Abstract
Cancer is one of the leading causes of death in the world, with breast cancer being the most prevalent cancer. Chemotherapy-induced nausea and vomiting (CINV) is one of the most serious side effects of chemotherapy. Because the current CINV treatment option has several flaws, alternative treatment options are required. Ginger has traditionally been used to treat nausea and vomiting, and it also has anticancer properties in breast cancer cells. Based on these findings, researchers investigated whether using ginger to treat CINV in breast cancer patients is both effective and safe. We searched PubMed, Embase, Cochrane Library, CNKI, and Wanfang from inception to June 2022. Outcomes included Rhodes Index Scores of Nausea, Vomiting, and Retching, severity and frequency of CINV. Five RCTs were included. We pooled all included data and performed subgroup analysis by types of CINV. Overall, authors found that ginger was associated with a reduction in CINV. Subgroup and sensitivity analysis revealed that managing severity of acute CINV in breast cancer patients with ginger was efficient. In terms of managing delayed CINV in breast cancer patients, ginger was also statistically significant. The authors concluded that ginger may be helpful in lowering both acute and delayed CINV in breast cancer patients. Since there were no serious side effects, ginger is thought to be safe.
1. Introduction
Cancer is one of the leading causes of death in the world. According to cancer statistics in 2022, there are expected to be 1,918,030 new cancer cases and 609,360 cancer-related deaths [1]. Among them, breast cancer has the highest estimated new cases of 287,850 patients (31%) [1]. Chemotherapy, like other types of cancer treatment, is widely used in the treatment of breast cancer. Even though it is a necessary conventional treatment for cancer patients, it has a few serious side effects that can be fatal to cancer patients both during and after treatment and chemotherapy-induced nausea and vomiting (CINV) is one of them [2]. Chemotherapy for cancer patients is divided into four categories: high emetic risk, moderate emetic risk, low emetic risk, and minimal emetic risk [3]. Specifically, in comparison to cisplatin, melphalan, cyclophosphamide, and dacarbazine, which have a high emetogenic potential, anthracyclines, methotrexate, oxaliplatin, and carboplatin have a moderate emetogenic potential [3].
CINV is generally classified into five categories, which are acute, delayed, anticipatory, breakthrough, and refractory [4]. Acute CINV develops within 24 h of starting chemotherapy. Delayed CINV develops after 24 h of chemotherapy. Anticipatory CINV is a general response to chemotherapy. Despite appropriate prophylaxis, breakthrough CINV occurs within 5 days of chemotherapy, and refractory CINV occurs in subsequent chemotherapy cycles after the occurrence of breakthrough CINV in prior cycles, excluding anticipatory CINV [4]. Treatment agents for CINV and a combination of the agents are categorized as minimal, low, moderate, or high and the prevention and treatment strategies are varied depending on the severity of CINV [3]. Different types of treatments include dexamethasone which is a first-line use in combination with other agents [4]. However, side effects including insomnia, indigestion/epigastric discomfort, agitation, increased appetite, weight gain, and acne were reported [5]. Untreated CINV is linked to treatment discontinuation, decreased quality of life, complications such as dehydration and electrolyte imbalances, and, ultimately, decreased treatment success and increased costs of care [5]. Therefore, efforts are being made to establish effective evidence-based clinical guidelines; for example, the ASCO (American Society for Clinical Oncology) has recognized acupuncture as an alternative therapy for CINV and is expected to provide additional alternative therapies as qualified evidence accumulates [6].
Ginger (Zingiber officinale) is a commonly used herb to treat nausea and vomiting, and several bioactive compounds, including shogaols, gingerols, zingerone, and paradols, have been identified within the ginger rhizome [7]. These compounds are thought to interact with a variety of areas involved in the development of CINV [8]. In cases of acute CINV, free radicals produced by toxic chemotherapy drugs stimulate enterochromaffin cells in the gastrointestinal tract, resulting in the production of serotonin [9,10]. Serotonin then binds to intestinal vagal afferent nerves via 5-HT3 receptors, causing the vomiting reflex to be triggered in the CNS via the nucleus of the solitary tract (NTS) and chemoreceptor trigger zone (CTZ) [9,10]. Moreover, 5-HT3 receptor signaling may be involved in delayed CINV, but to a lesser extent than in acute CINV [10]. Substance P is thought to be the main neurotransmitter involved in delayed CINV [11]. Chemotherapy drugs cause neurons in the central and peripheral nervous systems to release substance P, which then binds to neurokinin-1 (NK1) receptors, primarily in the NTS, to cause vomiting [10].
Ginger’s bioactivity has been studied to see how it affects the CINV mechanism. Ymahara was the first to demonstrate that the whole ginger including gingerols 6,8 and 10 can inhibit 5-HT3-induced contraction [12]. Followed by that, the 5-HT3 antagonistic effect of ginger using four major compounds of ginger (gingerol 6,8,10 and 6-shogaol) was found in animal experiments [13]. Clinical trials for humans also reported that daily addition of ginger with conventional chemotherapy resulted in a favorable effect on CINV with no adverse effects [14,15,16].
Using ginger for breast cancer patients not only to treat CINV but also to manage the disease has numerous benefits. According to a study conducted in 2017 by Martin and his colleagues, 10-gingerol significantly inhibited metastasis of TNBC (Triple-negative breast cancer) cells dose-dependently [17]. Another investigation found that ginger therapy reduced colony formation and proliferation in MCF-7 and MDA-MB-231 breast cancer cell lines [18]. The non-tumorigenic normal mammary epithelial cell line (MCF-10A) was not severely affected by it, but there was a loss of cell viability, chromatin condensation, DNA fragmentation, activation of caspase 3, and cleavage of poly (ADP-ribose) polymerase. At the molecular level, the upregulation of the Bax and the downregulation of the Bcl-2 proteins may contribute to the apoptotic cell death caused by ginger [19].
Given these overlapping benefits of using ginger in breast cancer treatment, the authors wanted to systematically examine the efficacy and safety of using ginger with CINV as the primary endpoint and others as secondary endpoints. Despite a recent study demonstrating that ginger use in CINV is beneficial [20], since most of the analysis looked at several cancer types, systematic and meta-analyses on breast cancer and CINV are limited [21,22]. Thus, the efficacy and safety of using ginger to manage the side effects of breast cancer treatment, as well as its potential benefits, will be examined and discussed in this study.
2. Results
2.1. Literature Search Results
We discovered a total of 165 records using search databases. We screened 115 remaining studies for eligible title and abstract after removing duplicates (n = 50), and 110 were excluded due to ineligibility or irrelevance (Figure 1). The abstract and title were read, and 47 articles were eliminated. After determining eligibility, 63 articles were excluded because 27 were “Review articles,” 3 were “Not human,” 5 were “Not intervention,” 4 were “Not population,” 9 were “Not study design,” and 15 were “Not full text.” As a result, we finally included five trials in this re-evaluation [23,24,25,26,27].
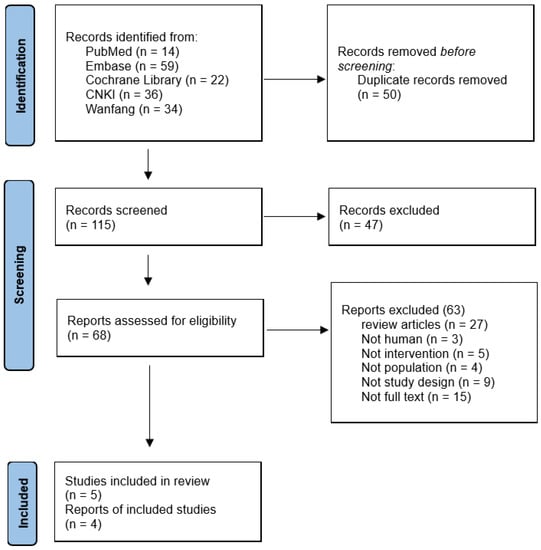
Figure 1.
Flow diagram of study selection.
2.2. Characteristics of Included Studies
This review included five randomized controlled trials, and the key characteristics of the included studies are listed in Table 1. The participants in the studies ranged in age from 41.8 to 52.1 years. Sample sizes ranged between 60 and 119 patients. The chemotherapy drugs used in the participants ranged from moderate to highly emetogenic, such as those based on doxorubicin and anthracycline [23,24,25,27]. The chemotherapy drugs used in one study were not reported [26].

Table 1.
Characteristics of included studies.
In three studies, ginger was used as an intervention in the form of capsules containing powdered ginger root; in one study, ginger powder was mixed with yogurt; and in one study, fresh ginger was sliced into 3 cm diameter and 0.2 cm thickness slices. The daily dose of ginger ranged from 0.5 g to 1.5 g, and the treatment period ranged from 3 to 6 days. Participants in the experiment group consumed ginger from three days before to thirty minutes after finishing chemotherapy. Four of the five studies’ participants received appropriate anti-vomiting treatments for both the ginger and control groups [23,24,25,27]. In one study, all subjects received 5-HT3 receptor antagonists palonosetron, dexamethasone, an antihistamine, ranitidine, and aprepitant, before the administration of chemotherapy [24]. Three studies used 5-HT3 receptor antagonist and/or dexamethasone to manage CINV [25,26,27].
2.3. Risk of Bias Associated with Included Trials
The risk of bias ranges from low to high, and Figure 2 shows a summary of the individual studies. One study had a low risk of bias, three studies had a high risk of bias, and one study’s risk of bias was unclear. In all studies, random sequence generation was adequate, but allocation concealment was deemed high risk in one study [24] and unclear in another [27]. In two studies [24,25], performance and detection bias were deemed high risk, while one study [27] was deemed unclear. In one study [23], attrition and reporting domains were rated as high risks. All studies were deemed to be free of other bias.

Figure 2.
Risk of bias associated with included trials [23,24,25,26,27].
2.4. Effects of Ginger Intake in Managing CINV on Breast Cancer Patients
A total of four studies were included in this meta-analysis. One study was excluded from quantitative analysis because it was combined with ginger intake and acupoint therapy [27]. The merged data revealed that intake of ginger was more effective with a small effect size than control in reducing the severity of CINV in breast cancer patients (SMD −0.32, 95% CI −0.59, −0.05, p = 0.02; I2 = 75%) [23,24,25,26]. However, because of heterogeneity between samples, we conducted a subgroup analysis and divided nausea, vomiting, and retching into acute or delayed.
2.4.1. Effects of Ginger Intake in Managing Acute CINV on Breast Cancer Patients
Three studies found that ginger consumption was significantly more effective than a control group in reducing the severity of acute CINV in breast cancer patients (SMD −0.48, 95% CI −0.84, −0.13, p = 0.008; I2 = 66%) [24,25,26]. Figure 3: A subgroup analysis of two studies found that ginger consumption was associated with a significant reduction in acute vomiting severity (SMD −0.56, 95% CI −0.89, −0.22, p = 0.001; I2 = 0%) [24,26]. One trial (n = 60) found that ginger consumption reduced acute nausea severity more than antiemetic treatment alone (SMD −1.59, 95% CI −2.36, −0.82, p < 0.0001) [24].
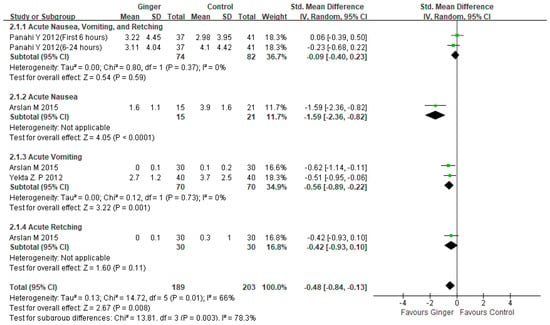
Figure 3.
Forest plot of acute CINV severity managements in ginger intake versus controls. CI: confidence interval; SD: standard deviation [24,25,26].
2.4.2. Effects of Ginger Intake in Managing Delayed CINV on Breast Cancer Patients
Although there was significant heterogeneity [24,25,26], the results showed a significant reduction in delayed CINV in the ginger group compared to the control group (SMD −0.48, 95%CI −0.82, −0.14, p = 0.006; I2 = 81%). (Figure 4). A pooled analysis of two studies found that the experimental group that took ginger was more effective than the control group in reducing delayed vomiting severity (SMD −0.82, 95%CI −1.36, −0.29, p = 0.002; I2 = 77%) [24,26]. One study found that ginger treatment, when compared to antiemetic treatment, significantly reduced the severity of delayed nausea in breast cancer patients (SMD −1.41, 95% CI −1.98, −0.84, p < 0.00001) [24]. Subgroup analysis on delayed retching revealed that ginger intake was more effective than antiemetic treatment alone in breast cancer patients (SMD −0.34, 95%CI −0.63, −0.04, p = 0.02; I2 = 0%) [24].
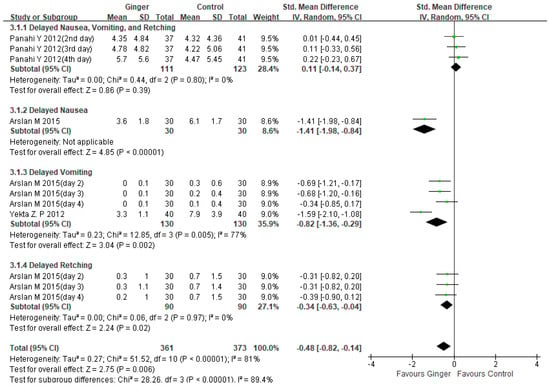
Figure 4.
Forest plot of delayed CINV severity managements in ginger intake versus controls. CI: confidence interval; SD: standard deviation [24,25,26].
2.5. Safety
In two studies [23,24], no patients reported adverse effects from ginger consumption. Panahi (2012) reported that taking ginger can cause heartburn, headaches, and vertigo. The only side effect reported by Yekta (2012) during the ginger intervention was heartburn. However, there was no statistically significant difference between the ginger and control groups (Table 1).
3. Discussion
Nausea and vomiting caused by chemotherapy are both psychologically and physically distressing symptoms. Different treatment regimens are required for acute, delayed, anticipatory, breakthrough, and refractory CINV, which frequently include 5-HT3 receptor antagonists, NK1 receptor antagonists, and corticosteroids [2,28]. Despite significant antiemetic agent research and development, CINV management remains a significant challenge, with many unmet needs, such as controlling non-acute CINV, developing appropriate CINV treatment protocols for multiple-day chemotherapy patients, and providing options for those who are prone to CINV despite treatment [28]. Furthermore, common antiemetic drug side effects include headache, constipation, and fatigue [29].
As a result, there is an ongoing demand for complementary and alternative therapies. One of the most promising and actively researched options is herbal medicine. Ginger has been used to treat nausea and vomiting for over 2000 years [30]. Several clinical trials [16,24,31,32] have shown that ginger has an antiemetic effect against both the acute and delayed phases of CINV. Mechanisms of how ginger’s components affect CINV are being researched. The main pungent constituents and fractions of ginger are 6-,8-,10-gingerol and 6-,8-,10-shogaol [33]. These components inhibit 5-HT3 receptors in the central and peripheral nervous systems [34,35]. These receptors play an important role in the regulation of peristalsis, pain transmission, and nausea and vomiting. The development of 5-HT3 receptor antagonists improved the treatment of CINV in cancer patients significantly [34].
Various methods may be used to improve ginger accessibility for CINV patients. Ginger partitioned moxibustion may be more effective than no treatment in reducing the severity and frequency of CINV (RR: 2.04, 95% CI: 1.42–2.93); moxibustion may be more effective than antiemetic drugs (RR: 1.87, 95% CI: 1.27–2.76) [35]. Another study found that ginger moxibustion combined with acupuncture reduced gastro-intestinal tract reactions to chemotherapy in cancer patients when compared to a control group [36]. Ginger slice consumption and ginger-applied acupoint therapy were combined in Liu et al. (2020). All levels of CINV (mild, moderate, severe, and very severe) were assessed from 0 to 5 days after chemotherapy, and the severity of CINV between the two groups was significantly reduced (p < 0.05), implying that ginger could effectively manage CINV in breast cancer when combined with acupoint therapy [27]. Another study found that direct inhalation of ginger aromatherapy was beneficial [37].
Uncontrolled CINV reduces patients’ quality of life (QOL), as well as their physical and social functioning. It can also result in medical complications such as poor nutrition, dehydration, and electrolyte imbalances [38]. It also leads to patients discontinuing potentially beneficial treatment regimens [38]. Uncontrolled CINV in a patient costs an extra USD 1300 per month in direct medical costs, according to one study [39]. However, current CINV treatments do not appear to be as effective as they could be [2]. Despite the existence of separate NCCN, ASOC, and MASCC/ESMO CINV practice guidelines, they share fundamental similarities and a lack of literature findings [6]. As a result, we determined that it was necessary to assemble evidence-based literature findings for CINV management, which could lead to the development of a new clinical practice guideline recommendation.
Although CINV can occur in any cancer patient receiving chemotherapy, we chose to focus on breast cancer patients for a number of reasons. First, ginger has been shown to be effective against breast cancer. Breast cancer is classified as either ER-positive (as in MCF-7 and T47D cell lines) or ER-negative (as in MDA-MB-231, MDA-MB-468, SKBR3 and MDA-MB-453 cell lines) [18]. Using additional biomarkers such as progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), breast cancer is further classified as luminal A, luminal B, basal-like, and HER2-positive [18]. Because these distinct subtypes of breast cancer respond differently to treatment, breast cancer is extremely difficult to treat. As a result, the search for complementary therapeutic methods is ongoing. In MDA-MB-231 cells, for example, methanolic extract of ginger inhibited proliferation and colony formation in a dose- and time-dependent manner [40]. In MCF-7 and MDA-MB-231 cells, ginger extract increased Bax levels while decreasing Bcl-2 proteins, NF-B, Bcl-X, Mcl-1, survivin, cyclin D1, and CDK-4. Furthermore, ginger extract inhibited the expression of two important cancer molecular targets, c-Myc and hTERT [41]. Gingerols were discovered to inhibit breast cancer cell proliferation and metastasis. By inhibiting cyclin-dependent kinases and cyclins, 10-gingerol inhibited MDA-MB-231 proliferation, resulting in a G1 phase arrest [42]. Moreover, 10-gingerol also inhibited cancer cell invasion by inhibiting the activation of Akt and p38 (MAPK) [43]. Furthermore, 6-gingerol inhibited MDA-MB-231 cell migration and motility in a concentration-dependent manner, as well as MMP-2 and 9 expression and activity [44]. Shogaols also inhibited breast cancer cell metastasis via a variety of mechanisms, including MMP-9 inhibition of NF-kB activation, invasion of MDA-MB-231 cells, and inhibiting invasion by decreasing levels of c-Src kinase, cortactin, and MT1-MMP, all of which inhibited the growth and sustainability of breast cancer cells [45,46,47]. Furthermore, because the current meta-analysis on ginger’s antiemetic effect on CINV focuses on all types of cancer and there is a lack of breast cancer focused analysis [20,48], we decided to focus on CINV in breast cancer patients for this study based on previous research demonstrating ginger’s anticancer effect in relieving CINV symptoms, as well as its beneficial effect on breast cancer itself, and the fact that breast cancer has the highest cancer prediction rate [1].
This meta-analysis included 337 patients from four randomized controlled trials, and we discovered that ginger was associated with a reduction in CINV (SMD −0.32, 95% CI −0.59, −0.05, p = 0.02; I2 = 75%). However, there was significant variation. As a result, we decided to conduct subgroup and sensitivity analysis. Subgroup analysis revealed that ginger was effective in reducing the severity of acute CINV in breast cancer patients (SMD −0.48, 95% CI −0.84, −0.13, p = 0.008; I2 = 66%). Another subgroup analysis of ginger’s efficacy in acute vomiting revealed statistical significance (SMD −0.56, 95% CI −0.89, −0.22, p = 0.001; I2 = 0%) (Figure 3). Ginger was also statistically significant in treating delayed CINV in breast cancer patients (SMD −0.48, 95%CI −0.82, −0.14, p = 0.006; I2 = 81%) (Figure 4). There have been no serious adverse effects reported as a result of ginger consumption. Based on our findings, we concluded that using ginger to treat CINV in breast cancer patients may be both effective and safe. However, because there were insufficient studies included in this analysis, evaluating publication bias using funnel plots was difficult. The sensitivity analysis comparing the fixed effect model and the random effect model yielded similar results. To advance the field and better inform clinicians and patients, more methodologically rigorous research on the safety and efficacy of ginger for CINV in breast cancer patients is required. CINV should be evaluated using validated tools for better meta-analysis. It must also be evaluated in terms of other side effects such as anxiety, depression, diarrhea, and quality of life as a result of chemotherapy.
4. Limitations
This research has limitations. First, we only included databases in English and Chinese; while ginger studies may appear in Japanese and Korean literatures, they are unlikely to be large in number and thus would not have significantly influenced our findings. Second, the clinical heterogeneity of the studies included in this review reduces the confidence in the results. Furthermore, the efficacy of ginger was assessed using various instruments; however, few publications reported the same results. As a result, it is critical to use validated tools that can be easily compared to other results. Finally, we were unable to assess publication bias due to the small number of included studies. As a result, more clinical trials in this field are required.
5. Methods
5.1. Objective
The purpose of this systematic and meta-analysis is to evaluate the efficacy and safety of ginger in the symptomatic management of chemotherapy side effects in breast cancer patients. The current systematic review and meta-analysis followed Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and Cochrane guidelines [21]. PROSPERO was used to register the study protocol (registration no. CRD42022344125).
5.2. Criteria to Select Articles
This review included randomized controlled trials of ginger for the treatment of CINV in breast cancer patients that were published in both English and Chinese. There were no restrictions on the study’s origin or publication year. Studies involving participants with CINV symptoms in breast cancer patients were considered eligible for inclusion. There were no restrictions on the participants’ geographic, socioeconomic, or ethnic backgrounds. Ginger consumption is one of the interventions to be considered. Both ginger (Zingiber officinale) alone and ginger combined with other herbs are included. The meta-analysis excludes studies in which ginger is used in conjunction with nonpharmacologic therapy such as acupuncture, massage, far infrared, physical therapy, thermotherapy, or magnetic therapy. There have been studies that compare the efficacy of various ginger formula modifications.
5.3. Search Strategy, Data Selection, and Data Extraction
PubMed, the Cochrane Library, Embase, CNKI, and Wanfang were searched until June 2022. Following terms were used: CINV[Title/Abstract], Chemotherapy induced nausea and Vomiting[Title/Abstract], Vomiting[Title/Abstract], Nausea[Title/Abstract], Vomiting[MeSH Terms], Nausea[MeSH Terms], 1 OR 2 OR 3 OR 4 OR 5 OR 6, Breast neoplasms[MeSH Terms], Breast cancer[Title/Abstract], Breast neoplasms[Title/Abstract], 8 OR 9 OR 10, ginger[MeSH Terms], ginger[Title/Abstract], zingiber officinale[Title/Abstract], ShengJiang[Title/Abstract], Zingiberis Rhizoma Recens[Title/Abstract], 12 OR 13 OR 14 OR 15 OR 16, 7 AND 11 AND 17 (Table 2). Endnote 20 (Clarivate Analytics, UK) was used to import search results from the original databases. To ensure objectivity, two authors (S.D.K. and E.B.K.) independently assessed each record’s eligibility using a study screening standard operating procedure. To identify additional eligible trials, reference lists from relevant systematic reviews and meta-analyses were reviewed. We began with the titles and worked our way down to the abstracts, excluding non-clinical trial studies and those that did not focus on ginger or breast cancer symptom management. The same two authors then reviewed the full-text article and documented the reason for exclusion, with disagreements resolved by consensus by another two authors (M.X.Y. and H.S.Y.).

Table 2.
Search strategy.
Using Excel, two authors (S.D.K. and E.B.K.) independently extracted detailed data from each study (study origin, year of publication, patient demographics, intervention, comparator, outcome and results, setting, AEs, etc.). Disagreements or disputes were settled through discussions with two additional reviewers (M.X.Y. and H.S.Y). The data required for meta-analysis were transferred from the data extraction form to RevMan software (The Cochrane. Collaboration, Oxford, UK) using a double entry method (version 5.4).
5.4. Risk of Bias
Two reviewers (S.D.K. and E.B.K.) used the Cochrane Collaboration’s tool to assess the risk of bias in each included study. Disputes were resolved by two additional reviewers through discussions and arbitration (M.X.Y. and H.S.Y.).
5.5. Meta-Analysis
We planned a meta-analysis of all studies that provided efficacy data on CINV in breast cancer patients and used a placebo, conventional medicine, ginger + conventional group as a comparator. We planned to pool data on CINV severity, CINV incidence, acute and delayed CINV efficacy, and safety. RevMan 5.4 was used to synthesize and statistically analyze the efficacy data. Because dichotomous data were unsuitable for synthesis, we decided to only use continuous data. Because the tools used to measure the outcome differed, the effect size was reported using the standard mean difference (SMD). Chi-square tests were performed in the forest plot using RevMan 5.4 to investigate statistical heterogeneity, and a p value of less than 0.10 was considered significant, according to the Cochrane Handbook [22]. The I2 value was calculated to quantify statistical heterogeneity. We used a fixed effects model for meta-analysis if there was no or low heterogeneity among studies (I2 = 50%); if statistical heterogeneity was high (I2 > 50%), we investigated sources of heterogeneity using subgroup or sensitivity analysis, and we used a random effects model for meta-analysis. The source of the high heterogeneity was identified using subgroup analysis or sensitivity analysis.
6. Conclusions
In conclusion, this review discovered that ginger could be a safe option for breast cancer patients who are suffering from CINV and is associated with symptom improvement in breast cancer patients suffering from CINV. To assess the full effects of ginger for CINV, more rigorous clinical trials focused on reliable outcome measurement and long-term effects are required.
Author Contributions
Conceptualization, S.-D.K. and E.-B.K.; Funding acquisition, S.-D.K.; Data curation, S.-D.K. and E.-B.K.; Investigation, M.-X.Y.; Methodology, H.-S.Y.; Supervision, H.-S.Y.; Writing—original draft, S.-D.K. and E.-B.K.; Writing—Review and amp and editing, M.-X.Y. and H.-S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI21C1261).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Adel, N. Overview of chemotherapy-induced nausea and vomiting and evidence-based therapies. Am. J. Manag. Care 2017, 23 (Suppl. 14), S259–S265. [Google Scholar]
- Natale, J.J. Overview of the prevention and management of CINV. Am. J. Manag. Care 2018, 24 (Suppl. 18), S391–S397. [Google Scholar]
- Wood, D.E.; Kazerooni, E.A.; Baum, S.L.; Eapen, G.A.; Ettinger, D.S.; Hou, L.; Jackman, D.M.; Klippenstein, D.; Kumar, R.; Lackner, R.P.; et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 412–441. [Google Scholar] [CrossRef]
- Vardy, J.; Chiew, K.S.; Galica, J.; Pond, G.R.; Tannock, I.F. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br. J. Cancer 2006, 94, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Razvi, Y.; Chan, S.; McFarlane, T.; McKenzie, E.; Zaki, P.; DeAngelis, C.; Pidduck, W.; Bushehri, A.; Chow, E.; Jerzak, K.J. ASCO, NCCN, MASCC/ESMO: A comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support. Care Cancer 2019, 27, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Haniadka, R.; Pereira, M.M.; D’Souza, J.J.; Pallaty, P.L.; Bhat, H.P.; Popuri, S. Update on the chemopreventive effects of ginger and its phytochemicals. Crit. Rev. Food Sci. Nutr. 2011, 51, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Ried, K.; McCarthy, A.L.; Vitetta, L.; Sali, A.; McKavanagh, D.; Isenring, L. Ginger-Mechanism of action in chemotherapy-induced nausea and vomiting: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 141–146. [Google Scholar] [CrossRef]
- Navari, R.M.; Qin, R.; Ruddy, K.J.; Liu, H.; Powell, S.F.; Bajaj, M.; Dietrich, L.; Biggs, D.; Lafky, J.M.; Loprinzi, C.L. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N. Engl. J. Med. 2016, 375, 134–142. [Google Scholar] [CrossRef]
- Rapoport, B.L. Delayed Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Incidence, and Current Management. Front. Pharmacol. 2017, 8, 19. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Tejani, M.A.; Kamen, C.; Peoples, A.R.; Mustian, K.M.; Morrow, G.R. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin. Pharmacother. 2013, 14, 757–766. [Google Scholar] [CrossRef]
- Yamahara, J.; Rong, H.Q.; Iwamoto, M.; Kobayashi, G.; Matsuda, H.; Fujimura, H. Active components of ginger exhibiting anti-serotonergic action. Phytother. Res. 1989, 3, 70–71. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.; Windeck, T.; Ploch, M.; Verspohl, E.J. Mode of action of gingerols and shogaols on 5-HT3 receptors: Binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur. J. Pharmacol. 2006, 530, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Cortinovis, D.; Fatigoni, S.; Cossu Rocca, M.; Fabi, A.; Seminara, P.; Ripamonti, C.; Alfieri, S.; Granata, R.; Bergamini, C.; et al. A randomized, double-blind, placebo-controlled, multicenter study of a ginger extract in the management of chemotherapy-induced nausea and vomiting (CINV) in patients receiving high-dose cisplatin. Ann. Oncol. 2017, 28, 2547–2551. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; McCarthy, A.L.; Ried, K.; McKavanagh, D.; Vitetta, L.; Sali, A.; Lohning, A.; Isenring, E. The Effect of a Standardized Ginger Extract on Chemotherapy-Induced Nausea-Related Quality of Life in Patients Undergoing Moderately or Highly Emetogenic Chemotherapy: A Double Blind, Randomized, Placebo Controlled Trial. Nutrients 2017, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.K.; Sharma, K.K.; Gupta, Y.K.; Bakhshi, S. Anti-emetic effect of ginger powder versus placebo as an add-on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatr. Blood Cancer 2011, 56, 234–238. [Google Scholar] [CrossRef]
- Martin, A.; Fuzer, A.M.; Becceneri, A.B.; da Silva, J.A.; Tomasin, R.; Denoyer, D.; Kim, S.H.; McIntyre, K.A.; Pearson, H.B.; Yeo, B.; et al. [10]-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer In Vivo. Oncotarget 2017, 8, 72260–72271. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Meng, X.; Gan, R.-Y.; Zhang, J.-J.; Li, H.-B. Dietary Natural Products for Prevention and Treatment of Breast Cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Elkady, A.I.; Abuzinadah, O.A.; Baeshen, N.A.; Rahmy, T.R. Differential control of growth, apoptotic activity, and gene expression in human breast cancer cells by extracts derived from medicinal herbs Zingiber officinale. J. Biomed. Biotechnol. 2012, 2012, 614356. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.P.; Peng, Y.X. Does the Oral Administration of Ginger Reduce Chemotherapy-Induced Nausea and Vomiting?: A Meta-analysis of 10 Randomized Controlled Trials. Cancer Nurs. 2019, 42, E14–E23. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Porouhan, P.; Mohammadianpanah, M.; Omidvari, S.; Mosalaei, A.; Ahmadloo, N.; Nasrollahi, H.; Hamedi, S.H. Efficacy of Ginger in Control of Chemotherapy Induced Nausea and Vomiting in Breast Cancer Patients Receiving Doxorubicin-Based Chemotherapy. Asian Pac. J. Cancer Prev. 2016, 17, 3877–3880. [Google Scholar] [PubMed]
- Arslan, M.; Ozdemir, L. Oral intake of ginger for chemotherapy-induced nausea and vomiting among women with breast cancer. Clin. J. Oncol. Nurs. 2015, 19, E92–E97. [Google Scholar] [CrossRef]
- Panahi, Y.; Saadat, A.; Sahebkar, A.; Hashemian, F.; Taghikhani, M.; Abolhasani, E. Effect of ginger on acute and delayed chemotherapy-induced nausea and vomiting: A pilot, randomized, open-label clinical trial. Integr. Cancer Ther. 2012, 11, 204–211. [Google Scholar] [CrossRef]
- Yekta, Z.P.; Ebrahimi, S.M.; Hosseini, M.; Nasrabadi, A.N.; Sedighi, S.; Surmaghi, M.H.; Madani, H. Ginger as a miracle against chemotherapy-induced vomiting. Iran. J. Nurs. Midwifery Res. 2012, 17, 325–329. [Google Scholar] [PubMed]
- Liu, C.; Su, Y.; Zhao, M.; You, H.; Wang, X. Curative effects of ginger combined with tropisetron on chemotherapy-induced nausea and vomiting of breast cancer. Chin. J. Mod. Nurs. 2020, 26, 4657–4660. [Google Scholar] [CrossRef]
- Gupta, K.; Walton, R.; Kataria, S.P. Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Recommendations, and New Trends. Cancer Treat. Res. Commun. 2021, 26, 100278. [Google Scholar] [CrossRef] [PubMed]
- Navari, R.M.; Aapro, M. Antiemetic Prophylaxis for Chemotherapy-Induced Nausea and Vomiting. N. Engl. J. Med. 2016, 374, 1356–1367. [Google Scholar] [CrossRef]
- Palatty, P.L.; Haniadka, R.; Valder, B.; Arora, R.; Baliga, M.S. Ginger in the prevention of nausea and vomiting: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Uthaipaisanwong, A.; Oranratanaphan, S.; Musigavong, N. Effects of ginger adjunct to the standard prophylaxis on reducing carboplatin and paclitaxel-induced nausea vomiting: A randomized controlled study. Support. Care Cancer 2020, 28, 3831–3838. [Google Scholar] [CrossRef]
- Levine, M.E.; Gillis, M.G.; Koch, S.Y.; Voss, A.C.; Stern, R.M.; Koch, K.L. Protein and ginger for the treatment of chemotherapy-induced delayed nausea. J. Altern. Complement. Med. 2008, 14, 545–551. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Al Kury, L.T.; Mahgoub, M.; Howarth, F.C.; Oz, M. Natural Negative Allosteric Modulators of 5-HT3 Receptors. Molecules 2018, 23, 3186. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Qin, Z.; Yao, Q.; Wang, Y.; Liu, Z. Moxibustion for Chemotherapy-Induced Nausea and Vomiting: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat. Med. 2017, 2017, 9854893. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Sun, S.; Dong, H.J.; Zhai, D.X.; Zhang, D.Y.; Shen, W.; Bai, L.L.; Yu, J.; Zhou, L.H.; Yu, C.Q. Wrist-ankle acupuncture and ginger moxibustion for preventing gastrointestinal reactions to chemotherapy: A randomized controlled trial. Chin. J. Integr. Med. 2015, 21, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, J.; Delaide, V.; Beloni, P. Effectiveness of Inhaled Aromatherapy on Chemotherapy-Induced Nausea and Vomiting: A Systematic Review. J. Altern. Complement. Med. 2021, 27, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.; Grunberg, S. Chemotherapy-induced nausea and vomiting: Challenges and opportunities for improved patient outcomes. Clin. J. Oncol. Nurs. 2009, 13, 54–64. [Google Scholar] [CrossRef]
- Tina Shih, Y.C.; Xu, Y.; Elting, L.S. Costs of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapy. Cancer 2007, 110, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.A.; Ahmad, M.K.; Khan, A.R.; Fatima, N.; Khan, H.J.; Rastogi, N.; Mishra, D.P.; Mahdi, A.A. Anticancer and Antioxidant activity of Zingiber officinale Roscoe rhizome. Indian J. Exp. Biol. 2016, 54, 767–773. [Google Scholar]
- Joo, J.H.; Hong, S.S.; Cho, Y.R.; Seo, D.W. 10-Gingerol inhibits proliferation and invasion of MDA-MB-231 breast cancer cells through suppression of Akt and p38MAPK activity. Oncol. Rep. 2016, 35, 779–784. [Google Scholar] [CrossRef]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef]
- Ling, H.; Yang, H.; Tan, S.H.; Chui, W.K.; Chew, E.H. 6-Shogaol, an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-κB activation. Br. J. Pharmacol. 2010, 161, 1763–1777. [Google Scholar] [CrossRef]
- Hong, B.H.; Wu, C.H.; Yeh, C.T.; Yen, G.C. Invadopodia-associated proteins blockade as a novel mechanism for 6-shogaol and pterostilbene to reduce breast cancer cell motility and invasion. Mol. Nutr. Food Res. 2013, 57, 886–895. [Google Scholar] [CrossRef]
- Ray, A.; Vasudevan, S.; Sengupta, S. 6-Shogaol Inhibits Breast Cancer Cells and Stem Cell-Like Spheroids by Modulation of Notch Signaling Pathway and Induction of Autophagic Cell Death. PLoS ONE 2015, 10, e0137614. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Chen, C.Y.; Hou, M.F.; Tsai, E.M.; Jong, Y.J.; Hung, C.H.; Kuo, P.L. 6-Dehydrogingerdione, an active constituent of dietary ginger, induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human breast cancer cells. Mol. Nutr. Food Res. 2010, 54, 1307–1317. [Google Scholar] [CrossRef]
- Lua, P.L.; Salihah, N.; Mazlan, N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement. Ther. Med. 2015, 23, 396–404. [Google Scholar] [CrossRef]
- Lee, J.; Oh, H. Ginger as an antiemetic modality for chemotherapy-induced nausea and vomiting: A systematic review and meta-analysis. Oncol. Nurs. Forum 2013, 40, 163–170. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).