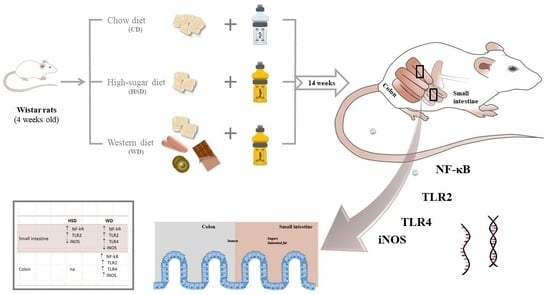
Unhealthy Diets Induce Distinct and Regional Effects on Intestinal Inflammatory Signalling Pathways and Long-Lasting Metabolic Dysfunction in Rats
Abstract
1. Introduction
2. Results
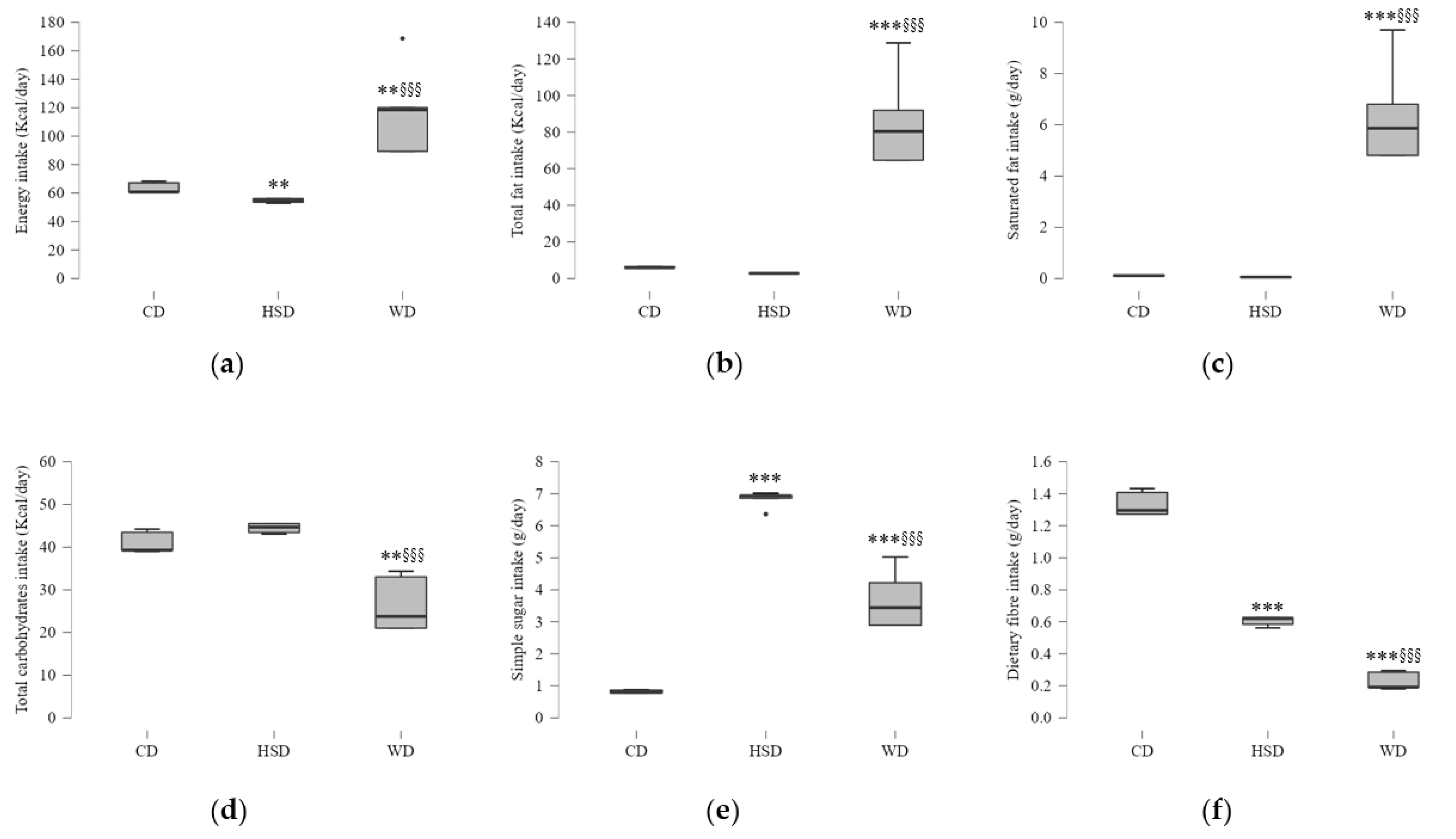
2.1. Effects of Energy and Macronutrient Intake on Anthropometric Parameters
2.2. Dietary Composition Effects on Serum Metabolic Parameters
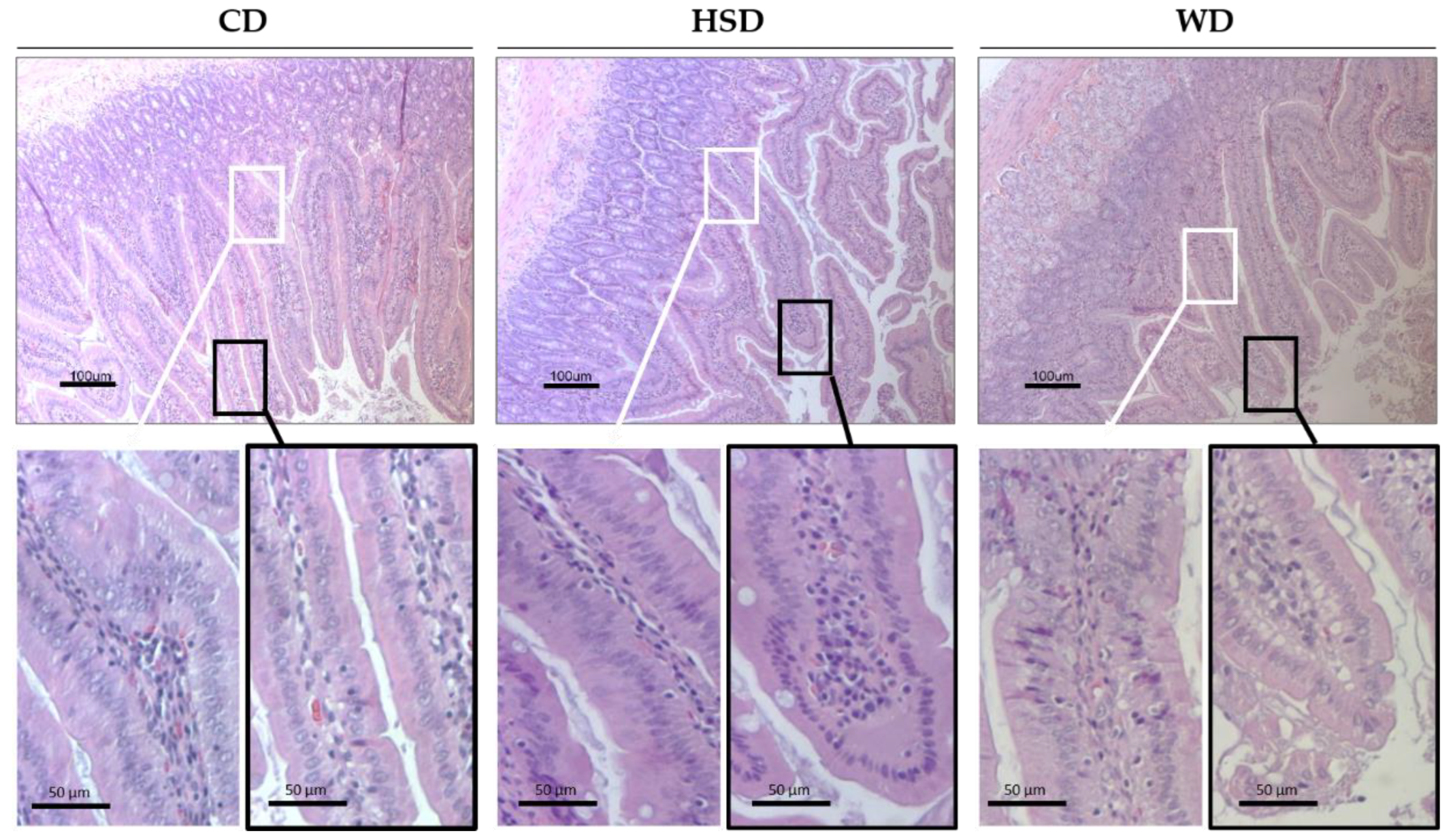
2.3. Effects of Obesogenic Diets on the Small Intestine Morphology
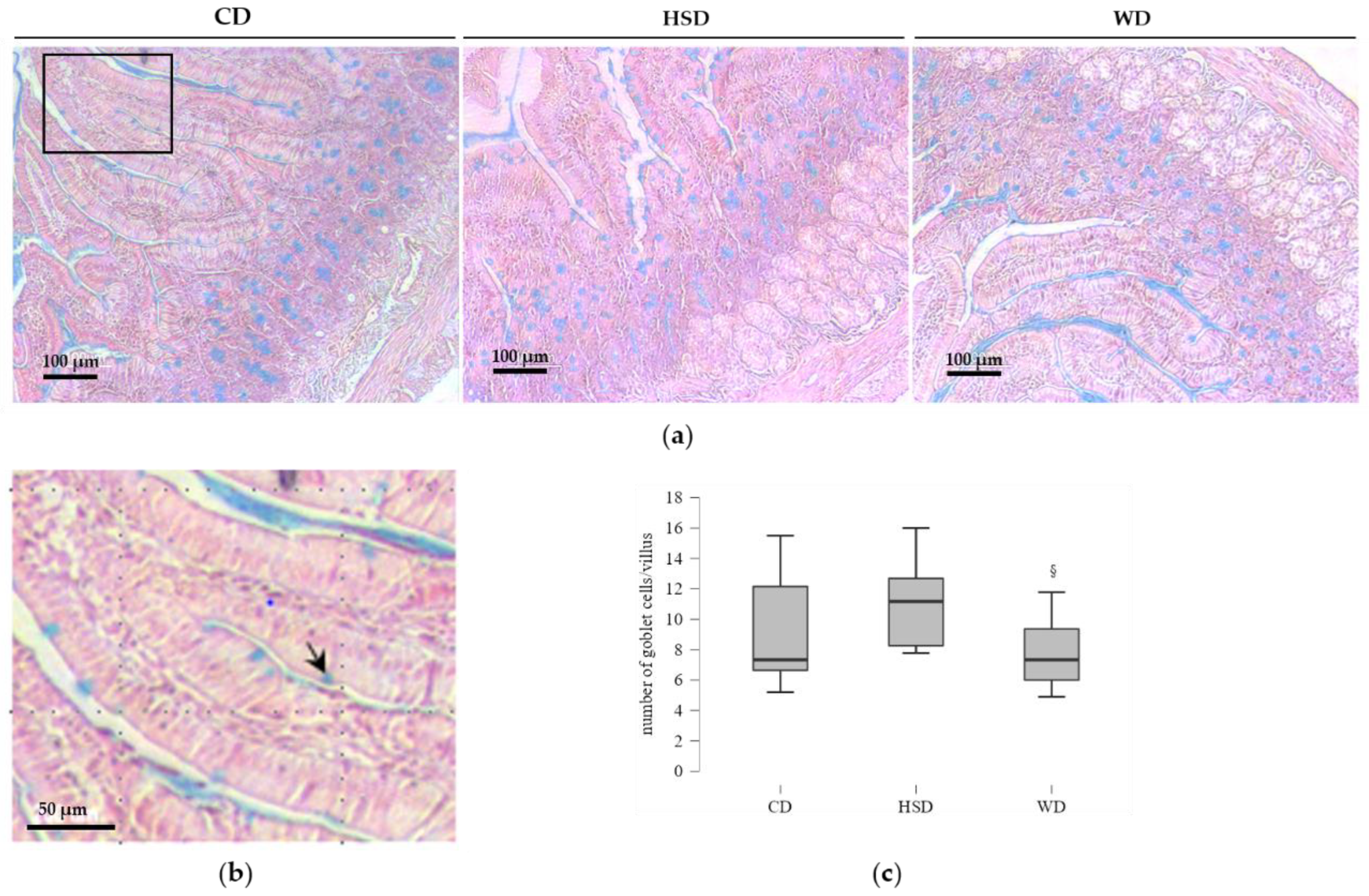
2.4. Distinct Effects of Obesogenic Diets on the Number of Goblet Cells
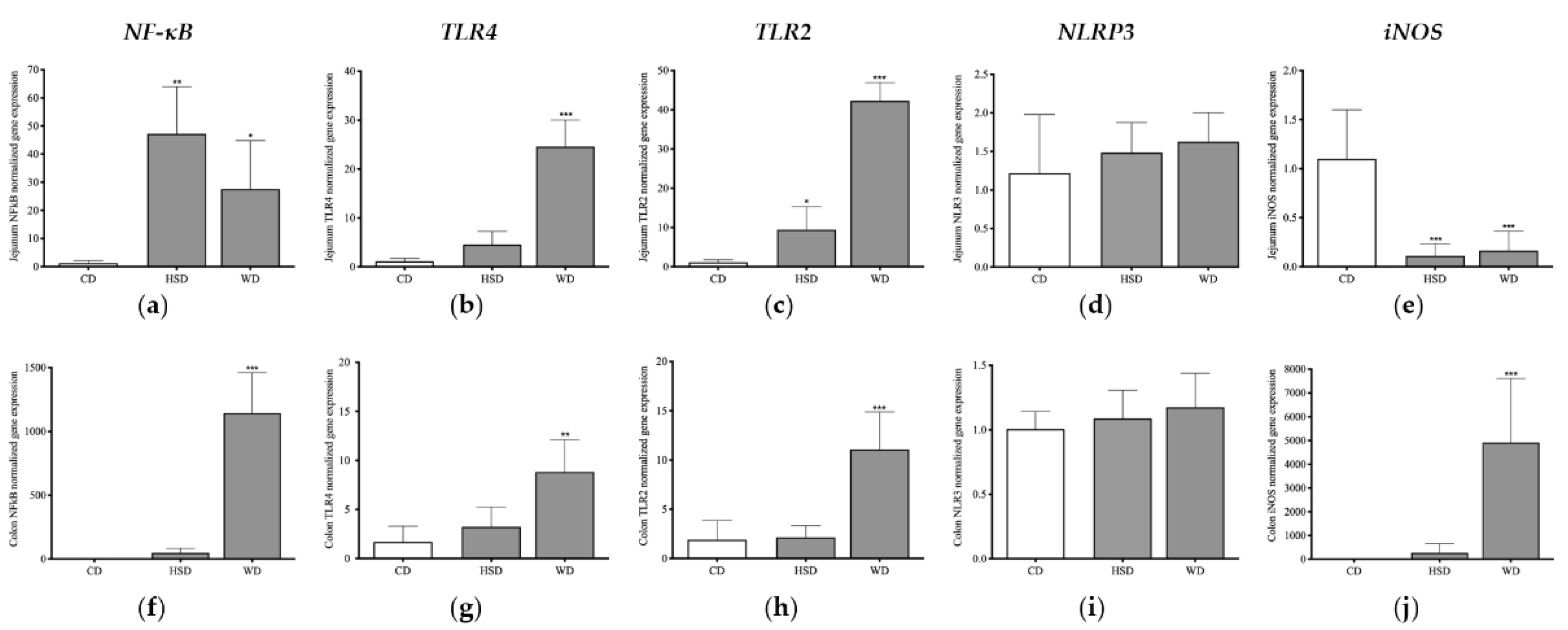
2.5. Distinct Effects of Obesogenic Diets on Inflammatory Signalling Pathways in the Small Intestine
2.6. Distinct Effects of Obesogenic Diets on Inflammatory Signalling Pathways in the Large Intestine
3. Discussion
4. Material and Methods
4.1. Animals and Experimental Diets
4.2. Tissue Collection
4.3. Histological Analysis and Goblet Cell Staining
4.4. Gene Expression Analysis
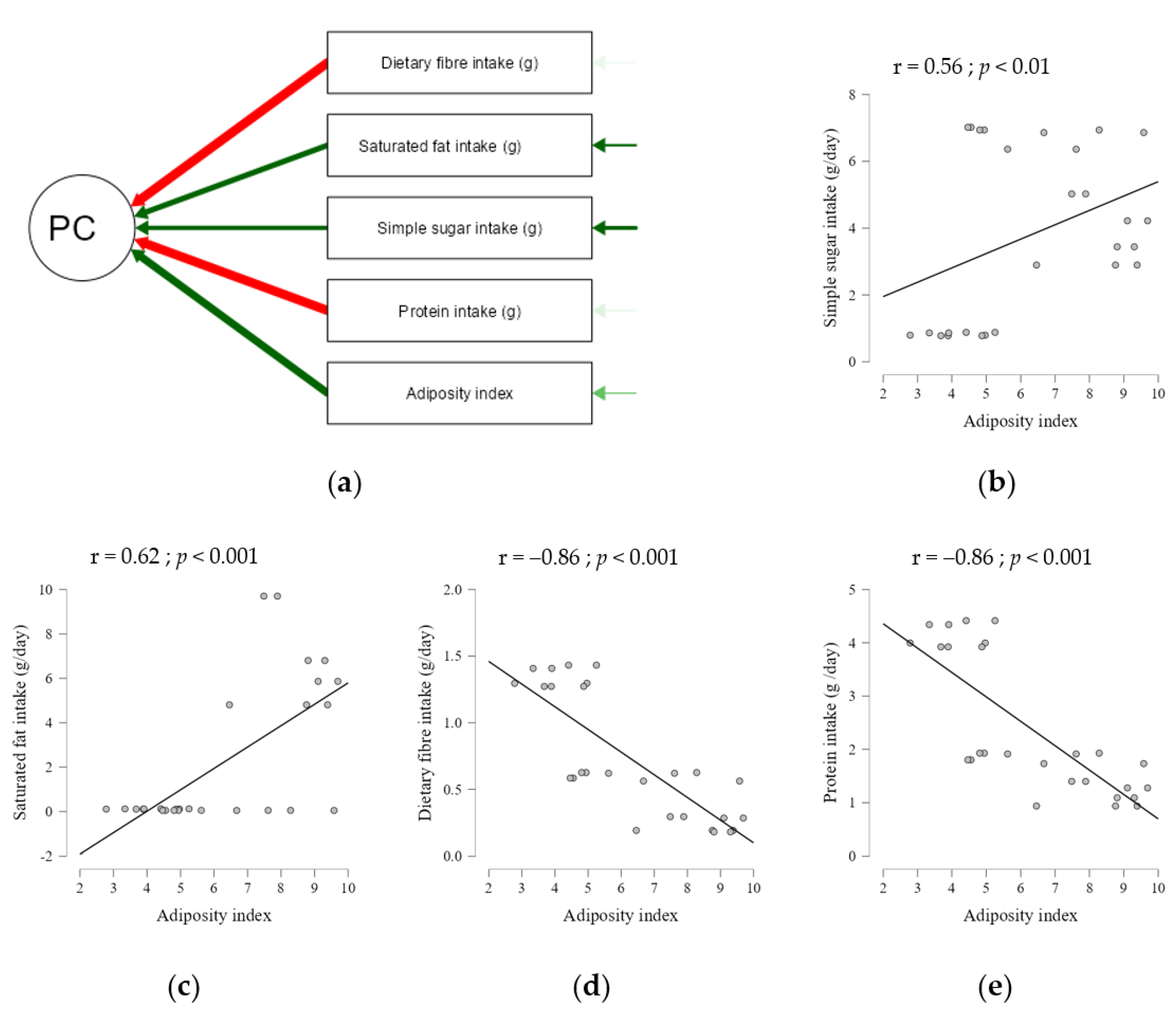
4.5. Correlation Analysis with Physiological Traits
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Della Corte, K.; Fife, J.; Gardner, A.; Murphy, B.L.; Kleis, L.; Della Corte, D.; Schwingshackl, L.; LeCheminant, J.D.; Buyken, A.E. World Trends in Sugar-Sweetened Beverage and Dietary Sugar Intakes in Children and Adolescents: A Systematic Review. Nutr. Rev. 2021, 79, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of Ultra-Processed Foods and Health Status: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2021, 125, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Debras, C.; Druesne-Pecollo, N.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultraprocessed Food Consumption and Risk of Type 2 Diabetes Among Participants of the NutriNet-Santé Prospective Cohort. JAMA Intern. Med. 2020, 180, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Everard, A. Talking Microbes: When Gut Bacteria Interact with Diet and Host Organs. Mol. Nutr. Food Res. 2016, 60, 58–66. [Google Scholar] [CrossRef]
- Nobs, S.P.; Zmora, N.; Elinav, E. Nutrition Regulates Innate Immunity in Health and Disease. Annu. Rev. Nutr. 2020, 40, 189–219. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial Dysbiosis-Induced Obesity: Role of Gut Microbiota in Homoeostasis of Energy Metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef]
- Golonka, R.M.; Xiao, X.; Abokor, A.A.; Joe, B.; Vijay-Kumar, M. Altered Nutrient Status Reprograms Host Inflammation and Metabolic Health via Gut Microbiota. J. Nutr. Biochem. 2020, 80, 108360. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.; Löber, U.; Adamek, K.; Węgrzyn, D.; Skonieczna-Żydecka, K.; Malinowski, D.; Łoniewski, I.; Markó, L.; Ulas, T.; Forslund, S.K.; et al. The Gut Microbiota Is Associated with the Small Intestinal Paracellular Permeability and the Development of the Immune System in Healthy Children during the First Two Years of Life. J. Transl. Med. 2021, 19, 177. [Google Scholar] [CrossRef]
- Alemao, C.A.; Budden, K.F.; Gomez, H.M.; Rehman, S.F.; Marshall, J.E.; Shukla, S.D.; Donovan, C.; Forster, S.C.; Yang, I.A.; Keely, S.; et al. Impact of Diet and the Bacterial Microbiome on the Mucous Barrier and Immune Disorders. Allergy 2021, 76, 714–734. [Google Scholar] [CrossRef]
- Hamilton, M.K.; Boudry, G.; Lemay, D.G.; Raybould, H.E. Changes in Intestinal Barrier Function and Gut Microbiota in High-Fat Diet-Fed Rats Are Dynamic and Region Dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G840–G851. [Google Scholar] [CrossRef] [PubMed]
- Martchenko, S.E.; Prescott, D.; Martchenko, A.; Sweeney, M.E.; Philpott, D.J.; Brubaker, P.L. Diurnal Changes in the Murine Small Intestine Are Disrupted by Obesogenic Western Diet Feeding and Microbial Dysbiosis. Sci. Rep. 2021, 11, 20571. [Google Scholar] [CrossRef] [PubMed]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. Bethesda Md. 2020, 11, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Birchenough, G.M.H.; Ståhlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Bäckhed, F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe 2018, 23, 27–40.e7. [Google Scholar] [CrossRef] [PubMed]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the Crossroads of Nutrients, Gut Microbiota, and Metabolic Inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like Receptors and Their Role in Gut Homeostasis and Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef]
- Furness, J.B.; Rivera, L.R.; Cho, H.-J.; Bravo, D.M.; Callaghan, B. The Gut as a Sensory Organ. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 729–740. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus Barrier, Mucins and Gut Microbiota: The Expected Slimy Partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Abreu, M.T. Toll-like Receptor Signalling in the Intestinal Epithelium: How Bacterial Recognition Shapes Intestinal Function. Nat. Rev. Immunol. 2010, 10, 131–144. [Google Scholar] [CrossRef]
- Anitha, M.; Reichardt, F.; Tabatabavakili, S.; Nezami, B.G.; Chassaing, B.; Mwangi, S.; Vijay-Kumar, M.; Gewirtz, A.; Srinivasan, S. Intestinal Dysbiosis Contributes to the Delayed Gastrointestinal Transit in High-Fat Diet Fed Mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 328–339. [Google Scholar] [CrossRef]
- Hörmann, N.; Brandão, I.; Jäckel, S.; Ens, N.; Lillich, M.; Walter, U.; Reinhardt, C. Gut Microbial Colonization Orchestrates TLR2 Expression, Signaling and Epithelial Proliferation in the Small Intestinal Mucosa. PLoS ONE 2014, 9, e113080. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, H.; Yao, H.; Wei, Q.; Mao, X.-M.; Jiang, T.; Xiang, J.; Dila, N. Expression and Activity of the TLR4/NF-ΚB Signaling Pathway in Mouse Intestine Following Administration of a Short-Term High-Fat Diet. Exp. Ther. Med. 2013, 6, 635–640. [Google Scholar] [CrossRef]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–688. [Google Scholar] [CrossRef]
- Price, A.E.; Shamardani, K.; Lugo, K.A.; Deguine, J.; Roberts, A.W.; Lee, B.L.; Barton, G.M. A Map of Toll-like Receptor Expression in the Intestinal Epithelium Reveals Distinct Spatial, Cell Type-Specific, and Temporal Patterns. Immunity 2018, 49, 560–575.e6. [Google Scholar] [CrossRef]
- Seguella, L.; Pesce, M.; Capuano, R.; Casano, F.; Pesce, M.; Corpetti, C.; Vincenzi, M.; Maftei, D.; Lattanzi, R.; Del Re, A.; et al. High-Fat Diet Impairs Duodenal Barrier Function and Elicits Glia-Dependent Changes along the Gut-Brain Axis That Are Required for Anxiogenic and Depressive-like Behaviors. J. Neuroinflammation 2021, 18, 115. [Google Scholar] [CrossRef]
- Arciszewski, M.B.; Sand, E.; Ekblad, E. Vasoactive Intestinal Peptide Rescues Cultured Rat Myenteric Neurons from Lipopolysaccharide Induced Cell Death. Regul. Pept. 2008, 146, 218–223. [Google Scholar] [CrossRef]
- Paveljšek, D.; Ivičak-Kocjan, K.; Treven, P.; Benčina, M.; Jerala, R.; Rogelj, I. Distinctive Probiotic Features Share Common TLR2-Dependent Signalling in Intestinal Epithelial Cells. Cell. Microbiol. 2021, 23, e13264. [Google Scholar] [CrossRef]
- Ou, Y.; Liu, R.; Wei, N.; Li, X.; Qiang, O.; Huang, W.; Tang, C. Effects of Octreotide on Nitric Oxide Synthase Expression in the Small Intestine of High Fat Diet-Induced Obese Rats. Obes. Res. Clin. Pract. 2012, 6, e280–e287. [Google Scholar] [CrossRef]
- Bódi, N.; Szalai, Z.; Bagyánszki, M. Nitrergic Enteric Neurons in Health and Disease—Focus on Animal Models. Int. J. Mol. Sci. 2019, 20, 2003. [Google Scholar] [CrossRef]
- Ralston, J.C.; Lyons, C.L.; Kennedy, E.B.; Kirwan, A.M.; Roche, H.M. Fatty Acids and NLRP3 Inflammasome–Mediated Inflammation in Metabolic Tissues. Annu. Rev. Nutr. 2017, 37, 77–102. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 Inflammasome Instigates Obesity-Induced Inflammation and Insulin Resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Muñoz-Planillo, R.; Núñez, G. Sensing and Reacting to Microbes through the Inflammasomes. Nat. Immunol. 2012, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef]
- An, J.; Zhao, X.; Wang, Y.; Noriega, J.; Gewirtz, A.T.; Zou, J. Western-Style Diet Impedes Colonization and Clearance of Citrobacter Rodentium. PLoS Pathog. 2021, 17, e1009497. [Google Scholar] [CrossRef]
- Reichardt, N.; Vollmer, M.; Holtrop, G.; Farquharson, F.M.; Wefers, D.; Bunzel, M.; Duncan, S.H.; Drew, J.E.; Williams, L.M.; Milligan, G.; et al. Specific Substrate-Driven Changes in Human Faecal Microbiota Composition Contrast with Functional Redundancy in Short-Chain Fatty Acid Production. ISME J. 2018, 12, 610–622. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Johansson, M.E.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New Developments in Goblet Cell Mucus Secretion and Function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef]
- Jensen, B.A.H.; Nielsen, T.S.; Fritzen, A.M.; Holm, J.B.; Fjære, E.; Serup, A.K.; Borkowski, K.; Risis, S.; Pærregaard, S.I.; Søgaard, I.; et al. Dietary Fat Drives Whole-Body Insulin Resistance and Promotes Intestinal Inflammation Independent of Body Weight Gain. Metabolism 2016, 65, 1706–1719. [Google Scholar] [CrossRef]
- Nakanishi, T.; Fukui, H.; Wang, X.; Nishiumi, S.; Yokota, H.; Makizaki, Y.; Tanaka, Y.; Ohno, H.; Tomita, T.; Oshima, T.; et al. Effect of a High-Fat Diet on the Small-Intestinal Environment and Mucosal Integrity in the Gut-Liver Axis. Cells 2021, 10, 3168. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.C.; Matheus, V.A.; Oliveira, R.B.; Tada, S.F.S.; Collares-Buzato, C.B. High-Fat Diet Induces Disruption of the Tight Junction-Mediated Paracellular Barrier in the Proximal Small Intestine Before the Onset of Type 2 Diabetes and Endotoxemia. Dig. Dis. Sci. 2021, 66, 3359–3374. [Google Scholar] [CrossRef] [PubMed]
- Selmin, O.I.; Papoutsis, A.J.; Hazan, S.; Smith, C.; Greenfield, N.; Donovan, M.G.; Wren, S.N.; Doetschman, T.C.; Snider, J.M.; Snider, A.J.; et al. N-6 High Fat Diet Induces Gut Microbiome Dysbiosis and Colonic Inflammation. Int. J. Mol. Sci. 2021, 22, 6919. [Google Scholar] [CrossRef] [PubMed]
- Aliluev, A.; Tritschler, S.; Sterr, M.; Oppenländer, L.; Hinterdobler, J.; Greisle, T.; Irmler, M.; Beckers, J.; Sun, N.; Walch, A.; et al. Diet-Induced Alteration of Intestinal Stem Cell Function Underlies Obesity and Prediabetes in Mice. Nat. Metab. 2021, 3, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.B.; Canuto, L.P.; Collares-Buzato, C.B. Intestinal Luminal Content from High-Fat-Fed Prediabetic Mice Changes Epithelial Barrier Function in Vitro. Life Sci. 2019, 216, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Olson, C.A.; Davis, E.; Tsan, L.; Chen, Y.-W.; Schade, R.; Liu, C.; Suarez, A.; Jones, R.B.; de La Serre, C.; et al. Gut Microbial Taxa Elevated by Dietary Sugar Disrupt Memory Function. Transl. Psychiatry 2021, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Togo, J.; Hu, S.; Li, M.; Niu, C.; Speakman, J.R. Impact of Dietary Sucrose on Adiposity and Glucose Homeostasis in C57BL/6J Mice Depends on Mode of Ingestion: Liquid or Solid. Mol. Metab. 2019, 27, 22–32. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef]
- Matziouridou, C.; Rocha, S.D.C.; Haabeth, O.A.; Rudi, K.; Carlsen, H.; Kielland, A. INOS- and NOX1-Dependent ROS Production Maintains Bacterial Homeostasis in the Ileum of Mice. Mucosal Immunol. 2018, 11, 774–784. [Google Scholar] [CrossRef]
- Qu, X.-W.; Wang, H.; De Plaen, I.G.; Rozenfeld, R.A.; Hsueh, W. Neuronal Nitric Oxide Synthase (NOS) Regulates the Expression of Inducible NOS in Rat Small Intestine via Modulation of Nuclear Factor Kappa B. FASEB J. 2001, 15, 439–446. [Google Scholar] [CrossRef]
- Byndloss, M.X.; Bäumler, A.J. The Germ-Organ Theory of Non-Communicable Diseases. Nat. Rev. Microbiol. 2018, 16, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia Drives Intestinal Barrier Dysfunction and Risk for Enteric Infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Kim, K.-A.; Gu, W.; Lee, I.-A.; Joh, E.-H.; Kim, D.-H. High Fat Diet-Induced Gut Microbiota Exacerbates Inflammation and Obesity in Mice via the TLR4 Signaling Pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated Fatty Acids Activate TLR-Mediated Proinflammatory Signaling Pathways [S]. J. Lipid Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef]
- Tomas, J.; Mulet, C.; Saffarian, A.; Cavin, J.-B.; Ducroc, R.; Regnault, B.; Kun Tan, C.; Duszka, K.; Burcelin, R.; Wahli, W.; et al. High-Fat Diet Modifies the PPAR-γ Pathway Leading to Disruption of Microbial and Physiological Ecosystem in Murine Small Intestine. Proc. Natl. Acad. Sci. USA 2016, 113, E5934–E5943. [Google Scholar] [CrossRef]
- Hu, S.; Togo, J.; Wang, L.; Wu, Y.; Yang, D.; Xu, Y.; Li, L.; Li, B.; Li, M.; Li, J.; et al. Effects of Dietary Macronutrients and Body Composition on Glucose Homeostasis in Mice. Natl. Sci. Rev. 2021, 8, nwaa177. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Hereu, M.; Atienza, L.; Casas, J.; Jáuregui, O.; Amézqueta, S.; Dasilva, G.; Medina, I.; Nogués, M.R.; Romeu, M.; et al. Mechanistically Different Effects of Fat and Sugar on Insulin Resistance, Hypertension, and Gut Microbiota in Rats. Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E552–E563. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Bäumler, A.J. Dysbiotic Proteobacteria Expansion: A Microbial Signature of Epithelial Dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- Grubišić, V.; Gulbransen, B.D. Enteric Glia: The Most Alimentary of All Glia. J. Physiol. 2017, 595, 557–570. [Google Scholar] [CrossRef]
- MacEachern, S.J.; Patel, B.A.; Keenan, C.M.; Dicay, M.; Chapman, K.; McCafferty, D.-M.; Savidge, T.C.; Beck, P.L.; MacNaughton, W.K.; Sharkey, K.A. Inhibiting Inducible Nitric Oxide Synthase in Enteric Glia Restores Electrogenic Ion Transport in Mice With Colitis. Gastroenterology 2015, 149, 445–455.e3. [Google Scholar] [CrossRef] [PubMed]
- De Marco, P.; Henriques, A.C.; Azevedo, R.; Sá, S.I.; Cardoso, A.; Fonseca, B.; Barbosa, J.; Leal, S. Gut Microbiome Composition and Metabolic Status Are Differently Affected by Early Exposure to Unhealthy Diets in a Rat Model. Nutrients 2021, 13, 3236. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nemoto, Y.; Takei, Y.; Morikawa, R.; Oshima, S.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; Nakamura, T.; Stutte, S.; et al. High-Fat Diet-Derived Free Fatty Acids Impair the Intestinal Immune System and Increase Sensitivity to Intestinal Epithelial Damage. Biochem. Biophys. Res. Commun. 2020, 522, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Grasset, E.; Puel, A.; Charpentier, J.; Collet, X.; Christensen, J.E.; Tercé, F.; Burcelin, R. A Specific Gut Microbiota Dysbiosis of Type 2 Diabetic Mice Induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell Metab. 2017, 25, 1075–1090.e5. [Google Scholar] [CrossRef]
- Nogueira, S.; Garcez, F.; Sá, S.; Moutinho, L.C.; Cardoso, A.; Soares, R.; Fonseca, B.M.; Leal, S. Early Unhealthy Eating Habits Underlie Morpho-Functional Changes in the Liver and Adipose Tissue in Male Rats. Histochem. Cell Biol. 2022, 157, 657–669. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, S.; Barbosa, J.; Faria, J.; Sá, S.I.; Cardoso, A.; Soares, R.; Fonseca, B.M.; Leal, S. Unhealthy Diets Induce Distinct and Regional Effects on Intestinal Inflammatory Signalling Pathways and Long-Lasting Metabolic Dysfunction in Rats. Int. J. Mol. Sci. 2022, 23, 10984. https://doi.org/10.3390/ijms231810984
Nogueira S, Barbosa J, Faria J, Sá SI, Cardoso A, Soares R, Fonseca BM, Leal S. Unhealthy Diets Induce Distinct and Regional Effects on Intestinal Inflammatory Signalling Pathways and Long-Lasting Metabolic Dysfunction in Rats. International Journal of Molecular Sciences. 2022; 23(18):10984. https://doi.org/10.3390/ijms231810984
Chicago/Turabian StyleNogueira, Sofia, Joana Barbosa, Juliana Faria, Susana I. Sá, Armando Cardoso, Raquel Soares, Bruno M. Fonseca, and Sandra Leal. 2022. "Unhealthy Diets Induce Distinct and Regional Effects on Intestinal Inflammatory Signalling Pathways and Long-Lasting Metabolic Dysfunction in Rats" International Journal of Molecular Sciences 23, no. 18: 10984. https://doi.org/10.3390/ijms231810984
APA StyleNogueira, S., Barbosa, J., Faria, J., Sá, S. I., Cardoso, A., Soares, R., Fonseca, B. M., & Leal, S. (2022). Unhealthy Diets Induce Distinct and Regional Effects on Intestinal Inflammatory Signalling Pathways and Long-Lasting Metabolic Dysfunction in Rats. International Journal of Molecular Sciences, 23(18), 10984. https://doi.org/10.3390/ijms231810984