Tryptophan Hydroxylase-2-Mediated Serotonin Biosynthesis Suppresses Cell Reprogramming into Pluripotent State
Abstract
1. Introduction
2. Results
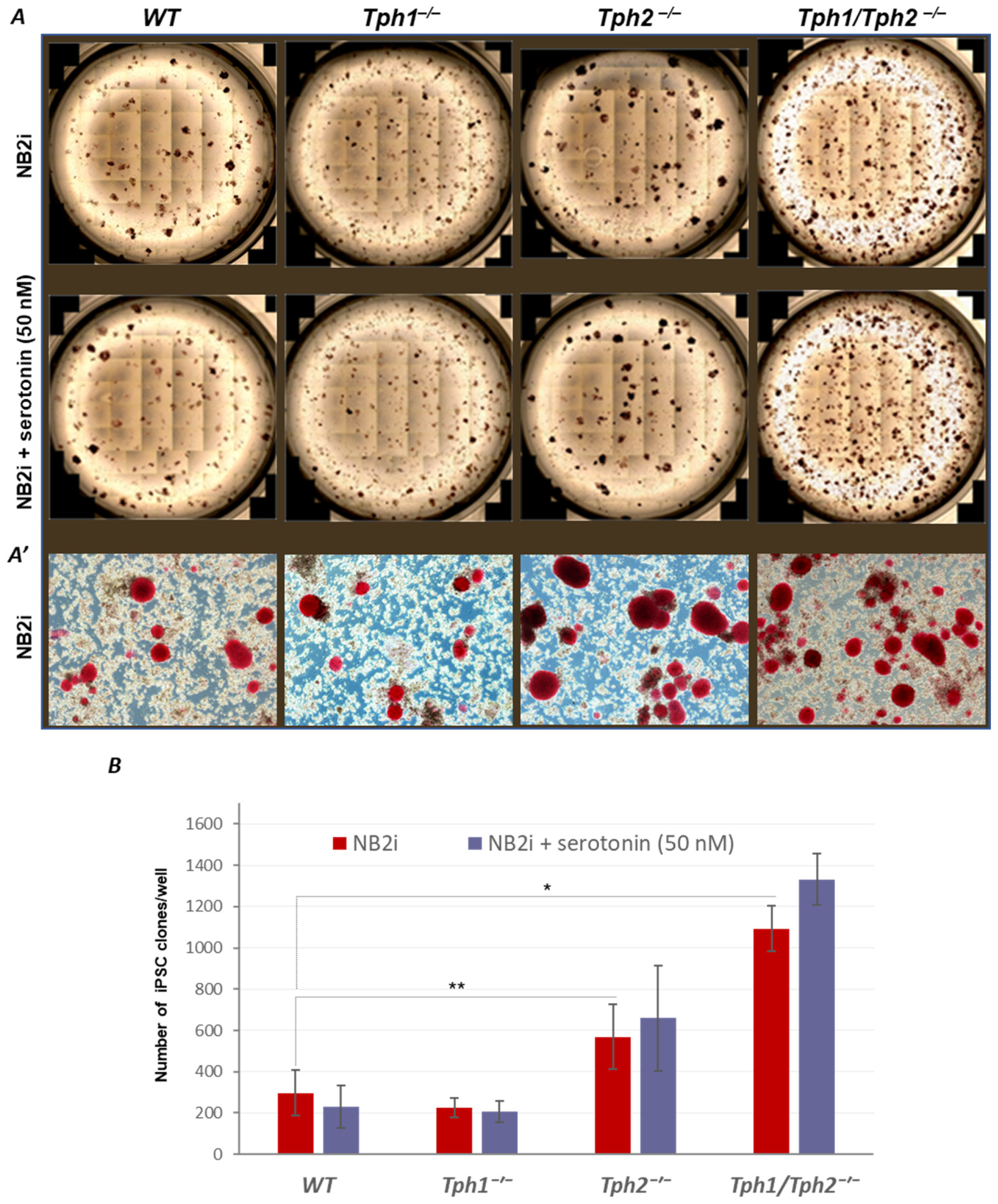
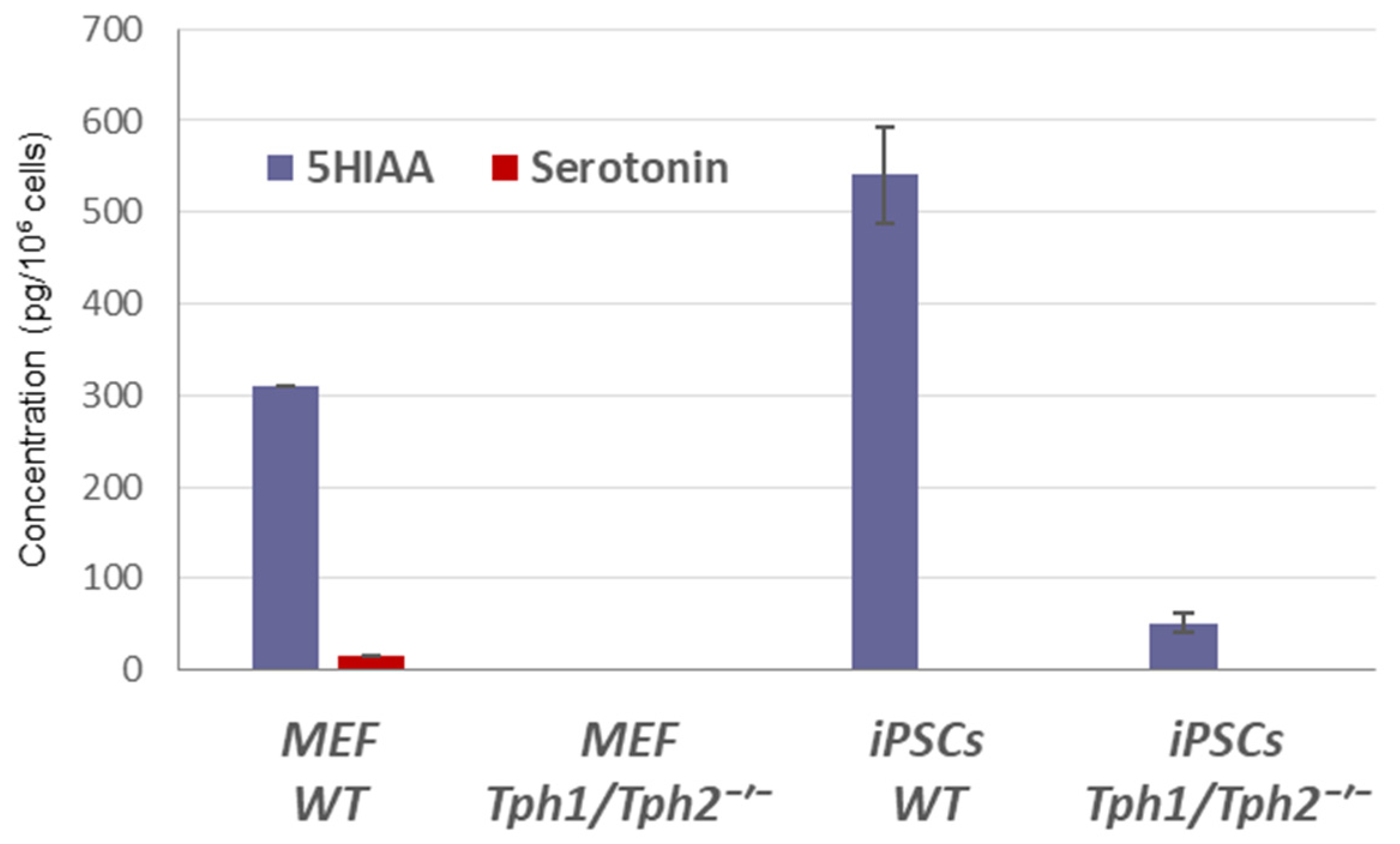
2.1. Loss of Serotonin Synthesis Improves the Efficiency of iPSC Generation
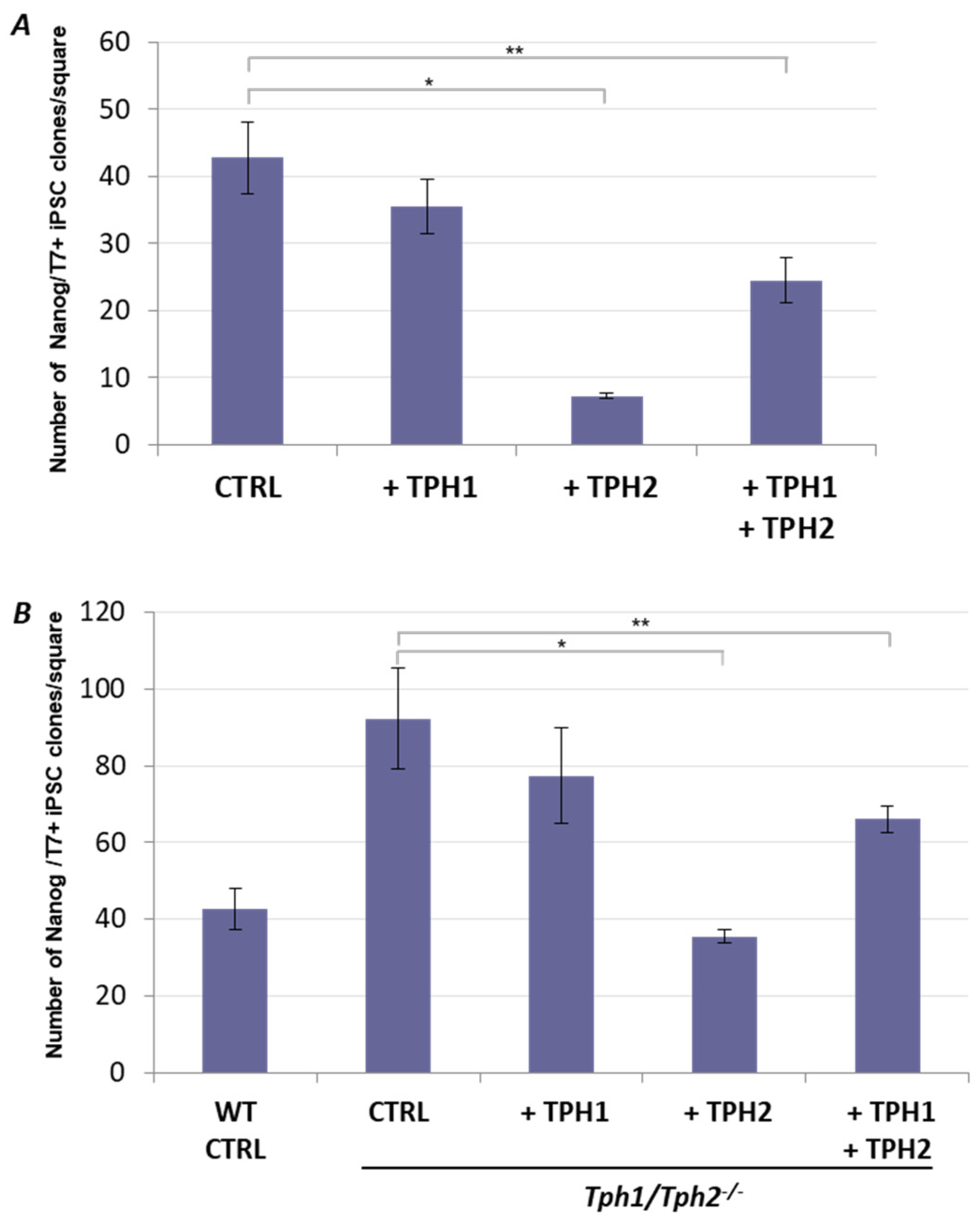
2.2. Ectopic Tph2 Expression Suppresses iPSCs Generation and Restores the Rate of Reprogramming of Tph1/Tph2−/− MEFs to the WT Level
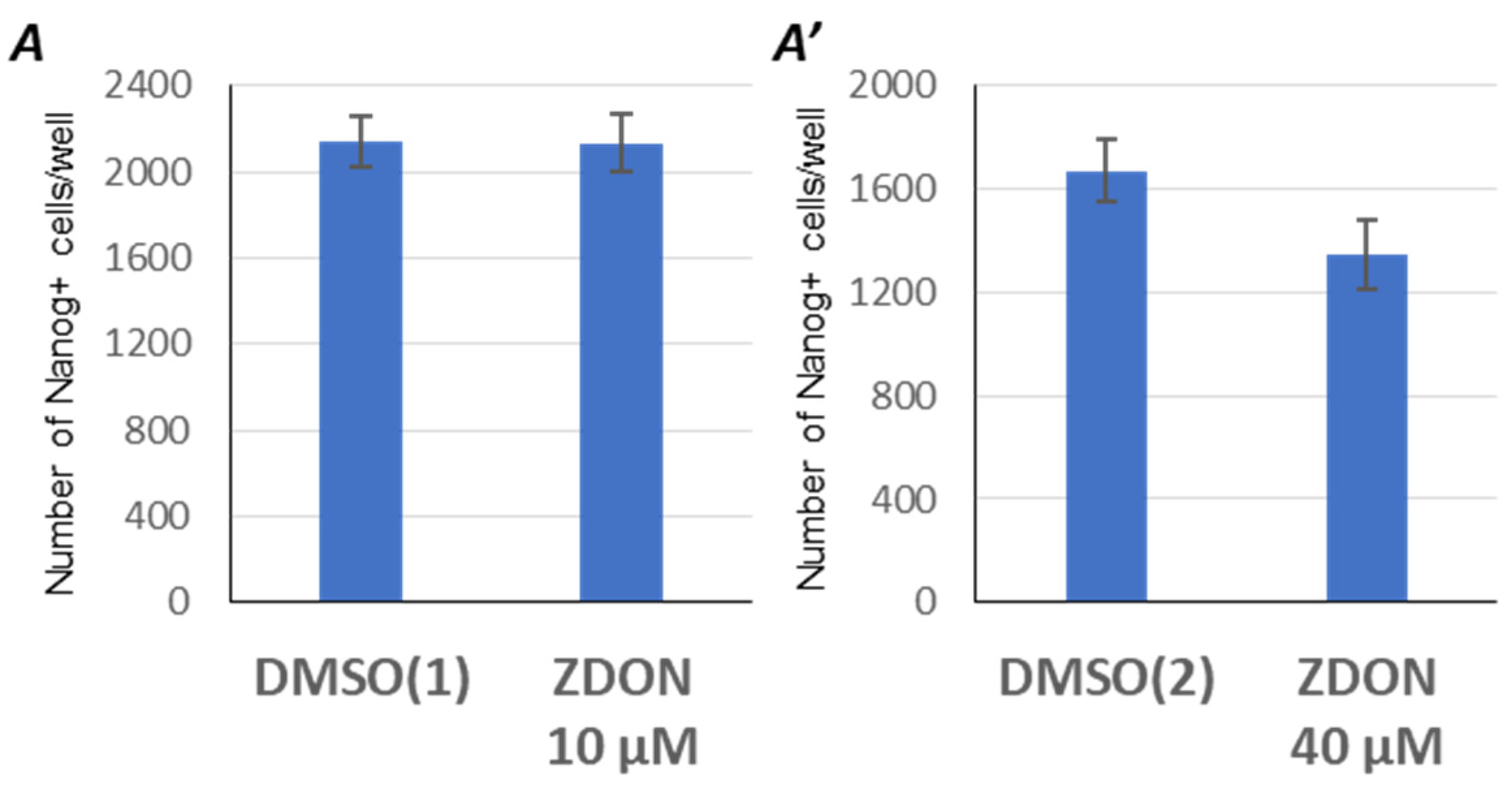
2.3. Suppression of Serotonylation by ZDON Does Not Enhance the Reprogramming Efficiency
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cells
4.3. Lentiviral Vectors
4.4. Lentivirus Packaging
4.5. Reprogramming MEFs into iPSCs
4.6. TPH Overexpression and Rescue Experiments during iPSC Generation
4.7. Immunostaining
4.8. Alkaline Phosphatase Staining
4.9. Immunoblotting
4.10. Teratoma Formation and Histological Analysis
4.11. HPLC
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hortnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Walther, D.J.; Bader, M. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 2003, 66, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, L.; Waider, J.; Kraft, S.; Kriegebaum, C.; Holtmann, B.; Reif, A.; Schmitt, A.; Lesch, K.P. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J. Neural Transm. 2008, 115, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Neal, K.B.; Parry, L.J.; Bornstein, J.C. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J. Physiol. 2009, 587, 567–586. [Google Scholar] [CrossRef] [PubMed]
- El-Merahbi, R.; Loffler, M.; Mayer, A.; Sumara, G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015, 589, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Filip, M.; Bader, M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol. Rep. PR 2009, 61, 761–777. [Google Scholar] [CrossRef]
- Filip, M.; Alenina, N.; Bader, M.; Przegalinski, E. Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict. Biol. 2010, 15, 227–249. [Google Scholar] [CrossRef]
- Eddahibi, S.; Hanoun, N.; Lanfumey, L.; Lesch, K.P.; Raffestin, B.; Hamon, M.; Adnot, S. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J. Clin. Investig. 2000, 105, 1555–1562. [Google Scholar] [CrossRef]
- Bader, M. Serotonylation: Serotonin Signaling and Epigenetics. Front. Mol. Neurosci. 2019, 12, 288. [Google Scholar] [CrossRef]
- Paulmann, N.; Grohmann, M.; Voigt, J.P.; Bert, B.; Vowinckel, J.; Bader, M.; Skelin, M.; Jevsek, M.; Fink, H.; Rupnik, M.; et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009, 7, e1000229. [Google Scholar] [CrossRef]
- Walther, D.J.; Peter, J.U.; Winter, S.; Holtje, M.; Paulmann, N.; Grohmann, M.; Vowinckel, J.; Alamo-Bethencourt, V.; Wilhelm, C.S.; Ahnert-Hilger, G.; et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 2003, 115, 851–862. [Google Scholar] [CrossRef]
- Farrelly, L.A.; Thompson, R.E.; Zhao, S.; Lepack, A.E.; Lyu, Y.; Bhanu, N.V.; Zhang, B.; Loh, Y.E.; Ramakrishnan, A.; Vadodaria, K.C.; et al. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature 2019, 567, 535–539. [Google Scholar] [CrossRef]
- Alenina, N.; Kikic, D.; Todiras, M.; Mosienko, V.; Qadri, F.; Plehm, R.; Boye, P.; Vilianovitch, L.; Sohr, R.; Tenner, K.; et al. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc. Natl. Acad. Sci. USA 2009, 106, 10332–10337. [Google Scholar] [CrossRef]
- Mosienko, V.; Bert, B.; Beis, D.; Matthes, S.; Fink, H.; Bader, M.; Alenina, N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl. Psychiatry 2012, 2, e122. [Google Scholar] [CrossRef]
- Klempin, F.; Beis, D.; Mosienko, V.; Kempermann, G.; Bader, M.; Alenina, N. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 8270–8275. [Google Scholar] [CrossRef]
- Mosienko, V.; Matthes, S.; Hirth, N.; Beis, D.; Flinders, M.; Bader, M.; Hansson, A.C.; Alenina, N. Adaptive changes in serotonin metabolism preserve normal behavior in mice with reduced TPH2 activity. Neuropharmacology 2014, 85, 73–80. [Google Scholar] [CrossRef]
- Song, N.N.; Jia, Y.F.; Zhang, L.; Zhang, Q.; Huang, Y.; Liu, X.Z.; Hu, L.; Lan, W.; Chen, L.; Lesch, K.P.; et al. Reducing central serotonin in adulthood promotes hippocampal neurogenesis. Sci. Rep. 2016, 6, 20338. [Google Scholar] [CrossRef]
- Song, N.N.; Huang, Y.; Yu, X.; Lang, B.; Ding, Y.Q.; Zhang, L. Divergent Roles of Central Serotonin in Adult Hippocampal Neurogenesis. Front. Cell Neurosci. 2017, 11, 185. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Lambert, H.W.; Lauder, J.M. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001, 305, 177–186. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Peterson, R.E.; Nikitina, L.A.; Bezuglov, V.V.; Lauder, J.M. The pre-nervous serotonergic system of developing sea urchin embryos and larvae: Pharmacologic and immunocytochemical evidence. Neurochem. Res. 2005, 30, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Bader, M. Serotonin synthesis in murine embryonic stem cells. Brain Res. Mol. Brain Res. 1999, 68, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Amireault, P.; Dube, F. Serotonin and its antidepressant-sensitive transport in mouse cumulus-oocyte complexes and early embryos. Biol. Reprod. 2005, 73, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Basu, B.; Desai, R.; Balaji, J.; Chaerkady, R.; Sriram, V.; Maiti, S.; Panicker, M.M. Serotonin in pre-implantation mouse embryos is localized to the mitochondria and can modulate mitochondrial potential. Reproduction 2008, 135, 657–669. [Google Scholar] [CrossRef]
- Amireault, P.; Dube, F. Intracellular cAMP and calcium signaling by serotonin in mouse cumulus-oocyte complexes. Mol. Pharmacol. 2005, 68, 1678–1687. [Google Scholar] [CrossRef]
- Beyer, T.; Danilchik, M.; Thumberger, T.; Vick, P.; Tisler, M.; Schneider, I.; Bogusch, S.; Andre, P.; Ulmer, B.; Walentek, P.; et al. Serotonin signaling is required for Wnt-dependent GRP specification and leftward flow in Xenopus. Curr. Biol. CB 2012, 22, 33–39. [Google Scholar] [CrossRef]
- Bhasin, N.; Kernick, E.; Luo, X.; Seidel, H.E.; Weiss, E.R.; Lauder, J.M. Differential regulation of chondrogenic differentiation by the serotonin2B receptor and retinoic acid in the embryonic mouse hindlimb. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2004, 230, 201–209. [Google Scholar] [CrossRef]
- Choi, D.S.; Ward, S.J.; Messaddeq, N.; Launay, J.M.; Maroteaux, L. 5-HT2B receptor-mediated serotonin morphogenetic functions in mouse cranial neural crest and myocardiac cells. Development 1997, 124, 1745–1755. [Google Scholar] [CrossRef]
- Fukumoto, T.; Kema, I.P.; Levin, M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr. Biol. CB 2005, 15, 794–803. [Google Scholar] [CrossRef]
- Kawakami, M.; Umeda, M.; Nakagata, N.; Takeo, T.; Yamamura, K. Novel migrating mouse neural crest cell assay system utilizing P0-Cre/EGFP fluorescent time-lapse imaging. BMC Dev. Biol. 2011, 11, 68. [Google Scholar] [CrossRef]
- Moiseiwitsch, J.R. The role of serotonin and neurotransmitters during craniofacial development. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2000, 11, 230–239. [Google Scholar] [CrossRef]
- Dube, F.; Amireault, P. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life Sci. 2007, 81, 1627–1637. [Google Scholar] [CrossRef]
- Bashammakh, S.; Würtele, M.; Kotnik, K.; Abdelilah-Seyfried, S.; Bader, M. Serotonin is required for pharyngeal arch morphogenesis in zebrafish. Sci. Res. 2014, 10, 1–9. [Google Scholar]
- Reisoli, E.; De Lucchini, S.; Nardi, I.; Ori, M. Serotonin 2B receptor signaling is required for craniofacial morphogenesis and jaw joint formation in Xenopus. Development 2010, 137, 2927–2937. [Google Scholar] [CrossRef]
- Narboux-Neme, N.; Angenard, G.; Mosienko, V.; Klempin, F.; Pitychoutis, P.M.; Deneris, E.; Bader, M.; Giros, B.; Alenina, N.; Gaspar, P. Postnatal growth defects in mice with constitutive depletion of central serotonin. ACS Chem. Neurosci. 2013, 4, 171–181. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Ponomartsev, S.V.; Tomilin, A.N. Pluripotent stem cell-based gene therapy approach: Human de novo synthesized chromosomes. Cell. Mol. Life Sci. CMLS 2021, 78, 1207–1220. [Google Scholar] [CrossRef]
- Hou, P.; Li, Y.; Zhang, X.; Liu, C.; Guan, J.; Li, H.; Zhao, T.; Ye, J.; Yang, W.; Liu, K.; et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 2013, 341, 651–654. [Google Scholar] [CrossRef]
- Mordhorst, A.; Dhandapani, P.; Matthes, S.; Mosienko, V.; Rothe, M.; Todiras, M.; Self, J.; Schunck, W.H.; Schutz, A.; Bader, M.; et al. Phenylalanine hydroxylase contributes to serotonin synthesis in mice. FASEB J. 2021, 35, e21648. [Google Scholar] [CrossRef]
- Bader, M. Inhibition of serotonin synthesis: A novel therapeutic paradigm. Pharmacol. Ther. 2020, 205, 107423. [Google Scholar] [CrossRef] [PubMed]
- Mosienko, V.; Beis, D.; Pasqualetti, M.; Waider, J.; Matthes, S.; Qadri, F.; Bader, M.; Alenina, N. Life without brain serotonin: Reevaluation of serotonin function with mice deficient in brain serotonin synthesis. Behav. Brain Res. 2015, 277, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, K.; Biri, B.; Schlosser, G.; Kiss, B.; Nyitray, L.; Fesus, L.; Kiraly, R. Real-time kinetic method to monitor isopeptidase activity of transglutaminase 2 on protein substrate. Anal. Biochem. 2016, 505, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Romero-Reyes, J.; Molina-Hernandez, A.; Diaz, N.F.; Camacho-Arroyo, I. Role of serotonin in vertebrate embryo development. Reprod Biol. 2021, 21, 100475. [Google Scholar] [CrossRef] [PubMed]
- Cote, F.; Fligny, C.; Bayard, E.; Launay, J.M.; Gershon, M.D.; Mallet, J.; Vodjdani, G. Maternal serotonin is crucial for murine embryonic development. Proc. Natl. Acad. Sci. USA 2007, 104, 329–334. [Google Scholar] [CrossRef]
- Coleman, C.M.; Neckameyer, W.S. Serotonin synthesis by two distinct enzymes in Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2005, 59, 12–31. [Google Scholar] [CrossRef]
- Qi, Y.X.; Huang, J.; Li, M.Q.; Wu, Y.S.; Xia, R.Y.; Ye, G.Y. Serotonin modulates insect hemocyte phagocytosis via two different serotonin receptors. Elife 2016, 5, e12241. [Google Scholar] [CrossRef]
- Fitzgerald, L.W.; Kaplinsky, L.; Kimelberg, H.K. Serotonin metabolism by monoamine oxidase in rat primary astrocyte cultures. J. Neurochem. 1990, 55, 2008–2014. [Google Scholar] [CrossRef]
- Visser, A.K.; van Waarde, A.; Willemsen, A.T.; Bosker, F.J.; Luiten, P.G.; den Boer, J.A.; Kema, I.P.; Dierckx, R.A. Measuring serotonin synthesis: From conventional methods to PET tracers and their (pre)clinical implications. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 576–591. [Google Scholar] [CrossRef]
- Lee, G.S.; Simpson, C.; Sun, B.H.; Yao, C.; Foer, D.; Sullivan, B.; Matthes, S.; Alenina, N.; Belsky, J.; Bader, M.; et al. Measurement of plasma, serum, and platelet serotonin in individuals with high bone mass and mutations in LRP5. J. Bone Miner Res. 2014, 29, 976–981. [Google Scholar] [CrossRef]
- Berendzen, K.M.; Durieux, J.; Shao, L.W.; Tian, Y.; Kim, H.E.; Wolff, S.; Liu, Y.; Dillin, A. Neuroendocrine Coordination of Mitochondrial Stress Signaling and Proteostasis. Cell 2016, 166, 1553–1563.e10. [Google Scholar] [CrossRef]
- Fanibunda, S.E.; Deb, S.; Maniyadath, B.; Tiwari, P.; Ghai, U.; Gupta, S.; Figueiredo, D.; Weisstaub, N.; Gingrich, J.A.; Vaidya, A.D.B.; et al. Serotonin regulates mitochondrial biogenesis and function in rodent cortical neurons via the 5-HT2A receptor and SIRT1-PGC-1alpha axis. Proc. Natl. Acad. Sci. USA 2019, 116, 11028–11037. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Lee, J.; Lee, J.Y.; Kim, H.; Lee, S.; Oh, C.M. A Systems Biology Approach to Investigating the Interaction between Serotonin Synthesis by Tryptophan Hydroxylase and the Metabolic Homeostasis. Int. J. Mol. Sci. 2021, 22, 2452. [Google Scholar] [CrossRef]
- Genet, N.; Billaud, M.; Rossignol, R.; Dubois, M.; Gillibert-Duplantier, J.; Isakson, B.E.; Marthan, R.; Savineau, J.P.; Guibert, C. Signaling Pathways Linked to Serotonin-Induced Superoxide Anion Production: A Physiological Role for Mitochondria in Pulmonary Arteries. Front. Physiol. 2017, 8, 76. [Google Scholar] [CrossRef]
- Sola-Penna, M.; Paixao, L.P.; Branco, J.R.; Ochioni, A.C.; Albanese, J.M.; Mundim, D.M.; Baptista-de-Souza, D.; Figueiredo, C.P.; Coelho, W.S.; Marcondes, M.C.; et al. Serotonin activates glycolysis and mitochondria biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively. Br. J. Cancer 2020, 122, 194–208. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef]
- Tsogtbaatar, E.; Landin, C.; Minter-Dykhouse, K.; Folmes, C.D.L. Energy Metabolism Regulates Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 87. [Google Scholar] [CrossRef]
- Skvortsova, E.V.; Nazarov, I.B.; Tomilin, A.N.; Sinenko, S.A. Dual Mode of Mitochondrial ROS Action during Reprogramming to Pluripotency. Int. J. Mol. Sci. 2022, 23, 10924. [Google Scholar] [CrossRef]
- Kida, Y.S.; Kawamura, T.; Wei, Z.; Sogo, T.; Jacinto, S.; Shigeno, A.; Kushige, H.; Yoshihara, E.; Liddle, C.; Ecker, J.R.; et al. ERRs Mediate a Metabolic Switch Required for Somatic Cell Reprogramming to Pluripotency. Cell Stem Cell 2015, 16, 547–555. [Google Scholar] [CrossRef]
- Someya, S.; Tohyama, S.; Kameda, K.; Tanosaki, S.; Morita, Y.; Sasaki, K.; Kang, M.I.; Kishino, Y.; Okada, M.; Tani, H.; et al. Tryptophan Metabolism Regulates Proliferative Capacity of Human Pluripotent Stem Cells. iScience 2021, 24, 102090. [Google Scholar] [CrossRef]
- Romano, M.; Elgueta, R.; McCluskey, D.; Ortega-Prieto, A.M.; Stolarczyk, E.; Dazzi, F.; Lucendo-Villarin, B.; Meseguer-Ripolles, J.; Williams, J.; Fanelli, G.; et al. Pluripotent Stem Cell-Derived Hepatocytes Inhibit T Cell Proliferation In Vitro through Tryptophan Starvation. Cells 2021, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hatabayashi, K.; Arita, M.; Yajima, N.; Takenaka, C.; Suzuki, T.; Takahashi, M.; Oshima, Y.; Hara, K.; Kagawa, K.; et al. Kynurenine signaling through the aryl hydrocarbon receptor maintains the undifferentiated state of human embryonic stem cells. Sci. Signal. 2019, 12, eaaw3306. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Guo, Y.; Li, C.; Li, S.; Wan, X. Small Molecules that Promote Self-Renewal of Stem Cells and Somatic Cell Reprogramming. Stem Cell Rev. Rep. 2020, 16, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shen, L.; Yu, J.; Wan, H.; Guo, A.; Chen, J.; Long, Y.; Zhao, J.; Pei, G. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell 2011, 10, 908–911. [Google Scholar] [CrossRef]
- Zhu, S.; Li, W.; Zhou, H.; Wei, W.; Ambasudhan, R.; Lin, T.; Kim, J.; Zhang, K.; Ding, S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 2010, 7, 651–655. [Google Scholar] [CrossRef]
- Bakhmet, E.I.; Nazarov, I.B.; Gazizova, A.R.; Vorobyeva, N.E.; Kuzmin, A.A.; Gordeev, M.N.; Sinenko, S.A.; Aksenov, N.D.; Artamonova, T.O.; Khodorkovskii, M.A.; et al. hnRNP-K Targets Open Chromatin in Mouse Embryonic Stem Cells in Concert with Multiple Regulators. Stem Cells 2019, 37, 1018–1029. [Google Scholar] [CrossRef]
- Sommer, C.A.; Stadtfeld, M.; Murphy, G.J.; Hochedlinger, K.; Kotton, D.N.; Mostoslavsky, G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells 2009, 27, 543–549. [Google Scholar] [CrossRef]
- Malashicheva, A.; Kanzler, B.; Tolkunova, E.; Trono, D.; Tomilin, A. Lentivirus as a tool for lineage-specific gene manipulations. Genesis 2007, 45, 456–459. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Skvortsova, E.V.; Liskovykh, M.A.; Ponomartsev, S.V.; Kuzmin, A.A.; Khudiakov, A.A.; Malashicheva, A.B.; Alenina, N.; Larionov, V.; Kouprina, N.; et al. Transfer of Synthetic Human Chromosome into Human Induced Pluripotent Stem Cells for Biomedical Applications. Cells 2018, 7, 261. [Google Scholar] [CrossRef]
- Skvortsova, E.V.; Sinenko, S.A.; Tomilin, A. Immortalized murine fibroblast cell lines are refractory to reprogramming to pluripotent state. Oncotarget 2018, 9, 35241–35250. [Google Scholar] [CrossRef]
- Kuzmin, A.A.; Ermakova, V.V.; Sinenko, S.A.; Ponomartsev, S.V.; Starkova, T.Y.; Skvortsova, E.V.; Cherepanova, O.; Tomilin, A.N. Genetic tool for fate mapping of Oct4 (Pou5f1)-expressing cells and their progeny past the pluripotency stage. Stem Cell Res. Ther. 2019, 10, 391. [Google Scholar] [CrossRef]
- Ponomartsev, S.V.; Sinenko, S.A.; Skvortsova, E.V.; Liskovykh, M.A.; Voropaev, I.N.; Savina, M.M.; Kuzmin, A.A.; Kuzmina, E.Y.; Kondrashkina, A.M.; Larionov, V.; et al. Human Alphoid(tetO) Artificial Chromosome as a Gene Therapy Vector for the Developing Hemophilia A Model in Mice. Cells 2020, 9, 879. [Google Scholar] [CrossRef]
- Yang, C.S.; Sinenko, S.A.; Thomenius, M.J.; Robeson, A.C.; Freel, C.D.; Horn, S.R.; Kornbluth, S. The deubiquitinating enzyme DUBAI stabilizes DIAP1 to suppress Drosophila apoptosis. Cell Death Differ. 2014, 21, 604–611. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinenko, S.A.; Kuzmin, A.A.; Skvortsova, E.V.; Ponomartsev, S.V.; Efimova, E.V.; Bader, M.; Alenina, N.; Tomilin, A.N. Tryptophan Hydroxylase-2-Mediated Serotonin Biosynthesis Suppresses Cell Reprogramming into Pluripotent State. Int. J. Mol. Sci. 2023, 24, 4862. https://doi.org/10.3390/ijms24054862
Sinenko SA, Kuzmin AA, Skvortsova EV, Ponomartsev SV, Efimova EV, Bader M, Alenina N, Tomilin AN. Tryptophan Hydroxylase-2-Mediated Serotonin Biosynthesis Suppresses Cell Reprogramming into Pluripotent State. International Journal of Molecular Sciences. 2023; 24(5):4862. https://doi.org/10.3390/ijms24054862
Chicago/Turabian StyleSinenko, Sergey A., Andrey A. Kuzmin, Elena V. Skvortsova, Sergey V. Ponomartsev, Evgeniya V. Efimova, Michael Bader, Natalia Alenina, and Alexey N. Tomilin. 2023. "Tryptophan Hydroxylase-2-Mediated Serotonin Biosynthesis Suppresses Cell Reprogramming into Pluripotent State" International Journal of Molecular Sciences 24, no. 5: 4862. https://doi.org/10.3390/ijms24054862
APA StyleSinenko, S. A., Kuzmin, A. A., Skvortsova, E. V., Ponomartsev, S. V., Efimova, E. V., Bader, M., Alenina, N., & Tomilin, A. N. (2023). Tryptophan Hydroxylase-2-Mediated Serotonin Biosynthesis Suppresses Cell Reprogramming into Pluripotent State. International Journal of Molecular Sciences, 24(5), 4862. https://doi.org/10.3390/ijms24054862





