Molecular Insights into mRNA Polyadenylation and Deadenylation
Abstract
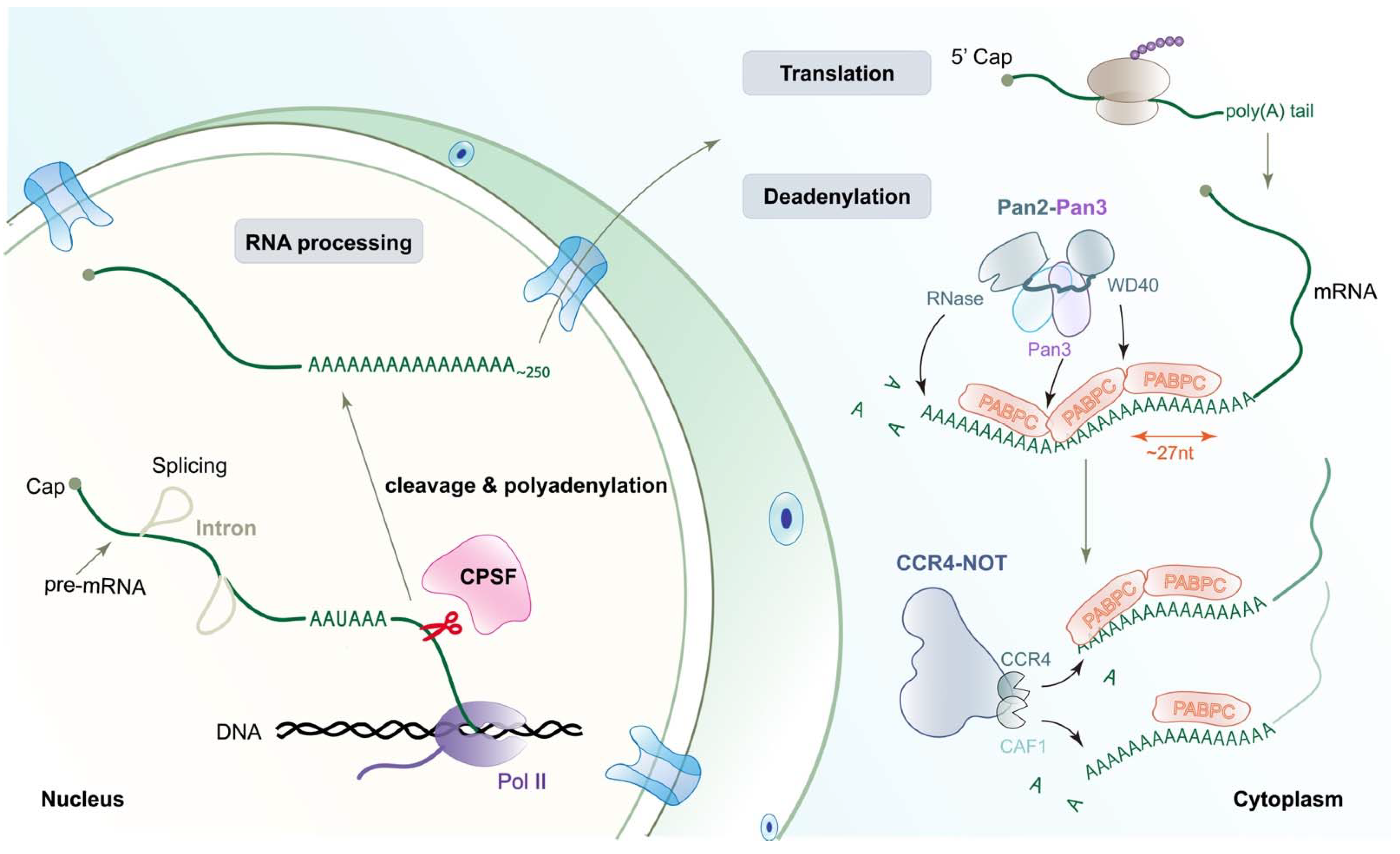
1. Introduction
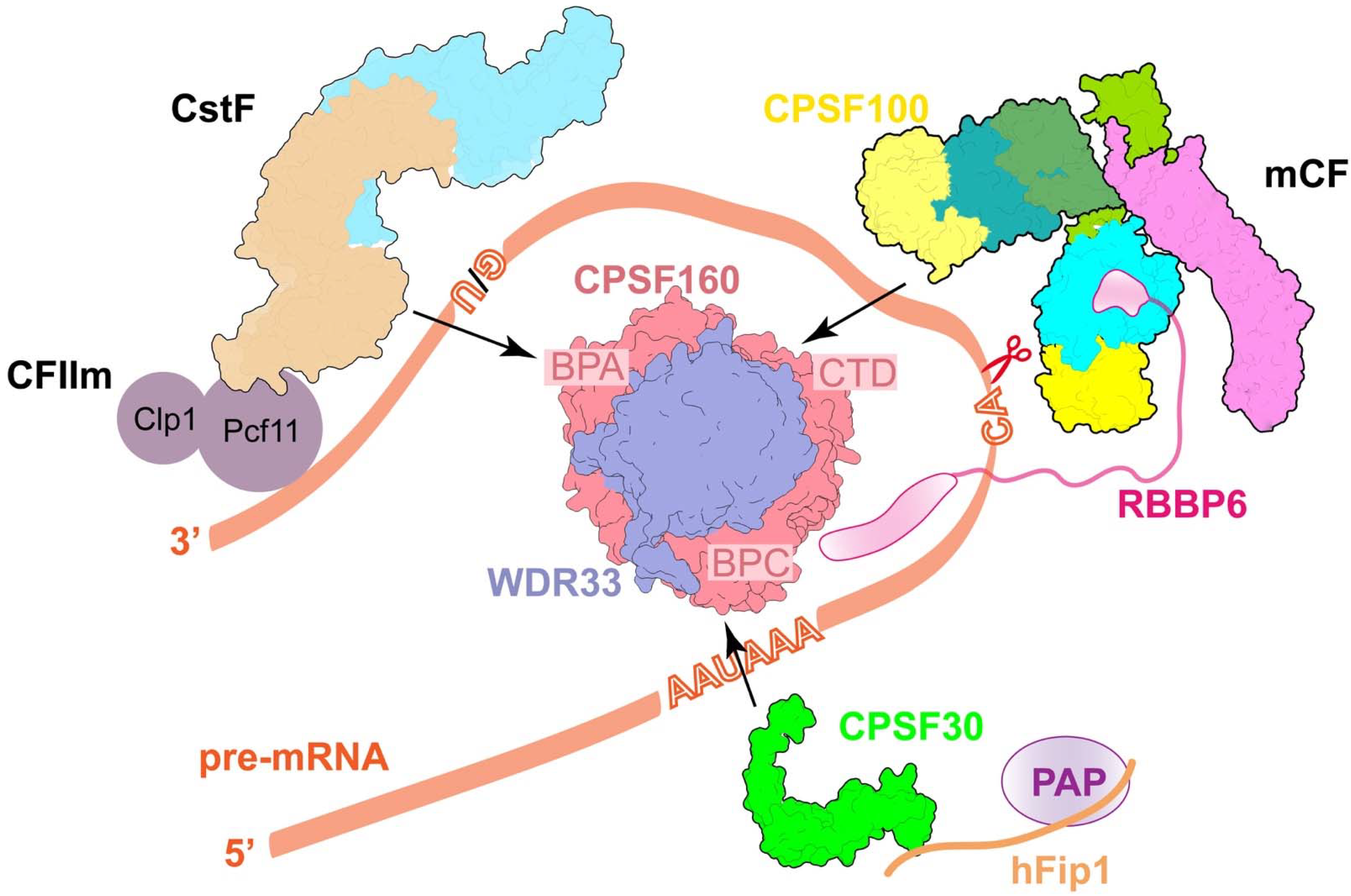
2. The Cleavage Step in Polyadenylation
2.1. Essential Components in Canonical Pre-mRNA 3′-End Processing Machinery
2.2. CPSF160-WDR33 Complex Acts as a Rigid Interaction Platform
2.3. The Recognition of PAS
2.4. The Role of hFip1
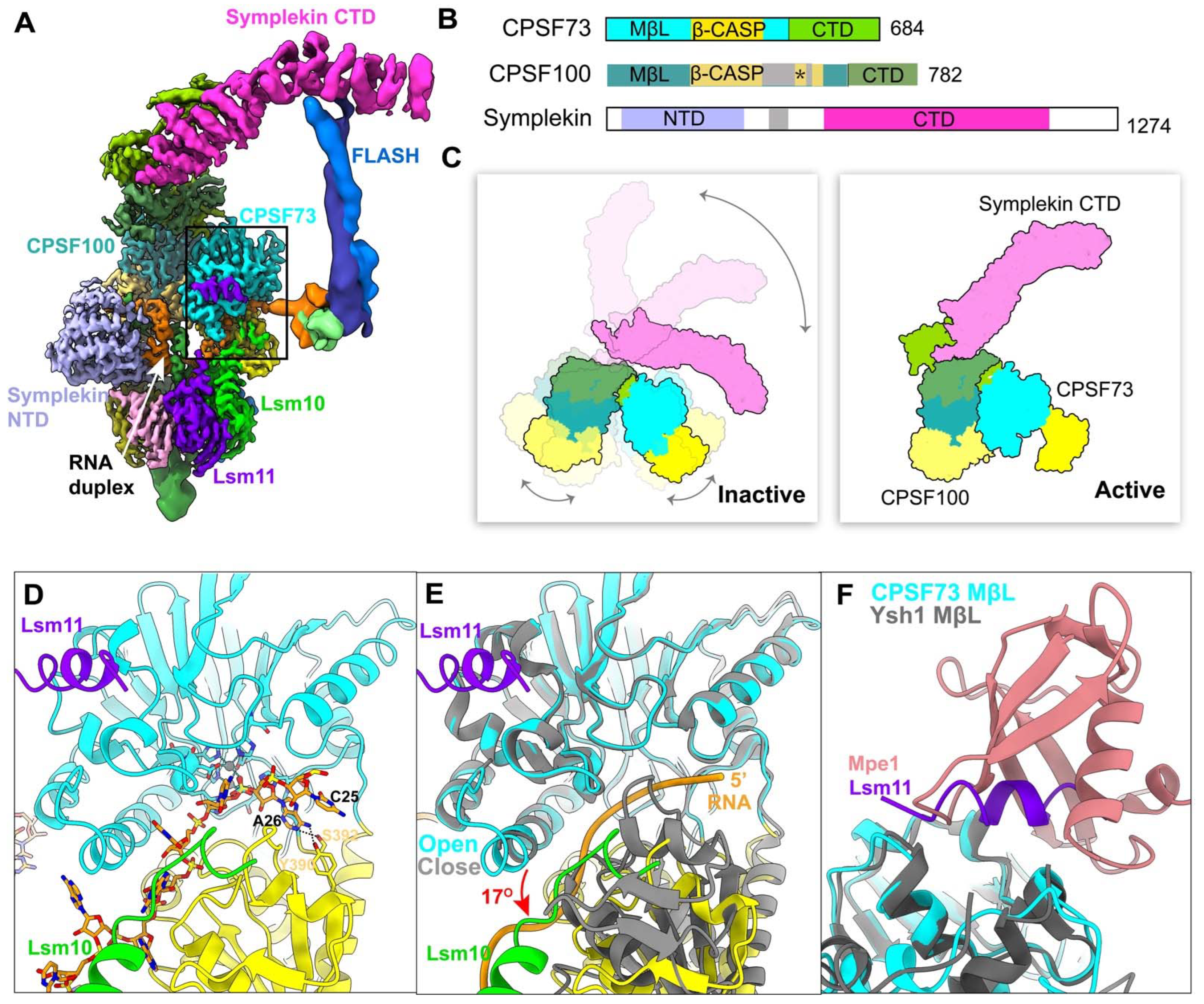
2.5. Molecular Architecture of CPSF
2.6. Accessory Factors CstF and CF IIm
2.7. Activation of the Pre-mRNA 3′-End Cleavage
3. The Biphasic Model of Deadenylation
3.1. PABPC’s Role in Deadenylation
3.2. The Architecture and Recognition Mechanism of Poly(A) RNP by Pan2-Pan3 Complex
3.3. The Roles of Non-Enzymatic Components in CCR4-NOT Complex
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Bienroth, S.; Keller, W.; Wahle, E. Assembly of a processive messenger RNA polyadenylation complex. EMBO J. 1993, 12, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Wahle, E. A novel poly(A)-binding protein acts as a specificity factor in the second phase of messenger RNA polyadenylation. Cell 1991, 66, 759–768. [Google Scholar] [CrossRef]
- Kühn, U.; Buschmann, J.; Wahle, E. The nuclear poly(A) binding protein of mammals, but not of fission yeast, participates in mRNA polyadenylation. RNA 2017, 23, 473–482. [Google Scholar] [CrossRef]
- Keller, R.W.; Kühn, U.; Aragón, M.; Bornikova, L.; Wahle, E.; Bear, D.G. The nuclear poly(A) binding protein, PABP2, forms an oligomeric particle covering the length of the poly(A) tail. J. Mol. Biol. 2000, 297, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E.; Wall, R.; Tushinski, R.J. An Adenylic Acid-Rich Sequence in Messenger RNA of HeLa Cells and Its Possible Relationship to Reiterated Sites in DNA. Proc. Natl. Acad. Sci. USA 1971, 68, 1321–1325. [Google Scholar] [CrossRef]
- Edmonds, M.; Vaughan, M.H., Jr.; Nakazato, H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: Possible evidence for a precursor relationship. Proc. Natl. Acad. Sci. USA 1971, 68, 1336–1340. [Google Scholar] [CrossRef]
- Berkovits, B.D.; Mayr, C. Alternative 3′ UTRs act as scaffolds to regulate membrane protein localization. Nature 2015, 522, 363–367. [Google Scholar] [CrossRef]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, L.; Chen, C.; Ge, Y.; Kang, M.; Song, Z.; Li, J.; Feng, Y.; Huo, Z.; He, G.; et al. Crosstalk between alternative polyadenylation and miRNAs in the regulation of protein translational efficiency. Genome Res. 2018, 28, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.S.; Warner, J.R.; Edmonds, M.; Nakazato, H.; Vaughan, M.H. Polyadenylic acid sequences in yeast messenger ribonucleic acid. J. Biol. Chem. 1973, 248, 508–519. [Google Scholar] [CrossRef]
- Curinha, A.; Braz, S.O.; Pereira-Castro, I.; Cruz, A.; Moreira, A. Implications of polyadenylation in health and disease. Nucleus 2014, 5, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Sun, W.; Xiao, X.; Li, S.; Jia, X.; Zhou, L.; Wang, P.; Zhang, Q. CPSF1 mutations are associated with early-onset high myopia and involved in retinal ganglion cell axon projection. Hum. Mol. Genet. 2019, 28, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Virtanen, A.; Kleiman, F.E. To polyadenylate or to deadenylate: That is the question. Cell Cycle 2010, 9, 4437–4449. [Google Scholar] [CrossRef]
- Balbo, P.B.; Bohm, A. Mechanism of poly(A) polymerase: Structure of the enzyme-MgATP-RNA ternary complex and kinetic analysis. Structure 2007, 15, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Mandel, C.R.; Kaneko, S.; Zhang, H.; Gebauer, D.; Vethantham, V.; Manley, J.L.; Tong, L. Polyadenylation factor CPSF-73 is the pre-mRNA 3′-end-processing endonuclease. Nature 2006, 444, 953–956. [Google Scholar] [CrossRef]
- Desterro, J.; Bak-Gordon, P.; Carmo-Fonseca, M. Targeting mRNA processing as an anticancer strategy. Nat. Rev. Drug Discov. 2019, 19, 112–129. [Google Scholar] [CrossRef]
- Sonoiki, E.; Ng, C.L.; Lee, M.C.S.; Guo, D.; Zhang, Y.-K.; Zhou, Y.; Alley, M.R.K.; Ahyong, V.; Sanz, L.M.; Lafuente-Monasterio, M.J.; et al. A potent antimalarial benzoxaborole targets a Plasmodium falciparum cleavage and polyadenylation specificity factor homologue. Nat. Commun. 2017, 8, 14574. [Google Scholar] [CrossRef]
- Liu, H.; Moore, C.L. On the Cutting Edge: Regulation and Therapeutic Potential of the mRNA 3′ End Nuclease. Trends Biochem. Sci. 2021, 46, 772–784. [Google Scholar] [CrossRef]
- Ross, N.T.; Lohmann, F.; Carbonneau, S.; Fazal, A.; Weihofen, W.A.; Gleim, S.; Salcius, M.; Sigoillot, F.; Henault, M.; Carl, S.H.; et al. CPSF3-dependent pre-mRNA processing as a druggable node in AML and Ewing’s sarcoma. Nat. Chem. Biol. 2020, 16, 50–59. [Google Scholar] [CrossRef]
- Murphy, M.R.; Doymaz, A.; Kleiman, F.E. Poly(A) tail dynamics: Measuring polyadenylation, deadenylation and poly(A) tail length. Methods Enzymol. 2021, 655, 265–290. [Google Scholar] [CrossRef]
- Marzluff, W.F.; Wagner, E.J.; Duronio, R.J. Metabolism and regulation of canonical histone mRNAs: Life without a poly(A) tail. Nat. Rev. Genet. 2008, 9, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.; Steiniger, M.; Marzluff, W.F. A Core Complex of CPSF73, CPSF100, and Symplekin May Form Two Different Cleavage Factors for Processing of Poly(A) and Histone mRNAs. Mol. Cell 2009, 34, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.S.; Platt, T. RNA Processing Generates the Mature 3′ End of Yeast CYC1 Messenger RNA in Vitro. Science 1988, 242, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.L.; Sharp, P.A. Accurate cleavage and polyadenylation of exogenous RNA substrate. Cell 1985, 41, 845–855. [Google Scholar] [CrossRef]
- Gutierrez, P.A.; Baughman, K.; Sun, Y.; Tong, L. A real-time fluorescence assay for CPSF73, the nuclease for pre-mRNA 3′-end processing. RNA 2021, 27, 1148–1154. [Google Scholar] [CrossRef]
- Yang, X.-C.; Sun, Y.; Aik, W.S.; Marzluff, W.F.; Tong, L.; Dominski, Z. Studies with recombinant U7 snRNP demonstrate that CPSF73 is both an endonuclease and a 5′-3′ exonuclease. RNA 2020, 26, 1345–1359. [Google Scholar] [CrossRef]
- Boreikaite, V.; Elliott, T.S.; Chin, J.W.; Passmore, L.A. RBBP6 activates the pre-mRNA 3′ end processing machinery in humans. Genes Dev. 2022, 36, 210–224. [Google Scholar] [CrossRef]
- Hill, C.H.; Boreikaite, V.; Kumar, A.; Casañal, A.; Kubík, P.; Degliesposti, G.; Maslen, S.; Mariani, A.; von Loeffelholz, O.; Girbig, M.; et al. Activation of the Endonuclease that Defines mRNA 3′ Ends Requires Incorporation into an 8-Subunit Core Cleavage and Polyadenylation Factor Complex. Mol. Cell 2019, 73, 1217–1231.e11. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Kluge, F.; Sandmeir, F.; Kühn, U.; Schäfer, P.; Tüting, C.; Ihling, C.; Conti, E.; Wahle, E. Reconstitution of 3′ end processing of mammalian pre-mRNA reveals a central role of RBBP6. Genes Dev. 2022, 36, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Aik, W.S.; Yang, X.C.; Marzluff, W.F.; Walz, T.; Dominski, Z.; Tong, L. Structure of an active human histone pre-mRNA 3′-end processing machinery. Science 2020, 367, 700–703. [Google Scholar] [CrossRef]
- Yoon, Y.; Shi, Y. Human pre-mRNA 3′ end processing: Reconstituting is believing. Genes Dev. 2022, 36, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Di Giammartino, D.C.; Li, W.; Ogami, K.; Yashinskie, J.J.; Hoque, M.; Tian, B.; Manley, J.L. RBBP6 isoforms regulate the human polyadenylation machinery and modulate expression of mRNAs with AU-rich 3′ UTRs. Genes Dev. 2014, 28, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Di Giammartino, D.C.; Taylor, D.; Sarkeshik, A.; Rice, W.J.; Yates, J.R., 3rd; Frank, J.; Manley, J.L. Molecular architecture of the human pre-mRNA 3′ processing complex. Mol. Cell 2009, 33, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Molina, J.B.; O’Reilly, F.J.; Fagarasan, H.; Sheekey, E.; Maslen, S.; Skehel, J.M.; Rappsilber, J.; Passmore, L.A. Mpe1 senses the binding of pre-mRNA and controls 3′ end processing by CPF. Mol. Cell 2022, 82, 2490–2504.e12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, X.; Forouzmand, E.; Jeong, J.; Qiao, F.; Sowd, G.A.; Engelman, A.N.; Xie, X.; Hertel, K.J.; Shi, Y. Molecular Mechanisms for CFIm-Mediated Regulation of mRNA Alternative Polyadenylation. Mol. Cell 2017, 69, 62–74.e4. [Google Scholar] [CrossRef]
- Hardy, J.G.; Norbury, C.J. Cleavage factor Im (CFIm) as a regulator of alternative polyadenylation. Biochem. Soc. Trans. 2016, 44, 1051–1057. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Shi, Y.; Walz, T.; Tong, L. Structural Insights into the Human Pre-mRNA 3′-End Processing Machinery. Mol. Cell 2020, 77, 800–809.e6. [Google Scholar] [CrossRef]
- Kumar, A.; Yu, C.W.; Rodríguez-Molina, J.B.; Li, X.-H.; Freund, S.M.; Passmore, L.A. Dynamics in Fip1 regulate eukaryotic mRNA 3′ end processing. Genes Dev. 2021, 35, 1510–1526. [Google Scholar] [CrossRef]
- Scrima, A.; Koníčková, R.; Czyzewski, B.K.; Kawasaki, Y.; Jeffrey, P.D.; Groisman, R.; Nakatani, Y.; Iwai, S.; Pavletich, N.P.; Thomä, N.H. Structural Basis of UV DNA-Damage Recognition by the DDB1–DDB2 Complex. Cell 2008, 135, 1213–1223. [Google Scholar] [CrossRef]
- Angers, S.; Li, T.; Yi, X.; MacCoss, M.J.; Moon, R.T.; Zheng, N. Molecular architecture and assembly of the DDB1–CUL4A ubiquitin ligase machinery. Nature 2006, 443, 590–593. [Google Scholar] [CrossRef]
- Clerici, M.; Faini, M.; Muckenfuss, L.M.; Aebersold, R.; Jinek, M. Structural basis of AAUAAA polyadenylation signal recognition by the human CPSF complex. Nat. Struct. Mol. Biol. 2018, 25, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Hamilton, K.; Manley, J.L.; Shi, Y.; Walz, T.; Tong, L. Molecular basis for the recognition of the human AAUAAA polyadenylation signal. Proc. Natl. Acad. Sci. 2017, 115, E1419–E1428. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.; Tong, L. Molecular mechanism for the interaction between human CPSF30 and hFip1. Genes Dev. 2020, 34, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Beaudoing, E.; Freier, S.; Wyatt, J.R.; Claverie, J.-M.; Gautheret, D. Patterns of Variant Polyadenylation Signal Usage in Human Genes. Genome Res. 2000, 10, 1001–1010. [Google Scholar] [CrossRef]
- Tian, B.; Hu, J.; Zhang, H.; Lutz, C.S. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res. 2005, 33, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.; Sun, Y.; Tong, L. Biophysical characterizations of the recognition of the AAUAAA polyadenylation signal. RNA 2019, 25, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Clerici, M.; Muckenfuss, L.M.; A Passmore, L.; Jinek, M. Mechanistic insights into mRNA 3′-end processing. Curr. Opin. Struct. Biol. 2019, 59, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hamilton, K.; Tong, L. Recent molecular insights into canonical pre-mRNA 3′-end processing. Transcription 2020, 11, 83–96. [Google Scholar] [CrossRef]
- Thore, S.; Fribourg, S. Structural insights into the 3′-end mRNA maturation machinery: Snapshot on polyadenylation signal recognition. Biochimie 2019, 164, 105–110. [Google Scholar] [CrossRef]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2017, 27, 14–25. [Google Scholar] [CrossRef]
- Russo, P.; Li, W.Z.; Guo, Z.; Sherman, F. Signals that produce 3' termini in CYC1 mRNA of the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 1993, 13, 7836–7849. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.; Faini, M.; Aebersold, R.; Jinek, M. Structural insights into the assembly and polyA signal recognition mechanism of the human CPSF complex. eLife 2017, 6, e33111. [Google Scholar] [CrossRef]
- Chan, S.L.; Huppertz, I.; Yao, C.; Weng, L.; Moresco, J.J.; Yates, J.R.; Ule, J.; Manley, J.L.; Shi, Y. CPSF30 and Wdr33 directly bind to AAUAAA in mammalian mRNA 3′ processing. Genes Dev. 2014, 28, 2370–2380. [Google Scholar] [CrossRef]
- Schönemann, L.; Kühn, U.; Martin, G.; Schäfer, P.; Gruber, A.R.; Keller, W.; Zavolan, M.; Wahle, E. Reconstitution of CPSF active in polyadenylation: Recognition of the polyadenylation signal by WDR33. Genes Dev. 2014, 28, 2381–2393. [Google Scholar] [CrossRef]
- Paulson, A.R.; Tong, L. Crystal structure of the Rna14–Rna15 complex. RNA 2012, 18, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hsu, P.L.; Yang, F.; Song, J.-E.; Varani, G. Reconstitution of the CstF complex unveils a regulatory role for CstF-50 in recognition of 3′-end processing signals. Nucleic Acids Res. 2017, 46, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, P.; Tüting, C.; Schönemann, L.; Kühn, U.; Treiber, T.; Treiber, N.; Ihling, C.; Graber, A.; Keller, W.; Meister, G.; et al. Reconstitution of mammalian cleavage factor II involved in 3′ processing of mRNA precursors. RNA 2018, 24, 1721–1737. [Google Scholar] [CrossRef]
- Jolles, B.; Jean-Jean, O. Poly(A) tail degradation in human cells: ATF4 mRNA as a model for biphasic deadenylation. Biochimie 2021, 185, 128–134. [Google Scholar] [CrossRef]
- Passmore, L.A.; Coller, J. Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat. Rev. Mol. Cell Biol. 2021, 23, 93–106. [Google Scholar] [CrossRef]
- Schäfer, I.B.; Yamashita, M.; Schuller, J.M.; Schüssler, S.; Reichelt, P.; Strauss, M.; Conti, E. Molecular Basis for poly(A) RNP Architecture and Recognition by the Pan2-Pan3 Deadenylase. Cell 2019, 177, 1619–1631.e21. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Park, J.; Ha, M.; Lim, J.; Chang, H.; Kim, V.N. PABP Cooperates with the CCR4-NOT Complex to Promote mRNA Deadenylation and Block Precocious Decay. Mol. Cell 2018, 70, 1081–1088.e5. [Google Scholar] [CrossRef] [PubMed]
- Deo, R.C.; Bonanno, J.B.; Sonenberg, N.; Burley, S.K. Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein. Cell 1999, 98, 835–845. [Google Scholar] [CrossRef]
- Burd, C.G.; Matunis, E.L.; Dreyfuss, G. The multiple RNA-binding domains of the mRNA poly(A)-binding protein have different RNA-binding activities. Mol. Cell. Biol. 1991, 11, 3419–3424. [Google Scholar] [CrossRef] [PubMed]
- Kühn, U.; Pieler, T. XenopusPoly(A) Binding Protein: Functional Domains in RNA Binding and Protein–Protein Interaction. J. Mol. Biol. 1996, 256, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Baer, B.W.; Kornberg, R.D. Repeating structure of cytoplasmic poly(A)-ribonucleoprotein. Proc. Natl. Acad. Sci. USA 1980, 77, 1890–1892. [Google Scholar] [CrossRef]
- Smith, B.L.; Gallie, D.R.; Leb, H.; Hansma, P.K. Visualization of Poly(A)-Binding Protein Complex Formation with Poly(A) RNA Using Atomic Force Microscopy. J. Struct. Biol. 1997, 119, 109–117. [Google Scholar] [CrossRef]
- Sawazaki, R.; Imai, S.; Yokogawa, M.; Hosoda, N.; Hoshino, S.-I.; Mio, M.; Mio, K.; Shimada, I.; Osawa, M. Characterization of the multimeric structure of poly(A)-binding protein on a poly(A) tail. Sci. Rep. 2018, 8, 1455. [Google Scholar] [CrossRef]
- Ford, L.P.; Bagga, P.S.; Wilusz, J. The poly(A) tail inhibits the assembly of a 3'-to-5' exonuclease in an in vitro RNA stability system. Mol. Cell. Biol. 1997, 17, 398–406. [Google Scholar] [CrossRef]
- Gallie, D.R. The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev. 1991, 5, 2108–2116. [Google Scholar] [CrossRef]
- Webster, M.W.; Chen, Y.-H.; Stowell, J.A.; Alhusaini, N.; Sweet, T.; Graveley, B.R.; Coller, J.; Passmore, L.A. mRNA Deadenylation Is Coupled to Translation Rates by the Differential Activities of Ccr4-Not Nucleases. Mol. Cell 2018, 70, 1089–1100.e8. [Google Scholar] [CrossRef]
- Uchida, N.; Hoshino, S.-I.; Katada, T. Identification of a Human Cytoplasmic Poly(A) Nuclease Complex Stimulated by Poly(A)-binding Protein. J. Biol. Chem. 2004, 279, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Mace, P.D.; Murphy, J.M. There’s more to death than life: Noncatalytic functions in kinase and pseudokinase signaling. J. Biol. Chem. 2021, 296, 100705. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.T.L.; Stowell, J.A.W.; Hill, C.H.; Passmore, L.A. The intrinsic structure of poly(A) RNA determines the specificity of Pan2 and Caf1 deadenylases. Nat. Struct. Mol. Biol. 2019, 26, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ukleja, M.; Cuéllar, J.; Siwaszek, A.; Kasprzak, J.M.; Czarnocki-Cieciura, M.; Bujnicki, J.M.; Dziembowski, A.; Valpuesta, J. The architecture of the Schizosaccharomyces pombe CCR4-NOT complex. Nat. Commun. 2016, 7, 10433. [Google Scholar] [CrossRef]
- Albert, T.K. Isolation and characterization of human orthologs of yeast CCR4-NOT complex subunits. Nucleic Acids Res. 2000, 28, 809–817. [Google Scholar] [CrossRef][Green Version]
- Bawankar, P.; Loh, B.; Wohlbold, L.; Schmidt, S.; Izaurralde, E. NOT10 and C2orf29/NOT11 form a conserved module of the CCR4-NOT complex that docks onto the NOT1 N-terminal domain. RNA Biol. 2013, 10, 228–244. [Google Scholar] [CrossRef]
- Chen, J.; Rappsilber, J.; Chiang, Y.-C.; Russell, P.; Mann, M.; Denis, C.L. Purification and characterization of the 1.0 MDa CCR4-NOT complex identifies two novel components of the complex. J. Mol. Biol. 2001, 314, 683–694. [Google Scholar] [CrossRef]
- Temme, C.; Zhang, L.; Kremmer, E.; Ihling, C.; Chartier, A.; Sinz, A.; Simonelig, M.; Wahle, E. Subunits of the Drosophila CCR4-NOT complex and their roles in mRNA deadenylation. RNA 2010, 16, 1356–1370. [Google Scholar] [CrossRef]
- Mostafa, D.; Takahashi, A.; Yanagiya, A.; Yamaguchi, T.; Abe, T.; Kureha, T.; Kuba, K.; Kanegae, Y.; Furuta, Y.; Yamamoto, T.; et al. Essential functions of the CNOT7/8 catalytic subunits of the CCR4-NOT complex in mRNA regulation and cell viability. RNA Biol. 2020, 17, 403–416. [Google Scholar] [CrossRef]
- Boland, A.; Chen, Y.; Raisch, T.; Jonas, S.; Ozturk, D.K.; Wohlbold, L.; Weichenrieder, O.; Izaurralde, E. Structure and assembly of the NOT module of the human CCR4–NOT complex. Nat. Struct. Mol. Biol. 2013, 20, 1289–1297. [Google Scholar] [CrossRef]
- Basquin, J.; Roudko, V.V.; Rode, M.; Basquin, C.; Séraphin, B.; Conti, E. Architecture of the Nuclease Module of the Yeast Ccr4-Not Complex: The Not1-Caf1-Ccr4 Interaction. Mol. Cell 2012, 48, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Keskeny, C.; Raisch, T.; Sgromo, A.; Igreja, C.; Bhandari, D.; Weichenrieder, O.; Izaurralde, E. A conserved CAF40-binding motif in metazoan NOT4 mediates association with the CCR4–NOT complex. Genes Dev. 2019, 33, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, L.; Hall, B.; Airhihen, B.; Winkler, G.S. The central region of CNOT1 and CNOT9 stimulates deadenylation by the Ccr4–Not nuclease module. Biochem. J. 2018, 475, 3437–3450. [Google Scholar] [CrossRef] [PubMed]
- Mauxion, F.; Preve, B.; Seraphin, B. C2ORF29/CNOT11 and CNOT10 form a new module of the CCR4-NOT complex. RNA Biol. 2013, 10, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.-C.; Kolkman, A.; Van Schaik, F.M.A.; Mulder, K.W.; Pijnappel, W.W.M.P.; Heck, A.; Timmers, H.T.M. Human Ccr4–Not complexes contain variable deadenylase subunits. Biochem. J. 2009, 422, 443–453. [Google Scholar] [CrossRef]
- Raisch, T.; Chang, C.-T.; Levdansky, Y.; Muthukumar, S.; Raunser, S.; Valkov, E. Reconstitution of recombinant human CCR4-NOT reveals molecular insights into regulated deadenylation. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Chen, Y.; Khazina, E.; Izaurralde, E.; Weichenrieder, O. Crystal structure and functional properties of the human CCR4-CAF1 deadenylase complex. Nucleic Acids Res. 2021, 49, 6489–6510. [Google Scholar] [CrossRef]
- Zhang, Q.; Pavanello, L.; Potapov, A.; Bartlam, M.; Winkler, G.S. Structure of the human Ccr4-Not nuclease module using X-ray crystallography and electron paramagnetic resonance spectroscopy distance measurements. Protein Sci. 2021, 31, 758–764. [Google Scholar] [CrossRef]
- Nicholson, A.L.; Pasquinelli, A.E. Tales of Detailed Poly(A) Tails. Trends Cell Biol. 2018, 29, 191–200. [Google Scholar] [CrossRef]
- Eisen, T.J.; Eichhorn, S.W.; Subtelny, A.O.; Lin, K.S.; McGeary, S.E.; Gupta, S.; Bartel, D.P. The Dynamics of Cytoplasmic mRNA Metabolism. Mol. Cell 2020, 77, 786–799.e10. [Google Scholar] [CrossRef]
- Charlesworth, A.; Meijer, H.A.; De Moor, C.H. Specificity factors in cytoplasmic polyadenylation. Wiley Interdiscip. Rev. RNA 2013, 4, 437–461. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.K. The emerging role of triple helices in RNA biology. Wiley Interdiscip. Rev. RNA 2013, 5, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Torabi, S.-F.; Chen, Y.-L.; Zhang, K.; Wang, J.; DeGregorio, S.J.; Vaidya, A.T.; Su, Z.; Pabit, S.A.; Chiu, W.; Pollack, L.; et al. Structural analyses of an RNA stability element interacting with poly(A). Proc. Natl. Acad. Sci. USA 2021, 118, e2026656118. [Google Scholar] [CrossRef] [PubMed]
- Torabi, S.-F.; Vaidya, A.T.; Tycowski, K.T.; DeGregorio, S.J.; Wang, J.; Shu, M.-D.; Steitz, T.A.; Steitz, J.A. RNA stabilization by a poly(A) tail 3′-end binding pocket and other modes of poly(A)-RNA interaction. Science 2021, 371, eabe6523. [Google Scholar] [CrossRef]



| Human | Yeast | |||
|---|---|---|---|---|
| Complex | Subunit | Role | Complex | Subunit |
| CPSF | mPSF 1 including | PAS Recognition | CPF | |
| CPSF160 | Scaffold | Cft1 | ||
| WDR33 | Scaffold, RNA binding | Pfs2 | ||
| CPSF30 | Recruits hFip1, RNA binding | Yth1 | ||
| hFip1 | Binds PAP | Fip1 | ||
| mCF 2 including | Cleavage | |||
| CPSF73 | Endonuclease | Ysh1 | ||
| CPSF100 | (Pseudo-)endonuclease, assembles | Cft2 | ||
| Symplekin | Scaffold | Pta1 * | ||
| RBBP6 | Activates cleavage, RNA binding | Mpe1 | ||
| PAP | Poly(A) polymerase | Pap1 | ||
| CstF | CstF50 | Scaffold | CFIA | n.d. |
| CstF64 | RNA binding | Rna15 | ||
| CstF77 | Scaffold, assembles | Rna14 | ||
| CF IIm | hPcf11 | Binds Pol II | Pcf11 | |
| hClp1 | RNA kinase | Clp1 | ||
| CF Im * | CFIm68 | RNA binding | n.d. | |
| CFIm25 | RNA binding | n.d. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Lu, X.; Zhang, S.; Yuan, L.; Sun, Y. Molecular Insights into mRNA Polyadenylation and Deadenylation. Int. J. Mol. Sci. 2022, 23, 10985. https://doi.org/10.3390/ijms231910985
Liu J, Lu X, Zhang S, Yuan L, Sun Y. Molecular Insights into mRNA Polyadenylation and Deadenylation. International Journal of Molecular Sciences. 2022; 23(19):10985. https://doi.org/10.3390/ijms231910985
Chicago/Turabian StyleLiu, Junjie, Xubing Lu, Siyu Zhang, Ling Yuan, and Yadong Sun. 2022. "Molecular Insights into mRNA Polyadenylation and Deadenylation" International Journal of Molecular Sciences 23, no. 19: 10985. https://doi.org/10.3390/ijms231910985
APA StyleLiu, J., Lu, X., Zhang, S., Yuan, L., & Sun, Y. (2022). Molecular Insights into mRNA Polyadenylation and Deadenylation. International Journal of Molecular Sciences, 23(19), 10985. https://doi.org/10.3390/ijms231910985






