4-Arylthiosemicarbazide Derivatives as Toxoplasmic Aromatic Amino Acid Hydroxylase Inhibitors and Anti-inflammatory Agents
Abstract
:1. Introduction
2. Results
2.1. Rationale
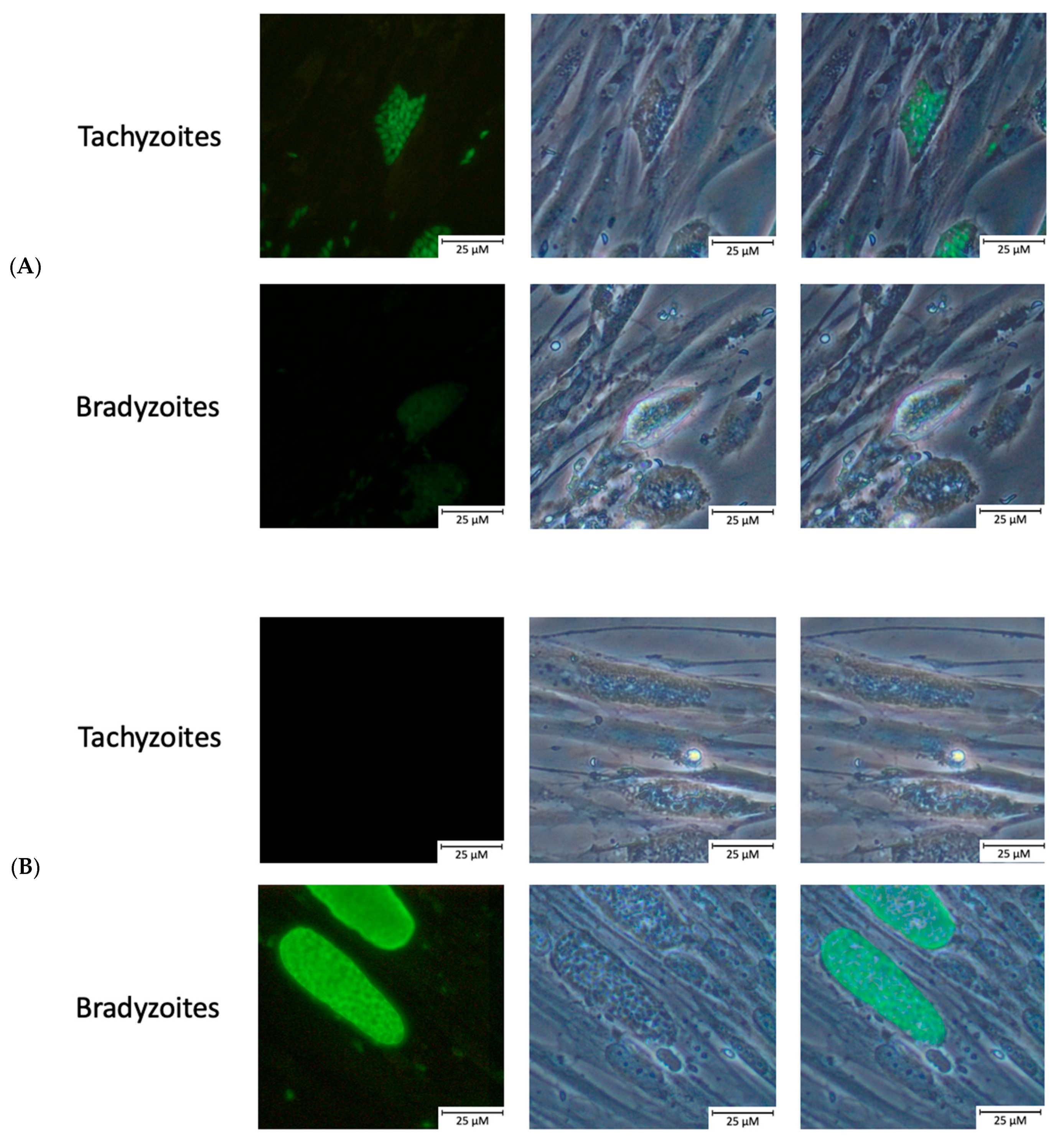
2.2. Confirmation of Bradyzoite Differentiation
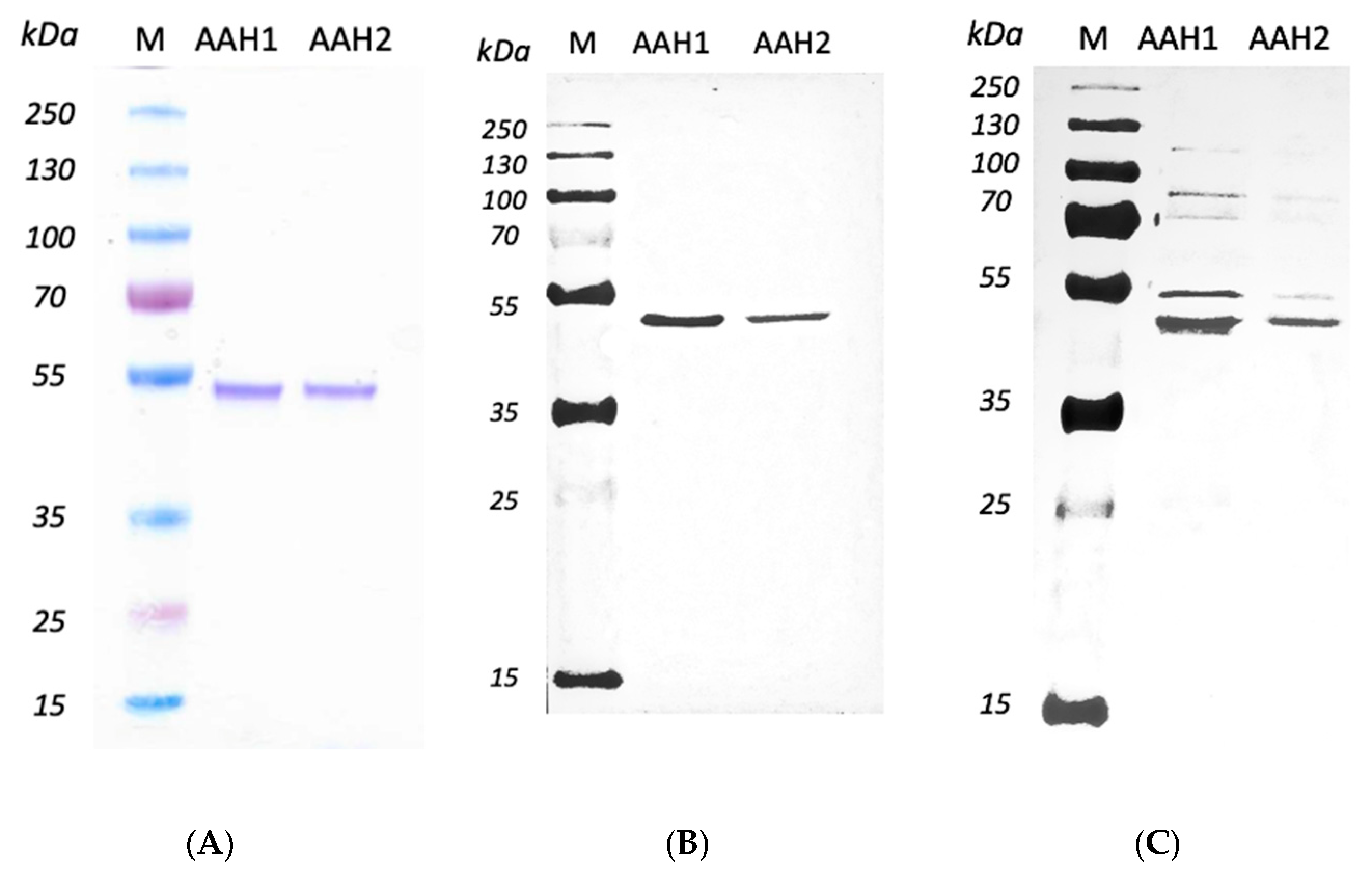
2.3. Expressing and Purifying Recombined AAHs
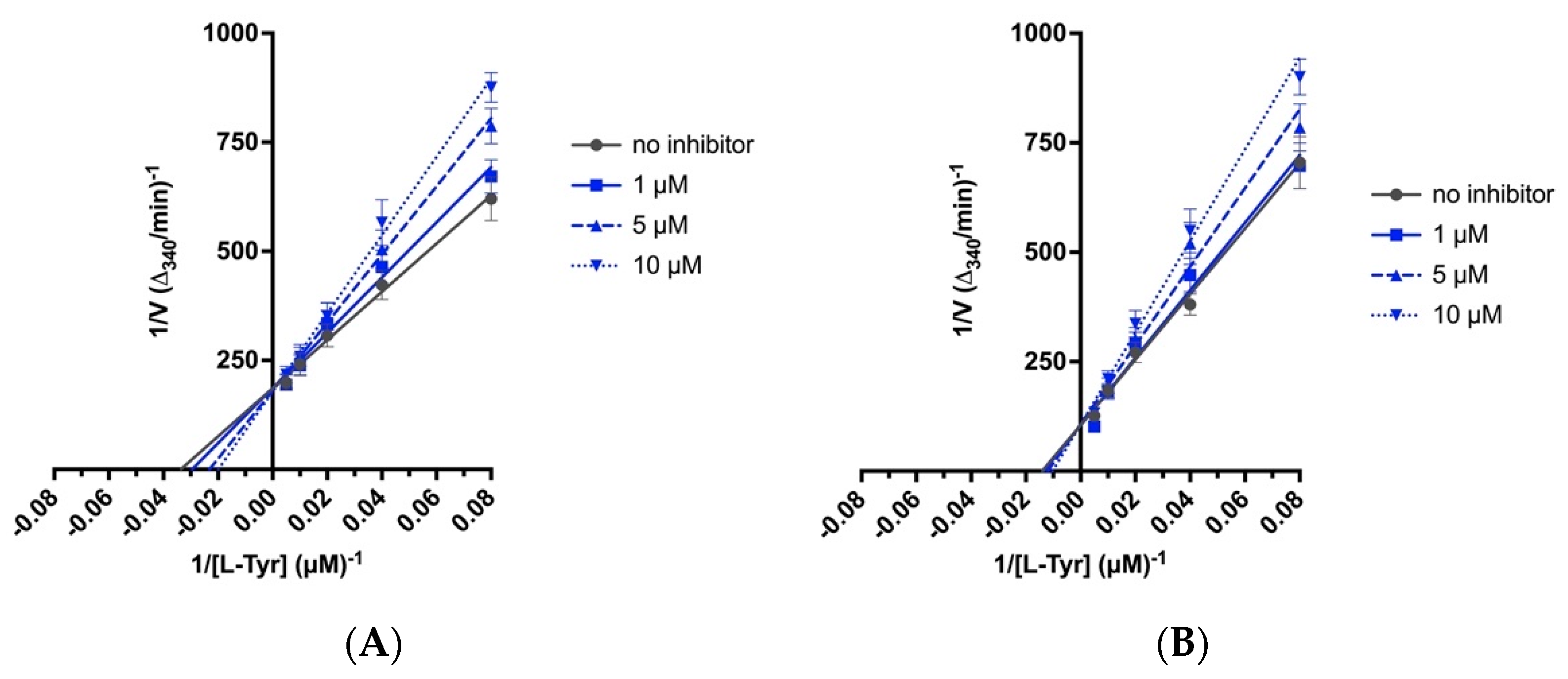
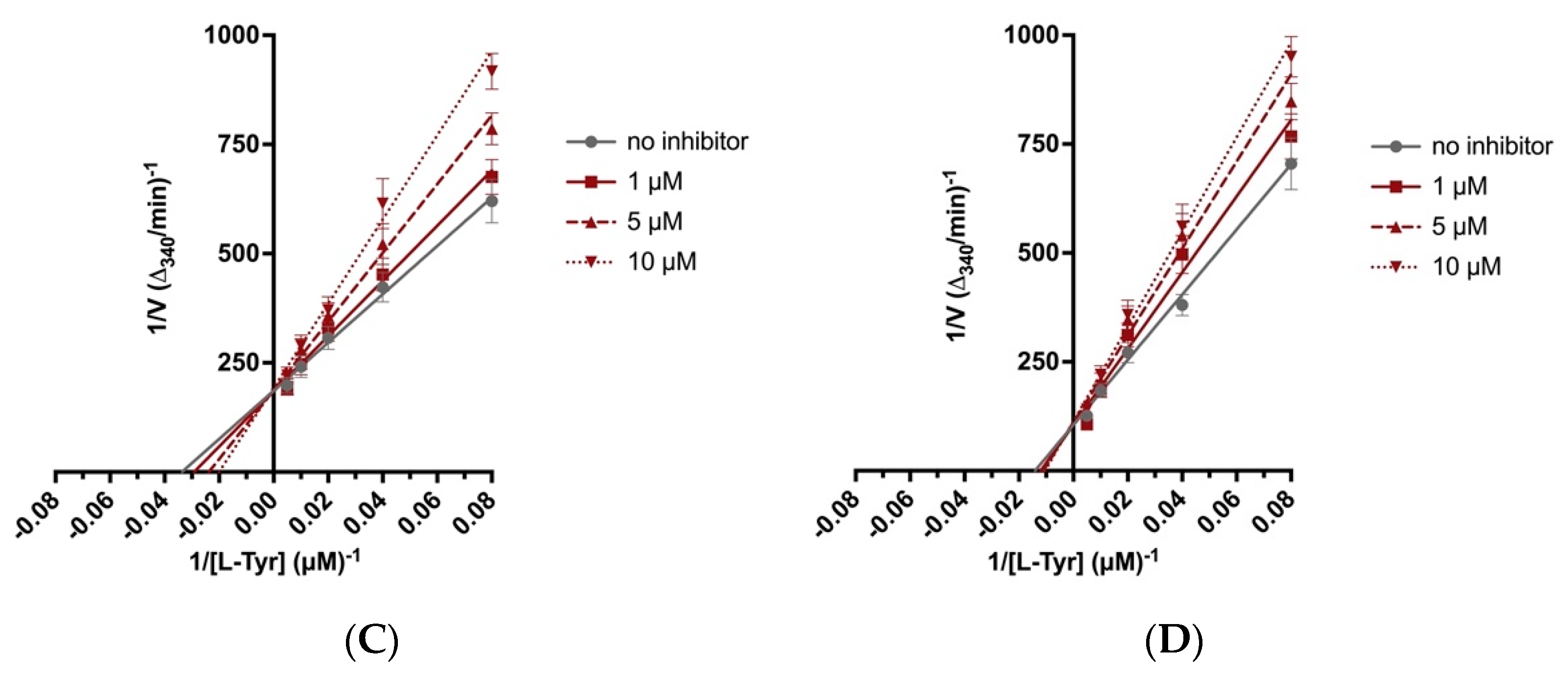
2.4. Biochemical Assays
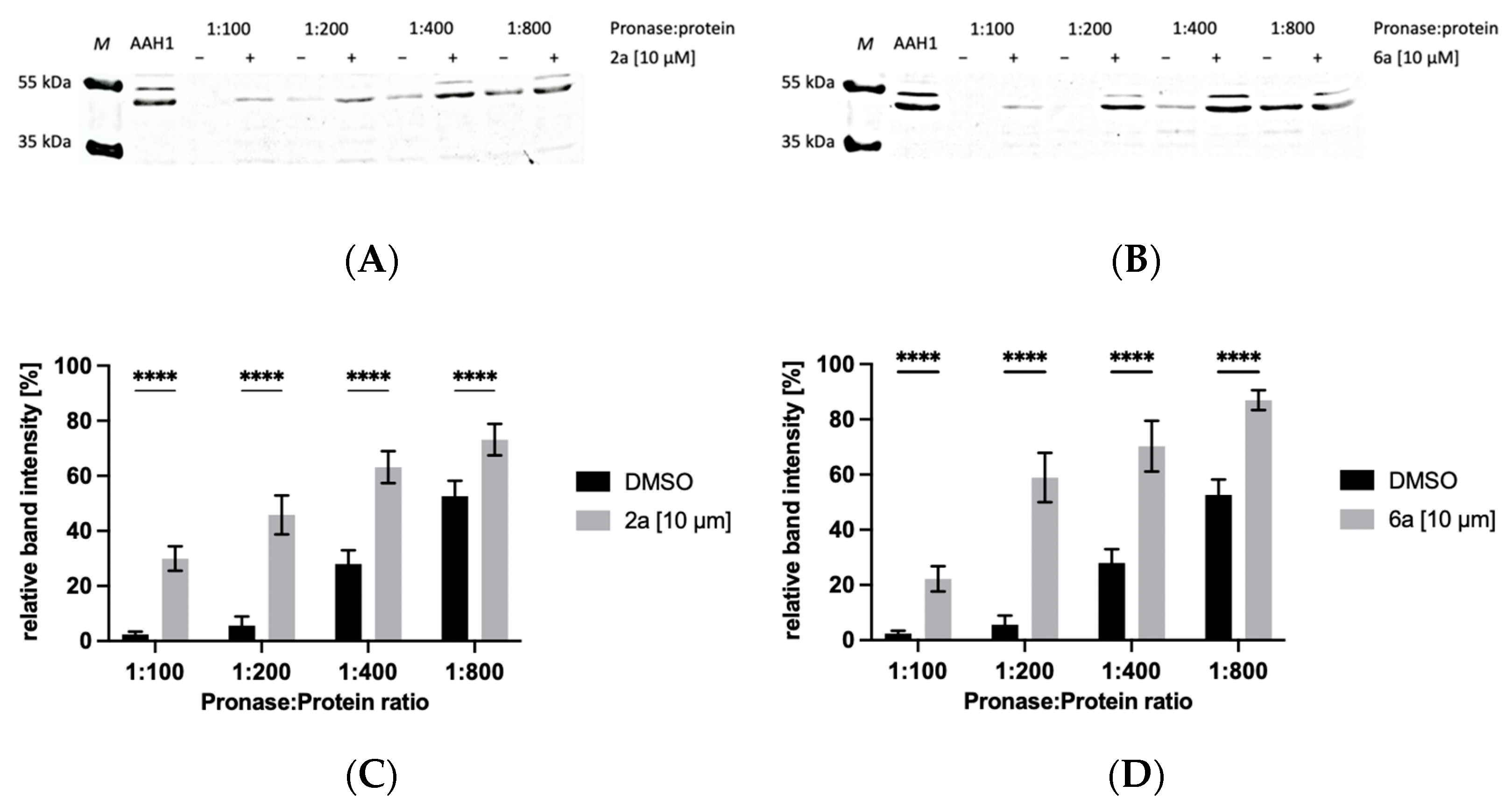
2.5. Drug Affinity Responsive Target Stability Assay
2.5.1. Protection of AAH1 from Proteolysis by 4-Arylthiosemicarbazide Derivatives in the Presence of 10 µM of Compound
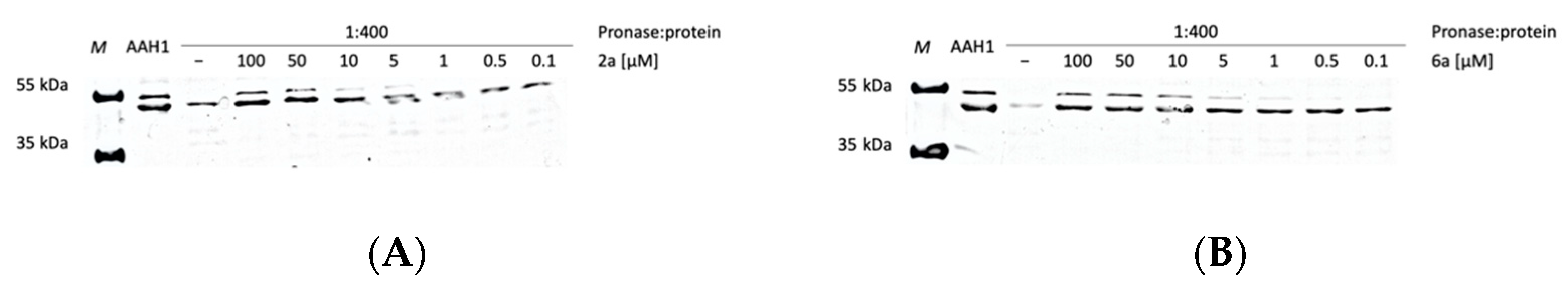
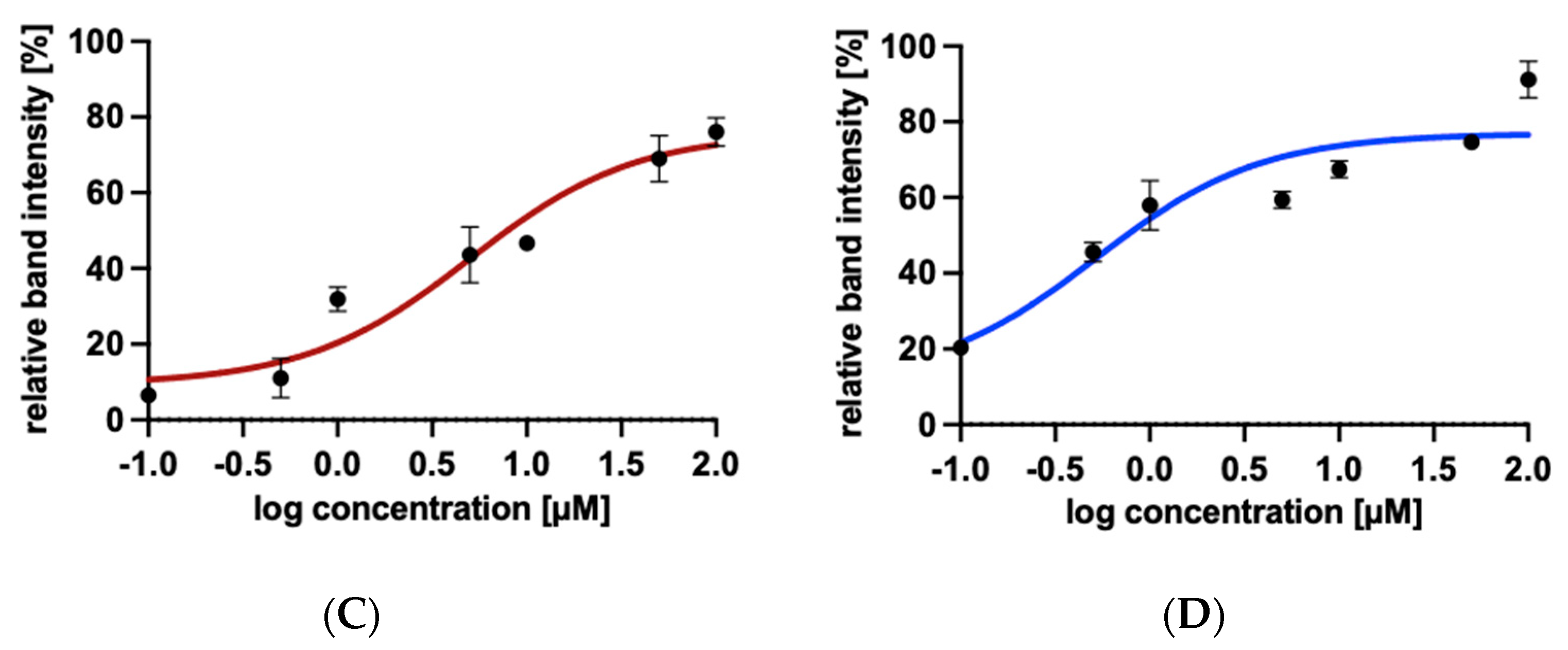
2.5.2. Protection of AAH1 from Proteolysis by 4-Arylthiosemicarbazide Derivatives in the Presence of Increasing Concentrations of Compounds
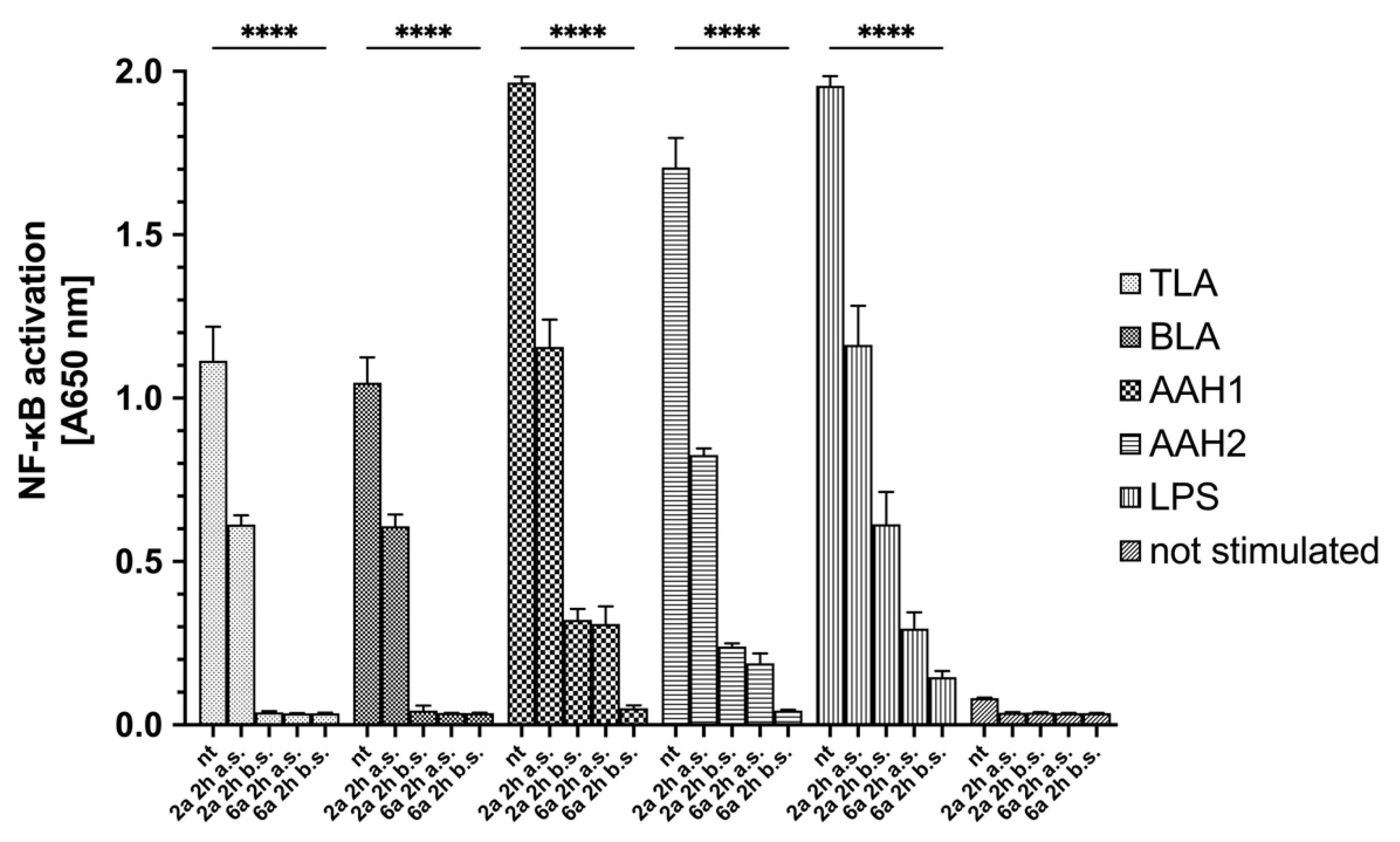
2.6. Anti-inflammatory Activity of 4-Arylthiosemicarbazide Derivatives
3. Discussion
4. Materials and Methods
4.1. Chemistry and Compound Preparation
4.2. Cell Culture
4.3. Parasite Culture
4.4. Development of Anti-SAG1 and Anti-MAG1 Rabbit Polyclonal Antibodies
4.5. Immunofluorescence Staining of Tachyzoites/Bradyzoites
4.6. Expression and Purification of Recombinant AAHs
4.7. Biochemical Assays
4.8. Drug Affinity Responsive Target Stability Assay
4.8.1. Protection of AAH1 from Proteolysis by 4-Arylthiosemicarbazide Derivatives in the Presence of 10 µM Compound
4.8.2. Protection of AAH1 from Proteolysis by 4-Arylthiosemicarbazide Derivatives in the Presence of Increasing Concentrations of Compound
4.9. Preparation of Soluble Tachyzoite and Bradyzoite Antigens
4.10. Quantification of NF-κB Induction
4.11. Cytokine Determination
4.12. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Djurković-Djaković, O.; Dupouy-Camet, J.; Van der Giessen, J.; Dubey, J.P. Toxoplasmosis: Overview from a One Health perspective. Food Waterborne Parasitol. 2019, 15, e00054. [Google Scholar] [CrossRef] [PubMed]
- Checkley, W.; White, A.C.; Jaganath, D.; Arrowood, M.J.; Chalmers, R.M.; Chen, X.M.; Fayer, R.; Griffiths, J.K.; Guerrant, R.L.; Hedstrom, L.; et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect. Dis. 2015, 15, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Battle, K.E.; Gething, P.W.; Elyazar, I.R.; Moyes, C.L.; Sinka, M.E.; Howes, R.E.; Guerra, C.A.; Price, R.N.; Baird, K.J.; Hay, S.I. The global public health significance of Plasmodium vivax. Adv. Parasitol. 2012, 80, 1–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torgerson, P.R.; Mastroiacovo, P. The global burden of congenital toxoplasmosis: A systematic review. Bull. World Health Organ. 2013, 91, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, S.; Basso, W.; Benavides Silván, J.; Ortega-Mora, L.M.; Maksimov, P.; Gethmann, J.; Conraths, F.J.; Schares, G. infection and toxoplasmosis in farm animals: Risk factors and economic impact. Food Waterborne Parasitol. 2019, 15, e00037. [Google Scholar] [CrossRef] [PubMed]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, A.; Soldati-Favre, D. Amino Acid Metabolism in Apicomplexan Parasites. Metabolites 2021, 11, 61. [Google Scholar] [CrossRef]
- Blume, M.; Seeber, F. Metabolic interactions between Toxoplasma gondii and its host. F1000Research 2018, 7, 1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, K.E.R.; Fairweather, S.J.; Rajendran, E.; Blume, M.; McConville, M.J.; Bröer, S.; Kirk, K.; van Dooren, G.G. The tyrosine transporter of Toxoplasma gondii is a member of the newly defined apicomplexan amino acid transporter (ApiAT) family. PLoS Pathog. 2019, 15, e1007577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, N.D.; Boothroyd, J.C. Toxoplasma growth in vitro is dependent on exogenous tyrosine and is independent of AAH2 even in tyrosine-limiting conditions. Exp. Parasitol. 2017, 176, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.T.; Verma, S.K.; Dubey, J.P.; Sibley, L.D. The aromatic amino acid hydroxylase genes AAH1 and AAH2 in Toxoplasma gondii contribute to transmission in the cat. PLoS Pathog. 2017, 13, e1006272. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, E.A.; Smith, J.E.; Pinney, J.W.; Westhead, D.R.; McConkey, G.A. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS ONE 2009, 4, e4801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belli, S.I.; Wallach, M.G.; Luxford, C.; Davies, M.J.; Smith, N.C. Roles of tyrosine-rich precursor glycoproteins and dityrosine- and 3,4-dihydroxyphenylalanine-mediated protein cross-linking in development of the oocyst wall in the coccidian parasite Eimeria maxima. Eukaryot. Cell 2003, 2, 456–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekier, A.; Węglińska, L.; Paneth, A.; Paneth, P.; Dzitko, K. 4-Arylthiosemicarbazide derivatives as a new class of tyrosinase inhibitors and anti-Toxoplasma gondii agents. J. Enzyme Inhib. Med. Chem. 2021, 36, 1145–1164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Li, H.; Song, X.; Ma, Z.; Lu, H.; Liu, S.; Zhao, Y.; Tan, M.; Wang, S.; et al. Identification of Toxoplasma gondii tyrosine hydroxylase (TH) activity and molecular immunoprotection against toxoplasmosis. Vaccines 2020, 8, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarland, R.; Wang, Z.T.; Jouroukhin, Y.; Li, Y.; Mychko, O.; Coppens, I.; Xiao, J.; Jones-Brando, L.; Yolken, R.H.; Sibley, L.D.; et al. AAH2 gene is not required for dopamine-dependent neurochemical and behavioral abnormalities produced by Toxoplasma infection in mouse. Behav. Brain Res. 2018, 347, 193–200. [Google Scholar] [CrossRef]
- Afonso, C.; Paixão, V.B.; Klaus, A.; Lunghi, M.; Piro, F.; Emiliani, C.; Di Cristina, M.; Costa, R.M. Toxoplasma-induced changes in host risk behaviour are independent of parasite-derived AaaH2 tyrosine hydroxylase. Sci. Rep. 2017, 7, 13822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.X.; Wei, S.S.; Lindsay, D.S.; Peng, H.J. A Systematic Review and Meta-Analysis of the Efficacy of Anti-Toxoplasma gondii Medicines in Humans. PLoS ONE 2015, 10, e0138204. [Google Scholar] [CrossRef] [PubMed]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse Event Profile of Pyrimethamine-Based Therapy in Toxoplasmosis: A Systematic Review. Drugs RD 2017, 17, 523–544. [Google Scholar] [CrossRef] [Green Version]
- Joynson, D.H.M.; Wreghitt, T.G. Toxoplasmosis: A Comprehensive Clinical Guide; Cambridge University Press: New York, NY, USA, 2001. [Google Scholar]
- Kortagere, S. Novel Molecules to Treat Chronic Central Nervous System Toxoplasmosis. J. Med. Chem. 2017, 60, 9974–9975. [Google Scholar] [CrossRef]
- Piper, J.R.; Johnson, C.A.; Krauth, C.A.; Carter, R.L.; Hosmer, C.A.; Queener, S.F.; Borotz, S.E.; Pfefferkorn, E.R. Lipophilic antifolates as agents against opportunistic infections. 1. Agents superior to trimetrexate and piritrexim against Toxoplasma gondii and Pneumocystis carinii in in vitro evaluations. J. Med. Chem. 1996, 39, 1271–1280. [Google Scholar] [CrossRef]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef] [Green Version]
- Mayers, D.L.; Sobel, J.D.; Ouellette, M.; Kaye, K.S.; Marchaim, D. Antimicrobial Drug Resistance: Mechanisms of Drug Resistance, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 1. [Google Scholar]
- He, J.; Wang, X.; Zhao, X.; Liang, Y.; He, H.; Fu, L. Synthesis and antitumor activity of novel quinazoline derivatives containing thiosemicarbazide moiety. Eur. J. Med. Chem. 2012, 54, 925–930. [Google Scholar] [CrossRef]
- Šarkanj, B.; Molnar, M.; Čačić, M.; Gille, L. 4-Methyl-7-hydroxycoumarin antifungal and antioxidant activity enhancement by substitution with thiosemicarbazide and thiazolidinone moieties. Food Chem. 2013, 139, 488–495. [Google Scholar] [CrossRef]
- Nazarbahjat, N.; Nordin, N.; Abdullah, Z.; Abdulla, M.A.; Yehye, W.A.; Halim, S.N.; Kee, C.H.; Ariffin, A. New thiosemicarbazides and 1,2,4-triazolethiones derived from 2-(ethylsulfanyl) benzohydrazide as potent antioxidants. Molecules 2014, 19, 11520–11537. [Google Scholar] [CrossRef] [Green Version]
- Sardari, S.; Feizi, S.; Rezayan, A.H.; Azerang, P.; Shahcheragh, S.M.; Ghavami, G.; Habibi, A. Synthesis and Biological Evaluation of Thiosemicarbazide Derivatives Endowed with High Activity toward Mycobacterium bovis. Iran J. Pharm. Res. 2017, 16, 1128–1140. [Google Scholar]
- Chudzik-Rząd, B.; Malm, A.; Trotsko, N.; Wujec, M.; Plech, T.; Paneth, A. Synergistic Effects of Thiosemicarbazides with Clinical Drugs against S. aureus. Molecules 2020, 25, 2302. [Google Scholar] [CrossRef]
- Bekier, A.; Kawka, M.; Lach, J.; Dziadek, J.; Paneth, A.; Gatkowska, J.; Dzitko, K.; Dziadek, B. Imidazole-Thiosemicarbazide Derivatives as Potent Anti-Mycobacterium tuberculosis Compounds with Antibiofilm Activity. Cells 2021, 10, 3476. [Google Scholar] [CrossRef]
- Kanso, F.; Khalil, A.; Noureddine, H.; El-Makhour, Y. Therapeutic perspective of thiosemicarbazones derivatives in inflammatory pathologies: A summary of in vitro/in vivo studies. Int. Immunopharmacol. 2021, 96, 107778. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.; Ahmed, M.; Ali, B.; Khan, K.M.; Ali, M.; Kobarfard, F.; Arshia; Ayatollahi, S.A.; Hassan, A.; Jabeen, A.; et al. Thiosemicarbazone and thiazolylhydrazones of 1-indanone: As a new class of nonacidic anti-inflammatory and antiplatelet aggregation agents. Pak. J. Pharm. Sci. 2019, 32, 15–19. [Google Scholar] [PubMed]
- Jacob, Í.; Gomes, F.O.S.; de Miranda, M.D.S.; de Almeida, S.M.V.; da Cruz-Filho, I.J.; Peixoto, C.A.; da Silva, T.G.; Moreira, D.R.M.; de Melo, C.M.L.; de Oliveira, J.F.; et al. Anti-inflammatory activity of novel thiosemicarbazone compounds indole-based as COX inhibitors. Pharmacol. Rep. 2021, 73, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Shawish, H.B.; Wong, W.Y.; Wong, Y.L.; Loh, S.W.; Looi, C.Y.; Hassandarvish, P.; Phan, A.Y.; Wong, W.F.; Wang, H.; Paterson, I.C.; et al. Nickel(II) complex of polyhydroxybenzaldehyde N4-thiosemicarbazone exhibits anti-inflammatory activity by inhibiting NF-κB transactivation. PLoS ONE 2014, 9, e100933. [Google Scholar] [CrossRef] [Green Version]
- Molestina, R.E.; Payne, T.M.; Coppens, I.; Sinai, A.P. Activation of NF-kappaB by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IkappaB to the parasitophorous vacuole membrane. J. Cell Sci. 2003, 116, 4359–4371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paneth, A.; Węglińska, L.; Bekier, A.; Stefaniszyn, E.; Wujec, M.; Trotsko, N.; Dzitko, K. Systematic Identification of Thiosemicarbazides for Inhibition of Toxoplasma gondii Growth In Vitro. Molecules 2019, 24, 614. [Google Scholar] [CrossRef] [Green Version]
- Paneth, A.; Węglińska, L.; Bekier, A.; Stefaniszyn, E.; Wujec, M.; Trotsko, N.; Hawrył, A.; Hawrył, M.; Dzitko, K. Discovery of Potent and Selective Halogen-Substituted Imidazole-Thiosemicarbazides for Inhibition of Toxoplasma gondii Growth In Vitro via Structure-Based Design. Molecules 2019, 24, 1618. [Google Scholar] [CrossRef] [Green Version]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [Green Version]
- De, B.; Adhikari, I.; Nandy, A.; Saha, A.; Goswami, B.B. In silico modelling of azole derivatives with tyrosinase inhibition ability: Application of the models for activity prediction of new compounds. Comput. Biol. Chem. 2018, 74, 105–114. [Google Scholar] [CrossRef]
- Liu, J.; Yi, W.; Wan, Y.; Ma, L.; Song, H. 1-(1-Arylethylidene)thiosemicarbazide derivatives: A new class of tyrosinase inhibitors. Bioorg. Med. Chem. 2008, 16, 1096–1102. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Yi, W.; Ma, C.; Wan, Y.; Zhou, B.; Ma, L.; Song, H. A class of potent tyrosinase inhibitors: Alkylidenethiosemicarbazide compounds. Eur. J. Med. Chem. 2009, 44, 1773–1778. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, M.; Cui, Y.; Hettinghouse, A.; Liu, C.J. A Semi-Quantitative Drug Affinity Responsive Target Stability (DARTS) assay for studying Rapamycin/mTOR interaction. J. Vis. Exp. 2019, 150, e59656. [Google Scholar] [CrossRef] [PubMed]
- Rosowski, E.E.; Lu, D.; Julien, L.; Rodda, L.; Gaiser, R.A.; Jensen, K.D.; Saeij, J.P. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med. 2011, 208, 195–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangaré, L.O.; Yang, N.; Konstantinou, E.K.; Lu, D.; Mukhopadhyay, D.; Young, L.H.; Saeij, J.P.J. GRA15 Activates the NF-κB Pathway through Interactions with TNF Receptor-Associated Factors. mBio 2019, 10, e00808-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liesenfeld, O.; Kosek, J.; Remington, J.S.; Suzuki, Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 1996, 184, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Fuchs, D.; Struck, D.; Niebergall, J.; Jahn, H.K.; Dunay, I.R.; Moter, A.; Gescher, D.M.; et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006, 177, 8785–8795. [Google Scholar] [CrossRef] [Green Version]
- Hatter, J.A.; Kouche, Y.M.; Melchor, S.J.; Ng, K.; Bouley, D.M.; Boothroyd, J.C.; Ewald, S.E. Toxoplasma gondii infection triggers chronic cachexia and sustained commensal dysbiosis in mice. PLoS ONE 2018, 13, e0204895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayoral, J.; Di Cristina, M.; Carruthers, V.B.; Weiss, L.M. Toxoplasma gondii: Bradyzoite Differentiation In Vitro and In Vivo. Methods Mol. Biol. 2020, 2071, 269–282. [Google Scholar] [CrossRef]
- Dziadek, B.; Gatkowska, J.; Brzostek, A.; Dziadek, J.; Dzitko, K.; Grzybowski, M.; Dlugonska, H. Evaluation of three recombinant multi-antigenic vaccines composed of surface and secretory antigens of Toxoplasma gondii in murine models of experimental toxoplasmosis. Vaccine 2011, 29, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Gatkowska, J.M.; Dziadek, B.; Dziadek, J.; Dzitko, K.; Długońska, H. Recombinant MAG1 Protein of Toxoplasma gondii as a Diagnostic Antigen. Pol. J. Microbiol. 2015, 64, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Pai, M.Y.; Lomenick, B.; Hwang, H.; Schiestl, R.; McBride, W.; Loo, J.A.; Huang, J. Drug affinity responsive target stability (DARTS) for small-molecule target identification. Methods Mol. Biol. 2015, 1263, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Tobias, R.; McClure, S.; Styba, G.; Shi, Q.; Jackowski, G. Removal of endotoxin from recombinant protein preparations. Clin. Biochem. 1997, 30, 455–463. [Google Scholar] [CrossRef]

| Compound | IC50 a | CC30 b,c | CC30 b,d | SR e | KITYR f |
|---|---|---|---|---|---|
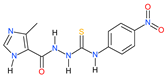 2a | 67.49 | 682.98 | 697.67 | 10.34 | 9.35 |
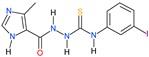 6a | 25.67 | 213.27 | 209.45 | 8.16 | 12.4 |
| Enzyme | Inhibitor | Vmax/KM a | KM b | KI c |
|---|---|---|---|---|
| AAH1 | none | 0.1817 | 0.030 | - |
| 2a | 0.1272 | 0.042 | 1.487 | |
| 6a | 0.1282 | 0.042 | 1.265 | |
| AAH2 | none | 0.1311 | 0.071 | - |
| 2a | 0.1094 | 0.085 | 3.691 | |
| 6a | 0.1038 | 0.089 | 3.227 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekier, A.; Brzostek, A.; Paneth, A.; Dziadek, B.; Dziadek, J.; Gatkowska, J.; Dzitko, K. 4-Arylthiosemicarbazide Derivatives as Toxoplasmic Aromatic Amino Acid Hydroxylase Inhibitors and Anti-inflammatory Agents. Int. J. Mol. Sci. 2022, 23, 3213. https://doi.org/10.3390/ijms23063213
Bekier A, Brzostek A, Paneth A, Dziadek B, Dziadek J, Gatkowska J, Dzitko K. 4-Arylthiosemicarbazide Derivatives as Toxoplasmic Aromatic Amino Acid Hydroxylase Inhibitors and Anti-inflammatory Agents. International Journal of Molecular Sciences. 2022; 23(6):3213. https://doi.org/10.3390/ijms23063213
Chicago/Turabian StyleBekier, Adrian, Anna Brzostek, Agata Paneth, Bożena Dziadek, Jarosław Dziadek, Justyna Gatkowska, and Katarzyna Dzitko. 2022. "4-Arylthiosemicarbazide Derivatives as Toxoplasmic Aromatic Amino Acid Hydroxylase Inhibitors and Anti-inflammatory Agents" International Journal of Molecular Sciences 23, no. 6: 3213. https://doi.org/10.3390/ijms23063213
APA StyleBekier, A., Brzostek, A., Paneth, A., Dziadek, B., Dziadek, J., Gatkowska, J., & Dzitko, K. (2022). 4-Arylthiosemicarbazide Derivatives as Toxoplasmic Aromatic Amino Acid Hydroxylase Inhibitors and Anti-inflammatory Agents. International Journal of Molecular Sciences, 23(6), 3213. https://doi.org/10.3390/ijms23063213







