance spectroscopy, tristimulus colorimetry) based on the interaction of the light beam with pigmented plant tissues. Thirdly, the differences in spectral characteristics between the unbound and chelated forms of bioligands are the markers for identifying the in vivo binding. Such specific analytical techniques could be exemplified by identifying ACN binding to Men+ in flowers and roots [13];
- (3)
- Structural analysis of metal binding by phenolic compounds: This research field includes investigating the structural features of PCs from various subclasses along with their chelation sites [77], the structure–activity relations (antioxidant and metal chelating properties) [237], in silico prediction of the binding sites’ structure, and the potential biological activities of metallocomplexes [238];
- (4)
- Elucidation of the reaction mechanisms of the metallophenolome using model phenol–metal complexes: Phenolic–Men+ complexation can alter the antioxidant activity of PCs due to the reduction of transition metal ions and the induction of Fenton reactions [237]. Therefore, the iron or copper chelating properties of PCs are considered to be a variant of the antioxidative effect [188,189,239]. The antioxidant activity of some phenolic–metal complexes is superior to that of the parent ligands [77]. The effect of metal binding on the antioxidant activity of the molecule was studied in ferrous flavonoid mixtures [148]. An important element of redox processes in biological systems is also the prooxidant activity of phenolics, which is stimulated by Men+ [240,241]. Flavonoid–metal complexes may exhibit superoxide dismutase activity, and their radical-scavenging activity is superior to the unbound flavonoid [242]. Phenolic–metal complexes are used in elucidating the reaction mechanisms with biologically important molecules such as DNA [243,244], proteins [243], pectins [245], and lipids [77]. Model phenolic metallocomplexes are employed in biomimetic studies of the mechanisms of action of metalloenzymes, which use flavonoids as a substrate [77];
- (5)
- Identification of unknown metallophenolics: An important problem in this research field remains the identification of stoichiometric and non-stoichiometric anthocyanin metallocomplexes in blue flowers [192] and phenolic–Men+ complexes in plant tissues and the rhizosphere under the toxic impact of metals [13,203]. An example of such a novel approach is the identification of metal binding PC ligands in wine [246];
- (6)
- Targeted analysis of metal chelators in phenolic metabolism: Metabolomic profiling enables identifying the effects of various metals on the qualitative composition and quantitative content of individual phenolic metabolites as potential chelators. Thus, Cu’s effect on Cucumber sativus significantly upregulates 4-hydroxycinnamic acid compared to other phenolic metabolites [247]. The treatment of Helianthus annuus with Cr enabled identifying ten isocoumarin derivatives as target metal chelating compounds [248]. Different changes in the metabolic profiles of flavonols and hydroxicinnamic acids were identified for two metallicolous populations of Arabidopsis halleri, which demonstrated different mechanisms of Cr tolerance [249]. The higher tolerance of a red- versus a green-leafed cultivar of sweet basil against boron toxicity was hypothesized to be partially related to the capability of anthocyanins to act as B chelators [250,251,252,253];
- (7)
- Medical diagnosis of health and diseases related to trace metals: The chelating capacities of PCs are employed in numerous medical diagnostic techniques. Tannic acid may be applied for chelation and imaging of labile iron in iron-associated cancer or other iron-overload disorders [234]. The complex of morin with 68Ga was proposed as a novel radiopharmaceutical for diagnostic purposes and kidney cancer cell labelling [254]. The morin metal complexes with DNA were confirmed as an effective tool for the discrimination of anticancer drugs’ binding mechanism to DNA [255];
- (8)
- Metallodrug design: PC–Men+ complexes demonstrate a broad spectrum of biological activities (anti-/pro-oxidant, antimicrobial, antiviral, anti-inflammatory, anti-diabetic, anticonvulsant, anticancer) [77,78,79,80]. An option to use various ligands differing in their binding sites and the universal property of chelating multiple Men+ corroborates the prospects of PCs in metallodrug design. Flavonoid–Men+ complexes are viewed as a novel class of therapeutical agents [76]. The complexation of PCs with Men+ enables obtaining metallocomplexes with improved biological activity compared to their parent ligands [77,79]. An essential advantage of those complexes is the use of non-toxic natural chelators, thus reducing the potential toxicity of metallodrugs in chemotherapy [256];
- (9)
- Chemical evolution of the living systems and organisms on Earth: The evolution and sophistication of mechanisms for chelating different elements are deemed as a factor of the evolutionary development of organisms [257]. Plant systems’ evolution involves metal homeostasis networks [4,258,259]. One of the important evolutionary aspects of elemental hyperaccumulation is based on the involvement of multiple kinds of chelators in plant tolerance mechanisms [260]. The important evolutionary role of flavonoids with antioxidant and chelating capacities was shown in the adaptation of metallicolous populations, wherein divergent strategies were revealed for Cd uptake, translocation, and detoxifying in different genetic units of Arabidopsis halleri [249]. The chelation mechanism, which was formed during evolutionary development toward essential elements, is utilized in plants to detoxify non-essential metal(loid)s as pollutants from anthropogenic sources [13]. The universality of this tolerance mechanism was demonstrated in relation to a new class of contaminants—metal-containing nanoparticles (NPs) [222]. ACN–Men+ complexation is the important factor of the colour evolution of flowers, which enhances plant polychroism in plant–pollinator interactions [192];
- (10)
- Mission-oriented biometal sciences. Environmental science: One of the adaptive mechanisms in plant tolerance strategies against metal(loid) toxicity is deemed to be the chelation process involving different kinds of chelators [261,262]. The chelating properties of phenolic metabolites are integrated in the omics approach to study plant responses to metal stress (transcriptomics, proteomics, metabolomics, ionomics) [263]. To improve plant metal tolerance, various techniques are utilized, including pre-treatment by phenolic-rich plant extracts with chelating capacity [264,265]. Chelation processes are crucial in phytoremediation technologies for metal-contaminated areas [266]. The phytoremediation potency of plants depends on various factors including the content of phenolic chelators in plant tissues [267]. Chelating capacity determines the efficacy of phenolics as natural removing agents (biosorbents) from contaminated soils and wastewaters [268,269,270,271]. The defensive role of phenolic metabolites in plant responses to metal-containing NPs as a new class of contaminants has already been demonstrated [272,273,274].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richards, C.L.; Hanzawa, Y.; Katari, M.S.; Ehrenreich, I.M.; Engelmann, K.E.; Purugganan, M.D. Perspective on ecological and evolutionary systems biology. Annu. Rev. Plant Biol. 2009, 35, 331–351. [Google Scholar] [CrossRef]
- Hill, M.K. Understanding Environmental Pollution, 3rd ed.; Cambridge University Press: New York, NY, USA, 2010; 534p, ISBN 978-0-521-73669-5. [Google Scholar]
- Hasanuzzaman, M.; Fujita, M. Heavy metals in the environment: Current status, toxic effects on plants and phytoremediation. In Phytotechnologies: Remediation of Environmental Contaminants; Anjum, N.A., Pereira, M.E., Ahmad, I., Duarte, A.C., Umar, S., Khan, N.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 7–73. ISBN 1439875189. [Google Scholar]
- Sharma, A.; Kapoor, D.; Gautam, S.; Landi, M.; Kandhol, N.; Araniti, F.; Ramakrishnan, M.; Satish, L.; Singh, V.P.; Sharma, P.; et al. Heavy metal induced regulation of plant biology: Recent insights. Physiol. Plant. 2022, 174, e13688. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Corso, M.; de la Torre, V.S.G. Biomolecular approaches to understanding metal tolerance and hyperaccumulation in plants. Metallomics 2020, 12, 840–859. [Google Scholar] [CrossRef]
- Hall, J.Á. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Rauser, W.E. Structure and function of metal chelators produced by plants. Cell Biochem. Biophys. 1999, 31, 19–48. [Google Scholar] [CrossRef]
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Rodrigo, M.A.M.; Adam, V.; Fujita, M.; Kizek, R.; et al. Jacks of metal/metalloid chelation trade in plants—An overview. Front. Plant Sci. 2015, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Fedenko, V.S. Cyanidin as endogenous chelator of metal ions in maize seedling roots. Ukr. Biochem. J. 2008, 80, 102–106. [Google Scholar]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Fedenko, V.S.; Shemet, S.A.; Landi, M. UV–vis spectroscopy and colorimetric models for detecting anthocyanin-metal complexes in plants: An overview of in vitro and in vivo techniques. J. Plant Physiol. 2017, 212, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.A.; Dias, D.A.; Callahan, D.L.; Kouremenos, K.A.; Beale, D.J.; Roessner, U. The use of metabolomics in the study of metals in biological systems. Metallomics 2015, 7, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Pirzadah, T.B.; Malik, B.; Hakeem, K.R. Integration of “omic” approaches to unravel the heavy metal tolerance in plants. In Essentials of Bioinformatics; Hakeem, K., Shaik, N., Banaganapalli, B., Elango, R., Eds.; Springer: Cham, Switzerland, 2019; Volume III, pp. 79–92. ISBN 978-3-030-19318-8. [Google Scholar] [CrossRef]
- Hanus-Fajerska, E.; Wiszniewska, A.; Kamińska, I.; Koźmińska, A. Metallomic approach to enhance agricultural application of halophytes. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021; pp. 1953–1969. ISBN 978-3-030-57635-6. [Google Scholar] [CrossRef]
- Jamla, M.; Khare, T.; Joshi, S.; Patil, S.; Penna, S.; Kumar, V. Omics approaches for understanding heavy metal responses and tolerance in plants. Curr. Plant Biol. 2021, 27, 100213. [Google Scholar] [CrossRef]
- Raza, A.; Tabassum, J.; Zahid, Z.; Charagh, S.; Bashir, S.; Barmukh, R.; Khan, R.S.A.; Barbosa, F., Jr.; Zhang, C.; Chen, H.; et al. Advances in “omics” approaches for improving toxic metals/metalloids tolerance in plants. Front. Plant Sci. 2022, 12, 794373. [Google Scholar] [CrossRef]
- Lay, J.O., Jr.; Liyanage, R.; Borgmann, S.; Wilkins, C.L. Problems with the “omics”. TrAC Trends Anal. Chem. 2006, 25, 1046–1056. [Google Scholar] [CrossRef]
- Williams, R.J.P. Chemical selection of elements by cells. Coord. Chem. Rev. 2001, 216, 583–595. [Google Scholar] [CrossRef]
- Haraguchi, H. Metallomics as integrated biometal science. J. Anal. At. Spectrom. 2004, 19, 5–14. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Anjitha, K.S.; Sameena, P.P.; Puthur, J.T. Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress 2021, 2, 100038. [Google Scholar] [CrossRef]
- Fedenko, V.S.; Shemet, S.A.; Guidi, L.; Landi, M. Metal/metalloid-induced accumulation of phenolic compounds in plants. In Metal Toxicity in Higher Plants; Landi, M., Shemet, S.A., Fedenko, V.S., Eds.; Nova Science Publishers: New York, NY, USA, 2020; pp. 67–115. ISBN 978-1-53616-790-0. [Google Scholar]
- Haraguchi, H. Metallomics research―Good luck on new publication. Met. Res. 2021, 1, rev-1–rev-13. [Google Scholar] [CrossRef]
- Lobinski, R.; Becker, J.S.; Haraguchi, H.; Sarkar, B. Metallomics: Guidelines for terminology and critical evaluation of analytical chemistry approaches (IUPAC Technical Report). Pure Appl. Chem. 2010, 82, 493–504. [Google Scholar] [CrossRef]
- Jakubowski, N.; Lobinski, R.; Moens, L. Metallobiomolecules. The basis of life, the challenge of atomic spectroscopy. J. Anal. At. Spectrom. 2004, 19, 1–4. [Google Scholar] [CrossRef]
- Maret, W. Metallomics: A Primer of Integrated Biometal Sciences; Imperial College Press: London, UK, 2016; 156p, ISBN 178326830. [Google Scholar]
- Szpunar, J. Metallomics: A new frontier in analytical chemistry. Anal. Bioanal. Chem. 2004, 378, 54–56. [Google Scholar] [CrossRef]
- Mounicou, S.; Szpunar, J.; Lobinski, R. Metallomics: The concept and methodology. Chem. Soc. Rev. 2009, 38, 1119–1138. [Google Scholar] [CrossRef]
- Maret, W. Metallomics: The Science of Biometals and Biometalloids. In Metallomics: The Science of Biometals; Arruda, M., Ed.; Springer: Cham, Switzerland, 2018; pp. 1–20. ISBN 978-3-319-90143-5. [Google Scholar] [CrossRef]
- Maret, W. An Appraisal of the Field of Metallomics and the Roles of Metal Ions in Biochemistry and Cell Signaling. Appl. Sci. 2021, 11, 10846. [Google Scholar] [CrossRef]
- Metallomics: Recent Analytical Techniques and Applications; Ogra, Y., Hirata, T., Eds.; Springer: Tokyo, Japan, 2017; p. 372. ISBN 4431567933. [Google Scholar] [CrossRef]
- Metallomics: The Science of Biometals; Arruda, M.A.Z., Ed.; Springer: Cham, Switzerland, 2018; p. 279. ISBN 978-3-319-90143-5. [Google Scholar] [CrossRef]
- Singh, V.; Verma, K. Metals from cell to environment: Connecting metallomics with other omics. Open J. Plant Sci. 2018, 3, 001–014. [Google Scholar]
- Lahner, B.; Gong, J.; Mahmoudian, M.; Smith, E.L.; Abid, K.B.; Rogers, E.E.; Guerinot, M.L.; Harper, J.F.; Ward, J.M.; McIntyre, L.; et al. Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat. Biotechnol. 2003, 21, 1215–1221. [Google Scholar] [CrossRef]
- Salt, D.E.; Baxter, I.; Lahner, B. Ionomics and the study of the plant ionome. Annu. Rev. Plant Biol. 2008, 59, 709–733. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Sinha, R.P.; Chauhan, D.K.; Tripathi, D.K.; Pathak, J. Role of ionomics in plant abiotic stress tolerance. In Plant Life Under Changing Environment: Responses and Management; Tripathi, D.K., Chauhan, D.K., Prasad, S.M., Ramawat, N., Singh, V.P., Sharma, S., Dubey, N.K., Eds.; Academic Press: London, UK, 2020; pp. 835–860. ISBN 978-0-12-818204-8. [Google Scholar] [CrossRef]
- Singh, A.; Jaiswal, A.; Singh, A.; Tomar, R.S.; Kumar, A. Plant ionomics: Toward high-throughput nutrient profiling. In Plant Nutrition and Food Security in the Era of Climate Change; Kumar, V., Srivastava, A.K., Suprasanna, P., Eds.; Academic Press: London, UK, 2022; pp. 227–254. ISBN 978-0-12-822916-3. [Google Scholar] [CrossRef]
- Li, Y.F.; Chen, C.; Qu, Y.; Gao, Y.; Li, B.; Zhao, Y.; Chai, Z. Metallomics, elementomics, and analytical techniques. Pure Appl. Chem. 2008, 80, 2577–2594. [Google Scholar] [CrossRef]
- Tripathi, R.D.; Tripathi, P.; Dwivedi, S.; Dubey, S.; Chakrabarty, D. Arsenomics: Omics of arsenic metabolism in plants. Front. Physiol. 2012, 3, 275. [Google Scholar] [CrossRef]
- Saeed, M.; Quraishi, U.M.; Malik, R.N. Arsenic uptake and toxicity in wheat (Triticum aestivum L.): A review of multi-omics approaches to identify tolerance mechanisms. Food Chem. 2021, 355, 129607. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castillo, J.I.; Saldaña-Robles, A.; Ozuna, C. Arsenic stress in plants: A metabolomic perspective. Plant Stress 2022, 3, 100055. [Google Scholar] [CrossRef]
- Zhang, P.; Georgiou, C.A.; Brusic, V. Elemental metabolomics. Briefings Bioinf. 2018, 19, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Outten, F.W.; Twining, B.S. Metal homeostasis. In Wiley Encyclopedia of Chemical Biology; Tadhg, P., Begley, T.P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 1–10. ISBN 9780471754770. [Google Scholar] [CrossRef]
- Li, Y.F.; Sun, H. Metallomics in Multidisciplinary Research and the Analytical Advances. At. Spectrosc. 2021, 42, 227–230. [Google Scholar] [CrossRef]
- Van der Ent, A.; Harris, H.H. Phytometallomics. Metallomics 2020, 12, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, T.; Chang, C.; Lei, Y.; Mao, X. Analytical Methodologies for Agrometallomics: A Critical Review. J. Agric. Food Chem. 2021, 69, 6100–6118. [Google Scholar] [CrossRef]
- López-Barea, J.; Gómez-Ariza, J.L. Environmental proteomics and metallomics. Proteomics 2006, 6, S51–S62. [Google Scholar] [CrossRef]
- Blasco, J.; Rodríguez-Moro, G.; Callejón-Leblic, B.; Ramírez-Acosta, S.; Arellano-Beltrán, F.; Arias-Borrego, A.; García-Barrera, T.; Gómez-Ariza, J.L. Environmental metallomics and metabolomics in free-living and model organisms: An approach for unraveling metal exposure mechanisms. In Environmental Metabolomics; Álvarez-Muñoz, D., Farré, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–119. ISBN 978-0-12-818196-6. [Google Scholar] [CrossRef]
- Chen, B.; Hu, L.; He, B.; Luan, T.; Jiang, G. Environmetallomics: Systematically investigating metals in environmentally relevant media. TrAC Trends Anal. Chem. 2020, 126, 115875. [Google Scholar] [CrossRef]
- Albarède, F.; Télouk, P.; Balter, V. Medical applications of isotope metallomics. Rev. Mineral. Geochem. 2017, 82, 851–885. [Google Scholar] [CrossRef]
- Mahan, B.; Chung, R.S.; Pountney, D.L.; Moynier, F.; Turner, S. Isotope metallomics approaches for medical research. Cell. Mol. Life Sci. 2020, 77, 3293–3309. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, Y.; Li, H.; Bai, X.; Yan, X.; Zhao, J.; Gao, Y. Advances of Synchrotron Radiation-based Radiometallomics for the Study of Uranium. At. Spectrosc. 2021, 42, 254–261. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhao, J.; Gao, Y.; Chen, C. Nanometallomics: New Approach on Analyzing Biological Effects of Metal-Related Nanomaterials. In Toxicology of Nanomaterials; Zhao, Y., Zhang, Z., Feng, W., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2016; pp. 220–232. ISBN 978-3-527-68913-2. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Cui, L.; Li, Y.F.; Li, B.; Chen, C. Comparative nanometallomics as a new tool for nanosafety evaluation. Metallomics 2021, 13, mfab013. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, P.A.; Holmes-Hampton, G.P. Biophysical probes of iron metabolism in cells and organelles. Curr. Opin. Chem. Biol. 2011, 15, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Dlouhy, A.C.; Outten, C.E. The iron metallome in eukaryotic organisms. In Metallomics and the Cell. Metal Ions in Life Sciences; Banci, L., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 12, pp. 241–278. ISBN 978-94-007-5560-4. [Google Scholar] [CrossRef]
- Sydor, A.M.; Zamble, D.B. Nickel metallomics: General themes guiding nickel homeostasis. In Metallomics and the Cell. Metal Ions in Life Sciences; Banci, L., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 12, pp. 375–416. ISBN 978-94-007-5560-4. [Google Scholar] [CrossRef]
- Rensing, C.; McDevitt, S.F. The Copper Metallome in Prokaryotic Cells. In Metallomics and the Cell. Metal Ions in Life Sciences; Banci, L., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 12, pp. 417–450. ISBN 978-94-007-5560-4. [Google Scholar] [CrossRef]
- Vest, K.E.; Hashemi, H.F.; Cobine, P.A. The copper metallome in eukaryotic cells. In Metallomics and the Cell. Metal Ions in Life Sciences; Banci, L., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 12, pp. 451–478. ISBN 978-94-007-5560-4. [Google Scholar] [CrossRef]
- Colvin, R.A.; Stork, C.J.; Li, Y.V.; Lai, B. Exploring the zinc metallome of cultured cortical neurons using synchroton radiation X-ray fluorescence microscopy. In Metal Ion in Stroke; Li, Y., Zhang, J., Eds.; Springer: New York, NY, USA, 2012; pp. 227–237. ISBN 978-1-4419-9662-6. [Google Scholar] [CrossRef]
- Park, J.; McCormick, S.P.; Chakrabarti, M.; Lindahl, P.A. Insights into the iron-ome and manganese-ome of Δmtm1 Saccharomyces cerevisiae mitochondria. Metallomics 2013, 5, 656–672. [Google Scholar] [CrossRef]
- Szpunar, J. Advances in analytical methodology for bioinorganic speciation analysis: Metallomics, metalloproteomics and heteroatom-tagged proteomics and metabolomics. Analyst 2005, 130, 442–465. [Google Scholar] [CrossRef]
- Shi, W.; Chance, M.R. Metallomics and metalloproteomics. Cell. Mol. Life Sci. 2008, 65, 3040–3048. [Google Scholar] [CrossRef]
- Zeng, X.; Cheng, Y.; Wang, C. Global mapping of metalloproteomes. Biochemistry 2021, 60, 3507–3514. [Google Scholar] [CrossRef]
- Wesenberg, D.; Krauss, G.J.; Schaumlöffel, D. Metallo-thiolomics: Investigation of thiol peptide regulated metal homeostasis in plants and fungi by liquid chromatography-mass spectrometry. Int. J. Mass Spectrom. 2011, 307, 46–54. [Google Scholar] [CrossRef]
- Schaumlöffel, D. The position of metallomics within other omics fields. In Metallomics: Analytical Techniques and Speciation Methods; Michalke, B., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 2016; pp. 1–16. ISBN 978-3-527-33969-3. [Google Scholar] [CrossRef]
- Codd, R. Metalloglycomics: A new perspective upon competitive metal–carbohydrate binding using EPR spectroscopy. Chem. Commun. 2004, 23, 2653–2655. [Google Scholar] [CrossRef] [PubMed]
- Farrell, N.P.; Gorle, A.K.; Peterson, E.J.; Berners-Price, S.J. Metalloglycomics. In Metallo-Drugs: Development and Action of Anticancer Agents. Metal Ions in Life Sciences; Sigel, A., Sigel, H., Freisinger, E., Sigel, R.K.O., Eds.; Walter de Gruyter GmbH: Berlin, Germany, 2018; Volume 18, pp. 109–140. ISBN 978-3-11-046984-4. [Google Scholar] [CrossRef]
- Gorle, A.K.; Rajaratnam, P.; Chang, C.W.; von Itzstein, M.; Berners-Price, S.J.; Farrell, N.P. Glycans as ligands in bioinorganic chemistry. Probing the interaction of a trinuclear platinum anticancer complex with defined monosaccharide fragments of heparan sulfate. Inorg. Chem. 2019, 58, 7146–7155. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.T.; Borthakur, D. Methods for metal chelation in plant homeostasis. Plant Physiol. Biochem. 2021, 163, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, Y.-F.; Bai, R.; Chen, C.; Chai, Z. Introduction. In Nuclear Analytical Techniques for Metallomics and Metalloproteomics; Chen, C., Chai, Z., Gao, Y., Eds.; Royal Society of Chemistry: Cambridge, UK, 2010; pp. 1–43. ISBN 1847559018. [Google Scholar] [CrossRef]
- Grazul, M.; Budzisz, E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord. Chem. Rev. 2009, 253, 2588–2598. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Flavonoid–metal ion complexes: A novel class of therapeutic agents. Med. Res. Rev. 2014, 34, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E. Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Uivarosi, V.; Munteanu, A. Flavonoid complexes as promising anticancer metallodrugs. In Flavonoids-from Biosynthesis to Human Health; Justino, G.C., Ed.; InTech: Rijeka, Croatia, 2017; pp. 305–337. ISBN 978-953-51-3423-7. [Google Scholar] [CrossRef]
- Borowska, S.; Brzoska, M.M.; Tomczyk, M. Complexation of bioelements and toxic metals by polyphenolic compounds–implications for health. Curr. Drug Targets 2018, 19, 1612–1638. [Google Scholar] [CrossRef] [PubMed]
- Khater, M.; Ravishankar, D.; Greco, F.; Osborn, H.M. Metal complexes of flavonoids: Their synthesis, characterization and enhanced antioxidant and anticancer activities. Future Med. Chem. 2019, 11, 2845–2867. [Google Scholar] [CrossRef] [PubMed]
- Malacaria, L.; Corrente, G.A.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. A Review on Coordination Properties of Al (III) and Fe (III) toward Natural Antioxidant Molecules: Experimental and Theoretical Insights. Molecules 2021, 26, 2603. [Google Scholar] [CrossRef] [PubMed]
- Rossberg, A.; Reich, T.; Bernhard, G. Complexation of uranium (VI) with protocatechuic acid—Application of iterative transformation factor analysis to EXAFS spectroscopy. Anal. Bioanal. Chem. 2003, 376, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Cornard, J.P.; Lapouge, C.; André, E. pH influence on the complexation site of Al (III) with protocatechuic acid. A spectroscopic and theoretical approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 108, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Kula, A. Thermal analysis of lanthanide (III) and Y(III) complexes with 4-hydroxy-3-methoxybenzoic acid. J. Therm. Anal. Calorim. 2005, 81, 381–385. [Google Scholar] [CrossRef]
- Vulpius, D.; Geipel, G.; Baraniak, L.; Bernhard, G. Complex formation of neptunium (V) with 4-hydroxy-3-methoxybenzoic acid studied by time-resolved laser-induced fluorescence spectroscopy with ultra-short laser pulses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Oke, I.M.; Ramorobi, L.M.; Mashele, S.S.; Bonnet, S.L.; Makhafola, T.J.; Eze, K.C.; Noreljaleel, A.E.M.; Chukwuma, C.I. Vanillic acid–Zn (II) complex: A novel complex with antihyperglycaemic and anti-oxidative activity. J. Pharm. Pharmacol. 2021, 73, 1703–1714. [Google Scholar] [CrossRef]
- Fazary, A.E.; Taha, M.; Ju, Y.H. Iron complexation studies of gallic acid. J. Chem. Eng. Data 2009, 54, 35–42. [Google Scholar] [CrossRef]
- Taha, M.; Khan, I.; Coutinho, J.A. Complexation and molecular modeling studies of europium (III)–gallic acid–amino acid complexes. J. Inorg. Biochem. 2016, 157, 25–33. [Google Scholar] [CrossRef]
- Motloung, D.M.; Mashele, S.S.; Matowane, G.R.; Swain, S.S.; Bonnet, S.L.; Noreljaleel, A.E.; Oyedemi, S.O.; Chukwuma, C.I. Synthesis, characterization, antidiabetic and antioxidative evaluation of a novel Zn (II)-gallic acid complex with multi-facet activity. J. Pharm. Pharmacol. 2020, 72, 1412–1426. [Google Scholar] [CrossRef]
- Frešer, F.; Hostnik, G.; Tošović, J.; Bren, U. Dependence of the Fe (II)-Gallic Acid Coordination Compound Formation Constant on the pH. Foods 2021, 10, 2689. [Google Scholar] [CrossRef]
- Iwan, M.; Kula, A.; Rzączyńska, Z.; Pikus, S.; Flisiuk, D.; Gomoła, M. Synthesis and properties of lanthanide (III) complexes with 4-hydroxy-3, 5-dimethoxybenzoic acid. Chem. Pap. 2007, 61, 376–382. [Google Scholar] [CrossRef]
- Świsłocka, R. Experimental (FT-IR, FT-Raman, 1H, 13C NMR) and theoretical study of alkali metal syringates. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 111, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, D.; Kumari, K.; Mkhize, Z.; Seru, L.K.; Bahadur, I.; Singh, P. Metal-ligand complex formation between ferrous or ferric ion with syringic acid and their anti-oxidant and anti-microbial activities: DFT and molecular docking approach. J. Mol. Liq. 2021, 322, 114872. [Google Scholar] [CrossRef]
- Allan, J.R.; Carson, B.R.; Gerrard, D.L.; Hoey, S. Thermal, spectral and magnetic studies of some compounds of cobalt (II), nickel (II) and copper (II) with cinnamic acid. Thermochim. Acta 1989, 154, 315–322. [Google Scholar] [CrossRef]
- Kalinovskaya, I.V.; Karasev, V.E.; Zadorozhnaya, A.N.; Lifar, L.I. Luminescence spectral properties of europium (III) and terbium (III) complexes with cinnamic acid. Russ. J. Coord. Chem. 2001, 27, 516–519. [Google Scholar] [CrossRef]
- Ferrer, E.G.; Salinas, M.V.; Correa, M.J.; Vrdoljak, F.; Williams, P.A. ALP inhibitors: Vanadyl (IV) complexes of ferulic and cinnamic acid. Z. Naturforsch. B 2005, 60, 305–311. [Google Scholar] [CrossRef]
- Kalinowska, M.; Świsłocka, R.; Lewandowski, W. The spectroscopic (FT-IR, FT-Raman and 1H, 13C NMR) and theoretical studies of cinnamic acid and alkali metal cinnamates. J. Mol. Struct. 2007, 834, 572–580. [Google Scholar] [CrossRef]
- Kalinowska, M.; Lewandowski, W.; Świsłocka, R.; Regulska, E. The FT-IR, FT-Raman, 1H and 13C NMR study on molecular structure of sodium (I), calcium (II), lanthanum (III) and thorium (IV) cinnamates. Spectroscopy 2010, 24, 277–281. [Google Scholar] [CrossRef][Green Version]
- Kalinowska, M.; Świsłocka, R.; Lewandowski, W. Zn (II), Cd (II) and Hg (I) complexes of cinnamic acid: FT-IR, FT-Raman, 1H and 13C NMR studies. J. Mol. Struct. 2011, 993, 404–409. [Google Scholar] [CrossRef]
- Graminha, A.E.; Honorato, J.; Dulcey, L.L.; Godoy, L.R.; Barbosa, M.F.; Cominetti, M.R.; Menezes, A.C.; Batista, A.A. Evaluation of the biological potential of ruthenium (II) complexes with cinnamic acid. J. Inorg. Biochem. 2020, 206, 111021. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, C.I.; Mashele, S.S.; Swain, S.S. Antidiabetic and Antioxidative Properties of Novel Zn (II)-cinnamic Acid Complex. Med. Chem. 2021, 17, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Świsłocka, R.; Kowczyk-Sadowy, M.; Kalinowska, M.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) and theoretical studies of p-coumaric acid and alkali metal p-coumarates. Spectroscopy 2012, 27, 35–48. [Google Scholar] [CrossRef]
- Koç, S.; Köse, D.A.; Avcı, E.; Köse, K. Synthesis and Thermal Characterization of p-Coumaric Acid Complexes of CoII, NiII, CuII and ZnII Metal Cations and Biological Applications. Hittite J. Sci. Eng. 2016, 3, 15–22. [Google Scholar] [CrossRef]
- Khvan, A.M.; Kristallovich, E.L.; Abduazimov, K.A. Complexation of caffeic and ferulic acids by transition-metal ions. Chem. Nat. Compd. 2001, 37, 72–75. [Google Scholar] [CrossRef]
- Fazary, A.E.; Ju, Y.H.; Al-Shihri, A.S.; Bani-Fwaz, M.Z.; Alfaifi, M.Y.; Alshehri, M.A.; Saleh, K.A.; Elbehairi, S.E.I.; Fawy, K.F.; Abd-Rabboh, H.S. Platinum and vanadate bioactive complexes of glycoside naringin and phenolates. Open Chem. 2017, 15, 189–199. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, A. Kinetics of complex formation of Fe (III) with caffeic acid: Experimental and theoretical study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 211, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Arciszewska, Ż.; Gama, S.; Kalinowska, M.; Świderski, G.; Świsłocka, R.; Gołębiewska, E.; Naumowicz, M.; Worobiczuk, M.; Cudowski, A.; Pietryczuk, A.; et al. Caffeic Acid/Eu (III) Complexes: Solution Equilibrium Studies, Structure Characterization and Biological Activity. Int. J. Mol. Sci. 2022, 23, 888. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Piekut, J.; Bruss, A.; Follet, C.; Sienkiewicz-Gromiuk, J.; Świsłocka, R.; Rzączyńska, Z.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H, 13C NMR, UV/VIS), Thermogravimetric and Antimicrobial Studies of Ca (II), Mn (II), Cu (II), Zn (II) and Cd (II) Complexes of Ferulic Acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 122, 631–638. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołębiewska, E.; Mazur, L.; Lewandowska, H.; Pruszyński, M.; Świderski, G.; Wyrwas, M.; Pawluczuk, N.; Lewandowski, W. Crystal structure, spectroscopic characterization, antioxidant and cytotoxic activity of new Mg (II) and Mn (II)/Na (I) complexes of isoferulic acid. Materials 2021, 14, 3236. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Pal, U.; Mondal, P.; Bose, A. Multi-spectroscopic and computational evaluation on the binding of sinapic acid and its Cu (II) complex with bovine serum albumin. Food Chem. 2019, 301, 125254. [Google Scholar] [CrossRef] [PubMed]
- Naso, L.G.; Valcarcel, M.; Roura-Ferrer, M.; Kortazar, D.; Salado, C.; Lezama, L.; González-Baró, A.C.; Williams, P.A.M.; Ferrer, E.G. Promising antioxidant and anticancer (human breast cancer) oxidovanadium (IV) complex of chlorogenic acid. Synthesis, characterization and spectroscopic examination on the transport mechanism with bovine serum albumin. J. Inorg. Biochem. 2014, 135, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Bajko, E.; Matejczyk, M.; Kaczyński, P.; Łozowicka, B.; Lewandowski, W. The study of anti-/pro-oxidant, lipophilic, microbial and spectroscopic properties of new alkali metal salts of 5-o-caffeoylquinic acid. Int. J. Mol. Sci. 2018, 19, 463. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Sienkiewicz-Gromiuk, J.; Świderski, G.; Pietryczuk, A.; Cudowski, A.; Lewandowski, W. Zn (II) complex of plant phenolic chlorogenic acid: Antioxidant, antimicrobial and structural studies. Materials 2020, 13, 3745. [Google Scholar] [CrossRef] [PubMed]
- Palierse, E.; Przybylski, C.; Brouri, D.; Jolivalt, C.; Coradin, T. Interactions of Calcium with Chlorogenic and Rosmarinic Acids: An Experimental and Theoretical Approach. Int. J. Mol. Sci. 2020, 21, 4948. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Tian, J.; Liu, Y.; Zhu, L.; Sun, J.; Meng, D.; Wang, Z.; Wang, C.; Zhou, Z.; Chen, L. Interaction mechanism of ferritin protein with chlorogenic acid and iron ion: The structure, iron redox, and polymerization evaluation. Food Chem. 2021, 349, 129144. [Google Scholar] [CrossRef]
- Świsłocka, R.; Regulska, E.; Karpińska, J.; Świderski, G.; Lewandowski, W. Molecular structure and antioxidant properties of alkali metal salts of rosmarinic acid. Experimental and DFT studies. Molecules 2019, 24, 2645. [Google Scholar] [CrossRef] [PubMed]
- Kola, A.; Hecel, A.; Lamponi, S.; Valensin, D. Novel Perspective on Alzheimer’s Disease Treatment: Rosmarinic Acid Molecular Interplay with Copper (II) and Amyloid β. Life 2020, 10, 118. [Google Scholar] [CrossRef]
- Świderski, G.; Jabłońska-Trypuć, A.; Kalinowska, M.; Świsłocka, R.; Karpowicz, D.; Magnuszewska, M.; Lewandowski, W. Spectroscopic, Theoretical and antioxidant study of 3D-transition metals (Co (II), Ni (II), Cu (II), Zn (II)) complexes with cichoric acid. Materials 2020, 13, 3102. [Google Scholar] [CrossRef]
- Manolov, I.; Kostova, I.; Netzeva, T.; Konstantinov, S.; Karaivanova, M. Cytotoxic activity of cerium complexes with coumarin derivatives. Molecular modeling of the ligands. Arch. Pharm. 2000, 333, 93–98. [Google Scholar] [CrossRef]
- Pi, J.; Zeng, J.; Luo, J.J.; Yang, P.H.; Cai, J.Y. Synthesis and biological evaluation of Germanium (IV)–polyphenol complexes as potential anti-cancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2902–2908. [Google Scholar] [CrossRef]
- Sulpizio, C.; Müller, S.T.; Zhang, Q.; Brecker, L.; Rompel, A. Synthesis, characterization, and antioxidant activity of Zn2+ and Cu2+ coordinated polyhydroxychalcone complexes. Monatsh. Chem. 2016, 147, 1871–1881. [Google Scholar] [CrossRef]
- Jin, G.; Zhao, Z.; Chakraborty, T.; Mandal, A.; Roy, A.; Roy, S.; Guo, Z. Decrypting the molecular mechanistic pathways delineating the chemotherapeutic potential of ruthenium-phloretin complex in colon carcinoma correlated with the oxidative status and increased apoptotic events. Oxid. Med.Cell. Longev. 2020, 2020, 7690845. [Google Scholar] [CrossRef] [PubMed]
- Shubina, V.S.; Shatalina, Y.V. Absorption spectroscopy study of acid-base and metal-binding properties of flavanones. J. Appl. Spectrosc. 2013, 80, 761–766. [Google Scholar] [CrossRef]
- Alexiou, A.D.; Decandio, C.C.; Almeida, S.D.N.; Ferreira, M.J.; Romoff, P.; Rocha, R.C. Metal-ligand coordination and antiradical activity of a trichromium (III) complex with the flavonoid naringenin. J. Coord. Chem. 2017, 70, 2148–2160. [Google Scholar] [CrossRef]
- Restrepo-Guerrero, A.G.; Goitia-Semenco, H.; Naso, L.G.; Rey, M.; Gonzalez, P.J.; Ferrer, E.G.; Williams, P.A. Antioxidant and Anticancer Activities and Protein Interaction of the Oxidovanadium (IV) Naringin Complex. Inorganics 2022, 10, 13. [Google Scholar] [CrossRef]
- Bijlsma, J.; de Bruijn, W.J.; Velikov, K.P.; Vincken, J.P. Unravelling discolouration caused by iron-flavonoid interactions: Complexation, oxidation, and formation of networks. Food Chem. 2022, 370, 131292. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Y.; Chen, X.; Peng, M. Investigation of flavonoids bearing different substituents on ring C and their Cu2+ complex binding with bovine serum albumin: Structure–affinity relationship aspects. J. Agric. Food Chem. 2011, 59, 10761–10769. [Google Scholar] [CrossRef]
- Lutoshkin, M.A.; Kuznetsov, B.N.; Levdansky, V.A. Spectrophotometric and quantum-chemical study of acid-base and complexing properties of (±)-taxifolin in aqueous solution. Heterocycl. Commun. 2017, 23, 395–400. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Guo, Q.; Yuan, J.; Zeng, J.; He, X.; Li, D. Synthesis of dihydromyricetin–manganese (II) complex and interaction with DNA. J. Mol. Struct. 2012, 1027, 64–69. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, M.; He, L.; Wang, Y.; Chen, S. Evaluation of General Synthesis Procedures for Bioflavonoid–Metal Complexes in Air-Saturated Alkaline Solutions. Front. Chem. 2020, 8, 589. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Kuntić, V.S.; Malešev, D.L.; Radović, Z.V.; Kosanić, M.M.; Mioč, U.B.; Vukojević, V.B. Spectrophotometric Investigation of Uranil (II)− Rutin Complex in 70 Ethanol. J. Agric. Food Chem. 1998, 46, 5139–5142. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Pękal, A. Flavonoids as analytical reagents. Crit. Rev. Anal. Chem. 2011, 41, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Atabey-Ozdemir, B.; Demirkiran, O.; Yildiz, U.; Tekin, I.O.; Coban, B. Cytotoxicity and DNA binding of copper (II) and zinc (II) complexes of flavonoids: Quercitrin, myricitrin, rutin. Bulg. Chem. Commun. 2017, 49, 901–907. [Google Scholar]
- Catapano, M.C.; Tvrdý, V.; Karlíčková, J.; Migkos, T.; Valentová, K.; Křen, V.; Mladěnka, P. The stoichiometry of isoquercitrin complex with iron or copper is highly dependent on experimental conditions. Nutrients 2017, 9, 1193. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, V.T.; de Menezes, J.B.; Santos, J.C.C.; de Assis Bastos, M.L.; de Araújo-Júnior, J.X.; do Nascimento, T.G.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B. Characterization and stability of the antimony-quercetin complex. Adv. Pharm. Bull. 2019, 9, 432. [Google Scholar] [CrossRef]
- Wongso, H. Natural product-based Radiopharmaceuticals: Focus on curcumin and its analogs, flavonoids, and marine peptides. J. Pharm. Anal. 2021, 12, 380–393. [Google Scholar] [CrossRef]
- Sahyon, H.A.; Althobaiti, F.; Ramadan, A.E.M.M.; Fathy, A.M. Quercetin-Based Rhodium (III) Complex: Synthesis, Characterization and Diverse Biological Potentials. J. Mol. Struct. 2022, 1257, 132584. [Google Scholar] [CrossRef]
- Lomozová, Z.; Catapano, M.C.; Hrubša, M.; Karlíčková, J.; Macáková, K.; Kučera, R.; Mladěnka, P. Chelation of iron and copper by quercetin B-ring methyl metabolites, isorhamnetin and tamarixetin, and their effect on metal-based Fenton chemistry. J. Agric. Food Chem. 2021, 69, 5926–5937. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Wu, H.; Li, W. Synthesis, in vitro, and in silico studies of fisetin and quercetin and their metal complexes as inhibitors of α-glucosidase and thrombin. J. Mol. Liq. 2022, 349, 118164. [Google Scholar] [CrossRef]
- Cruz, M.A.; Tovani, C.B.; Favarin, B.Z.; Soares, M.P.; Fukada, S.Y.; Ciancaglini, P.; Ramos, A.P. Synthesis of Sr–morin complex and its in vitro response: Decrease in osteoclast differentiation while sustaining osteoblast mineralization ability. J. Mater. Chem. B 2019, 7, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Bodini, M.E.; Del Valle, M.A.; Tapia, R.; Leighton, F.; Berrios, P. Zinc catechin complexes in aprotic medium. Redox chemistry and interaction with superoxide radical anion. Polyhedron 2001, 20, 1005–1009. [Google Scholar] [CrossRef]
- Hynes, M.J.; Coinceanainn, M.Ó. The kinetics and mechanisms of the reaction of iron (III) with gallic acid, gallic acid methyl ester and catechin. J. Inorg. Biochem. 2001, 85, 131–142. [Google Scholar] [CrossRef]
- Inoue, M.B.; Inoue, M.; Fernando, Q.; Valcic, S.; Timmermann, B.N. Potentiometric and 1H NMR studies of complexation of Al3+ with (−)-epigallocatechin gallate, a major active constituent of green tea. J. Inorg. Biochem. 2002, 88, 7–13. [Google Scholar] [CrossRef]
- Ansari, A.A.; Sharma, R.K. Synthesis and characterization of a biologically active lanthanum (III)–catechin complex and DNA binding spectroscopic studies. Spectrosc. Lett. 2009, 42, 178–185. [Google Scholar] [CrossRef]
- Grzesik, M.; Namiesnik, J.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of ferrous catechin complexes. Free Radic. Biol. Med. 2018, 120, S111. [Google Scholar] [CrossRef]
- Alasady, S.A.; Muhamad, Y.H.; Ahmed, R.S. Theoretical and Thermodynamics Studies of Complexes Formation between Natural Flavonoids and Hg (II) Ion. Syst. Rev. Pharm. 2020, 11, 2393–2404. [Google Scholar] [CrossRef]
- Fathima, A.; Manikandamathavan, V.M.; Jonnalagadda, R.R.; Nair, B.U. Chromium-catechin complex, synthesis and toxicity check using bacterial models. Heliyon 2020, 6, e04563. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiao, X.; Li, K.; Li, X.; Shi, B.; Liao, X. Synthesis of Catechin-Rare Earth Complex with Efficient and Broad-Spectrum Anti-Biofilm Activity. Chem. Biodivers. 2020, 17, e1900734. [Google Scholar] [CrossRef]
- Navarro, R.E.; Santacruz, H.; Inoue, M. Complexation of epigallocatechin gallate (a green tea extract, egcg) with Mn2+: Nuclear spin relaxation by the paramagnetic ion. J. Inorg. Biochem. 2005, 99, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Guo, Z.; Zhao, L.; Wei, Y. Metal-phenolic networks: Facile assembled complexes for cancer theranostics. Theranostics 2021, 11, 6407. [Google Scholar] [CrossRef] [PubMed]
- O’Coinceanainn, M.; Astill, C.; Baderschneider, B. Coordination of aluminium with purpurogallin and theaflavin digallate. J. Inorg. Biochem. 2003, 96, 463–468. [Google Scholar] [CrossRef]
- O’Coinceanainn, M.; Bonnely, S.; Baderschneider, B.; Hynes, M.J. Reaction of iron (III) with theaflavin: Complexation and oxidative products. J. Inorg. Biochem. 2004, 98, 657–663. [Google Scholar] [CrossRef]
- Naso, L.G.; Martínez, V.R.; Ferrer, E.G.; Williams, P.A. Antimetastatic effects of VOflavonoid complexes on A549 cell line. J. Trace Elem. Med. Biol. 2021, 64, 126690. [Google Scholar] [CrossRef] [PubMed]
- Malacaria, L.; La Torre, C.; Furia, E.; Fazio, A.; Caroleo, M.C.; Cione, E.; Marino, T.; Plastina, P. Aluminum (III), iron (III) and copper (II) complexes of luteolin: Stability, antioxidant, and anti-inflammatory properties. J. Mol. Liq. 2022, 345, 117895. [Google Scholar] [CrossRef]
- Frański, R. Influence of iron redox abilities on the electrospray ionization collision induced dissociation of iron complexes with methoxylated flavonoids. Int. J. Mass Spectrom. 2019, 446, 116216. [Google Scholar] [CrossRef]
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098. [Google Scholar] [CrossRef]
- Fedenko, V.S. Cyanidin complexation with metal ions. Ukr. Biochem. J. 2006, 78, 149–153. [Google Scholar]
- Khaodee, W.; Aeungmaitrepirom, W.; Tuntulani, T. Effectively simultaneous naked-eye detection of Cu (II), Pb (II), Al (III) and Fe (III) using cyanidin extracted from red cabbage as chelating agent. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 126, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Ike, J.N.; Tyopine, A.A.; Okoye, C.O.B. Application of Cyanidin in Quantitative Estimation of Metals in Fish Samples. Am. J. Anal. Chem. 2019, 10, 621–628. [Google Scholar] [CrossRef]
- Torrini, F.; Renai, L.; Scarano, S.; Del Bubba, M.; Palladino, P.; Minunni, M. Colorimetric selective quantification of anthocyanins with catechol/pyrogallol moiety in edible plants upon zinc complexation. Talanta 2022, 240, 123156. [Google Scholar] [CrossRef] [PubMed]
- Mollaamin, F.; Mohammadian, N.T.; Najaflou, N.; Monajjemi, M. Iranian Qara Qat fruit (redcurrant) in Arasbaran forests as the resource of anthocyanin pigments in formation of [ACN-Mg2+/Al3+/Ga3+/Sn2+/Cr3+/Fe3+] chelation clusters. SN Appl. Sci. 2021, 3, 1–18. [Google Scholar] [CrossRef]
- Tang, P.; Giusti, M.M. Metal chelates of petunidin derivatives exhibit enhanced color and stability. Foods 2020, 9, 1426. [Google Scholar] [CrossRef] [PubMed]
- Andreu, G.P.; Delgado, R.; Velho, J.A.; Curti, C.; Vercesi, A.E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur. J. Pharmacol. 2005, 513, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Selles, A.J.; Nuevas-Paz, L.; Martínez-Sánchez, G. Inhibition of Peroxidation Potential and Protein Oxidative Damage by Metal Mangiferin Complexes. Appl. Sci. 2022, 12, 2240. [Google Scholar] [CrossRef]
- Dias, K.; Nikolaou, S. Does the combination of resveratrol with Al (III) and Zn (II) improve its antioxidant activity? Nat. Prod. Commun. 2011, 6, 1673–1676. [Google Scholar] [CrossRef]
- Chiavarino, B.; Crestoni, M.E.; Fornarini, S.; Taioli, S.; Mancini, I.; Tosi, P. Infrared spectroscopy of copper-resveratrol complexes: A joint experimental and theoretical study. J. Chem. Phys. 2012, 137, 024307. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Panahi, Y.; Johnston, T.P.; Sahebkar, A. Biological properties of metal complexes of curcumin. BioFactors 2019, 45, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Vergara, V.B.; Kalinich, J.F. Nutraceuticals as Potential Radionuclide Decorporation Agents. Nutrients 2021, 13, 2545. [Google Scholar] [CrossRef]
- Fucassi, F.; Heikal, A.; Mikhalovska, L.I.; Standen, G.; Allan, I.U.; Mikhalovsky, S.V.; Cragg, P.J. Metal chelation by a plant lignan, secoisolariciresinol diglucoside. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 345–351. [Google Scholar] [CrossRef]
- Borsari, M.; Gabbi, C.; Ghelfi, F.; Grandi, R.; Saladini, M.; Severi, S.; Borella, F. Silybin, a new iron-chelating agent. J. Inorg. Biochem. 2001, 85, 123–129. [Google Scholar] [CrossRef]
- Tvrdý, V.; Catapano, M.C.; Rawlik, T.; Karlíčková, J.; Biedermann, D.; Křen, V.; Mladěnka, P.; Valentová, K. Interaction of isolated silymarin flavonolignans with iron and copper. J. Inorg. Biochem. 2018, 189, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Rajalakshmi, S.; Saravanan, S.; Preeth, D.R.; Vasanthi, R.L.; Shairam, M.; Chatterjee, S. Synthesis and characterization of zinc-silibinin complexes: A potential bioactive compound with angiogenic, and antibacterial activity for bone tissue engineering. Colloids Surf. B Biointerfaces 2018, 167, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Merdy, P.; Guillon, E.; Frapart, Y.M.; Aplincourt, M. Iron and manganese surface complex formation with extracted lignin. Part 2: Characterisation of magnetic interaction between transition metal and quinonic radical by EPR microwave power saturation experiments. New J. Chem. 2003, 27, 577–582. [Google Scholar] [CrossRef]
- Zeng, X.; Du, Z.; Xu, Y.; Sheng, Z.; Jiang, W. Characterization of the interactions between apple condensed tannins and biologically important metal ions [Fe2+ (3d6), Cu2+ (3d9) and Zn2+ (3d10)]. LWT 2019, 114, 108384. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, X.; Li, W.W.; Shi, Y.; Lai, S.; Zhuang, J.; Yao, S.; Liu, Y.; Hu, J.; Gao, L.; et al. Proanthocyanidin–aluminum complexes improve aluminum resistance and detoxification of Camellia sinensis. J. Agric. Food Chem. 2020, 68, 7861–7869. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Hashida, K.; Otsuka, Y.; Ohara, S.; Kojima, K.; Shinohara, K. Identification of a hydrolyzable tannin, oenothein B, as an aluminum-detoxifying ligand in a highly aluminum-resistant tree, Eucalyptus camaldulensis. Plant Physiol. 2014, 164, 683–693. [Google Scholar] [CrossRef]
- Przewloka, S.R.; Shearer, B.J. The further chemistry of ellagic acid. II. Ellagic acid and water-soluble ellagates as metal precipitants. Holzforschung 2002, 56, 13–19. [Google Scholar] [CrossRef]
- Kraal, P.; Jansen, B.; Nierop, K.G.; Verstraten, J.M. Copper complexation by tannic acid in aqueous solution. Chemosphere 2006, 65, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; von Elverfeldt, D.; Hagemeyer, C.E.; et al. Engineering multifunctional capsules through the assembly of metal–phenolic networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Liu, W.; Zeng, X.; Song, X.; Yang, X.; Zhang, X.; Feng, J. Metal ion/tannic acid assembly as a versatile photothermal platform in engineering multimodal nanotheranostics for advanced applications. ACS Nano 2018, 12, 3917–3927. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Chen, R. Study of Complexes of Tannic Acid with Fe (III) and Fe (II). J. Anal. Methods Chem. 2019, 2019, 3894571. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef]
- Nobahar, A.; Carlier, J.D.; Miguel, M.G.; Costa, M.C. A review of plant metabolites with metal interaction capacity: A green approach for industrial applications. BioMetals 2021, 34, 761–793. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Yoshida, K.; Mihoko, M.; Kondo, T. Blue flower color development byanthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 857–964. [Google Scholar] [CrossRef]
- Iwashina, T. Contribution to flower colors of flavonoids including anthocyanins: A review. Nat. Prod. Commun. 2015, 10, 529–544. [Google Scholar] [CrossRef]
- Trunschke, J.; Lunau, K.; Pyke, G.H.; Ren, Z.X.; Wang, H. Flower color evolution and the evidence of pollinator-mediated selection. Front. Plant Sci. 2021, 12, 617851. [Google Scholar] [CrossRef] [PubMed]
- Fedenko, V.S.; Shemet, S.A.; Struzhko, V.S. Complexation of cyanidin with cadmium ions in solution. Ukr. Biochem. J. 2005, 77, 104–109. [Google Scholar]
- Fedenko, V.S. Dose effect of cyanidin interaction with lead ions in roots of maize seedlings. Ukr. Biochem. J. 2007, 79, 24–29. [Google Scholar]
- Hale, K.L.; McGrath, S.P.; Lombi, E.; Stack, S.M.; Terry, N.; Pickering, I.J.; George, G.N.; Pilon-Smits, E.A.H. Molybdenum sequestration in Brassica: A role for anthocyanins? Plant Physiol. 2001, 126, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Hale, K.L.; Tufan, H.A.; Pickering, I.J.; George, G.N.; Terry, N.; Pilon, M.; Pilon-Smits, E.A.H. Anthocyanins facilitate tungsten accumulation in Brassica. Physiol. Plant. 2002, 116, 351–358. [Google Scholar] [CrossRef]
- Stoutjesdijk, P.A.; Sale, P.W.; Larkin, P.J. Possible involvement of condensed tannins in aluminium tolerance of Lotus pedunculatus. Funct. Plant Biol. 2001, 28, 1063–1074. [Google Scholar] [CrossRef]
- Lavid, N.; Schwartz, A.; Yarden, O.; Tel-Or, E. The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta 2001, 212, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.E.; Guedes, T.T.; Bezerra, C.F.; Costa, M.D.S.; Campina, F.F.; de Freitas, T.S.; Souza, A.K.; Souza, C.E.S.; de Matos, Y.M.L.S.; Pereira-Junior, F.N.; et al. Identification of the gallic acid mechanism of action on mercury chloride toxicity reduction using infrared spectroscopy and antioxidant assays. Int. Biodeterior. Biodegrad. 2019, 141, 24–29. [Google Scholar] [CrossRef]
- Wojcieszek, J.; Ruzik, L. Enzymatic extraction of copper complexes with phenolic compounds from Açaí (Euterpe oleracea Mart.) and bilberry (Vaccinium myrtillus L.) fruits. Food Anal. Methods 2016, 9, 2105–2114. [Google Scholar] [CrossRef]
- Kidd, P.S.; Llugany, M.; Poschenrieder, C.H.; Gunse, B.; Barcelo, J. The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J. Exp. Bot. 2001, 52, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Schmidt, W. Mobilization of iron by plant-borne coumarins. Trends Plant Sci. 2017, 22, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Rodríguez-Celma, J.; Lan, P.; Wu, Y.C.; Vélez-Bermúdez, I.C.; Schmidt, W. Scopoletin 8-hydroxylase-mediated fraxetin production is crucial for iron mobilization. Plant Physiol. 2018, 177, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Kalinowska, M.; Laderiere, B.; Champagne, P.; Kowczyk-Sadowy, M.; Lewandowski, W. Mn (II), Cu (II) and Cd (II) p-coumarates: FT-IR, FT-Raman, 1H and 13C NMR and thermogravimetric studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 103, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, H., Ed.; Academic press: London, UK, 2011; 672p, ISBN 978-0-12384905-2. [Google Scholar]
- Vatansever, R.; Ozyigit, I.I.; Filiz, E. Essential and beneficial trace elements in plants, and their transport in roots: A review. Appl. Biochem. Biotechnol. 2017, 181, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, E.; Ceccanti, C.; Guidi, L.; Landi, M. Role of beneficial elements in plants: Implications for the photosynthetic process. Photosynthetica 2021, 59, 349–360. [Google Scholar] [CrossRef]
- Maret, W. The quintessence of metallomics: A harbinger of a different life science based on the periodic table of the bioelements. Metallomics 2022, 14, mfac051. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Maejima, E.; Yoshimura, T.; Urayama, M.; Yamauchi, A.; Owadano, M.; Okada, R.; Osaki, M.; Kanayama, Y.; Shinano, T. The ionomic study of vegetable crops. PLoS ONE 2016, 11, e0160273. [Google Scholar] [CrossRef] [PubMed]
- Buruleanu, L.C.; Radulescu, C.; Georgescu, A.A.; Dulama, I.D.; Nicolescu, C.M.; Olteanu, L.R.; Stanescu, S.G. Chemometric assessment of the interactions between the metal contents, antioxidant activity, total phenolics, and flavonoids in mushrooms. Anal. Lett. 2019, 52, 1195–1214. [Google Scholar] [CrossRef]
- Tabassum, S.; Zaki, M.; Afzal, M.; Arjmand, F. New modulated design and synthesis of quercetin–CuII/ZnII–Sn2 IV scaffold as anticancer agents: In vitro DNA binding profile, DNA cleavage pathway and Topo-I activity. Dalton Trans. 2013, 42, 10029–10041. [Google Scholar] [CrossRef] [PubMed]
- Porkodi, J.; Raman, N. Synthesis, characterization and biological screening studies of mixed ligand complexes using flavonoids as precursors. Appl. Organomet. Chem. 2018, 32, e4030. [Google Scholar] [CrossRef]
- Rahim, M.A.; Björnmalm, M.; Bertleff-Zieschang, N.; Ju, Y.; Mettu, S.; Leeming, M.G.; Caruso, F. Multiligand metal–phenolic assembly from green tea infusions. ACS Appl. Mater. Interfaces 2017, 10, 7632–7639. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Nakabayashi, R.; Paunesku, T.; Suzuki, M.; Saito, K.; Woloschak, G.E.; Smalle, J.A. Direct isolation of flavonoids from plants using ultra-small anatase TiO2 nanoparticles. Plant J. 2014, 77, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Binkowska, I. Hesperidin: Synthesis and characterization of bioflavonoid complex. SN Appl. Sci. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Candela, R.G.; Lazzara, G.; Piacente, S.; Bruno, M.; Cavallaro, G.; Badalamenti, N. Conversion of Organic Dyes into Pigments: Extraction of Flavonoids from Blackberries (Rubus ulmifolius) and Stabilization. Molecules 2021, 26, 6278. [Google Scholar] [CrossRef] [PubMed]
- Widyasari, E.M.; Kusumawardhany, E.; Sugiharti, R.J.; Sriyani, M.E.; Marzuki, M. The Optimization Method for Synthesis of 99mTc-Rutin as Potential Radiotracer in The Development of Cancer Drugs from Flavonoid. Indones. J. Cancer Chemoprev. 2019, 10, 80–87. [Google Scholar] [CrossRef]
- El-Sharawy, D.M.; Khater, S.I.; Essam, H.M.; Sherif, N.H.; Hassan, H.M.; Elmaidomy, A.H. 99mTc-Luteolin: Radiolabeling, In Silico ADMET and Biological Evaluation as a Natural Tracer Tumor imaging. J. Radiat. Res. Appl. Sci. 2021, 14, 125–132. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Gu, F.L.; Zhan, Q.; Palvannan, T.; Yusoff, A.R.M. Flavonoids mediated ‘Green’nanomaterials: A novel nanomedicine system to treat various diseases–Current trends and future perspective. Mater. Lett. 2018, 210, 26–30. [Google Scholar] [CrossRef]
- Pappas, A.C.; Zoidis, E.; Goliomytis, M.; Simitzis, P.E.; Sotirakoglou, K.; Charismiadou, M.A.; Nikitas, C.; Danezis, G.; Deligeorgis, S.G.; Georgiou, C.A. Elemental Metabolomics: Modulation of egg metallome with flavonoids, an exploratory study. Antioxidants 2019, 8, 361. [Google Scholar] [CrossRef]
- Zoidis, E.; Pappas, A.C.; Goliomytis, M.; Simitzis, P.E.; Sotirakoglou, K.; Tavrizelou, S.; Danezis, G.; Georgiou, C.A. Quercetin and Egg Metallome. Antioxidants 2021, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Fedenko, V.S.; Shemet, S.A. Plant metallomics: Role of phenolic chelators. In Proceedings of the 5th International Scientific Conference Restoration of Disturbed Natural Ecosystems, Donetsk, Ukraine, 12–15 May 2014; pp. 404–405. [Google Scholar]
- Fedenko, V.S. Phenolic chelators and plant metallomics. In Plant Physiology: Achievements and New Trends for Development; Morgun, V.V., Ed.; Logos: Kyiv, Ukraine, 2017; Volume 2, pp. 582–589. ISBN 978-616-7442-01-0. [Google Scholar]
- Santin, M.; Lucini, L.; Castagna, A.; Rocchetti, G.; Hauser, M.T.; Ranieri, A. Comparative “phenol-omics” and gene expression analyses in peach (Prunus persica) skin in response to different postharvest UV-B treatments. Plant Physiol. Biochem. 2019, 135, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Gutsch, A.; Vandionant, S.; Sergeant, K.; Jozefczak, M.; Vangronsveld, J.; Hausman, J.F.; Cuypers, A. Systems biology of metal tolerance in plants: A case study on the effects of Cd exposure on two model plants. In Plant Metallomics and Functional Omics; Sablok, G., Ed.; Springer: Cham, Switzerland, 2019; pp. 23–37. ISBN 978-3-030-19103-0. [Google Scholar] [CrossRef]
- Guerriero, G.; Sergeant, K.; Hausman, J.F. Integrated-omics: A powerful approach to understanding the heterogeneous lignification of fibre crops. Int. J. Mol. Sci. 2013, 14, 10958–10978. [Google Scholar] [CrossRef] [PubMed]
- Eticha, D.; Staß, A.; Horst, W.J. Localization of aluminium in the maize root apex: Can morin detect cell wall-bound aluminium? J. Exp. Bot. 2005, 56, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Lei, G.J.; Wang, Z.W.; Shi, Y.Z.; Braam, J.; Li, G.X.; Zheng, S.J. Coordination between apoplastic and symplastic detoxification confers plant aluminum resistance. Plant Physiol. 2013, 162, 1947–1955. [Google Scholar] [CrossRef]
- Gei, V.; Erskine, P.D.; Harris, H.H.; Echevarria, G.; Mesjasz-Przybyłowicz, J.; Barnabas, A.D.; Przybyłowicz, W.J.; Kopittke, P.M.; van der Ent, A. Tools for the discovery of hyperaccumulator plant species and understanding their ecophysiology. In Agromining: Farming for Metals; Van der Ent, A., Echevarria, G., Baker, A., Morel, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 117–133. ISBN 978-3-319-61899-9. [Google Scholar] [CrossRef]
- Fedenko, V.S.; Landi, M.; Shemet, S.A. Detection of nickel in maize roots: A novel nondestructive approach by reflectance spectroscopy and colorimetric models. Ecol. Indic. 2017, 82, 463–469. [Google Scholar] [CrossRef]
- Phiwchai, I.; Yuensook, W.; Sawaengsiriphon, N.; Krungchanuchat, S.; Pilapong, C. Tannic acid (TA): A molecular tool for chelating and imaging labile iron. Eur. J. Pharm. Sci. 2018, 114, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, J.; Bluemlein, K.; Krupp, E.M.; Mueller, M.; Wood, B.A. Metallomics study in plants exposed to arsenic, mercury, selenium and sulphur. In Metallomics. Advances in Experimental Medicine and Biology; Arruda, M., Ed.; Springer: Cham, Switzerland, 2018; Volume 1055, pp. 67–100. ISBN 978-3-319-90143-5. [Google Scholar] [CrossRef]
- Ito, T.; Oyama, K.I.; Yoshida, K. Direct observation of hydrangea blue-complex composed of 3-O-glucosyldelphinidin, Al3+ and 5-O-acylquinic acid by ESI-mass spectrometry. Molecules 2018, 23, 1424. [Google Scholar] [CrossRef] [PubMed]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, E.; Janjua, N.K.; Ahmed, S.; Murtaza, I.; Ali, T.; Masood, N.; Rizvi, A.S.; Murtaza, G. DFT predictions, synthesis, stoichiometric structures and anti-diabetic activity of Cu (II) and Fe (III) complexes of quercetin, morin, and primuletin. J. Mol. Struct. 2017, 1150, 459–468. [Google Scholar] [CrossRef]
- Říha, M.; Karlíčková, J.; Filipský, T.; Macáková, K.; Rocha, L.; Bovicelli, P.; Silvestri, I.P.; Saso, L.; Jahodář, L.; Radomír Hrdina, R.; et al. In vitro evaluation of copper-chelating properties of flavonoids. RSC Adv. 2014, 4, 32628–32638. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.A.; Potapovich, A.I.; Strigunova, E.N.; Kostyuk, T.V.; Afanas’ev, I.B. Experimental evidence that flavonoid metal complexes may act as mimics of superoxide dismutase. Arch. Biochem. Biophys. 2004, 428, 204–208. [Google Scholar] [CrossRef]
- Munteanu, A.C.; Badea, M.; Olar, R.; Silvestro, L.; Dulea, C.; Negut, C.D.; Uivarosi, V. Synthesis and structural investigation of new bio-relevant complexes of lanthanides with 5-hydroxyflavone: DNA binding and protein interaction studies. Molecules 2016, 21, 1737. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, E.; Janjua, N.K.; Ahmed, S. Removal of metal ions using metal-flavonoid-DNA adduct protocol. J. Saudi Chem. Soc. 2019, 23, 118–126. [Google Scholar] [CrossRef]
- Chirug, L.; Okun, Z.; Ramon, O.; Shpigelman, A. Iron ions as mediators in pectin-flavonols interactions. Food Hydrocoll. 2018, 84, 441–449. [Google Scholar] [CrossRef]
- Fabjanowicz, M.; Płotka-Wasylka, J. Metals and metal-binding ligands in wine: Analytical challenges in identification. Trends Food Sci. Technol. 2021, 112, 382–390. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Paglia, K.; Vaniya, A.; Wancewicz, B.; Keller, A.A. Metabolomics Reveals the Molecular Mechanisms of Copper Induced Cucumber Leaf (Cucumis sativus) Senescence. Environ. Sci. Technol. 2018, 52, 7092–7100. [Google Scholar] [CrossRef]
- Gonzalez Ibarra, A.A.; Wrobel, K.; Yanez Barrientos, E.; Corrales Escobosa, A.R.; Gutierrez Corona, J.F.; Enciso Donis, I.; Wrobel, K. Changes of Metabolomic Profile in Helianthus annuus under Exposure to Chromium (VI) Studied by capHPLC-ESI-QTOF-MS and MS/MS. J. Anal. Methods Chem. 2017, 2017, 3568621. [Google Scholar] [CrossRef]
- Corso, M.; Schvartzman, M.; Guzzo, F.; Souard, F.; Malkowski, E.; Hanikenne, M.; Verbruggen, N. Contrasting cadmium resistance strategies in two metallicolous populations of Arabidopsis halleri. New Phytol. 2018, 218, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Landi, M. Can anthocyanins be part of the metal homeostasis network in plant? Am. J. Agric. Biol. Sci. 2015, 10, 170–177. [Google Scholar] [CrossRef]
- Landi, M.; Pardossi, A.; Remorini, D.; Guidi, L. Antioxidant and photosynthetic response of a purple-leaved and a green-leaved cultivar of sweet basil (Ocimum basilicum) to boron excess. Environ. Exp. Bot. 2013, 85, 64–75. [Google Scholar] [CrossRef]
- Landi, M.; Remorini, D.; Pardossi, A.; Guidi, L. Purple versus green-leafed Ocimum basilicum: Which differences occur with regard to photosynthesis under boron toxicity? J. Plant Nutr. Soil Sci. 2013, 176, 942–951. [Google Scholar] [CrossRef]
- Landi, M.; Margaritopoulou, T.; Papadakis, I.E.; Araniti, F. Boron toxicity in higher plants: An update. Planta 2019, 250, 1011–1032. [Google Scholar] [CrossRef]
- Sentkowska, A.; Kilian, K.; Kopeć, M.; Pyrzyńska, K.; Cheda, Ł. Ga (III) complex with morin for kidney cancer cell labelling. Appl. Organomet. Chem. 2017, 31, e3882. [Google Scholar] [CrossRef]
- Jamali, A.A.; Tavakoli, A.; Dolatabadi, J.E.N. Analytical overview of DNA interaction with Morin and its metal complexes. Eur. Food Res. Technol. 2012, 235, 367–373. [Google Scholar] [CrossRef]
- Guzowska, M.K.; Kalinowska, M.; Lewandowski, W. Good fashion is evolution, not revolution”-methods to enhance existing anticancer medicines, primarily with use of transition metal. Anticancer Agents Med. Chem. 2017, 18, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.P.; Rickaby, R.E. Evolution’s Destiny: Co-Evolving Chemistry of the Environment and Life; Royal Society of Chemistry: Cambridge, UK, 2012; p. 319. ISBN 978-1-84973-558-2. [Google Scholar]
- Hanikenne, M.; Nouet, C. Metal hyperaccumulation and hypertolerance: A model for plant evolutionary genomics. Curr. Opin. Plant Biol. 2011, 14, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U. Conceptualizing plant systems evolution. Curr. Opin. Plant Biol. 2018, 42, 66–75. [Google Scholar] [CrossRef]
- Cappa, J.J.; Pilon-Smits, E.A. Evolutionary aspects of elemental hyperaccumulation. Planta 2014, 239, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Regvar, M.; Vogel-Mikuš, K. Functional Significance of Metal Ligands in Hyperaccumulating Plants: What Do We Know? In Detoxification of Heavy Metals; Sherameti, I., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 59–72. ISBN 978-3-642-21408-0. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the Links between Heavy Metal Stress and Plant Signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed]
- Glińska, S.; Bartczak, M.; Oleksiak, S.; Wolska, A.; Gabara, B.; Posmyk, M.; Janas, K. Effects of anthocyanin-rich extract from red cabbage leaves on meristematic cells of Allium cepa L. roots treated with heavy metals. Ecotoxicol. Environ. Saf. 2007, 68, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Stingu, A.; Volf, I.; Popa, V.I.; Gostin, I. New approaches concerning the utilization of natural amendments in cadmium phytoremediation. Ind. Crops Prod. 2012, 35, 53–60. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Jia, X.; Wang, W.K.; Liu, T.; Huang, S.P.; Yang, M.Y. Growth under elevated air temperature alters secondary metabolites in Robinia pseudoacacia L. seedlings in Cd-and Pb-contaminated soils. Sci. Total Environ. 2016, 565, 586–594. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Naidu, R. Bioremediation potential of natural polyphenol rich green wastes: A review of current research and recommendations for future directions. Environ. Technol. Innov. 2015, 4, 17–28. [Google Scholar] [CrossRef]
- Vandenbossche, M.; Jimenez, M.; Casetta, M.; Traisnel, M. Remediation of heavy metals by biomolecules: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1644–1704. [Google Scholar] [CrossRef]
- Bacelo, H.A.; Santos, S.C.; Botelho, C.M. Tannin-based biosorbents for environmental applications—A review. Chem. Eng. J. 2016, 303, 575–587. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, S.; Thakkar, A.; Baldi, A.; Murthy, R.S.R.; Sharma, R.K. Physical, chemical and phytoremediation technique for removal of heavy metals. J. Heavy Met. Toxic. Dis. 2016, 1, 3–10. [Google Scholar] [CrossRef]
- Hatami, M.; Kariman, K.; Ghorbanpour, M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Sci. Total Environ. 2016, 571, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, R.; Maestri, E.; Pagano, L.; Marmiroli, M.; White, J.C.; Marmiroli, N. Plant response to metal-containing engineered nanomaterials: An omics-based perspective. Environ. Sci. Technol. 2018, 52, 2451–2467. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Kohno, Y.; Kato, Y.; Shibata, M.; Fukuhara, C.; Maeda, Y.; Tomita, Y.; Kobayashi, K. Enhanced stability of natural anthocyanin incorporated in Fe-containing mesoporous silica. Microporous Mesoporous Mater. 2015, 203, 232–237. [Google Scholar] [CrossRef]
- Jung, J.; Cavender, G.; Simonsen, J.; Zhao, Y. Investigation of the mechanisms of using metal complexation and cellulose nanofiber/sodium alginate layer-by-layer coating for retaining anthocyanin pigments in thermally processed blueberries in aqueous media. J. Agric. Food Chem. 2015, 63, 3031–3038. [Google Scholar] [CrossRef]
- Manini, P.; Panzella, L.; Eidenberger, T.; Giarra, A.; Cerruti, P.; Trifuoggi, M.; Napolitano, A. Efficient binding of heavy metals by black sesame pigment: Toward innovative dietary strategies to prevent bioaccumulation. J. Agric. Food Chem. 2016, 64, 890–897. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Avallone, L.; Menichini, F. Radical scavenging, antioxidant and metal chelating activities of Annona cherimola Mill.(cherimoya) peel and pulp in relation to their total phenolic and total flavonoid contents. J. Food Compos. Anal. 2012, 25, 179–184. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Xu, B. Phenolic profiles, antioxidant capacities and metal chelating ability of edible mushrooms commonly consumed in China. LWT-Food Sci. Technol. 2016, 72, 423–431. [Google Scholar] [CrossRef]
- Mladěnka, P.; Říha, M.; Martin, J.; Gorová, B.; Matějíček, A.; Spilková, J. Fruit extracts of 10 varieties of elderberry (Sambucus nigra L.) interact differently with iron and copper. Phytochem. Lett. 2016, 18, 232–238. [Google Scholar] [CrossRef]
- Zhai, Q.; Narbad, A.; Chen, W. Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients 2014, 7, 552–571. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Escobar-Cévoli, R.; Castro-Espín, C.; Béraud, V.; Buckland, G.; Zamora-Ros, R.; Béraud, G.B.V. An overview of global flavonoid intake and its food sources. In Flavonoids-From Biosynthesis to Human Health; Justino, G.C., Ed.; Intech: Rijeka, Croatia, 2017; pp. 371–391. ISBN 978-953-51-3424-4. [Google Scholar] [CrossRef]
- Scalbert, A.; Brennan, L.; Manach, C.; Andres-Lacueva, C.; Dragsted, L.O.; Draper, J.; Rappaport, S.M.; van der Hooft, J.J.; Wishart, D.S. The food metabolome: A window over dietary exposur. Am. J. Clin. Nutr. 2014, 99, 1286–1308. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Rodrigues, F.; Vinha, A.F.; Alves, R.C.; Oliveira, M.B.P. Nutrigenomics and polyphenols. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Duxford, UK, 2018; pp. 103–132. ISBN 978-0-12-813573-0. [Google Scholar] [CrossRef]
- Gao, R.; Hu, H.; Shi, T.; Bao, Y.; Sun, Q.; Wang, L.; Rena, Y.; Jin, W.; Yuan, L. Incorporation of gelatin and Fe2+ increases the pH-sensitivity of zein-anthocyanin complex films used for milk spoilage detection. Curr. Res. Food Sci. 2022, 5, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.C.; Singh, R.N.; Torti, F.M.; Torti, S.V. Synthetic and natural iron chelators: Therapeutic potential and clinical use. Future Med. Chem. 2009, 1, 1643–1670. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Saxena, D. Lead Toxicity and Flavonoids. In. Research Methodology in Chemical Sciences: Experimental and Theoretical Approach; Chakraborty, T., Ledwani, L., Eds.; Apple Academic Press: Oakville, ON, Canada, 2017; pp. 305–336. ISBN 978-1-4987-2860-7. [Google Scholar] [CrossRef]
- Brzóska, M.; Borowska, S.; Tomczyk, M. Antioxidants as a potential preventive and therapeutic strategy for cadmium. Curr. Drug Targets 2016, 17, 1350–1384. [Google Scholar] [CrossRef]
- Gomes de Moura, C.F.; Ribeiro, D.A. Are food compounds able to modulate noxious activities induced by cadmium exposure? Crit. Rev. Food Sci. Nutr. 2017, 57, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Rogalska, J. Effect of an Extract from Aronia melanocarpa L. Berries on the Body Status of Zinc and Copper under Chronic Exposure to Cadmium: An In Vivo Experimental Study. Nutrients 2017, 9, 1374. [Google Scholar] [CrossRef] [PubMed]
- Jalili-Baleh, L.; Babaei, E.; Abdpour, S.; Bukhari, S.N.A.; Foroumadi, A.; Ramazani, A.; Abdollahi, M.; Khoobi, M. A review on flavonoid-based scaffolds as multi-target-directed ligands (MTDLs) for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 152, 570–589. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; de la Luz Cádiz-Gurrea, M.; Nunes, M.A.; Pinto, D.; Vinha, A.F.; Linares, I.B.; Oliveira, M.B.P.P.; Carretero, A.S. Cosmetics. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Duxford, UK, 2018; pp. 393–427. ISBN 978-0-12-813573-0. [Google Scholar] [CrossRef]
- Giacomelli, L.; Togni, S.; Meneghin, M.; Eggenhöffner, R.; Maramaldi, G. In vivo validation of the multicomponent powder (Vitachelox®) against the deposition of polluting ions. Clin. Cosmet. Investig. Dermatol. 2018, 11, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.M.; Cantrill, V.; Benohoud, M.; Tidder, A.; Rayner, C.M.; Blackburn, R.S. Application of Anthocyanins from Blackcurrant (Ribes nigrum L.) Fruit Waste as Renewable Hair Dyes. J. Agric. Food Chem. 2018, 66, 6790–6798. [Google Scholar] [CrossRef]
- Vankar, P.S. Structure-mordant interaction, replacement by biomordants and enzymes. In Natural Dyes for Textiles: Sources, Chemistry and Applications; Woodhead Publishing: Duxford, UK, 2017; pp. 89–102. ISBN 978-0-08-101884-2. [Google Scholar] [CrossRef]
- Samanta, A.K.; Konar, A. Dyeing of textiles with natural dyes. In Natural Dyes; Kumbasar, E.A., Ed.; Intech: Rijeka, Croatia, 2011; pp. 29–56. ISBN 978-953-307-783-3. [Google Scholar] [CrossRef]
- Arora, J.; Agarwal, P.; Gupta, G. Rainbow of natural dyes on textiles using plants extracts: Sustainable and eco-friendly processes. Green Sustain. Chem. 2017, 7, 35–47. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Galán-Vidal, C.A.; Contreras-López, E.; Páez-Hernández, M. Purification of anthocyanins with o-dihydroxy arrangement by sorption in cationic resins charged with Fe (III). J. Chem. 2014, 2014, 367236. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Bernal, F.A.; Orduz-Diaz, L.L.; Coy-Barrera, E. Exploitation of the complexation reaction of ortho-dihydroxylated anthocyanins with aluminum (III) for their quantitative spectrophotometric determination in edible sources. Food Chem. 2015, 185, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Turner, R.J. Stability of biogenic metal (loid) nanomaterials related to the colloidal stabilization theory of chemical nanostructures. Crit. Rev. Biotechnol. 2018, 38, 1–20. [Google Scholar] [CrossRef]
- Wan, X.; Xiang, W.; Wan, N.; Yan, S.; Bao, Z.; Wang, Y. Complexation and reduction of iron by phenolic substances: Implications for transport of dissolved Fe from peatlands to aquatic ecosystems and global iron cycling. Chem. Geol. 2018, 498, 128–138. [Google Scholar] [CrossRef]
- Veys-Renaux, D.; Reguer, S.; Bellot-Gurlet, L.; Mirambet, F.; Rocca, E. Conversion of steel by polyphenolic model molecules: Corrosion inhibition mechanism by rutin, esculin, esculetol. Corros. Sci. 2018, 136, 1–8. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq. 2018, 266, 577–590. [Google Scholar] [CrossRef]
- Rahim, M.A.; Ejima, H.; Cho, K.L.; Kempe, K.; Müllner, M.; Best, J.P.; Caruso, F. Coordination-driven multistep assembly of metal–polyphenol films and capsules. Chem. Mater. 2014, 26, 1645–1653. [Google Scholar] [CrossRef]
- Halake, K.; Cho, S.; Kim, J.; Lee, T.; Cho, Y.; Chi, S.; Park, M.; Kim, K.; Lee, D.; Ju, H.; et al. Applications using the metal affinity of polyphenols with mussel-inspired chemistry. Macromol. Res. 2018, 26, 93–99. [Google Scholar] [CrossRef]
- Wei, J.; Wang, G.; Chen, F.; Bai, M.; Liang, Y.; Wang, H.; Zhao, D.; Zhao, Y. Sol-gel Synthesis of Metal-Phenolic Coordination Spheres and Their Derived Carbon Composites. Angew. Chem. Int. Ed. 2018, 130, 9986–9991. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, J.Y.; Lee, J.M.; Jeong, Y.C.; So, S.H.; Cho, Y.S.; Nam, S.; Park, C.R.; Yang, S.J. Macroscopically interconnected hierarchically porous carbon monolith by metal-phenolic coordination as an sorbent for multi-scale molecules. Carbon 2018, 126, 190–196. [Google Scholar] [CrossRef]
- Liao, Y.; Yao, Y.; Yu, Y.; Zeng, Y. Enhanced Antibacterial Activity of Curcumin by Combination with Metal Ions. Colloid Interface Sci. Commun. 2018, 25, 1–6. [Google Scholar] [CrossRef]
- Hug, H.; Bader, M.; Mair, P.; Glatzel, T. Biophotovoltaics: Natural pigments in dye-sensitized solar cells. Appl. Energy 2014, 115, 216–225. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.; Mohamad, A.B.; Ludin, N.A.; Kadhum, A.A.H.; Sopian, K. Dye-sensitised solar cells: Development, structure, operation principles, electron kinetics, characterisation, synthesis materials and natural photosensitisers. Renew. Sustain. Energy Rev. 2016, 65, 183–213. [Google Scholar] [CrossRef]
- Ludin, N.A.; Mahmoud, A.A.A.; Mohamad, A.B.; Kadhum, A.A.H.; Sopian, K.; Karim, N.S.A. Review on the development of natural dye photosensitizer for dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2014, 31, 386–396. [Google Scholar] [CrossRef]
- Kumara, N.T.R.N.; Lim, A.; Lim, C.M.; Petra, M.I.; Ekanayake, P. Recent progress and utilization of natural pigments in dye sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 78, 301–317. [Google Scholar] [CrossRef]
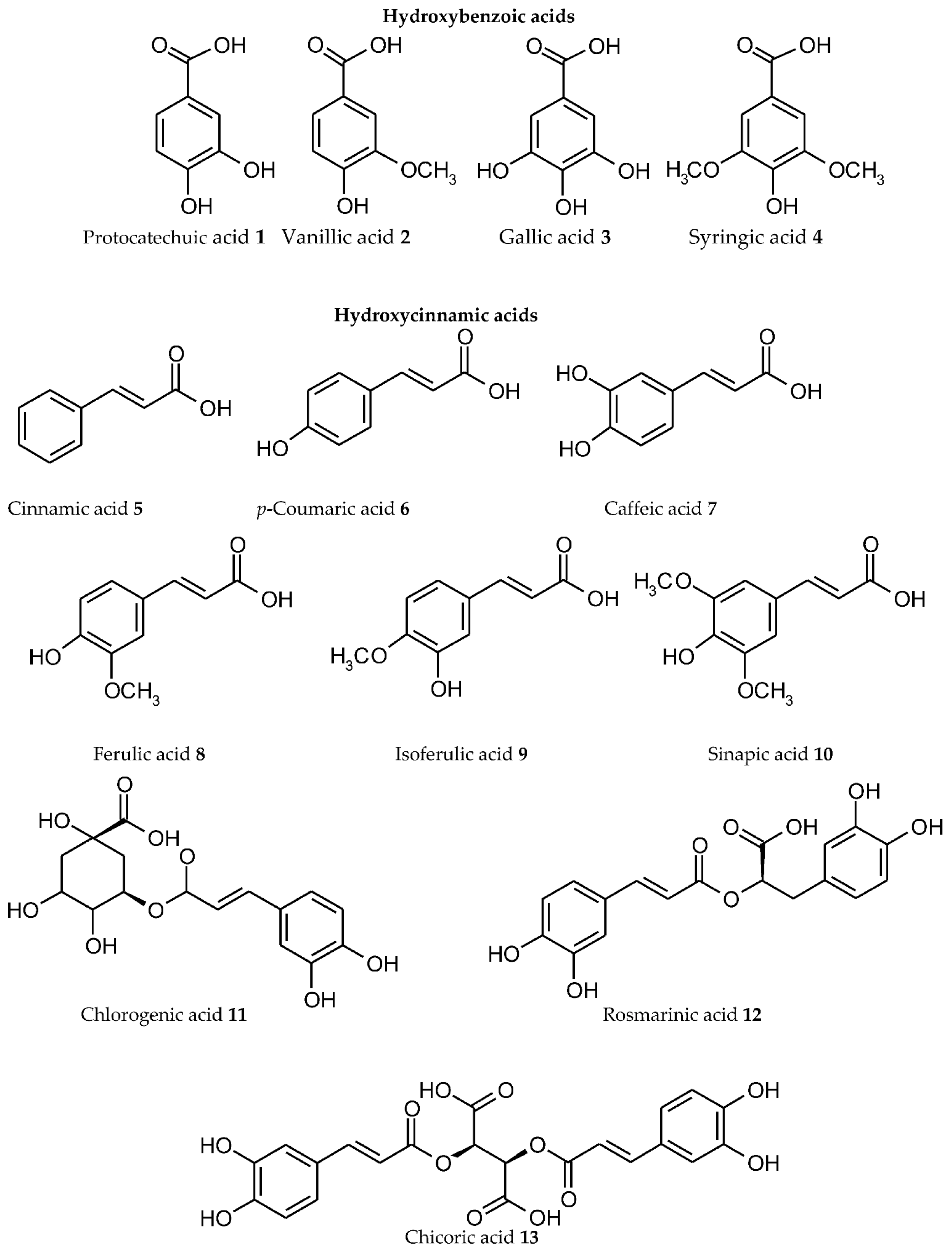
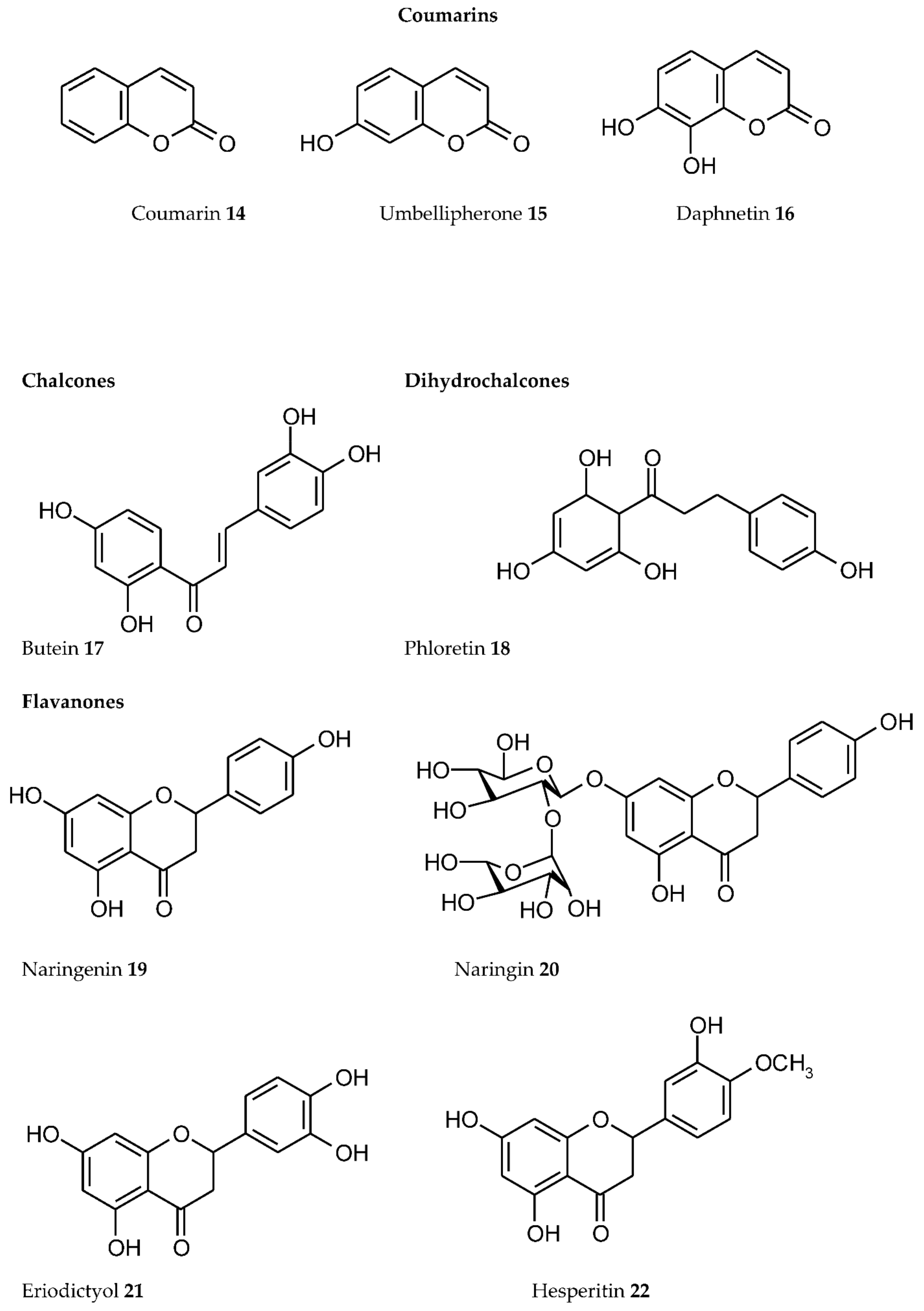
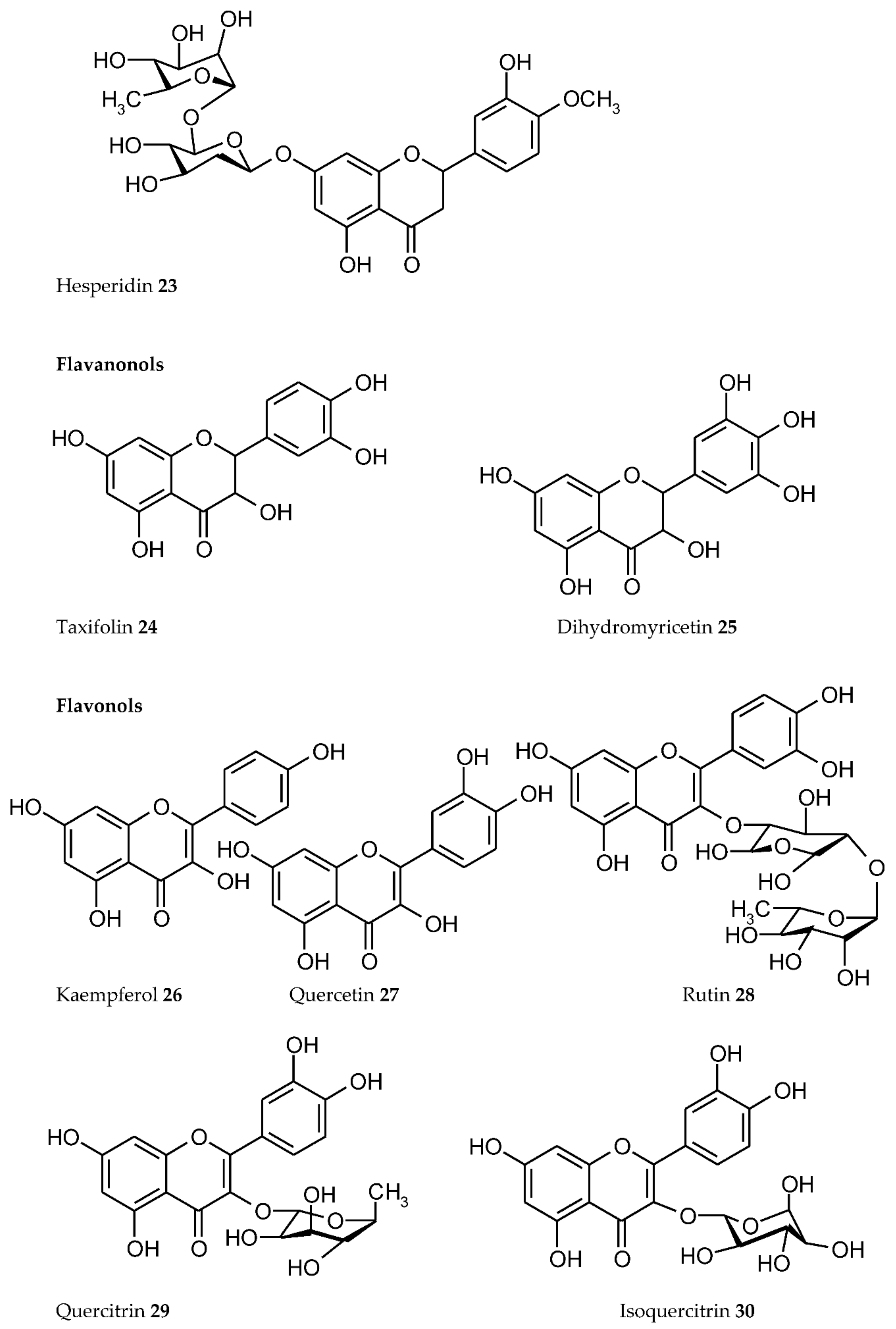
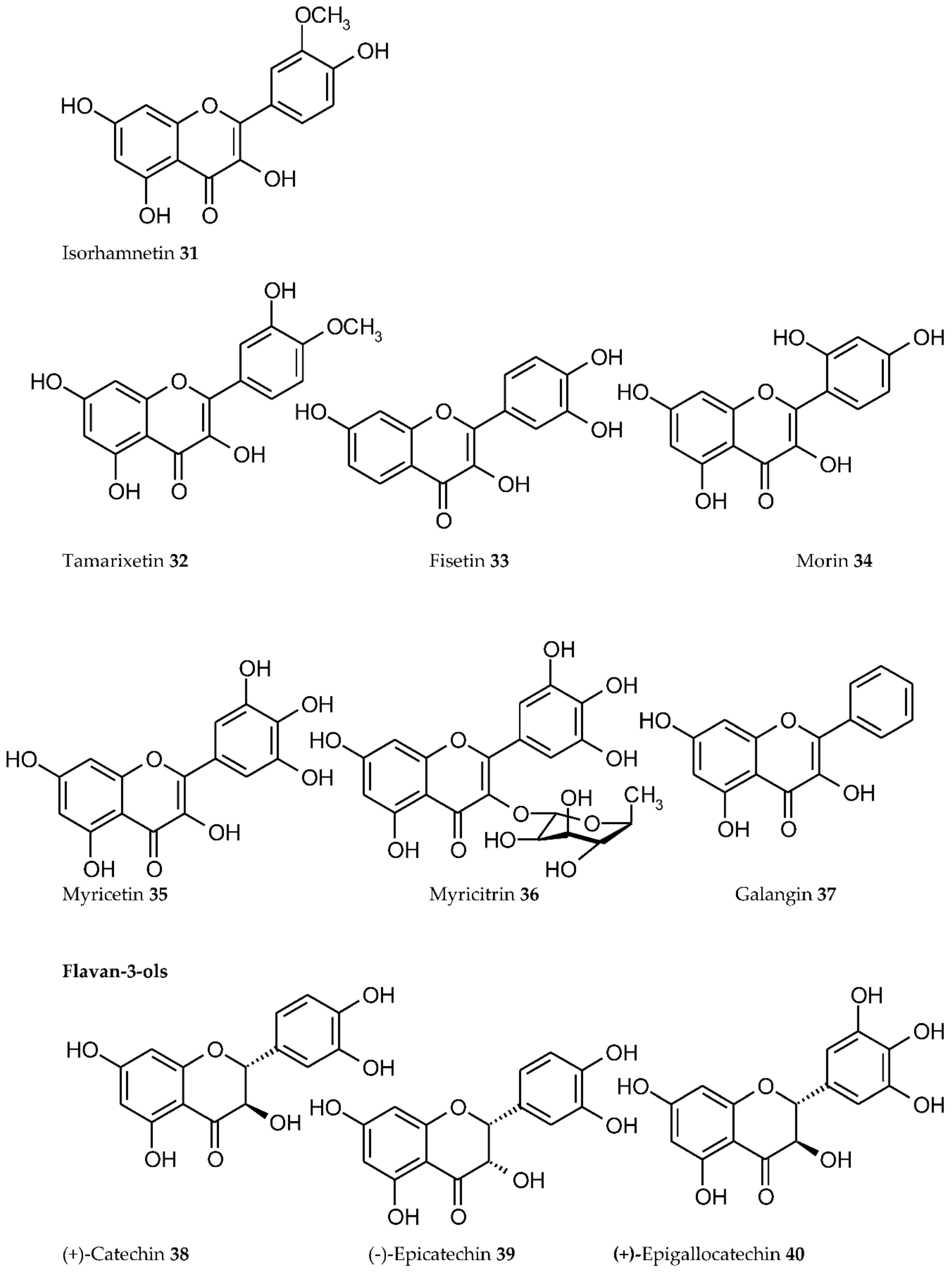
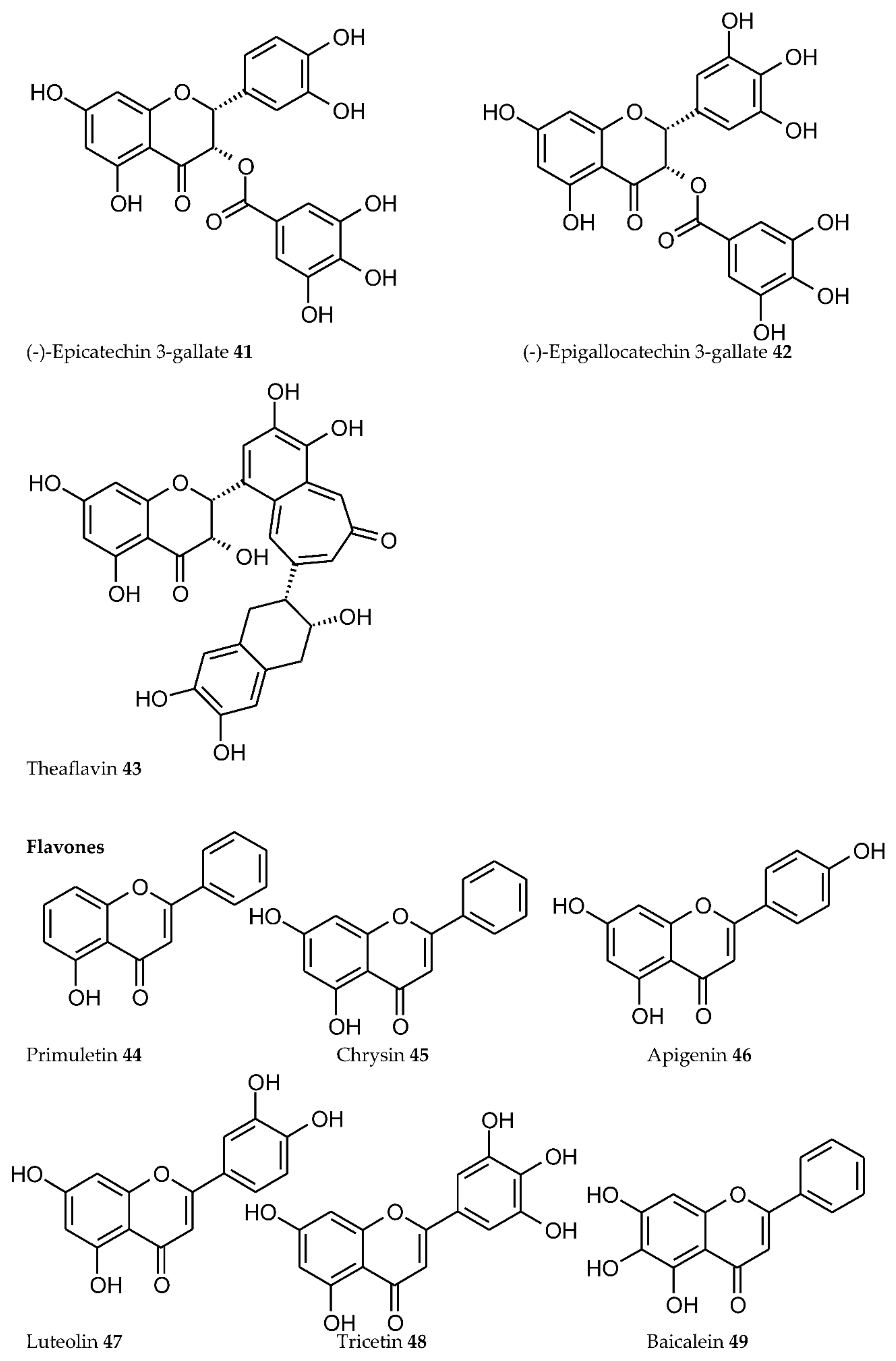
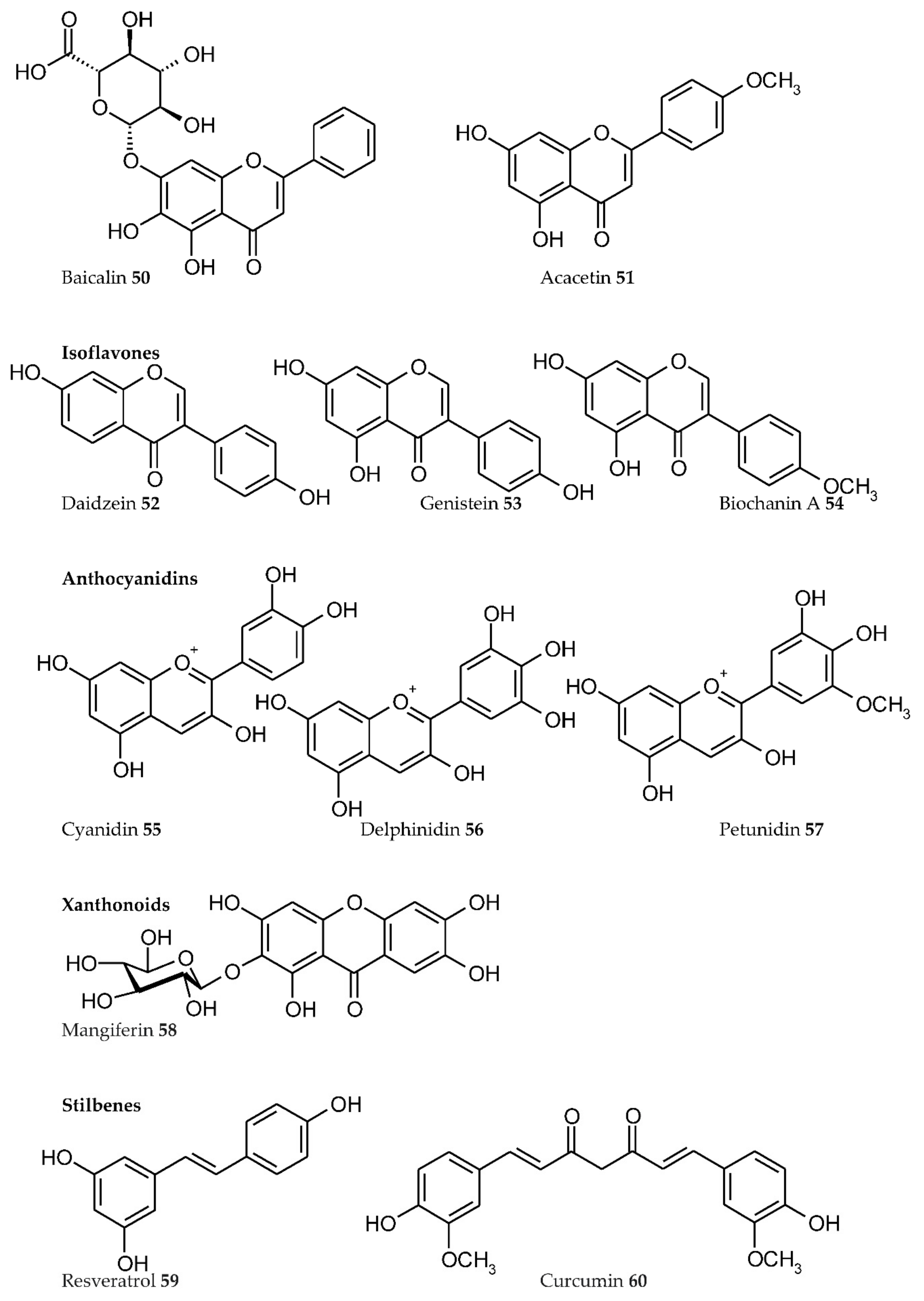
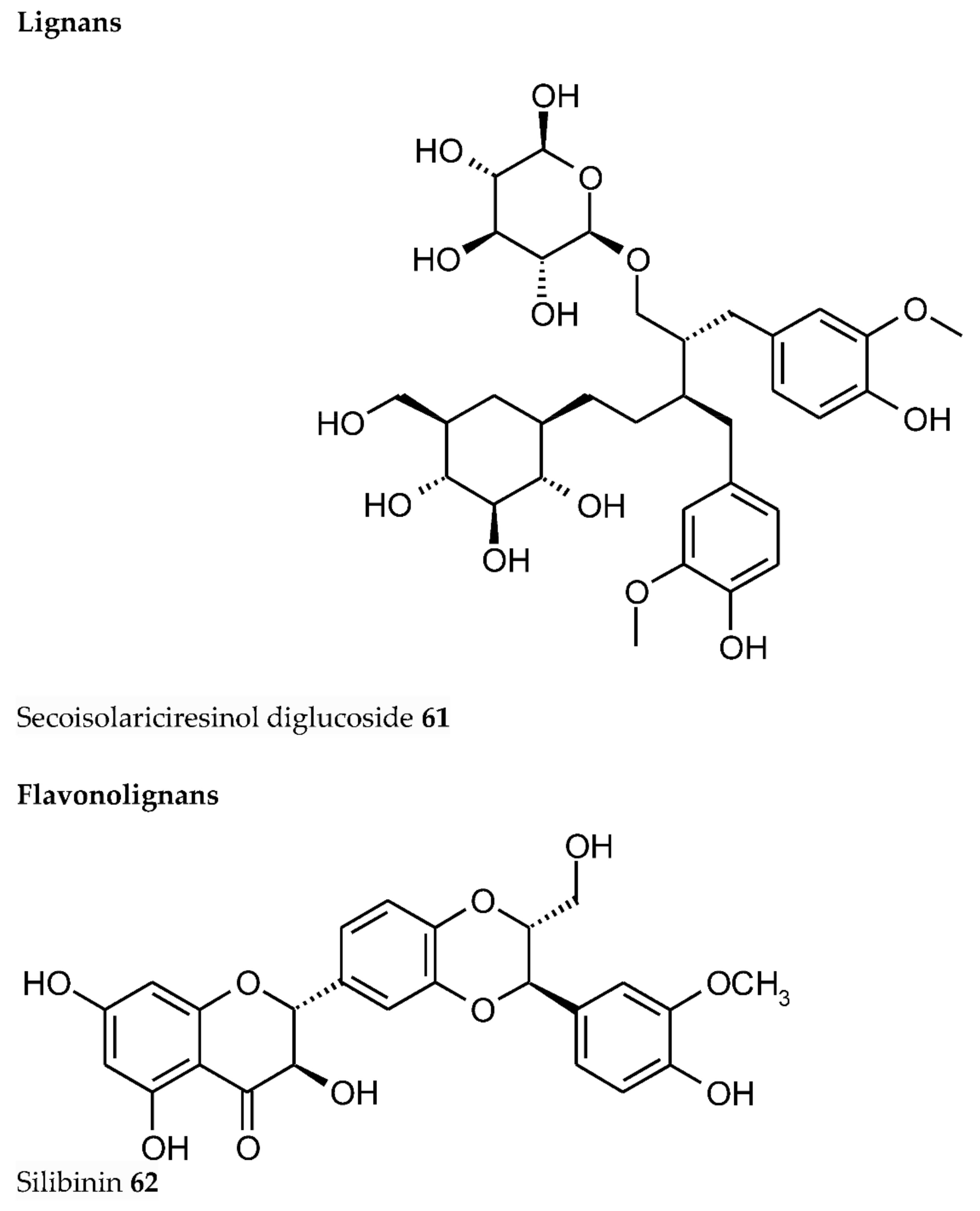

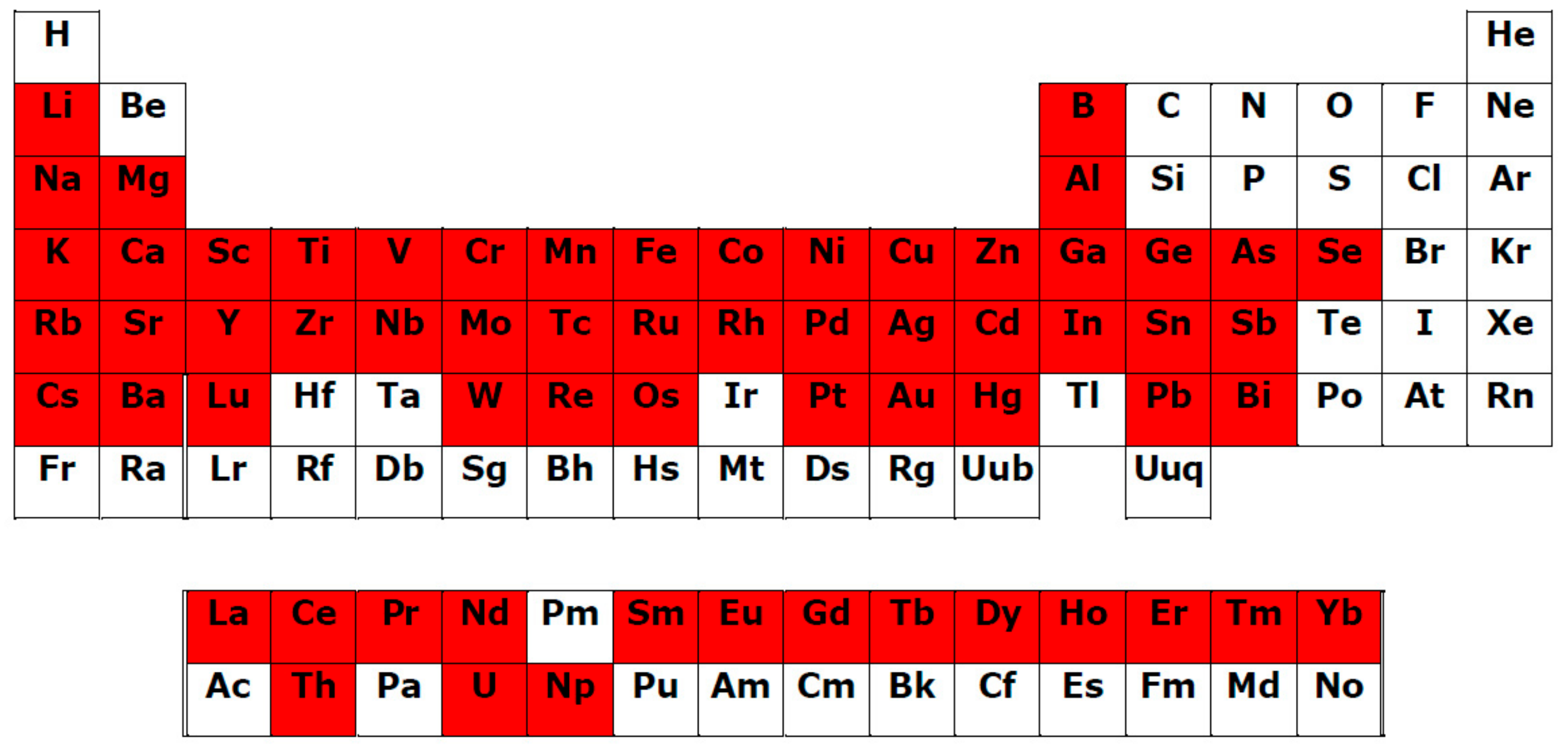
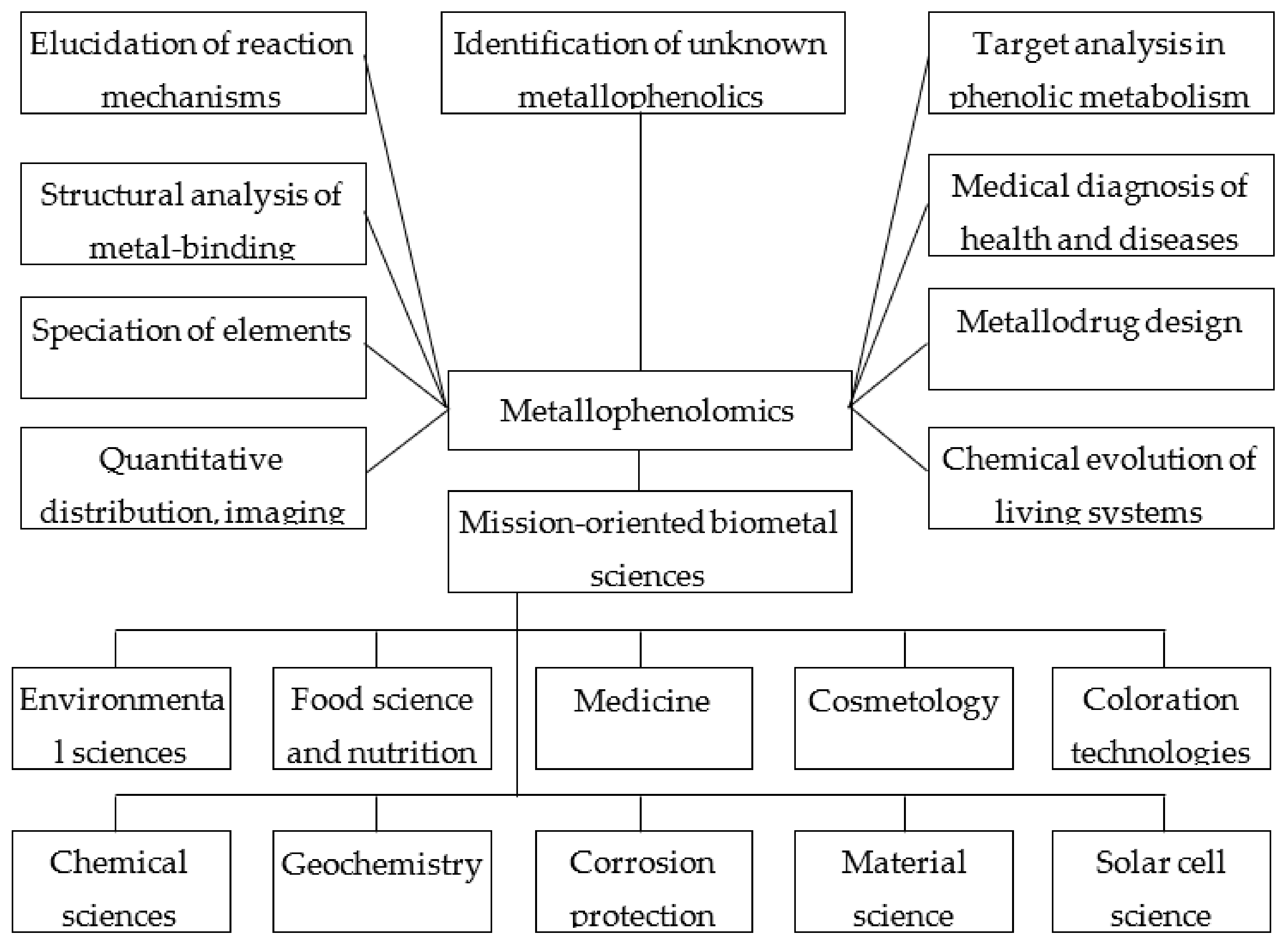
| Phenolic Ligand | Metal(loid) Ion | Number of Metal Ions | References |
|---|---|---|---|
| Phenolic acid | |||
| Hydroxybenzoic acids | |||
| Protocatechuic acid 1 | Al(III), U(VI) | 2 | [83,84] |
| Vanillic acid 2 | Zn(II), Y(III), La(III), Ce(III), Pr(III), Nd(III), Sm(III), Eu(III), Gd(III), Tb(III), Dy(III), Ho(III), Er(III), Tm(III), Yb(III), Lu(III), Np(V) | 17 | [85,86,87] |
| Gallic acid 3 | Fe(II), Zn(II), Fe(III), Eu(III) | 4 | [88,89,90,91] |
| Syringic acid 4 | Li(I), Na(I), K(I), Rb(I), Cs(I), Fe(II), Fe(III), Y(III), La(III), Ce(III), Pr(III), Nd(III), Sm(III), Eu(III), Gd(III), Tb(III), Dy(III), Ho(III), Er(III), Tm(III), Yb(III), Lu(III) | 22 | [92,93,94] |
| Hydroxycinnamic acids | |||
| Cinnamic acid 5 | Li(I), Na(I), K(I), Rb(I), Cs(I), Hg(I), Ca(II), Co(II), Ni(II), Cu(II), Zn(II), Ru(II), Cd(II), La(III), Eu(III), Tb(III), VO(IV), Th(IV) | 19 | [95,96,97,98,99,100,101,102] |
| p-Coumaric acid 6 | Li(I), Na(I), K(I), Rb(I), Cs(I), Co(II), Ni(II), Cu(II), Zn(II),Al(III) | 10 | [82,103,104] |
| Caffeic acid 7 | Li(I), Na(I), K(I), Rb(I), Cs(I), Cu(II), Pb(II), Pt(II), Al(III), Fe(III), Cr(III), Eu(III) | 12 | [82,105,106,107,108] |
| Ferulic acid 8 | Ca(II), Mn(II), Cu(II), Zn(II), Cd(II), Al(III), VO(IV), V(V) | 8 | [61,82,97,109] |
| Isoferulic acid 9 | Na(I), Mg(II), Mn(II) | 3 | [110] |
| Sinapic acid 10 | Cu(II), Pt(II), V(V) | 3 | [106,111] |
| Chlorogenic acid 11 | Li(I), Na(I), K(I), Rb(I), Cs(I), Ca(II), Zn (II), Fe(III), VO(IV) | 9 | [112,113,114,115,116] |
| Rosmarinic acid 12 | Li(I), Na(I), K(I), Rb(I), Cs(I), Ca(II), Cu(II) | 7 | [115,117,118] |
| Chicoric acid 13 | Co(II), Ni(II), Cu(II), Zn(II) | 4 | [119] |
| Coumarins | |||
| Coumarin 14 | La(III), Ce(III), Nd(III), Sm(III), Dy(III) | 5 | [108] |
| Umbellipherone 15 | Ce(III) | 1 | [120] |
| Daphnetin 16 | Cu(II), Zn(II), Ge(IV) | 3 | [121] |
| Chalcones | |||
| Butein 17 | Cu(II), Zn(II) | 2 | [122] |
| Dihydrochalcones | |||
| Phloretin 18 | Ru(III) | 1 | [123] |
| Flavanones | |||
| Naringenin 19, naringin 20 | Fe(II), Cu(II), Ni(II), Zn(II), Pt(II), Fe(III), Cr(III), La(III), Y(III), Eu(III), Ce(IV), VO(IV), V(V) | 12 | [76,77,106,124,125,126] |
| Eriodictyol 21 | Fe(II), Fe(III) | 2 | [127] |
| Hesperitin 22, hesperidin 23 | Ni(II), Cu(II), Zn(II), Al(III), VO(IV), | 5 | [76] |
| Flavanonols | |||
| Taxifolin 24 | Fe(II), Ni(II), Cu(II), Zn(II), Fe(III) | 5 | [77,124,128,129,130] |
| Dihydromyricetin 25 | Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Zn(II) | 6 | [131,132] |
| Flavonols | |||
| Kaempferol 26 | Fe(II), Cu(II), Zn(II), Pb(II), Fe(III), VO(IV) | 6 | [76,77,81,133] |
| Quercetin 27, rutin 28, quercitrin 29, isoquercitrin 30 | Mg(II), Ca(II), Sc(II), Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Zn(II), Mo(II), Pd(II), Cd(II), Hg(II), Sn(II), Pb(II), Al(III), Cr(III), Fe(III), Ga(III), Y(III), Rh(III), Sb(III), La(III), Pr(III), Nd(III), Eu(III), Gd(III), Tb(III), Dy(III), Tm(III), Au(III), Ge(IV), Zr(IV), Ru(IV), Sn(IV), Os(IV), Cr(VI), Mo(VI), W(VI), Tc(VII), Os(VIII), VO(IV), UO2(II), | 43 | [76,77,78,81,134,135,136,137,138,139,140] |
| Isorhamnetin 31 | Fe(II), Cu(II) | 2 | [141] |
| Tamarixetin 32 | Fe(II), Cu(II) | 2 | [141] |
| Fisetin 33 | Fe(II), Cu(II), Zn(II), Fe(III), VO(IV) | 4 | [77,142] |
| Morin 34 | Mg(II), Ca(II), Mn(II), Co(II), Ni(II), Cu(II), Zn(II), Sr(II), Pd(II), Cd(II), Ba(II), Sn(II), Pt(II), Al(III), Cr(III), Fe(III), Au(III), La(III), Eu(III), Gd(III), Lu(III), Zr(IV), VO(IV), Mo(VI), W(VI), Ti(COO)2 2+ | 26 | [76,77,78,143] |
| Myricetin 35, myricitrin 36 | Cu(II), Zn(II), Al(III), Fe(III) | 4 | [76,78,136] |
| Galangin 37 | Fe(II), Cu(II), Zn(II), Al(III) | 4 | [133] |
| Flavan-3-ols | |||
| (+)-Catechin 38, (-)-epicatechin 39 | Fe(II), Cu(II), Zn(II), Hg(II), Al(III), Fe(III), Cr(III), La(III), Yb(III), Gd(III) | 10 | [77,144,145,146,147,148,149,150,151] |
| (+)-Epigallocatechin 40 | Fe(II) | 1 | [148] |
| (-)-Epicatechin 3-gallate 41 | Fe(II), Cu(II), Zn(II), Al(III), Fe(III) | 3 | [80,146] |
| (-)-Epigallocatechin 3-gallate 42 | Fe(II), Mn(II), Cu(II), Zn(II), Pt(II), Al(III), Fe(III) | 7 | [80,146,148,152,153] |
| Theaflavin 43 | Al(III), Fe(III) | 2 | [154,155] |
| Flavones | |||
| Primuletin 44 | Zn(II), Cu(II); Pb(II), Al(III), Fe(III) | 5 | [77,133] |
| Chrysin 45 | Cu(II), Pd(II), Al(III), Fe(III), La(III), Ho(III), Er(III), Yb(III), Ce(IV), VO(IV) | 10 | [76,77,133] |
| Apigenin 46 | Cu(II), Pb(II), VO(IV) | 3 | [76,133,156] |
| Luteolin 47 | Mn(II), Fe(II), Cu(II), Al(III), Fe(III), Y(III), Ho(III), Yb(III), Lu(III), VO(IV) | 10 | [76,77,81,156,157] |
| Tricetin 48 | Fe(II), Fe(III) | 2 | [127] |
| Baicalein 49, baicalin 50 | Fe(II), Cu(II), Fe(III), VO(IV) | 4 | [76,77,156] |
| Acacetin 51 | Fe(III) | 1 | [158] |
| Isoflavones | |||
| Daidzein 52 | Ce(IV) | 1 | [77] |
| Genistein 53 | Cu(II), Fe(III) | 2 | [76,159] |
| Biochanin A 54 | Cu(II), Fe(III) | 2 | [159] |
| Anthocyanidins | |||
| Cyanidin 55 and its glycosides | Cs(I), Mg(II), Ca(II), Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Sr(II), Zn(II), Cd(II), Sn(II), Ba(II), Hg(II), Pb(II), B(III), Al(III), V(III), Cr(III), Fe(III), Ga(III), As(III), Bi(III), Ge(IV), VO3−, MoO42−, WO42− | 27 | [13,160,161,162,163] |
| Delphinidin 56 and its glycosides | Mg(II), Zn(II), Sn(II), Al(III), Cr(III), Fe(III), Ga(III) | 7 | [13,163,164] |
| Petunidin 57 and its glycosides | Mg(II), Sn(II), Al(III), Cr(III), Fe(III), Ga(III) | 6 | [164,165] |
| Xanthonoids | |||
| Mangiferin 58 | Fe(II), Cu(II), Zn(II), Fe(III), Se(IV), Ge(IV) | 6 | [121,166,167] |
| Stilbenes | |||
| Resveratrol 59 | Fe(II), Cu(II), Zn(II), Al(III), Fe(III) | 5 | [80,130,168,169] |
| Curcuminoids | |||
| Curcumin 60 | Mg(II), Ca(II), Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Zn(II), Se(II), Pd(II), Cd(II), Sn(II), Hg(II), Pb(II), Al(III), Cr(III), Fe(III), Ga(III), Y(III), Ru(III), In(III), Re(III), Sm(III), Eu(III), Dy(III), Au(III), VO(IV), Nb(V) | 28 | [130,170,171,172] |
| Lignans | |||
| Secoisolariciresinol diglucoside 61 | Ag(I), Ca(II), Fe(II), Ni(II), Cu(II), Pb(II) | 6 | [173] |
| Flavonolignans | |||
| Silibinin(silybin) 62 | Ni(II), Cu(II), Zn(II), Fe(III), Ga(III), VO(IV) | 6 | [156,174,175,176] |
| Lignins | |||
| Ligno-cellulosic substrate | Mn(II), Cu(II), Fe(III) | 3 | [177] |
| Tannins | |||
| Condensed tannins | Fe(II), Cu(II), Zn(II), Al(III) | 4 | [178,179] |
| Oenothein B 63 | Al(III) | 1 | [180] |
| Ellagic acid 64 | Mg(II), Ca(II), Mn(II), Fe(II), Co(II), Cu(II), Fe(III) | 7 | [181] |
| Tannic acid 65 | Mg(II), Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Zn(II), Mo(II), Cd(II), Al(III), V(III), Cr(III), Fe(III), Ru(III), Rh(III), Ce(III), Eu(III), Gd(III), Tb(III), Ti(IV), Zr(IV) | 21 | [153,182,183,184,185] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedenko, V.S.; Landi, M.; Shemet, S.A. Metallophenolomics: A Novel Integrated Approach to Study Complexation of Plant Phenolics with Metal/Metalloid Ions. Int. J. Mol. Sci. 2022, 23, 11370. https://doi.org/10.3390/ijms231911370
Fedenko VS, Landi M, Shemet SA. Metallophenolomics: A Novel Integrated Approach to Study Complexation of Plant Phenolics with Metal/Metalloid Ions. International Journal of Molecular Sciences. 2022; 23(19):11370. https://doi.org/10.3390/ijms231911370
Chicago/Turabian StyleFedenko, Volodymyr S., Marco Landi, and Sergiy A. Shemet. 2022. "Metallophenolomics: A Novel Integrated Approach to Study Complexation of Plant Phenolics with Metal/Metalloid Ions" International Journal of Molecular Sciences 23, no. 19: 11370. https://doi.org/10.3390/ijms231911370
APA StyleFedenko, V. S., Landi, M., & Shemet, S. A. (2022). Metallophenolomics: A Novel Integrated Approach to Study Complexation of Plant Phenolics with Metal/Metalloid Ions. International Journal of Molecular Sciences, 23(19), 11370. https://doi.org/10.3390/ijms231911370