Advances in Anti-Cancer Activities of Flavonoids in Scutellariae radix: Perspectives on Mechanism
Abstract
1. Introduction
2. Induction of Cancer Cell Apoptosis by Major Flavonoids in SR
3. Regulation of Canonical Tumor-Associated Signaling Pathway
4. Inhibition of Cell Cycle Transition
5. Inhibition of Tumor DNA Damage Repair
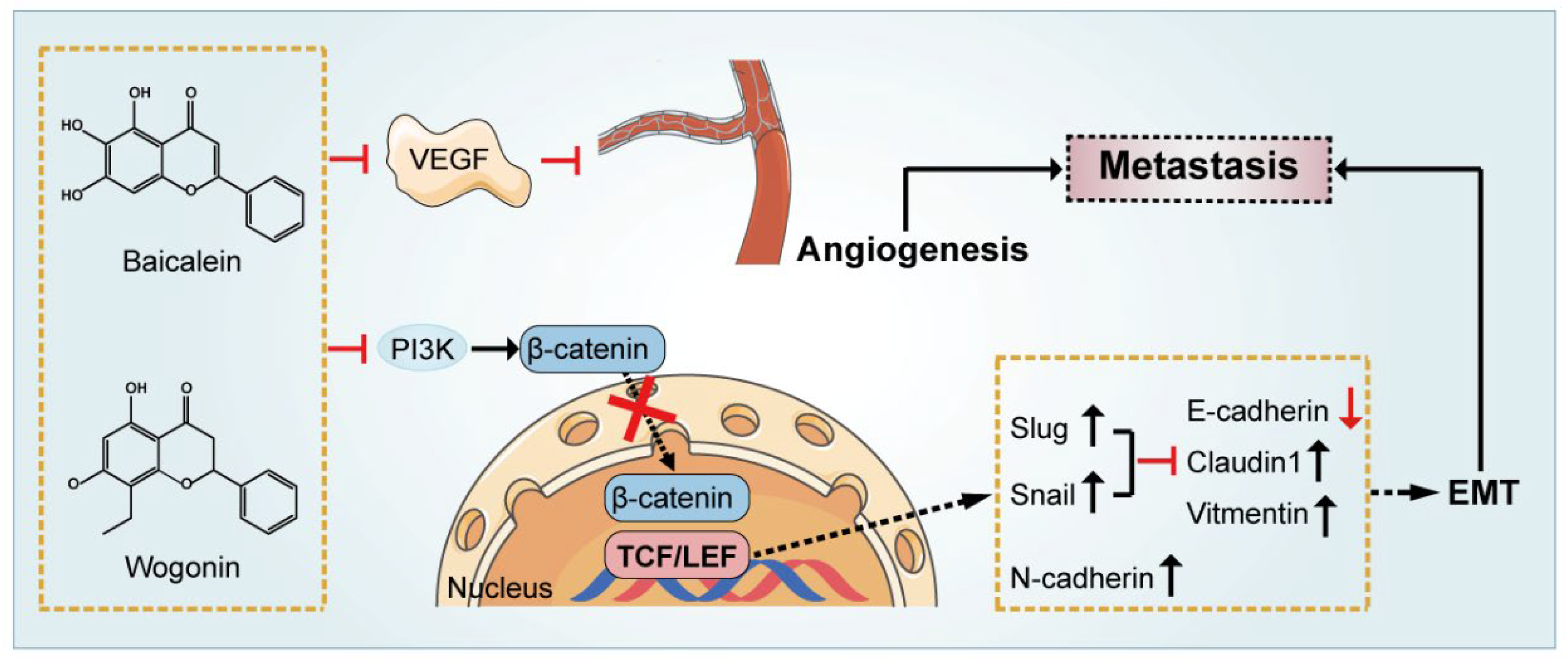
6. Inhibition of Tumor Metastasis and Angiogenesis
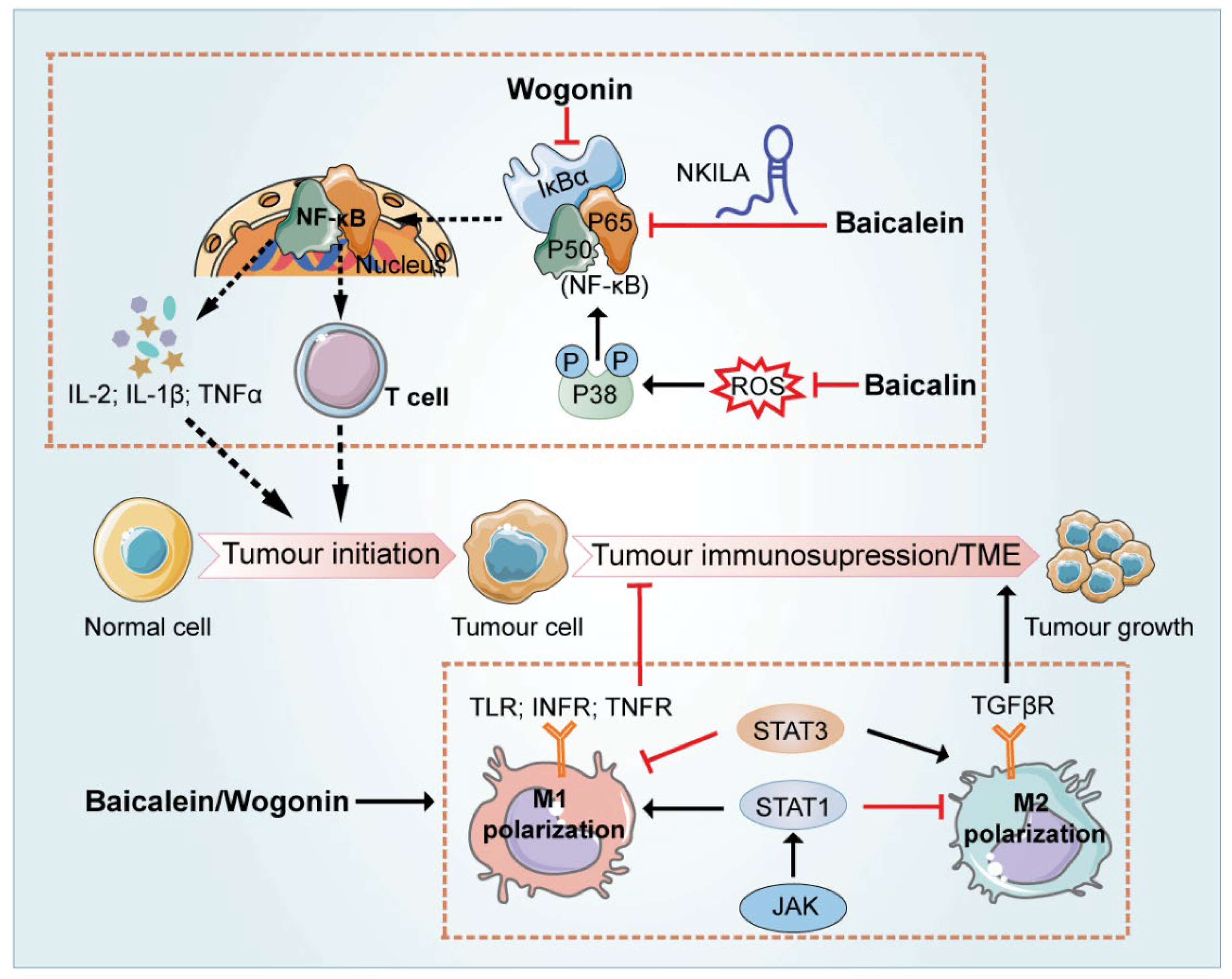
7. Regulation of the Immune-Related Tumor Microenvironment
8. Applications of Flavonoids in SR in Reversing Drug-Resistance and Sensitizing Chemotherapy
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zheng, Z.; Liu, H.; Zhai, S.; Zhang, H.; Shan, G.; Kwok, R.T.K.; Ma, C.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; et al. Highly Efficient Singlet Oxygen Generation, Two-Photon Photodynamic Therapy and Melanoma Ablation by Rationally Designed Mitochondria-Specific near-Infrared AIEgens. Chem. Sci. 2020, 11, 2494–2503. [Google Scholar] [CrossRef] [PubMed]
- Orangio, G.R. The Economics of Colon Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.J.; Sottoriva, A.; Graham, T.A. Measuring Clonal Evolution in Cancer with Genomics. Annu. Rev. Genom. Hum. Genet. 2019, 20, 309–329. [Google Scholar] [CrossRef]
- Han, S.; Wang, W.; Wang, S.; Yang, T.; Zhang, G.; Wang, D.; Ju, R.; Lu, Y.; Wang, H.; Wang, L. Tumor Microenvironment Remodeling and Tumor Therapy Based on M2-like Tumor Associated Macrophage-Targeting Nano-Complexes. Theranostics 2021, 11, 2892–2916. [Google Scholar] [CrossRef]
- Tajan, M.; Vousden, K.H. Dietary Approaches to Cancer Therapy. Cancer Cell 2020, 37, 767–785. [Google Scholar] [CrossRef]
- Cheng, C.-S.; Chen, J.; Tan, H.-Y.; Wang, N.; Chen, Z.; Feng, Y. Scutellaria baicalensis and Cancer Treatment: Recent Progress and Perspectives in Biomedical and Clinical Studies. Am. J. Chin. Med. 2018, 46, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, X.-Y.; Martin, C. Scutellaria baicalensis, the Golden Herb from the Garden of Chinese Medicinal Plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, P.; Liu, S.; Liu, Q.; Li, Y.; Sun, Y.; He, C.; Xiao, P. Traditional Uses, Ten-Years Research Progress on Phytochemistry and Pharmacology, and Clinical Studies of the Genus Scutellaria. J. Ethnopharmacol. 2021, 265, 113198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Wang, S.; Kuang, Y.; Hu, Z.-M.; Qiao, X.; Ye, M. A Comprehensive Review on Phytochemistry, Pharmacology, and Flavonoid Biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, S.; Li, X.; Liu, R. Advances in the Study of Emodin: An Update on Pharmacological Properties and Mechanistic Basis. Chin. Med. 2021, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Apoptosis in Cancer: From Pathogenesis to Treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.-Y.; Lin, L.-T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad Targeting of Resistance to Apoptosis in Cancer. Semin. Cancer Biol. 2015, 3, S78–S103. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as Anticancer Mechanism: Function and Dysfunction of Its Modulators and Targeted Therapeutic Strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. New Therapeutic Aspects of Flavones: The Anticancer Properties of Scutellaria and Its Main Active Constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Ye, C.; Yu, X.; Zeng, J.; Dai, M.; Zhang, B. Effects of Baicalein on Proliferation, Apoptosis, Migration and Invasion of Ewing’s Sarcoma Cells. Int. J. Oncol. 2017, 51, 1785–1792. [Google Scholar] [CrossRef]
- Yu, Y.; Pei, M.; Li, L. Baicalin Induces Apoptosis in Hepatic Cancer Cells In Vitro and Suppresses Tumor Growth In Vivo. Int. J. Clin. Exp. Med. 2015, 8, 8958–8967. [Google Scholar] [PubMed]
- Kalpage, H.A.; Bazylianska, V.; Recanati, M.A.; Fite, A.; Liu, J.; Wan, J.; Mantena, N.; Malek, M.H.; Podgorski, I.; Heath, E.I.; et al. Tissue-Specific Regulation of Cytochrome c by Post-Translational Modifications: Respiration, the Mitochondrial Membrane Potential, ROS, and Apoptosis. FASEB J. 2019, 33, 1540–1553. [Google Scholar] [CrossRef] [PubMed]
- Peña, Q.; Sciortino, G.; Maréchal, J.-D.; Bertaina, S.; Simaan, A.J.; Lorenzo, J.; Capdevila, M.; Bayón, P.; Iranzo, O.; Palacios, Ò. Copper(II) N,N,O-Chelating Complexes as Potential Anticancer Agents. Inorg. Chem. 2021, 60, 2939–2952. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Choi, E.-O.; Park, C.; Hwang, H.-J.; Hong, S.H.; Kim, G.-Y.; Cho, E.-J.; Kim, W.-J.; Choi, Y.H. Baicalein Induces Apoptosis via ROS-Dependent Activation of Caspases in Human Bladder Cancer 5637 Cells. Int. J. Oncol. 2016, 49, 1009–1018. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Yang, C.-X.; Zhang, L.; Yang, C.-Y.; Xu, X.-Q. Baicalein, as a Prooxidant, Triggers Mitochondrial Apoptosis in MCF-7 Human Breast Cancer Cells Through Mobilization of Intracellular Copper and Reactive Oxygen Species Generation. Oncotargets Ther. 2019, 12, 10749–10761. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Wen, X.; Bell, C.; Appiah, S. Liposome-delivered Baicalein Induction of Myeloid Leukemia K562 Cell Death via Reactive Oxygen Species Generation. Mol. Med. Rep. 2018, 17, 4524–4530. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Han, J.; Lee, N.; Yoon, J.-H.; Youn, K.; Ha, H.J.; Yoon, E.; Kim, D.H.; Jun, M. Neuroprotective Effects of Baicalein, Wogonin, and Oroxylin A on Amyloid Beta-Induced Toxicity via NF-ΚB/MAPK Pathway Modulation. Molecules 2020, 25, 5087. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Lu, N.; Zhang, H.; Dai, Q.; Wei, L.; Li, Z.; You, Q.; Guo, Q. Wogonin Induced Cytotoxicity in Human Hepatocellular Carcinoma Cells by Activation of Unfolded Protein Response and Inactivation of AKT. Hepatol. Res. 2013, 43, 890–905. [Google Scholar] [CrossRef]
- Ge, W.; Yin, Q.; Xian, H. Wogonin Induced Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Human Malignant Neuroblastoma Cells Via IRE1α-Dependent Pathway. J. Mol. Neurosci. 2015, 56, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.S.; Blower, M.D. The Endoplasmic Reticulum: Structure, Function and Response to Cellular Signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef]
- Chang, H.-T.; Chou, C.-T.; Kuo, D.-H.; Shieh, P.; Jan, C.-R.; Liang, W.-Z. The Mechanism of Ca2+ Movement in the Involvement of Baicalein-Induced Cytotoxicity in ZR-75-1 Human Breast Cancer Cells. J. Nat. Prod. 2015, 78, 1624–1634. [Google Scholar] [CrossRef]
- Bar, J.; Moskovits, N.; Oren, M. Involvement of Stromal P53 in Tumor-Stroma Interactions. Semin. Cell Dev. Biol. 2010, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Wu, Y.; Dai, Q.; Zhou, Y.; Li, Z.; Yang, L.; Guo, Q.; Lu, N. Selective Anti-Tumor Activity of Wogonin Targeting the Warburg Effect through Stablizing P53. Pharmacol. Res. 2018, 135, 49–59. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, L.; Chakraborty, T.; Ghosh, T.; Chanda, P.; Roy, S. Construing the Biochemical and Molecular Mechanism Underlying the In Vivo and In Vitro Chemotherapeutic Efficacy of Ruthenium-Baicalein Complex in Colon Cancer. Int. J. Biol. Sci. 2019, 15, 1052–1071. [Google Scholar] [CrossRef] [PubMed]
- Advani, S.H. Targeting MTOR Pathway: A New Concept in Cancer Therapy. Indian J. Med. Paediatr. Oncol. 2010, 31, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Lobe, C.; Tahraoui, S.; Clapéron, A.; Mergey, M.; Merabtene, F.; Wendum, D.; Coulouarn, C.; Housset, C.; Desbois-Mouthon, C.; et al. The IGF2/IR/IGF1R Pathway in Tumor Cells and Myofibroblasts Mediates Resistance to EGFR Inhibition in Cholangiocarcinoma. Clin. Cancer Res. 2018, 24, 4282–4296. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Mei, J.; Tan, Y. Baicalin Attenuates DDP (Cisplatin) Resistance in Lung Cancer by Downregulating MARK2 and p-Akt. Int. J. Oncol. 2017, 50, 93–100. [Google Scholar] [CrossRef]
- Hou, Y.; Pi, C.; Feng, X.; Wang, Y.; Fu, S.; Zhang, X.; Zhao, L.; Wei, Y. Antitumor Activity In Vivo and Vitro of New Chiral Derivatives of Baicalin and Induced Apoptosis via the PI3K/Akt Signaling Pathway. Mol. Ther. Oncolytics 2020, 19, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, Y.; Li, Y.; Cao, Y.; Tang, L.; Chen, F.; Xia, J. Baicalein Inhibits Cervical Cancer Progression via Downregulating Long Noncoding RNA BDLNR and Its Downstream PI3K/Akt Pathway. Int. J. Biochem. Cell Biol. 2018, 94, 107–118. [Google Scholar] [CrossRef]
- Gurney, A.; Axelrod, F.; Bond, C.J.; Cain, J.; Chartier, C.; Donigan, L.; Fischer, M.; Chaudhari, A.; Ji, M.; Kapoun, A.M.; et al. Wnt Pathway Inhibition via the Targeting of Frizzled Receptors Results in Decreased Growth and Tumorigenicity of Human Tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 11717–11722. [Google Scholar] [CrossRef] [PubMed]
- Karaca, B.; Bakır, E.; Yerer, M.B.; Cumaoğlu, A.; Hamurcu, Z.; Eken, A. Doxazosin and Erlotinib Have Anticancer Effects in the Endometrial Cancer Cell and Important Roles in ERα and Wnt/β-Catenin Signaling Pathways. J. Biochem. Mol. Toxicol. 2021, 35, e22905. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xia, J.; Yang, H.; Li, Y.; Liu, S.; Cao, Y.; Tang, L.; Yu, X. Baicalein Blocked Cervical Carcinoma Cell Proliferation by Targeting CCND1 via Wnt/β-Catenin Signaling Pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2729–2736. [Google Scholar] [CrossRef]
- Abu-Shahba, A.G.; Gebraad, A.; Kaur, S.; Paananen, R.O.; Peltoniemi, H.; Seppänen-Kaijansinkko, R.; Mannerström, B. Proangiogenic Hypoxia-Mimicking Agents Attenuate Osteogenic Potential of Adipose Stem/Stromal Cells. Tissue Eng. Regen. Med. 2020, 17, 477–493. [Google Scholar] [CrossRef]
- Salcher, S.; Hagenbuchner, J.; Geiger, K.; Seiter, M.A.; Rainer, J.; Kofler, R.; Hermann, M.; Kiechl-Kohlendorfer, U.; Ausserlechner, M.J.; Obexer, P. C10ORF10/DEPP, a Transcriptional Target of FOXO3, Regulates ROS-Sensitivity in Human Neuroblastoma. Mol. Cancer 2014, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Nonoguchi, K.; Sakurai, T.; Masuda, T.; Itoh, K.; Fujita, J. A Novel Protein Depp, Which Is Induced by Progesterone in Human Endometrial Stromal Cells Activates Elk-1 Transcription Factor. Mol. Hum. Reprod. 2005, 11, 471–476. [Google Scholar] [CrossRef]
- Su, M.-Q.; Zhou, Y.-R.; Rao, X.; Yang, H.; Zhuang, X.-H.; Ke, X.-J.; Peng, G.-Y.; Zhou, C.-L.; Shen, B.-Y.; Dou, J. Baicalein Induces the Apoptosis of HCT116 Human Colon Cancer Cells via the Upregulation of DEPP/Gadd45a and Activation of MAPKs. Int. J. Oncol. 2018, 53, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Michowski, W.; Kolodziejczyk, A.; Sicinski, P. The Cell Cycle in Stem Cell Proliferation, Pluripotency and Differentiation. Nat. Cell Biol. 2019, 21, 1060–1067. [Google Scholar] [CrossRef]
- Pennycook, B.R.; Barr, A.R. Restriction Point Regulation at the Crossroads between Quiescence and Cell Proliferation. FEBS Lett. 2020, 594, 2046–2060. [Google Scholar] [CrossRef] [PubMed]
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Jiang, W.; Yang, J.; Huang, C.; Li, Z. Baicalein: A Review of Its Anti-Cancer Effects and Mechanisms in Hepatocellular Carcinoma. Biomed. Pharmacother. 2017, 93, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Chamorro, L.; Felip, E.; Ezeonwumelu, I.J.; Margelí, M.; Ballana, E. Cyclin-Dependent Kinases as Emerging Targets for Developing Novel Antiviral Therapeutics. Trends Microbiol. 2021, 29, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-P.; He, L.; Zhang, Q.-P.; Zeng, X.-T.; Liu, S.-Q. Baicalein Inhibits Proliferation of Myeloma U266 Cells by Downregulating IKZF1 and IKZF3. Med. Sci. Monit. 2018, 24, 2809–2817. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Sokolosky, M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; et al. GSK-3 as Potential Target for Therapeutic Intervention in Cancer. Oncotarget 2014, 5, 2881–2911. [Google Scholar] [CrossRef]
- Orzechowska, B.U.; Wróbel, G.; Turlej, E.; Jatczak, B.; Sochocka, M.; Chaber, R. Antitumor Effect of Baicalin from the Scutellaria baicalensis Radix Extract in B-Acute Lymphoblastic Leukemia with Different Chromosomal Rearrangements. Int. Immunopharmacol. 2020, 79, 106114. [Google Scholar] [CrossRef]
- Kuo, H.-M.; Tsai, H.-C.; Lin, Y.-L.; Yang, J.-S.; Huang, A.-C.; Yang, M.-D.; Hsu, S.-C.; Chung, M.-C.; Gibson Wood, W.; Chung, J.-G. Mitochondrial-Dependent Caspase Activation Pathway Is Involved in Baicalein-Induced Apoptosis in Human Hepatoma J5 Cells. Int. J. Oncol. 2009, 35, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, E.; Umemura, N. Induced Overexpression of CD44 Associated with Resistance to Apoptosis on DNA Damage Response in Human Head and Neck Squamous Cell Carcinoma Cells. Int. J. Oncol. 2017, 50, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, X.; Yang, W.H.; Kang, Y. A Flavone, Wogonin from Scutellaria baicalensis Inhibits the Proliferation of Human Colorectal Cancer Cells by Inducing of Autophagy, Apoptosis and G2/M Cell Cycle Arrest via Modulating the PI3K/AKT and STAT3 Signalling Pathways. J. BUON 2019, 24, 1143–1149. [Google Scholar] [PubMed]
- Chao, J.-I.; Su, W.-C.; Liu, H.-F. Baicalein Induces Cancer Cell Death and Proliferation Retardation by the Inhibition of CDC2 Kinase and Survivin Associated with Opposite Role of P38 Mitogen-Activated Protein Kinase and AKT. Mol. Cancer Ther. 2007, 6, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.H.; Fung, T.K.; Poon, R.Y.C. Cyclin A in Cell Cycle Control and Cancer. Cell. Mol. Life Sci. 2002, 59, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Calway, T.D.; Wen, X.-D.; Smith, J.; Yu, C.; Wang, Y.; Mehendale, S.R.; Yuan, C.-S. Hydrophobic Flavonoids from Scutellaria Baicalensis Induce Colorectal Cancer Cell Apoptosis through a Mitochondrial-Mediated Pathway. Int. J. Oncol. 2013, 42, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Cramer-Morales, K.; Nieborowska-Skorska, M.; Scheibner, K.; Padget, M.; Irvine, D.A.; Sliwinski, T.; Haas, K.; Lee, J.; Geng, H.; Roy, D.; et al. Personalized Synthetic Lethality Induced by Targeting RAD52 in Leukemias Identified by Gene Mutation and Expression Profile. Blood 2013, 122, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Gillman, R.; Lopes Floro, K.; Wankell, M.; Hebbard, L. The Role of DNA Damage and Repair in Liver Cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188493. [Google Scholar] [CrossRef]
- Mojiri, A.; Walther, B.K.; Jiang, C.; Matrone, G.; Holgate, R.; Xu, Q.; Morales, E.; Wang, G.; Gu, J.; Wang, R.; et al. Telomerase Therapy Reverses Vascular Senescence and Extends Lifespan in Progeria Mice. Eur. Heart J. 2021, 42, 4352–4369. [Google Scholar] [CrossRef]
- Huang, S.-T.; Wang, C.-Y.; Yang, R.-C.; Chu, C.-J.; Wu, H.-T.; Pang, J.-H.S. Wogonin, an Active Compound in Scutellaria baicalensis, Induces Apoptosis and Reduces Telomerase Activity in the HL-60 Leukemia Cells. Phytomedicine 2010, 17, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, Z.; Tian, J.; Wang, H.; Song, G.; Guo, Q.; Tian, J.; Han, Y.; Liao, Q.; Liu, G.; et al. The Downregulation of C-Myc and Its Target Gene HTERT Is Associated with the Antiproliferative Effects of Baicalin on HL-60 Cells. Oncol. Lett. 2017, 14, 6833–6840. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, T.; Cao, Z.; Zhang, J.; Tang, M.; Tian, Y.; Li, Y.; Lu, X.; Chen, Y.; Wang, H.; Wei, F.-Z.; et al. SIRT6 Coordinates with CHD4 to Promote Chromatin Relaxation and DNA Repair. Nucleic Acids Res. 2020, 48, 2982–3000. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Dutt, S.; Xu, R.; Graves, K.; Juszczynski, P.; Manis, J.P.; Shipp, M.A. BBAP Monoubiquitylates Histone H4 at Lysine 91 and Selectively Modulates the DNA Damage Response. Mol. Cell 2009, 36, 110–120. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Kawaguchi, K.; Toi, M. DNA Damage Repair Functions and Targeted Treatment in Breast Cancer. Breast Cancer 2020, 27, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Pratz, K.W.; Koh, B.; Patel, A.G.; Flatten, K.S.; Poh, W.; Herman, J.G.; Dilley, R.; Harrell, M.I.; Smith, B.D.; Karp, J.E.; et al. Poly(ADP-Ribose) Polymerase Inhibitor Hypersensitivity in Aggressive Myeloproliferative Neoplasms. Clin. Cancer Res. 2016, 22, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Xu, Y.-L.; Tang, Z.-H.; Li, T.; Zhang, L.-L.; Chen, X.; Lu, J.-H.; Leung, C.-H.; Ma, D.-L.; Qiang, W.-A.; et al. Baicalein Induces Beclin 1- and Extracellular Signal-Regulated Kinase-Dependent Autophagy in Ovarian Cancer Cells. Am. J. Chin. Med. 2017, 45, 123–136. [Google Scholar] [CrossRef]
- Chen, M.; Wu, H.L.; Wong, T.S.; Chen, B.; Gong, R.-H.; Wong, H.L.X.; Xiao, H.; Bian, Z.; Kwan, H.Y. Combination of Wogonin and Artesunate Exhibits Synergistic Anti-Hepatocellular Carcinoma Effect by Increasing DNA-Damage-Inducible Alpha, Tumor Necrosis Factor α and Tumor Necrosis Factor Receptor-Associated Factor 3-Mediated Apoptosis. Front. Pharmacol. 2021, 12, 657080. [Google Scholar] [CrossRef]
- Tuli, H.S.; Aggarwal, V.; Kaur, J.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Tuorkey, M.; Kaur, G.; Savla, R.; Sak, K.; et al. Baicalein: A Metabolite with Promising Antineoplastic Activity. Life Sci. 2020, 259, 118183. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Ma, X.; Yan, W.; Dai, Z.; Gao, X.; Ma, Y.; Xu, Q.; Jiang, J.; Zhang, S. Baicalein Suppresses Metastasis of Breast Cancer Cells by Inhibiting EMT via Downregulation of SATB1 and Wnt/β-Catenin Pathway. Drug Des. Dev. Ther. 2016, 10, 1419–1441. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, A.; Kuang, G.; Gong, X.; Jiang, R.; Lin, D.; Li, J.; Li, H.; Zhang, X.; Wan, J.; et al. Baicalin Inhibits the Metastasis of Highly Aggressive Breast Cancer Cells by Reversing Epithelial-to-Mesenchymal Transition by Targeting β-Catenin Signaling. Oncol. Rep. 2017, 38, 3599–3607. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, Y.; Gao, M.; Zhang, F. Wogonoside Attenuates Cutaneous Squamous Cell Carcinoma by Reducing Epithelial-Mesenchymal Transition/Invasion and Cancer Stem-Like Cell Property. Oncotargets Ther. 2020, 13, 10097–10109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qu, J.; Liu, X.; Wang, J.; Ma, X.; Zhao, X.; Yang, Q.; Yan, W.; Zhao, Z.; Hui, Y.; et al. Baicalein Suppress EMT of Breast Cancer by Mediating Tumor-Associated Macrophages Polarization. Am. J. Cancer Res. 2018, 8, 1528–1540. [Google Scholar] [PubMed]
- Zhu, L.; Fu, X.; Chen, X.; Han, X.; Dong, P. M2 Macrophages Induce EMT through the TGF-β/Smad2 Signaling Pathway. Cell Biol. Int. 2017, 41, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Zhou, R.; Zhong, W.; Lu, S.; Ma, Z.; Chai, Y. Baicalin Inhibits Human Osteosarcoma Cells Invasion, Metastasis, and Anoikis Resistance by Suppressing the Transforming Growth Factor-Β1-Induced Epithelial-to-Mesenchymal Transition. Anticancer Drugs 2017, 28, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qi, B.; Xiaoxiang, W.; Xu, J.; Liu, X. Baicalein Increases Cisplatin Sensitivity of A549 Lung Adenocarcinoma Cells via PI3K/Akt/NF-ΚB Pathway. Biomed. Pharmacother. 2017, 90, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Itatani, Y.; Kawada, K.; Yamamoto, T.; Sakai, Y. Resistance to Anti-Angiogenic Therapy in Cancer-Alterations to Anti-VEGF Pathway. Int. J. Mol. Sci. 2018, 19, E1232. [Google Scholar] [CrossRef]
- Hegde, P.S.; Wallin, J.J.; Mancao, C. Predictive Markers of Anti-VEGF and Emerging Role of Angiogenesis Inhibitors as Immunotherapeutics. Semin. Cancer Biol. 2018, 52, 117–124. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, K.; Hu, Y.; Zhou, Y.; Luo, X.; Li, X.; Wei, L.; Li, Z.; You, Q.; Guo, Q.; et al. Wogonoside Inhibits Angiogenesis in Breast Cancer via Suppressing Wnt/β-Catenin Pathway. Mol. Carcinog. 2016, 55, 1598–1612. [Google Scholar] [CrossRef]
- Yan, Y.; Yao, L.; Sun, H.; Pang, S.; Kong, X.; Zhao, S.; Xu, S. Effects of Wogonoside on Invasion and Migration of Lung Cancer A549 Cells and Angiogenesis in Xenograft Tumors of Nude Mice. J. Thorac. Dis. 2020, 12, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Dürr, C.; Hanna, B.S.; Schulz, A.; Lucas, F.; Zucknick, M.; Benner, A.; Clear, A.; Ohl, S.; Öztürk, S.; Zenz, T.; et al. Tumor Necrosis Factor Receptor Signaling Is a Driver of Chronic Lymphocytic Leukemia That Can Be Therapeutically Targeted by the Flavonoid Wogonin. Haematologica 2018, 103, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.Z.; Hasmim, M.; Lequeux, A.; Xiao, M.; Duhem, C.; Chouaib, S.; Berchem, G.; Janji, B. Improving Cancer Immunotherapy by Targeting the Hypoxic Tumor Microenvironment: New Opportunities and Challenges. Cells 2019, 8, E1083. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Komar, C.; Tooker, G.M.; Liu, M.; Lee, J.W.; Gladney, W.L.; Ben-Josef, E.; Beatty, G.L. Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2017, 23, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Capece, D.; Verzella, D.; Tessitore, A.; Alesse, E.; Capalbo, C.; Zazzeroni, F. Cancer Secretome and Inflammation: The Bright and the Dark Sides of NF-ΚB. Semin. Cell Dev. Biol. 2018, 78, 51–61. [Google Scholar] [CrossRef]
- Patel, M.; Horgan, P.G.; McMillan, D.C.; Edwards, J. NF-ΚB Pathways in the Development and Progression of Colorectal Cancer. Transl. Res. 2018, 197, 43–56. [Google Scholar] [CrossRef]
- Yu, X.; Tang, W.; Yang, Y.; Tang, L.; Dai, R.; Pu, B.; Feng, C.; Xia, J. Long Noncoding RNA NKILA Enhances the Anti-Cancer Effects of Baicalein in Hepatocellular Carcinoma via the Regulation of NF-ΚB Signaling. Chem. Biol. Interact. 2018, 285, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.-F.; Shi, W.-W.; Yu, C.-H.; Jin, X.-Y.; Zhang, H.-H.; Zhang, W.-Y.; Wang, L.-C.; Yu, B. Baicalein Attenuates Vinorelbine-Induced Vascular Endothelial Cell Injury and Chemotherapeutic Phlebitis in Rabbits. Toxicol. Appl. Pharmacol. 2017, 318, 23–32. [Google Scholar] [CrossRef]
- Clara, J.A.; Monge, C.; Yang, Y.; Takebe, N. Targeting Signalling Pathways and the Immune Microenvironment of Cancer Stem Cells—A Clinical Update. Nat. Rev. Clin. Oncol. 2020, 17, 204–232. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The Cancer Metabolic Reprogramming and Immune Response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef]
- Li, L.; Yu, R.; Cai, T.; Chen, Z.; Lan, M.; Zou, T.; Wang, B.; Wang, Q.; Zhao, Y.; Cai, Y. Effects of Immune Cells and Cytokines on Inflammation and Immunosuppression in the Tumor Microenvironment. Int. Immunopharmacol. 2020, 88, 106939. [Google Scholar] [CrossRef] [PubMed]
- Puthenveetil, A.; Dubey, S. Metabolic Reprograming of Tumor-Associated Macrophages. Ann. Transl. Med. 2020, 8, 1030. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Rao, L.; Yao, H.; Wang, Z.; Ning, P.; Chen, X. Engineering Macrophages for Cancer Immunotherapy and Drug Delivery. Adv. Mater. 2020, 32, e2002054. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, L.; Zhu, J.; Zhang, Y.; Yang, R.; Yan, J.; Huang, R.; Zheng, C.; Xiao, W.; Huang, C.; et al. Predicting the Herbal Medicine Triggering Innate Anti-Tumor Immunity from a System Pharmacology Perspective. Biomed. Pharmacother. 2021, 143, 112105. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Man, K.; Tsao, S.-W.; Che, C.-M.; Feng, Y. Autophagy-Induced RelB/P52 Activation Mediates Tumour-Associated Macrophage Repolarisation and Suppression of Hepatocellular Carcinoma by Natural Compound Baicalin. Cell Death Dis. 2015, 6, e1942. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, W.; Zhou, X.; Liu, M.; Hou, X.; Cheng, Z.; Chen, D. Development of Dual-Targeted Nano-Dandelion Based on an Oligomeric Hyaluronic Acid Polymer Targeting Tumor-Associated Macrophages for Combination Therapy of Non-Small Cell Lung Cancer. Drug Deliv. 2019, 26, 1265–1279. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Yang, D.; Guo, Q.; Liang, Y.; Zhao, Y.; Tian, X.; Ye, Y.; Tian, J.; Wu, T.; Lu, N. Wogonin Induces Cellular Senescence in Breast Cancer via Suppressing TXNRD2 Expression. Arch. Toxicol. 2020, 94, 3433–3447. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Zhang, Z.; Xu, B.; Zhao, S.; Ding, Y.; Wu, X.; Wu, R.; Lv, Y.; Dong, J. Baicalein and Baicalin Promote Antitumor Immunity by Suppressing PD-L1 Expression in Hepatocellular Carcinoma Cells. Int. Immunopharmacol. 2019, 75, 105824. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer Prevention and Treatment Using Combination Therapy with Natural Compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Zhang, M.; Song, Y.; Zhang, Y.; Fan, S.; Ren, S.; Fu, L.; Zhang, N.; Hui, H.; et al. Baicalein Resensitizes Tamoxifen-Resistant Breast Cancer Cells by Reducing Aerobic Glycolysis and Reversing Mitochondrial Dysfunction via Inhibition of Hypoxia-Inducible Factor-1α. Clin. Transl. Med. 2021, 11, e577. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Li, J.-H.; Luo, N.; Zhong, M.-Z.; Xiao, Z.-Q.; Wang, J.-X.; Yao, X.-Y.; Peng, Y.; Cao, J. Inhibition of MicroRNA-196a Might Reverse Cisplatin Resistance of A549/DDP Non-Small-Cell Lung Cancer Cell Line. Tumour Biol. 2016, 37, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Jang, H.; Shin, D.; Baek, S.H.; Roh, J.-L. Targeting Nrf2 with Wogonin Overcomes Cisplatin Resistance in Head and Neck Cancer. Apoptosis 2016, 21, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.-P.; Wang, L.-G.; Wang, H.-J.; Ye, W.-F.; Wang, X.-Z. Wogonin Exacerbates the Cytotoxic Effect of Oxaliplatin by Inducing Nitrosative Stress and Autophagy in Human Gastric Cancer Cells. Phytomedicine 2018, 39, 168–175. [Google Scholar] [CrossRef]
- Xing, F.; Sun, C.; Luo, N.; He, Y.; Chen, M.; Ding, S.; Liu, C.; Feng, L.; Cheng, Z. Wogonin Increases Cisplatin Sensitivity in Ovarian Cancer Cells Through Inhibition of the Phosphatidylinositol 3-Kinase (PI3K)/Akt Pathway. Med. Sci. Monit. 2019, 25, 6007–6014. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xu, G.; Chen, F.; Fang, L.; Gou, S. Novel Platinum(IV) Complexes Conjugated with a Wogonin Derivative as Multi-Targeted Anticancer Agents. Bioorg. Med. Chem. 2017, 25, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wei, Y.; Huang, Y.; He, B.; Zhou, Y.; Fu, J. Nanoemulsion Improves the Oral Bioavailability of Baicalin in Rats: In Vitro and In Vivo Evaluation. Int. J. Nanomed. 2013, 8, 3769–3779. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.K.; Li, C.R.; Wo, S.K.; Wang, S.; Zhou, L.; Zhang, L.; Lin, G.; Zuo, Z. In Vitro and In Situ Evaluation of Herb-Drug Interactions during Intestinal Metabolism and Absorption of Baicalein. J. Ethnopharmacol. 2012, 141, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.-S.; Na, Y.-G.; Cho, C.-W. Sustained Cytotoxicity of Wogonin on Breast Cancer Cells by Encapsulation in Solid Lipid Nanoparticles. Nanomaterials 2018, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.-F.; Li, Y.; Wan, J.; Wang, Y.; Yang, M.; Zhu, W.-F.; Wang, C.-H.; Yuan, H.-L. Process Optimization and Evaluation of Novel Baicalin Solid Nanocrystals. Int. J. Nanomed. 2013, 8, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.A.; Patwardhan, R.S.; Sharma, D.; Sandur, S.K.; Devarajan, P.V. Pre-Clinical Evaluation of an Innovative Oral Nano-Formulation of Baicalein for Modulation of Radiation Responses. Int. J. Pharm. 2021, 595, 120181. [Google Scholar] [CrossRef]
- Meng, L.; Xia, X.; Yang, Y.; Ye, J.; Dong, W.; Ma, P.; Jin, Y.; Liu, Y. Co-Encapsulation of Paclitaxel and Baicalein in Nanoemulsions to Overcome Multidrug Resistance via Oxidative Stress Augmentation and P-Glycoprotein Inhibition. Int. J. Pharm. 2016, 513, 8–16. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Salazar, L.A.; Shaheen, S.; Ayatollahi, S.A.; Kobarfard, F.; Imran, M.; Imran, A.; Custódio, L.; López, M.D.; et al. The Therapeutic Potential of Wogonin Observed in Preclinical Studies. Evid. Based Complement. Alternat. Med. 2021, 2021, 9935451. [Google Scholar] [CrossRef]
- Tian, J.; Wang, L.; Wang, L.; Ke, X. A Wogonin-Loaded Glycyrrhetinic Acid-Modified Liposome for Hepatic Targeting with Anti-Tumor Effects. Drug Deliv. 2014, 21, 553–559. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Zhang, Y.; Chen, Y.; Cao, J.; An, R.; Wang, X. LC-MS/MS Analysis of Gegen Qinlian Decoction and Its Pharmacokinetics after Oral Administration to Rats. Biomed. Chromatogr. 2015, 29, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cui, H.; Li, T.; Qi, J.; Chen, H.; Gao, F.; Tian, X.; Mu, Y.; He, R.; Lv, S.; et al. Synergistic Effect of Berberine-Based Chinese Medicine Assembled Nanostructures on Diarrhea-Predominant Irritable Bowel Syndrome In Vivo. Front. Pharmacol. 2020, 11, 1210. [Google Scholar] [CrossRef]
- Xiang, Y.; Guo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese Medicine as a Cancer Treatment: Modern Perspectives of Ancient but Advanced Science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lu, R.; Kapur, P.; Jaiswal, B.S.; Hannan, R.; Zhang, Z.; Pedrosa, I.; Luke, J.J.; Zhang, H.; Goldstein, L.D.; et al. An Empirical Approach Leveraging Tumorgrafts to Dissect the Tumor Microenvironment in Renal Cell Carcinoma Identifies Missing Link to Prognostic Inflammatory Factors. Cancer Discov. 2018, 8, 1142–1155. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Chen, Y.; Liang, C.-L.; Liu, H.; Qiu, F.; Dai, Z. Antitumor Effects of Immunity-Enhancing Traditional Chinese Medicine. Biomed. Pharmacother. 2020, 121, 109570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-B.; Zhou, Y.-L.; Fang, J.-Y. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer 2021, 7, 647–660. [Google Scholar] [CrossRef]
- Foegeding, N.J.; Byndloss, M.X. TAKing on Cancer. Cell Host Microbe 2021, 29, 851–853. [Google Scholar] [CrossRef]
- Wu, M.; Bai, J.; Ma, C.; Wei, J.; Du, X. The Role of Gut Microbiota in Tumor Immunotherapy. J. Immunol. Res. 2021, 2021, 5061570. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, W.; Wu, Z.; Tian, X.; Xiang, J.; Li, L.; Li, Z.; Peng, X.; Wei, S.; Ma, X.; et al. Baicalin and the Liver-Gut System: Pharmacological Bases Explaining Its Therapeutic Effects. Pharmacol. Res. 2021, 165, 105444. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, L.-Z.; Zhao, S.; Shen, Z.-F.; Shen, H.; Zhan, L.-B. Protective Effect of Baicalin on the Regulation of Treg/Th17 Balance, Gut Microbiota and Short-Chain Fatty Acids in Rats with Ulcerative Colitis. Appl. Microbiol. Biotechnol. 2020, 104, 5449–5460. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Zheng, Q.; Fan, G.; Liu, R. Advances in Anti-Cancer Activities of Flavonoids in Scutellariae radix: Perspectives on Mechanism. Int. J. Mol. Sci. 2022, 23, 11042. https://doi.org/10.3390/ijms231911042
Gu Y, Zheng Q, Fan G, Liu R. Advances in Anti-Cancer Activities of Flavonoids in Scutellariae radix: Perspectives on Mechanism. International Journal of Molecular Sciences. 2022; 23(19):11042. https://doi.org/10.3390/ijms231911042
Chicago/Turabian StyleGu, Yiqing, Qi Zheng, Guifang Fan, and Runping Liu. 2022. "Advances in Anti-Cancer Activities of Flavonoids in Scutellariae radix: Perspectives on Mechanism" International Journal of Molecular Sciences 23, no. 19: 11042. https://doi.org/10.3390/ijms231911042
APA StyleGu, Y., Zheng, Q., Fan, G., & Liu, R. (2022). Advances in Anti-Cancer Activities of Flavonoids in Scutellariae radix: Perspectives on Mechanism. International Journal of Molecular Sciences, 23(19), 11042. https://doi.org/10.3390/ijms231911042




