The Efficacy and Biopharmaceutical Properties of a Fixed-Dose Combination of Disulfiram and Benzyl Benzoate
Abstract
1. Introduction
2. Results and Discussion
2.1. Analytical Method Validation
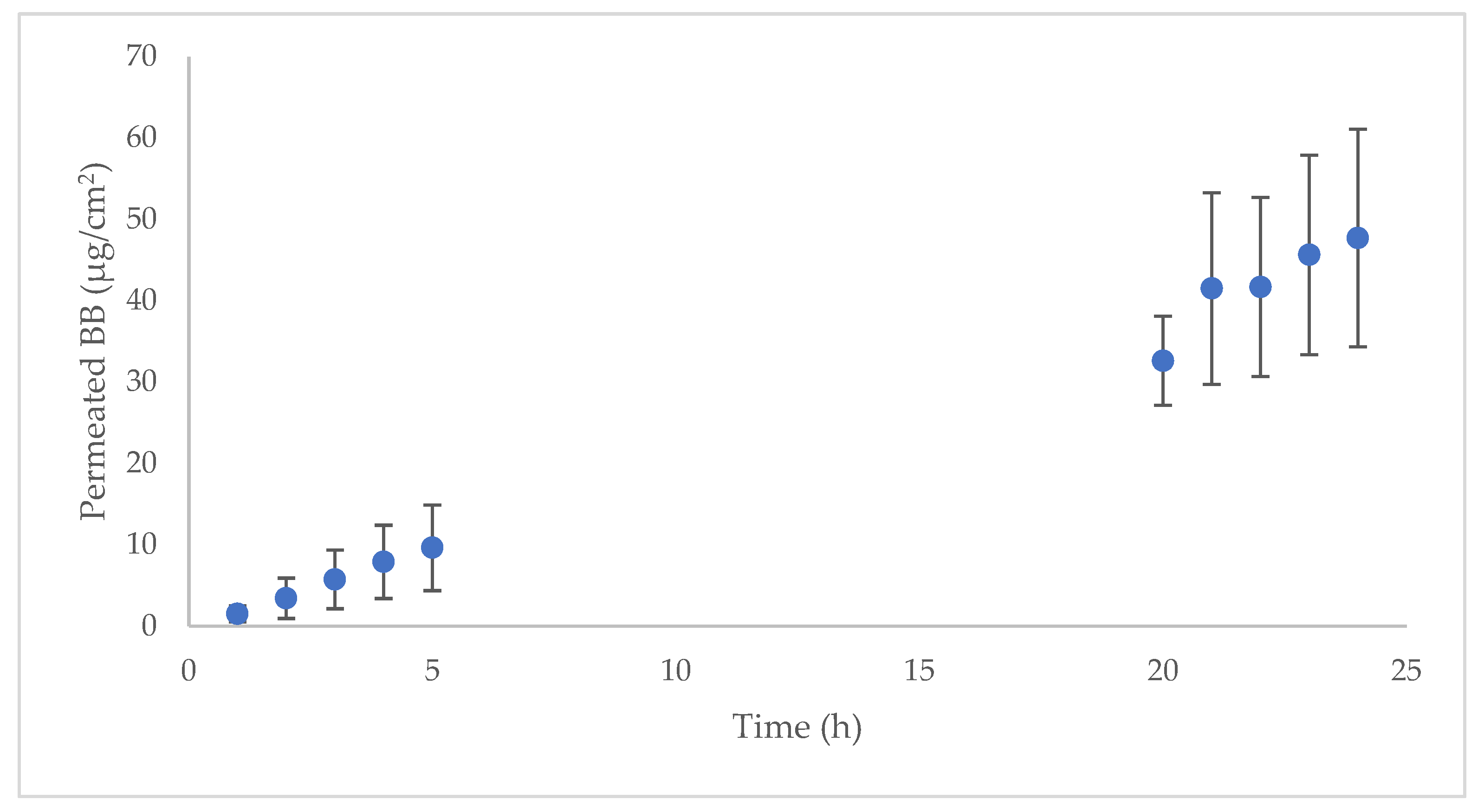
2.2. Human Skin Permeation
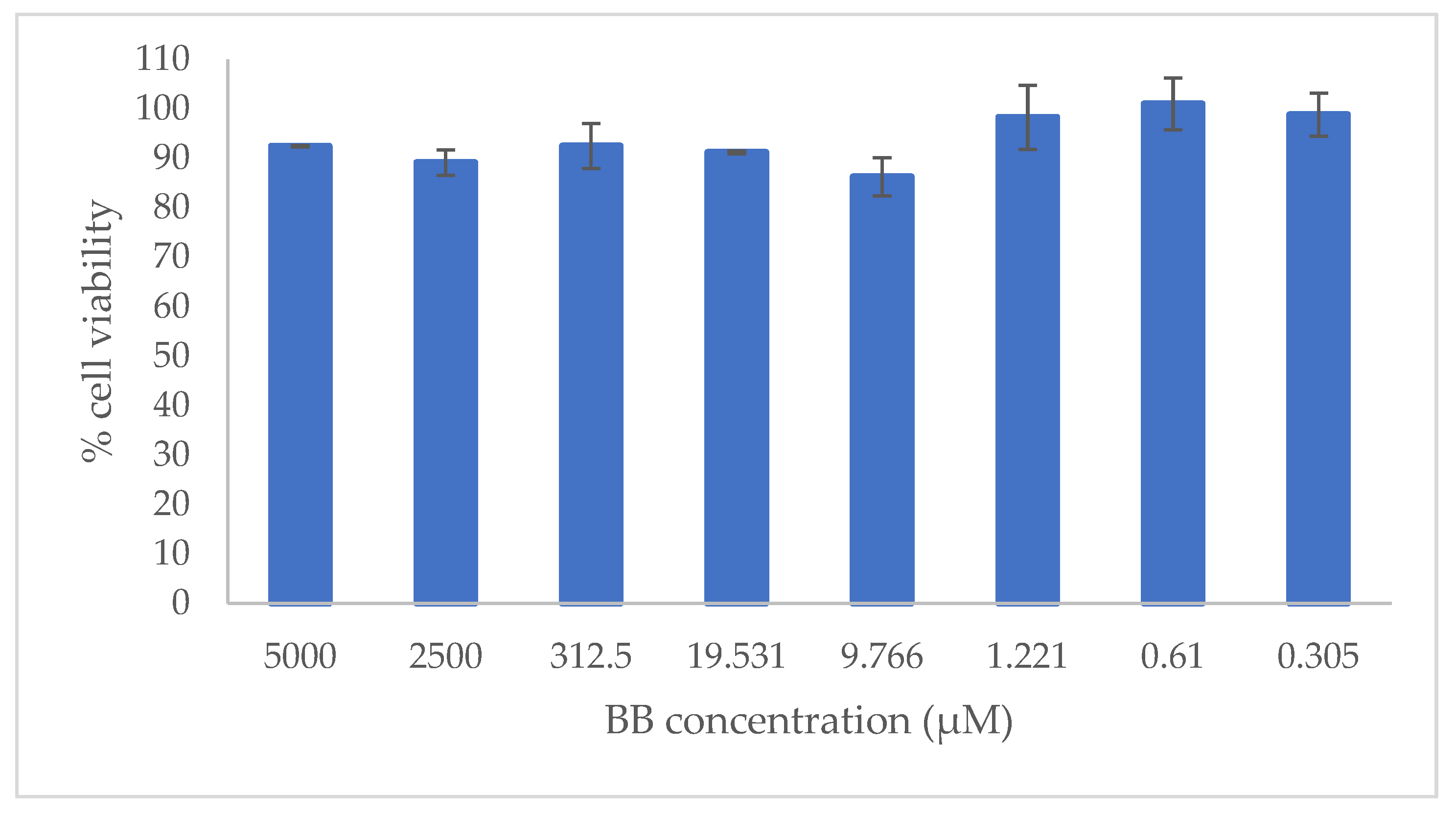
2.3. In Vitro Cytotoxicity Evaluation
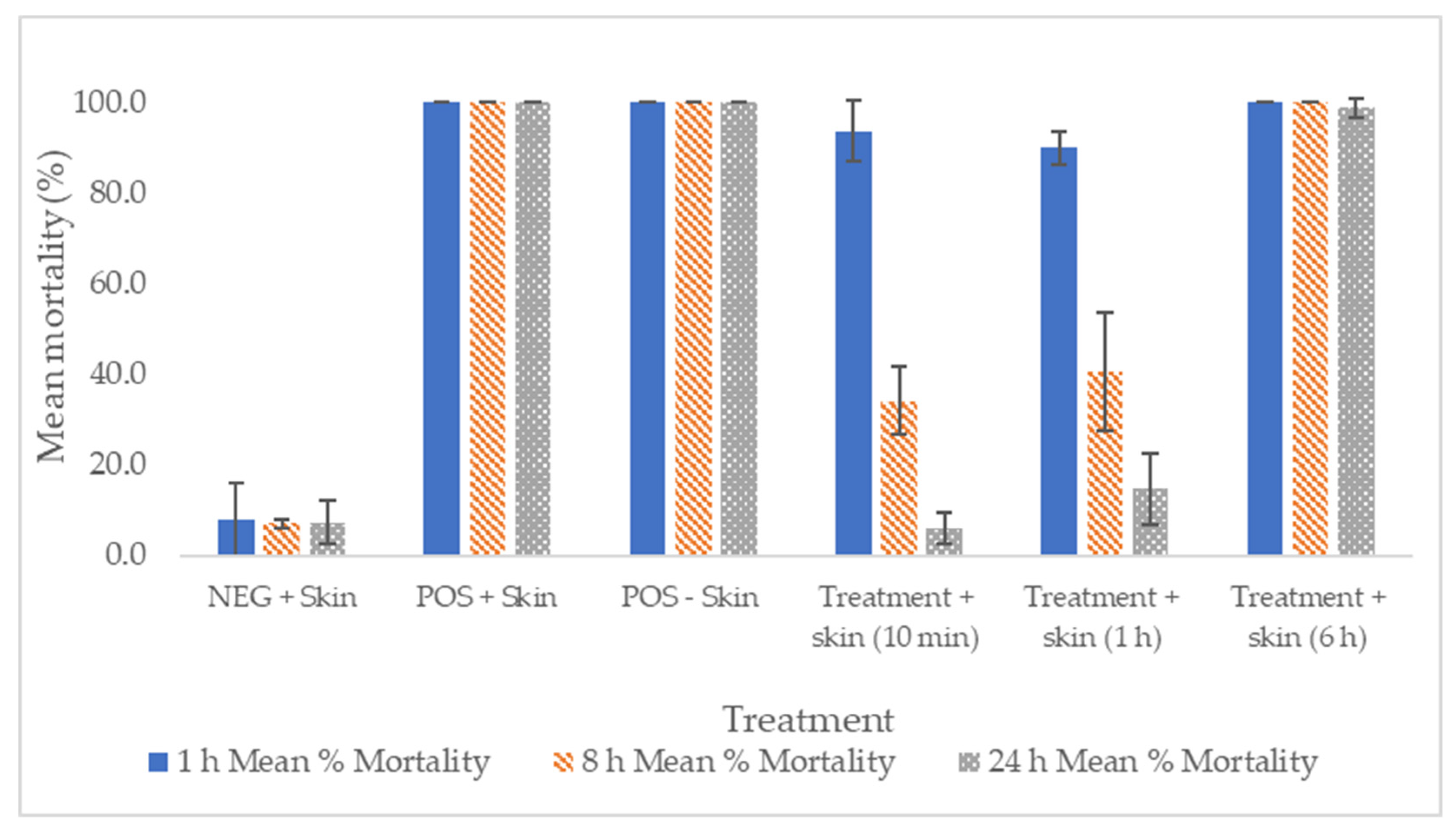
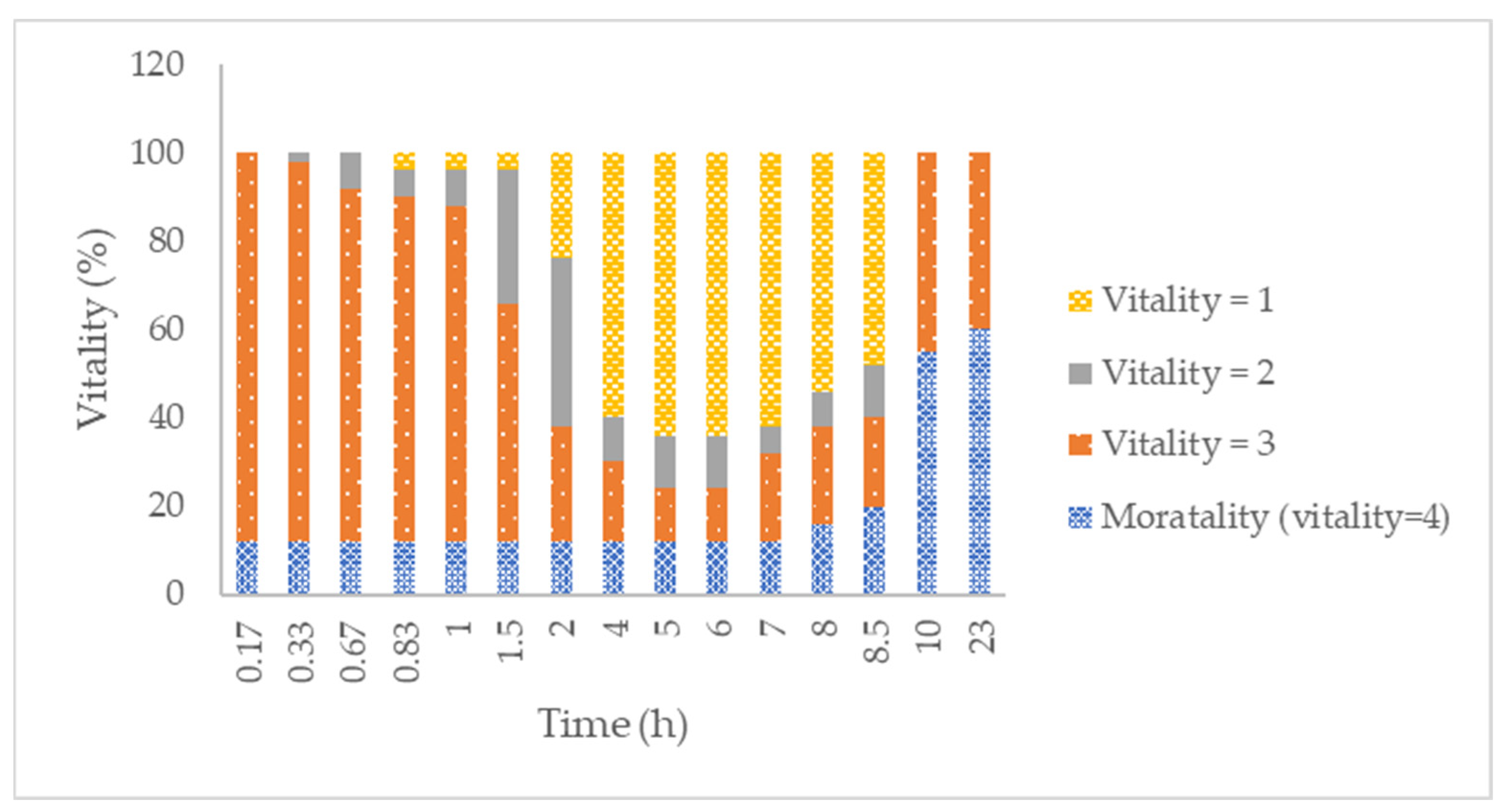
2.4. In Vitro and Ex Vivo Efficacy against Scabies
2.5. In Vitro Efficacy against Adults and Eggs of the Human BH-RL Strain of Lice, Resistant to Both Permethrin and Malathion
3. Materials and Methods
3.1. Materials
3.2. High-Performance Liquid Chromatography Quantification and Validation of Disulfiram and Benzyl Benzoate
3.3. Ex Vivo Skin Absorption Experiment with Human Skin
3.4. In Vitro Cytotoxicity Evaluation
3.5. In Vitro and Ex Vivo Efficacy against Scabies
3.6. In Vitro Efficacy against Permethrin-Resistant Head Lice and Its Eggs
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dressler, C.; Rosumeck, S.; Sunderkötter, C.; Werner, R.N.; Nast, A. The Treatment of Scabies: A Systematic Review of Randomized Controlled Trials. Dtsch. Arztebl. Int. 2016, 113, 757. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fitzgerald, D.; Grainger, R.J.; Reid, A. Interventions for Preventing the Spread of Infestation in Close Contacts of People with Scabies. Cochrane Database Syst. Rev. 2014, 2014, CD009943. [Google Scholar] [CrossRef] [PubMed]
- Rosumeck, S.; Nast, A.; Dressler, C. Ivermectin and Permethrin for Treating Scabies. Cochrane Database Syst. Rev. 2018, 2018, CD012994. [Google Scholar] [CrossRef] [PubMed]
- Kühne, A.; Gilsdorf, A. Infectious Disease Outbreaks in Centralized Homes for Asylum Seekers in Germany from 2004–2014. Bundesgesundheitsblatt-Gesundheitsforsch.-Gesundh. 2016, 59, 570–577. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strong, M.; Johnstone, P.; Group, C.I.D. Interventions for Treating Scabies. Cochrane Database Syst. Rev. 2007, 2007, CD000320. [Google Scholar] [CrossRef]
- Gunning, K.; College, U.; City, S.L. AAFP: Lice and Scabies. Am. Fam. Physician 2019, 99, 635–642. [Google Scholar]
- Arlian, L.G.; Morgan, M.S. A Review of Sarcoptes Scabiei: Past, Present and Future. Parasites Vectors 2017, 10, 297. [Google Scholar] [CrossRef]
- Salavastru, C.M.; Chosidow, O.; Boffa, M.J.; Janier, M.; Tiplica, G.S. European Guideline for the Management of Scabies. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1248–1253. [Google Scholar] [CrossRef]
- Mang, R.; Kremer, A.; Lehmann, P.; Assmann, T. Scabies-Clinical Resistance to Permethrin Therapy: Case Reports and a Critical Discussion of Current Treatment Recommendations. Der Hautarzt 2021, 72, 595–599. [Google Scholar] [CrossRef]
- Meyersburg, D.; Kaiser, A.; Bauer, J.W. Loss of Efficacy of Topical 5% Permethrin for Treating Scabies: An Austrian Single-Center Study. J. Dermatol. Treat. 2022, 33, 774–777. [Google Scholar] [CrossRef]
- Elsner, E.; Uhlmann, T.; Krause, S.; Hartmann, R. Increase of Scabies and Therapy Resistance among German Military Personnel: An 8-Year Follow-up Study in the Department of Dermatology of the Armed Forces Hospital Berlin, Germany (2012–2019). Hautarzt 2020, 71, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Zargari, O.; Aghazadeh, N.; Moeineddin, F. Clinical Applications of Topical Ivermectin in Dermatology. Dermatol. Online J. 2016, 22, 9. [Google Scholar] [CrossRef]
- Soerensen, C.A.S.; Pallesen, K.A.U.; Munk, N.T.; Vestergaard, C. Eleven Danish Patients Diagnosed with Scabies and Treated with Tenutex®. Clin. Case Rep. 2021, 9, 1688–1690. [Google Scholar] [CrossRef] [PubMed]
- de Pablo Màrquez, B. Actualización En Pediculosis Capitis. Med. Fam. Semer. 2019, 45, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Fong, J.H.S.; Pinto-Rojas, A. Pediculosis Capitis. J. Pediatr. Health Care 2005, 19, 369–373. [Google Scholar] [CrossRef]
- Salavastru, C.M.; Chosidow, O.; Janier, M.; Tiplica, G.S. European Guideline for the Management of Pediculosis Pubis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1425–1428. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Lam, J.M.; Leong, K.F.; Barankin, B.; Hon, K.L. Paediatrics: How to Manage Pediculosis Capitis. Drugs Context 2022, 11. [Google Scholar] [CrossRef]
- Durand, R.; Bouvresse, S.; Berdjane, Z.; Izri, A.; Chosidow, O.; Clark, J.M. Insecticide Resistance in Head Lice: Clinical, Parasitological and Genetic Aspects. Clin. Microbiol. Infect. 2012, 18, 338–344. [Google Scholar] [CrossRef]
- Burgess, I.F.; Kay, K.; Burgess, N.A.; Brunton, E.R. Soya Oil-Based Shampoo Superior to 0.5% Permethrin Lotion for Head Louse Infestation. Med. Devices Evid. Res. 2011, 4, 35–42. [Google Scholar] [CrossRef]
- Kristensen, M.; Knorr, M.; Rasmussen, A.-M.; Jespersen, J.B. Survey of Permethrin and Malathion Resistance in Human Head Lice Populations from Denmark. J. Med. Entomol. 2006, 43, 533–538. [Google Scholar] [CrossRef]
- Izri, M.A.; Brière, C. Premiers Cas de Résistance de Pediculus Capitis Linné 1758 Au Malathion En France. Presse Med. 1995, 24, 1444. [Google Scholar] [PubMed]
- Downs, A.M.R.; Stafford, K.A.; Harvey, I.; Coles, G.C. Evidence for Double Resistance to Permethrin and Malathion in Head Lice. Br. J. Dermatol. 1999, 141, 508–511. [Google Scholar] [CrossRef]
- Hunter, J.A.; Barker, S.C. Susceptibility of Head Lice (Pediculus Humanus Capitis) to Pediculicides in Australia. Parasitol. Res. 2003, 90, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Kucirka, S.A.; Parish, L.C.; Witkowski, J.A. The Story of Lindane Resistance and Head Lice. Int. J. Dermatol. 1983, 22, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Burgess, I.F.; Brown, C.M.; Peock, S.; Kaufman, J. Head Lice Resistant to Pyrethroid Insecticides in Britain. BMJ 1995, 311, 752. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, D.M.; Knipple, D.C. The Molecular Biology of Knockdown Resistance to Pyrethroid Insecticides. Insect Biochem. Mol. Biol. 2003, 33, 563–577. [Google Scholar] [CrossRef]
- Tenutex®-FASS Allmänhet. Available online: https://www.fass.se/LIF/product?userType=2&nplId=19821022000063&docType=6&focus=tab_produktresume&autoScroll=true&scrollPosition=400 (accessed on 18 May 2022).
- Landegren, J.; Borglund, E.; Storgards, K. Treatment of Scabies with Disulfiram and Benzyl Benzoate Emulsion: A Controlled Study. Acta Derm. Venereol. 1979, 59, 274–276. [Google Scholar]
- Tenurid-FASS Allmänhet. Available online: https://www.fass.se/LIF/product?userType=2&nplId=AP_00003680 (accessed on 18 May 2022).
- Karamanakos, P.N.; Pappas, P.; Boumba, V.A.; Thomas, C.; Malamas, M.; Vougiouklakis, T.; Marselos, M. Pharmaceutical Agents Known to Produce Disulfiram-like Reaction: Effects on Hepatic Ethanol Metabolism and Brain Monoamines. Int. J. Toxicol. 2007, 26, 423–432. [Google Scholar] [CrossRef]
- Frazier, K.R.; Moore, J.A.; Long, T.E. Antibacterial Activity of Disulfiram and Its Metabolites. J. Appl. Microbiol. 2019, 126, 79–86. [Google Scholar] [CrossRef]
- Das, S.; Garg, T.; Chopra, S.; Dasgupta, A. Repurposing Disulfiram to Target Infections Caused by Non-Tuberculous Mycobacteria. J. Antimicrob. Chemother. 2019, 74, 1317–1322. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wu, C.; Wang, L.; Chen, Z.S.; Cui, W. The Combination of Disulfiram and Copper for Cancer Treatment. Drug Discov. Today 2020, 25, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, E.; Rohondia, S.; Khan, R.; Dou, Q.P. Repurposing Disulfiram as An Anti-Cancer Agent: Updated Review on Literature and Patents. Recent Pat. Anticancer. Drug Discov. 2019, 14, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Brinck-Lindroth, G.; Lundqvist, L.; Nilsson, A. Control of the Human Head Louse with Disulfiram and Benzyl Benzoate Emulsions. Acta Derm. Venereol. 1984, 64, 325–330. [Google Scholar] [PubMed]
- Agency, E.M. Validation of Analytical Procedures: Text and Methodology. ICH Harmon. Tripart. Guidel. 2011, 20, 278. [Google Scholar]
- European Commission The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation. Sccs 2016, 1564, 151.
- Lipinski, C.A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information Benzyl Benzoate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Benzyl-benzoate (accessed on 18 May 2022).
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Benzyl Alcohol, Benzoic Acid and Its Salts, and Benzyl Benzoate. Int. J. Toxicol. 2017, 36, 5S–30S. [Google Scholar] [CrossRef]
- Jimbo, Y. Penetration of Fragrance Compounds through Human Epidermis. J. Dermatol. 1983, 10, 229–239. [Google Scholar] [CrossRef]
- Bronaugh, R.L.; Wester, R.C.; Bucks, D.; Maibach, H.I.; Sarason, R. In Vivo Percutaneous Absorption of Fragrance Ingredients in Rhesus Monkeys and Humans. Food Chem. Toxicol. 1990, 28, 369–373. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information Disulfiram: Chemical and Physical Properties. PubChem Compd. Summ. CID 3117 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Disulfiram (accessed on 18 May 2022).
- Qian, L.; Cantrell, F.L. Disulfiram. Encycl. Toxicol. Third Ed. 2021, 208–209. [Google Scholar] [CrossRef]
- Niles, A.L.; Moravec, R.A.; Riss, T.L. In Vitro Viability and Cytotoxicity Testing and Same-Well Multi-Parametric Combinations for High Throughput Screening. Curr. Chem. Genom. 2009, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.F.; Myerscough, M.R.; Currie, B.J. Studies in Vitro on the Relative Efficacy of Current Acaricides for Sarcoptes Scabiei Var. Hominis. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 92–96. [Google Scholar] [CrossRef]
- Currie, B.J.; Harumal, P.; McKinnon, M.; Walton, S.F. First Documentation of in Vivo and in Vitro Ivermectin Resistance in Sarcoptes Scabiei. Clin. Infect. Dis. 2004, 39, e8–e12. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Candy, K.; Melloul, E.; Bernigaud, C.; Chai, L.; Darmon, C.; Durand, R.; Botterel, F.; Chosidow, O.; Izri, A.; et al. In Vitro Activity of Ten Essential Oils against Sarcoptes Scabiei. Parasites Vectors 2016, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Andriantsoanirina, V.; Guillot, J.; Ratsimbason, M.; Mekhloufi, G.; Randriamialinoro, F.; Ranarivelo, L.; Ariey, F.; Durand, R. In Vitro Efficacy of Essential Oils against Sarcoptes Scabiei. Sci. Rep. 2022, 12, 7176. [Google Scholar] [CrossRef]
- Morgan, M.S.; Arlian, L.G.; Rider, S.D.; Grunwald, W.C.; Cool, D.R. A Proteomic Analysis of Sarcoptes Scabiei (Acari: Sarcoptidae). J. Med. Entomol. 2016, 53, 553–561. [Google Scholar] [CrossRef]
- Ploemen, J.P.H.T.M.; Van Iersel, M.L.P.S.; Wormhoudt, L.W.; Commandeur, J.N.M.; Vermeulen, N.P.E.; Van Bladeren, P.J. In Vitro Inhibition of Rat and Human Glutathione S-Transferase Isoenzymes by Disulfiram and Diethyldithiocarbamate. Biochem. Pharmacol. 1996, 52, 197–204. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Peng, X.H.; Nandigama, K.; Tekle, S.; Ambudkar, S.V. The Molecular Basis of the Action of Disulfiram as a Modulator of the Multidrug Resistance-Linked ATP Binding Cassette Transporters MDR1 (ABCB1) and MRP1 (ABCC1). Mol. Pharmacol. 2004, 65, 675–684. [Google Scholar] [CrossRef]
- Jay, D. Inhibition of Membrane-Bound Succinate Dehydrogenase by Disulfiram. J. Bioenerg. Biomembr. 1991, 23, 335–343. [Google Scholar] [CrossRef]
- Subahar, R.; Susanto, L.; Aidilla, R.; Aulia, A.P.; Yulhasri, Y.; Winita, R.; Lubis, N.S.; Sari, I.P. In Vitro Experiments of Pediculus Humanus Capitis (Phthiraptera: Pediculidae) Resistance to Permethrin and 6-Paradol in East Jakarta: Detoxification Enzyme Activity and Electron Microscopic Changes in Lice. Vet. World 2021, 14, 3065–3075. [Google Scholar] [CrossRef]
- Gao, J.R.; Yoon, K.S.; Frisbie, R.K.; Coles, G.C.; Clark, J.M. Esterase-Mediated Malathion Resistance in the Human Head Louse, Pediculus Capitis (Anoplura: Pediculidae). Pestic. Biochem. Physiol. 2006, 85, 28–37. [Google Scholar] [CrossRef]
- Shirley, D.A.; Sharma, I.; Warren, C.A.; Moonah, S. Drug Repurposing of the Alcohol Abuse Medication Disulfiram as an Anti-Parasitic Agent. Front. Cell. Infect. Microbiol. 2021, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.S.; Strycharz, J.P.; Gao, J.R.; Takano-Lee, M.; Edman, J.D.; Clark, J.M. An Improved in Vitro Rearing System for the Human Head Louse Allows the Determination of Resistance to Formulated Pediculicides. Pestic. Biochem. Physiol. 2006, 86, 195–202. [Google Scholar] [CrossRef]
- Heukelbach, J.; Wolf, D.; Clark, J.M.; Dautel, H.; Roeschmann, K. High Efficacy of a Dimeticone-Based Pediculicide Following a Brief Application: In Vitro Assays and Randomized Controlled Investigator-Blinded Clinical Trial. BMC Dermatol. 2019, 19, 14. [Google Scholar] [CrossRef]
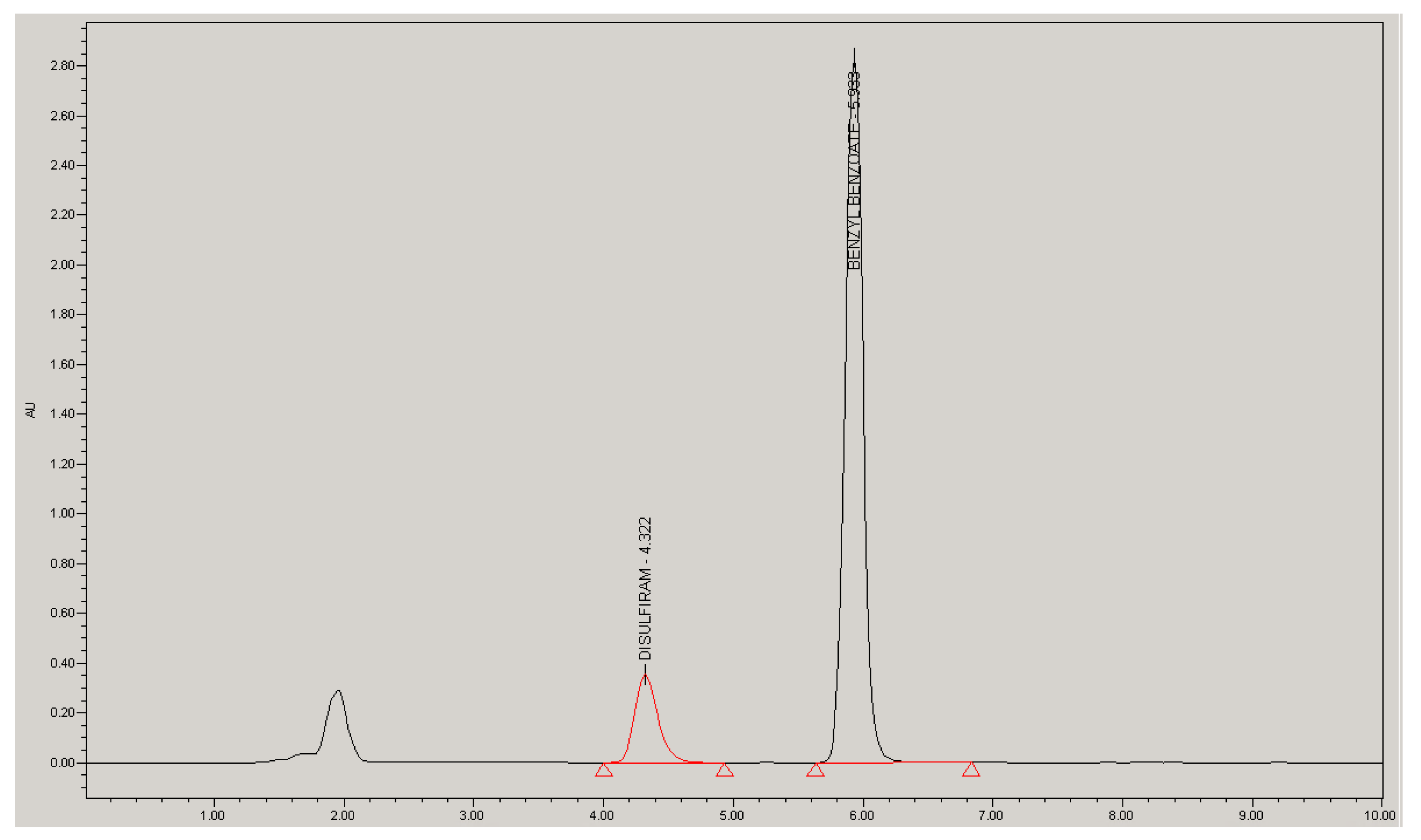

| Parameter | D | BB | Specification | |
|---|---|---|---|---|
| Range | 0.08–15.70 µg/mL | 0.90–182.70 µg/mL | - | |
| Linearity | Slope | 137,322.71 (p < 0.05) | 84,127.02 (p < 0.05) | p < 0.05 |
| Intercept | –7049.71 (p = 0.04) | –16,389.67 (p > 0.05) | p > 0.05 | |
| R2 | 0.99980 | 0.99997 | >0.990 | |
| CV response factor | 8.71% | 0.71% | <10% | |
| Homoskedasticity (Levene’s test) | p = 0.31 | p = 0.83 | p > 0.05 | |
| Residual plot | No tendency | No tendency | No tendency | |
| Concentration Level | Sample ID | Disulfiram | ||
|---|---|---|---|---|
| 0.08 μg/mL | Analyst 1 day 1 Analyst 2 day 2 | Mean: 101.20% CV = 2.52% Mean:105.00% CV = 2.54% | Mean: 103.12% CV = 3.03% | Mean: 104.60% CV: 2.61% |
| 0.38 μg/mL | Analyst 1 day 1 | Mean:104.20% CV = 1.92% | Mean: 103.95% CV = 1.37% | |
| Analyst 2 day 2 | Mean: 103.70% CV = 0.94% | |||
| 6.35 μg/mL | Analyst 1 day 1 | Mean: 104.80% CV = 0.27% | Mean: 106.62% CV = 2.19% | |
| Analyst 2 day 2 | Mean: 108.40% CV = 1.84% | |||
| Concentration Level | Sample Id. | Benzyl Benzoate | ||
|---|---|---|---|---|
| 0.89 μg/mL | Analyst 1 day 1 Analyst 2 day 2 | Mean: 91.70% CV = 1.98% Mean: 90.30% CV = 1.81% | Mean: 91.02% CV = 1.88% | Mean: 95.80% CV = 4.01% |
| 3.57 μg/mL | Analyst 1 day 1 Analyst 2 day 2 | Mean: 96.30% CV = 0.37% Mean: 97.60% CV = 0.49% | Mean: 98.90% CV = 0.59% | |
| 71.42 μg/mL | Analyst 1 day 1 | Mean: 104.80% CV = 0.27% | Mean: 100.10% CV = 0.86% | |
| Analyst 2 day 2 | Mean: 108.40% CV = 1.84% | |||
| Mean | SD | |
|---|---|---|
| Jsup (µg/hcm2) | 1.3014 | 0.3366 |
| R2 | 0.9963 | 0.0067 |
| Kp (cm/h) | 5.78 × 10−6 | 1.50 × 10−6 |
| Tlag (h) | 0.7755 | 0.7059 |
| P (cm/h2) | 3.5978 × 10−5 | 1.3657 × 10−5 |
| Dif (1/h) | 0.1252 | 0.0183 |
| Test Solution | Mean % Mortality | |||
|---|---|---|---|---|
| 0 h | 1 h | 8 h | 24 h | |
| Placebo—thin layer (n = 91 mites) | 0 | 0 | 0 | 0 |
| Placebo—thick layer (n = 88 mites) | 0 | 0 | 0 | 0 |
| Tenutex emulsion—thin layer (n = 91 mites) | 0 | 100 | 100 | 100 |
| Tenutex emulsion—thick layer (n = 90 mites) | 0 | 100 | 100 | 100 |
| Average % Hatchability ± SD | |
|---|---|
| Tenutex, 1 h | 15 ± 18 |
| ddH2O, 1 h | 96 ± 2 |
| Tenutex, 8 h | 0.2 ± 0.4 |
| ddH2O, 8 h | 98 ± 1 |
| Tenutex, 24 h | 0 ± 0 |
| ddH2O, 24 h | 87 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajarin-Reinares, M.; Martinez-Esteve, E.; Pena-Rodríguez, E.; Cañellas-Santos, M.; Bulut, S.; Karabelas, K.; Clauss, A.; Nieto, C.; Mallandrich, M.; Fernandez-Campos, F. The Efficacy and Biopharmaceutical Properties of a Fixed-Dose Combination of Disulfiram and Benzyl Benzoate. Int. J. Mol. Sci. 2022, 23, 10969. https://doi.org/10.3390/ijms231810969
Lajarin-Reinares M, Martinez-Esteve E, Pena-Rodríguez E, Cañellas-Santos M, Bulut S, Karabelas K, Clauss A, Nieto C, Mallandrich M, Fernandez-Campos F. The Efficacy and Biopharmaceutical Properties of a Fixed-Dose Combination of Disulfiram and Benzyl Benzoate. International Journal of Molecular Sciences. 2022; 23(18):10969. https://doi.org/10.3390/ijms231810969
Chicago/Turabian StyleLajarin-Reinares, Maria, Elia Martinez-Esteve, Eloy Pena-Rodríguez, Mariona Cañellas-Santos, Sanja Bulut, Kostas Karabelas, Adam Clauss, Carles Nieto, Mireia Mallandrich, and Francisco Fernandez-Campos. 2022. "The Efficacy and Biopharmaceutical Properties of a Fixed-Dose Combination of Disulfiram and Benzyl Benzoate" International Journal of Molecular Sciences 23, no. 18: 10969. https://doi.org/10.3390/ijms231810969
APA StyleLajarin-Reinares, M., Martinez-Esteve, E., Pena-Rodríguez, E., Cañellas-Santos, M., Bulut, S., Karabelas, K., Clauss, A., Nieto, C., Mallandrich, M., & Fernandez-Campos, F. (2022). The Efficacy and Biopharmaceutical Properties of a Fixed-Dose Combination of Disulfiram and Benzyl Benzoate. International Journal of Molecular Sciences, 23(18), 10969. https://doi.org/10.3390/ijms231810969








