Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair
Abstract
1. Introduction
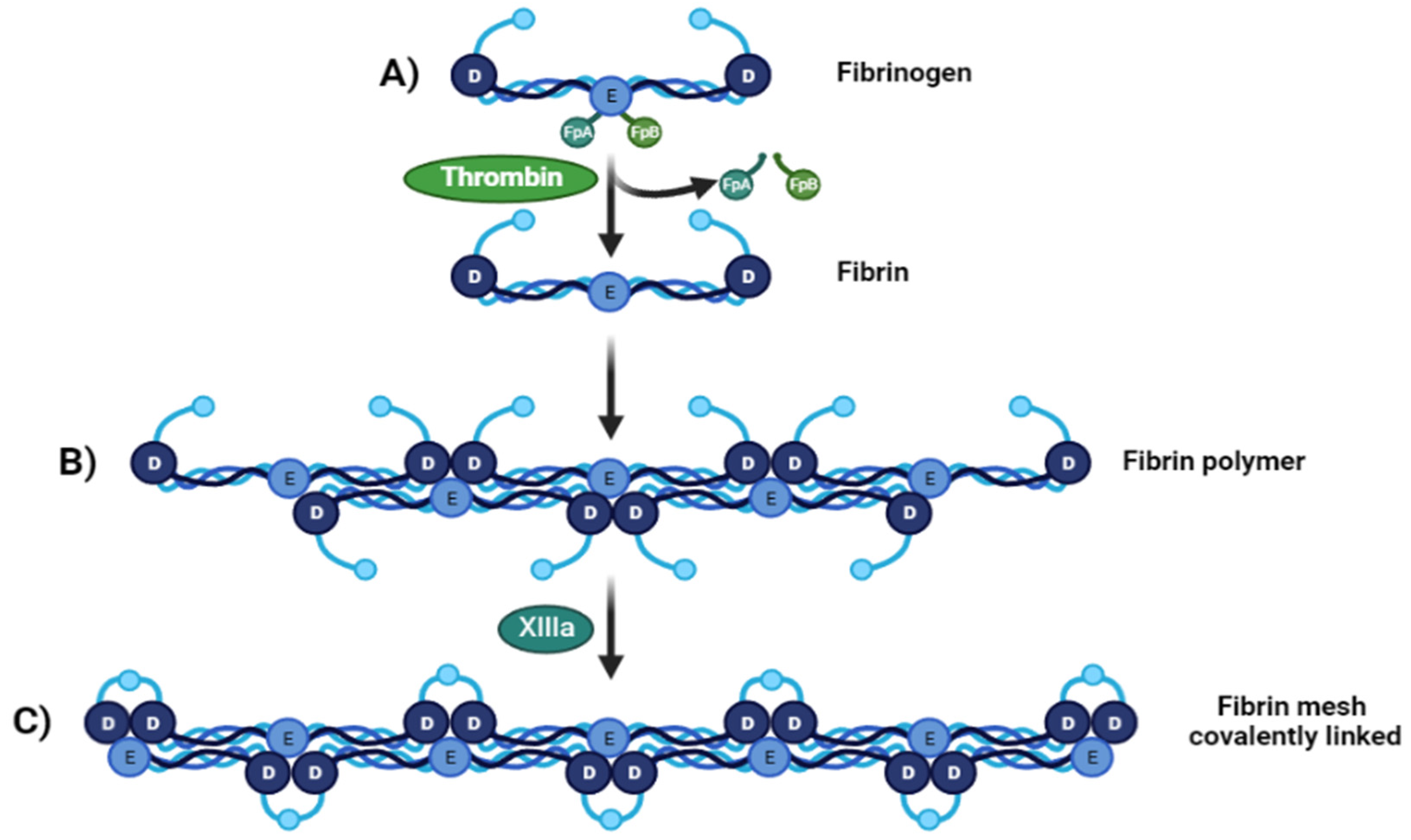
2. Fibrin: Structure and Molecular Interactions
3. Mechanical and Physical Properties of the Fibrin Scaffolds
| Scaffold | Fibrin/Fibrinogen Content (mg/mL) | Pore Size (µm) | Mechanical Strength (Mpa) | Longitudinal Elasticity (Youngs Modulus) | Reference | ||
|---|---|---|---|---|---|---|---|
| Other Component Content | Elastic Modulus (kPa) | Elongation at Break (%) | |||||
| Fibrin glue (Tiseel) | 67–106 | - | - | ≈0.0029 | 15 | - | [45] |
| Fibrin glue (EVICEL) | 55–85 | - | - | 0.0135 | 38 | - | [45] |
| Fibrin hydrogel | 5 | - | 9.7 ± 7.1 | 0.0034 | - | - | [31] |
| Fibrin hydrogel | 12.5 | - | 8.1 ± 5.3 | 0.0054 | - | - | [31] |
| Fibrin hydrogel | 25 | - | 6.4 ± 3.4 | 0.0109 | - | - | [31] |
| Fibrin hydrogel | 50 | - | - | ≈0.01 | 20 | - | [46] |
| Hydrogel: Fibrin-PAAm | 50 | 44.46% PAAm | - | ≈0.052 | 120 | ≈55 | [46] |
| Composite: Fibrin-PAAm-PCL | 44.46% PAAm PCL as a core | - | ≈0.16 | 150 | ≈22 | [46] | |
| Composite: Fibrin-collagen sponge | 110 | - | ≈110 | ≈12 | - | - | [47] |
| Composite: Fibrin-genipin crosslinked DCM-PVA | - | Genipin = 0.04 g/g DMC-PVA 70:30 | 22–95 | - | 14.7 ± 2.7 | 62.39 ± 6.56 | [16] |
| Htdrogel: Fibrin-PLC-ECM | - | PCL = 28% ECM = 2%, 5%, and 10% | 250–400 | 0.13–0.20 | - | - | [37] |
| Hydrogel: Fibrin-PLC-ECM (salt leached) | - | PCL = 28% ECM = 2%, 5%, and 10% | <400 | 0.02–0.05 | - | - | [37] |
| Advanced platelet-rich fibrin glue | - | - | - | 0.17 | ≈70 | ≈25 | [48] |
| Platelet.poor plasma-derived fibrin glue | - | - | - | 0.13 | ≈70 | ≈15 | [48] |
4. Biodegradation
| Polymer Type | Material | Properties | Advantages | Disadvantages | Ref. | ||
|---|---|---|---|---|---|---|---|
| Toxicity | Biocompatibility | Immunogenicity | |||||
| Natural | Fibrin | Not reported | High | Non-immunogenicity | Properties of cell adhesive/binding | Quick rate of degradation; Poor biomechanical strength | [42,54] |
| Collagen | Low | High | Low | Favorable for cell adhesion, proliferation, and ECM secretion | Physical and chemical variable properties Variable degradation | [54,55] | |
| Silk fibroin | Non-toxicity | High | Prolongated presence of silk may induce degradation that may prompt the immune response | Support for cell adhesion, proliferation, and vascularization | Moderately degradable | [54,56] | |
| Gelatin | Low toxicity | High | Non immunogenicity | Better infiltration, adhesion, spreading, and proliferation of cells | Low stability in physiological conditions | [54,57] | |
| Chitosan | Non-toxicity | Hemostatic potential | Low immunogenicity | Promotes adhesion, accelerates repair, and prevents formation of scar tissue | Poor mechanical strength and stability Low solubility Quick rate degradation in vivo | [54,58] | |
| Alginate | Non-toxicity | High | Non-immunogenic | Mimicking function of the extracellular matrix | Low adhesion, poor mechanical characteristics | [59] | |
| Hyaluronic acid | Non-toxicity | High | Non-immunogenic | Supporting migration of mesenchymal stem cells and epithelial cells Fill irregular defects | Poor biomechanical strength Low biodegradability in the crystalline phase | [60] | |
| Synthetic | Polylactic acid (PLA) | Non-toxicity | High | Non-immunogenicity | High stress resistance High Young’s modulus Possibility of synthesizing in different forms | His depolymerization require excessive heating Local acidosis caused by biodegradation products | [54,61,62] |
| Poly(ƹ-caprolactone) (PCL) | Non-toxicity | High | Low immunogenicity | Good mechanical properties Controls cell proliferation and angiogenesis | Low bioactivity | [54,63] | |
| Polyvinyl alcohol (PVA) | Non-toxicity | High | Low immunogenicity | Higher elasticity Similar tensile strength to human articular cartilage | Lack of cell-adhesive property. | [54,64] | |
| Poly(ethylene glycol) (PEG) | Non-toxicity | High | Non-immunogenicity | Elastic Bioadhesive | Creates insoluble networks | [55,65] | |
5. Biocompatibility
6. Cells on Fibrin Scaffolds
7. Growth Factors on Fibrin Scaffolds
- Platelet-rich fibrin (PRF) is prepared from blood samples collected without anticoagulants or biological agents. PRF has been further modified into an advanced form called advanced platelet-rich fibrin (A-PRF), which has a fibrin clot softer than PRF and more platelet cells than PRF [98]. PRF has a solid fibrin matrix that contains a higher concentration of platelets, leucocytes, growth factors (18–148 ng/mL of PDGF, 390–560 ng/mL of TGF-b, around 126.86 ng/mL of b-FGF and around 274 ng/mL of IGF) and adhesive proteins such as fibronectin, fibrinogen, vitronectin, and thrombospondin-1 compared to PRP [99].
- Concentrated growth factors (CGF) (20–175 ng/mL of PDGF, 390–584 ng/mL of TGF-b, around 130.56 ng/mL of b-FGF, 321 pg/mL of IGF, 7.5 pg/mL IL-6) [100]. They can be considered a modified form of PRF. CGF is produced by centrifugation of a blood sample using alternating speed rates. This process leads to a dense fibrin matrix that can promote cell migration, such as fibroblast and endothelial cells [101]. They contain more growth factors than PRP and PRF.
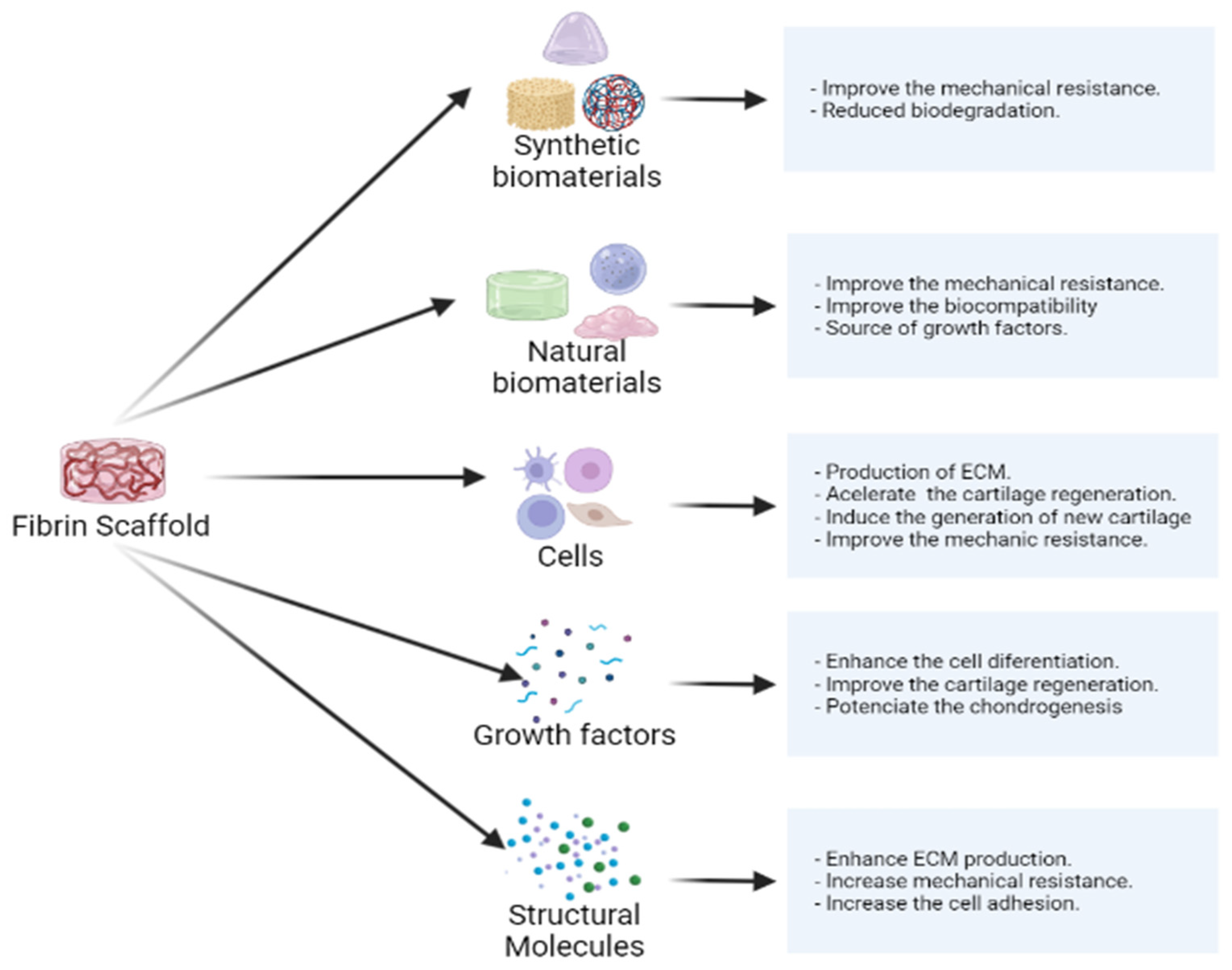
8. Modifications That Promote the Advantages of Fibrin Scaffolds
9. Applications of Fibrin Scaffolds in Cartilage Engineering
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madeira, C.; Santhagunam, A.; Salgueiro, J.B.; Cabral, J.M.S. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 2015, 33, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Burdick, J.A. Engineering Cartilage Tissue. Adv. Drug Deliv. Rev. 2008, 60, 243. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Bonhome-Espinosa, A.B.; Chato-Astrain, J.; Sánchez-Porras, D.; García-García, Ó.D.; Carmona, R.; López-López, M.T.; Alaminos, M.; Carriel, V.; Rodriguez, I.A. Evaluation of Fibrin-Agarose Tissue-Like Hydrogels Biocompatibility for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8, 596. [Google Scholar] [CrossRef]
- Zylińska, B.; Silmanowicz, P.; Sobczyńska-Rak, A.; Jarosz, Ł.; Szponder, T. Treatment of Articular Cartilage Defects: Focus on Tissue Engineering. In Vivo Brooklyn 2018, 32, 1289–1300. [Google Scholar] [CrossRef]
- Khan, M.; Adili, A.; Winemaker, M.; Bhandari, M. Management of osteoarthritis of the knee in younger patients. Can. Med. Assoc. J. 2018, 190, E72. [Google Scholar] [CrossRef]
- Hussain, S.M.; Neilly, D.W.; Baliga, S.; Patil, S.; Meek, R.M.D. Knee osteoarthritis: A review of management options. Scott. Med. J. 2016, 61, 7–16. [Google Scholar] [CrossRef]
- Bruyère, O.; Cooper, C.; Pelletier, J.P.; Branco, J.; Luisa Brandi, M.; Guillemin, F.; Hochberg, M.C.; Kanis, J.A.; Kvien, T.K.; Martel-Pelletier, J.; et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: A report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin. Arthritis Rheum. 2014, 44, 253–263. [Google Scholar] [CrossRef]
- Iannitti, T.; Lodi, D.; Palmieri, B. Intra-Articular Injections for the Treatment of Osteoarthritis: Focus on the Clinical Use of Hyaluronic Acid. Drugs R D 2011, 11, 13. [Google Scholar] [CrossRef]
- Epanomeritakis, I.E.; Lee, E.; Lu, V.; Khan, W. The Use of Autologous Chondrocyte and Mesenchymal Stem Cell Implants for the Treatment of Focal Chondral Defects in Human Knee Joints—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 4065. [Google Scholar] [CrossRef]
- Lavoie, J.R.; Rosu-Myles, M. Uncovering the secretes of mesenchymal stem cells. Biochimie 2013, 95, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M.; et al. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 603444. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Titan, A.L.; Stafford, M.; Zheng, C.H.; Levenston, M.E. Variations in chondrogenesis of human bone marrow-derived mesenchymal stem cells in fibrin/alginate blended hydrogels. Acta Biomater. 2012, 8, 3754–3764. [Google Scholar] [CrossRef] [PubMed]
- Snyder, T.N.; Madhavan, K.; Intrator, M.; Dregalla, R.C.; Park, D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014, 8, 1–11. [Google Scholar] [CrossRef]
- Setayeshmehr, M.; Esfandiari, E.; Hashemibeni, B.; Tavakoli, A.H.; Rafienia, M.; Samadikuchaksaraei, A.; Moroni, L.; Joghataei, M.T. Chondrogenesis of human adipose-derived mesenchymal stromal cells on the [devitalized costal cartilage matrix/poly(vinyl alcohol)/fibrin] hybrid scaffolds. Eur. Polym. J. 2019, 118, 528–541. [Google Scholar] [CrossRef]
- Freytes, D.O.; Godier-Furnemont, A.; Duan, Y.; O’Neill, J.D.; Vunjak-Novakovic, G. Biomaterial scaffolds for cardiac regeneration and repair derived from native heart matrix. In Cardiac Regeneration and Repair; Li, R.-K., Weisel, R.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 201–224. [Google Scholar] [CrossRef]
- Kohane, D.S.; Langer, R. Polymeric Biomaterials in Tissue Engineering. Pediatr. Res. 2008, 63, 487–491. [Google Scholar] [CrossRef]
- Sukaryo, S.G.; Purnama, A.; Hermawan, H. Structure and properties of biomaterials. Adv. Struct. Mater. 2016, 58, 1–22. [Google Scholar] [CrossRef]
- Pourentezari, M.; Anvari, M.; Yadegari, M.; Abbasi, A.; Dortaj, H. A Review of Tissue-Engineered Cartilage Utilizing Fibrin and Its Composite. Int. J. Med. Lab. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Park, C.H.; Woo, K.M. Fibrin-based biomaterial applications in tissue engineering and regenerative medicine. Adv. Exp. Med. Biol. 2018, 1064, 253–261. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Pieters, M.; de Lange-Loots, Z.; Weisel, J.W. Fibrinogen and Fibrin. In Macromolecular Protein Complexes III: Structure and Function; Harris, J.R., Marles-Wright, J., Eds.; Springer: Berlin, Germany, 2021; pp. 471–501. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, R.I.; Weisel, J.W. Not fibrin(ogen), but fibrinogen or fibrin. Blood 2015, 126, 1977–1978. [Google Scholar] [CrossRef] [PubMed]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Subcell. Biochem. 2017, 82, 405–456. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, M.W. Structure and Functions of Fibrin. In Recent Advances in Thrombosis and Hemostasis 2008; Springer: Japan, Tokyo, 2009; pp. 3–26. [Google Scholar] [CrossRef]
- Helms, C.C.; Ariëns, R.A.S.; Uitte De Willige, S.; Standeven, K.F.; Guthold, M. α-α Cross-links increase fibrin fiber elasticity and stiffness. Biophys. J. 2012, 102, 168–175. [Google Scholar] [CrossRef]
- Roura, S.; Gálvez-Montón, C.; Bayes-Genis, A. Fibrin, the preferred scaffold for cell transplantation after myocardial infarction? An old molecule with a new life. J. Tissue Eng. Regen. Med. 2017, 11, 2304–2313. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937. [Google Scholar] [CrossRef]
- Mooney, R.; Tawil, B.; Mahoney, M. Specific Fibrinogen and Thrombin Concentrations Promote Neuronal Rather Than Glial Growth When Primary Neural Cells Are Seeded Within Plasma-Derived Fibrin Gels. Tissue Eng. Part A 2010, 16, 1607–1619. [Google Scholar] [CrossRef]
- Hashemibeni, B.; Mardani, M.; Bahrami, M.; Valiani, A.; Setayesh Mehr, M.; Pourentezari, M. Comparison of Fibrin and PLGA/fibrin Scaffolds for Chondrogenesis of Human Adipose Derived Stem Cells by Icariin. J. Kerman Univ. Med. Sci. 2020, 27, 14–23. [Google Scholar] [CrossRef]
- Zhao, T.; Qi, Y.; Xiao, S.; Ran, J.; Wang, J.; Ghamor-Amegavi, E.P.; Zhou, X.; Li, H.; He, T.; Gou, Z.; et al. Integration of mesenchymal stem cell sheet and bFGF-loaded fibrin gel in knitted PLGA scaffolds favorable for tendon repair. J. Mater. Chem. B 2019, 7, 2201–2211. [Google Scholar] [CrossRef]
- Ciardulli, M.C.; Marino, L.; Lovecchio, J.; Giordano, E.; Forsyth, N.R.; Selleri, C.; Maffulli, N.; Porta, G. Della Tendon and Cytokine Marker Expression by Human Bone Marrow Mesenchymal Stem Cells in a Hyaluronate/Poly-Lactic-Co-Glycolic Acid (PLGA)/Fibrin Three-Dimensional (3D) Scaffold. Cells 2020, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Brouillette, M.J.; Seol, D.; Zheng, H.; Buckwalter, J.A.; Martin, J.A. Use of Recombinant Human Stromal Cell–Derived Factor 1α–Loaded Fibrin/Hyaluronic Acid Hydrogel Networks to Achieve Functional Repair of Full-Thickness Bovine Articular Cartilage Via Homing of Chondrogenic Progenitor Cells. Arthritis Rheumatol. 2015, 67, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.; Wu, B.M. Biological and mechanical characterization of chitosan-alginate scaffolds for growth factor delivery and chondrogenesis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Honarvar, A.; Karbasi, S.; Hashemibeni, B.; Setayeshmehr, M.; Kazemi, M.; Valiani, A. Effects of cartilage acellular solubilised ECM on physicomechanical and biological properties of polycaprolactone/fibrin hybrid scaffold fabricated by 3D-printing and salt-leaching methods. Mater. Technol. 2020, 37, 204–212. [Google Scholar] [CrossRef]
- Little, C.J.; Bawolin, N.K.; Chen, X. Mechanical Properties of Natural Cartilage and Tissue-Engineered Constructs. Tissue Eng. Part B Rev. 2011, 17, 213–227. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Shi, Y.; Meng, X.; Qiu, Z.; Qu, X.; Dang, J.; Zhang, Y.; Sun, L.; Wang, L.; et al. Effect of electrohydrodynamic printing scaffold with different spacing on chondrocyte dedifferentiation. Ann. Transl. Med. 2022, 10, 743. [Google Scholar] [CrossRef]
- Han, Y.; Lian, M.; Wu, Q.; Qiao, Z.; Sun, B.; Dai, K. Effect of Pore Size on Cell Behavior Using Melt Electrowritten Scaffolds. Front. Bioeng. Biotechnol. 2021, 9, 495. [Google Scholar] [CrossRef]
- Brown, A.C.; Barker, T.H. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2014, 10, 1502. [Google Scholar] [CrossRef]
- Rajangam, T.; An, S.S.A. Fibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedical applications. Int. J. Nanomed. 2013, 8, 3641. [Google Scholar] [CrossRef]
- Valdoz, J.C.; Johnson, B.C.; Jacobs, D.J.; Franks, N.A.; Dodson, E.L.; Sanders, C.; Cribbs, C.G.; Van Ry, P.M. The ECM: To Scaffold, or Not to Scaffold, That Is the Question. Int. J. Mol. Sci. 2021, 22, 12690. [Google Scholar] [CrossRef]
- Black, L.D.; Allen, P.G.; Morris, S.M.; Stone, P.J.; Suki, B. Mechanical and Failure Properties of Extracellular Matrix Sheets as a Function of Structural Protein Composition. Biophys. J. 2008, 94, 1916. [Google Scholar] [CrossRef] [PubMed]
- Hickerson, W.L.; Nur, I.; Meidler, R. A comparison of the mechanical, kinetic, and biochemical properties of fibrin clots formed with two different fibrin sealants. Blood Coagul. Fibrinolysis 2011, 22, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Liao, I.C.; Moutos, F.T.; Estes, B.T.; Zhao, X.; Guilak, F. Composite Three-Dimensional Woven Scaffolds with Interpenetrating Network Hydrogels to Create Functional Synthetic Articular Cartilage. Adv. Funct. Mater. 2013, 23, 5833–5839. [Google Scholar] [CrossRef] [PubMed]
- Deponti, D.; Giancamillo, A.D.; Gervaso, F.; Domenicucci, M.; Domeneghini, C.; Sannino, A.; Peretti, G.M. Collagen scaffold for cartilage tissue engineering: The benefit of fibrin glue and the proper culture time in an infant cartilage model. Tissue Eng. Part A 2014, 20, 1113–1126. [Google Scholar] [CrossRef]
- Isobe, K.; Watanebe, T.; Kawabata, H.; Kitamura, Y.; Okudera, T.; Okudera, H.; Uematsu, K.; Okuda, K.; Nakata, K.; Tanaka, T.; et al. Mechanical and degradation properties of advanced platelet-rich fibrin (A-PRF), concentrated growth factors (CGF), and platelet-poor plasma-derived fibrin (PPTF). Int. J. Implant Dent. 2017, 3, 4–9. [Google Scholar] [CrossRef]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. Sci. World J. 2015, 2015, 685690. [Google Scholar] [CrossRef]
- Kołodziejczyk, J.; Ponczek, M.B. The role of fibrinogen, fibrin and fibrin(ogen) degradation products (FDPs) in tumor progression. Contemp. Oncol. 2013, 17, 113. [Google Scholar] [CrossRef]
- Grassl, E.D.; Oegema, T.R.; Tranquillo, R.T. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J. Biomed. Mater. Res. 2002, 60, 607–612. [Google Scholar] [CrossRef]
- Wozniak, G. Fibrin sealants in supporting surgical techniques: The importance of individual components. Cardiovasc. Surg. 2003, 11, 17–21. [Google Scholar] [CrossRef]
- Shaikh, F.M.; Callanan, A.; Kavanagh, E.G.; Burke, P.E.; Grace, P.A.; McGloughlin, T.M. Fibrin: A natural biodegradable scaffold in vascular tissue engineering. Cells. Tissues Organs 2008, 188, 333–346. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, A.S.; Mahapatro, A. Natural-Based Polymers for Biomedical Applications. In ACS Symposium Series; Woodhead Pub.: Hamilton, UK, 2008; Volume 977, pp. 1–7. [Google Scholar] [CrossRef]
- Santi, S.; Mancini, I.; Dirè, S.; Callone, E.; Speranza, G.; Pugno, N.; Migliaresi, C.; Motta, A. A Bio-inspired Multifunctionalized Silk Fibroin. ACS Biomater. Sci. Eng. 2021, 7, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Radhakrishnan, S.; Kalkura, S.N.; Balme, S.; Miele, P.; Bechelany, M. Overview of Protein-Based Biopolymers for Biomedical Application. Macromol. Chem. Phys. 2019, 220, 1900126. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef]
- Farokhi, M.; Jonidi Shariatzadeh, F.; Solouk, A.; Mirzadeh, H. Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 230–247. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Ghalia, M.A.; Dahman, Y. Biodegradable poly(lactic acid)-based scaffolds: Synthesis and biomedical applications. J. Polym. Res. 2017, 24, 1–22. [Google Scholar] [CrossRef]
- Bistolfi, A.; Ferracini, R.; Galletta, C.; Tosto, F.; Sgarminato, V.; Digo, E.; Vernè, E.; Massè, A. Regeneration of articular cartilage: Scaffold used in orthopedic surgery. A short handbook of available products for regenerative joints surgery. Clin. Sci. Res. Rep. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2016, 66, 159–182. [Google Scholar] [CrossRef]
- Han, D.K.; Park, K.D.; Hubbell, J.A.; Kim, Y.H. Surface characteristics and biocompatibility of lactide-based poly(ethylene glycol) scaffolds for tissue engineering. J. Biomater. Sci. Polym. Ed. 1998, 9, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Cassaro, C.V.; Justulin, L.A.; De Lima, P.R.; De Assis Golim, M.; Biscola, N.P.; De Castro, M.V.; De Oliveira, A.L.R.; Doiche, D.P.; Pereira, E.J.; Ferreira, R.S.; et al. Fibrin biopolymer as scaffold candidate to treat bone defects in rats. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, 20190027. [Google Scholar] [CrossRef] [PubMed]
- Creste, C.F.Z.; Orsi, P.R.; Landim-Alvarenga, F.C.; Justulin, L.A.; Golim, M.d.A.; Barraviera, B.; Ferreira, R.S. Highly Effective Fibrin Biopolymer Scaffold for Stem Cells Upgrading Bone Regeneration. Materials 2020, 13, 2747. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Wu, Y.; Du, P.; Sun, L.; Yu, Z.; Song, S.; Yin, J.; Ma, X.; Jing, C.; et al. Preparation of PU/Fibrin Vascular Scaffold with Good Biomechanical Properties and Evaluation of Its Performance in vitro and in vivo. Int. J. Nanomed. 2020, 15, 8697. [Google Scholar] [CrossRef] [PubMed]
- Hashemibeni, B.; Izadi, M.A.; Valiani, A.; Esfandiari, I.; Bahramian, H.; Dortaj, H.; Pourentezari, M. Investigation and Comparison of the Effect of TGF-β3, kartogenin and Avocado/Soybean Unsaponifiables on the In-vitro and In-vivo Chondrogenesis of Human Adipose-Derived Stem Cells on Fibrin Scaffold. Iran. J. Pharm. Res. 2021, 20, 368. [Google Scholar] [CrossRef]
- Kreuz, P.C.; Gentili, C.; Samans, B.; Martinelli, D.; Krüger, J.P.; Mittelmeier, W.; Endres, M.; Cancedda, R.; Kaps, C. Scaffold-assisted cartilage tissue engineering using infant chondrocytes from human hip cartilage. Osteoarthr. Cartil. 2013, 21, 1997–2005. [Google Scholar] [CrossRef][Green Version]
- Diederichs, S.; Renz, Y.; Hagmann, S.; Lotz, B.; Seebach, E.; Richter, W. Stimulation of a calcified cartilage connecting zone by GDF-5-augmented fibrin hydrogel in a novel layered ectopic in vivo model. J. Biomed. Mater. Res. 2018, 106, 2214–2224. [Google Scholar] [CrossRef]
- Bianchi, V.J.; Lee, A.; Anderson, J.; Parreno, J.; Theodoropoulos, J.; Backstein, D.; Kandel, R. Redifferentiated Chondrocytes in Fibrin Gel for the Repair of Articular Cartilage Lesions. Am. J. Sports Med. 2019, 47, 2348–2359. [Google Scholar] [CrossRef]
- Radeloff, K.; Weiss, D.; Hagen, R.; Kleinsasser, N.; Radeloff, A. Differentiation Behaviour of Adipose-Derived Stromal Cells (ASCs) Seeded on Polyurethane-Fibrin Scaffolds In Vitro and In Vivo. Biomedicines 2021, 9, 982. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546. [Google Scholar] [CrossRef]
- Liu, J.; Song, W.; Yuan, T.; Xu, Z.; Jia, W.; Zhang, C. A Comparison between Platelet-Rich Plasma (PRP) and Hyaluronate Acid on the Healing of Cartilage Defects. PLoS ONE 2014, 9, e97293. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Wang, D.; Mu, S.; Lv, W.; Hao, Y.; Lu, X.; Zhang, G.; Nan, W.; Chen, H.; et al. Improved mechanical properties by modifying fibrin scaffold with PCL and its biocompatibility evaluation. J. Biomater. Sci. Polym. Ed. 2020, 31, 658–678. [Google Scholar] [CrossRef] [PubMed]
- Menzies, K.L.; Jones, L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sánchez-López, J.D.; Schaub, S.; Durán, J.D.G.; Lopez-Lopez, M.T.; Carriel, V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef] [PubMed]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef]
- Aleman, M.M.; Walton, B.L.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and red blood cells in venous thrombosis. Thromb. Res. 2014, 133, S38–S40. [Google Scholar] [CrossRef]
- Lin, F.-Y.; Zhu, J.; Eng, E.T.; Hudson, N.E.; Springer, T.A. β-Subunit Binding Is Sufficient for Ligands to Open the Integrin αIIbβ3 Headpiece. J. Biol. Chem. 2016, 291, 4537–4546. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Urlić, I.; Ivković, A. Cell Sources for Cartilage Repair—Biological and Clinical Perspective. Cells 2021, 10, 2496. [Google Scholar] [CrossRef]
- Apelgren, P.; Amoroso, M.; Lindahl, A.; Brantsing, C.; Rotter, N.; Gatenholm, P.; Kölby, L. Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLoS ONE 2017, 12, e0189428. [Google Scholar] [CrossRef]
- Grimaud, E.; Heymann, D.; Rédini, F. Recent advances in TGF-β effects on chondrocyte metabolism: Potential therapeutic roles of TGF-β in cartilage disorders. Cytokine Growth Factor Rev. 2002, 13, 241–257. [Google Scholar] [CrossRef]
- Wu, L.; Leijten, J.; Van Blitterswijk, C.A.; Karperien, M. Fibroblast Growth Factor-1 Is a Mesenchymal Stromal Cell-Secreted Factor Stimulating Proliferation of Osteoarthritic Chondrocytes in Co-Culture. Stem Cells Dev. 2013, 22, 2356. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Chen, K.; Lou, Y.; Jiang, Y.; Zhang, D. Small molecule compounds promote the proliferation of chondrocytes and chondrogenic differentiation of stem cells in cartilage tissue engineering. Biomed. Pharmacother. 2020, 131, 110652. [Google Scholar] [CrossRef] [PubMed]
- Zapata, N.M.; Zuluaga, N.J.; Betancur, S.N.; López, L.E. Cultivo de Tejido Cartilaginoso Articular: Acercamiento Conceptual. Rev. EIA Scieloco 2007, 4, 117–129. [Google Scholar]
- Smolka, W.; Ptas, M.; Panek, A.; Krok-Borkowicz, M.; Zambrzycki, M.; Gubernat, M.; Markowski, J.; Fraczek-Szczypta, A. Surface Modification of Carbon Nanofibers to Improve Their Biocompatibility in Contact with Osteoblast and Chondrocytes Cell Lines. Materials 2021, 14, 6370. [Google Scholar] [CrossRef] [PubMed]
- Perrier-Groult, E.; Pérès, E.; Pasdeloup, M.; Gazzolo, L.; Dodon, M.D.; Mallein-Gerin, F. Evaluation of the biocompatibility and stability of allogeneic tissue-engineered cartilage in humanized mice. PLoS ONE 2019, 14, e0217183. [Google Scholar] [CrossRef]
- Van Susante, J.L.C.; Pieper, J.; Buma, P.; Van Kuppevelt, T.H.; Van Beuningen, H.; Van Der Kraan, P.M.; Veerkamp, J.H.; Van Den Berg, W.B.; Veth, R.P.H. Linkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro. Biomaterials 2001, 22, 2359–2369. [Google Scholar] [CrossRef]
- Gurusinghe, S.; Strappe, P. Gene modification of mesenchymal stem cells and articular chondrocytes to enhance chondrogenesis. BioMed Res. Int. 2014, 2014, 369528. [Google Scholar] [CrossRef]
- Kim, Y.S.; Chien, A.J.; Guo, J.L.; Smith, B.T.; Watson, E.; Pearce, H.A.; Koons, G.L.; Navara, A.M.; Lam, J.; Scott, D.W.; et al. Chondrogenesis of cocultures of mesenchymal stem cells and articular chondrocytes in poly(l-lysine)-loaded hydrogels. J. Control. Release 2020, 328, 710–721. [Google Scholar] [CrossRef]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The Role of Growth Factors in Cartilage Repair. Clin. Orthop. Relat. Res. 2011, 469, 2706. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 1259. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A short overview of certain bioactive components. Open Med. 2016, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Shin, J.; Bhang, S.H.; Shin, J.Y.; Park, J.; Im, G.; Kim, C.S.; Kim, B.S. Enhanced skin wound healing by a sustained release of growth factors contained in platelet-rich plasma. Exp. Mol. Med. 2011, 43, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-rich fibrin: Basics of biological actions and protocol modifications. Open Med. 2021, 16, 446. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zhang, Y. Autologous liquid platelet rich fibrin: A novel drug delivery system. Acta Biomater. 2018, 75, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wan, M.; Li, Z.; Zhong, N.; Liang, D.; Ge, L. A comparative study of the effects of concentrated growth factors in two different forms on osteogenesis in vitro. Mol. Med. Rep. 2019, 20, 1039. [Google Scholar] [CrossRef]
- Stanca, E.; Calabriso, N.; Giannotti, L.; Nitti, P.; Damiano, F.; Stanca, B.D.C.; Carluccio, M.A.; De Benedetto, G.E.; Demitri, C.; Palermo, A.; et al. Analysis of CGF Biomolecules, Structure and Cell Population: Characterization of the Stemness Features of CGF Cells and Osteogenic Potential. Int. J. Mol. Sci. 2021, 22, 8867. [Google Scholar] [CrossRef]
- Singh, C.; Kumar, A.; Mehta, G.S. Efficacy of concentrated growth factors (CGF) vs normal saline dressing in chronic non healing ulcers. IP J. Surg. Allied Sci. 2022, 4, 1–9. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant Dent. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Pötter, N.; Westbrock, F.; Grad, S.; Alini, M.; Stoddart, M.J.; Schmal, H.; Kubosch, D.; Salzmann, G.; Kubosch, E.J. Evaluation of the influence of platelet-rich plasma (PRP), platelet lysate (PL) and mechanical loading on chondrogenesis in vitro. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, H. A Comprehensive Review of Concentrated Growth Factors and Their Novel Applications in Facial Reconstructive and Regenerative Medicine. Aesthetic Plast. Surg. 2020, 44, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Grasman, J.M.; Page, R.L.; Dominko, T.; Pins, G.D. Crosslinking strategies facilitate tunable structural properties of fibrin microthreads. Acta Biomater. 2012, 8, 4020–4030. [Google Scholar] [CrossRef] [PubMed]
- Abbasgholizadeh, R.; Islas, J.F.; Navran, S.; Potaman, V.N.; Schwartz, R.J.; Birla, R.K. A Highly Conductive 3D Cardiac Patch Fabricated Using Cardiac Myocytes Reprogrammed from Human Adipogenic Mesenchymal Stem Cells. Cardiovasc. Eng. Technol. 2020, 11, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, T.; Song, Y.; Sun, W. Assessment of various crosslinking agents on collagen/chitosan scaffolds for myocardial tissue engineering. Biomed. Mater. 2020, 15, 045003. [Google Scholar] [CrossRef] [PubMed]
- de Melo, B.A.G.; Jodat, Y.A.; Mehrotra, S.; Calabrese, M.A.; Kamperman, T.; Mandal, B.B.; Santana, M.H.A.; Alsberg, E.; Leijten, J.; Shin, S.R. 3D Printed Cartilage-Like Tissue Constructs with Spatially Controlled Mechanical Properties. Adv. Funct. Mater. 2019, 29, 1–13. [Google Scholar] [CrossRef]
- Jiang, B.; Waller, T.M.; Larson, J.C.; Appel, A.A.; Brey, E.M. Fibrin-Loaded Porous Poly(Ethylene Glycol) Hydrogels as Scaffold Materials for Vascularized Tissue Formation. Tissue Eng. Part A 2013, 19, 224–234. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter 2012, 8, 3280. [Google Scholar] [CrossRef]
- Rampichová, M.; Filová, E.; Varga, F.; Lytvynets, A.; Prosecká, E.; Koláčná, L.; Motlík, J.; Nečas, A.; Vajner, L.; Uhlík, J.; et al. Fibrin/Hyaluronic Acid Composite Hydrogels as Appropriate Scaffolds for In Vivo Artificial Cartilage Implantation. ASAIO J. 2010, 56, 563–568. [Google Scholar] [CrossRef]
- Attalla, R.; Puersten, E.; Jain, N.; Selvaganapathy, P.R. 3D bioprinting of heterogeneous bi- and tri-layered hollow channels within gel scaffolds using scalable multi-axial microfluidic extrusion nozzle. Biofabrication 2018, 11, 015012. [Google Scholar] [CrossRef]
- Brougham, C.M.; Levingstone, T.J.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Incorporation of fibrin into a collagen–glycosaminoglycan matrix results in a scaffold with improved mechanical properties and enhanced capacity to resist cell-mediated contraction. Acta Biomater. 2015, 26, 205–214. [Google Scholar] [CrossRef]
- Geer, C.B.; Tripathy, A.; Schoenfisch, M.H.; Lord, S.T.; Gorkun, O.V. Role of “B-b” knob-hole interactions in fibrin binding to adsorbed fibrinogen. J. Thromb. Haemost. 2007, 5, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.; Cederholm-Williams, S.A. Fibrinogen and fibrin: Characterization, processing and medical applications. In Handbook of Biodegradable Polymers; Domb, A., Kost, J., Wiseman, D., Eds.; Harwood: Amsterdam, The Netherlands, 1997; pp. 347–365. ISBN 9781501511981. [Google Scholar]
- Munirah, S.; Samsudin, O.C.; Chen, H.C.; Salmah, S.H.S.; Aminuddin, B.S.; Ruszymah, B.H.I. Articular cartilage restoration in load-bearing osteochondral defects by implantation of autologous chondrocyte-fibrin constructs. J. Bone Jt. Surg. Br. 2007, 89-B, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Choi, S.W.; Kim, S.R.; Oh, I.S.; Won, M.H. Autologous chondrocyte implantation in the knee using fibrin. Knee Surg. Sport. Traumatol. Arthrosc. 2010, 18, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.B.; Ning, L.J.; Lian, Q.Z.; Xia, Z.; Xin, Y.; Sen, B.H.; Fei, N.F. A study on repair of porcine articular cartilage defects with tissue-engineered cartilage constructed in vivo by composite scaffold materials. Ann. Plast. Surg. 2010, 65, 430–436. [Google Scholar] [CrossRef]
- Goodrich, L.R.; Chen, A.C.; Werpy, N.M.; Williams, A.A.; Kisiday, J.D.; Su, A.W.; Cory, E.; Morley, P.S.; Mcilwrait, C.W.; Sah, R.L.; et al. Addition of mesenchymal stem cells to autologous platelet-enhanced fibrin scaffolds in chondral defects: Does it enhance repair? J. Bone Jt. Surg. Am. Vol. 2016, 98, 23–34. [Google Scholar] [CrossRef]
- Kazemnejad, S.; Khanmohammadi, M.; Mobini, S.; Taghizadeh-Jahed, M.; Khanjani, S.; Arasteh, S.; Golshahi, H.; Torkaman, G.; Ravanbod, R.; Heidari-Vala, H.; et al. Comparative repair capacity of knee osteochondral defects using regenerated silk fiber scaffolds and fibrin glue with/without autologous chondrocytes during 36 weeks in rabbit model. Cell Tissue Res. 2016, 364, 559–572. [Google Scholar] [CrossRef]
- Koh, Y.G.; Kwon, O.R.; Kim, Y.S.; Choi, Y.J.; Tak, D.H. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 97–109. [Google Scholar] [CrossRef]
- Sheu, S.Y.; Wang, C.H.; Pao, Y.H.; Fu, Y.T.; Liu, C.H.; Yao, C.H.; Kuo, T.F. The effect of platelet-rich fibrin on autologous osteochondral transplantation: An in vivo porcine model. Knee 2017, 24, 1392–1401. [Google Scholar] [CrossRef]
- Choi, S.; Kim, G.M.; Maeng, Y.H.; Kang, H.; Teong, C.T.; Lee, E.E.; Yoo, S.J.; Dlima, D.D.; Kim, M.K. Autologous Bone Marrow Cell Stimulation and Allogenic Chondrocyte Implantation for the Repair of Full-Thickness Articular Cartilage Defects in a Rabbit Model. Cartilage 2018, 9, 402–409. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Golshahi, H.; Saffarian, Z.; Montazeri, S.; Khorasani, S.; Kazemnejad, S. Repair of Osteochondral Defects in Rabbit Knee Using Menstrual Blood Stem Cells Encapsulated in Fibrin Glue: A Good Stem Cell Candidate for the Treatment of Osteochondral Defects. Tissue Eng. Regen. Med. 2019, 16, 311–324. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, J.S.; Jeon, Y.M.; Jeon, Y.S. Clinical, radiological, and histological outcomes after the fibrin-matrix autologous chondrocyte implantation for chondral lesions of the knee in patients more than 50 years old: A prospective case series with minimum 2-year follow-up. J. Orthop. Surg. Hong Kong 2020, 28, 2309499019893509. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-C.; Ou, K.-L.; Lin, Y.-H.; Lin, M.-F.; Yang, T.-L.; Chen, C.-H.; Chan, W.P. Platelet-Rich Fibrin Facilitates One-Stage Cartilage Repair by Promoting Chondrocytes Viability, Migration, and Matrix Synthesis. Int. J. Mol. Sci. 2020, 21, 577. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.; Zha, K.; Tian, Z.; Liu, S.; Sui, X.; Wang, Z.; Zheng, J.; Wang, J.; Tian, X.; et al. Porcine fibrin sealant combined with autologous chondrocytes successfully promotes full-thickness cartilage regeneration in a rabbit model. J. Tissue Eng. Regen. Med. 2021, 15, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Hashimoto, Y.; Orita, K.; Nishida, Y.; Nishino, K.; Nakamura, H. Autologous Platelet-Rich Fibrin Membrane to Augment Healing of Microfracture Has Better Macroscopic and Histologic Grades Compared with Microfracture Alone on Chondral Defects in a Rabbit Model. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 417–426. [Google Scholar] [CrossRef]
| Scaffold | Animal Model | Specific Sites of Implantation | Time of Evaluation | Results | Ref. |
|---|---|---|---|---|---|
| Fibrin scaffold | Male rats | Subcutaneously transplanted in skin (ectopic) | 14 days | After 14 days of in vitro chondrogenic differentiation of human adipose derived stem cells, the fibrin scaffold, in which the cells were differentiated, were implanted. In vivo differentiation of cells under the skin increased the amount of cartilage matrix constituents such as proteoglycans. | [69] |
| Polyglycolic acid (PGA)-fibrin scaffolds | Immuno-compromised athymic mice | Subcutaneously transplanted in skin (ectopic) | 4 weeks | Subcutaneous transplantation of human infant hip chondrocytes loaded in PGA-fibrin scaffolds, added with human platelet-rich plasma, in nude mice, showed the formation of hyaline-like cartilage, rich in type II and type X collagen. | [70] |
| Fibrin hydrogel | Immune deficient SCID mice | Subcutaneously transplanted in back skin (ectopic) | 4 weeks | Pro-chondrogenic and pro-hypertrophic bioactive fibrin hydrogel with high potential to promote the integration of cartilage scaffolds with bone. Fibrin hydrogel promises to overcome poor fixation of biomaterials in cartilage defects facilitating their long-term regeneration. | [71] |
| Fibrin gel | 5–6 months age New Zealand white rabbit | Implantation in injured knee joint | 6 weeks | Fibrocartilaginous repair tissues, containing the hyaline cartilage marker collagen type 2. | [72] |
| Polyurethane fibrin composite | Female adult New Zealand White Rabbits | Injured auricular cartilage | 4 and 12 weeks | After 12 weeks in vivo, there was a production of cartilage extracellular matrix components. Also, there is gene expression of specific marker genes for mature cartilage, such as SOX-9 and collagen II. | [73] |
| Study Model | Implant Used | Follow-Up | Results | Ref. |
|---|---|---|---|---|
| Sheep Full-thickness chondral defects on femoral condyle | Autologous fibrin scaffold | 12 weeks | Good integration with surrounding cartilage. Nearly normal appearance. Cells resembled chondrocytes embedded within cartilaginous-like matrix. Histological section revealed accumulated proteoglycans. | [117] |
| Case series (human) Deep cartilage defects on femoral condyle | Fibrin gel/autologous chondrocytes | 24 months (clinical) 12 months (second look arthroscopy/histological) | Most patients had excellent/good results. Clinical scores improved. Synthesis of GAG and type II collagen in implants. Grafted areas with good filling; some grafts with fibrillations or mild hypertrophy; most tissue repair well integrated. | [118] |
| Minipigs Full-thickness chondral defects on femoral condyle | Fibrin matrix/acellular cartilage matrix/autologous chondrocytes | 12 weeks | Surface of the repaired joint cartilage (fibrin, cartilage matrix, and chondrocytes) was porcelain white, slightly transparent, and smooth but thinner than normal cartilage. Comparable tissue thickness in the repaired region with the surrounding tissue; few fibrous connective tissues distributed on the boundary. Complete and homogeneous distribution of GAGs and type II collagen, less than normal cartilage. | [119] |
| Minipigs Full-thickness chondral defects on femoral trochlea | Commercial fibrin matrix/commercial hyaluronic acid/autologous chondrocytes | 24 weeks | Type II collagen revealed positivity in the newly formed cartilage on the borders of the defects. Type II collagen was less present in the center of the defect. Biomechanical properties of fibrin/HA composite hydrogel at 6 months comparable with native cartilage. Presence of a noncellular transient zone followed by a layer of isogenous groups of chondrocytes merged with fibrocartilaginous tissue at the center. | [112] |
| Adult horses Full-thickness chondral defects on femoral condyle | Autologous platelet-enriched fibrin/bone-marrow-derived mesenchymal stem cells | 12 months | The addition of BMDMSCs to APEF did not enhance cartilage repair and stimulated bone formation in some cartilage defects. The middle-to-superficial part of the repair had a more fibrous, hypocellular appearance with an absence of GAG staining. | [120] |
| Rabbit Osteochondral defects on femoral trochlear groove | Autologous platelet-rich plasma gel/allogenic chondrocytes | 12 and 36 weeks | Similarity of repaired tissue with normal cartilage. Relatively complete integration with surrounding cartilage. Defects were mainly filled by fibrocartilaginous tissue. Lateral and basal integration was relatively suitable. Presence of type II collagen and proteoglycans less than normal cartilage. | [121] |
| Randomized clinical trial Symptomatic cartilage lesion on the femoral condyle | Commercial fibrin scaffold/adipose-derived stem cells | 24 months | Significantly more patients with fibrin/stem cells (80%) exhibited normal or nearly normal repair tissue signal intensity (MRI findings). Intermediate degree of staining for safranin O (proteoglycan) and type II collagen. | [122] |
| Minipigs Osteochondral defects on femoral condyle | Autologous platelet-rich fibrin/autologous cartilage fragments (0.25 cm3) 12 months | 6 months | Healing almost complete, reparative tissue appeared to be well integrated at the margins of the repair site, flush and smooth surfaces were observed on the repaired cartilage. Better stiffness compared with controls. Relatively smooth repaired hyaline-like cartilage containing columnar arrangements of chondrocytes. The regenerated tissues appeared to be integrated with the normal hyaline cartilage as well as with the underlying subchondral bone. | [123] |
| Rabbits Full-thickness osteochondral defects | Commercial fibrin matrix/allogenic chondrocytes or autologous bone-marrow-derived mesenchymal stem cells | 12 weeks | Regenerated tissue showed a mixture of hyaline cartilage and fibrocartilage, well connected to the surrounding normal cartilage. Higher expression of type II collagen, clearer configuration and distribution of chondrocytes and collagen; higher concentrations of GAG regarding controls. | [124] |
| Rabbits Full-thickness osteochondral defects on trochlear groove | Commercial fibrin gel/autologous redifferentiated chondrocytes | 6 weeks | Repair tissues from dedifferentiated cell implants resembled fibrocartilage. They contained both Col 1 and Col 2 as well as ACAN. Average ratio of Col 2:Col 1 was greater for tissues formed by dedifferentiated cells than for tissues formed by redifferentiated cells. Redifferentiation of passaged chondrocytes does not improve defect repair in the first 6 weeks. | [72] |
| Rabbits Full-thickness osteochondral defects on trochlear groove | Autologous fibrin glue/menstrual blood-derived stem cells | 12–24 weeks | Defects were filled with hyaline cartilage-like tissue with proper integration, high content of glycosaminoglycan, and the existence of collagen fibers, especially collagen type II. | [125] |
| Case series (human) Full thickness cartilage lesion in medial femoral condyle | Commercial fibrinogen and thrombin/autologous chondrocytes | 12 months | Arthroscopic evaluation indicated that cartilage repair was adequate (mild hypertrophy existed). Histological analysis indicated high deposition of GAGs, adequate type II collagen expression, and higher type II collagen expression over type I collagen. | [126] |
| Minipigs Full thickness cartilage defects in medial femoral condyle | Platelet-rich fibrin/diced cartilage autografts | 6 months | Most of the repair tissue stained positively for Col II but negatively for Col I. Repair tissue integrated with contiguous native tissue and the subchondral bone. Repair tissue integrated with contiguous native tissue and the subchondral bone. | [127] |
| Rabbits Full thickness cartilage defects in femoral trochlear groove | Platelet-rich fibrin membrane alone | 24 weeks | Repaired cartilage covered the defect well, both edges of the repaired cartilage well integrated, smooth surface. Defect was not filled with chondrocyte-like cells and cartilage matrix. Margins of the repaired cartilage were well integrated. Type II collagen staining in the area of repair was observed. | [128] |
| Rabbits Full thickness cartilage defects in femoral trochlear groove | Commercial xenogeneic porcine fibrin sealant/autologous chondrocytes | 6 months | GAG content and type II collagen expression were consistent with the surrounding normal cartilage, and the integration of the new tissue was continuous and smooth. best reparative effect in the fibrin matrix plus autologous chondrocytes. Better mechanical properties than fibrin matrix alone. | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Murillo, J.A.; Simental-Mendía, M.A.; Moncada-Saucedo, N.K.; Delgado-Gonzalez, P.; Islas, J.F.; Roacho-Pérez, J.A.; Garza-Treviño, E.N. Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair. Int. J. Mol. Sci. 2022, 23, 9879. https://doi.org/10.3390/ijms23179879
Rojas-Murillo JA, Simental-Mendía MA, Moncada-Saucedo NK, Delgado-Gonzalez P, Islas JF, Roacho-Pérez JA, Garza-Treviño EN. Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair. International Journal of Molecular Sciences. 2022; 23(17):9879. https://doi.org/10.3390/ijms23179879
Chicago/Turabian StyleRojas-Murillo, Juan Antonio, Mario A. Simental-Mendía, Nidia K. Moncada-Saucedo, Paulina Delgado-Gonzalez, José Francisco Islas, Jorge A. Roacho-Pérez, and Elsa N. Garza-Treviño. 2022. "Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair" International Journal of Molecular Sciences 23, no. 17: 9879. https://doi.org/10.3390/ijms23179879
APA StyleRojas-Murillo, J. A., Simental-Mendía, M. A., Moncada-Saucedo, N. K., Delgado-Gonzalez, P., Islas, J. F., Roacho-Pérez, J. A., & Garza-Treviño, E. N. (2022). Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair. International Journal of Molecular Sciences, 23(17), 9879. https://doi.org/10.3390/ijms23179879







