The Clinical Application of Growth Hormone and Its Biological and Molecular Mechanisms in Assisted Reproduction
Abstract
1. Introduction
2. The Application of Growth Hormone in Clinical Practice
2.1. The Application of Growth Hormone in Poor Ovarian Responders
2.2. The Application of Growth Hormone in Women with Polycystic Ovary Syndrome
2.3. The Application of Growth Hormone in Women with Poor Embryonic Development
2.4. The Application of Growth Hormone in Normal Ovarian Responders during ART Treatment
3. Molecular Mechanisms of Growth Hormone in Ovarian Functions
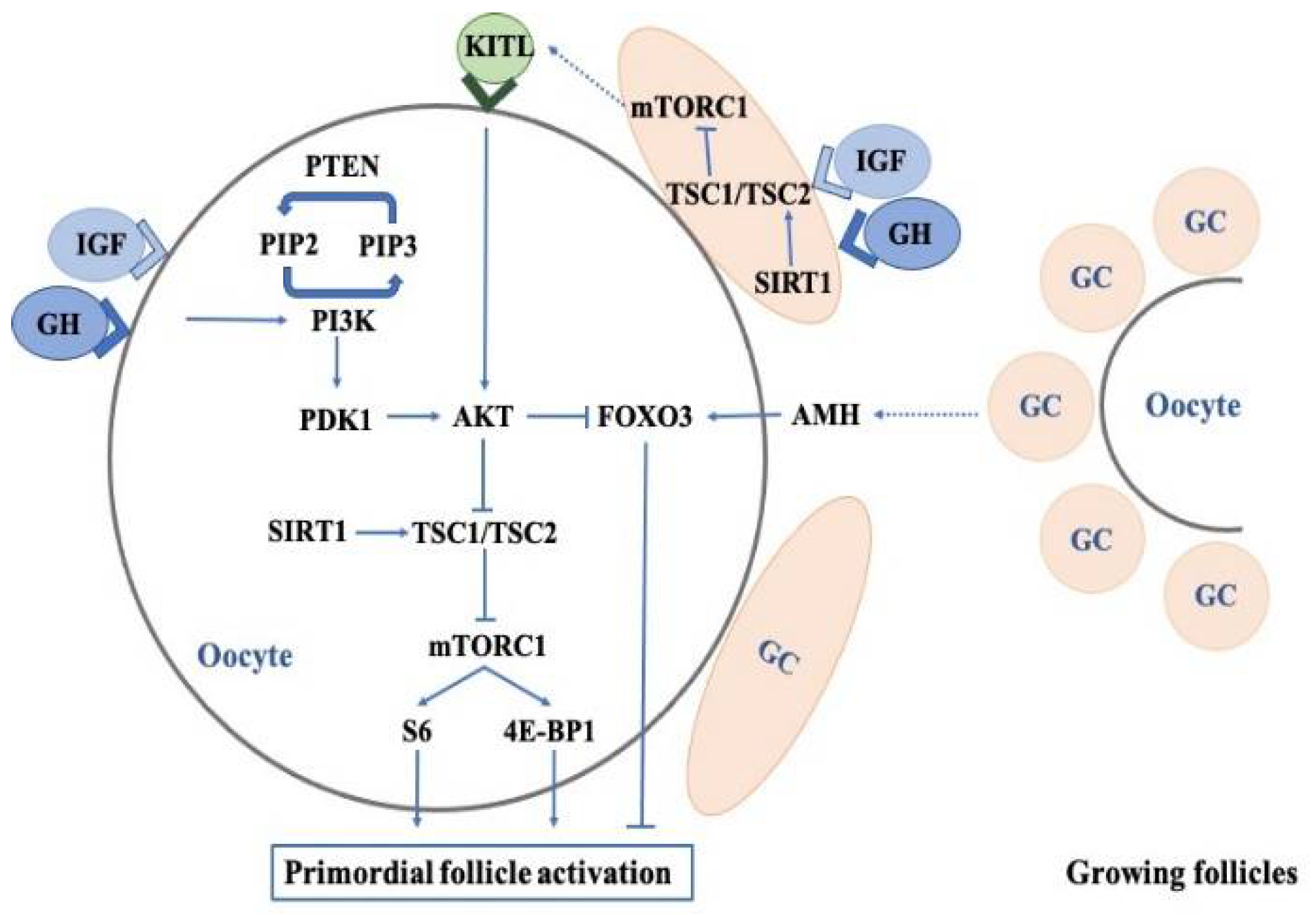
3.1. Effects of Growth Hormone on the Regulation of Primordial Follicles
3.1.1. PI3K-PTEN-AKT-FOXO3 Signaling
3.1.2. The mTOR Pathway
3.1.3. Anti-Mullerian Hormone
3.2. Effects of Growth Hormone on Oocyte Quality
3.2.1. Nuclear Maturation
3.2.2. Cytoplasmic Maturation
3.3. Effects of Growth Hormone on the Sensitivity of Oocytes to Gonadotropins
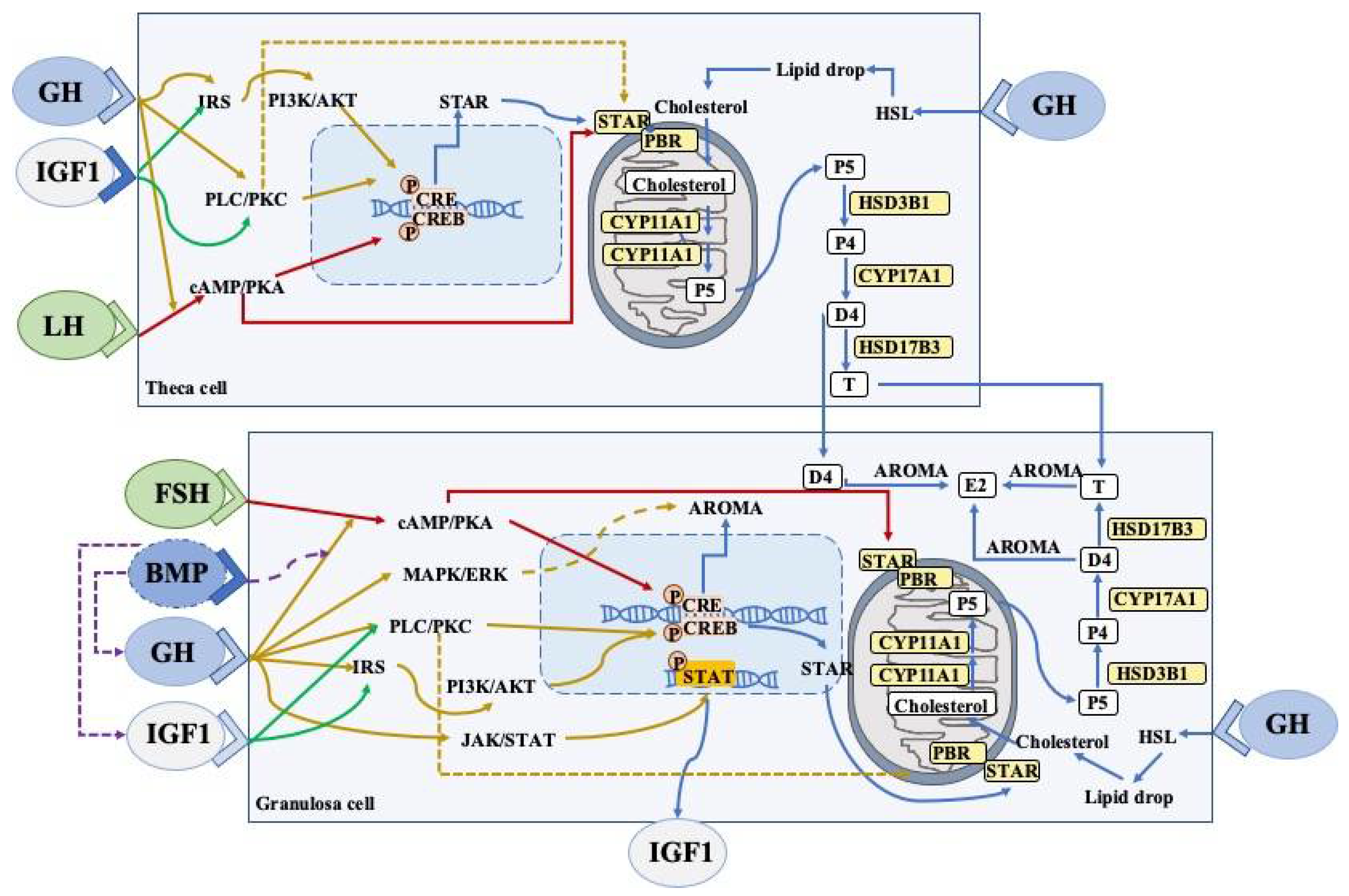
3.4. Effects of Growth Hormone on Granulosa Cells and Thecal Cells
3.4.1. Steroidogenesis
3.4.2. JAK2-Dependent Signaling Pathway
3.4.3. JAK2-Independent Signaling Pathway
3.4.4. FSHR Pathway
3.4.5. GH/IGFs Signaling Pathway
3.5. Mitochondrial Functions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dosouto, C.; Calaf, J.; Polo, A.; Haahr, T.; Humaidan, P. Growth Hormone and Reproduction: Lessons Learned From Animal Models and Clinical Trials. Front. Endocrinol. 2019, 10, 404. [Google Scholar] [CrossRef]
- Kolibianakis, E.M.; Venetis, C.A.; Diedrich, K.; Tarlatzis, B.C.; Griesinger, G. Addition of growth hormone to gonadotrophins in ovarian stimulation of poor responders treated by in-vitro fertilization: A systematic review and meta-analysis. Hum. Reprod. Update 2009, 15, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Shu, J.; Guo, J.; Chang, H.-M.; Leung, P.C.K.; Sheng, J.-Z.; Huang, H. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: A systematic review and network meta-analysis. Hum. Reprod. Update 2020, 26, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, A.; Monget, P.; Imbert-Bolloré, P.; Coshigano, K.; Kopchick, J.J.; Kelly, P.A.; Binart, N. Growth hormone is required for ovarian follicular growth. Endocrinology 2002, 143, 4104–4112. [Google Scholar] [CrossRef]
- Banerjee, S.; Chaturvedi, C.M. Specific neural phase relation of serotonin and dopamine modulate the testicular activity in Japanese quail. J. Cell. Physiol. 2019, 234, 2866–2879. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Caicedo, D. The Role of Growth Hormone on Ovarian Functioning and Ovarian Angiogenesis. Front. Endocrinol. 2019, 10, 450. [Google Scholar] [CrossRef]
- Kajimura, S.; Kawaguchi, N.; Kaneko, T.; Kawazoe, I.; Hirano, T.; Visitacion, N.; Grau, E.G.; Aida, K. Identification of the growth hormone receptor in an advanced teleost, the tilapia (Oreochromis mossambicus) with special reference to its distinct expression pattern in the ovary. J. Endocrinol. 2004, 181, 65–76. [Google Scholar] [CrossRef]
- Ahumada-Solórzano, S.M.; Carranza, M.E.; Pedernera, E.; Rodríguez-Méndez, A.J.; Luna, M.; Arámburo, C. Local expression and distribution of growth hormone and growth hormone receptor in the chicken ovary: Effects of GH on steroidogenesis in cultured follicular granulosa cells. Gen. Comp. Endocrinol. 2012, 175, 297–310. [Google Scholar] [CrossRef]
- Zhao, J.; Taverne, M.A.M.; van der Weijden, G.C.; Bevers, M.M.; van den Hurk, R. Immunohistochemical localisation of growth hormone (GH), GH receptor (GHR), insulin-like growth factor I (IGF-I) and type I IGF-I receptor, and gene expression of GH and GHR in rat pre-antral follicles. Zygote 2002, 10, 85–94. [Google Scholar] [CrossRef]
- Steffl, M.; Schweiger, M.; Mayer, J.; Amselgruber, W.M. Expression and localization of growth hormone receptor in the oviduct of cyclic and pregnant pigs and mid-implantation conceptuses. Histochem. Cell Biol. 2009, 131, 773–779. [Google Scholar] [CrossRef]
- Marchal, R.; Caillaud, M.; Martoriati, A.; Gérard, N.; Mermillod, P.; Goudet, G. Effect of growth hormone (GH) on in vitro nuclear and cytoplasmic oocyte maturation, cumulus expansion, hyaluronan synthases, and connexins 32 and 43 expression, and GH receptor messenger RNA expression in equine and porcine species. Biol. Reprod. 2003, 69, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- de Prada, J.K.N.; VandeVoort, C.A. Growth hormone and in vitro maturation of rhesus macaque oocytes and subsequent embryo development. J. Assist. Reprod. Genet. 2008, 25, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Abir, R.; Garor, R.; Felz, C.; Nitke, S.; Krissi, H.; Fisch, B. Growth hormone and its receptor in human ovaries from fetuses and adults. Fertil. Steril. 2008, 90, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-M.; Hao, G.-M.; Gao, B.-L. Application of Growth Hormone in in vitro Fertilization. Front. Endocrinol. 2019, 10, 502. [Google Scholar] [CrossRef]
- Hrabia, A. Growth hormone production and role in the reproductive system of female chicken. Gen. Comp. Endocrinol. 2015, 220, 112–118. [Google Scholar] [CrossRef]
- Owen, E.J.; West, C.; Mason, B.A.; Jacobs, H.S. Co-treatment with growth hormone of sub-optimal responders in IVF-ET. Hum. Reprod. 1991, 6, 524–528. [Google Scholar] [CrossRef]
- Bassiouny, Y.A.; Dakhly, D.M.R.; Bayoumi, Y.A.; Hashish, N.M. Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil. Steril. 2016, 105, 697–702. [Google Scholar] [CrossRef]
- Li, X.-L.; Wang, L.; Lv, F.; Huang, X.-M.; Wang, L.-P.; Pan, Y.; Zhang, X.-M. The influence of different growth hormone addition protocols to poor ovarian responders on clinical outcomes in controlled ovary stimulation cycles: A systematic review and meta-analysis. Medicine 2017, 96, e6443. [Google Scholar] [CrossRef]
- Chu, K.; Pang, W.; Sun, N.; Zhang, Q.; Li, W. Outcomes of poor responders following growth hormone co-treatment with IVF/ICSI mild stimulation protocol: A retrospective cohort study. Arch. Gynecol. Obstet. 2018, 297, 1317–1321. [Google Scholar] [CrossRef]
- Cai, M.-H.; Liang, X.-Y.; Wu, Y.-Q.; Huang, R.; Yang, X. Six-week pretreatment with growth hormone improves clinical outcomes of poor ovarian responders undergoing in vitro fertilization treatment: A self-controlled clinical study. J. Obstet. Gynaecol. Res. 2019, 45, 376–381. [Google Scholar] [CrossRef]
- Choe, S.-A.; Kim, M.J.; Lee, H.J.; Kim, J.; Chang, E.M.; Kim, J.W.; Park, H.M.; Lyu, S.W.; Lee, W.S.; Yoon, T.K.; et al. Increased proportion of mature oocytes with sustained-release growth hormone treatment in poor responders: A prospective randomized controlled study. Arch. Gynecol. Obstet. 2018, 297, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Homburg, R.; Levy, T.; Ben-Rafael, Z. Adjuvant growth hormone for induction of ovulation with gonadotrophin-releasing hormone agonist and gonadotrophins in polycystic ovary syndrome: A randomized, double-blind, placebo controlled trial. Hum. Reprod. 1995, 10, 2550–2553. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Luo, S.; Fan, P.; Jin, S.; Zhu, H.; Deng, T.; Quan, Y.; Huang, W. Growth hormone alleviates oxidative stress and improves oocyte quality in Chinese women with polycystic ovary syndrome: A randomized controlled trial. Sci. Rep. 2020, 10, 18769. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Wang, J.; Huang, G.; Ye, H. Does growth hormone supplementation improve oocyte competence and IVF outcomes in patients with poor embryonic development? A randomized controlled trial. BMC Pregnancy Childbirth 2020, 20, 310. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Shuai, J.; Chen, W.-H.; Zhang, X.-D.; Pei, L.; Huang, G.-N.; Ye, H. Impact of growth hormone supplementation on improving oocyte competence in unexplained poor embryonic development patients of various ages. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2021, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gonda, K.J.; Domar, A.D.; Gleicher, N.; Marrs, R.P. Insights from clinical experience in treating IVF poor responders. Reprod. Biomed. Online 2018, 36, 12–19. [Google Scholar] [CrossRef]
- Eftekhar, M.; Aflatoonian, A.; Mohammadian, F.; Eftekhar, T. Adjuvant growth hormone therapy in antagonist protocol in poor responders undergoing assisted reproductive technology. Arch. Gynecol. Obstet. 2013, 287, 1017–1021. [Google Scholar] [CrossRef]
- Lattes, K.; Brassesco, M.; Gomez, M.; Checa, M.A. Low-dose growth hormone supplementation increases clinical pregnancy rate in poor responders undergoing in vitro fertilisation. Gynecol. Endocrinol. 2015, 31, 565–568. [Google Scholar]
- Bergh, C.; Hillensjo, T.; Wikland, M.; Nilsson, L.; Borg, G.; Hamberger, L. Adjuvant growth hormone treatment during in vitro fertilization: A randomized, placebo-controlled study. Fertil. Steril. 1994, 62, 113–120. [Google Scholar] [CrossRef]
- Bosch, E.; Labarta, E.; Kolibianakis, E.; Rosen, M.; Meldrum, D. Regimen of ovarian stimulation affects oocyte and therefore embryo quality. Fertil. Steril. 2016, 105, 560–570. [Google Scholar] [CrossRef]
- Dunne, C.; Seethram, K.; Roberts, J. Growth Hormone Supplementation in the Luteal Phase Before Microdose GnRH Agonist Flare Protocol for In Vitro Fertilization. J. Obstet. Gynaecol. Can. 2015, 37, 810–815. [Google Scholar] [CrossRef]
- Norman, R.; Alvino, H.; Hart, R. A randomised double blind placebo controlled study of recombinant human growth hormone (r-HGH) on live birth rates in women who are poor responders. Hum. Reprod. 2016, 38, 908–915. [Google Scholar]
- Tesarik, J.; Hazout, A.; Mendoza, C. Improvement of delivery and live birth rates after ICSI in women aged >40 years by ovarian co-stimulation with growth hormone. Hum. Reprod. 2005, 20, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Dakhly, D.M.R.; Bassiouny, Y.A.; Bayoumi, Y.A.; Hassan, M.A.; Gouda, H.M.; Hassan, A.A. The addition of growth hormone adjuvant therapy to the long down regulation protocol in poor responders undergoing in vitro fertilization: Randomized control trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Dor, J.; Seidman, D.S.; Amudai, E.; Bider, D.; Levran, D.; Mashiach, S. Adjuvant growth hormone therapy in poor responders to in-vitro fertilization: A prospective randomized placebo-controlled double-blind study. Hum. Reprod. 1995, 10, 40–43. [Google Scholar] [CrossRef]
- Jirge, P.R.; Chougule, S.M.; Gavali, V.G.; Bhomkar, D.A. Impact of dehydroepiandrosterone on clinical outcome in poor responders: A pilot study in women undergoing in vitro fertilization, using bologna criteria. J. Hum. Reprod. Sci. 2014, 7, 175–180. [Google Scholar] [CrossRef]
- Ob’edkova, K.; Kogan, I.; Krikheli, I.; Dzhemlikhanova, L.; Muller, V.; Mekina, I.; Lesik, E.; Komarova, E.; Mazilina, M.; Niauri, D.; et al. Growth hormone co-treatment in IVF/ICSI cycles in poor responders. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2017, 33, 15–17. [Google Scholar] [CrossRef]
- Bayoumi, Y.A.; Dakhly, D.M.R.; Bassiouny, Y.A.; Hashish, N.M. Addition of growth hormone to the microflare stimulation protocol among women with poor ovarian response. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2015, 131, 305–308. [Google Scholar] [CrossRef]
- Cai, M.-H.; Gao, L.-Z.; Liang, X.-Y.; Fang, C.; Wu, Y.-Q.; Yang, X. The Effect of Growth Hormone on the Clinical Outcomes of Poor Ovarian Reserve Patients Undergoing in vitro Fertilization/Intracytoplasmic Sperm Injection Treatment: A Retrospective Study Based on POSEIDON Criteria. Front. Endocrinol. 2019, 10, 775. [Google Scholar] [CrossRef]
- Keane, K.N.; Yovich, J.L.; Hamidi, A.; Hinchliffe, P.M.; Dhaliwal, S.S. Single-centre retrospective analysis of growth hormone supplementation in IVF patients classified as poor-prognosis. BMJ Open 2017, 7, e018107. [Google Scholar] [CrossRef]
- Ferraretti, A.P.; La Marca, A.; Fauser, B.C.J.M.; Tarlatzis, B.; Nargund, G.; Gianaroli, L. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum. Reprod. 2011, 26, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Klinkert, E.R.; Broekmans, F.J.M.; Looman, C.W.N.; Te Velde, E.R. A poor response in the first in vitro fertilization cycle is not necessarily related to a poor prognosis in subsequent cycles. Fertil. Steril. 2004, 81, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.J.; Alvino, H.; Hull, L.M.; Mol, B.W.; Hart, R.J.; Kelly, T.-L.; Rombauts, L. Human growth hormone for poor responders: A randomized placebo-controlled trial provides no evidence for improved live birth rate. Reprod. Biomed. Online 2019, 38, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Alviggi, C.; Andersen, C.Y.; Buehler, K.; Conforti, A.; De Placido, G.; Esteves, S.C.; Fischer, R.; Galliano, D.; Polyzos, N.P.; Sunkara, S.K.; et al. A new more detailed stratification of low responders to ovarian stimulation: From a poor ovarian response to a low prognosis concept. Fertil. Steril. 2016, 105, 1452–1453. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, J.; Bi, L.; Liu, P.; Jiao, X. Growth Hormone Cotreatment for Low-Prognosis Patients According to the POSEIDON Criteria. Front. Endocrinol. (Lausanne) 2021, 12, 790160. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Chen, L.; Liu, P.; Li, R.; Qiao, J. Growth Hormone Supplementation May Not Improve Live Birth Rate in Poor Responders. Front. Endocrinol. (Lausanne) 2020, 11, 1. [Google Scholar] [CrossRef]
- Liu, F.-T.; Hu, K.-L.; Li, R. Effects of Growth Hormone Supplementation on Poor Ovarian Responders in Assisted Reproductive Technology: A Systematic Review and Meta-analysis. Reprod. Sci. 2021, 28, 936–948. [Google Scholar] [CrossRef]
- Duffy, J.M.; Ahmad, G.; Mohiyiddeen, L.; Nardo, L.G.; Watson, A. Growth hormone for in vitro fertilization. Cochrane Database Syst. Rev. 2010, 2010, CD000099. [Google Scholar] [CrossRef]
- Kyrou, D.; Kolibianakis, E.M.; Venetis, C.A.; Papanikolaou, E.G.; Bontis, J.; Tarlatzis, B.C. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: A systematic review and meta-analysis. Fertil. Steril. 2009, 91, 749–766. [Google Scholar] [CrossRef]
- Yang, P.; Wu, R.; Zhang, H. The effect of growth hormone supplementation in poor ovarian responders undergoing IVF or ICSI: A meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2020, 18, 76. [Google Scholar] [CrossRef]
- Cozzolino, M.; Cecchino, G.N.; Troiano, G.; Romanelli, C. Growth hormone cotreatment for poor responders undergoing in vitro fertilization cycles: A systematic review and meta-analysis. Fertil. Steril. 2020, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J.; Rombauts, L.; Norman, R.J. Growth hormone in IVF cycles: Any hope? Curr. Opin. Obstet. Gynecol. 2017, 29, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-X.; Shen, M.-S.; Tzeng, C.-R. Low Dose Growth Hormone Adjuvant Treatment with Ultra-Long Ovarian Stimulation Protocol in Poor Responders Showed Non-inferior Pregnancy Outcome Compared with Normal Responders. Front. Endocrinol. 2019, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Safdarian, L.; Aghahosseini, M.; Alyasin, A.; Samaei Nouroozi, A.; Rashidi, S.; Shabani Nashtaei, M.; Najafian, A.; Lak, P. Growth Hormone (GH) Improvement of Ovarian Responses and Pregnancy Outcome in Poor Ovarian Responders: A Randomized Study. Asian Pac. J. Cancer Prev. 2019, 20, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, T.; Kozinoglu, H.; Kaba, A. Growth hormone co-treatment within a GnRH agonist long protocol in patients with poor ovarian response: A prospective, randomized, clinical trial. J. Assist. Reprod. Genet. 2008, 25, 123–127. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.E.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Prim. 2016, 2, 16057. [Google Scholar] [CrossRef]
- Qi, L.; Liu, B.; Chen, X.; Liu, Q.; Li, W.; Lv, B.; Xu, X.; Wang, L.; Zeng, Q.; Xue, J.; et al. Single-Cell Transcriptomic Analysis Reveals Mitochondrial Dynamics in Oocytes of Patients With Polycystic Ovary Syndrome. Front. Genet. 2020, 11, 396. [Google Scholar] [CrossRef]
- Qiao, J.; Feng, H.L. Extra- and intra-ovarian factors in polycystic ovary syndrome: Impact on oocyte maturation and embryo developmental competence. Hum. Reprod. Update 2011, 17, 17–33. [Google Scholar] [CrossRef]
- Hart, R.J. Physiological Aspects of Female Fertility: Role of the Environment, Modern Lifestyle, and Genetics. Physiol. Rev. 2016, 96, 873–909. [Google Scholar] [CrossRef]
- Premoli, A.C.; Santana, L.F.; Ferriani, R.A.; de Moura, M.D.; De Sá, M.F.S.; Reis, R.M. dos Growth hormone secretion and insulin-like growth factor-1 are related to hyperandrogenism in nonobese patients with polycystic ovary syndrome. Fertil. Steril. 2005, 83, 1852–1855. [Google Scholar] [CrossRef]
- Bentov, Y.; Yavorska, T.; Esfandiari, N.; Jurisicova, A.; Casper, R.F. The contribution of mitochondrial function to reproductive aging. J. Assist. Reprod. Genet. 2011, 28, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, H.; Corachán, A.; Aguilar, A.; Quiñonero, A.; Carbajo-García, M.C.; Alamá, P.; Tejera, A.; Taboas, E.; Muñoz, E.; Pellicer, A.; et al. Single-cell RNA sequencing of oocytes from ovarian endometriosis patients reveals a differential transcriptomic profile associated with lower quality. Hum. Reprod. 2019, 34, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Ogino, M.; Tsubamoto, H.; Sakata, K.; Oohama, N.; Hayakawa, H.; Kojima, T.; Shigeta, M.; Shibahara, H. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J. Assist. Reprod. Genet. 2016, 33, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Oron, G.; Son, W.-Y.; Buckett, W.; Tulandi, T.; Holzer, H. The association between embryo quality and perinatal outcome of singletons born after single embryo transfers: A pilot study. Hum. Reprod. 2014, 29, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lian, Y.; Li, M.; Chen, L.; Liu, P.; Qiao, J. Does IVF cleavage stage embryo quality affect pregnancy complications and neonatal outcomes in singleton gestations after double embryo transfers? J. Assist. Reprod. Genet. 2014, 31, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.J.; Richter, K.S.; Heitmann, R.J.; Graham, J.R.; Tucker, M.J.; DeCherney, A.H.; Browne, P.E.; Levens, E.D. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil. Steril. 2013, 99, 1283–1289.e1. [Google Scholar] [CrossRef] [PubMed]
- Younis, J.S.; Simon, A.; Koren, R.; Dorembus, D.; Schenker, J.G.; Laufer, N. The effect of growth hormone supplementation on in vitro fertilization outcome: A prospective randomized placebo-controlled double-blind study. Fertil. Steril. 1992, 58, 575–580. [Google Scholar] [CrossRef]
- Skillern, A.; Leonard, W.; Pike, J.; Mak, W. Growth hormone supplementation during ovarian stimulation improves oocyte and embryo outcomes in IVF/PGT-A cycles of women who are not poor responders. J. Assist. Reprod. Genet. 2021, 38, 1055–1060. [Google Scholar] [CrossRef]
- Gallardo, T.D.; John, G.B.; Bradshaw, K.; Welt, C.; Reijo-Pera, R.; Vogt, P.H.; Touraine, P.; Bione, S.; Toniolo, D.; Nelson, L.M.; et al. Sequence variation at the human FOXO3 locus: A study of premature ovarian failure and primary amenorrhea. Hum. Reprod. 2008, 23, 216–221. [Google Scholar] [CrossRef]
- Orisaka, M.; Miyazaki, Y.; Shirafuji, A.; Tamamura, C.; Tsuyoshi, H.; Tsang, B.K.; Yoshida, Y. The role of pituitary gonadotropins and intraovarian regulators in follicle development: A mini-review. Reprod. Med. Biol. 2021, 20, 169–175. [Google Scholar] [CrossRef]
- Carlsson, I.B.; Scott, J.E.; Visser, J.A.; Ritvos, O.; Themmen, A.P.N.; Hovatta, O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum. Reprod. 2006, 21, 2223–2227. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Abbott, J.A.; Walters, K.A.; Ledger, W.L. Translational Physiology of Anti-Müllerian Hormone: Clinical Applications in Female Fertility Preservation and Cancer Treatment. Front. Endocrinol. 2021, 12, 689532. [Google Scholar] [CrossRef] [PubMed]
- Zaczek, D.; Hammond, J.; Suen, L.; Wandji, S.; Service, D.; Bartke, A.; Chandrashekar, V.; Coschigano, K.; Kopchick, J. Impact of growth hormone resistance on female reproductive function: New insights from growth hormone receptor knockout mice. Biol. Reprod. 2002, 67, 1115–1124. [Google Scholar] [CrossRef]
- Chandrashekar, V.; Zaczek, D.; Bartke, A. The consequences of altered somatotropic system on reproduction. Biol. Reprod. 2004, 71, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Saccon, T.D.; Rovani, M.T.; Garcia, D.N.; Mondadori, R.G.; Cruz, L.A.X.; Barros, C.C.; Bartke, A.; Masternak, M.M.; Schneider, A. Primordial follicle reserve, DNA damage and macrophage infiltration in the ovaries of the long-living Ames dwarf mice. Exp. Gerontol. 2020, 132, 110851. [Google Scholar] [CrossRef] [PubMed]
- Saccon, T.D.; Moreira, F.; Cruz, L.A.; Mondadori, R.G.; Fang, Y.; Barros, C.C.; Spinel, L.; Bartke, A.; Masternak, M.M.; Schneider, A. Ovarian aging and the activation of the primordial follicle reserve in the long-lived Ames dwarf and the short-lived bGH transgenic mice. Mol. Cell. Endocrinol. 2017, 455, 23–32. [Google Scholar] [CrossRef]
- Liu, X.; Andoh, K.; Yokota, H.; Kobayashi, J.; Abe, Y.; Yamada, K.; Mizunuma, H.; Ibuki, Y. Effects of growth hormone, activin, and follistatin on the development of preantral follicle from immature female mice. Endocrinology 1998, 139, 2342–2347. [Google Scholar] [CrossRef][Green Version]
- Martins, F.S.; Celestino, J.J.H.; Saraiva, M.V.A.; Chaves, R.N.; Rossetto, R.; Silva, C.M.G.; Lima-Verde, I.B.; Lopes, C.A.P.; Campello, C.C.; Figueiredo, J.R. Interaction between growth differentiation factor 9, insulin-like growth factor I and growth hormone on the in vitro development and survival of goat preantral follicles. Braz. J. Med. Biol. Res. 2010, 43, 728–736. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Ebbert, K.; Cordeiro, M.H.; Romero, M.M.; Whelan, K.A.; Suarez, A.A.; Woodruff, T.K.; Kurita, T. Constitutive Activation of PI3K in Oocyte Induces Ovarian Granulosa Cell Tumors. Cancer Res. 2016, 76, 3851–3861. [Google Scholar] [CrossRef]
- Maidarti, M.; Anderson, R.A.; Telfer, E.E. Crosstalk between PTEN/PI3K/Akt Signalling and DNA Damage in the Oocyte: Implications for Primordial Follicle Activation, Oocyte Quality and Ageing. Cells 2020, 9, 200. [Google Scholar] [CrossRef]
- John, G.B.; Gallardo, T.D.; Shirley, L.J.; Castrillon, D.H. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev. Biol. 2008, 321, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Adhikari, D.; Zheng, W.; Liang, S.; Hämäläinen, T.; Tohonen, V.; Ogawa, W.; Noda, T.; Volarevic, S.; Huhtaniemi, I.; et al. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum. Mol. Genet. 2009, 18, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Su, Y.; He, Y.; Zhang, J.; Liu, W.; Zhang, H.; Hou, Z.; Liu, J.; Li, J. New strategy for in vitro activation of primordial follicles with mTOR and PI3K stimulators. Cell Cycle 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Liu, L.; Adhikari, D.; Jagarlamudi, K.; Rajareddy, S.; Shen, Y.; Du, C.; Tang, W.; Hämäläinen, T.; Peng, S.L.; et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008, 319, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Zhi, X.; Moreira, F.; Lucia, T.J.; Mondadori, R.G.; Masternak, M.M. Primordial follicle activation in the ovary of Ames dwarf mice. J. Ovarian Res. 2014, 7, 120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castrillon, D.H.; Miao, L.; Kollipara, R.; Horner, J.W.; DePinho, R.A. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003, 301, 215–218. [Google Scholar] [CrossRef]
- Bezerra, M.É.S.; Barberino, R.S.; Menezes, V.G.; Gouveia, B.B.; Macedo, T.J.S.; Santos, J.M.S.; Monte, A.P.O.; Barros, V.R.P.; Matos, M.H.T. Insulin-like growth factor-1 (IGF-1) promotes primordial follicle growth and reduces DNA fragmentation through the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signalling pathway. Reprod. Fertil. Dev. 2018, 30, 1503–1513. [Google Scholar] [CrossRef]
- Zhang, H.; Risal, S.; Gorre, N.; Busayavalasa, K.; Li, X.; Shen, Y.; Bosbach, B.; Brännström, M.; Liu, K. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr. Biol. 2014, 24, 2501–2508. [Google Scholar] [CrossRef]
- Adhikari, D.; Flohr, G.; Gorre, N.; Shen, Y.; Yang, H.; Lundin, E.; Lan, Z.; Gambello, M.J.; Liu, K. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol. Hum. Reprod. 2009, 15, 765–770. [Google Scholar] [CrossRef]
- Adhikari, D.; Zheng, W.; Shen, Y.; Gorre, N.; Hämäläinen, T.; Cooney, A.J.; Huhtaniemi, I.; Lan, Z.-J.; Liu, K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum. Mol. Genet. 2010, 19, 397–410. [Google Scholar] [CrossRef]
- Mok-Lin, E.; Ascano, M.J.; Serganov, A.; Rosenwaks, Z.; Tuschl, T.; Williams, Z. Premature recruitment of oocyte pool and increased mTOR activity in Fmr1 knockout mice and reversal of phenotype with rapamycin. Sci. Rep. 2018, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A.; Quainoo, N. Impact of Growth Hormone-Related Mutations on Mammalian Aging. Front. Genet. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A. Growth Hormone and Aging: Updated Review. World J. Mens. Health 2019, 37, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Dominick, G.; Berryman, D.E.; List, E.O.; Kopchick, J.J.; Li, X.; Miller, R.A.; Garcia, G.G. Regulation of mTOR activity in Snell dwarf and GH receptor gene-disrupted mice. Endocrinology 2015, 156, 565–575. [Google Scholar] [CrossRef]
- Schneider, A.; Zhi, X.; Bartke, A.; Kopchick, J.J.; Masternak, M.M. Effect of growth hormone receptor gene disruption and PMA treatment on the expression of genes involved in primordial follicle activation in mice ovaries. Age 2014, 36, 9701. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Dekanová, P.; Harrath, A.H. FSH, oxytocin and IGF-I regulate the expression of sirtuin 1 in porcine ovarian granulosa cells. Physiol. Res. 2020, 69, 461–466. [Google Scholar] [CrossRef]
- Long, G.-Y.; Yang, J.-Y.; Xu, J.-J.; Ni, Y.-H.; Zhou, X.-L.; Ma, J.-Y.; Fu, Y.-C.; Luo, L.-L. SIRT1 knock-in mice preserve ovarian reserve resembling caloric restriction. Gene 2019, 686, 194–202. [Google Scholar] [CrossRef]
- Tatone, C.; Di Emidio, G.; Barbonetti, A.; Carta, G.; Luciano, A.M.; Falone, S.; Amicarelli, F. Sirtuins in gamete biology and reproductive physiology: Emerging roles and therapeutic potential in female and male infertility. Hum. Reprod. Update 2018, 24, 267–289. [Google Scholar] [CrossRef]
- Josso, N. WOMEN IN REPRODUCTIVE SCIENCE: Anti-Müllerian hormone: A look back and ahead. Reproduction 2019, 158, F81–F89. [Google Scholar] [CrossRef]
- Weenen, C.; Laven, J.S.E.; Von Bergh, A.R.M.; Cranfield, M.; Groome, N.P.; Visser, J.A.; Kramer, P.; Fauser, B.C.J.M.; Themmen, A.P.N. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 2004, 10, 77–83. [Google Scholar] [CrossRef]
- Liu, Y.; Masternak, M.M.; Schneider, A.; Zhi, X. Dwarf mice as models for reproductive ageing research. Reprod. Biomed. Online 2021, 44, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.T.; Mira, M.F.; Hamed, A.I.; Ezzat, T.; Sallam, M.T. Anti-Müllerian hormone levels in patients with turner syndrome: Relation to karyotype, spontaneous puberty, and replacement therapy. Am. J. Med. Genet. A 2018, 176, 1929–1934. [Google Scholar] [CrossRef]
- Visser, J.A.; Hokken-Koelega, A.C.S.; Zandwijken, G.R.J.; Limacher, A.; Ranke, M.B.; Flück, C.E. Anti-Müllerian hormone levels in girls and adolescents with Turner syndrome are related to karyotype, pubertal development and growth hormone treatment. Hum. Reprod. 2013, 28, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Lem, A.J.; Boonstra, V.H.; Renes, J.S.; Breukhoven, P.E.; De Jong, F.H.; Laven, J.S.E.; Hokken-Koelega, A.C.S. Anti-Müllerian hormone in short girls born small for gestational age and the effect of growth hormone treatment. Hum. Reprod. 2011, 26, 898–903. [Google Scholar] [CrossRef]
- Hou, H.-Y.; Wang, X.; Yu, Q.; Li, H.-Y.; Li, S.-J.; Tang, R.-Y.; Guo, Z.-X.; Chen, Y.-Q.; Hu, C.-X.; Yang, Z.-J.; et al. Evidence that growth hormone can improve mitochondrial function in oocytes from aged mice. Reproduction 2018, 157, 345–358. [Google Scholar] [CrossRef]
- Ménézo, Y.J.; el Mouatassim, S.; Chavrier, M.; Servy, E.J.; Nicolet, B. Human oocytes and preimplantation embryos express mRNA for growth hormone receptor. Zygote 2003, 11, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.R.; Lorenzo, P.L.; Carneiro, G.F.; Ball, B.A.; Bilodeau-Goeseels, S.; Kastelic, J.; Pegoraro, L.M.C.; Pimentel, C.A.; Esteller-Vico, A.; Illera, J.C.; et al. The involvement of growth hormone in equine oocyte maturation, receptor localization and steroid production by cumulus-oocyte complexes in vitro. Res. Vet. Sci. 2013, 95, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Yu, Q.; Liu, H.; Huang, T.; Zhao, S.; Ma, J.; Zhao, H. Growth Hormone Promotes in vitro Maturation of Human Oocytes. Front. Endocrinol. 2019, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.; Ruiz-Requena, E.; Ortega, E.; Cremades, N.; Martinez, F.; Bernabeu, R.; Greco, E.; Tesarik, J. Follicular fluid markers of oocyte developmental potential. Hum. Reprod. 2002, 17, 1017–1022. [Google Scholar] [CrossRef]
- Hazout, A.; Junca, A.M.; Ménézo, Y.; Demouzon, J.; Cohen-Bacrie, P. Effect of growth hormone on oocyte competence in patients with multiple IVF failures. Reprod. Biomed. Online 2009, 18, 664–670. [Google Scholar] [CrossRef]
- Hassan, H.A.; Azab, H.; Rahman, A.A.; Nafee, T.M. Effects of growth hormone on in vitro maturation of germinal vesicle of human oocytes retrieved from small antral follicles. J. Assist. Reprod. Genet. 2001, 18, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.R.; Lorenzo, P.L.; Carneiro, G.F.; Bilodeau-Goeseels, S.; Kastelic, J.P.; Esteller-Vico, A.; Lopez-Bejar, M.; Liu, I.K.M. Selection of developmentally competent immature equine oocytes with brilliant cresyl blue stain prior to in vitro maturation with equine growth hormone. Zygote 2014, 22, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Kiapekou, E.; Loutradis, D.; Drakakis, P.; Zapanti, E.; Mastorakos, G.; Antsaklis, A. Effects of GH and IGF-I on the in vitro maturation of mouse oocytes. Hormones 2005, 4, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Izadyar, F.; Colenbrander, B.; Bevers, M.M. In vitro maturation of bovine oocytes in the presence of growth hormone accelerates nuclear maturation and promotes subsequent embryonic development. Mol. Reprod. Dev. 1996, 45, 372–377. [Google Scholar] [CrossRef]
- Lin, Y.; Xie, B.; Li, X.; Li, R.; Ma, C.; Zhu, J.; Qiao, J. Supplementation of the In Vitro Maturation Culture Medium of Mouse Oocytes with Growth Hormone Improves Pregnancy Outcomes. Reprod. Sci. 2021, 28, 2540–2549. [Google Scholar] [CrossRef] [PubMed]
- Uzbekova, S.; Arlot-Bonnemains, Y.; Dupont, J.; Dalbiès-Tran, R.; Papillier, P.; Pennetier, S.; Thélie, A.; Perreau, C.; Mermillod, P.; Prigent, C.; et al. Spatio-temporal expression patterns of Aurora kinases A, B, and C and cytoplasmic polyadenylation-element-binding protein in bovine oocytes during meiotic maturation. Biol. Reprod. 2008, 78, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.J.; Sun, Q.Y. Characterization of Aurora-A in porcine oocytes and early embryos implies its functional roles in the regulation of meiotic maturation, fertilization and cleavage. Zygote 2005, 13, 23–30. [Google Scholar] [CrossRef]
- Yao, L.J.; Zhong, Z.S.; Zhang, L.S.; Chen, D.Y.; Schatten, H.; Sun, Q.Y. Aurora-A Is a Critical Regulator of Microtubule Assembly and Nuclear Activity in Mouse Oocytes, Fertilized Eggs, and Early Embryos. Biol. Reprod. 2004, 70, 1392–1399. [Google Scholar] [CrossRef][Green Version]
- Nishimura, Y.; Endo, T.; Kano, K.; Naito, K. Porcine Aurora A accelerates Cyclin B and Mos synthesis and promotes meiotic resumption of porcine oocytes. Anim. Reprod. Sci. 2009, 113, 114–124. [Google Scholar] [CrossRef]
- Duesbery, N.S.; Choi, T.; Brown, K.D.; Wood, K.W.; Resau, J.; Fukasawa, K.; Cleveland, D.W.; Vande Woude, G.F. CENP-E is an essential kinetochore motor in maturing oocytes and is masked during mos-dependent, cell cycle arrest at metaphase II. Proc. Natl. Acad. Sci. USA 1997, 94, 9165–9170. [Google Scholar] [CrossRef]
- Pawlak, P.; Cieslak, A.; Warzych, E.; Zejden, Z.; Szumacher-Strabel, M.; Molinska-Glura, M.; Lechniak, D. No single way to explain cytoplasmic maturation of oocytes from prepubertal and cyclic gilts. Theriogenology 2012, 78, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Jochems, R.; Gaustad, A.H.; Zak, L.J.; Grindflek, E.; Zeremichael, T.T.; Oskam, I.C.; Myromslien, F.D.; Kommisrud, E.; Krogenaes, A.K. Ovarian characteristics and in vitro nuclear and cytoplasmic oocyte maturation in Duroc and Landrace pigs. Vet. Med. Sci. 2021, 7, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.R.; Lorenzo, P.L.; Carneiro, G.F.; Ball, B.A.; Gonçalves, P.B.D.; Pegoraro, L.M.C.; Bilodeau-Goeseels, S.; Kastelic, J.P.; Casey, P.J.; Liu, I.K.M. The effect of growth hormone (GH) and insulin-like growth factor-I (IGF-I) on in vitro maturation of equine oocytes. Zygote 2012, 20, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.R.; Lorenzo, P.L.; Carneiro, G.F.; Bilodeau-Goeseels, S.; Kastelic, K.P.; Pegoraro, L.C.; Pimentel, C.A.; Esteller-Vico, A.; Illera, J.C.; Silvan, G.; et al. Effect of equine growth hormone (eGH) on in vitro maturation of equine oocytes and on steroidogenesis by their cumulus-oocyte complexes. Anim. Reprod. Sci. 2006, 94, 1–4. [Google Scholar]
- Eichenlaub-Ritter, U.; Wieczorek, M.; Lüke, S.; Seidel, T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion 2011, 11, 783–796. [Google Scholar] [CrossRef]
- Coticchio, G.; Dal Canto, M.; Mignini Renzini, M.; Guglielmo, M.C.; Brambillasca, F.; Turchi, D.; Novara, P.V.; Fadini, R. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 2015, 21, 427–454. [Google Scholar] [CrossRef]
- Feng, P.; Xie, Q.; Liu, Z.; Guo, Z.; Tang, R.; Yu, Q. Study on the Reparative Effect of PEGylated Growth Hormone on Ovarian Parameters and Mitochondrial Function of Oocytes from Rats with Premature Ovarian Insufficiency. Front. Cell Dev. Biol. 2021, 9, 649005. [Google Scholar] [CrossRef]
- Weall, B.M.; Al-Samerria, S.; Conceicao, J.; Yovich, J.L.; Almahbobi, G. A direct action for GH in improvement of oocyte quality in poor-responder patients. Reproduction 2015, 149, 147–154. [Google Scholar] [CrossRef]
- Short, K.R.; Moller, N.; Bigelow, M.L.; Coenen-Schimke, J.; Nair, K.S. Enhancement of muscle mitochondrial function by growth hormone. J. Clin. Endocrinol. Metab. 2008, 93, 597–604. [Google Scholar] [CrossRef]
- Dalton, C.M.; Szabadkai, G.; Carroll, J. Measurement of ATP in single oocytes: Impact of maturation and cumulus cells on levels and consumption. J. Cell. Physiol. 2014, 229, 353–361. [Google Scholar] [CrossRef]
- Li, H.-K.; Kuo, T.-Y.; Yang, H.-S.; Chen, L.-R.; Li, S.S.-L.; Huang, H.-W. Differential gene expression of bone morphogenetic protein 15 and growth differentiation factor 9 during in vitro maturation of porcine oocytes and early embryos. Anim. Reprod. Sci. 2008, 103, 312–322. [Google Scholar] [CrossRef]
- Lima, I.M.T.; Brito, I.R.; Rossetto, R.; Duarte, A.B.G.; Rodrigues, G.Q.; Saraiva, M.V.A.; Costa, J.J.N.; Donato, M.A.M.; Peixoto, C.A.; Silva, J.R.V.; et al. BMPRIB and BMPRII mRNA expression levels in goat ovarian follicles and the in vitro effects of BMP-15 on preantral follicle development. Cell Tissue Res. 2012, 348, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, M.; Machado, S.A.; Stojkovic, P.; Zakhartchenko, V.; Hutzler, P.; Gonçalves, P.B.; Wolf, E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: Correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol. Reprod. 2001, 64, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Cicek, O.S.Y.; Demir, M.; Yalcinkaya, L.; Sertel, E. The effect of growth hormone adjuvant therapy on assisted reproductive technologies outcomes in patients with diminished ovarian reserve or poor ovarian response. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101982. [Google Scholar] [CrossRef] [PubMed]
- Regan, S.L.P.; Knight, P.G.; Yovich, J.L.; Arfuso, F.; Dharmarajan, A. Growth hormone during in vitro fertilization in older women modulates the density of receptors in granulosa cells, with improved pregnancy outcomes. Fertil. Steril. 2018, 110, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, G.G.; Kölle, S.; Boie, G.; Sinowatz, F.; Palma, G.A.; Alberio, R.H. In vivo effect of growth hormone on the expression of connexin-43 in bovine ovarian follicles. Mol. Reprod. Dev. 2006, 73, 600–606. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Kobayashi, J.; Mizunuma, H.; Kikuchi, N.; Liu, X.; Andoh, K.; Abe, Y.; Yokota, H.; Yamada, K.; Ibuki, Y.; Hagiwara, H. Morphological assessment of the effect of growth hormone on preantral follicles from 11-day-old mice in an in vitro culture system. Biochem. Biophys. Res. Commun. 2000, 268, 36–41. [Google Scholar] [CrossRef]
- Nakamura, E.; Otsuka, F.; Inagaki, K.; Miyoshi, T.; Matsumoto, Y.; Ogura, K.; Tsukamoto, N.; Takeda, M.; Makino, H. Mutual regulation of growth hormone and bone morphogenetic protein system in steroidogenesis by rat granulosa cells. Endocrinology 2012, 153, 469–480. [Google Scholar] [CrossRef]
- Lanzone, A.; Fortini, A.; Fulghesu, A.M.; Soranna, L.; Caruso, A.; Mancuso, S. Growth hormone enhances estradiol production follicle-stimulating hormone-induced in the early stage of the follicular maturation. Fertil. Steril. 1996, 66, 948–953. [Google Scholar] [CrossRef]
- Ferreira, A.C.A.; Maside, C.; Sá, N.A.R.; Guerreiro, D.D.; Correia, H.H.V.; Leiva-Revilla, J.; Lobo, C.H.; Araújo, V.R.; Apgar, G.A.; Brandão, F.Z.; et al. Balance of insulin and FSH concentrations improves the in vitro development of isolated goat preantral follicles in medium containing GH. Anim. Reprod. Sci. 2016, 165, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahumada-Solórzano, S.M.; Martínez-Moreno, C.G.; Carranza, M.; Ávila-Mendoza, J.; Luna-Acosta, J.L.; Harvey, S.; Luna, M.; Arámburo, C. Autocrine/paracrine proliferative effect of ovarian GH and IGF-I in chicken granulosa cell cultures. Gen. Comp. Endocrinol. 2016, 234, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kopchick, J.J.; Parkinson, C.; Stevens, E.C.; Trainer, P.J. Growth hormone receptor antagonists: Discovery, development, and use in patients with acromegaly. Endocr. Rev. 2002, 23, 623–646. [Google Scholar] [CrossRef]
- Takahashi, Y. The Role of Growth Hormone and Insulin-Like Growth Factor-I in the Liver. Int. J. Mol. Sci. 2017, 18, 1447. [Google Scholar] [CrossRef]
- Dehkhoda, F.; Lee, C.M.M.; Medina, J.; Brooks, A.J. The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects. Front. Endocrinol. 2018, 9, 35. [Google Scholar] [CrossRef]
- Kang, S.K.; Tai, C.J.; Cheng, K.W.; Leung, P.C. Gonadotropin-releasing hormone activates mitogen-activated protein kinase in human ovarian and placental cells. Mol. Cell. Endocrinol. 2000, 170, 143–151. [Google Scholar] [CrossRef]
- Seger, R.; Hanoch, T.; Rosenberg, R.; Dantes, A.; Merz, W.E.; Strauss, J.F., 3rd; Amsterdam, A. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J. Biol. Chem. 2017, 292, 8847. [Google Scholar] [CrossRef]
- Moore, R.K.; Otsuka, F.; Shimasaki, S. Role of ERK1/2 in the differential synthesis of progesterone and estradiol by granulosa cells. Biochem. Biophys. Res. Commun. 2001, 289, 796–800. [Google Scholar] [CrossRef]
- Rowlinson, S.W.; Yoshizato, H.; Barclay, J.L.; Brooks, A.J.; Behncken, S.N.; Kerr, L.M.; Millard, K.; Palethorpe, K.; Nielsen, K.; Clyde-Smith, J.; et al. An agonist-induced conformational change in the growth hormone receptor determines the choice of signalling pathway. Nat. Cell Biol. 2008, 10, 740–747. [Google Scholar] [CrossRef]
- Manna, P.R.; Huhtaniemi, I.T.; Stocco, D.M. Mechanisms of protein kinase C signaling in the modulation of 3’,5’-cyclic adenosine monophosphate-mediated steroidogenesis in mouse gonadal cells. Endocrinology 2009, 150, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Niswender, G.D. Molecular control of luteal secretion of progesterone. Reproduction 2002, 123, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Crépieux, P. Molecular Mechanisms of Action of FSH. Front. Endocrinol. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Little-Ihrig, L.; Chandran, U.; Law, N.C.; Hunzicker-Dunn, M.; Zeleznik, A.J. Protein Kinase A: A Master Kinase of Granulosa Cell Differentiation. Sci. Rep. 2016, 6, 28132. [Google Scholar] [CrossRef]
- Liang, A.; Plewes, M.R.; Hua, G.; Hou, X.; Blum, H.R.; Przygrodzka, E.; George, J.W.; Clark, K.L.; Bousfield, G.R.; Butnev, V.Y.; et al. Bioactivity of recombinant hFSH glycosylation variants in primary cultures of porcine granulosa cells. Mol. Cell. Endocrinol. 2020, 514, 110911. [Google Scholar] [CrossRef]
- Mukherjee, A.; Park-Sarge, O.K.; Mayo, K.E. Gonadotropins induce rapid phosphorylation of the 3’,5’-cyclic adenosine monophosphate response element binding protein in ovarian granulosa cells. Endocrinology 1996, 137, 3234–3245. [Google Scholar] [CrossRef]
- Słuczanowska-Głąbowska, S.; Laszczyńska, M.; Piotrowska, K.; Głąbowski, W.; Kopchick, J.J.; Bartke, A.; Kucia, M.; Ratajczak, M.Z. Morphology of ovaries in laron dwarf mice, with low circulating plasma levels of insulin-like growth factor-1 (IGF-1), and in bovine GH-transgenic mice, with high circulating plasma levels of IGF-1. J. Ovarian Res. 2012, 5, 18. [Google Scholar] [CrossRef][Green Version]
- Isola, J.V.V.; Zanini, B.M.; Sidhom, S.; Kopchick, J.J.; Bartke, A.; Masternak, M.M.; Stout, M.B.; Schneider, A. 17α-Estradiol promotes ovarian aging in growth hormone receptor knockout mice, but not wild-type littermates. Exp. Gerontol. 2020, 129, 110769. [Google Scholar] [CrossRef]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar]
- Baumgarten, S.C.; Armouti, M.; Ko, C.; Stocco, C. IGF1R Expression in Ovarian Granulosa Cells Is Essential for Steroidogenesis, Follicle Survival, and Fertility in Female Mice. Endocrinology 2017, 158, 2309–2318. [Google Scholar] [CrossRef]
- Zhou, J.; Kumar, T.R.; Matzuk, M.M.; Bondy, C. Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol. Endocrinol. 1997, 11, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Lavranos, T.C.; O’Leary, P.C.; Rodgers, R.J. Effects of insulin-like growth factors and binding protein 1 on bovine granulosa cell division in anchorage-independent culture. J. Reprod. Fertil. 1996, 107, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Spicer, L.J.; Aad, P.Y. Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: Role of follicle-stimulating hormone and IGF2 receptor. Biol. Reprod. 2007, 77, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Sauceda, C.; Webster, N.J.G. Mitochondrial Dysfunction in Obesity and Reproduction. Endocrinology 2021, 162, bqaa158. [Google Scholar] [CrossRef]
- Roth, Z. Symposium review: Reduction in oocyte developmental competence by stress is associated with alterations in mitochondrial function. J. Dairy Sci. 2018, 101, 3642–3654. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Ostadmohammadi, V.; Jamilian, M.; Bahmani, F.; Asemi, Z. Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J. Ovarian Res. 2019, 12, 5. [Google Scholar] [CrossRef]
- Samimi, M.; Pourhanifeh, M.H.; Mehdizadehkashi, A.; Eftekhar, T.; Asemi, Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J. Cell. Physiol. 2019, 234, 19384–19392. [Google Scholar] [CrossRef]
- Gong, Y.; Luo, S.; Fan, P.; Zhu, H.; Li, Y.; Huang, W. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2020, 18, 121. [Google Scholar] [CrossRef]
- John, G.B.; Shidler, M.J.; Besmer, P.; Castrillon, D.H. Kit signaling via PI3K promotes ovarian follicle maturation but is dispensable for primordial follicle activation. Dev. Biol. 2009, 331, 292–299. [Google Scholar] [CrossRef]
- Grosbois, J.; Demeestere, I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum. Reprod. 2018, 33, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, D.; Díaz, O.; Devesa, P.; Devesa, J. Growth Hormone (GH) and Cardiovascular System. Int. J. Mol. Sci. 2018, 19, 290. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-Y.; Kim, H.-J.; Kim, M. The protective effect of growth hormone on Cu/Zn superoxide dismutase-mutant motor neurons. BMC Neurosci. 2015, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, J.; Zhang, Y.; Zhang, J.; Xu, W.; Wu, C.; Zhou, P. Growth hormone protects against ovarian granulosa cell apoptosis: Alleviation oxidative stress and enhancement mitochondrial function. Reprod. Biol. 2021, 21, 100504. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Y.; Li, L.; Wang, H.-H.; Ma, X.-S.; Qian, W.-P.; Shen, W.; Schatten, H.; Sun, Q.-Y. SIRT1, 2, 3 protect mouse oocytes from postovulatory aging. Aging 2016, 8, 685–696. [Google Scholar] [CrossRef]
- Mason, H.D.; Martikainen, H.; Beard, R.W.; Anyaoku, V.; Franks, S. Direct gonadotrophic effect of growth hormone on oestradiol production by human granulosa cells in vitro. J. Endocrinol. 1990, 126, R1–R4. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z.; Zhang, H.; Jin, L.; Li, Y.; Ai, J.; Zhu, G. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: An analysis of more than 10,000 cycles. Fertil. Steril. 2012, 97, 1321–1324. [Google Scholar] [CrossRef]
| ART (Year) | Inclusion Criteria | Main Exclusion Criteria | GH Usage /Stimulation Protocol | Clinical Outcomes |
|---|---|---|---|---|
| IVF (2018) | 127 females (according to the Bologna consensus criteria [41]) | BMI > 30 mg/m2, and women with other causes of infertility | Sustained-release of 20 mg of GH three times before and during COS/GnRH-Ant protocol | GH elevated the follicles number and the proportion of metaphase II oocytes; there was no significant differences in the percentages of clinical and ongoing pregnancy, or miscarriage [21]. |
| IVF (2019) | 184 females (according to the Bologna consensus criteria [41]) | No description | Injection of GH at dosages of 4, 4, and 2 IU over three successive days, along with the ovulation induction/GnRH-a ultra-long protocol | GH elevated the ovarian response, the number of retrieved oocytes, the number of embryos available for transfer, clinical pregnancy rates, and ongoing pregnancy rates; there were no significant differences between groups regarding miscarriage rates [53]. |
| IVF/ ICSI (2019) | 130 females (≤1 IVF cycle, with ≤5 oocytes, rFSH dosage > 250 IU/d, basal FSH ≤ 15 IU, BMI < 33 kg/m2, and age < 41 years) | Females with a history of malignant diseases or pituitary/hypothalamic diseases; current ovarian cysts (>3 cm) or chronic infectious diseases, PCOS, or AUB | Daily injection of 12 IU GH from day 1 of COS until the day of hCG/GnRH-Ant protocol | GH increased the number of retrieved oocytes and did not signficantly affect the LBRs or the number of transferred embryos [43]. |
| IVF/ ICSI (2019) | 105 females (according to the Bologna consensus criteria [41]) | FSH > 20 IU/L, a history of infertility due to non-POR causes | Daily subcutaneous injection of 2.5mg of GH from the eighth day of the cycle until the injection of HCG /GnRH-Ant protocol | GH significantly increased the number of retrieved oocytes, MII oocytes, fertilized oocytes, transferred embryos, and clinical PRs [54]. |
| IVF/ ICSI (2015) | 145 females (according to the Bologna consensus criteria [41]) | FSH > 20 IU/L, women with other causes of infertility, and severe male factors | Daily injection of 2.5 mg of GH from day 6 of COS until the day of hCG/GnRH-a long protocol | GH increased the number of retrieved oocytes, MII oocytes, and the mean number of fertilized oocytes and elevated PRs without any significant differences between groups [38]. |
| IVF/ ICSI (2017) | 50 females (according to the Bologna consensus criteria [41]) | FSH > 20 IU/L, BMI ≥ 35 kg/m2, and severe male factors | Daily injection of 4 IU of GH from day 1 of COS until the day of hCG/GnRH-Ant protocol | GH lowered the effective dose of Gn and the duration of stimulation while elevating the total number of oocytes, as well as the numbers of MII oocytes, 2PN zygotes, and good-quality transferred embryos and the probability of pregnancy [37]. |
| IVF/ ICSI (2018) | 240 females (according to the Bologna consensus criteria [41]) | >45 years, FSH > 20 IU/L, tubal occlusion, and severe male factors | Daily injection of 7.5 IU of GH from day 21 of the previous cycle until the day of hCG/GnRH-a long protocol | GH improved the numbers of retrieved oocytes, MII oocytes, fertilized oocytes, transferred embryos, and cryopreserved embryos; there were no significant differences in the LBRs, whether fresh or cumulative [34]. |
| IVF/ ICSI (2016) | 141 females (according to the Bologna consensus criteria [41]) | FSH > 20 IU/L, and women with other causes of infertility | Daily injection of 7.5 IU of GH from day 6 of COS until the day of hCG/GnRH-Ant protocol | GH lowered the effective dose of Gn and the duration of GnRH-Ant treatment while increasing the numbers of collected oocytes, MII oocytes, fertilized oocytes, and transferred embryos, and as well as the mean E2 levels on the day of hCG; there were no significant differences in clinical PRs per cycle or LBRs per cycle [17]. |
| IVF/ ICSI (2013) | 82 females (≥1 previous failed IVF-ET cycles with ≤3 retrieved oocytes and ≤3 subsequently obtained embryos using GnRH-a long protocol, and/or E2 levels ≤ 500 pg/mL on the day of hCG) | BMI ≥ 30 mg/m2, FSH > 15 IU/L, women with other causes of infertility, and azoospermia | Daily injection of 4 IU of GH from day 21 of the previous cycle until the day of hCG/GnRH-Ant protocol | GH increased the numbers of retrieved oocytes and obtained embryos; there were no significant differences between groups regarding implantation, or chemical and clinical PRs [27]. |
| IVF (2015) | 64 females (according to the Bologna consensus criteria [41]) | BMI ≥ 30kg/m2, women with other causes of infertility, altered karyotype in couples, and severe male factors | Daily injection of 0.5 IU of GH from day 1 of the agonist until the day of hCG/GnRH-a long protocol | GH increased the numbers of top-quality embryos and cryopreserved embryos[28]. |
| ICSI (2008) | 61 females who responded poorly to high doses of gonadotropin treatment during their first cycles in the same center | D3 FSH > 20 IU/L | Daily injection of 12 IU of GH from day 21 of the precious cycle until the day of hCG/GnRH-a long protocol | GH increased zygotes; although more pregnancies and more clinical pregnancies with fetal heart beat were achieved in the GH group (12/31), compared to the control group (6/30), the difference was not statistically significant [55]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, P.; Huang, X. The Clinical Application of Growth Hormone and Its Biological and Molecular Mechanisms in Assisted Reproduction. Int. J. Mol. Sci. 2022, 23, 10768. https://doi.org/10.3390/ijms231810768
Pan P, Huang X. The Clinical Application of Growth Hormone and Its Biological and Molecular Mechanisms in Assisted Reproduction. International Journal of Molecular Sciences. 2022; 23(18):10768. https://doi.org/10.3390/ijms231810768
Chicago/Turabian StylePan, Peipei, and Xuefeng Huang. 2022. "The Clinical Application of Growth Hormone and Its Biological and Molecular Mechanisms in Assisted Reproduction" International Journal of Molecular Sciences 23, no. 18: 10768. https://doi.org/10.3390/ijms231810768
APA StylePan, P., & Huang, X. (2022). The Clinical Application of Growth Hormone and Its Biological and Molecular Mechanisms in Assisted Reproduction. International Journal of Molecular Sciences, 23(18), 10768. https://doi.org/10.3390/ijms231810768





