Abstract
Antiviral type I interferons (IFN) produced in the early phase of viral infections effectively inhibit viral replication, prevent virus-mediated tissue damages and promote innate and adaptive immune responses that are all essential to the successful elimination of viruses. As professional type I IFN producing cells, plasmacytoid dendritic cells (pDC) have the ability to rapidly produce waste amounts of type I IFNs. Therefore, their low frequency, dysfunction or decreased capacity to produce type I IFNs might increase the risk of severe viral infections. In accordance with that, declined pDC numbers and delayed or inadequate type I IFN responses could be observed in patients with severe coronavirus disease (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as compared to individuals with mild or no symptoms. Thus, besides chronic diseases, all those conditions, which negatively affect the antiviral IFN responses lengthen the list of risk factors for severe COVID-19. In the current review, we would like to briefly discuss the role and dysregulation of pDC/type I IFN axis in COVID-19, and introduce those type I IFN-dependent factors, which account for an increased risk of COVID-19 severity and thus are responsible for the different magnitude of individual immune responses to SARS-CoV-2.
1. Introduction
Coronavirus disease (COVID-19) caused by a single-stranded RNA virus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) came from the Chinese city of Wuhan in 2019, and caused pandemic around the world [1]. Since the beginning of the pandemic, more infectious novel virus variants have emerged and led to newer waves of the pandemic [2].
The symptoms of COVID-19 can range from asymptomatic to mild and to severe symptoms in humans indicating that the individual’s immunological state greatly influences the course and outcome of the disease [3].
In general, the course of COVID-19 can be divided into 3 distinct stages based on the clinical manifestation and recommended therapy [4]. The first stage is the early phase of infection, which begins immediately after infection and includes an incubation period. This stage is usually asymptomatic or is associated with mild and often non-specific symptoms such as malaise, fever and dry cough. In patients in whom COVID-19 is restricted to this stage, the prognosis is excellent. The second stage is already associated with pulmonary involvement, which can occur without or with hypoxia. Patients develop viral pneumonia with cough, fever, and possibly hypoxia. At this stage, most patients with COVID-19 require hospitalization for close monitoring or treatment. The severe third stage of the disease manifests as an extrapulmonary, systemic inflammation resulting in a cytokine storm. At this stage, lymphopenia may develop and a decrease in helper, regulatory, and memory T cell counts [5], an increase in neutrophil counts and a significant increase in inflammatory cytokines and biomarkers can be observed [5,6]. In patients with this advanced stage of the disease, a cytokine profile resembling secondary haemophagocytic lymphohistiocytosis may be observed, which is characterized by elevated levels of IL-2, IL-7, granulocyte colony stimulating factor (G-CSF), IFN-γ inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α) and tumor necrosis factor-α (TNF-α) [7]. Furthermore, septic shock, vasoplegia, acute respiratory distress syndrome, cardiopulmonary collapse, as well as systemic organ involvement or even myocarditis may occur at this stage [4]. Overall, the prognosis in this phase of the disease is rather poor, with only a few patients recovering from the critical stage of the disease.
Besides the well-known respiratory pathology, various extrapulmonary manifestations of COVID-19 have already been reported highlighting the involvement of cardiovascular, genitourinary, gastrointestinal and central nervous system as well as the skin [8]. The multi-organ involvement can be manifested by various symptoms including thrombotic complications, myocardial dysfunction, arrhythmia, acute coronary syndrome, acute kidney injury, gastrointestinal symptoms, hepatocellular injury, hyperglycemia and ketosis, neurologic illnesses, ocular symptoms, dermatologic complications, preeclampsia and fertility problems [8,9,10,11,12,13].
In addition, those patients who recovered from COVID-19 may suffer from post-COVID-19 syndrome, which negatively affects their quality of life for months after recovery. The post-COVID-19 syndrome is characterized by a wide variety of clinical symptoms including pulmonary embolism, deep vein thrombosis, acute myocardial infarction, depression, anxiety, myalgia, dyspnea, fatigue, defects in memory and concentration and a variety of neuropsychiatric syndromes [14,15,16,17].
In general the incidence of post-COVID-19 syndrome is about 10–35%; however, this rate can reach up to 85% for those patients who required hospitalization during acute SARS-CoV-2 infection [15].The severity and mortality of COVID-19 is higher in patients with chronic conditions such as diabetes, hypertension, and cardiovascular diseases [18]. A growing body of evidence indicates that individuals with disturbed antiviral interferon (IFN) response are more likely to develop severe COVID-19 symptoms. So far, it seems that mortality rates are higher in seniors, men, pregnant women, and obese patients [19,20,21,22] that might be explained by the impaired or dysregulated type I IFN response, which is a vital component of antiviral immunity. In addition, the pandemic does not spare the young with no underlying medical conditions either that might be related to genetic defects in the IFN signaling pathways or autoantibodies generated against type I IFNs, which neutralize the direct inhibitory effect of type I IFNs on viral replication [23]. Therefore, the individual’s type I IFN signature greatly contributes to the variability of COVID-19 outcome, and conditions associated with decreased type I IFN production indicate poorer prognosis. The main sources of type I IFNs upon viral infections are plasmacytoid dendritic cells (pDCs), which are specialized for the recognition of viral nucleic acids and subsequent release of huge amount of type I IFNs [24]. pDCs and pDC-derived IFNs are central players in the antiviral immune responses against SARS-CoV-2, thus a number of excellent papers have already reviewed the importance of antiviral IFNs [25] and pDCs [26] in COVID-19.
Therefore, in this review, we aimed to briefly summarize the role of IFNs and pDCs in COVID-19 based on the newest available data on this field. Nevertheless, we want to give a deep insight into those risk factors for COVID-19 severity, which are associated with impaired type I IFN responses and reduced pDC number to highlight that low type I IFN signature of individuals due to different inborn or acquired conditions predicts a more severe disease outcome.
3. The Role of pDCs in COVID-19
In the previous section we have emphasized the importance of optimal type I IFN response in COVID-19. As previously mentioned pDCs are the main producers of IFNα and according to the newest studies well-functioning pDCs are crucial to overcome SARS-CoV-2 infection.
In vitro studies showed that human pDCs are resistant to SARS-CoV-2 viruses due to the lack of ACE-2 and TMPRSS2 receptors, which are required for the cellular entry of SARS-CoV-2. However, instead of these proteins SARS-CoV-2 can use the transmembrane neuropilin 1 receptor (NRP1, also called blood dendritic cell antigen 4 [BDCA4]), the specific cell surface marker of pDCs, to enter the cells [49]. Previously it was observed that antibody ligation of BDCA4 impaired type I IFN production of pDCs [50,51], and lately it was also revealed that the binding of SARS-CoV-2 to BDCA4 on pDCs also decreased the type I IFN responses of pDCs that can serve as an evasion mechanism for the virus [26].
Another study also revealed that pDCs are refractory to SARS-CoV-2 infection under in vitro conditions. Upon SARS-CoV-2 stimulation, the signs of viral replication can not be observed in pDCs [52], and more interestingly SARS-CoV-2 stimulation increased the viability of pDCs compared to medium condition [52]. In addition, upon SARS-CoV-2 stimulation pDCs mount a robust type I IFN response by effectively producing type I IFNs. For instance, the concentration of IFNα2 can even reach 80 ng/mL [52]. Furthermore, the SARS-CoV-2-exposed pDCs can be divided into 3 activated subpopulations based on their cell surface co-stimulatory molecule expression, and this diversification can also be observed upon coculture with SARS-CoV-2 infected cells [52]. Upon SARS-CoV-2 stimulation pDCs mainly diversify into P1-pDCs (PD-L1+CD80−), which subset is characterized by high type I IFN production [49]. Infected cells also efficiently induce a P1 dominant diversification [53]. The type I IFN production of activated pDCs seems to be dependent on TLR7 signaling upon SARS-CoV-2 stimulation [49], and their diversification and cytokine production is related to the adaptor molecules IRAK4 and UNC93B1 [52]. The TLR7 activation-induced signaling also promotes the effective antiviral action of pDCs via the so-called interferogenic synapse in response to SARS-CoV-2 infected cells [54]. Overall, these in vitro studies indicate that pDCs can induce an effective type I IFN-dependent antiviral response against SARS-CoV-2 by inducing an antiviral state in the host cells to inhibit viral replication and by facilitating the antiviral actions of various innate and adaptive immune cells (Figure 1).
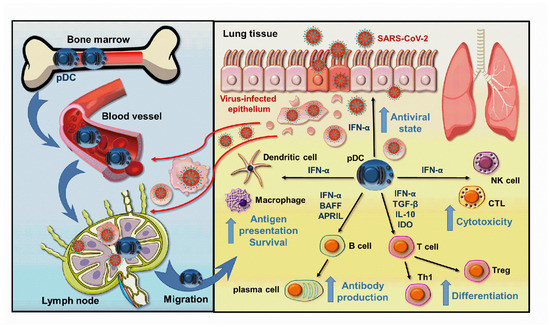
Figure 1.
Beneficial effects of pDC-derived type I IFNs help to overcome SARS-CoV-2 infection. When the SARS-CoV-2 viruses break through the first line of defense ensured by the epithelial cells, viral particles or cell debris derived from virus-infected cells are delivered to the draining lymph nodes, where pDCs are stimulated to migrate to the entry site of the viruses. Here, the pDC-derived type I IFNs initiate an antiviral state in the host cells, which effectively blocks viral replication, and also promotes the activation and function of both innate and adaptive immune cells thereby creating an effective antiviral response. APRIL: A proliferation-inducing ligand; BAFF: B-cell activating factor; IL: interleukin; IDO: Indoleamine 2, 3-dioxygenase; IFN: interferon; NK: natural killer; pDC: plasmacytoid dendritic cell; TGF: Transforming Growth Factor; Th: T helper; Treg: T regulatory; CTL: cytotoxic T cell.
Besides the in vitro experiments, numerous in vivo studies proved that pDCs are crucial to mount an effective antiviral response against SARS-CoV-2. Several studies showed that pDC number is reduced in COVID-19 patients [55,56,57]. Furthermore, the decreased pDC number in COVID-19 patients negatively correlates with disease severity [57,58,59,60]. It was found that the immune landscape of patients differs depending on the severity of the disease. pDC frequency was lower in severe cases and that correlated well with disease severity. Moreover, clinical improvement of patients went hand in hand with increasing pDC frequency showing a dynamic process [61]. Another study also found that pDC frequency and number are decreased in asymptomatic patients compared to healthy donors and in hospitalized patients their level is dramatically reduced. Moreover, in asymptomatic patients, the high type I IFN producing P1-pDC population was dominant, while in hospitalized patients mainly the P2 subgroup (PD-L1+CD80+) with lower capacity to produce antiviral IFNs was observed [49,52]. Single-cell RNA sequencing showed that in the pDCs of severe COVID-19 patients the expression of pro-apoptotic molecules is increased, whereas their TLR7 and DHX36 expression are lost, and their antiviral effects and cytotoxic functions are decreased compared to pDCs from patients with moderate disease or cells from healthy controls [62]. The pDCs of hospitalized patients are characterized by decreased type I IFN and increased pro-inflammatory cytokine (TNF-α, IL-6) production compared to asymptomatic individuals [49].
Single-cell RNA sequencing of bronchoalveolar lavage fluid (BALF) from COVID-19 patients showed that in the BALF of severe/critical COVID-19 patients lower proportions of pDCs can be found compared to those with moderate infection [63]. Sánchez-Cerrillo et al. also found that pDCs are diminished from the blood of critical patients, and no pDCs were found in bronchoscopy infiltrates as well [64]. The decreased pDC number in the blood can be explained by the fact that pDCs migrate to the lungs upon inflammation; however, in critical cases, pDCs are also depleted in the lung. In case of extremely severe infection, it is due to the hyper-inflammatory landscape of the lung, which milieu impairs the viability and type I IFN producing capacity of pDCs.
In the context of chronic viral infections, high viral load induces pDC exhaustion, which means that pDCs tune down their type I IFN secretion and eventually die by apoptosis. This promotes viral replication and decreases the efficiency of innate immune responses [65]. In line with that, a study showed that pDCs from COVID-19 patients are characterized by decreased mTOR signaling and IFN production [58]. Moreover, pDCs displayed an apoptotic gene signature, which positively correlated with disease status and severity [57].
The type I IFN producing ability of pDCs is highly affected by the cytokine milieu of the inflamed lung and it is negatively regulated by pro-inflammatory mediators such as prostaglandin E2 (PGE2), IL-1β, IL-10 and TNF-α [66,67,68,69,70,71,72], which are highly elevated in COVID-19 patients [60,73].
It is also important to note that IL-3, which is mainly produced by T cells is an essential survival factor for pDCs and is also depleted in COVID-19 [74]. Benard et al. identified IL-3 as a prognostic marker for COVID-19 severity and outcome. Low IL-3 level correlates with increased viral load, mortality and severity. Non-survivors had lower T cell numbers and in COVID-19 patients the number of T cells correlates with pDC number in the plasma and BALF as well. In addition, in the BALF of COVID-19 patients with pulmonary manifestation a positive correlation was found between IL-3 and CXCL12 levels. The authors found that IL-3 derived from T cells induced the secretion of CXCL12 by epithelial cells and this chemokine mediated the recruitment of pDCs into the lung [75] (Figure 2).
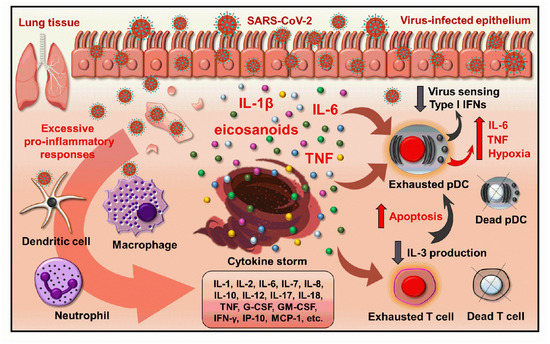
Figure 2.
The inflammatory microenvironment in severe COVID-19 leads to the exhaustion and decreased antiviral potential of pDCs. The overactivation of immune cells in severe COVID-19 leads to an excessive production of pro-inflammatory cytokines and eventually cascades into a cytokine storm. This inflammatory environment drives pDC exhaustion that is characterized by high hypoxia and functional abnormalities of pDCs. However, the inflammatory environment has an inhibitory effect on the virus sensing ability and type I IFN production of pDCs, and their pro-inflammatory cytokine production comes to the fore that can further fuel the detrimental inflammatory circuit. The excessive inflammatory milieu also causes T cell exhaustion and leads to increased T cell death. Consequently, the levels of T cell-derived IL-3, which is an essential survival factor for pDCs, also drop and that leads to pDCs apoptosis. Thus, severe COVID-19 is associated with a reduced number of pDCs. G-CSF: granulocyte colony stimulating factor; GM-CSF: granulocyte-macrophage colony stimulating factor; IFN: interferon; IP: interferon gamma-induced protein, MCP: monocyte chemoattractant protein; pDC: plasmacytoid dendritic cell; TNF: tumor necrosis factor.
In conclusion, these studies highlight that optimal pDC number and type I IFN response are vital to control SARS-CoV-2 infection and prevent the development of severe disease. Thus, all kind of diseases and conditions, which are characterized by low pDC frequency and decreased type I IFN production are risk factors for severe/critical COVID-19. The features of pDCs in COVID-19 are extensively reviewed in a recent paper [26], which thoroughly details the positive correlation between pDC function and COVID-19 severity, and provides a summary table about the observations regarding the fate of pDCs during COVID-19.
4. Risk Factors of COVID-19
While the mortality rate of COVID-19 is 0.9% in the healthy population, this ratio is significantly higher in patients with cardiovascular disease (10.5%), diabetes (7.3%) and hypertension (6%) [76]. The reason for the more severe disease course is that these conditions are all associated with chronic inflammation [18,77]. In diabetes, persistent inflammation due to hyperglycemia promotes easier entry of the virus into cells, and inhibits T-cell functions leading to a higher viral load. In addition, an exaggerated immune response predisposes to the development of a cytokine storm [78]. Excessive baseline activity of immune cells is also observed in hypertension, which reduces the efficiency of virus elimination and leads to more severe airway inflammation [79]. Heart disease is also associated with a poor prognosis of COVID-19, since infection associated fever and tachycardia increase the body’s need for oxygen that puts a heavy strain on a sick heart, and the virus can also damage the heart muscle directly or by inducing a cytokine storm indirectly [80].
In addition to the above mentioned diseases, which correlate with a more severe prognosis of COVID-19, there are a number of other risk factors that are closely linked to the body’s dysregulated type I IFN response or decreased type I IFN production due to various congenital or acquired causes [23,25]. The factors or conditions, which negatively affect the body’s type I IFN production result in a higher viral load due to inadequate virus elimination and thus lead to a more severe course of COVID-19. Such factors influencing type I IFN responses may include genetic defects of the antiviral immune response, or factors modulating baseline IFN signature, such as sex, age, and the condition of the microbiome, which promotes a steady-state basal IFN production. In addition, obesity, pregnancy, and viral infection-induced autoantibody production also result in an altered IFN response of the body. Furthermore, immunosuppression-associated chronic conditions that result from endogenous immunodysfunctions or immunosuppressive therapies are also risk factors for COVID-19. These conditions are also mostly associated with impaired functionality of pDCs, the main sources of type I IFNs in antiviral responses. In the following section, we detail those type I IFN response-associated risk factors, which may contribute to the development of more severe symptoms of COVID-19 (Figure 3).
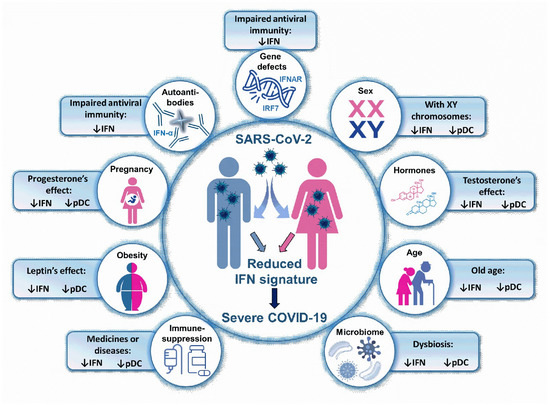
Figure 3.
Type I IFN related risk factors in COVID-19. A low IFN signature predicts a more severe COVID-19 outcome. Thus, those conditions, which are associated with impaired type I IFN response or decreased pDC number may be risk factors for severe COVID-19. IFN: interferon; pDC: plasmacytoid dendritic cell, IRF: interferon regulatory factor; IFNAR: interferon-α/β receptor.
5. Type I IFN-Associated Risk Factors in COVID-19
5.1. Genetic and Congenital Factors Associated with Reduced Antiviral IFN Production
It has long been known that defective variants at 13 genetic loci contribute to the development of influenza virus-induced severe pneumonia (IRF7, IRF9 and TLR3 genes), adverse events to live attenuated virus vaccines (IFNAR1, IFNAR2, STAT2 genes) or herpes simplex encephalitis (TLR3, UNC93B1, TICAM1, TRAF3, TBK1, IKBKG, IRF3, IFNAR1, STAT1 genes). These congenital gene defects impair the TLR3- and IRF7-dependent type I IFN responses [81,82]. In an international cohort, it has been shown that approximately 3.5% of patients with critical COVID-19 carry loss of function mutations at these loci [83]. In 23 people from the 659 patients with severe COVID-19 autosomal recessive (IRF7, IFNAR1) and autosomal dominant (TLR3, UNC93B1, TICAM1, TBK1, IRF7, IRF3, IFNAR1, IFNAR2) deficiencies were found. 10 patients were also characterized by low IFNα levels. It seems that the penetration values are higher for autosomal recessive mutations than for autosomal dominant gene defects [83,84]. TLR3-, IRF7-, or IFNAR1-deficient cells are highly sensitive to SARS-CoV-2, and IRF7-deficient pDCs are unable to produce type I IFNs upon viral exposure [83]. Furthermore, IFNAR1-deficient cells do not respond to type I IFN stimulation. The studies identified two patients (49 and 50 years old) with autosomal recessive IRF7 deficiency and two other patients (26 and 38 years old) with IFNAR1 mutation. Prior to COVID-19 pneumonia, none of the four patients required hospitalization for severe viral illness, highlighting that in contrast to seasonal influenza viruses these mutations have a higher penetrance for COVID-19 [84]. Moreover, van der Made et al. identified four young male patients, who carried the loss-of-function variants of TLR7. They all suffered from severe COVID-19 and were characterized by impaired type I and type II IFN production [85]. Another study found that in men under 60 years recessive TLR7 deficiency accounts for 1% of critical COVID-19 cases [86]. In addition, the interferon-induced transmembrane protein 3 (IFITM3) gene encodes a protein, which is critical to restrict viral replication and to inhibit membrane fusion. A study found that homozygosity for the C allele of the rs12252 SNP in IFITIM3 gene leads to more severe disease in an age-dependent manner [87]. This genetic variant is also associated with COVID-19 mortality in the Arab population [88].
Genome-level association studies (GWAS) have so far identified 4 chromosomal regions that may be associated with severe COVID-19. The first such region is located on chromosome three, and all that is currently known is that it encodes six genes (SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6, and XCR1); however, their functions are still unexplored [89,90]. Furthermore, three other regions found in a recent GWAS analyzing 2244 critically ill patients from UK intensive care units were also identified in an international GWAS comparing hospitalized COVID-19 patients with other members of the population [90]. The odds ratio for heterozygous susceptibility alleles is between 1.2 and 1.4. Two of these three regions also contain genes involved in the antiviral immune response. The first region at chr12q24.13 includes the OAS1, OAS2, and OAS3 genes and a group of ISGs required for RNase L enzyme activation. The second region, chr21q22.1, contains IFNAR2, which encodes the second chain of the IFN receptor [90].
We could expect that more alleles of the genes involved in the type I IFN response might be beneficial against COVID-19. However, according to the latest data it is not the case. People with Down syndrome have an extra chromosome, which encodes several gene involved in type I IFN response, for example the IFN receptor is also triplicated in trisomic cells. In the initial phase of the infection, the overactive type I IFN response might be advantageous, but later it fuels a detrimental inflammatory response due to the pleiotropic effects of type I IFNs [91].
Besides genetic defects, autoantibodies against type I IFNs are also associated with severe COVID-19. It was discovered that at least 10% of patients with critical COVID-19 pneumonia had autoantibodies that were able to neutralize large amounts of at least one, but typically more, type I IFN subtypes in vitro and in vivo. These IgG antibodies mainly neutralized IFNω, IFNα, or both, however, some patients had autoantibodies against all 13 IFNα subtypes. These autoantibodies were not found in any of the tested individuals with asymptomatic or mild SARS-CoV-2 and were present only in 0.33% of healthy individuals. Interestingly, these autoantibodies already existed in the patients prior to SARS-CoV-2 infection, and were the cause of severe disease rather than the consequence of the infection. The presence of these antibodies was associated with poor clinical outcome and increased mortality. It is noteworthy that 94% of patients with autoantibodies were male, half of them were over 65 years of age, and more than a third of them died from COVID-19. Overall, autoantibodies against type I IFNs are present in at least 3.5% of women and 12.5% of men with critical COVID-19 [92]. Another study showed that in vitro non-neutralizing anti-IFN antibodies were detected in 16% of patients, who were admitted to the intensive care unit (ICU) due to non-viral respiratory infection. However, neutralizing autoantibodies were only detectable in severe SARS-CoV-2 infected patients and their presence was associated with higher mortality and the development of multiple organ failure [93]. Wang and his colleagues also screened COVID-19 patients and healthy individuals for autoantibodies against extracellular and secreted proteins. They identified autoantibodies against type I IFNs in 5.2% of patients, who were hospitalized with COVID-19. Autoantibodies against type III IFNs (IFNλ2 and IFNλ3) were also found. Patients with type I IFN neutralizing antibodies were characterized by higher average viral load and extended durations of hospital admission [94]. It should be noted that only 2% of individuals with autoantibodies against type I IFNs produce autoantibodies against IFNβ [92]. However, autoantibodies are likely to be more common against the 13 IFNα subtypes and IFNω. Furthermore, genes encoding some of these IFN subtypes underwent strong negative selection, suggesting that they play an extremely important role in the antiviral response of the population [95]. Increased autoantibody production is probably due to an X chromosome-linked defect that is indicated by the increased involvement of men and the fact that one of the autoantibody-producing women suffered from a disease called incontinentia pigmenti, in which the inactivation of the X chromosome is skewed, and not random [92]. After the age of 65, autoantibody production is more likely because the composition of the immune system also changes with age. For instance, an atypical B-cell subpopulation might arise known as age-associated B cells (ABC), which differentiate into abnormal plasma cells characterized by increased autoantibody production [96]. In line with that, the incidence of neutralizing antibodies sharply rises after the age of 70. Neutralizing antibodies account for approximately 20% of both critical COVID-19 cases in the over 80s, and total lethal COVID-19 cases [97].
5.2. Influence of Biological Sex and Sex Hormones on Antiviral IFN Signature
Sexual dimorphism is observed not only in the physical appearance and behaviour of the sexes, but also in the context of autoimmunity and antiviral immunity [98,99,100]. It has long been known that women are less susceptible to viral infections than men due to their ability to develop a more effective antiviral response. Today, unfortunately, we can see how this observation is confirmed, as the currently raging COVID-19 pandemic affects men much more severely than women. For men, the mortality rate is 1.7 times higher in COVID-19, and sex differences are even more pronounced in the population over 30 years of age [101,102]. In a cohort longitudinal analysis of COVID-19 patients, higher level of IFNα was found in female patients [103]. The different type I IFN-producing capacity of pDCs in men and women plays a major role in this phenomenon. Genes involved in the antiviral response are often located on sex chromosomes or contain a hormone response element (HRE), so their expression is regulated by sex hormones and depends on the inactivation of sex chromosomes.
X chromosome number affects the type I IFN response of pDCs. In a humanized mouse model, it has been shown that when CD34+ human stem cells isolated from women or men are transplanted into female or male mice, pDCs derived from female stem cells produce higher amounts of type I IFNs upon TLR7 stimulation than pDCs from male donor cells regardless of the sex of the recipient mice. These data suggest that the double X chromosomes in women provide immunological benefits as it may contribute to an enhanced immune response against infections [104]. A similar study examined the effect of X chromosome number and sex hormones on the TLR7-induced IFNα response of primary pDCs in healthy women, patients suffering from Turner syndrome, men, and transgender volunteers receiving hormone therapy. It has been found that the antiviral effect induced by type I IFNs is much more pronounced in healthy women than in men or women with Turner syndrome, where one of the X chromosomes is absent. However, the strength of the antiviral response did not correlate with serum sex hormone levels [105]. Furthermore, it is known that several genes encoded on the X chromosome involved in the TLR signaling pathway can avoid X chromosome inactivation and thus contribute to a stronger antiviral and humoral immune response. It has been observed that TLR7 encoded on the X chromosome is biallelically expressed in the pDCs, B cells and monocytes not only of women (XX) but also of men with Klinefelter’s syndrome (XXY), and that immune cells with biallelic TLR7 expression show greater transcriptional activity compared to monoallellic cells [106]. This was supported by another study showing that pDCs from women with biallelic TLR7 expression are capable of greater IFN production than pDCs expressing only one TLR7 allele [107]. These data may also explain why men with a single X chromosome have a higher mortality rate for COVID-19 compared to women [108,109]. PDCs in women with biallelic TLR7 expression may produce a higher amount of type I IFNs and respond more rapidly to SARS-CoV-2 infection that may result in a better control of the infection in women [107]. These data are further supported by a recent study showing that loss-of-function mutation of TLR7 on the X chromosome results in severe COVID-19 symptoms in young men that also indicates that the corresponding TLR7-mediated type I IFN response can play an essential role in overcoming the disease [85]. In addition, it is important to mention that pDC-derived type I IFNs regulate B cell activation and differentiation into plasma cells and are therefore essential to elicit an optimal antibody response against viral infections, therefore women may have an advantage over men in terms of antibody response regarding SARS-CoV-2 infection [102].
Sex hormones also affect antiviral immune responses. Due to their lipophilic nature, steroid hormones readily cross the plasma membranes of cells and, by binding to nuclear receptors, are able to affect the functions of immune cells, including pDCs [110]. Oestrogen is known to play an important role in regulating TLR-mediated immune responses in human and mouse pDCs. In mice, 17β-oestradiol (E2) has been reported to significantly increase CpG-B-induced IFNα production by spleen pDCs [111]. Consistent with this observation, E2 treatment in postmenopausal women also significantly increased TLR7/9 activation-induced IFNα production by primary pDCs. It has also been shown that E2 directly targets pDCs, as deletion of the oestrogen receptor α (ERα) in mouse pDCs suspended the positive effect of E2 treatment on TLR-induced IFNα induction [112]. In addition, impairment of oestrogen receptor signaling significantly reduces TLR7-induced IFNα expression in human pDCs from umbilical cord blood [104]. In another study, the ERα signaling pathway was found to induce increased IFNα secretion in TLR7-stimulated mouse pDCs through activation of the transcription factor IRF5, which is a positive regulator of the IFNα response of pDCs [113].
So far, only one study has examined the effect of androgens on the functions of pDCs. Dihydrotestosterone (DHT) has been shown to reduce TLR7-mediated IFNα production by pDCs isolated from the blood of healthy women. It was also found that pDCs in male infants produced less IFNα in response to TLR7 stimulation compared to female infants that can be explained by the early postnatal testosterone surge in 1–6-month-old male infants [114].
Based on the above data, it can be concluded that while oestrogens positively regulate the type I IFN response of pDCs, testosterone may negatively affect these processes. Thus, gender differences can greatly determine the strength of an individual’s immune response to viral infections as well as the efficacy of vaccines [115].
Observations to date have shown that COVID-19 is also more dangerous for pregnant women, which might be explained by the effects of progesterones. Pregnant women are less likely to have typical symptoms of SARS-CoV-2 infection such as fever, dyspnoea, and muscle pain, but are more likely to be admitted to the ICU or require invasive ventilation than other non-pregnant women of childbearing age. Of course, other risk factors for COVID-19, such as pre-existing comorbidities, ethnicity, chronic hypertension, pre-existing diabetes, high maternal age, and high body mass index (BMI), also carry the potential for more severe viral infections during pregnancy. Pregnant women with COVID-19 are at increased risk of preterm birth, gestational toxaemia, caesarean section, maternal mortality, and admission to the ICU. Newborns are also more likely to require neonatal intensive care [21,116].
During pregnancy, a number of physiological changes occur in the body, including changes in the function of the immune system. It is known that in pregnant women, from the start of implantation, the immune response shifts towards a Th2 type tolerogenic immune response that provides the optimal microenvironment for the development of the foetus in the maternal uterus. The predominant Th2 immunity then switches to a Th1 dominance at the end of pregnancy that is required for labour induction [117]. Along with the number of circulating NK cells, the number of pDCs also decreases as pregnancy progresses [118,119]. Furthermore, in vitro experiments have already showed that after H1N1 infection, pDCs of pregnant women produce less IFNα compared to non-pregnant women [119]. This may explain why pregnant women are more severely affected during influenza as well as COVID-19 pandemics [120]. Progesterone hormone levels also increase in women during pregnancy, and their immunosuppressive properties and negative effects on the functions of pDCs are well known [121]. In contrast to oestrogen, progesterone and its synthetic analogues inhibit the activity of innate immune cells and negatively regulate the secretion of type I IFNs in human pDCs [122]. In vitro experiments have shown that progesterone and depo-medroxyprogesterone acetate (DMPA), a synthetic form of progesterone, inhibit TLR9 activation-induced IFNα secretion in mouse and human pDCs. In vivo vesicular stomatitis virus (VSV) infection has also been shown to significantly lower serum IFNα levels in DMPA-treated mice compared to DMPA-untreated mice. The inhibitory effect of progesterone may be due to the inhibition of TLR9 activation-induced nuclear translocation of the transcription factor IRF7 in pDCs [123,124]. These data indicate that the enhanced tolerogenic responses to protect the foetus during pregnancy and the negative effect of progesterone on pDCs’ type I IFN production make pregnant women more vulnerable to viral infections including SARS-CoV-2 infection.
The question may arise whether it is safe to use type I IFN therapy in pregnant women. A meta-analysis concluded that IFNα did not significantly increase the risk of developmental abnormalities, miscarriages, stillbirths, or preterm births in women exposed to IFNs during pregnancy [125]. Thus, in pregnant women suffering from severe COVID-19, if the possibility of IFN therapy arises, it may be safe to use.
5.3. The Role of Age in Impaired IFN Production
Age is a very prominent clinical risk factor of COVID-19 mortality [126]. This is supported by the fact that the mortality rate of COVID-19 was found to be lower in patients under 60 years of age (1.4%) than in those over 60 years of age (4.5%) [127]. Increased morbidity and mortality in the elderly are likely to be caused by a shift in the innate immune system towards inflammation, as well as age-related cellular changes and abnormalities in antiviral signaling pathways leading to delayed, prolonged type I IFN production. In the elderly, the basic inflammatory phenotype may result in a late type I IFN response during viral infections that has been previously observed in the case of SARS-CoV infection as well [128]. Delayed antiviral type I IFN response leads to increased tissue damage and cytokine storm, which is also the characteristic of severe COVID-19 [128,129]. With regard to SARS-CoV infection, it has also been previously reported that the frequency of pro-inflammatory macrophages and alveolar macrophages in the lung may also shift due to the disruption of IFN production [130]. Furthermore, during viral infections, type I IFNs support NK cell activation, while inhibit pathological responses mediated by neutrophil granulocytes and type II innate lymphoid cells (ILC2) in the infected mucosa [129,131,132].
In addition, the efficiency of the early type I IFN response is decreased with age due to the descending number of IFN-producing macrophages and DCs, and impairment of signaling pathways implicated in IFN production [133,134,135,136]. While the myeloid DC population persists with advancing age, a decline in the number and function of pDCs was reported in association with aging [135,136,137,138,139,140]. In the elderly, the decline in IFN-producing ability of pDCs is partly due to the decreased TLR7/9 expression [135] and functional impairment of IRF7 [138]. These processes are associated with increased reactive oxygen species (ROS) levels and cell damage observed in aging cells [141]. Furthermore, aging also affects the RIG-I/MDA-5 signaling pathway, as proteasomal degradation of TRAF3 is increased in elderly human monocytes, making IRF3 activation less efficient and thus results in lower production of antiviral IFNs [133]. In contrast to adults, the nasal epithelial cells, macrophages and DCs of children are abundant in receptors such as RIG-I and MDA5. The high baseline expression of these sensors results in a stronger, immediate antiviral response against SARS-CoV-2 that can partially explain the lower sensitivity of children to the more severe symptoms of COVID-19 [142].
Thus, impairment of type I IFN production pathways, delayed IFN response, and pDC dysfunction in elderly individuals greatly reduce the chances of overcoming SARS-CoV-2 infection [129,143].
5.4. The Role of Microbiome in Antiviral IFN Production
A healthy gut microbiome is essential to support the host’s immune responses. On the one hand, it prevents the activation of pro-inflammatory cascades, on the other hand, it prepares the body for future viral infections [144,145]. However, in the state of dysbiosis, these protective functions are impaired. Many studies suggest that the clinical manifestation and severity of COVID-19 may be linked to gut dysbiosis [144,145,146,147]. Furthermore, SARS-CoV-2 infection can also alter the microbial composition of the lung indicating that serious inflammation occurs in lung tissues. The level of inflammation detected in the lung was significantly correlated with the levels of pathogenic microorganisms and SARS-CoV-2 [148]. Dysbiosis of the respiratory tract in hospitalized COVID-19 patients leads to accelerated destabilization over time and correlates with disease severity and systemic immune activation [149]. In intubated patients an enrichment of Staphylococcus species can be observed. Moreover, the small commensal DNA viruses, Anelloviridae and Redondoviridae showed increased titer and colonization in severe COVID-19 as well [149]. In the upper respiratory tract the bacterial load, bacterial richness consistently increased, while the abundance of an amplicon sequence variant, Corynebacterium_unclassified.ASV0002 decreased, as disease severity increased [150].
Various commensal intestinal bacteria with beneficial immunomodulatory potential, such as Faecalibacterium prausnitzii, Eubacterium rectale, and bifidobacteria were reduced in COVID-19 patients and their frequency remained low even after 30 days of recovery from COVID-19. The decline in beneficial gut bacteria was correlated with increased disease severity, and elevated levels of inflammatory markers and cytokines in the patients ’plasma. This may suggest that the microbial disturbance that persists after disease resolution may contribute to post-COVID syndrome [151]. Another study also found that decreased commensal species and increased opportunistic pathogenic species characterize the gut of COVID-19 patients. Severe illness was associated with the abundance of Burkholderia contaminans, Bacteroides nordii and Blautia sp. CAG 257. The abundance of Burkholderia contaminans was correlated with higher levels of inflammation and lower number of immune cells [152]. In addition, a decrease in Lactobacillus and Bifidobacterium species, which play important roles in protecting against intestinal infections by stimulating intestinal functions, promoting immune responses, and preventing the overgrowth of pathogenic species, has been observed in COVID-19 patients with intestinal dysbiosis [153].
Since the commensal microbial flora is vital to maintain the baseline IFN secretion in the human body, dysbiosis might lead to a decreased antiviral immune response. The stimulatory signals from commensal bacteria keep the immune cells as well as the stromal cells in constant state of antiviral readiness. Among others, they maintain the constitutive, low-level IFN production of pDCs [154], the baseline activity of mononuclear phagocytes and NK cells [155], the baseline production of IFN by lung stromal cells, and thus the constitutive expression of antiviral Mx proteins [40]. It is important to note that antibiotic therapy can easily destroy this vulnerable system, since antibiotics not only target pathogenic bacteria but also kill or drastically reduce the numbers of commensal bacteria, which are responsible for sustaining tonic levels of IFN signals, and thus antibiotics eliminate the body’s baseline antiviral state [40,41], and increase the risk of viral infections and inflammatory conditions [39]. This phenomenon was elegantly demonstrated using a mouse model. When mice with healthy intestinal flora were infected with influenza virus, 80% of the mice survived. However, with antibiotic pre-treatment, only one-third of the mice survived the infection, but faecal transplantation could rescue mice from pathogen-induced death/sepsis. These results indicate that a healthy intestinal flora provide a strong protection against influenza, as the gut microbiota-driven systemic antiviral immunity was already active when the virus entered the body. On the contrary, in the absence of intestinal bacteria, the antiviral genes only turn on when the immune response is triggered. However, this sometimes happens too late, when the virus has already multiplied in the body and thus the high viral load leads to an exaggerated, detrimental immune response [40]. In line with that, a significant correlation was found between previous antibiotic exposure and increased severity of COVID-19 in Spain [156]. Thus, it can be assumed that among many other factors, dysbiosis caused by the overuse of antibiotics, may be listed as a risk factor for severe COVID-19.
These data suggest that the use of probiotics as a prophylaxis may be advisable to reduce the incidence of respiratory infections [157,158]. Several data indicate that prebiotics and probiotics are able to enhance the type I IFN response of pDCs through TLR9 stimulation and thus provide a more effective antiviral response [159,160,161,162]. Besides probiotics, it may be advisable to increase the intake of anti-inflammatory foods, such as vegetables and fruits, as a high-fiber diet serves as a good source of carbohydrates for beneficial bacteria. In addition, foods with a high polyphenol content, such as vegetables, fruits, cereals, tea, coffee, dark chocolate or cocoa powder, have prebiotic or antimicrobial properties and thus can effectively inhibit the replication of pathogens in the body [163,164,165]. Therefore, a proper, personalized diet might help to prevent coronavirus infection and might contribute to patients’ recovery, as well as might help to eliminate dysbiosis caused by the infection and restore the gut microbiota after recovery from COVID-19.
5.5. Obesity and Antiviral IFNs
So far it seems, that obesity also predisposes to a more severe course of SARS-CoV-2 infection [166]. Diabetes, hypertension, and cardiovascular diseases, which are risk factors of COVID-19, are commonly associated with obesity. For example, one study found that 74% of diabetics were obese, which may further exacerbate the severity of COVID-19 in this disease group [167]. When obesity, diabetes, hypertension, and dyslipidemia occur together, it is called metabolic syndrome, a disease, which is also associated with increased COVID-19 mortality [168]. Thus, obesity is not only a single risk factor, but by acting synergistically with other underlying diseases it may further increase the incidence of critical SARS-CoV-2 infection.
According to a comprehensive study examining data from 5700 hospitalized patients infected with SARS-CoV-2, obesity (41.7%) is the second most common comorbidity in COVID-19 after hypertension (56.6%) [169]. According to a French study, 47.6% of patients in the ICU had a BMI above 30 kg/m2, while 28.2% had a BMI above 35 kg/m2 [170]. Reports from two Spanish ICUs also confirmed that obesity is the most common comorbidity that occurred in half of the patients admitted to hospital [171]. However, data from 6 New York University hospitals show an inverse correlation between BMI and age among those admitted to the ICU. Although the risk of severe disease in SARS-CoV-2 infection increases with age, younger patients with critical disease were more likely to be obese [172]. A meta-analysis found that obese people were 113% more likely to be hospitalized, 74% more likely to be admitted to an ICU, and 48% more likely to die [173].
More severe COVID-19 symptoms in obese individuals may be caused by a weaker and prolonged type I IFN response that results in a decreased antiviral immune response. In obese people, the serum level of the hormone leptin produced by fat cells is high, which may indicate leptin resistance. Leptin may induce the expression and activation of suppressor of cytokine signaling (SOCS) 3 and while decrease the type I IFN response in obese individuals [174,175,176]. Type I IFNs and leptin use the same JAK–STAT signaling pathway that can be inhibited by SOCS3 and that results in a lower IFN response to viral infections in obese individuals [174,177]. It has recently been shown that the baseline SOCS3 expression is increased and correlates with a decreased type I IFN response in obese patients [177]. Due to this reason, obese individuals are also much more susceptible to infections and are characterized by higher mortality during seasonal influenza epidemics [178,179]. In addition, increased inflammatory cytokines levels, enhanced M1 polarization of lung macrophages and impaired IFN response and ISG induction by respiratory epithelial cells and macrophages can be observed in obesity that can eventually lead to more severe pneumonia and lung damage in obese individuals [178,179]. Furthermore, the diet of obese individuals is generally high in fat, which can also lead to dysbiosis, thereby further reducing the intensity of the type I IFN response [174]. Collectively, obese patients are characterized by reduced IFN production, and thus might provide a microenvironment that allows the emergence of novel virulent variants of the virus [178].
5.6. Underlying Chronic Medical Conditions Associated with Impaired IFN Response Causing Immunosuppression
We had already mentioned that chronic diseases such as diabetes, hypertension, obesity are listed as the underlying cause in the majority of COVID-19 mortality. However, the proportion of another group of diseases, the immunosuppression-associated chronic diseases, which are caused by either endogenous immunodysfunctions or immunosuppressive treatments, is also remarkably high [180]. This group includes, but is not limited to, primary and secondary immune deficiencies, cancers, chronic renal failure, post-transplant organ status, and autoimmune diseases. Particular attention should be paid to this group of diseases, as not only the patients themselves are at increased risk, but also their immediate environment, as immunosuppressed individuals can serve as “reservoirs” for viruses and might remain infectious for up to several months [181,182,183]. Furthermore, it may also be of concern that viral pneumonia may occur atypically with low inflammatory markers in these patients, but later can be associated with a more severe disease course [184].
Studies have shown that patients with primary and secondary immunodeficiencies are characterized by increased morbidity and mortality from COVID-19 compared to the general population [185]. A meta-analysis also supports increased mortality in patients with chronic renal failure associated with immunosuppression [186,187]. Cancer patients with COVID-19 are 3.5 times more likely to be admitted to the ICU and to need mechanical ventilation, and are more prone to infections with SARS-CoV-2, which is eliminated later from their bodies compared to the general population [188,189]. In the case of autoimmune diseases, however, the situation is more complicated. Although COVID-19 is more severe compared to influenza in autoimmune patients [190], it appears that low-dose immunosuppressive therapy may provide protection against the complications of COVID-19 in these patients [191,192].
Immunosuppressive agents used to treat certain autoimmune conditions also affect the production of type I IFNs, as well as the functions of pDCs, and may predispose to more severe viral infections. For example, steroids have been reported to reduce the number and type I IFN responses of pDCs in systemic lupus erythematosus (SLE) patients; however, it is important to note that after the discontinuation of glucocorticoids, both pDC number and IFNα levels recovered rapidly in the patients [193,194]. Hydrochloroquine also reduces type I IFN production of TLR7 or TLR9 activated pDCs in patients with SLE [195] and also inhibits TLR9 activation-induced type I IFN production by pDCs of cutaneous lupus erythematosus patients [196]. Furthermore, the active form of mycophenolate mofetil, mycophenolic acid, is also able to dose-dependently reduce CpG-induced type I IFN secretion in pDCs of SLE patients by inhibiting nuclear translocation of IRF7 [197]. Furthermore, baricitinib, which inhibits the JAK/STAT pathway, is able to inhibit IFN secretion by pDCs and thus increases the risk of varicella reactivation as well [198,199].
It is important to note that besides the above mentioned immunomodulatory effects, some IFN response inhibitors, also exhibit direct antiviral activity as well. For example, chloroquine interferes with different stages of the viral life cycle including viral entry, uncoating, assembly and budding. Via increasing endosomal pH chloroquine blocks virus-endosome fusion and is also able to inhibit posttranslational modifications of viral proteins by interfering with proteolytic processes [200,201]. Furthermore, mycophenolate mofetil was able to inhibit SARS-COV-2 replication in vitro. Similar antiviral activity was observed for calcineurin and mTOR inhibitors as well as thiopurine analogs against SARS-CoV and MERS-CoV strains [202]. Nevertheless, immunosuppressive agents may still be detrimental in the initial phase of COVID-19, since the weakened immune system cannot adequately control viral replication. However, in the later stages of the disease, the immunosuppressive effects of these drugs may be particularly beneficial, as they may prevent an overzealous immune response, the development of cytokine storm, and multi-organ failure. Thus, as previously mentioned, low-dose immunosuppression may have a beneficial effect in autoimmune patients, as it may alleviate the severe symptoms of COVID-19 caused by the body’s overactivated immune response [191,192].
6. Discussion
The severity of viral infections can greatly vary among individuals, as a wide array of endogenous and exogenous factors can affect an individual’s type I IFN response, which is one of the most important weapons of our immune system that can rapidly inhibit the replication of viruses [24]. Our body prepares to defend against viral infections long before viral exposure, owing to the constituent baseline type I IFN production by various tissues and cell types that creates a general antiviral state in the host [24]. In accordance with that, it was observed that the incidence and mortality of severe COVID-19 caused by SARS-CoV-2 is also significantly higher in individuals with an inadequate type I IFN response [203,204,205].
Interestingly, a robust type I IFN response can be observed in some patients that may result in early control of the infection and thus in a mild course. The sign of an effective IFN response in these patients may be reflected by the appearance of a skin condition called “COVID toes” [206]. The lesion is reminiscent of perniosis, which is an inflammatory condition caused by cold, and associated with red-purple discoloration and blistering of the acral areas. Histologically, edema of the epidermis as well as perivascular and perieccrine lymphocytic infiltration are present, and even microthrombi may form in the blood vessels. Similar changes can be observed in a rare cutaneous form of lupus, the so-called familiar chilblain lupus (FCL), which can be classified as an interferonopathy and is associated with increased type I IFN production [207]. Therefore, one might suppose that COVID toes are probably caused by the increased systemic type I IFN secretion and thus might serve as a marker for patients with efficient viral clearance and mild course of COVID-19 [206].
An individual’s IFN signature may also be adversely affected by viral evasion mechanisms as well as by host-dependent factors, which result in low or delayed IFN response and high viral load that impacts the severity of COVID-19 symptoms [203,208]. Thus, individuals with reduced IFN signature, such as patients with congenital defects of the IFN pathway, men, the elderly, people suffering from dysbiosis, obese or immunosuppressed individuals and pregnant women may benefit from type I IFN therapy in the early phase of the disease or as prophylaxis. Thus, the therapeutic application of type I IFNs in COVID-19 is in the focus of several ongoing clinical studies [209]. However, in order to treat COVID-19 more effectively, the type, dose, route and frequency of administration of the most optimal therapeutic IFNs subtype and the time of intervention needs to be optimized. PEGylated forms may be preferred over unmodified IFNs, since those can be administered by subcutaneous injection once a week. It should be noted that IFN injection elicits a systemic response and induces antiviral, pro-inflammatory and anti-inflammatory effects simultaneously. In contrast, the local action of inhaled type I IFNs in the airways may compensate for the production of IFNβ by epithelial cells and may have excellent preventive potential when used as a nasal drop [210,211,212,213,214]. Another important issue is the subtype of IFN used in therapy, since while IFNα has a strong antiviral effect, IFNβ also has immunomodulatory and antiproliferative effects as well [215,216]. It is also important to emphasize that the use of IFNs in patients with severe or critical COVID-19 is not advised, since IFNs significantly enhance the inflammatory state in the later stages of the infection and exacerbate cytokine storm and lung injury similar to the inflammation boosting effect of delayed type I IFN responses in severe COVID-19 patients [217].
The results of clinical trials regarding IFN therapies are encouraging [209]; however, prior to use it is essential to consider the condition of the patient, the effects of the treatment, and the possible side effects of the IFN subtype applied in these therapies.
7. Conclusions
In summary, an individual’s IFN signature is a major factor influencing the severity of COVID-19 outcome, which is strongly associated with the activity and number of pDCs. If lifestyle-related factors, which are detrimental to type I IFN responses or normal pDC functions, accumulate in a given population, a more severe epidemic can be expected.
The COVID-19 pandemic shed light on the importance of adequate/sufficient IFN responses and on the benefits of early or prophylactic IFN therapies. Therefore, a comprehensive analysis of the complex and pleiotropic effects of type I IFNs are needed to gain a clear understanding on the specific functions, kinetic profiles, tissue- and cell-type specific effects of all subtypes of type I IFNs (including the 13 subtypes of IFN-α along with IFN-β, IFN-ε, IFN-κ, IFN-ω, IFN-δ, IFN-ζ, and IFN-τ) and their efficacy against novel virus variants. In addition to SARS-CoV-2 infection, data obtained from such studies might also apply to other viral infections. Thus, patient with known risk factors might be targeted by the most optimal type I IFN therapy in the early stage of the infection that could significantly reduce disease severity and mortality upon the possible emergence of a new pandemic.
Author Contributions
D.B., T.F. and K.P. have a substantial contribution to the literature search and manuscript writing. All authors approved the submitted version of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research, Development and Innovation Office (NKFIH FK 128294 to K.P., NKFIH PD 135193 to T.F.). The work was also supported by GINOP-2.3.2-15-2016-00050 project. The project is co-financed by the European Union and the European Regional Development Fund. K.P. and D.B. were supported by ÚNKP-21-05-DE-170 and ÚNKP-21-3-II-DE-21 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund. K.P. was further supported by the János Bolyai Research Scholarship from the Hungarian Academy of Sciences (bo_122_19).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, A.; Singh, R.; Kaur, J.; Pandey, S.; Sharma, V.; Thakur, L.; Thakur, L.; Sati, S.; Mani, S.; Asthana, S.; et al. Wuhan to World: The COVID-19 Pandemic. Front. Cell Infect. Microbiol. 2021, 11, 596201. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, C.; Malani, P.N. COVID-19 in 2022-The Beginning of the End or the End of the Beginning? JAMA 2022, 327, 2389–2390. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.-L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; on behalf of the HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Husain, M. COVID and the brain. Brain 2021, 144, 3545–3546. [Google Scholar] [CrossRef]
- Delli Muti, N.; Finocchi, F.; Tossetta, G.; Salvio, G.; Cutini, M.; Marzioni, D.; Balercia, G. Could SARS-CoV-2 infection affect male fertility and sexuality? APMIS 2022, 130, 243–252. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Delli Muti, N.; Balercia, G.; Ciavattini, A.; Giannubilo, S.R.; Marzioni, D. Preeclampsia and severe acute respiratory syndrome coronavirus 2 infection: A systematic review. J. Hypertens. 2022, 40, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E. Infection of liver hepatocytes with SARS-CoV-2. Nat. Metab. 2022, 4, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L.; et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Mehandru, S.; Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Pavli, A.; Tsakris, A. Post-COVID Syndrome: An Insight on Its Pathogenesis. Vaccines 2021, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2021, 20. [Google Scholar] [CrossRef]
- Abdel-Gawad, M.; Zaghloul, M.S.; Abd-Elsalam, S.; Hashem, M.; Lashen, S.A.; Mahros, A.M.; Mohammed, A.Q.; Hassan, A.M.; Bekhit, A.N.; Mohammed, W.; et al. Post-COVID-19 Syndrome Clinical Manifestations: A Systematic Review. Antiinflamm. Antiallergy Agents Med. Chem. 2022, 28. [Google Scholar] [CrossRef]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Levin, A.T.; Hanage, W.P.; Owusu-Boaitey, N.; Cochran, K.B.; Walsh, S.P.; Meyerowitz-Katz, G. Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 2020, 35, 1123–1138. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Zhou, D.; Coomar, D.; Sheikh, J.; Lawson, H.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- Kompaniyets, L.; Goodman, A.B.; Belay, B.; Freedman, D.S.; Sucosky, M.S.; Lange, S.J.; Gundlapalli, A.V.; Boehmer, T.K.; Blanck, H.M. Body Mass Index and Risk for COVID-19-Related Hospitalization, Intensive Care Unit Admission, Invasive Mechanical Ventilation, and Death—United States, March-December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Zhang, Q.; Zhang, S.Y.; Jouanguy, E.; Casanova, J.L. Type I interferons and SARS-CoV-2: From cells to organisms. Curr. Opin. Immunol. 2022, 74, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Bencze, D.; Fekete, T.; Pazmandi, K. Type I Interferon Production of Plasmacytoid Dendritic Cells under Control. Int. J. Mol. Sci. 2021, 22, 4190. [Google Scholar] [CrossRef]
- Palermo, E.; Di Carlo, D.; Sgarbanti, M.; Hiscott, J. Type I Interferons in COVID-19 Pathogenesis. Biology 2021, 10, 829. [Google Scholar] [CrossRef]
- Van der Sluis, R.M.; Holm, C.K.; Jakobsen, M.R. Plasmacytoid dendritic cells during COVID-19: Ally or adversary? Cell Rep. 2022, 40, 111148. [Google Scholar] [CrossRef]
- Bridges, J.P.; Vladar, E.K.; Huang, H.; Mason, R.J. Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax 2022, 77, 203–209. [Google Scholar] [CrossRef]
- Guo, M.; Tao, W.; Flavell, R.A.; Zhu, S. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 269–283. [Google Scholar] [CrossRef]
- Simsek, C.; Erul, E.; Balaban, H.Y. Role of gastrointestinal system on transmission and pathogenesis of SARS-CoV-2. World J. Clin. Cases 2021, 9, 5427–5434. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, J.; Ou, X.; Cheng, W.; Qin, Y.; Guo, Y.; Jiang, Y. Alimentary system is directly attacked by SARS-COV-2 and further prevents immune dysregulation caused by COVID-19. Int. J. Clin. Pract. 2021, 75, e13893. [Google Scholar] [CrossRef]
- Denney, L.; Ho, L.P. The role of respiratory epithelium in host defence against influenza virus infection. Biomed. J. 2018, 41, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Schnepf, D.; Staeheli, P. Interferon-lambda orchestrates innate and adaptive mucosal immune responses. Nat. Rev. Immunol. 2019, 19, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Hatton, C.F.; Botting, R.A.; Duenas, M.E.; Haq, I.J.; Verdon, B.; Thompson, B.J.; Spegarova, J.S.; Gothe, F.; Stephenson, E.; Gardner, A.I.; et al. Delayed induction of type I and III interferons mediates nasal epithelial cell permissiveness to SARS-CoV-2. Nat. Commun. 2021, 12, 7092. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.Q.; Huang, M.; Sun, X.; Deng, F.; Wang, H.; Ning, Y.J. Immune evasion of SARS-CoV-2 from interferon antiviral system. Comput. Struct. Biotechnol. J. 2021, 19, 4217–4225. [Google Scholar] [CrossRef]
- Beyer, D.K.; Forero, A. Mechanisms of Antiviral Immune Evasion of SARS-CoV-2. J. Mol. Biol. 2022, 434, 167265. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Barrett, B.S.; Mickens, K.L.; Vladar, E.K.; Morrison, J.H.; Hasenkrug, K.J.; Poeschla, E.M.; Santiago, M.L. Interferon Resistance of Emerging SARS-CoV-2 Variants. bioRxiv 2021, 10. preprint. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Del-Barco, D.; Risco-Acevedo, D.; Berlanga-Acosta, J.; Martos-Benitez, F.D.; Guillen-Nieto, G. Revisiting Pleiotropic Effects of Type I Interferons: Rationale for Its Prophylactic and Therapeutic Use Against SARS-CoV-2. Front. Immunol. 2021, 12, 655528. [Google Scholar] [CrossRef]
- Kawashima, T.; Kosaka, A.; Yan, H.; Guo, Z.; Uchiyama, R.; Fukui, R.; Kaneko, D.; Kumagai, Y.; You, D.-J.; Carreras, J.; et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-beta. Immunity 2013, 38, 1187–1197. [Google Scholar] [CrossRef]
- Bradley, K.C.; Finsterbusch, K.; Schnepf, D.; Crotta, S.; Llorian, M.; Davidson, S.; Fuchs, S.Y.; Staeheli, P.; Wack, A. Microbiota-Driven Tonic Interferon Signals in Lung Stromal Cells Protect from Influenza Virus Infection. Cell Rep. 2019, 28, 245–256.e4. [Google Scholar] [CrossRef]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.B.; Ferraro, M.; Zheng, H.M.; Patel, N.; Gould-Fogerite, S.; Fitzgerald-Bocarsly, P. Viral induction of low frequency interferon-alpha producing cells. Virology 1994, 204, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald-Bocarsly, P. Natural interferon-alpha producing cells: The plasmacytoid dendritic cells. Biotechniques 2002, 33, S16–S29. [Google Scholar] [CrossRef]
- Yin, Z.; Dai, J.; Deng, J.; Sheikh, F.; Natalia, M.; Shih, T.; Lewis-Antes, A.; Amrute, S.B.; Garrigues, U.; Doyle, S.; et al. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J. Immunol. 2012, 189, 2735–2745. [Google Scholar] [CrossRef]
- Sposito, B.; Broggi, A.; Pandolfi, L.; Crotta, S.; Clementi, N.; Ferrarese, R.; Sisti, S.; Criscuolo, E.; Spreafico, R.; Long, J.M.; et al. The interferon landscape along the respiratory tract impacts the severity of COVID-19. Cell 2021, 184, 4953–4968.e16. [Google Scholar] [CrossRef]
- Park, A.; Iwasaki, A. Type I and Type III Interferons—Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe 2020, 27, 870–878. [Google Scholar] [CrossRef]
- Kim, Y.M.; Shin, E.C. Type I and III interferon responses in SARS-CoV-2 infection. Exp. Mol. Med. 2021, 53, 750–760. [Google Scholar] [CrossRef]
- Choi, H.; Shin, E.C. Roles of Type I and III Interferons in COVID-19. Yonsei Med. J. 2021, 62, 381–390. [Google Scholar] [CrossRef]
- Severa, M.; Diotti, R.A.; Etna, M.P.; Rizzo, F.; Fiore, S.; Ricci, D.; Iannetta, M.; Sinigaglia, A.; Lodi, A.; Mancini, N.; et al. Differential plasmacytoid dendritic cell phenotype and type I Interferon response in asymptomatic and severe COVID-19 infection. PLoS Pathog. 2021, 17, e1009878. [Google Scholar] [CrossRef]
- Fanning, S.L.; George, T.C.; Feng, D.; Feldman, S.B.; Megjugorac, N.J.; Izaguirre, A.G.; Fitzgerald-Bocarsly, P. Receptor cross-linking on human plasmacytoid dendritic cells leads to the regulation of IFN-alpha production. J. Immunol. 2006, 177, 5829–5839. [Google Scholar] [CrossRef]
- Grage-Griebenow, E.; Loseke, S.; Kauth, M.; Gehlhar, K.; Zawatzky, R.; Bufe, A. Anti-BDCA-4 (neuropilin-1) antibody can suppress virus-induced IFN-alpha production of plasmacytoid dendritic cells. Immunol. Cell Biol. 2007, 85, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Onodi, F.; Bonnet-Madin, L.; Meertens, L.; Karpf, L.; Poirot, J.; Zhang, S.Y.; Picard, C.; Puel, A.; Jouanguy, E.; Zhang, Q.; et al. SARS-CoV-2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4. J. Exp. Med. 2021, 218, e20201387. [Google Scholar] [CrossRef] [PubMed]
- Venet, M.; Ribeiro, M.S.; Décembre, E.; Bellomo, A.; Joshi, G.; Villard, M.; Cluet, D.; Perret, M.; Pescamona, R.; Paidassi, H.; et al. SARS-CoV-2 infected cells trigger an acute antiviral response mediated by Plasmacytoid dendritic cells in mild but not severe COVID-19 patients. medRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Assil, S.; Coleon, S.; Dong, C.; Decembre, E.; Sherry, L.; Allatif, O.; Webster, B.; Dreux, M. Plasmacytoid Dendritic Cells and Infected Cells Form an Interferogenic Synapse Required for Antiviral Responses. Cell Host Microbe 2019, 25, 730–745.e6. [Google Scholar] [CrossRef] [PubMed]
- Peruzzi, B.; Bencini, S.; Capone, M.; Mazzoni, A.; Maggi, L.; Salvati, L.; Vanni, A.; Orazzini, C.; Nozzoli, C.; Morettini, A.; et al. Quantitative and qualitative alterations of circulating myeloid cells and plasmacytoid DC in SARS-CoV-2 infection. Immunology 2020, 161, 345–353. [Google Scholar] [CrossRef]
- Laing, A.G.; Lorenc, A.; Del Molino Del Barrio, I.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef]
- Liu, C.; Martins, A.J.; Lau, W.W.; Rachmaninoff, N.; Chen, J.; Imberti, L.; Mostaghimi, D.; Fink, D.L.; Burbelo, P.D.; Dobbs, K.; et al. Time-resolved systems immunology reveals a late juncture linked to fatal COVID-19. Cell 2021, 184, 1836–1857.e22. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tsang, O.T.-Y.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020, 5, eabd7114. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Bolouri, H.; Speake, C.; Skibinski, D.; Long, S.A.; Hocking, A.M.; Campbell, D.J.; Hamerman, J.A.; Malhotra, U.; Buckner, J.H. The COVID-19 immune landscape is dynamically and reversibly correlated with disease severity. J. Clin. Investig. 2021, 131, e143648. [Google Scholar] [CrossRef] [PubMed]
- Saichi, M.; Ladjemi, M.Z.; Korniotis, S.; Rousseau, C.; Ait Hamou, Z.; Massenet-Regad, L.; Amblard, E.; Noel, F.; Marie, Y.; Bouteiller, D.; et al. Single-cell RNA sequencing of blood antigen-presenting cells in severe COVID-19 reveals multi-process defects in antiviral immunity. Nat. Cell Biol. 2021, 23, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cerrillo, I.; Landete, P.; Aldave, B.; Sanchez-Alonso, S.; Sanchez-Azofra, A.; Marcos-Jimenez, A.; Ávalos, E.; Alcaraz-Serna, A.; Santos, I.D.L.; Mateu-Albero, T.; et al. COVID-19 severity associates with pulmonary redistribution of CD1c+ DCs and inflammatory transitional and nonclassical monocytes. J. Clin. Investig. 2020, 130, 6290–6300. [Google Scholar] [CrossRef]
- Greene, T.T.; Zuniga, E.I. Type I Interferon Induction and Exhaustion during Viral Infection: Plasmacytoid Dendritic Cells and Emerging COVID-19 Findings. Viruses 2021, 13, 1839. [Google Scholar] [CrossRef]
- Bekeredjian-Ding, I.; Schafer, M.; Hartmann, E.; Pries, R.; Parcina, M.; Schneider, P.; Giese, T.; Endres, S.; Wollenberg, B.; Hartmann, G. Tumour-derived prostaglandin E and transforming growth factor-beta synergize to inhibit plasmacytoid dendritic cell-derived interferon-alpha. Immunology 2009, 128, 439–450. [Google Scholar] [CrossRef]
- Palucka, A.K.; Blanck, J.P.; Bennett, L.; Pascual, V.; Banchereau, J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc. Natl. Acad. Sci. USA 2005, 102, 3372–3377. [Google Scholar] [CrossRef]
- Sisirak, V.; Vey, N.; Goutagny, N.; Renaudineau, S.; Malfroy, M.; Thys, S.; Treilleux, I.; Labidi-Galy, S.I.; Bachelot, T.; Dezutter-Dambuyant, C.; et al. Breast cancer-derived transforming growth factor-beta and tumor necrosis factor-alpha compromise interferon-alpha production by tumor-associated plasmacytoid dendritic cells. Int. J. Cancer. 2013, 133, 771–778. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Sisirak, V.; Meeus, P.; Gobert, M.; Treilleux, I.; Bajard, A.; Combes, J.-D.; Faget, J.; Mithieux, F.; Cassignol, A.; et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 2011, 71, 5423–5434. [Google Scholar] [CrossRef]
- Son, Y.; Ito, T.; Ozaki, Y.; Tanijiri, T.; Yokoi, T.; Nakamura, K.; Takebayashi, M.; Amakawa, R.; Fukuhara, S. Prostaglandin E2 is a negative regulator on human plasmacytoid dendritic cells. Immunology 2006, 119, 36–42. [Google Scholar] [CrossRef]
- Fabricius, D.; Neubauer, M.; Mandel, B.; Schutz, C.; Viardot, A.; Vollmer, A.; Jahrsdörfer, B.; Debatin, K.-M. Prostaglandin E2 inhibits IFN-alpha secretion and Th1 costimulation by human plasmacytoid dendritic cells via E-prostanoid 2 and E-prostanoid 4 receptor engagement. J. Immunol. 2010, 184, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Georgel, P. Crosstalk between Interleukin-1beta and Type I Interferons Signaling in Autoinflammatory Diseases. Cells 2021, 10, 1134. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Niemeyer, C.M.; Sieff, C.A.; Mathey-Prevot, B.; Wimperis, J.Z.; Bierer, B.E.; Clark, S.C.; Nathan, D. Expression of human interleukin-3 (multi-CSF) is restricted to human lymphocytes and T-cell tumor lines. Blood 1989, 73, 945–951. [Google Scholar] [CrossRef]
- Benard, A.; Jacobsen, A.; Brunner, M.; Krautz, C.; Klosch, B.; Swierzy, I.; Naschberger, E.; Podolska, M.J.; Kouhestani, D.; David, P.; et al. Interleukin-3 is a predictive marker for severity and outcome during SARS-CoV-2 infections. Nat. Commun. 2021, 12, 1112. [Google Scholar] [CrossRef]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 145–151. [Google Scholar]
- Rod, J.E.; Oviedo-Trespalacios, O.; Cortes-Ramirez, J. A brief-review of the risk factors for COVID-19 severity. Rev. Saude Publica 2020, 54, 60. [Google Scholar] [CrossRef]
- Muniyappa, R.; Gubbi, S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E736–E741. [Google Scholar] [CrossRef]
- Trump, S.; Lukassen, S.; Anker, M.S.; Chua, R.L.; Liebig, J.; Thurmann, L.; Corman, V.M.; Binder, M.; Loske, J.; Klasa, C.; et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat. Biotechnol. 2021, 39, 705–716. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef]
- Casanova, J.L.; Su, H.C.; Effort, C.H.G. A Global Effort to Define the Human Genetics of Protective Immunity to SARS-CoV-2 Infection. Cell 2020, 181, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Elhabyan, A.; Elyaacoub, S.; Sanad, E.; Abukhadra, A.; Elhabyan, A.; Dinu, V. The role of host genetics in susceptibility to severe viral infections in humans and insights into host genetics of severe COVID-19: A systematic review. Virus Res. 2020, 289, 198163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bastard, P.; Bolze, A.; Jouanguy, E.; Zhang, S.Y.; Cobat, A.; Notarangelo, L.D.; Su, H.C.; Abel, L.; Casanova, J.-L. Life-Threatening COVID-19: Defective Interferons Unleash Excessive Inflammation. Med 2020, 1, 14–20. [Google Scholar] [CrossRef]
- van der Made, C.I.; Simons, A.; Schuurs-Hoeijmakers, J.; van den Heuvel, G.; Mantere, T.; Kersten, S.; Van Deuren, R.C.; Steehouwer, M.; Van Reijmersdal, S.V.; Jaeger, M.; et al. Presence of Genetic Variants among Young Men with Severe COVID-19. JAMA 2020, 324, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Boisson, B.; Onodi, F.; Matuozzo, D.; Moncada-Velez, M.; Maglorius Renkilaraj, M.R.L.; Zhang, P.; Meertens, L.; Bolze, A.; Materna, M.; et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 2021, 6, eabl4348. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Zhao, Y.; Liu, N.; Peng, Y.-C.; Giannoulatou, E.; Jin, R.-H.; Yan, H.-P.; Wu, H.; Liu, J.-H.; Wang, D.-Y.; et al. Interferon-Induced Transmembrane Protein 3 Genetic Variant rs12252-C Associated with Disease Severity in Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 34–37. [Google Scholar] [CrossRef]
- Alghamdi, J.; Alaamery, M.; Barhoumi, T.; Rashid, M.; Alajmi, H.; Aljasser, N.; lhendi, Y.; Alkhalaf, H.; Alqahtani, H.; Algablan, O.; et al. Interferon-induced transmembrane protein-3 genetic variant rs12252 is associated with COVID-19 mortality. Genomics 2021, 113, 1733–1741. [Google Scholar] [CrossRef]
- Severe COVID-19 GWAS Group. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [CrossRef]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D.; et al. Genetic mechanisms of critical illness in COVID-19. Nature 2021, 591, 92–98. [Google Scholar] [CrossRef]
- De Toma, I.; Dierssen, M. Network analysis of Down syndrome and SARS-CoV-2 identifies risk and protective factors for COVID-19. Sci. Rep. 2021, 11, 1930. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Koning, R.; Bastard, P.; Casanova, J.L.; Brouwer, M.C.; van de Beek, D.; Amsterdam, U.M.C. COVID-19 Biobank Investigators. Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 2021, 47, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Manry, J.; Laval, G.; Patin, E.; Fornarino, S.; Itan, Y.; Fumagalli, M.; Sironi, M.; Tichit, M.; Bouchier, C.; Casanova, J.-L.; et al. Evolutionary genetic dissection of human interferons. J. Exp. Med. 2011, 208, 2747–2759. [Google Scholar] [CrossRef]
- Ma, S.; Wang, C.; Mao, X.; Hao, Y. B Cell Dysfunction Associated with Aging and Autoimmune Diseases. Front. Immunol. 2019, 10, 318. [Google Scholar] [CrossRef]
- Bastard, P.; Gervais, A.; Le Voyer, T.; Rosain, J.; Philippot, Q.; Manry, J.; Michailidis, E.; Hoffmann, H.-H.; Eto, S.; Garcia-Prat, M.; et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci. Immunol. 2021, 6, eabl4340. [Google Scholar] [CrossRef]
- Ghosh, S.; Klein, R.S. Sex Drives Dimorphic Immune Responses to Viral Infections. J. Immunol. 2017, 198, 1782–1790. [Google Scholar] [CrossRef]
- Kadel, S.; Kovats, S. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front. Immunol. 2018, 9, 1653. [Google Scholar] [CrossRef]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Scully, E.P.; Haverfield, J.; Ursin, R.L.; Tannenbaum, C.; Klein, S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020, 20, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, L.; Fragale, A.; Romagnoli, G.; Parlato, S.; Lapenta, C.; Santini, S.M.; Ozato, K.; Capone, I. Type I IFN-dependent antibody response at the basis of sex dimorphism in the outcome of COVID-19. Cytokine Growth Factor Rev. 2021, 58, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Laffont, S.; Rouquie, N.; Azar, P.; Seillet, C.; Plumas, J.; Aspord, C.; Guéry, J.-C. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-alpha production of plasmacytoid dendritic cells from women. J. Immunol. 2014, 193, 5444–5452. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.; Peckham, H.; Radziszewska, A.; Menon, M.; Oliveri, P.; Simpson, F.; Deakin, C.T.; Lee, S.; Ciurtin, C.; Butler, G.; et al. Sex and Pubertal Differences in the Type 1 Interferon Pathway Associate with Both X Chromosome Number and Serum Sex Hormone Concentration. Front. Immunol. 2018, 9, 3167. [Google Scholar] [CrossRef]
- Souyris, M.; Cenac, C.; Azar, P.; Daviaud, D.; Canivet, A.; Grunenwald, S.; Pienkowski, C.; Chaumeil, J.; Mejía, J.E.; Guéry, J.-C. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 2018, 3, eaap8855. [Google Scholar] [CrossRef]
- Hagen, S.H.; Henseling, F.; Hennesen, J.; Savel, H.; Delahaye, S.; Richert, L.; Ziegler, S.M.; Altfeld, M. Heterogeneous Escape from X Chromosome Inactivation Results in Sex Differences in Type I IFN Responses at the Single Human pDC Level. Cell Rep. 2020, 33, 108485. [Google Scholar] [CrossRef]
- Gebhard, C.; Regitz-Zagrosek, V.; Neuhauser, H.K.; Morgan, R.; Klein, S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020, 11, 29. [Google Scholar] [CrossRef]
- Yanez, N.D.; Weiss, N.S.; Romand, J.A.; Treggiari, M.M. COVID-19 mortality risk for older men and women. BMC Public Health 2020, 20, 1742. [Google Scholar] [CrossRef]
- Bereshchenko, O.; Bruscoli, S.; Riccardi, C. Glucocorticoids, Sex Hormones, and Immunity. Front. Immunol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Ma, L.; Sun, L.; Fu, G.; Hou, Y. 17beta-estradiol enhances the response of plasmacytoid dendritic cell to CpG. PLoS ONE 2009, 4, e8412. [Google Scholar] [CrossRef] [PubMed]
- Seillet, C.; Laffont, S.; Tremollieres, F.; Rouquie, N.; Ribot, C.; Arnal, J.F.; Douin-Echinard, V.; Gourdy, P.; Guéry, J.-C. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood 2012, 119, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, M.; Ziegler, S.; Laffont, S.; Smith, N.; Chauveau, L.; Tomezsko, P.; Sharei, A.; Kourjian, G.; Porichis, F.; Hart, M.; et al. Sex Differences in Plasmacytoid Dendritic Cell Levels of IRF5 Drive Higher IFN-alpha Production in Women. J. Immunol. 2015, 195, 5327–5336. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Zhang, L.; Madera, R.F.; Woda, M.; Libraty, D.H. Plasmacytoid dendritic cell interferon-alpha production to R-848 stimulation is decreased in male infants. BMC Immunol. 2012, 13, 35. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Narang, K.; Enninga, E.A.L.; Gunaratne, M.; Ibirogba, E.R.; Trad, A.T.A.; Elrefaei, A.; Theiler, R.N.; Ruano, R.; Szymanski, L.M.; Chakraborty, R.; et al. SARS-CoV-2 Infection and COVID-19 During Pregnancy: A Multidisciplinary Review. Mayo Clin. Proc. 2020, 95, 1750–1765. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Veenstra van Nieuwenhoven, A.L.; Heineman, M.J.; Faas, M.M. The immunology of successful pregnancy. Hum. Reprod. Update 2003, 9, 347–357. [Google Scholar] [CrossRef]
- Vanders, R.L.; Gibson, P.G.; Murphy, V.E.; Wark, P.A. Plasmacytoid dendritic cells and CD8 T cells from pregnant women show altered phenotype and function following H1N1/09 infection. J. Infect. Dis. 2013, 208, 1062–1070. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Honein, M.A.; Rasmussen, S.A.; Williams, J.L.; Swerdlow, D.L.; Biggerstaff, M.S.; Lindstrom, S.; Louie, J.K.; Christ, C.M.; Bohm, S.R.; et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009, 374, 451–458. [Google Scholar] [CrossRef]
- Druckmann, R.; Druckmann, M.A. Progesterone and the immunology of pregnancy. J. Steroid Biochem. Mol. Biol. 2005, 97, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Mackern-Oberti, J.P.; Jara, E.L.; Riedel, C.A.; Kalergis, A.M. Hormonal Modulation of Dendritic Cells Differentiation, Maturation and Function: Implications for the Initiation and Progress of Systemic Autoimmunity. Arch. Immunol. Ther. Exp. 2017, 65, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.C.; Thomas, S.; Li, C.; Kaja, M.K.; Clark, E.A. Cutting edge: Progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J. Immunol. 2008, 180, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Huijbregts, R.P.; Helton, E.S.; Michel, K.G.; Sabbaj, S.; Richter, H.E.; Goepfert, P.A.; Hel, Z. Hormonal contraception and HIV-1 infection: Medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology 2013, 154, 1282–1295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yazdani Brojeni, P.; Matok, I.; Garcia Bournissen, F.; Koren, G. A systematic review of the fetal safety of interferon alpha. Reprod. Toxicol. 2012, 33, 265–268. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 2020, 20, 669–677. [Google Scholar] [CrossRef]
- Nikolich-Zugich, J.; Knox, K.S.; Rios, C.T.; Natt, B.; Bhattacharya, D.; Fain, M.J. SARS-CoV-2 and COVID-19 in older adults: What we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience 2020, 42, 505–514. [Google Scholar] [CrossRef]
- Acharya, D.; Liu, G.; Gack, M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020, 20, 397–398. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Zheng, J.; Wohlford-Lenane, C.; Abrahante, J.E.; Mack, M.; Sompallae, R.; McCray, P.B.; Meyerholz, D.K.; Perlman, S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Investig. 2019, 129, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Duerr, C.U.; McCarthy, C.D.; Mindt, B.C.; Rubio, M.; Meli, A.P.; Pothlichet, J.; Eva, M.M.; Gauchat, J.-F.; Qureshi, S.T.; Mazer, B.D.; et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat. Immunol. 2016, 17, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Molony, R.D.; Nguyen, J.T.; Kong, Y.; Montgomery, R.R.; Shaw, A.C.; Iwasaki, A. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci. Signal. 2017, 10, eaan2392. [Google Scholar] [CrossRef] [PubMed]
- Pillai, P.S.; Molony, R.D.; Martinod, K.; Dong, H.; Pang, I.K.; Tal, M.C.; Solis, A.G.; Bielecki, P.; Mohanty, S.; Trentalange, M.; et al. Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science 2016, 352, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cabezas, B.; Naranjo-Gomez, M.; Fernandez, M.A.; Grifols, J.R.; Pujol-Borrell, R.; Borras, F.E. Reduced numbers of plasmacytoid dendritic cells in aged blood donors. Exp. Gerontol. 2007, 42, 1033–1038. [Google Scholar] [CrossRef]
- Jing, Y.; Shaheen, E.; Drake, R.R.; Chen, N.; Gravenstein, S.; Deng, Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 2009, 70, 777–784. [Google Scholar] [CrossRef]
- Canaday, D.H.; Amponsah, N.A.; Jones, L.; Tisch, D.J.; Hornick, T.R.; Ramachandra, L. Influenza-induced production of interferon-alpha is defective in geriatric individuals. J. Clin. Immunol. 2010, 30, 373–383. [Google Scholar] [CrossRef]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef]
- Qian, F.; Wang, X.; Zhang, L.; Lin, A.; Zhao, H.; Fikrig, E.; Montgomery, R.R. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J. Infect. Dis. 2011, 203, 1415–1424. [Google Scholar] [CrossRef]
- Agrawal, A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: A mini-review. Gerontology 2013, 59, 421–426. [Google Scholar] [CrossRef]
- Stout-Delgado, H.W.; Yang, X.; Walker, W.E.; Tesar, B.M.; Goldstein, D.R. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J. Immunol. 2008, 181, 6747–6756. [Google Scholar] [CrossRef] [PubMed]
- Loske, J.; Rohmel, J.; Lukassen, S.; Stricker, S.; Magalhaes, V.G.; Liebig, J.; Chua, R.L.; Thürmann, L.; Messingschlager, M.; Seegebarth, A.; et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 2022, 40, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Piccoli, G.; Stocchi, V.; Sestili, P. Gut Microbiota Status in COVID-19: An Unrecognized Player? Front. Cell Infect. Microbiol. 2020, 10, 576551. [Google Scholar] [CrossRef]
- Rajput, S.; Paliwal, D.; Naithani, M.; Kothari, A.; Meena, K.; Rana, S. COVID-19 and Gut Microbiota: A Potential Connection. Indian J. Clin. Biochem. 2021, 36, 266–277. [Google Scholar] [CrossRef]
- Dhar, D.; Mohanty, A. Gut microbiota and Covid-19—Possible link and implications. Virus Res. 2020, 285, 198018. [Google Scholar] [CrossRef]
- Chhibber-Goel, J.; Gopinathan, S.; Sharma, A. Interplay between severities of COVID-19 and the gut microbiome: Implications of bacterial co-infections? Gut Pathog. 2021, 13, 14. [Google Scholar] [CrossRef]
- Han, Y.; Jia, Z.; Shi, J.; Wang, W.; He, K. The active lung microbiota landscape of COVID-19 patients through the metatranscriptome data analysis. Bioimpacts 2022, 12, 139–146. [Google Scholar] [CrossRef]
- Merenstein, C.; Liang, G.; Whiteside, S.A.; Cobian-Guemes, A.G.; Merlino, M.S.; Taylor, L.J.; Glascock, A.; Bittinger, K.; Tanes, C.; Graham-Wooten, J.; et al. Signatures of COVID-19 severity and immune response in the respiratory tract microbiome. medRxiv 2021, 12, e01777-21. [Google Scholar] [CrossRef]
- Shilts, M.H.; Rosas-Salazar, C.; Strickland, B.A.; Kimura, K.S.; Asad, M.; Sehanobish, E.; Freeman, M.H.; Wessinger, B.C.; Gupta, V.; Brown, H.M.; et al. Severe COVID-19 Is Associated with an Altered Upper Respiratory Tract Microbiome. Front. Cell Infect. Microbiol. 2021, 11, 781968. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Song, Z.G.; Liu, C.; Tan, S.; Lin, S.; Zhu, J.; Dai, F.-H.; Gao, J.; She, J.-L.; Mei, Z.; et al. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 2022, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. Management of COVID-19: The Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020, 49, 147–157. [Google Scholar] [PubMed]
- Schaupp, L.; Muth, S.; Rogell, L.; Kofoed-Branzk, M.; Melchior, F.; Lienenklaus, S.; Ganal-Vonarburg, S.C.; Klein, M.; Guendel, F.; Hain, T.; et al. Microbiota-Induced Type I Interferons Instruct a Poised Basal State of Dendritic Cells. Cell 2020, 181, 1080–1096.e19. [Google Scholar] [CrossRef]
- Ganal, S.C.; Sanos, S.L.; Kallfass, C.; Oberle, K.; Johner, C.; Kirschning, C.; Lienenklaus, S.; Weiss, S.; Staeheli, P.; Aichele, P.; et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 2012, 37, 171–186. [Google Scholar] [CrossRef]
- Llor, C.; Ouchi, D.; Giner-Soriano, M.; Garcia-Sangenis, A.; Bjerrum, L.; Morros, R. Correlation between Previous Antibiotic Exposure and COVID-19 Severity. A Population-Based Cohort Study. Antibiotics 2021, 10, 1364. [Google Scholar] [CrossRef]
- Kiousi, D.E.; Karapetsas, A.; Karolidou, K.; Panayiotidis, M.I.; Pappa, A.; Galanis, A. Probiotics in Extraintestinal Diseases: Current Trends and New Directions. Nutrients 2019, 11, 788. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Ge, T.; Xiao, Y.; Liao, Y.; Cui, Y.; Zhang, Y.; Ho, W.; Yu, G.; Zhang, T. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e4509. [Google Scholar] [CrossRef]
- Jounai, K.; Ikado, K.; Sugimura, T.; Ano, Y.; Braun, J.; Fujiwara, D. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS ONE 2012, 7, e32588. [Google Scholar] [CrossRef]
- Sugimura, T.; Jounai, K.; Ohshio, K.; Tanaka, T.; Suwa, M.; Fujiwara, D. Immunomodulatory effect of Lactococcus lactis JCM5805 on human plasmacytoid dendritic cells. Clin. Immunol. 2013, 149, 509–518. [Google Scholar] [CrossRef]
- Jounai, K.; Sugimura, T.; Ohshio, K.; Fujiwara, D. Oral administration of Lactococcus lactis subsp. lactis JCM5805 enhances lung immune response resulting in protection from murine parainfluenza virus infection. PLoS ONE 2015, 10, e0119055. [Google Scholar]
- Sugimura, T.; Takahashi, H.; Jounai, K.; Ohshio, K.; Kanayama, M.; Tazumi, K.; anihata, Y.; Miura, Y.; Fujiwara, D.; Yamamoto, N. Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br. J. Nutr. 2015, 114, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; McInnes, I.B.; McMurray, J.J.V. Obesity Is a Risk Factor for Severe COVID-19 Infection: Multiple Potential Mechanisms. Circulation 2020, 142, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Crouse, A.B.; Grimes, T.; Li, P.; Might, M.; Ovalle, F.; Shalev, A. Metformin Use Is Associated with Reduced Mortality in a Diverse Population with COVID-19 and Diabetes. Front. Endocrinol. 2020, 11, 600439. [Google Scholar] [CrossRef]
- Lohia, P.; Kapur, S.; Benjaram, S.; Pandey, A.; Mir, T.; Seyoum, B. Metabolic syndrome and clinical outcomes in patients infected with COVID-19: Does age, sex, and race of the patient with metabolic syndrome matter? J. Diabetes 2021, 13, 420–429. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef]
- Barrasa, H.; Rello, J.; Tejada, S.; Martin, A.; Balziskueta, G.; Vinuesa, C.; Fernández-Miret, B.; Villagra, A.; Vallejo, A.; Sebastián, A.S.; et al. SARS-CoV-2 in Spanish Intensive Care Units: Early experience with 15-day survival in Vitoria. Anaesth. Crit. Care Pain Med. 2020, 39, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Kass, D.A.; Duggal, P.; Cingolani, O. Obesity could shift severe COVID-19 disease to younger ages. Lancet 2020, 395, 1544–1545. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jennings, J.; Gong, Y.; Sang, Y. Viral Infections and Interferons in the Development of Obesity. Biomolecules 2019, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Namkoong, H. Susceptibility of the obese population to COVID-19. Int. J. Infect. Dis. 2020, 101, 380–381. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attina, A.; Leggeri, C.; Cinelli, G.; Tarsitano, M.G.; Caparello, G.; Carrano, E.; et al. COVID-19: Is there a role for immunonutrition in obese patient? J. Transl. Med. 2020, 18, 415. [Google Scholar] [CrossRef]
- Teran-Cabanillas, E.; Hernandez, J. Role of Leptin and SOCS3 in Inhibiting the Type I Interferon Response During Obesity. Inflammation 2017, 40, 58–67. [Google Scholar] [CrossRef]
- Honce, R.; Schultz-Cherry, S. Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Easterbrook, J.D.; Dunfee, R.L.; Schwartzman, L.M.; Jagger, B.W.; Sandouk, A.; Kash, J.C.; Memoli, M.J.; Taubenberger, J.K. Obese mice have increased morbidity and mortality compared to non-obese mice during infection with the 2009 pandemic H1N1 influenza virus. Influenza Other Respir. Viruses 2011, 5, 418–425. [Google Scholar] [CrossRef]
- Vaid, N.; Ardissino, M.; Reed, T.A.N.; Goodall, J.; Utting, P.; Miscampbell, M.; Condurache, D.G.; Cohen, D.L. Clinical characteristics and outcomes of immunosuppressed patients hospitalized with COVID-19: Experience from London. J. Intern. Med. 2021, 289, 385–394. [Google Scholar] [CrossRef]
- Aydillo, T.; Gonzalez-Reiche, A.S.; Aslam, S.; van de Guchte, A.; Khan, Z.; Obla, A.; Dutta, J.; van Bakel, H.; Aberg, J.; García-Sastre, A.; et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N. Engl. J. Med. 2020, 383, 2586–2588. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Rodriguez, T.E.; Beck, E.T.; Relich, R.F.; Udeoji, D.U.; Petrak, R.; Chundi, V.V. Persistent SARS-CoV-2 infectivity greater than 50 days in a case series of allogeneic peripheral blood stem cell transplant recipients. Curr. Probl. Cancer Case Rep. 2021, 3, 100057. [Google Scholar] [CrossRef] [PubMed]
- Avanzato, V.A.; Matson, M.J.; Seifert, S.N.; Pryce, R.; Williamson, B.N.; Anzick, S.L.; Barbian, K.; Judson, S.D.; Fischer, E.R.; Martens, C.; et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell 2020, 183, 1901–1912.e9. [Google Scholar] [CrossRef] [PubMed]
- Manuel, O.; Estabrook, M. American Society of Transplantation Infectious Diseases Community of P. RNA respiratory viral infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13511. [Google Scholar] [CrossRef]
- Shields, A.M.; Burns, S.O.; Savic, S.; Richter, A.G.; UK PIN COVID-19 Consortium. COVID-19 in patients with primary and secondary immunodeficiency: The United Kingdom experience. J. Allergy Clin. Immunol. 2021, 147, 870–875.e1. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, J.; Zhu, Y.; Liu, L.; Liu, Y.; He, Q. Mortality in chronic kidney disease patients with COVID-19: A systematic review and meta-analysis. Int. Urol. Nephrol. 2021, 53, 1623–1629. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef]
- Gosain, R.; Abdou, Y.; Singh, A.; Rana, N.; Puzanov, I.; Ernstoff, M.S. COVID-19 and Cancer: A Comprehensive Review. Curr. Oncol. Rep. 2020, 22, 53. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Yuan, Y.; Mei, Q.; Yuan, X. Clinical challenges in cancer patients with COVID-19: Aging, immunosuppression, and comorbidities. Aging 2020, 12, 24462–24474. [Google Scholar] [CrossRef]
- Tan, E.H.; Sena, A.G.; Prats-Uribe, A.; You, S.C.; Ahmed, W.U.; Kostka, K.; Reich, C.; Duvall, S.L.; Lynch, K.; Matheny, M.; et al. COVID-19 in patients with autoimmune diseases: Characteristics and outcomes in a multinational network of cohorts across three countries. Rheumatology 2021, 60, SI37–SI50. [Google Scholar] [CrossRef]
- Sarmiento-Monroy, J.C.; Espinosa, G.; Londono, M.C.; Meira, F.; Caballol, B.; Llufriu, S.; Carrasco, J.L.; Moll-Udina, A.; Quintana, L.F.; Giavedoni, P.; et al. A multidisciplinary registry of patients with autoimmune and immune-mediated diseases with symptomatic COVID-19 from a single center. J. Autoimmun. 2021, 117, 102580. [Google Scholar] [CrossRef] [PubMed]
- Monreal, E.; Sainz de la Maza, S.; Fernandez-Velasco, J.I.; Natera-Villalba, E.; Rita, C.G.; Rodriguez-Jorge, F.; Beltrán-Corbellini, A.; Iturrieta-Zuazo, I.; De Santiago, E.R. The Impact of Immunosuppression and Autoimmune Disease on Severe Outcomes in Patients Hospitalized with COVID-19. J. Clin. Immunol. 2021, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Shodell, M.; Shah, K.; Siegal, F.P. Circulating human plasmacytoid dendritic cells are highly sensitive to corticosteroid administration. Lupus 2003, 12, 222–230. [Google Scholar] [CrossRef]
- Shodell, M.; Siegal, F.P. Corticosteroids depress IFN-alpha-producing plasmacytoid dendritic cells in human blood. J. Allergy Clin. Immunol. 2001, 108, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Sacre, K.; Criswell, L.A.; McCune, J.M. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res. Ther. 2012, 14, R155. [Google Scholar] [CrossRef] [PubMed]
- Gardet, A.; Pellerin, A.; McCarl, C.A.; Diwanji, R.; Wang, W.; Donaldson, D.; Franchimont, N.; Werth, V.P.; Rabah, D. Effect of in vivo Hydroxychloroquine and ex vivo Anti-BDCA2 mAb Treatment on pDC IFNalpha Production from Patients Affected with Cutaneous Lupus Erythematosus. Front. Immunol. 2019, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Shigesaka, M.; Ito, T.; Inaba, M.; Imai, K.; Yamanaka, H.; Azuma, Y.; Tanaka, A.; Amuro, H.; Nishizawa, T.; Son, Y.; et al. Mycophenolic acid, the active form of mycophenolate mofetil, interferes with IRF7 nuclear translocation and type I IFN production by plasmacytoid dendritic cells. Arthritis Res. Ther. 2020, 22, 264. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Sakata, K.; Kitanaga, Y.; Ma, X.; Lee, S.; Ishii, A.; Yamagata, K.; Nakano, K.; Tanaka, Y. Janus Kinase Inhibitor Baricitinib Modulates Human Innate and Adaptive Immune System. Front. Immunol. 2018, 9, 1510. [Google Scholar] [CrossRef]
- Sunzini, F.; McInnes, I.; Siebert, S. JAK inhibitors and infections risk: Focus on herpes zoster. Ther. Adv. Musculoskelet Dis. 2020, 12, 1759720X20936059. [Google Scholar] [CrossRef]
- Hashem, A.M.; Alghamdi, B.S.; Algaissi, A.A.; Alshehri, F.S.; Bukhari, A.; Alfaleh, M.A.; Memish, Z.A. Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: A narrative review. Travel Med. Infect. Dis. 2020, 35, 101735. [Google Scholar] [CrossRef]
- Devaux, C.A.; Rolain, J.M.; Colson, P.; Raoult, D. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int. J. Antimicrob Agents. 2020, 55, 105938. [Google Scholar] [CrossRef] [PubMed]
- Schoot, T.S.; Kerckhoffs, A.P.M.; Hilbrands, L.B.; van Marum, R.J. Immunosuppressive Drugs and COVID-19: A Review. Front. Pharmacol. 2020, 11, 1333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, C.; Zhao, W. Virus Caused Imbalance of Type I IFN Responses and Inflammation in COVID-19. Front. Immunol. 2021, 12, 633769. [Google Scholar] [CrossRef]
- Carvalho, T. Interferon linked to COVID-19 severity. Nat. Med. 2020, 26, 1806. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.P.; Goncalves, J.I.B.; Zanin, R.F.; Schuch, F.B.; de Souza, A.P.D. Circulating Type I Interferon Levels and COVID-19 Severity: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 657363. [Google Scholar] [CrossRef]
- Damsky, W.; Peterson, D.; King, B. When interferon tiptoes through COVID-19: Pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J. Am. Acad. Dermatol. 2020, 83, e269–e270. [Google Scholar] [CrossRef]
- Fiehn, C. Familial Chilblain Lupus—What Can We Learn from Type I Interferonopathies? Curr. Rheumatol. Rep. 2017, 19, 61. [Google Scholar] [CrossRef]
- Gu, W.; Gan, H.; Ma, Y.; Xu, L.; Cheng, Z.J.; Li, B.; Zhang, X.; Jiang, W.; Sun, J.; Sun, B.; et al. The molecular mechanism of SARS-CoV-2 evading host antiviral innate immunity. Virol. J. 2022, 19, 49. [Google Scholar] [CrossRef]
- Sodeifian, F.; Nikfarjam, M.; Kian, N.; Mohamed, K.; Rezaei, N. The role of type I interferon in the treatment of COVID-19. J. Med. Virol. 2022, 94, 63–81. [Google Scholar] [CrossRef]
- Monk, P.D.; Marsden, R.J.; Tear, V.J.; Brookes, J.; Batten, T.N.; Mankowski, M.; Gabbay, F.J.; Davies, D.; Holgate, S.T.; Ho, L.-P.; et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 196–206. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, T.; Chen, L.; Chen, X.; Li, L.; Qin, X.; Li, H.; Luo, J. An experimental trial of recombinant human interferon alpha nasal drops to prevent COVID-19 in medical staff in an epidemic area. Curr. Top Med Chem. 2021, 21, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhan, Y.; Zhu, L.; Hou, Z.; Liu, F.; Song, P.; Qiu, F.; Wang, X.; Zou, X.; Wan, D. Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with Favorable Clinical Responses in COVID-19 Patients. Cell Host Microbe 2020, 28, 455–464.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, V.; Shannon, C.P.; Wei, X.S.; Xiang, X.; Wang, X.; Wang, Z.-H.; Tebbutt, S.J.; Kollmann, T.R.; Fish, E.N. Interferon-alpha2b Treatment for COVID-19. Front. Immunol. 2020, 11, 1061. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liu, Y.; Liu, L.; Hu, H.; Cheng, X.; Liu, P.; Song, Z.; Zha, L.; Bai, S.; Xu, T.; et al. An open-label, randomized trial of the combination of IFN-kappa plus TFF2 with standard care in the treatment of patients with moderate COVID-19. EClinicalMedicine 2020, 27, 100547. [Google Scholar] [CrossRef]
- Li, S.F.; Gong, M.J.; Zhao, F.R.; Shao, J.J.; Xie, Y.L.; Zhang, Y.G.; Chang, H.-Y. Type I Interferons: Distinct Biological Activities and Current Applications for Viral Infection. Cell Physiol. Biochem. 2018, 51, 2377–2396. [Google Scholar] [CrossRef]
- Wittling, M.C.; Cahalan, S.R.; Levenson, E.A.; Rabin, R.L. Shared and Unique Features of Human Interferon-Beta and Interferon-Alpha Subtypes. Front. Immunol. 2020, 11, 605673. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, E.C. The type I interferon response in COVID-19: Implications for treatment. Nat. Rev. Immunol. 2020, 20, 585–586. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).