Proteases and Their Potential Role as Biomarkers and Drug Targets in Dry Eye Disease and Ocular Surface Dysfunction
Abstract
:1. Introduction
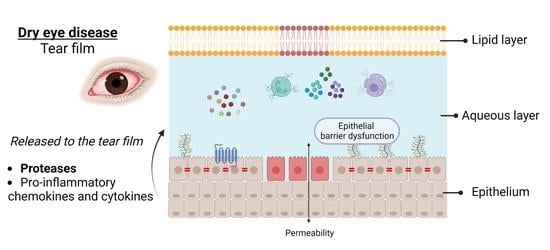
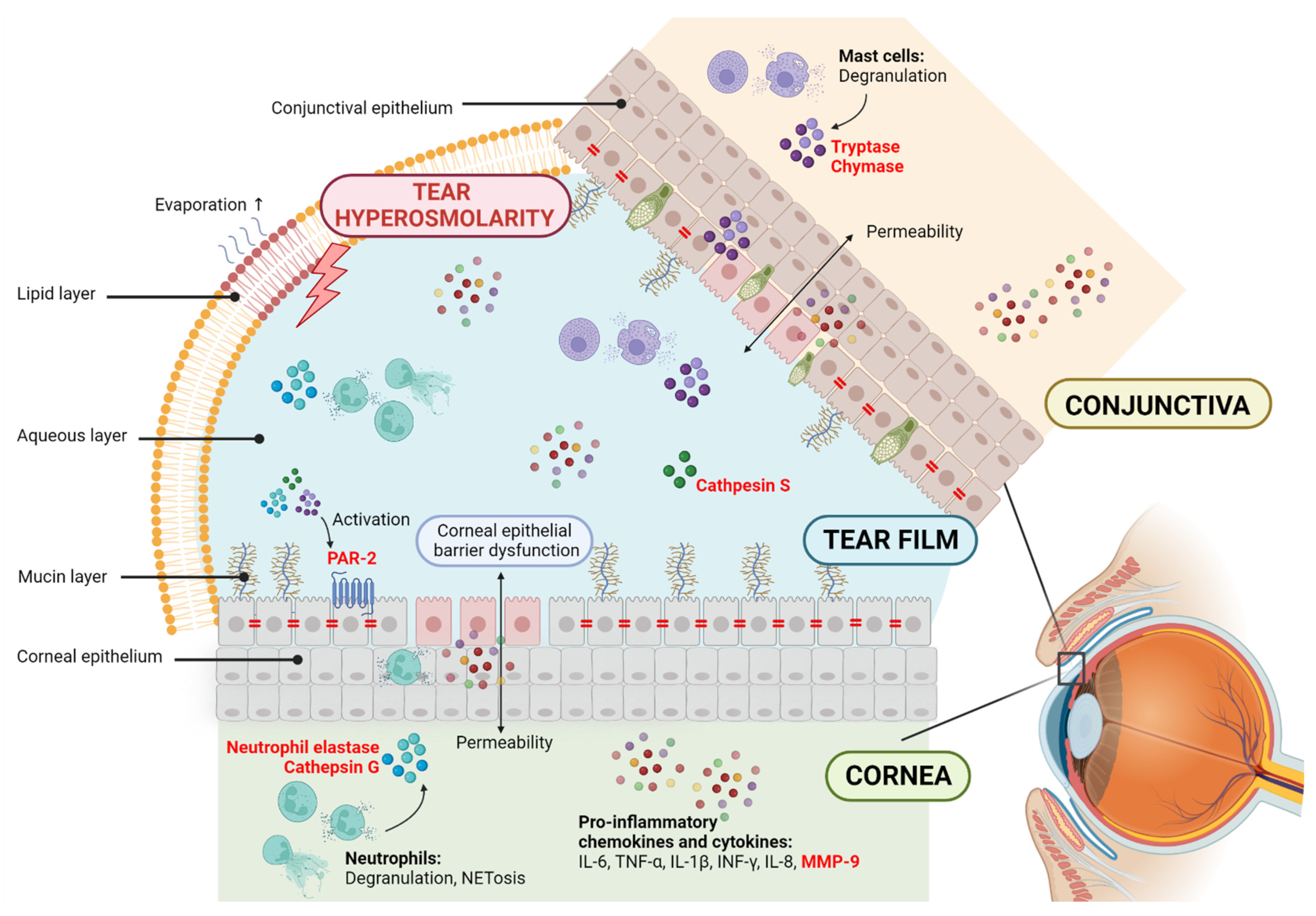
2. Proteases and Dry Eye Disease
2.1. Matrix Metalloproteases
2.2. Serine Proteases
2.3. Cysteine Proteases
3. Protease-Activated Receptors and Dry Eye Disease
4. Protease Inhibitors and Dry Eye Disease
4.1. MMP-9 Inhibitors
4.2. Serine Protease Inhibitors
5. Conclusions and Future Directions/Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABP | activity-based probes |
| ABPP | activity-based protein profiling |
| ADDE | aqueous deficient dry eye |
| BAC | benzalkonium chloride |
| CyA | cyclosporin A |
| DED | dry eye disease |
| DEWS | dry eye workshop |
| EDE | evaporative dry eye |
| ELISA | enzyme-linked immunosorbent assay |
| FDA | Food and Drug Administration |
| GPCR | G protein-coupled receptor |
| HCE | human corneal epithelial cells |
| ICAM-1 | intracellular adhesion molecule-1 |
| ICAT | isotope-coded affinity tag labelling |
| IL | interleukin |
| INF | interferon |
| IP3 | inositol 1,4,5-triphosphate |
| JNK | c-Jun N-terminal kinase |
| LFA | lymphocyte function-associated antigen |
| MAPK | mitogen-activated protein kinase |
| MGD | meibomian gland dysfunction |
| MMP | matric metalloprotease |
| NET | neutrophil extracellular trap |
| NFκB | nuclear factor kappa beta |
| NSSDE | non-Sjögren syndrome dry eye |
| OSDI | ocular surface disease index |
| PAR | protease-activated receptor |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| SERPINA3K | serine protease inhibitor A3K |
| SILAC | stable isotope labelling by amino acids in cell culture |
| SSDE | Sjögren syndrome dry eye |
| TIMPs | tissue inhibitors of MMPs |
| TFOS | Tear Film and Ocular Society |
| TNF | tumor necrosis factor |
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; Santo, R.M. The Impact of Dry Eye Disease Treatment on Patient Satisfaction and Quality of Life: A Review. Ocul. Surf. 2019, 17, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Labetoulle, M.; Rolando, M.; Messmer, E.M. Understanding Symptoms and Quality of Life in Patients With Dry Eye Syndrome. Ocul. Surf. 2016, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Rouen, P.A.; White, M.L. Dry Eye Disease. Home Healthc. Now 2018, 36, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Choi, Y.-H.; Paik, H.J.; Kim, M.K.; Wee, W.R.; Kim, D.H. Sex Differences in the Effect of Aging on Dry Eye Disease. Clin. Interv. Aging 2017, 12, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Verjee, M.A.; Brissette, A.R.; Starr, C.E. Dry Eye Disease: Early Recognition with Guidance on Management and Treatment for Primary Care Family Physicians. Ophthalmol. Ther. 2020, 9, 877–888. [Google Scholar] [CrossRef]
- Krolo, I.; Blazeka, M.; Merdzo, I.; Vrtar, I.; Sabol, I.; Vickovic, I. Mask-Associated Dry Eye During COVID-19 Pandemic-How Face Masks Contribute to Dry Eye Disease Symptoms. Med. Arch. 2021, 75, 144. [Google Scholar] [CrossRef]
- Boccardo, L. Self-Reported Symptoms of Mask-Associated Dry Eye: A Survey Study of 3605 People. Contact Lens Anterior Eye 2022, 45, 101408. [Google Scholar] [CrossRef]
- Reyes, J.L.; Vannan, D.T.; Eksteen, B.; Avelar, I.J.; Rodríguez, T.; González, M.I.; Mendoza, A.V. Innate and Adaptive Cell Populations Driving Inflammation in Dry Eye Disease. Mediators Inflamm. 2018, 2018, 2532314. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Sullivan, B.D.; Evans, J.E.; Schirra, F.; Yamagami, H.; Liu, M.; Richards, S.M.; Suzuki, T.; Schaumberg, D.A.; Sullivan, R.M.; et al. Androgen Deficiency, Meibomian Gland Dysfunction, and Evaporative Dry Eye. Ann. N. Y. Acad. Sci. 2002, 966, 211–222. [Google Scholar] [CrossRef]
- Bai, Y.; Ngo, W.; Khanal, S.; Nichols, K.K.; Nichols, J.J. Human Precorneal Tear Film and Lipid Layer Dynamics in Meibomian Gland Dysfunction. Ocul. Surf. 2021, 21, 250–256. [Google Scholar] [CrossRef]
- Messmer, E.M. The Pathophysiology, Diagnosis, and Treatment of Dry Eye Disease. Dtsch. Arztebl. Int. 2015, 112, 71–82. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjögren Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benítez-del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the Vicious Circle of Dry Eye Disease: A Focus on the Pathophysiology of Meibomian Gland Dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef]
- Stern, M.E.; Pflugfelder, S.C. Inflammation in Dry Eye. Ocul. Surf. 2004, 2, 124–130. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II Pathophysiology Report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology Report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Stevenson, W. Dry Eye Disease. Arch. Ophthalmol. 2012, 130, 90. [Google Scholar] [CrossRef]
- Fong, P.; Shih, K.; Lam, P.; Chan, T.Y.; Jhanji, V.; Tong, L. Role of Tear Film Biomarkers in the Diagnosis and Management of Dry Eye Disease. Taiwan J. Ophthalmol. 2019, 9, 150. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C. Antiinflammatory Therapy for Dry Eye. Am. J. Ophthalmol. 2004, 137, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kunert, K.S.; Tisdale, A.S.; Stern, M.E.; Smith, J.A.; Gipson, I.K. Analysis of Topical Cyclosporine Treatment of Patients with Dry Eye SyndromeEffect on Conjunctival Lymphocytes. Arch. Ophthalmol. 2000, 118, 1489. [Google Scholar] [CrossRef] [PubMed]
- Semba, C.; Gadek, T. Development of Lifitegrast: A Novel T-Cell Inhibitor for the Treatment of Dry Eye Disease. Clin. Ophthalmol. 2016, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.M. Loteprednol Etabonate: A Formulation for Short-Term Use in Inflammatory Flares in Dry Eye Disease. Drugs of Today 2022, 58, 77. [Google Scholar] [CrossRef] [PubMed]
- De Souza, G.A.; Godoy, L.M.F.; Mann, M. Identification of 491 Proteins in the Tear Fluid Proteome Reveals a Large Number of Proteases and Protease Inhibitors. Genome Biol. 2006, 7, R72. [Google Scholar] [CrossRef]
- Ehrmann, M.; Clausen, T. Proteolysis as a Regulatory Mechanism. Annu. Rev. Genet. 2004, 38, 709–724. [Google Scholar] [CrossRef]
- Bond, J.S. Proteases: History, Discovery, and Roles in Health and Disease. J. Biol. Chem. 2019, 294, 1643–1651. [Google Scholar] [CrossRef]
- Puente, X.S.; Sánchez, L.M.; Overall, C.M.; López-Otín, C. Human and Mouse Proteases: A Comparative Genomic Approach. Nat. Rev. Genet. 2003, 4, 544–558. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The Database of Proteolytic Enzymes, Their Substrates and Inhibitors. Nucleic Acids Res. 2012, 40, D343–D350. [Google Scholar] [CrossRef] [Green Version]
- Rawlings, N.D.; Barrett, A.J. Evolutionary Families of Peptidases. Biochem. J. 1993, 290, 205–218. [Google Scholar] [CrossRef]
- López-Otín, C.; Overall, C.M. Protease Degradomics: A New Challenge for Proteomics. Nat. Rev. Mol. Cell Biol. 2002, 3, 509–519. [Google Scholar] [CrossRef]
- López-Otín, C.; Bond, J.S. Proteases: Multifunctional Enzymes in Life and Disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef]
- Turk, B. Targeting Proteases: Successes, Failures and Future Prospects. Nat. Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef]
- Heuberger, D.M.; Schuepbach, R.A. Protease-Activated Receptors (PARs): Mechanisms of Action and Potential Therapeutic Modulators in PAR-Driven Inflammatory Diseases. Thromb. J. 2019, 17, 4. [Google Scholar] [CrossRef]
- Fu, R.; Klinngam, W.; Heur, M.; Edman, M.C.; Hamm-Alvarez, S.F. Tear Proteases and Protease Inhibitors: Potential Biomarkers and Disease Drivers in Ocular Surface Disease. Eye Contact Lens Sci. Clin. Pract. 2020, 46, S70–S83. [Google Scholar] [CrossRef]
- Yu, Z.; Li, J.; Govindarajan, G.; Hamm-Alvarez, S.F.; Alam, J.; Li, D.-Q.; de Paiva, C.S. Cathepsin S Is a Novel Target for Age-Related Dry Eye. Exp. Eye Res. 2022, 214, 108895. [Google Scholar] [CrossRef]
- Joossen, C.; Baán, A.; Moreno-Cinos, C.; Joossens, J.; Cools, N.; Lanckacker, E.; Moons, L.; Lemmens, K.; Lambeir, A.-M.M.; Fransen, E.; et al. A Novel Serine Protease Inhibitor as Potential Treatment for Dry Eye Syndrome and Ocular Inflammation. Sci. Rep. 2020, 10, 17268. [Google Scholar] [CrossRef]
- Decraecker, L.; Boeckxstaens, G.; Denadai-Souza, A. Inhibition of Serine Proteases as a Novel Therapeutic Strategy for Abdominal Pain in IBS. Front. Physiol. 2022, 13, 948. [Google Scholar] [CrossRef]
- Bleuez, C.; Koch, W.F.; Urbach, C.; Hollfelder, F.; Jermutus, L. Exploiting Protease Activation for Therapy. Drug Discov. Today 2022, 27, 1743–1754. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix Metalloproteinases (MMPs): Chemical–Biological Functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix Metalloproteinases and the Regulation of Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Van Wart, H.E.; Birkedal-Hansent, H. The Cysteine Switch: A Principle of Regulation of Metalloproteinase Activity with Potential Applicability to the Entire Matrix Metalloproteinase Gene Family (Collagenase/Gelatinase/Stromelysin/Zinc Enzyme). Proc. Natl. Acad. Sci. USA 1990, 87, 5578–5582. [Google Scholar] [CrossRef]
- Xu, I.; Thériault, M.; Brunette, I.; Rochette, P.J.; Proulx, S. Matrix Metalloproteinases and Their Inhibitors in Fuchs Endothelial Corneal Dystrophy. Exp. Eye Res. 2021, 205, 108500. [Google Scholar] [CrossRef]
- Corrales, R.M.; Stern, M.E.; De Paiva, C.S.; Welch, J.; Li, D.-Q.; Pflugfelder, S.C. Desiccating Stress Stimulates Expression of Matrix Metalloproteinases by the Corneal Epithelium. Investig. Opthalmol. Vis. Sci. 2006, 47, 3293. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, D.-Q.Q.; Doshi, A.; Farley, W.; Corrales, R.M.; Pflugfelder, S.C. Experimental Dry Eye Stimulates Production of Inflammatory Cytokines and MMP-9 and Activates MAPK Signaling Pathways on the Ocular Surface. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4293–4301. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Corrales, R.M.; Villarreal, A.L.; Farley, W.J.; Li, D.-Q.; Stern, M.E.; Pflugfelder, S.C. Corticosteroid and Doxycycline Suppress MMP-9 and Inflammatory Cytokine Expression, MAPK Activation in the Corneal Epithelium in Experimental Dry Eye. Exp. Eye Res. 2006, 83, 526–535. [Google Scholar] [CrossRef]
- Qu, M.; Qi, X.; Wang, Q.; Wan, L.; Li, J.; Li, W.; Li, Y.; Zhou, Q. Therapeutic Effects of STAT3 Inhibition on Experimental Murine Dry Eye. Investig. Opthalmol. Vis. Sci. 2019, 60, 3776. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Kim, J.M.; Lee, M.J.; Sohn, Y.S.; Kang, K.K.; Yoo, M. The Therapeutic Effect of DA-6034 on Ocular Inflammation via Suppression of MMP-9 and Inflammatory Cytokines and Activation of the MAPK Signaling Pathway in an Experimental Dry Eye Model. Curr. Eye Res. 2010, 35, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Farley, W.; Luo, L.; Chen, L.Z.; de Paiva, C.S.; Olmos, L.C.; Li, D.-Q.; Fini, M.E. Matrix Metalloproteinase-9 Knockout Confers Resistance to Corneal Epithelial Barrier Disruption in Experimental Dry Eye. Am. J. Pathol. 2005, 166, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Bang, S.-P.; Shim, K.-Y.; Son, M.-J.; Kim, H.; Jun, J.H. Association of Tear Matrix Metalloproteinase 9 Immunoassay with Signs and Symptoms of Dry Eye Disease: A Cross-Sectional Study Using Qualitative, Semiquantitative, and Quantitative Strategies. PLoS ONE 2021, 16, e0258203. [Google Scholar] [CrossRef]
- Schargus, M.; Ivanova, S.; Kakkassery, V.; Dick, H.B.; Joachim, S. Correlation of Tear Film Osmolarity and 2 Different MMP-9 Tests With Common Dry Eye Tests in a Cohort of Non–Dry Eye Patients. Cornea 2015, 34, 739–744. [Google Scholar] [CrossRef]
- Kang, M.-J.; Kim, H.S.; Kim, M.S.; Kim, E.C. The Correlation between Matrix Metalloproteinase-9 Point-of-Care Immunoassay, Tear Film Osmolarity, and Ocular Surface Parameters. J. Ophthalmol. 2022, 2022, 6132016. [Google Scholar] [CrossRef]
- Kook, K.Y.; Jin, R.; Li, L.; Yoon, H.J.; Yoon, K.C. Tear Osmolarity and Matrix Metallopeptidase-9 in Dry Eye Associated with Sjögren’s Syndrome. Korean J. Ophthalmol. 2020, 34, 179–186. [Google Scholar] [CrossRef]
- Lanza, N.L.; Valenzuela, F.; Perez, V.L.; Galor, A. The Matrix Metalloproteinase 9 Point-of-Care Test in Dry Eye. Ocul. Surf. 2016, 14, 189–195. [Google Scholar] [CrossRef]
- Di Cera, E. Serine Proteases. IUBMB Life 2009, 61, 510–515. [Google Scholar] [CrossRef]
- Perona, J.J.; Craik, C.S. Structural Basis of Substrate Specificity in the Serine Proteases. Protein Sci. 2008, 4, 337–360. [Google Scholar] [CrossRef]
- Drag, M.; Salvesen, G.S. Emerging Principles in Protease-Based Drug Discovery. Nat. Rev. Drug Discov. 2010, 9, 690–701. [Google Scholar] [CrossRef]
- Vergnolle, N. Protease Inhibition as New Therapeutic Strategy for GI Diseases. Gut 2016, 65, 1215–1224. [Google Scholar] [CrossRef]
- Van Gent, D.; Sharp, P.; Morgan, K.; Kalsheker, N. Serpins: Structure, Function and Molecular Evolution. Int. J. Biochem. Cell Biol. 2003, 35, 1536–1547. [Google Scholar] [CrossRef]
- Heutinck, K.M.; ten Berge, I.J.M.; Hack, C.E.; Hamann, J.; Rowshani, A.T. Serine Proteases of the Human Immune System in Health and Disease. Mol. Immunol. 2010, 47, 1943–1955. [Google Scholar] [CrossRef]
- Safavi, F.; Rostami, A. Role of Serine Proteases in Inflammation: Bowman–Birk Protease Inhibitor (BBI) as a Potential Therapy for Autoimmune Diseases. Exp. Mol. Pathol. 2012, 93, 428–433. [Google Scholar] [CrossRef]
- Denadai-Souza, A.; Bonnart, C.; Tapias, N.S.; Marcellin, M.; Gilmore, B.; Alric, L.; Bonnet, D.; Burlet-Schiltz, O.; Hollenberg, M.D.; Vergnolle, N.; et al. Functional Proteomic Profiling of Secreted Serine Proteases in Health and Inflammatory Bowel Disease. Sci. Rep. 2018, 8, 7834. [Google Scholar] [CrossRef]
- Sathe, S.; Sakata, M.; Beaton, A.R.; Sack, R.A. Identification, Origins and the Diurnal Role of the Principal Serine Protease Inhibitors in Human Tear Fluid. Curr. Eye Res. 1998, 17, 348–362. [Google Scholar] [CrossRef]
- Mun, Y.; Hwang, J.S.; Shin, Y.J. Role of Neutrophils on the Ocular Surface. Int. J. Mol. Sci. 2021, 22, 10386. [Google Scholar] [CrossRef]
- Döring, G. The Role of Neutrophil Elastase in Chronic Inflammation. Am. J. Respir. Crit. Care Med. 1994, 150, S114–S117. [Google Scholar] [CrossRef]
- Pham, C.T.N. Neutrophil Serine Proteases Fine-Tune the Inflammatory Response. Int. J. Biochem. Cell Biol. 2008, 40, 1317–1333. [Google Scholar] [CrossRef]
- Sonawane, S.; Khanolkar, V.; Namavari, A.; Chaudhary, S.; Gandhi, S.; Tibrewal, S.; Jassim, S.H.; Shaheen, B.; Hallak, J.; Horner, J.H.; et al. Ocular Surface Extracellular DNA and Nuclease Activity Imbalance: A New Paradigm for Inflammation in Dry Eye Disease. Investig. Opthalmol. Vis. Sci. 2012, 53, 8253. [Google Scholar] [CrossRef]
- Nair, A.P.; D’Souza, S.; Shetty, R.; Ahuja, P.; Kundu, G.; Khamar, P.; Dadachanji, Z.; Paritekar, P.; Patel, P.; Dickman, M.M.; et al. Altered Ocular Surface Immune Cell Profile in Patients with Dry Eye Disease. Ocul. Surf. 2021, 21, 96–106. [Google Scholar] [CrossRef]
- Postnikoff, C.K.; Held, K.; Viswanath, V.; Nichols, K.K. Enhanced Closed Eye Neutrophil Degranulation in Dry Eye Disease. Ocul. Surf. 2020, 18, 841–851. [Google Scholar] [CrossRef]
- Tibrewal, S.; Ivanir, Y.; Sarkar, J.; Nayeb-Hashemi, N.; Bouchard, C.S.; Kim, E.; Jain, S. Hyperosmolar Stress Induces Neutrophil Extracellular Trap Formation: Implications for Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7961–7969. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Hasíková, L.; Hampel, U.; Grüneboom, A.; Shan, X.; Herrmann, I.; Garreis, F.; Bock, F.; Knopf, J.; Singh, J.; et al. Aggregated Neutrophil Extracellular Traps Occlude Meibomian Glands during Ocular Surface Inflammation. Ocul. Surf. 2021, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Magrini, L.; Bonini, S.; Centofanti, M.; Schiavone, M.; Bonini, S. Tear Tryptase Levels and Allergic Conjunctivitis. Allergy 1996, 51, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H. Mast Cell Tryptases and Chymases in Inflammation and Host Defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef]
- Butrus, S.I.; Ochsner, K.I.; Abelson, M.B.; Schwartz, L.B. The Level of Tryptase in Human Tears. Ophthalmology 1990, 97, 1678–1683. [Google Scholar] [CrossRef]
- Li, Q.; Jie, Y.; Wang, C.; Zhang, Y.; Guo, H.; Pan, Z. Tryptase Compromises Corneal Epithelial Barrier Function. Cell Biochem. Funct. 2014, 32, 183–187. [Google Scholar] [CrossRef]
- Ebihara, N.; Funaki, T.; Murakami, A.; Takai, S.; Miyazaki, M. Mast Cell Chymase Decreases the Barrier Function and Inhibits the Migration of Corneal Epithelial Cells. Curr. Eye Res. 2005, 30, 1061–1069. [Google Scholar] [CrossRef]
- Villani, E.; Rabbiolo, G.; Nucci, P. Ocular Allergy as a Risk Factor for Dry Eye in Adults and Children. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 398–403. [Google Scholar] [CrossRef]
- Turk, B.; Turk, D.; Salvesen, G. Regulating Cysteine Protease Activity: Essential Role of Protease Inhibitors As Guardians and Regulators. Curr. Pharm. Des. 2002, 8, 1623–1637. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS Database of Proteolytic Enzymes, Their Substrates and Inhibitors in 2017 and a Comparison with Peptidases in the PANTHER Database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [Green Version]
- Tušar, L.; Usenik, A.; Turk, B.; Turk, D. Mechanisms Applied by Protein Inhibitors to Inhibit Cysteine Proteases. Int. J. Mol. Sci. 2021, 22, 997. [Google Scholar] [CrossRef]
- Roush, W.R.; Gwaltney, S.L.; Cheng, J.; Scheidt, K.A.; McKerrow, J.H.; Hansell, E. Vinyl Sulfonate Esters and Vinyl Sulfonamides: Potent, Irreversible Inhibitors of Cysteine Proteases. J. Am. Chem. Soc. 1998, 120, 10994–10995. [Google Scholar] [CrossRef]
- Im, E.; Kazlauskas, A. The Role of Cathepsins in Ocular Physiology and Pathology. Exp. Eye Res. 2007, 84, 383–388. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chen, S.-J.; Shen, M.-R.; Huang, Y.-T.; Hsieh, H.-P.; Lin, S.-Y.; Lin, C.-C.; Chang, W.-S.W.; Chang, J.-Y. Lysosomal Cysteine Protease Cathepsin S Is Involved in Cancer Cell Motility by Regulating Store-Operated Ca2+ Entry. Biochim. Biophys. Acta-Mol. Cell Res. 2019, 1866, 118517. [Google Scholar] [CrossRef]
- Wolters, P.J.; Chapman, H.A. Importance of Lysosomal Cysteine Proteases in Lung Disease. Respir. Res. 2000, 1, 170–177. [Google Scholar] [CrossRef]
- Li, X.; Wu, K.; Edman, M.; Schenke-Layland, K.; MacVeigh-Aloni, M.; Janga, S.R.; Schulz, B.; Hamm-Alvarez, S.F. Increased Expression of Cathepsins and Obesity-Induced Proinflammatory Cytokines in Lacrimal Glands of Male NOD Mouse. Investig. Opthalmol. Vis. Sci. 2010, 51, 5019. [Google Scholar] [CrossRef]
- Hamm-Alvarez, S.F.; Janga, S.R.; Edman, M.C.; Madrigal, S.; Shah, M.; Frousiakis, S.E.; Renduchintala, K.; Zhu, J.; Bricel, S.; Silka, K.; et al. Tear Cathepsin S as a Candidate Biomarker for Sjögren’s Syndrome. Arthritis Rheumatol. 2014, 66, 1872–1881. [Google Scholar] [CrossRef]
- Edman, M.C.; Janga, S.R.; Meng, Z.; Bechtold, M.; Chen, A.F.; Kim, C.; Naman, L.; Sarma, A.; Teekappanavar, N.; Kim, A.Y.; et al. Increased Cathepsin S Activity Associated with Decreased Protease Inhibitory Capacity Contributes to Altered Tear Proteins in Sjögren’s Syndrome Patients. Sci. Rep. 2018, 8, 11044. [Google Scholar] [CrossRef]
- Ossovskaya, V.S.; Bunnett, N.W. Protease-Activated Receptors: Contribution to Physiology and Disease. Physiol. Rev. 2004, 84, 579–621. [Google Scholar] [CrossRef]
- Adams, M.N.; Ramachandran, R.; Yau, M.-K.; Suen, J.Y.; Fairlie, D.P.; Hollenberg, M.D.; Hooper, J.D. Structure, Function and Pathophysiology of Protease Activated Receptors. Pharmacol. Ther. 2011, 130, 248–282. [Google Scholar] [CrossRef]
- Sébert, M.; Sola-Tapias, N.; Mas, E.; Barreau, F.; Ferrand, A. Protease-Activated Receptors in the Intestine: Focus on Inflammation and Cancer. Front. Endocrinol. 2019, 10, 717. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ansuini, H.; Celli, N.; De Blasi, A.; O’Brien, P.; Brass, L.; Molino, M. Neutrophil Proteases Can Inactivate Human PAR3 and Abolish the Co-Receptor Function of PAR3 on Murine Platelets. Thromb. Haemost. 2001, 85, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Price, R.; Mercuri, N.B.; Ledonne, A. Emerging Roles of Protease-Activated Receptors (PARs) in the Modulation of Synaptic Transmission and Plasticity. Int. J. Mol. Sci. 2021, 22, 869. [Google Scholar] [CrossRef] [PubMed]
- Rothmeier, A.S.; Ruf, W. Protease-Activated Receptor 2 Signaling in Inflammation. Semin. Immunopathol. 2012, 34, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.R.; Sloss, C.M.; Cameron, P.; Kanke, T.; McKenzie, R.C.; Plevin, R. The Role of Intracellular Ca2+ in the Regulation of Proteinase-Activated Receptor-2 Mediated Nuclear Factor Kappa B Signalling in Keratinocytes. Br. J. Pharmacol. 2005, 145, 535–544. [Google Scholar] [CrossRef]
- Chandrabalan, A.; Ramachandran, R. Molecular Mechanisms Regulating Proteinase-Activated Receptors (PARs). FEBS J. 2021, 288, 2697–2726. [Google Scholar] [CrossRef]
- Ramachandran, R.; Mihara, K.; Chung, H.; Renaux, B.; Lau, C.S.; Muruve, D.A.; DeFea, K.A.; Bouvier, M.; Hollenberg, M.D. Neutrophil Elastase Acts as a Biased Agonist for Proteinase-Activated Receptor-2 (PAR2). J. Biol. Chem. 2011, 286, 24638–24648. [Google Scholar] [CrossRef]
- Stern, M.E.; Schaumburg, C.S.; Pflugfelder, S.C. Dry Eye as a Mucosal Autoimmune Disease. Int. Rev. Immunol. 2013, 32, 19–41. [Google Scholar] [CrossRef]
- Valdez-Morales, E.E.; Overington, J.; Guerrero-Alba, R.; Ochoa-Cortes, F.; Ibeakanma, C.O.; Spreadbury, I.; Bunnett, N.W.; Beyak, M.; Vanner, S.J. Sensitization of Peripheral Sensory Nerves by Mediators From Colonic Biopsies of Diarrhea-Predominant Irritable Bowel Syndrome Patients: A Role for PAR2. Am. J. Gastroenterol. 2013, 108, 1634–1643. [Google Scholar] [CrossRef]
- Bucci, M.; Roviezzo, F.; Cirino, G. Protease-Activated Receptor-2 (PAR2) in Cardiovascular System. Vascul. Pharmacol. 2005, 43, 247–253. [Google Scholar] [CrossRef]
- Sokolova, E.; Reiser, G. A Novel Therapeutic Target in Various Lung Diseases: Airway Proteases and Protease-Activated Receptors. Pharmacol. Ther. 2007, 115, 70–83. [Google Scholar] [CrossRef]
- Lang, R.; Song, P.I.; Legat, F.J.; Lavker, R.M.; Harten, B.; Kalden, H.; Grady, E.F.; Bunnett, N.W.; Armstrong, C.A.; Ansel, J.C. Human Corneal Epithelial Cells Express Functional PAR-1 and PAR-2. Investig. Opthalmol. Vis. Sci. 2003, 44, 99–105. [Google Scholar] [CrossRef]
- Tripathi, T.; Alizadeh, H. Role of Protease-Activated Receptors 2 (PAR2) in Ocular Infections and Inflammation. Recept. Clin. Investig. 2014, 2, e2991. [Google Scholar] [CrossRef]
- Zhao, P.; Lieu, T.; Barlow, N.; Metcalf, M.; Veldhuis, N.A.; Jensen, D.D.; Kocan, M.; Sostegni, S.; Haerteis, S.; Baraznenok, V.; et al. Cathepsin S Causes Inflammatory Pain via Biased Agonism of PAR2 and TRPV4. J. Biol. Chem. 2014, 289, 27215–27234. [Google Scholar] [CrossRef]
- Klinngam, W.; Fu, R.; Janga, S.; Edman, M.; Hamm-Alvarez, S. Cathepsin S Alters the Expression of Pro-Inflammatory Cytokines and MMP-9, Partially through Protease—Activated Receptor-2, in Human Corneal Epithelial Cells. Int. J. Mol. Sci. 2018, 19, 3530. [Google Scholar] [CrossRef]
- Mori, M.; De Lorenzo, E.; Torre, E.; Fragai, M.; Nativi, C.; Luchinat, C.; Arcangeli, A. A Highly Soluble Matrix Metalloproteinase-9 Inhibitor for Potential Treatment of Dry Eye Syndrome. Basic Clin. Pharmacol. Toxicol. 2012, 111, 289–295. [Google Scholar] [CrossRef]
- Richichi, B.; Baldoneschi, V.; Burgalassi, S.; Fragai, M.; Vullo, D.; Akdemir, A.; Dragoni, E.; Louka, A.; Mamusa, M.; Monti, D.; et al. A Divalent PAMAM-Based Matrix Metalloproteinase/Carbonic Anhydrase Inhibitor for the Treatment of Dry Eye Syndrome. Chem. Eur. J. 2016, 22, 1714–1721. [Google Scholar] [CrossRef]
- Shoari, A.; Rasaee, M.J.; Rezaei Kanavi, M.; Afsar Aski, S.; Tooyserkani, R. In Vivo Effect of RSH-12, a Novel Selective MMP-9 Inhibitor Peptide, in the Treatment of Experimentally Induced Dry Eye Model. Curr. Eye Res. 2021, 46, 7–13. [Google Scholar] [CrossRef]
- Liu, X.; Lin, Z.; Zhou, T.; Zong, R.; He, H.; Liu, Z.; Ma, J.; Liu, Z.; Zhou, Y. Anti-Angiogenic and Anti-Inflammatory Effects of SERPINA3K on Corneal Injury. PLoS ONE 2011, 6, e16712. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Xie, H.; Chen, L.; Zhou, Y.; Chen, W.; Liu, Z. Serine Protease Inhibitor A3K Protects Rabbit Corneal Endothelium From Barrier Function Disruption Induced by TNF-α. Investig. Opthalmol. Vis. Sci. 2013, 54, 5400. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Zhou, Y.; Wang, Y.; Zhou, T.; Li, J.; Luo, P.; He, H.; Wu, H.; Liu, Z. Serine Protease Inhibitor A3K Suppressed the Formation of Ocular Surface Squamous Metaplasia in a Mouse Model of Experimental Dry Eye. Investig. Opthalmol. Vis. Sci. 2014, 55, 5813. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, X.; Zhou, T.; Wang, Y.; Bai, L.; He, H.; Liu, Z. A Mouse Dry Eye Model Induced by Topical Administration of Benzalkonium Chloride. Mol. Vis. 2011, 17, 257–264. [Google Scholar]
- Steele, F.R.; Chader, G.J.; Johnson, L.V.; Tombran-Tink, J. Pigment Epithelium-Derived Factor: Neurotrophic Activity and Identification as a Member of the Serine Protease Inhibitor Gene Family. Proc. Natl. Acad. Sci. USA 1993, 90, 1526–1530. [Google Scholar] [CrossRef]
- Karakousis, P.C.; John, S.K.; Behling, K.C.; Surace, E.M.; Smith, J.E.; Hendrickson, A.; Tang, W.X.; Bennett, J.; Milam, A.H. Localization of Pigment Epithelium Derived Factor (PEDF) in Developing and Adult Human Ocular Tissues. Mol. Vis. 2001, 7, 154–163. [Google Scholar]
- Singh, R.B.; Blanco, T.; Mittal, S.K.; Taketani, Y.; Chauhan, S.K.; Chen, Y.; Dana, R. Pigment Epithelium-Derived Factor Secreted by Corneal Epithelial Cells Regulates Dendritic Cell Maturation in Dry Eye Disease. Ocul. Surf. 2020, 18, 460–469. [Google Scholar] [CrossRef]
- Ma, B.; Zhou, Y.; Liu, R.; Zhang, K.; Yang, T.; Hu, C.; Gao, Y.; Lan, Q.; Liu, Y.; Yang, X.; et al. Pigment Epithelium-Derived Factor (PEDF) Plays Anti-Inflammatory Roles in the Pathogenesis of Dry Eye Disease. Ocul. Surf. 2021, 20, 70–85. [Google Scholar] [CrossRef]
- Joossens, J.; Ali, O.M.; El-Sayed, I.; Surpateanu, G.; Van der Veken, P.; Lambeir, A.-M.; Setyono-Han, B.; Foekens, J.A.; Schneider, A.; Schmalix, W.; et al. Small, Potent, and Selective Diaryl Phosphonate Inhibitors for Urokinase-Type Plasminogen Activator with In Vivo Antimetastatic Properties. J. Med. Chem. 2007, 50, 6638–6646. [Google Scholar] [CrossRef]
- Joossen, C.; Lanckacker, E.; Zakaria, N.; Koppen, C.; Joossens, J.; Cools, N.; De Meester, I.; Lambeir, A.-M.; Delputte, P.; Maes, L.; et al. Optimization and Validation of an Existing, Surgical and Robust Dry Eye Rat Model for the Evaluation of Therapeutic Compounds. Exp. Eye Res. 2016, 146, 172–178. [Google Scholar] [CrossRef]
- Singh, R.B.; Blanco, T.; Mittal, S.K.; Alemi, H.; Chauhan, S.K.; Chen, Y.; Dana, R. Pigment Epithelium–Derived Factor Enhances the Suppressive Phenotype of Regulatory T Cells in a Murine Model of Dry Eye Disease. Am. J. Pathol. 2021, 191, 720–729. [Google Scholar] [CrossRef]
- Saarinen, N.V.V.; Stone, V.M.; Hankaniemi, M.M.; Mazur, M.A.; Vuorinen, T.; Flodström-Tullberg, M.; Hyöty, H.; Hytönen, V.P.; Laitinen, O.H. Antibody Responses against Enterovirus Proteases Are Potential Markers for an Acute Infection. Viruses 2020, 12, 78. [Google Scholar] [CrossRef]
- Costanzi, E.; Kuzikov, M.; Esposito, F.; Albani, S.; Demitri, N.; Giabbai, B.; Camasta, M.; Tramontano, E.; Rossetti, G.; Zaliani, A.; et al. Structural and Biochemical Analysis of the Dual Inhibition of MG-132 against SARS-CoV-2 Main Protease (Mpro/3CLpro) and Human Cathepsin-L. Int. J. Mol. Sci. 2021, 22, 11779. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.S.; Dean, R.A.; Morrison, C.J.; Overall, C.M. Identification of Cellular MMP Substrates Using Quantitative Proteomics: Isotope-Coded Affinity Tags (ICAT) and Isobaric Tags for Relative and Absolute Quantification (ITRAQ). In Matrix Metalloproteinase Protocols; Clark, I.M., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; pp. 451–470. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine Protease Mechanism and Specificity. Chem. Rev. 2002, 102, 4501–4523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yu, Z.; Ye, Z.; Su, M. Multiplex Analyses of the Changes of Aromatic Compounds during the Development of Peach Fruit Using GC–MS and ITRAQ Proteomic Techniques. Sci. Hortic. 2018, 236, 96–105. [Google Scholar] [CrossRef]
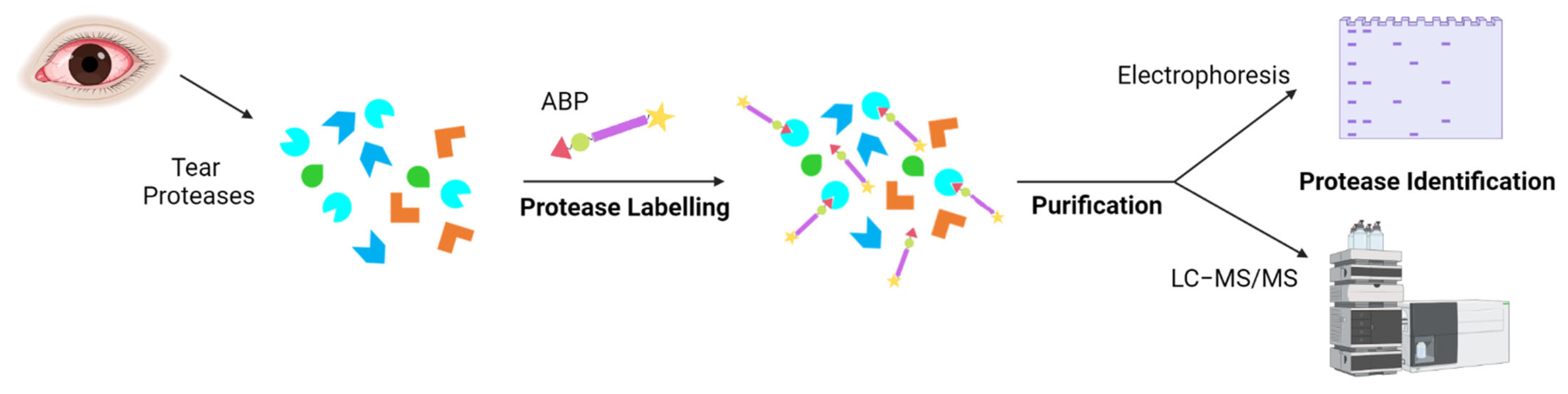
- Cravatt, B.F.; Wright, A.T.; Kozarich, J.W. Activity-Based Protein Profiling: From Enzyme Chemistry to Proteomic Chemistry. Annu. Rev. Biochem. 2008, 77, 383–414. [Google Scholar] [CrossRef]
- Peng, H.; Hulleman, J.D. Prospective Application of Activity-Based Proteomic Profiling in Vision Research-Potential Unique Insights into Ocular Protease Biology and Pathology. Int. J. Mol. Sci. 2019, 20, 3855. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal Structure of SARS-CoV-2 Main Protease Provides a Basis for Design of Improved a-Ketoamide Inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [Green Version]

| Inhibitor | Target | Experimental Setting | Effect a | Ref. |
|---|---|---|---|---|
| PES_103 | MMP-9 | Dry eye mice model Transdermal scopolamine patches | ↑ Tear production | [106] |
| Divalent PAMAM | MMP-9 | Dry eye rabbit model Atropine sulfate | ↑ Tear production ↓ Corneal damage | [107] |
| RSH-12 | MMP-9 | Dry eye rabbit model Atropine sulfate | ↑ Tear volume ↓ Tear breakup time | [108] |
| SERPINA3K | Serine proteases | Dry eye mice model BAC induced | ↓ Epithelial damage ↓ TNF-α | [111] |
| PEDF | Serine protease | Dry eye mice model Controlled environment chamber | ↓ DCs, Th17 ↓ Proinflammatory cytokines ↓ Fluorescein score | [115,119] |
| UAMC-00050 | Serine proteases | Dry eye rat model Surgical removal exorbital lacrimal gland | ↓ IL-1α, TNF-α, MMP-9 ↓ CD3+, CD45+ | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Llorca, A.; Scarpellini, C.; Augustyns, K. Proteases and Their Potential Role as Biomarkers and Drug Targets in Dry Eye Disease and Ocular Surface Dysfunction. Int. J. Mol. Sci. 2022, 23, 9795. https://doi.org/10.3390/ijms23179795
Ramos-Llorca A, Scarpellini C, Augustyns K. Proteases and Their Potential Role as Biomarkers and Drug Targets in Dry Eye Disease and Ocular Surface Dysfunction. International Journal of Molecular Sciences. 2022; 23(17):9795. https://doi.org/10.3390/ijms23179795
Chicago/Turabian StyleRamos-Llorca, Alba, Camilla Scarpellini, and Koen Augustyns. 2022. "Proteases and Their Potential Role as Biomarkers and Drug Targets in Dry Eye Disease and Ocular Surface Dysfunction" International Journal of Molecular Sciences 23, no. 17: 9795. https://doi.org/10.3390/ijms23179795
APA StyleRamos-Llorca, A., Scarpellini, C., & Augustyns, K. (2022). Proteases and Their Potential Role as Biomarkers and Drug Targets in Dry Eye Disease and Ocular Surface Dysfunction. International Journal of Molecular Sciences, 23(17), 9795. https://doi.org/10.3390/ijms23179795