Abstract
Abnormal activation of the kynurenine and serotonin pathways of tryptophan metabolism is linked to a host of neuropsychiatric disorders. Concurrently, noninvasive brain stimulation (NIBS) techniques demonstrate high therapeutic efficacy across neuropsychiatric disorders, with indications for modulated neuroplasticity underlying such effects. We therefore conducted a scoping review with meta-analysis of eligible studies, conforming with the PRISMA statement, by searching the PubMed and Web of Science databases for clinical and preclinical studies that report the effects of NIBS on biomarkers of tryptophan metabolism. NIBS techniques reviewed were electroconvulsive therapy (ECT), transcranial magnetic stimulation (TMS), and transcranial direct current stimulation (tDCS). Of the 564 search results, 65 studies were included with publications dating back to 1971 until 2022. The Robust Bayesian Meta-Analysis on clinical studies and qualitative analysis identified general null effects by NIBS on biomarkers of tryptophan metabolism, but moderate evidence for TMS effects on elevating serum serotonin levels. We cannot interpret this as evidence for or against the effects of NIBS on these biomarkers, as there exists several confounding methodological differences in this literature. Future controlled studies are needed to elucidate the effects of NIBS on biomarkers of tryptophan metabolism, an under-investigated question with substantial implications to clinical research and practice.
1. Introduction
1.1. Rationale
The products of tryptophan (TRP) metabolism have vital physiological roles. These bioactive metabolites include serotonin (5-HT), melatonin, and the kynurenines, all with diverse regulatory functions. Although TRP itself is an essential amino acid, with its availability dependent on dietary intake, only a small amount is used for protein synthesis while most of it is degraded via the 5-HT or kynurenine pathways (Figure 1). The biomarkers of interest in the present review, whose concentrations in various media inform about the activation of TRP metabolism are: TRP, kynurenine (KYN), formylkynurenine, kynurenic acid (KA), quinolinic acid (QA), NAD+, 3-hydroxykynurenine (3-HK), xanthurenic acid (XA), picolinic acid (PA), anthranilic acid (AA), 5-HT, oxitriptan, 5-hydroxytryptamine (5-HT), 5-hydroxyindoleacetic acid (5-HIAA), N-acetylserotonin, and melatonin.
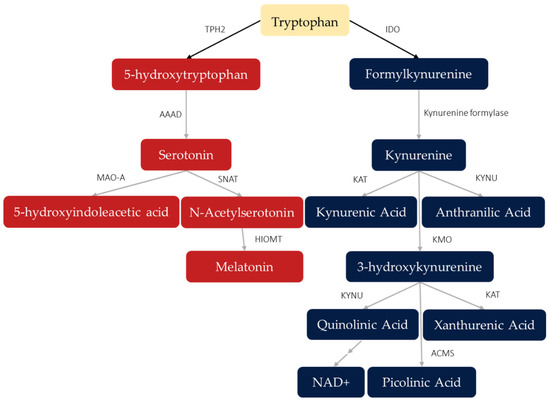
Figure 1.
Products of tryptophan metabolism via serotonin (red) and kynurenine (blue) pathways. Arrows point toward the direction of metabolism as mediated by different enzymes or other catalysts, with fields showing metabolites produced along these pathways. Double arrows indicate multiple metabolic steps (catalysts not shown). Abbreviations: TPH2: tryptophan hydroxylase, isoenzyme 2; AAAD: aromatic acid decarboxylase; MAO-A: monoamine oxidase A; HIOMT: hydroxyindole O-methyl transferase; SNAT: serotonin-N-acetyltransferase; IDO: indoleamine 2,3-dioxyenase; KAT: kynurenine aminotransferase; KMO: kynurenine 3-monooxygenase; KYNU: kynurinase; ACMS: 2-amino-3-carboxymuconic-6-semialdehyde decarboxylase.
Healthy intake of dietary TRP and production of these metabolic products are linked to cardiovascular health [1], gut-brain homeostasis [2], cognitive and mood regulation [3,4], and immune and inflammation mediation [5]. To reach the central nervous system (CNS), a portion of dietary TRP is absorbed in the small intestine to be released into the blood stream. It then traverses through the bloodstream and is subsequently transported across the blood–brain barrier by the L-type amino acid transporter, which is also expressed by nerve cells within the CNS. This dietary TRP is a vital substrate for 5-HT synthesis by serotonergic neurons in the CNS, which project from the rostral raphe nuclei to the cerebral cortex. These cortices ubiquitously express critical proteins for 5-HT neurotransmission, such as the highly studied receptors 5-HT1A and 5HT2A, and monoamine oxidase-A (MAO-A) [6], among several more receptor subtypes and transporters. Serotonergic neurons also project to limbic and sub-cortical structures, other brain stem regions and the spinal cord—providing an anatomical link between the role of serotonin in behavioral, emotional, cognitive, and motor functions mediated by the nervous system. Activation of the kynurenine pathway generates molecules that are neuroactive and mediate inflammatory response [5,7]. Several highly prevalent neuropsychiatric disorders, such as major depressive disorder (MDD), bipolar disorder, and schizophrenia, present with biomarker concentrations that indicate abnormal activation of these pathways [8,9,10]. Further, there is evidence that the restoration of these abnormal activations coincides with therapeutic effects. For instance, antidepressant effects following anti-inflammatory medication are observed in patients with inflammatory conditions [11] and with depressive disorders [12]. Further, the antidepressant effects of the selective serotonin reuptake inhibitor (SSRI), escitalopram, have been observed to negatively correlate with kynurenic acid (KA) plasma levels, with lower levels predicting greater therapeutic outcomes in patients diagnosed with MDD [13]. In a meta-analysis examining peripheral levels of kynurenines in psychiatric patients compared to healthy controls, Marx et al. [9] observed significantly lower TRP levels in patients with MDD and schizophrenia compared to healthy controls, in addition to elevated kynurenine (KYN) to TRP ratios. This latter observation suggests that the diminished TRP availability may be due to over-activation of the kynurenine pathway. Moreover, compared to healthy controls, KA levels were lower in MDD patients but schizophrenia patients had KA levels comparable to controls [9]. Such unique patterns are proposed to underlie the specific syndromes of these disorders [7], supporting efforts to target these abnormal pathway activations for intervention or to inform treatment selection [5,7]. In further support of these efforts, Haroon et al. [14] assessed the association between blood plasma and cerebrospinal fluid (CSF) biomarkers of immune and kynurenine pathway activation, finding elevations in both mediums in depressed patients compared to healthy controls; these elevations also corresponded to more severe motivation and anhedonia symptoms. As elevated kynurenine pathway activation catabolizes more available TRP, serotonin synthesis is consequently diminished due to lack of substrate. Such findings link over-activation of the kynurenine pathway to psychiatric symptoms that are associated with diminished serotonin activity, such as impaired neuroplasticity [15] and disrupted “re-learning” of appropriate emotion processing in depression [4]. Additionally, disrupted serotonin synthesis following kynurenine pathway over-activation lends further credence to the association between psychiatric symptoms and seemingly distant events that alter TRP degradation toward the kynurenine pathway, such as chronic stress [16] and inflammation [7]. As the authors above, Haroon et al. [14] envisioned using biomarkers of TRP metabolism to guide psychiatric treatment.
Noninvasive brain stimulation (NIBS) techniques have demonstrated therapeutic utility across neuropsychiatric disorders, with varied levels of efficacy and quality of evidence [17]. Those techniques under review here are repetitive transcranial magnetic stimulation (rTMS) [17,18,19,20], transcranial direct current stimulation (tDCS) [17,18], and electroconvulsive therapy (ECT) [17]—each considered non-invasive as no breaching or implantation occurs during treatment [21]. Current efforts are aimed at developing precise protocols for these techniques [22]. However, while elucidating the mechanisms of action of NIBS is critical toward these aims, current theories need to be broadened or supported by molecular evidence to account for the enduring therapeutic effects of brain stimulation [23].
In light of theories predicting a link between improved clinical outcomes and recovered pathway activation, and the need to specify molecular mechanisms of NIBS to optimize protocol selection, efforts to investigate the influence of NIBS on tryptophan metabolism are re-emerging [24]. While there exists reviews relevant to this research question [24,25,26], there is a current need for systematic reviews which assess the effects of NIBS on specific biomarkers of tryptophan metabolism in humans and animal models. This examination is critical to understanding the therapeutic mechanisms of brain stimulation across clinical diagnoses and informing efforts toward precision medicine [22].
1.2. Objective
This scoping review systematically surveys the current literature investigating the effects of therapeutic NIBS on biomarkers of tryptophan metabolism and synthesizes findings by qualitative- and meta-analyses. Our findings are discussed in light of current etiological theories of neuropsychiatric disorders and whether they are supported by the observed therapeutic and biomarker effects of NIBS. Our search criteria and research question are summarized in Table 1. Briefly, we sought published studies utilizing therapeutic NIBS, and which probed levels of kynurenine or serotonin pathway metabolites in healthy or diagnosed humans and animal models. No further restrictions were considered.

Table 1.
PICO statement.
2. Methods
This invited review was designed to conform with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) [27].
2.1. Eligibility Criteria
Inclusion criteria were (1) treatment with ECT, rTMS, or tDCS; (2) participants were human and non-human species; (3) levels of the following biomarkers were assessed using any form of biological sample collection from NIBS-treated participants: tryptophan (TRP), kynurenine (KYN), formylkynurenine, quinolinic acid (QA), NAD+, 3-hydroxykynurenine (3-HK), xanthurenic acid (XA), picolinic acid (PA), anthranilic acid (AA), serotonin (5-HT), oxitriptan, 5-hydroxytryptamine (5-HT), 5-hydroxyindoleacetic acid (5-HIAA), N-acetyl-5-HT, and melatonin; 4) effects of NIBS on biomarkers of interest levels was assessed by comparing baseline with concurrent or post-NIBS levels, without restriction to timepoints of sampling. Exclusion criteria were (1) no new findings were published or data was previously reported. Otherwise, there were no further restrictions on experimental design, the language the manuscript was written-in, NIBS parameters or targets, participant characteristics such as age or diagnosis, nor restrictions on sampling method were considered. The rationale for these relaxed restrictions was to maximize the number of included studies for this survey, as we anticipated this literature to be sparse.
2.2. Information Sources
We searched the NCBI PubMed and Web of Science databases for studies published from inception until 15 July 2022. Included studies and relevant reviews in our search results were also screened for relevant studies.
2.3. Search Strategy
Search terms for NIBS were initially based on techniques in a recent expert review [22], however we focused our search here to ECT, rTMS, and tDCS because preliminary searches to test the feasibility of this review could not identify relevant studies for other NIBS techniques and these techniques are frequently discussed in reviews on the therapeutic efficacy of NIBS and mechanisms of action, e.g, [17]. The subset of TRP metabolites, and corresponding terms for the products of the serotonin and kynurenine pathways that we reviewed were based on recent expert reviews [5,7]. The search queries used for the PubMed and Web of Science search are shown in Table S1.
2.4. Selection and Data Collection
Two independent reviewers (C.G.G. and T.T.Z.L.) conducted the search and initial screening by title and abstract. Both reviewers independently retrieved the identified potential studies, conducted full-article screening, and then extracted data for review. These results were then merged, with any disagreements settled through discussion with G.S.K. No automation tools were used for screening or data extraction.
2.5. Data Items
A customized form was used to report relevant data from our included studies: study design; NIBS protocol parameters (e.g., stimulation site, session count); participant characteristics (e.g., species, health condition, age); control group characteristics when available; biomarkers of TRP metabolism including metabolite concentration levels, ratios, sampling source, and time points; and significant and non-significant effects of NIBS on biomarkers and direction of effect (Table 1). These significant effects were retrieved from one-group pretest-posttest designs or group differences in parallel or crossover designs. For studies using a parallel design, we preferred to report results based on group*time interaction over group differences in posttests.
For quantitative analysis, we extracted numerical results necessary for the calculation of the standardized mean difference (SMD). If only statistical graphs were provided, we extracted the values using WebPlotDigitizer (https://automeris.io/WebPlotDigitizer/, accessed on 15 July 2022). Clinical measures of human participants (e.g., effects on clinical assessment scales) were also extracted. If there were multiple clinical measures, we sought the primary outcome in the study.
2.6. Qualitative Synthesis Methods
Results from clinical and preclinical studies were visualized as bar charts where the counts for significant changes, in either direction, and nonsignificant changes were stacked to present an overview of the effects of NIBS and biomarkers of TRP metabolism. In this synthesis, we stacked studies assessing the same metabolite and NIBS technique (i.e., ECT, rTMS, or tDCS). That is, we differentiated clinical and preclinical studies and NIBS, but did not perform subgroup analyses of all ways in which these studies vary, for example, dissimilar NIBS parameters. Instead, when patterns suggesting heterogeneity or true effects emerged, we agreed to follow them up by examining the characteristics of the relevant studies.
Studies may report findings from multiple experimental groups, for instance, effects on various health conditions. In these cases, we counted experimental groups instead of the study count towards synthesis. Furthermore, within experimental groups, there may be multiple sampling time points. We determined that the time points associated with significant changes would represent the groups, presuming that those time points were most sensitive to effects by NIBS. Experimental groups with significant changes in opposite directions at different time points would be excluded from the bar charts and followed up specifically. The bar charts and other similar graphs were created using the Python library on Plotly (https://plotly.com/python/, accessed on 15 July 2022).
2.7. Quantitative Synthesis Methods by Bayesian Meta-Analysis
Only clinical studies with calculable SMDs underwent meta-analysis. We used the Robust Bayesian Meta-Analysis (RoBMA) method, which applied to the data variety of models resting on different assumptions about effects, heterogeneity, and publication bias [28]. These models were weighted according to their predictability of the data and averaged to draw final inferences. The Bayesian framework allows quantification of evidence for null findings, while more traditional, frequentist approaches cannot distinguish support for null findings from the absence of evidence (in the case of p > 0.05). The qualitative analysis of significant and nonsignificant effects, as described above, and classical meta-analysis methods are examples of frequentist approaches. Besides the above advantage given by the framework, RoBMA benefits from its model-averaging feature such that it does not require all-or-none decisions about publication bias and works well under high heterogeneity [28], well-suited for this scoping review. The resulting inferences were presented as inclusion Bayes factors (BF10), representing the strength of evidence for the presence of the meta-analytic item relative to the absence. For example, a BF10 of effects equal to three means the data are three times more likely to have occurred if effects exist, or three times less likely when BF10 equals 1/3. Such a BF10 larger than three or less than 1/3 is tentatively interpreted as moderate evidence supporting or against the presence of the meta-analytic item, respectively, as per [29]. Additionally, >10 or <1/10 indicates strong evidence; >30 or <1/30 indicates very strong evidence; >100 or <1/100 indicates extreme evidence. As we expected a substantial number of clinical studies that used a one-group pretest-posttest or crossover design, we decided not to exclude them from this meta-analysis and calculate the SMD as the change or difference divided by the pretest or control standard deviation (SD). Computing the standard error of such SMDs requires the pretest-posttest correlation (r), and as few studies reported this, we adopted Rosenthal’s estimate of r = 0.7 [30], and used r = 0.5 and 0.9 for sensitivity testing. For studies with a parallel design, we preferred to calculate the SMD as the group difference in posttests divided by the pooled SD. Similar to the approach in our qualitative analysis above, when there were multiple SMD values within an experimental group, for example, due to multiple sampling time points, we chose the one with the largest absolute value, assuming it is most sensitive to the change.
Statistics measures other than the mean and SD were converted to them as per the Cochrane Handbook [31], except the median and first and third quartile were converted according to [32]. This quantitative analysis was executed using JASP version 0.16.2 (https://jasp-stats.org/, accessed on 15 July 2022).
3. Results
3.1. Selection of Sources of Evidence
A total of 65 studies (Table 2 and Table 3) [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97] were included after screening 307 records in PubMed and 257 records in Web of Science, with seven of our included studies identified in the references of studies included from databases. A total of 29 studies could not be retrieved or were excluded for not meeting inclusion criteria during full-text screening (Table S2) [36,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125]. Further details of our screening results are shown in the PRISMA flow diagram (Figure 2). All the included studies were published in English.

Table 2.
Clinical studies included: characteristics and individual results.

Table 3.
Preclinical studies included: characteristics and individual results.
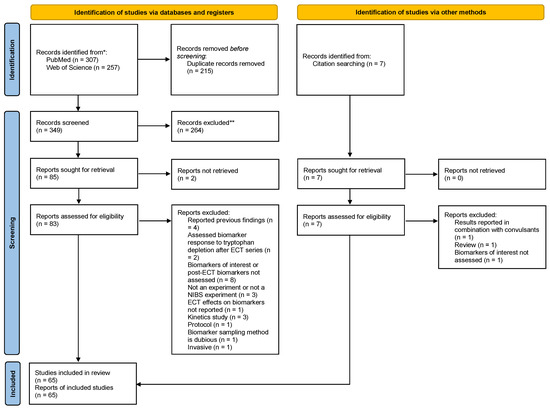
Figure 2.
PRISMA flowchart. *: for each database in our search; **: no automation tools were used.
3.2. Characteristics of Included Studiess
38 included studies recruited humans (Table 2) [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] and 27 studied animal models (Table 3) [71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97]. Clinical assessment outcomes for human participants are available in the supplementary spreadsheets.
A histogram of the study count versus publication year (Figure 3) shows that ECT research, both clinical and preclinical, dominated the 1970s and 1980s. Around the year 2000, there was an emergence of preclinical TMS studies followed by clinical research. In the past decade, there have been no preclinical ECT studies, while the number of clinical ECT and TMS studies have been growing, in addition to the appearance of tDCS studies. Of the 37 clinical studies (excluding case reports [49]), 25 (68%) used ECT, and 11 (30%) used TMS. A lower percentage of preclinical studies (12 of 27, 44%) employed ECT, while more (14, 52%) used TMS. An overview of the effects of NIBS on the concentrations of each biomarker of interest are shown Figure 4.
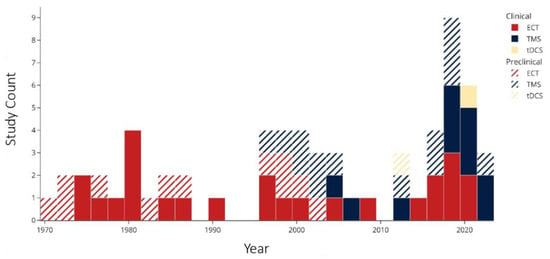
Figure 3.
Publication year of included studies. Abbreviations: ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation; tDCS: transcranial direct current stimulation.
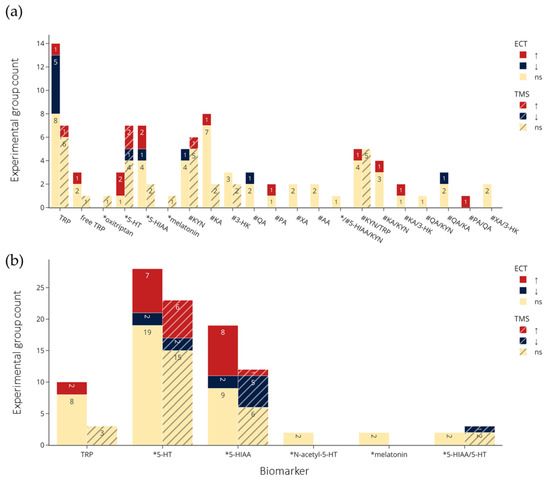
Figure 4.
Summary of (a) clinical and (b) preclinical trials findings, including direction of effects by NIBS and by biomarker. Colors indicate statistical effects on biomarkers by NIBS: red = significant increase; blue = significant decrease; yellow = no significant changes to biomarker levels following NIBS. ↑: Significantly increased or the experimental group levels were significantly larger than the control group; ↓: significantly decreased or the experimental group levels significantly smaller than the control group, *: biomarkers in the serotonin pathway; #: biomarkers in the kynurenine pathway. TRP refers to total TRP when not specified, and for brain tissue and microdialysis the regions are collapsed. Abbreviations: TRP: tryptophan; 5-HT: serotonin; 5-HIAA: 5-hydroxyindoleacetic acid; KYN: kynurenine; KA: kynurenic acid; 3-HK: 3-hydroxykynurenine; QA: quinolinic acid; XA: xanthurenic acid; AA: anthranilic acid; PA: picolinic acid; ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation; ns: not significant.
The clinical and preclinical research on the effects of NIBS on biomarkers of TRP metabolism show overlapping and distinct interests in terms of studied metabolites and sampling sources (Figure 5). Overall, the most frequently investigated were TRP, 5-HT and 5-HIAA. In clinical studies, metabolites in the kynurenine pathway, notably KYN and KA, were frequently studied, but missing in preclinical studies.
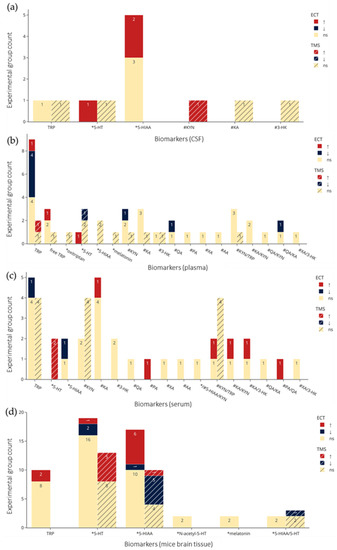
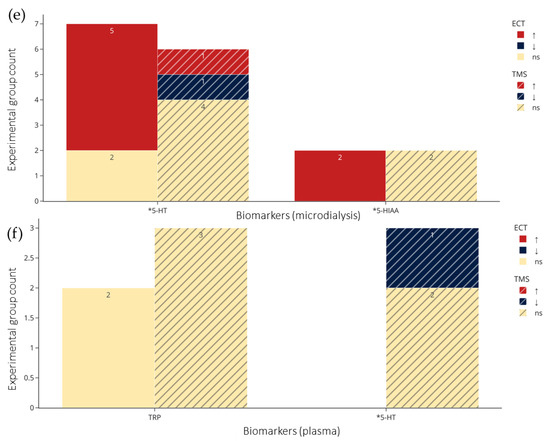
Figure 5.
Count of experimental groups reporting significant increases, decreases, or non-significant changes in the metabolites by NIBS. (a–c) From CSF, plasma, and serum in clinical studies respectively. (d–f) from brain tissue, microdialysis, and plasma in preclinical studies respectively. Colors indicate statistical effects on biomarkers by NIBS: red = significant increase; blue = significant decrease; yellow = no significant changes to biomarker levels following NIBS. *: biomarkers in the serotonin pathway; #: biomarkers in the kynurenine pathway. TRP refers to total TRP when not specified, and for brain tissue and microdialysis the regions are collapsed. Abbreviations: TRP: tryptophan; 5-HT: serotonin; 5-HIAA: 5-hydroxyindoleacetic acid; KYN: kynurenine; KA: kynurenic acid; 3-HK: 3-hydroxykynurenine; QA: quinolinic acid; XA: xanthurenic acid; AA: anthranilic acid; PA: picolinic acid; ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation; ns: not significant.
Clinical studies generally lacked randomized control designs and included pharmacotherapy-as-usual. Of the 37 clinical studies reporting statistical results (thus excluding case reports [49]), 31 used a one-group pretest-posttest design, while six employed a parallel or crossover design, of which four were randomized, and two were not. Regarding preclinical studies, 25 of the 27 (93%) used a parallel design, and no concurrent pharmacotherapy.
The heterogeneity of NIBS study protocols and participant characteristics were also assessed. Large differences across study protocols was the number of sessions, biomarker sampling time-points and, for TMS, stimuli schedule (see supplementary text). In clinical studies (excluding case reports [49]), 30 of 37 treated patients diagnosed with depression (supplementary text). The majority of clinical studies recruited more females than males (25 of the 35 provided the gender ratio, 71%) or recruited equal amounts (two, 6%), with the age mean or median ranging between 40 and 60 (with 27 of the 36 offering such information, 72%). By contrast, in all but two preclinical studies providing sex ratios, animals were all males. Most preclinical studies used healthy animals (22 of 27, 81%). Of 27 preclinical studies, 24 (89%) used rats, two (7%) mice, and one study used dogs (4%). Lastly, biomarker sampling time points appeared arbitrarily chosen or is vaguely described. For a more detailed discussion of these heterogenous methods of our included studies, see supplementary text.
3.3. Qualitative Synthesis Results
Figure 4a,b and Figure 5a–f visualize results of the counts of experimental groups with significant or nonsignificant changes in biomarkers of TRP metabolism, with the latter organized according to sampling medium. Figure 5a–c show examinations of CSF (a), plasma (b), serum (c) in clinical studies, and Figure 5d–f show brain tissue (d), microdialysis (e), and plasma (f) in preclinical studies. The sampling results of brain tissue and microdialysis involved various regions but are collapsed in Figure 5d and e for brevity; they are shown individually in Figure S1. Moreover, for brevity, only ECT and TMS results are visualized, and readers are referred to the bottom of Table 2 and Table 3 for the single clinical or preclinical tDCS study, respectively. For a more detailed report on the qualitative analysis of the methods and results in clinical and preclinical studies, see supplementary text.
3.4. Quantitative Synthesis Results by Bayesian Meta-Analysis
Table 4 lists the BF10 values of effects, heterogeneity, and publication bias for the changes in the biomarker levels by NIBS, where r is set to 0.5, 0.7, or 0.9, in addition to the counts of experimental groups used for calculation. Here, only the outcomes with at least moderate evidence for effects or null effects (BF10 > 3 or <1/3, respectively) across the three r’s are shown. The rest are available in the supplementary spreadsheets. Consistent with our observations from the qualitative analysis, there was strong evidence of heterogeneity of studies assessing ECT effects on TRP plasma levels (BF10>10 across the three r’s), based on the eight studies with calculable SMDs [35,39,43,48,52,53,56,58]. However, these studies also gave moderate evidence for null effects. Furthermore, the same two studies as in the qualitative analysis provided moderate evidence for TMS increasing serum levels of 5-HT (supplementary text) [62,63].

Table 4.
Outcomes of robust Bayesian meta-analysis.
Null effects on three other biomarkers were also observed. Allen et al. [35] and Ryan et al. [52] gave moderate evidence for no change in plasma KA levels and KA/KYN ratio in response to ECT. Both studies were multi-session and recruited depressed patients, with the timing of post-ECT sampling on the scale of days (4–7 days in Allen et al. [35] and 1–3 days in Ryan et al. [52]). Furthermore, Sibon et al. [67] and Tateishi et al. [68] provided moderate evidence for no effects on plasma levels of TRP in response to TMS. However, while Tateishi et al. [68] applied multiple treatment sessions on depressed patients, Sibon et al. [67] entailed one session with young and healthy volunteers. Neither study offered the timing of post-TMS sampling.
SMDs for changes in the levels of the biomarkers were calculable in 32 of 37 clinical studies (86%, excluding case reports [49]), while SMDs for changes in the clinical measures were calculable for 22 (59%; values available in the supplementary spreadsheets). Compared to the effects of NIBS on biomarker concentrations, the BF10 values for ECT and TMS improving clinical measures were both larger than 100, indicating high probably of therapeutic outcomes in response to NIBS (other data are available in the supplementary spreadsheets).
4. Discussion
We conducted a scoping review, which conformed with the PRISMA-ScR statement [27], with qualitative and meta-analysis to assess effects of therapeutic NIBS on biomarkers of TRP metabolism. We used the RoBMA [28] to synthesize the findings of studies with calculable SMDs (Table 4) and found moderate evidence for no effects on plasma TRP levels following ECT, with strong evidence for heterogeneity of these results. These findings suggest the absence of an effect by ECT on biomarkers of interest, but these results are inconclusive as they are more-than-not likely due to differences between ECT protocols or varied time points of post-ECT biomarker sampling. We found moderate evidence for TMS elevating effects on serum levels of 5-HT [62,63], but no effects on plasma KA levels and KA/KYN ratio post-ECT [35,52], and no effects on plasma TRP following TMS [67,68]—however, these latter findings were based on two studies each. Overall, the outcomes of NIBS on these biomarkers of interest (Figure 1) are highly heterogenous, with most studies finding null effects across biomarkers (Figure 4) and sampling method (Figure 5), and reporting insufficient statistics for quantitative synthesis, characterized by high methodological heterogeneity, with only a small number using adequately randomized controlled designs (supplementary text). In summary, we cannot conclusively claim or rule out the effects of NIBS on TRP metabolism given the current literature. However, taken at face-value, our findings provide preliminary evidence that informs current hypotheses for the mechanism of action by therapeutic NIBS across neuropsychiatric disorders, specifically mechanisms influenced by TRP metabolism and its molecular targets. To wit, below we discuss our findings in the context of evidence of NIBS’s impact on substrate availability and cytokine expression that mediate TRP metabolism, followed by changes to neurotransmitter potency critical to the induction of neuroplasticity.
As many studies on NIBS conclude, its mechanism of action need further investigation, but current evidence suggests that the observed therapeutic effects may be underlined by changes to long-term potentiation (LTP) and long-term depression (LTD) induction throughout the CNS. Evidence for these NIBS effects, in association with effects on TRP metabolism, are findings of stimulation-induced molecular and morphological changes in the CNS. For example, Peng et al. [94] investigated the effects of low to high frequency (HF) rTMS over the vertex of unpredictable-stress treated rats, which elevated prefrontal catecholamine levels and reduced MAO-A activity. HF-rTMS at 5 Hz had the highest elevating effects on prefrontal 5-HT levels, while also reducing 5-HIAA, and MAO-A activity [94]—this latter protein being critical to the degradation of 5-HT to 5-HIAA (Figure 1). Our meta-analysis showed elevated serum 5-HT levels following TMS, with null or mixed findings across NIBS and biomarker sampling methods. Indeed, clinical and preclinical studies that assessed the effects of TMS [62,63,64,65,68,69,83,84,85,86,87,88,89,90,91,92,93,94,95,96] and ECT [44,57,71,72,73,74,75,76,77,78,79,80] on 5-HT concentrations reported mostly heterogenous results, with 61% of comparisons reporting null effects (Figure 4a,b), with similar mixed results when examining effects by biomarker source (Figure 5a–f). ECT did not have a significant effect on 5-HT levels, suggesting that the therapeutic mechanisms of ECT differ from rTMS. Indeed, in a recent PET study examining TRD patients treated with ECT, Baldinger-Melich et al. [25] reported negligible changes to MAO-A expression in the cerebral cortex of patients after compared to before ECT, despite high antidepressant effects. Regarding the kynurenines, a recent pilot study examined the effects of rTMS on inflammatory cytokines, finding no effects; nor did cytokine levels correlate with depression severity, although significant antidepressants effects were observed [126]. Our results are consistent with these findings, as null effects of rTMS on KYN/TRP (Figure 4a), a biomarker for the rate of TRP metabolism toward the kynurenine pathway, were observed. Such findings suggest that the therapeutic effects of rTMS may not involve changes to inflammatory cytokine expression that lead to increased activation of the kynurenine pathway.
An alternative and exploratory link between NIBS and TRP metabolism is the research thread on the stimulation effects on the autonomic nervous system (ANS). Heart rate variability is a proxy of ANS health, with high variability interpreted as indicating abnormal ANS functioning in depressed patients [127,128]. Excitatory stimulation of the left DLPFC, a common therapeutic rTMS protocol, has restorative effects on this variability compared to sham stimulation [129]. As absorption in the small intestine is also a function of the ANS, the therapeutic effects of rTMS may affect TRP absorption. A consequence of this hypothesis is abnormal TRP levels in peripheral circulation following abnormal ANS function. Indeed, peripheral TRP levels are significantly lower in depressed patients compared to healthy controls [9]. Restoration of ANS function in depressed patients by NIBS would hypothetically increase peripheral TRP levels if symptoms and these biomarkers are associated—however, our included studies using rTMS mostly found null effects on TRP levels [59,60,69], with one finding significant elevated levels in plasma [68], and meta-analysis showing moderate evidence for no effects of ECT [35,39,43,48,52,53,56,58], despite the antidepressant efficacy of these techniques. This is an interesting line of research, and more direct experiments testing whether there is an association between ANS functioning and TRP availability are needed. The effects on TRP availability are critical, as this is the main source of substrate for the 5-HT and KYN pathways, critical for neuroplasticity [15], and for immune response mediation [1,2,7].
Despite the ubiquitous distribution and functional roles of 5-HT, most of its TRP substrate that enters the CNS is degraded toward the kynurenines (Figure 1). Various neuroactive metabolites are produced by this pathway, such as KA and QA, as they are not permeable across the blood–brain barrier—although, peripheral KYN may also be a major source for KA and QA in the CNS, as KYN has been observed to be highly permeable to the blood-brain barrier [130]. In the CNS, KA and QA have various roles, including in immune response, as both act on microglia. These have further effects on neuroplasticity, as KA acts as a receptor antagonist on neuronal N-methyl-D-aspartate (NMDA) whereas QA acts as an agonist, and with further mechanisms of each altering glutamate levels differentially. The effects of KA are typically considered neuroprotective, whereas QA effects are neurotoxic [7]. In support of these effects, low levels of the KA/QA ratio, indicating more neurotoxic and less neuroprotective products from the kynurenine pathway, were observed to coincide with more severe anhedonia symptoms and smaller cortical thickness of the right dorsal anterior cingulate cortex (ACC) in untreated depressed patients compared to controls [131]. In patients with bipolar disorder, KA/QA ratio has also been observed to be significantly lower than healthy controls [132]. More recently, a meta-analysis sought to identify which biomarkers of kynurenine pathway activity differentiate psychiatric disorders, finding KA/QA being significantly lower in MDD and bipolar disorder patients compared to healthy controls [9]. However, despite ECT having significant antidepressant effects (in uni- and bipolar patients) [17], two included studies reported null effects of ECT on QA/KA, an inversed form [33,52], but a third reported significant reductions of the QA/KA ratio [54]—suggesting less neurotoxic QA and more neuroprotective KA, and no studies investigating these ratios after tDCS nor rTMS (Figure 4). Consistent with these effects, our meta-analysis indicated no effects on plasma KA levels and KA/KYN ratio following ECT [35,52]. Moreover, two studies using rTMS found null effects on KA levels in plasma and CSF despite antidepressant effects [68,69]. This reinforces the possibility that the antidepressant effects of NIBS may not be associated with changes to the production of these neuroactive kynurenines but may instead target downstream molecular targets of TRP metabolism.
NIBS has been observed to potentiate signaling strength of TRP metabolites. For instance, in a PET study, HF-rTMS had significant antidepressant effects, with changes to symptom severity positively correlated with change in 5-HT2A receptor binding in bilateral DLPFC, but negatively correlated with right hippocampus receptor binding [133]. This receptor is thought to mediate expression of brain derived neurotrophic factor (BDNF), with 5-HT2A agonists decreasing BDNF mRNA expression in the hippocampus, but increasing expression in the rat neocortex [134]. In another PET study on healthy participants, the effects of HF-rTMS over the left DLPFC on limbic serotonin synthesis were assessed with the radioligand [(11)C]-alpha-methyl-tryptophan, thought to approximate the capacity of TRP metabolism toward the 5-HT pathway. Brain stimulation over the left DLPFC, compared to over the left occipital cortex, was followed by increases in 5-HT release in the right posterior cingulate cortex, but not cortical or sub-cortical regions, with the metabolite presumed to be from terminals of dorsal raphe nuclei [67]. Baldinger et al. [135] reviewed the literature on the effects of ECT on 5-HT neurotransmission, reporting mixed effects on 5-HT1A, including no effects [136] and diminished 5-HT1A receptor binding in the right subgenual ACC, orbitofrontal cortex, amygdala, and hippocampus [137]. In a separate PET study, 5-HT2A receptor binding was also found to be globally diminished, including in the bilateral occipital cortex, the medial parietal cortex, the limbic cortex, and the bilateral prefrontal cortex of depressed patients after ECT [138]. Thus, while the effects of NIBS on 5-HT and other biomarkers of TRP metabolism may not be directly observable, as indicated by our findings, modulations of the molecular targets of these metabolites may instead underlie the therapeutic effects of NIBS.
We observed several limitations with our review methods. First, biomarker keywords were not abbreviated in our search, nor were all names for the same metabolite used. Potentially, this may have caused some studies to have been missed. We attempted to mitigate this by screening the references of reviews and included studies. Second, some metabolites that may be relevant were excluded (such as 6-sulfatoxymelatonin, a product of melatonin metabolism—e.g., [105] was excluded; Table S2). We initially aimed to keep this review focused on TRP metabolites commonly discussed in light of their vital physiological roles, such as 5-HT, melatonin, kynurenine, and NAD+ [7]. Likewise for NIBS, not all techniques were considered, such as transcranial electric stimulation, though several more exist beyond those reviewed here [22]. We sought studies using ECT, rTMS, and tDCS as these are frequently discussed in reviews on the therapeutic efficacy of NIBS and mechanisms of action, e.g., [17,139]. Lastly, our univariate focus on biomarker levels may not be sufficient to elucidate the mechanisms of action by NIBS and the relation between TRP metabolism and health condition—this aim will require future studies to utilize cross-domain expertise, including biomarker effects. That is, research on the dynamics within and between multiple levels of analysis are needed to develop etiologies of neuropsychiatric disorders that capture real-world variability of patients [140] and to elucidate the dynamics between adaptive biological architecture and metabolic homeostasis. For instance, whether change in brain activity and noise following successful therapeutic NIBS coincides to changes in biomarker levels is an important research question to be investigated with careful signal analysis approaches, such as [141,142,143].
5. Conclusions
This scoping review investigated the effects of non-invasive brain stimulation on biomarkers of tryptophan metabolism and synthesized relevant studies using qualitative and meta-analyses. Although the amount of evidence for each biomarker in clinical and preclinical studies is sparse, we were able to conduct meta-analyses, although studies eligible for this synthesis were few. In agreement with Bayesian meta-analysis results, qualitative analysis revealed highly heterogenous methods and findings in this literature. Thus, more randomized controlled studies are needed to elucidate the effects of therapeutic non-invasive brain stimulation on tryptophan metabolism and the correspondence between biomarkers and abnormalities observed in several neuropsychiatric disorders. Such findings would inform optimal use of brain stimulation, in concurrence with other therapies. Specifically, as the nervous system functions by electrochemical principles, the optimal application or synergism of electrical (e.g., non-invasive and invasive brain stimulation) and chemical (e.g., antidepressant and anti-inflammatory treatments) therapies need further investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23179692/s1.
Author Contributions
Conceptualization, C.G.G. and G.S.K.; methodology, C.G.G. and T.T.Z.L.; software, C.G.G. and T.T.Z.L.; validation, C.G.G., T.T.Z.L. and G.S.K.; formal analysis, T.T.Z.L.; investigation, C.G.G., T.T.Z.L. and G.S.K.; resources, G.S.K.; data curation, C.G.G. and T.T.Z.L.; writing—original draft preparation and visualization, C.G.G. and T.T.Z.L.; writing—review and editing, C.G.G., T.T.Z.L., R.L.D.K., B.B.B.Z., S.Y.Y. and G.S.K.; supervision, G.S.K.; project administration, C.G.G. and T.T.Z.L.; funding acquisition, G.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
The work described in this paper was partly supported by the Mental Health Research Center (MHRC), The Hong Kong Polytechnic University, and by grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (PolyU15100120 and PolyU25100219).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Available data reviewed in this article is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Melhem, N.J.; Taleb, S. Tryptophan: From Diet to Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 9904. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef] [PubMed]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.-P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef] [PubMed]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158–160. [Google Scholar] [CrossRef]
- Kanova, M.; Kohout, P. Tryptophan: A Unique Role in the Critically III. Int. J. Mol. Sci. 2021, 22, 11714. [Google Scholar] [CrossRef]
- James, G.M.; Gryglewski, G.; Vanicek, T.; Berroterán-Infante, N.; Philippe, C.; Kautzky, A.; Nics, L.; Vraka, C.; Godbersen, G.M.; Unterholzner, J.; et al. Parcellation of the Human Cerebral Cortex Based on Molecular Targets in the Serotonin System Quantified by Positron Emission Tomography In vivo. Cereb. Cortex 2019, 29, 372–382. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Solmi, M.; Sanches, M.; Machado, M.O.; Stubbs, B.; Ajnakina, O.; Sherman, C.; Sun, Y.R.; Liu, C.S.; Brunoni, A.R.; et al. Evidence-based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl. Psychiatry 2020, 10, 152. [Google Scholar] [CrossRef]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 16–25. [Google Scholar] [CrossRef]
- Kappelmann, N.; Lewis, G.; Dantzer, R.; Jones, P.B.; Khandaker, G.M. Antidepressant activity of anti-cytokine treatment: A systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 2018, 23, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Köhler-Forsberg, O.; Benros, M.E.; Nordentoft, M.; Farkouh, M.E.; Iyengar, R.L.; Mors, O.; Krogh, J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2014, 71, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Erabi, H.; Okada, G.; Shibasaki, C.; Setoyama, D.; Kang, D.; Takamura, M.; Yoshino, A.; Fuchikami, M.; Kurata, A.; Kato, T.A.; et al. Kynurenic acid is a potential overlapped biomarker between diagnosis and treatment response for depression from metabolome analysis. Sci. Rep. 2020, 10, 16822. [Google Scholar] [CrossRef]
- Haroon, E.; Welle, J.R.; Woolwine, B.J.; Goldsmith, D.R.; Baer, W.; Patel, T.; Felger, J.C.; Miller, A.H. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology 2020, 45, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Kraus, C.; Castrén, E.; Kasper, S.; Lanzenberger, R. Serotonin and neuroplasticity—Links between molecular, functional and structural pathophysiology in depression. Neurosci. Biobehav. Rev. 2017, 77, 317–326. [Google Scholar] [CrossRef]
- Westfall, S.; Caracci, F.; Estill, M.; Frolinger, T.; Shen, L.; Pasinetti, G.M. Chronic Stress-Induced Depression and Anxiety Priming Modulated by Gut-Brain-Axis Immunity. Front. Immunol. 2021, 12, 670500. [Google Scholar] [CrossRef]
- Rosson, S.; de Filippis, R.; Croatto, G.; Collantoni, E.; Pallottino, S.; Guinart, D.; Brunoni, A.R.; Dell’Osso, B.; Pigato, G.; Hyde, J.; et al. Brain stimulation and other biological non-pharmacological interventions in mental disorders: An umbrella review. Neurosci. Biobehav. Rev. 2022, 139, 104743. [Google Scholar] [CrossRef]
- Hyde, J.; Carr, H.; Kelley, N.; Seneviratne, R.; Reed, C.; Parlatini, V.; Garner, M.; Solmi, M.; Rosson, S.; Cortese, S.; et al. Efficacy of neurostimulation across mental disorders: Systematic review and meta-analysis of 208 randomized controlled trials. Mol. Psychiatry 2022, 27, 2709–2719. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Kan, R.L.D.; Zhang, B.B.B.; Zhang, J.J.Q.; Kranz, G.S. Non-invasive brain stimulation for posttraumatic stress disorder: A systematic review and meta-analysis. Transl. Psychiatry 2020, 10, 168. [Google Scholar] [CrossRef]
- Kellner, C.H.; Greenberg, R.M.; Petrides, G.; Ahle, G.M.; Adams, D.A.; Liebman, L.S. Electroconvulsive Therapy Is a Noninvasive Brain Stimulation Technique. J. ECT 2016, 32, 70. [Google Scholar] [CrossRef] [PubMed]
- Padberg, F.; Bulubas, L.; Mizutani-Tiebel, Y.; Burkhardt, G.; Kranz, G.S.; Koutsouleris, N.; Kambeitz, J.; Hasan, A.; Takahashi, S.; Keeser, D.; et al. The intervention, the patient and the illness—Personalizing non-invasive brain stimulation in psychiatry. Exp. Neurol. 2021, 341, 113713. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, H.; Mizoguchi, Y.; Monji, A. Is the Therapeutic Mechanism of Repetitive Transcranial Magnetic Stimulation in Cognitive Dysfunctions of Depression Related to the Neuroinflammatory Processes in Depression? Front. Psychiatry 2022, 13, 834425. [Google Scholar] [CrossRef] [PubMed]
- Baldinger-Melich, P.; Gryglewski, G.; Philippe, C.; James, G.M.; Vraka, C.; Silberbauer, L.; Balber, T.; Vanicek, T.; Pichler, V.; Unterholzner, J.; et al. The effect of electroconvulsive therapy on cerebral monoamine oxidase A expression in treatment-resistant depression investigated using positron emission tomography. Brain Stimul. 2019, 12, 714–723. [Google Scholar] [CrossRef]
- Yrondi, A.; Sporer, M.; Péran, P.; Schmitt, L.; Arbus, C.; Sauvaget, A. Electroconvulsive therapy, depression, the immune system and inflammation: A systematic review. Brain Stimul. 2018, 11, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Maier, M.; Bartoš, F.; Wagenmakers, E.-J. Robust Bayesian meta-analysis: Addressing publication bias with model-averaging. Psychol. Methods 2022. [Google Scholar] [CrossRef]
- Lee, M.D.; Wagenmakers, E.-J. Bayesian Cognitive Modeling; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Rosenthal, R. Meta-Analytic Procedures for Social Research; SAGE Publications, Inc.: New York, NY, USA, 1991. [Google Scholar]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 143–176. [Google Scholar]
- McGrath, S.; Zhao, X.; Steele, R.; Thombs, B.D.; Benedetti, A.; Levis, B.; Riehm, K.E.; Saadat, N.; Levis, A.W.; Azar, M.; et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat. Methods Med. Res. 2020. [Google Scholar] [CrossRef]
- Aarsland, T.I.; Leskauskaite, I.; Midttun, O.; Ulvik, A.; Ueland, P.M.; Oltedal, L.; Erchinger, V.J.; Oedegaard, K.J.; Haavik, J.; Kessler, U. The effect of electroconvulsive therapy (ECT) on serum tryptophan metabolites. Brain Stimul. 2019, 12, 1135–1142. [Google Scholar] [CrossRef]
- Aberg-Wistedt, A.; Mårtensson, B.; Bertilsson, L.; Malmgren, R. Electroconvulsive Therapy Effects on Cerebrospinal Fluid Monoamine Metabolites and Platelet Serotonin Uptake In Melancholia. Convuls. Ther. 1986, 2, 91–98. [Google Scholar] [PubMed]
- Allen, A.P.; Naughton, M.; Dowling, J.; Walsh, A.; O’Shea, R.; Shorten, G.; Scott, L.; McLoughlin, D.M.; Cryan, J.F.; Clarke, G.; et al. Kynurenine pathway metabolism and the neurobiology of treatment-resistant depression: Comparison of multiple ketamine infusions and electroconvulsive therapy. J. Psychiatr. Res. 2018, 100, 24–32. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, G.; Lehmann, J.; Raotma, H. Evaluation of the combination of tryptophan and ECT in the treatment of depression. Acta Psychiatr. Scand. 1977, 56, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Guloksuz, S.; Arts, B.; Walter, S.; Drukker, M.; Rodriguez, L.; Myint, A.M.; Schwarz, M.J.; Ponds, R.; van Os, J.; Kenis, G.; et al. The impact of electroconvulsive therapy on the tryptophan-kynurenine metabolic pathway. Brain Behav. Immun. 2015, 48, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Hasani, P.A.M.; Moghadam, F.M.; Mokhtaree, M.; Nazer, M. Effect of Electroconvulsive Therapy on Serum Serotonin Level in Patients with Treatment- Resistant Major Depressive Disorder. J. Evol. Med. Dent. Sci. 2019, 8, 1283–1286. [Google Scholar] [CrossRef]
- Hoekstra, R.; van den Broek, W.W.; Fekkes, D.; Bruijn, J.A.; Mulder, P.G.; Pepplinkhuizen, L. Effect of electroconvulsive therapy on biopterin and large neutral amino acids in severe, medication-resistant depression. Psychiatry Res. 2001, 103, 115–123. [Google Scholar] [CrossRef]
- Hoffmann, G.; Linkowski, P.; Kerkhofs, M.; Desmedt, D.; Mendlewicz, J. Effects of ECT on sleep and CSF biogenic amines in affective illness. Psychiatry Res. 1985, 16, 199–206. [Google Scholar] [CrossRef]
- Hofmann, P.; Loimer, N.; Chaudhry, H.R.; Pfersmann, D.; Schmid, R.; Wieselmann, G. 5-Hydroxy-indolacetic-acid (5-HIAA) serum levels in depressive patients and ECT. J. Psychiatr. Res. 1996, 30, 209–216. [Google Scholar] [CrossRef]
- Jori, A.; Dolfini, E.; Casati, C.; Argenta, G. Effect of ECT and imipramine treatment on the concentration of 5-hydroxyindoleacetic acid (5HIAA) and homovanillic acid (HVA) in the cerebrospinal fluid of depressed patients. Psychopharmacologia 1975, 44, 87–90. [Google Scholar] [CrossRef]
- Kirkegaard, C.; Mosller, S.E.; Bjosrum, N. Addition of L-tryptophan to electroconvulsive treatment in endogenous depression. A double-blind study. Acta Psychiatr. Scand. 1978, 58, 457–462. [Google Scholar] [CrossRef]
- Lestra, C.; d’Amato, T.; Ghaemmaghami, C.; Perret-Liaudet, A.; Broyer, M.; Renaud, B.; Dalery, J.; Chamba, G. Biological parameters in major depression: Effects of paroxetine, viloxazine, moclobemide, and electroconvulsive therapy. Relation to early clinical outcome. Biol. Psychiatry 1998, 44, 274–280. [Google Scholar] [CrossRef]
- Mokhtar, A.S.E.; Morgan, C.J.; Bradley, D.M.; Badawy, A.A.B. No early effects of electroconvulsive therapy on tryptophan metabolism and disposition in endogenous depression. Biol. Psychiatry 1997, 42, 201–205. [Google Scholar] [CrossRef]
- Nikisch, G.; Mathé, A.A. CSF monoamine metabolites and neuropeptides in depressed patients before and after electroconvulsive therapy. Eur. Psychiatry 2008, 23, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Olajossy, M.; Olajossy, B.; Wnuk, S.; Potembska, E.; Urbańska, E. Blood serum concentrations of kynurenic acid in patients diagnosed with recurrent depressive disorder, depression in bipolar disorder, and schizoaffective disorder treated with electroconvulsive therapy. Psychiatr. Pol. 2017, 51, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Palmio, J.; Huuhka, M.; Saransaari, P.; Oja, S.S.; Peltola, J.; Leinonen, E.; Suhonen, J.; Keränen, T. Changes in plasma amino acids after electroconvulsive therapy of depressed patients. Psychiatry Res. 2005, 137, 183–190. [Google Scholar] [CrossRef]
- Rudorfer, M.V.; Risby, E.D.; Hsiao, J.K.; Linnoila, M.; Potter, W.Z. Disparate Biochemical Actions of Electroconvulsive Therapy and Antidepressant Drugs. Convuls. Ther. 1988, 4, 133–140. [Google Scholar]
- Rudorfer, M.V.; Risby, E.D.; Osman, O.T.; Gold, P.W.; Potter, W.Z. Hypothalamic-pituitary-adrenal axis and monoamine transmitter activity in depression: A pilot study of central and peripheral effects of electroconvulsive therapy. Biol. Psychiatry 1991, 29, 253–264. [Google Scholar] [CrossRef]
- Ryan, K.M.; Allers, K.A.; Harkin, A.; McLoughlin, D.M. Blood plasma B vitamins in depression and the therapeutic response to electroconvulsive therapy. Brain Behav. Immun. Health 2020, 4, 100063. [Google Scholar] [CrossRef]
- Ryan, K.M.; Allers, K.A.; McLoughlin, D.M.; Harkin, A. Tryptophan metabolite concentrations in depressed patients before and after electroconvulsive therapy. Brain Behav. Immun. 2020, 83, 153–162. [Google Scholar] [CrossRef]
- Sawa, Y. The effect of electroconvulsive therapy on plasma cyclic-AMP, non-esterified fatty acid, tryptophan and tyrosine in depression. Keio J. Med. 1981, 30, 193–204. [Google Scholar] [CrossRef][Green Version]
- Schwieler, L.; Samuelsson, M.; Frye, M.A.; Bhat, M.; Schuppe-Koistinen, I.; Jungholm, O.; Johansson, A.G.; Landén, M.; Sellgren, C.M.; Erhardt, S. Electroconvulsive therapy suppresses the neurotoxic branch of the kynurenine pathway in treatment-resistant depressed patients. J. Neuroinflamm. 2016, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Strömgren, L.S. Influence of unilateral ECT on tryptophan metabolism in endogenous depression. Pharmacopsychiatria 1981, 14, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Stelmasiak, Z.; Curzon, G. Effect of electroconvulsive therapy on plasma unesterified fatty acid and free tryptophan concentrations in man. J. Neurochem. 1974, 22, 603–604. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, M.A.; Subrahmanyam, T.S.; Ramanamurthy, P.S.V.; Haranath, P.S. Influence of single ECT on dopamine, noradrenaline and 5-hydroxytryptamine concentrations in CSF of schizophenics. Indian J. Med. Res. 1981, 74, 757–762. [Google Scholar] [PubMed]
- Whalley, L.J.; Yates, C.M.; Christie, J.E. Effect of electroconvulsive therapy (ECT) on plasma tryptophan. Psychol. Med. 1980, 10, 377–380. [Google Scholar] [CrossRef]
- Leblhuber, F.; Geisler, S.; Ehrlich, D.; Steiner, K.; Reibnegger, G.; Fuchs, D.; Kurz, K. Repetitive transcranial magnetic stimulation in the treatment of resistant depression: Changes of specific neurotransmitter precursor amino acids. J. Neural Transm. 2021, 128, 1225–1231. [Google Scholar] [CrossRef]
- Leblhuber, F.; Steiner, K.; Fuchs, D. Treatment of patients with geriatric depression with repetitive transcranial magnetic stimulation. J. Neural Transm. 2019, 126, 1105–1110. [Google Scholar] [CrossRef]
- Leblhuber, F.; Steiner, K.; Gostner, J.; Fuchs, D. Repetitive transcranial magnetic stimulation in patients with late life depression influences phenylalanine metabolism. Pteridines 2018, 29, 87–90. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Yu, R.; Sun, Y. Effect of transcranial magnetic stimulation on treatment effect and immune function. Saudi J. Biol. Sci. 2022, 29, 379–384. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, C.; Liu, Y.; Wang, L.; Chen, X.; Zhou, X. The effect of bilateral low-frequency rTMS over dorsolateral prefrontal cortex on serum brain-derived neurotropic factor and serotonin in patients with generalized anxiety disorder. Neurosci. Lett. 2018, 684, 67–71. [Google Scholar] [CrossRef]
- Maestú, C.; Blanco, M.; Nevado, A.; Romero, J.; Rodriguez-Rubio, P.; Galindo, J.; Bautista Lorite, J.; de las Morenas, F.; Fernández-Argüelles, P. Reduction of pain thresholds in fibromyalgia after very low-intensity magnetic stimulation: A double-blinded, randomized placebo-controlled clinical trial. Pain Res. Manag. 2013, 18, e101–e106. [Google Scholar] [CrossRef] [PubMed]
- Miniussi, C.; Bonato, C.; Bignotti, S.; Gazzoli, A.; Gennarelli, M.; Pasqualetti, P.; Tura, G.B.; Ventriglia, M.; Rossini, P.M. Repetitive transcranial magnetic stimulation (rTMS) at high and low frequency: An efficacious therapy for major drug-resistant depression? Clin. Neurophysiol. 2005, 116, 1062–1071. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niimi, M.; Ishima, T.; Hashimoto, K.; Hara, T.; Yamada, N.; Abo, M. Effect of repetitive transcranial magnetic stimulation on the kynurenine pathway in stroke patients. Neuroreport 2020, 31, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Sibon, I.; Strafella, A.P.; Gravel, P.; Ko, J.H.; Booij, L.; Soucy, J.P.; Leyton, M.; Diksic, M.; Benkelfat, C. Acute prefrontal cortex TMS in healthy volunteers: Effects on brain 11C-alphaMtrp trapping. Neuroimage 2007, 34, 1658–1664. [Google Scholar] [CrossRef]
- Tateishi, H.; Setoyama, D.; Kang, D.; Matsushima, J.; Kojima, R.; Fujii, Y.; Mawatari, S.; Kikuchi, J.; Sakemura, Y.; Fukuchi, J.; et al. The changes in kynurenine metabolites induced by rTMS in treatment-resistant depression: A pilot study. J. Psychiatr. Res. 2021, 138, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, H.; Setoyama, D.; Kato, T.A.; Kang, D.; Matsushima, J.; Nogami, K.; Mawatari, S.; Kojima, R.; Fujii, Y.; Sakemura, Y.; et al. Changes in the metabolites of cerebrospinal fluid induced by rTMS in treatment-resistant depression: A pilot study. Psychiatry Res. 2022, 313, 114636. [Google Scholar] [CrossRef] [PubMed]
- Hadoush, H.; Alqudah, A.; Banihani, S.A.; Al-Jarrah, M.; Amro, A.; Aldajah, S. Melatonin serum level, sleep functions, and depression level after bilateral anodal transcranial direct current stimulation in patients with Parkinson’s disease: A feasibility study. Sleep Sci. 2021, 14, 25–30. [Google Scholar] [CrossRef]
- Evans, J.P.; Grahame-Smith, D.G.; Green, A.R.; Tordoff, A.F. Electroconvulsive shock increases the behavioural responses of rats to brain 5-hydroxytryptamine accumulation and central nervous system stimulant drugs. Br. J. Pharmacol. 1976, 56, 193–199. [Google Scholar] [CrossRef]
- Gur, E.; Dremencov, E.; Garcia, F.; Van de Kar, L.D.; Lerer, B.; Newman, M.E. Functional effects of chronic electroconvulsive shock on serotonergic 5-HT(1A) and 5-HT(1B) receptor activity in rat hippocampus and hypothalamus. Brain Res. 2002, 952, 52–60. [Google Scholar] [CrossRef]
- Juckel, G.; Mendlin, A.; Jacobs, B.L. Electrical stimulation of rat medial prefrontal cortex enhances forebrain serotonin output: Implications for electroconvulsive therapy and transcranial magnetic stimulation in depression. Neuropsychopharmacology 1999, 21, 391–398. [Google Scholar] [CrossRef]
- Karoum, F.; Korpi, E.R.; Chuang, L.W.; Linnoila, M.; Wyatt, R.J. The effects of desipramine, zimelidine, electroconvulsive treatment and lithium on rat brain biogenic amines: A comparison with peripheral changes. Eur. J. Pharmacol. 1986, 121, 377–385. [Google Scholar] [CrossRef]
- Khanna, N.K.; Lauria, P.; Sharma, V.N. 5-hydroxytryptamine content of the dog myocardium after chronic electroconvulsive therapy. Indian J. Physiol. Pharmacol. 1971, 15, 187–188. [Google Scholar] [PubMed]
- Madhav, T.R.; Pei, Q.; Grahame-Smith, D.G.; Zetterström, T.S. Repeated electroconvulsive shock promotes the sprouting of serotonergic axons in the lesioned rat hippocampus. Neuroscience 2000, 97, 677–683. [Google Scholar] [CrossRef]
- McIntyre, I.M.; Oxenkrug, G.F. Electroconvulsive shock: Effect on pineal and hypothalamic indoles. J. Pineal Res. 1984, 1, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Shields, P.J. Effects of electroconvulsive shock on the metabolism of 5-hydroxytryptamine in the rat brain. J. Pharm. Pharmacol. 1972, 24, 919–920. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, M.F. Some effects of chronic antidepressant treatments on rat brain monoaminergic systems. J. Neural Transm. 1983, 57, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Tagliamonte, A.; Tagliamonte, P.; Di Chiara, G.; Gessa, R.; Gessa, G.L. Increase of brain tryptophan by electroconvulsive shock in rats. J. Neurochem. 1972, 19, 1509–1512. [Google Scholar] [CrossRef]
- Yoshida, K.; Higuchi, H.; Kamata, M.; Yoshimoto, M.; Shimizu, T.; Hishikawa, Y. Dopamine releasing response in rat striatum to single and repeated electroconvulsive shock treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 1997, 21, 707–715. [Google Scholar] [CrossRef]
- Yoshida, K.; Higuchi, H.; Kamata, M.; Yoshimoto, M.; Shimizu, T.; Hishikawa, Y. Single and repeated electroconvulsive shocks activate dopaminergic and 5-hydroxytryptaminergic neurotransmission in the frontal cortex of rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 1998, 22, 435–444. [Google Scholar] [CrossRef]
- Ben-Shachar, D.; Belmaker, R.H.; Grisaru, N.; Klein, E. Transcranial magnetic stimulation induces alterations in brain monoamines. J. Neural Transm. 1997, 104, 191–197. [Google Scholar] [CrossRef]
- Ben-Shachar, D.; Gazawi, H.; Riboyad-Levin, J.; Klein, E. Chronic repetitive transcranial magnetic stimulation alters beta-adrenergic and 5-HT2 receptor characteristics in rat brain. Brain Res. 1999, 816, 78–83. [Google Scholar] [CrossRef]
- El Arfani, A.; Parthoens, J.; Demuyser, T.; Servaes, S.; De Coninck, M.; De Deyn, P.P.; Van Dam, D.; Wyckhuys, T.; Baeken, C.; Smolders, I.; et al. Accelerated high-frequency repetitive transcranial magnetic stimulation enhances motor activity in rats. Neuroscience 2017, 347, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Gur, E.; Lerer, B.; Dremencov, E.; Newman, M.E. Chronic repetitive transcranial magnetic stimulation induces subsensitivity of presynaptic serotonergic autoreceptor activity in rat brain. Neuroreport 2000, 11, 2925–2929. [Google Scholar] [CrossRef]
- Heath, A.; Lindberg, D.R.; Makowiecki, K.; Gray, A.; Asp, A.J.; Rodger, J.; Choi, D.S.; Croarkin, P.E. Medium- and high-intensity rTMS reduces psychomotor agitation with distinct neurobiologic mechanisms. Transl. Psychiatry 2018, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Kanno, M.; Matsumoto, M.; Togashi, H.; Yoshioka, M.; Mano, Y. Effects of repetitive transcranial magnetic stimulation on behavioral and neurochemical changes in rats during an elevated plus-maze test. J. Neurol. Sci. 2003, 211, 5–14. [Google Scholar] [CrossRef]
- Kanno, M.; Matsumoto, M.; Togashi, H.; Yoshioka, M.; Mano, Y. Effects of acute repetitive transcranial magnetic stimulation on extracellular serotonin concentration in the rat prefrontal cortex. J. Pharmacol. Sci. 2003, 93, 451–457. [Google Scholar] [CrossRef][Green Version]
- Kanno, M.; Matsumoto, M.; Togashi, H.; Yoshioka, M.; Mano, Y. Effects of acute repetitive transcranial magnetic stimulation on dopamine release in the rat dorsolateral striatum. J. Neurol. Sci. 2004, 217, 73–81. [Google Scholar] [CrossRef]
- Keck, M.E.; Sillaber, I.; Ebner, K.; Welt, T.; Toschi, N.; Kaehler, S.T.; Singewald, N.; Philippu, A.; Elbel, G.K.; Wotjak, C.T.; et al. Acute transcranial magnetic stimulation of frontal brain regions selectively modulates the release of vasopressin, biogenic amines and amino acids in the rat brain. Eur. J. Neurosci. 2000, 12, 3713–3720. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.; Yu, S.L.; Jee, S.; Cheon, K.A.; Song, D.H.; Kim, S.J.; Im, W.Y.; Kang, J. Effects of high-frequency repetitive transcranial magnetic stimulation (rTMS) on spontaneously hypertensive rats, an animal model of attention-deficit/hyperactivity disorder. Int. J. Dev. Neurosci. 2016, 53, 83–89. [Google Scholar] [CrossRef]
- Löffler, S.; Gasca, F.; Richter, L.; Leipscher, U.; Trillenberg, P.; Moser, A. The effect of repetitive transcranial magnetic stimulation on monoamine outflow in the nucleus accumbens shell in freely moving rats. Neuropharmacology 2012, 63, 898–904. [Google Scholar] [CrossRef]
- Peng, Z.W.; Xue, F.; Zhou, C.H.; Zhang, R.G.; Wang, Y.; Liu, L.; Sang, H.F.; Wang, H.N.; Tan, Q.R. Repetitive transcranial magnetic stimulation inhibits Sirt1/MAO-A signaling in the prefrontal cortex in a rat model of depression and cortex-derived astrocytes. Mol. Cell. Biochem. 2018, 442, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Poh, E.Z.; Hahne, D.; Moretti, J.; Harvey, A.R.; Clarke, M.W.; Rodger, J. Simultaneous quantification of dopamine, serotonin, their metabolites and amino acids by LC-MS/MS in mouse brain following repetitive transcranial magnetic stimulation. Neurochem. Int. 2019, 131, 104546. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, P.; Yin, R.; Xiao, M.; Zhang, Y.; Reinhardt, J.D.; Wang, H.; Xu, G. Combination of repetitive transcranial magnetic stimulation and treadmill training reduces hyperreflexia by rebalancing motoneuron excitability in rats after spinal cord contusion. Neurosci. Lett. 2022, 775, 136536. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takano, Y.; Tanaka, S.; Hironaka, N.; Kobayashi, K.; Hanakawa, T.; Watanabe, K.; Honda, M. Transcranial direct-current stimulation increases extracellular dopamine levels in the rat striatum. Front. Syst. Neurosci. 2013, 7, 6. [Google Scholar] [CrossRef]
- Cassidy, F.; Murry, E.; Weiner, R.D.; Carroll, B.J. Lack of relapse with tryptophan depletion following successful treatment with ECT. Am. J. Psychiatry 1997, 154, 1151–1152. [Google Scholar] [CrossRef]
- Cassidy, F.; Weiner, R.D.; Cooper, T.B.; Carroll, B.J. Combined catecholamine and indoleamine depletion following response to ECT. Br. J. Psychiatry 2010, 196, 493–494. [Google Scholar] [CrossRef][Green Version]
- Chang, T.G.; Wang, C.H.; Chiu, N.Y.; Hsu, W.Y. Application of electroconvulsive therapy in treatment of retinitis pigmentosa comorbid with major depressive disorder and panic disorder. J. ECT 2011, 27, e57–e58. [Google Scholar] [CrossRef]
- Costain, D.W.; Grahame-Smith, D.G.; Green, A.R. Relevance of the enhanced 5-hydroxytryptamine behavioural responses in rats to electroconvulsive therapy [proceedings]. Br. J. Pharmacol. 1978, 62, 394P. [Google Scholar]
- D’Elia, G.; Lehmann, J.; Raotma, H. Influence of tryptophan on memory functions in depressive patients treated with unilateral ECT. Acta Psychiatr. Scand. 1978, 57, 259–268. [Google Scholar] [CrossRef]
- Green, A.R. Repeated exposure of rats to the convulsant agent flurothyl enhances 5-hydroxytryptamine- and dopamine-mediated behavioural responses. Br. J. Pharmacol. 1978, 62, 325–331. [Google Scholar] [CrossRef]
- Ikeda, T.; Kurosawa, M.; Uchikawa, C.; Kitayama, S.; Nukina, N. Modulation of monoamine transporter expression and function by repetitive transcranial magnetic stimulation. Biochem. Biophys. Res. Commun. 2005, 327, 218–224. [Google Scholar] [CrossRef]
- Krahn, L.E.; Gleber, E.; Rummans, T.A.; Pileggi, T.S.; Lucas, D.L.; Li, H. The effects of electroconvulsive therapy on melatonin. J. ECT 2000, 16, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Kranaster, L.; Hoyer, C.; Mindt, S.; Neumaier, M.; Müller, N.; Zill, P.; Schwarz, M.J.; Moll, N.; Lutz, B.; Bindila, L.; et al. The novel seizure quality index for the antidepressant outcome prediction in electroconvulsive therapy: Association with biomarkers in the cerebrospinal fluid. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiong, D.; Pang, R.; Deng, Q.; Sun, N.; Zheng, J.; Liu, J.; Xiang, W.; Chen, Z.; Lu, J.; et al. Effects of repetitive magnetic stimulation on motor function and GAP43 and 5-HT expression in rats with spinal cord injury. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef] [PubMed]
- Nordin, G.; Ottosson, J.O.; Roos, B.E. Influence of convulsive therapy on 5-hydroxyindoleacetic acid and homovanillic acid in cerebrospinal fluid in endogenous depression. Psychopharmacologia 1971, 20, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Olajossy, M.; Olajossy, B.; Potembska, E.; Skoczen, N.; Wnuk, S.; Urbanska, E. Differences in the dynamics of changes in the concentration of kynurenic acid in the blood serum of depressed patients treated with electroconvulsive therapy. Psychiatr. Danub. 2018, 30, 331–339. [Google Scholar] [CrossRef]
- Papakostas, Y.G.; Markianos, M.; Zervas, I.M.; Theodoropoulou, M.; Vaidakis, N.; Daras, M. Administration of Citalopram Before ECT: Seizure Duration and Hormone Responses. J. ECT 2000, 16, 356–360. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, Y.; Yu, N.W.; Liao, X.L.; Shi, L. The Clinical Efficacy and Possible Mechanism of Combination Treatment of Cerebral Ischemic Stroke with Ginkgo Biloba Extract and Low-Frequency Repetitive Transcranial Magnetic Stimulation. Sichuan Da Xue Xue Bao Yi Xue Ban 2021, 52, 883–889. [Google Scholar] [CrossRef]
- Potter, W.Z.; Rudorfer, M.V.; Pickar, D.; Linnoila, M. Effects of psychotropic drugs on neurotransmitters in man. Life Sci. 1987, 41, 817–820. [Google Scholar] [CrossRef]
- Price, W.A.; Zimmer, B. Effects of L-Tryptophan on Electroconvulsive Therapy Seizure Time. J. Nerv. Ment. Dis. 1985, 175, 636–638. [Google Scholar] [CrossRef]
- Rausch, J.L.; Rich, C.L.; Risch, S.C. Platelet serotonin transport after a single ECT. Psychopharmacology 1988, 95, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Shiiba, S.J.; Yamamoto, S.; Sasaki, H.; Nishi, M.; Ishikawa, K.; Yasuda, S.; Tokuda, N.; Nakanishi, O.; Ishikawa, T. Cutaneous magnetic stimulation reduces rat chronic pain via activation of the supra-spinal descending pathway. Cell. Mol. Neurobiol. 2012, 32, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wägner, A.; Aberg-Wistedt, A.; Åsberg, M.; Bertilsson, L.; Mårtensson, B.; Montero, D. Effects of antidepressant treatments on platelet tritiated imipramine binding in major depressive disorder. Arch. Gen. Psychiatry 1987, 44, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Abrams, R.; Essman, W.B.; Taylor, M.A.; Fink, M. Concentration of 5-hydroxyindoleacetic acid, homovanillic acid, and tryptophan in the cerebrospinal fluid of depressed patients before and after ECT. Biol. Psychiatry 1976, 11, 85–90. [Google Scholar] [PubMed]
- Belmaker, R.H.; Grisaru, N. Magnetic stimulation of the brain in animal depression models responsive to ECS. J. ECT 1998, 14, 194–205. [Google Scholar] [CrossRef]
- Mano, Y.; Funakawa, I.; Nakamuro, T.; Takayanagi, T.; Matsui, K. The kinesiological, chemical and pathological analysis in pulsed magnetic stimulation to the brain. Rinsho Shinkeigaku 1989, 29, 982–988. [Google Scholar]
- Molnár, L.; Degrell, I.; Rochlitz, S. Effect of bilateral and unilateral electroconvulsive therapy (ECT) on the composition of the cerebrospinal fluid (CSF). A possibility to calculate the intracellular redox changes of the brain in humans (author’s transl). Arch. Psychiatr. Nervenkr. 1979, 227, 159–169. [Google Scholar] [CrossRef]
- Mohamad Safiai, N.I.; Amir, N.A.; Basri, H.; Inche Mat, L.N.; Hoo, F.K.; Yusof Khan, A.H.K.; Loh, W.C.; Chia, P.K.; Ramachandran, V.; Mat Din, H.; et al. Effectiveness and tolerability of repetitive transcranial magnetic stimulation for preventive treatment of episodic migraine: A single-centre, randomised, double-blind, sham-controlled phase 2 trial (Magnet-EM). Trials 2020, 21, 923. [Google Scholar] [CrossRef]
- Papakostas, Y.; Markianos, M.; Papadimitriou, G.; Stefanis, C. Thyrotropin and Prolactin Secretion During ECT: Implications for the Mechanism of ECT Action. Convuls. Ther. 1990, 6, 214–220. [Google Scholar]
- Wang, M.; Li, Y.; Wang, X.; Guo, M. Study on the influence of simulative EEG modulation magnetic field on the discharge of median raphe nuclei. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2004, 21, 219–224. [Google Scholar]
- Dalal, P.K.; Lal, N.; Trivedi, J.K.; Seth, P.K.; Agarwal, A.K.; Khalid, A. Ect and platelet 5ht uptake in major depression. Indian J. Psychiatry 1997, 39, 272–277. [Google Scholar] [PubMed]
- D’Elia, G.; Lehmann, J.; Raotma, H. Bimodal distribution of serum trypotphan level. Acta Psychiatr. Scand. 1979, 60, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, H.; Mizoguchi, Y.; Kawaguchi, A.; Imamura, Y.; Matsushima, J.; Kunitake, H.; Murakawa, T.; Haraguchi, Y.; Kunitake, Y.; Maekawa, T.; et al. Changes in interleukin-1 beta induced by rTMS are significantly correlated with partial improvement of cognitive dysfunction in treatment-resistant depression: A pilot study. Psychiatry Res. 2020, 289, 112995. [Google Scholar] [CrossRef] [PubMed]
- Iseger, T.A.; van Bueren, N.E.R.; Kenemans, J.L.; Gevirtz, R.; Arns, M. A frontal-vagal network theory for Major Depressive Disorder: Implications for optimizing neuromodulation techniques. Brain Stimul. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Michael, J.A.; Kaur, M. The Heart-Brain Connection in Depression: Can it inform a personalised approach for repetitive transcranial magnetic stimulation (rTMS) treatment? Neurosci. Biobehav. Rev. 2021, 127, 136–143. [Google Scholar] [CrossRef]
- Iseger, T.A.; Arns, M.; Downar, J.; Blumberger, D.M.; Daskalakis, Z.J.; Vila-Rodriguez, F. Cardiovascular differences between sham and active iTBS related to treatment response in MDD. Brain Stimul. 2020, 13, 167–174. [Google Scholar] [CrossRef]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood-brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef]
- Meier, T.B.; Drevets, W.C.; Wurfel, B.E.; Ford, B.N.; Morris, H.M.; Victor, T.A.; Bodurka, J.; Teague, T.K.; Dantzer, R.; Savitz, J. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav. Immun. 2016, 53, 39–48. [Google Scholar] [CrossRef]
- Savitz, J.; Dantzer, R.; Wurfel, B.E.; Victor, T.A.; Ford, B.N.; Bodurka, J.; Bellgowan, P.S.; Teague, T.K.; Drevets, W.C. Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology 2015, 52, 200–211. [Google Scholar] [CrossRef]
- Baeken, C.; De Raedt, R.; Bossuyt, A.; Van Hove, C.; Mertens, J.; Dobbeleir, A.; Blanckaert, P.; Goethals, I. The impact of HF-rTMS treatment on serotonin(2A) receptors in unipolar melancholic depression. Brain Stimul. 2011, 4, 104–111. [Google Scholar] [CrossRef]
- Vaidya, V.A.; Marek, G.J.; Aghajanian, G.K.; Duman, R.S. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J. Neurosci. 1997, 17, 2785–2795. [Google Scholar] [CrossRef]
- Baldinger, P.; Lotan, A.; Frey, R.; Kasper, S.; Lerer, B.; Lanzenberger, R. Neurotransmitters and electroconvulsive therapy. J. ECT 2014, 30, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Saijo, T.; Takano, A.; Suhara, T.; Arakawa, R.; Okumura, M.; Ichimiya, T.; Ito, H.; Okubo, Y. Effect of electroconvulsive therapy on 5-HT1A receptor binding in patients with depression: A PET study with [11C]WAY 100635. Int. J. Neuropsychopharmacol. 2010, 13, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Lanzenberger, R.; Baldinger, P.; Hahn, A.; Ungersboeck, J.; Mitterhauser, M.; Winkler, D.; Micskei, Z.; Stein, P.; Karanikas, G.; Wadsak, W.; et al. Global decrease of serotonin-1A receptor binding after electroconvulsive therapy in major depression measured by PET. Mol. Psychiatry 2013, 18, 93–100. [Google Scholar] [CrossRef]
- Yatham, L.N.; Liddle, P.F.; Lam, R.W.; Zis, A.P.; Stoessl, A.J.; Sossi, V.; Adam, M.J.; Ruth, T.J. Effect of electroconvulsive therapy on brain 5-HT(2) receptors in major depression. Br. J. Psychiatry 2010, 196, 474–479. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Holtzheimer, P.E.; Gao, S.; Kirwin, D.S.; Price, R.B. Leveraging Neuroplasticity to Enhance Adaptive Learning: The Potential for Synergistic Somatic-Behavioral Treatment Combinations to Improve Clinical Outcomes in Depression. Biol. Psychiatry 2019, 85, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Winter, N.R.; Leenings, R.; Ernsting, J.; Sarink, K.; Fisch, L.; Emden, D.; Blanke, J.; Goltermann, J.; Opel, N.; Barkhau, C.; et al. Quantifying Deviations of Brain Structure and Function in Major Depressive Disorder Across Neuroimaging Modalities. JAMA Psychiatry 2022. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, M.; Buscarino, A.; Fortuna, L.; Gagliano, S. Can Noise in the Feedback Improve the Performance of a Control System? J. Phys. Soc. Jpn. 2021, 90, 075002. [Google Scholar] [CrossRef]
- Corradino, C.; Bucolo, M. Automatic preprocessing of EEG signals in long time scale. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; pp. 4110–4113. [Google Scholar]
- Fortuna, L.; Bucolo, M.; Frasca, M.; La Rosa, M.; Shannahoff-Khalsa, D.S.; Schult, R.L.; Wright, J.A. Independent component analysis of magnetoencephalography data. In Proceedings of the 23rd Annual International Conference of the IEEE, Istanbul, Turkey, 25–28 October 2001; pp. 1981–1984. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).